Abstract
Autosomal recessive (AR) complete Interferon-γ Receptor1 (IFN-γR1) deficiency is a rare variant of Mendelian susceptibility to mycobacterial disease (MSMD). Whilst hematopoietic stem cell transplantation (HSCT) remains the only curative treatment, outcomes are heterogeneous; delayed engraftment and/or graft rejection being commonly observed. This case report and literature review expands the knowledge about this rare but potentially fatal pathology, providing details regarding diagnosis, antimicrobial treatment, transplant performance and outcome that may help to guide physicians caring for patients with AR complete IFN-γR1 or IFN-γR2 deficiency.
Keywords: Primary immunodeficiency, atypical mycobacteria, interferon gamma receptor, transplantation, infant
Introduction
Autosomal recessive (AR) complete Interferon-γ Receptor1 (IFN-γR1) deficiency is a rare variant (8%) of Mendelian susceptibility to mycobacterial disease (MSMD) [1,2]. To date 32 patients with 26 different mutations have been reported [3,4]. AR complete IFN-γR1 deficiency is characterized by a lack of cytokine production such as Interleukin-12 (IL-12) or tumor necrosis factor α (TNF-α) in response to IFN-γ activation in vitro and high levels of circulating IFN-γ [3,5]. Clinical penetrance is complete with disseminated infections in early childhood, most commonly caused by BCG [3] or atypical mycobacteria including M. avium complex, M. abscessus M. chelonae, M. fortuitum, M. mageritense, M. peregrinum, M. smegmatis and M. scrofulaceum [3,5,6]. Other pathogens, such as Listeria monocytogenes, Salmonella spp and Toxoplasma spp as well as viral pathogens have also been described to cause disease in these patients [3]. Complete IFN-γR1 deficiency is fatal within the first two decades of life despite antibiotic treatment [3,5,7]. Hematopoietic stem cell transplantation (HSCT) remains the only curative treatment option; however, outcomes are heterogeneous [8–12]. Delayed engraftment and/or graft rejection are common observations [9]. The presence of high IFN-γ plasma levels of IFN-γR1 deficient patients might explain these potentially severe complications, as IFN-γ has been shown to have anti-hematopoietic properties in vivo [13,14]. We describe the clinical presentation, diagnosis, and successful transplant of a child with AR complete IFN-γR1 deficiency and review the current experience of this rare disorder. The informed consent of the legal tutors of our patient was obtained.
Case Report
A 4-year-old male born to non-consanguineous parents was admitted with generalized lymphadenopathy, rash and night sweats. Family history was unremarkable; personal medical history included a Salmonella gastroenteritis aged 12 months, requiring intravenous antibiotics. He had not received BCG vaccine.
A full blood count revealed neutrophilia (45,000/mm3) and raised C-reactive protein (CRP, 308 mg/l). Repeated blood cultures and an infectious disease screening including Mantoux tuberculin skin test, Brucella, Rickettsia, Bartonella, Coxiella, Leishmania, Cytomegalovirus, Epstein-Barr virus, viral hepatitis panel and HIV serology were negative. Immunological studies showed raised serum immunoglobulin (IgG 1,890mg/dl, IgA 227mg/dl, IgM 260mg/dl), whilst lymphocyte subsets, T cell response to mitogens and neutrophil respiratory burst test using dihydrorhodamine flow cytometry were normal for his age. Due to persistent fever and respiratory distress despite intravenous antibiotic therapy, bone marrow aspirates, lymph node and lung biopsies were performed, showing non-specific reactive changes, without signs of malignancy, hemophagocytosis or infectious agents despite prolonged culturing, acid-fast bacilli testing and molecular biology methods. Broad-spectrum antibiotics including Trimethoprim-Sulfamethoxazole (TMP-SMX), liposomal Amphotericin B, and Imipenem resulted in disappearance of fever and respiratory symptoms and subsequent hospital discharge.
Three months later he represented with fever, generalized lymphadenopathies, hepato-splenomegaly, disseminated rash and a marked edema of the right lower extremity (Figure 1A–D). Blood tests revealed high inflammatory parameters: neutrophilia: 54,380/mm3, subsequently 120,000/mm3, ESR: 102mm/h, CRP: 107mg/l, IgG: 4,744mg/dl, soluble CD25: 15,241U/ml (normal 158–623). Cultures of a repeated lymph node biopsy grew Mycobacterium fortuitum. Subsequently, treatment was initiated with Azithromycin (10mg/kg/day), TMP-SMX (TMP 10mg/kg/d), and Ciprofloxacin (30mg/kg/d).
Figure 1. Clinical presentation.
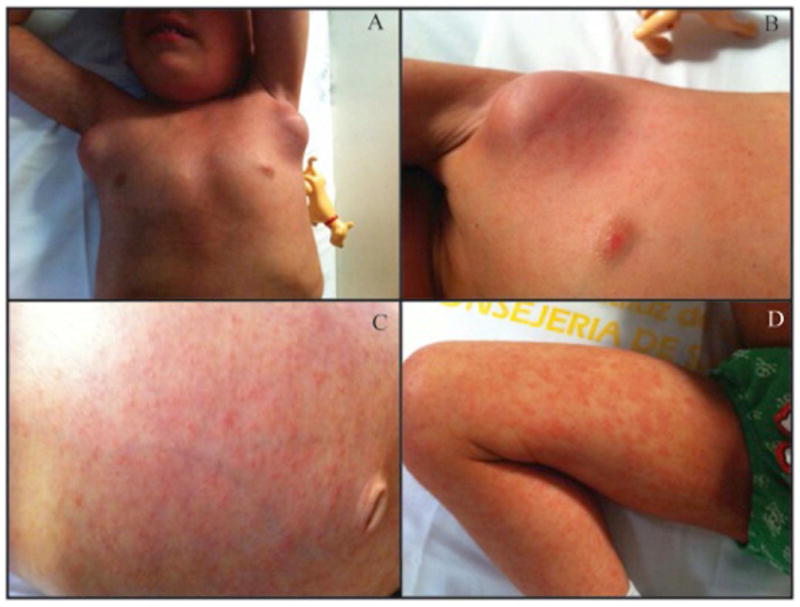
Marked lymphadenopathies (A, B) and generalized macupapular rash affecting torso and lower extremities (C, D).
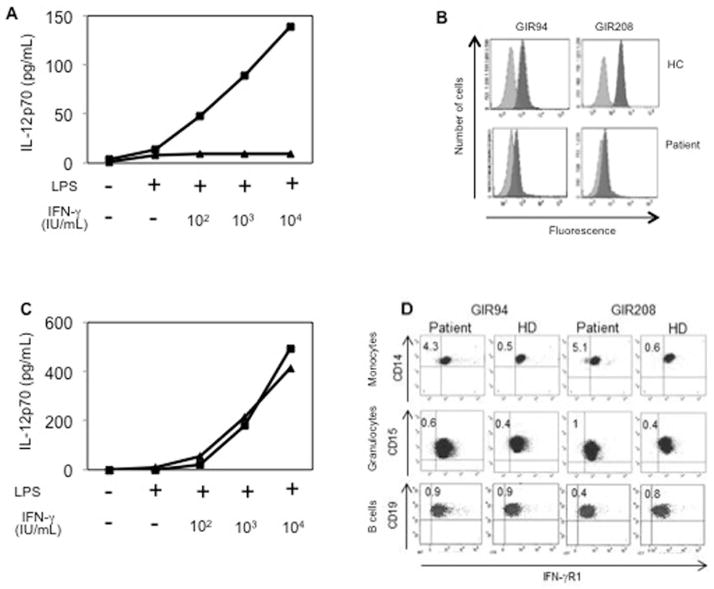
The atypical pathogen identification in context with the clinical picture led to functional studies of the IFN-γ axis showing markedly raised IFN-γ plasma levels (275pg/ml, normal<10) and normal IFN-γ production after whole blood stimulation with phytohaemagglutinin in the presence or absence of IL-12 (data not shown). However, whole blood culture cells showed no response to increasing doses of IFN-γ in vitro, in terms of IL-12p70 production (Figure 2A). Flow cytometry confirmed a marked decrease in the expression of IFN-γR1 in polymorphonuclear granulocytes (Figure 2B), compared with healthy controls suggesting a complete IFN-γR1 deficiency. Sequencing of the IFNGR1 gene identified a known compound heterozygous mutations (c.523delT/c.652del3) confirming the diagnosis of AR complete IFN-γR1 deficiency (supplementary Figure 1) [3]. Complete clinical recovery was achieved within two weeks of triple antibiotic therapy and IFN-γ plasma levels were normal 4 and 2 months pre-HSCT.
Figure 2. Cytokine responses to IFN-γ stimulation and expression of IFN-γR1 prior and after HSCT.
(A) IL-12p70 production in vitro in response to lipopolysaccharide plus various concentrations of IFN-γ before HSCT. The patient is represented by triangles and a healthy control (HC) with squares.
(B) Expression of IFN-γR1 before HSCT. Whole blood from the patient and from one HC was stained with two IFN-γR1-specific mAbs (dark gray; GIR94 and GIR208) and isotypic control antibodies (pale gray).
(C) IL-12p70 production in vitro response to lipopolysaccharide plus various concentrations of IFN-γ after HSCT as described in (A).
(D) Expression of IFN-γR1 after HSCT. Whole blood from the patient and from a HC was stained with two IFN-γR1-specific mAbs as done in (B). CD14+ monocytes, CD15+ granulocytes and CD19+ B cells were analyzed.
A fully myeloablative conditioning regimen included Cyclophosphamide and Busulfan (BUCY-200). Graft-versus-host-disease (GVHD) prophylaxis consisted of Cyclosporine A (pre dose serum levels 200–300ng/ml) and four doses of Methotrexate (15mg/m2 day+1, 10mg/m2 on days+3, +6 and +11 post HSCT). A non–T-cell-depleted HSCT from a fully matched sibling donor was performed (cell dose 5.3×106/kg CD34+ HSCs). Neutrophil (>1,000cells/mm3) and platelets recovery (>50,000cells/mm3) occurred on day+15; early engraftment studies revealed >98% donor cells on day+17 post HSCT. M. fortuitum triple therapy was maintained during transplant with temporary substitution of TMP-SMX for Amikacin during the aplastic phase. The patient did not suffer from fever or infections during and post HSCT. Donor chimerism was maintained >90% and IFN-γ plasma levels were <10pg/ml at 1, 2, 3 and 4 months post HSCT. In vitro cellular responses to increasing concentrations of IFN-γ were repeatedly measured at 4 and 8 months after HSCT showing similar IL-12p70 induction as his donor (Figure 2C). IFN-γR1 expression 4 months post HSCT was normal in 95% of patient’s CD14+ monocytes and 99% of CD15+ polymorphonuclear granulocytes and equal in donor and recipient CD19+ B cells (Figure 2D). Thirteen months after HSCT the patient is in an excellent clinical shape, with no signs of GVHD and a stable donor chimerism of 97%. GVHD prophylaxis was reduced at six and finally stopped at 10 months after HSCT. Treatment against M. fortuitum was maintained 12 months post HSCT.
Discussion
Mutations conferring a complete defect in IFN-γ response, e.g. AR IFN-γR1 and IFN-γR2 deficiency, lead to potentially fatal mycobacterial infections [1–7]. The detection of IFN-γR1 expression on nucleated cells and the performance of functional tests for analyzing IFN-γ responses facilitate targeted gene sequencing and the prediction of effectiveness of adjuvant therapies such as exogenous IFN-γ. Patients with AR complete IFN-γR1 deficiency require HSCT [5,15]. However, HSCT does not completely correct this pathology as the IFN-γR is also expressed on cells and tissue types not directly derived from lymphoid tissue or bone marrow [16]. Malignancies such as lymphoma, Kaposi sarcoma and a pineal germinoma have been reported [6,17,18]. Tumor surveillance seems reasonable for all patients with IFN-γR deficiency independently of their transplant status.
Our patient was diagnosed in the context of disseminated M. fortuitum infection, which was controlled by combined and long-term antibiotic therapy. Immunological results suggested AR complete IFN-γR1 deficiency allowing directed candidate gene sequencing thus saving time and economic resources.
To date, ten patients underwent HSCTs, all but one from HLA matched family donors [8–12]. Four of these patients died within the first 8 months of transplant, two survived despite autologous reconstitution and in four patients HSCT was curative [8,9,11,12]. Importantly, all these four patients received long-term antibiotic therapy, myeloablative conditioning, as well as non-T-cell-depleted grafts with good nucleated cell counts [8,9,11,12]. Mycobacterial control pre-transplant appears to be fundamental for successful transplant outcomes. M. fortuitum is known to be more susceptible to anti-mycobacterial therapy compared with M. avium complex or M. bovis and this may also have contributed to the favorable outcome in our case [19]. Pre-transplant, normalization of IFN-γ levels is warranted, as raised levels have been associated graft rejections [13,14]. HSCT was only performed in our patient, once IFN-γ levels had normalized using a complete myeloablative-conditioning regimen from a HLA identical brother. GVHD prophylaxis was maintained for 10 and antimycobacterial treatment for 12 months, respectively. This approach resulted in a fast and sustained engraftment without major complications. Follow up evaluations include graft function, conditioning toxicity, and recurrence of mycobacterial infection, and the development of malignancies.
Conclusions
AR complete IFN-γR1 deficiency is associated with high mortality. HSCT is the only curative treatment option and should always be considered. Ideal transplant conditions include control of mycobacterial infection, normalized IFN-γ levels, myeloablative conditioning and a non-T-cell-depleted graft with high number of nucleated cells obtained from an HLA matched sibling.
Supplementary Material
(A) The IFNGR1 genotypes (c.523delT and c.652del3, mutant alleles; wt, wild-type) of all family members are indicated. A black square and an arrow indicate the patient.
Acknowledgments
Acknowledgements and funding sources
National Institute of Allergy and Infectious Diseases grant number 5R01AI089970, the National Center for Research Resources and the National Center for Advancing Sciences of the National Institutes of Health grant number 8UL1TR000043, The Rockefeller University, the St. Giles Foundation, Institut National de la Santé et de la Recherche Médicale (INSERM), Paris Descartes University, Laboratoire d’Excellence Integrative Biology of Emerging Infectious Diseases (ANR-10-LABX-62-IBEID), the French National Research Agency (ANR) under the “Investments for the future” (grand number ANR-10-IAHU-01)
“Fondo de Investigaciones Sanitarias”, Ministerio de Economía y Competitividad (PI10/01718, PI13/1456), from the European Regional Development Fund-European Social Fund-FEDER-FSE
“Ayudas para contratos de formación en Investigación Río Hortega” Instituto de Salud Carlos III, Madrid, Spain (P. Olbrich)
Abbreviations
- AR
Autosomal recessive
- GVHD
Graft-versus-host-disease
- HSCT
Hematopoietic stem cell transplantation
- IFN-γR1
Interferon-γ Receptor1
- TMP-SMX
Trimethoprim-Sulfamethoxazole
Footnotes
Conflict of interest
The authors have no conflict of interest to declare.
References
- 1.Newport MJ, Huxley CM, Huston S, Hawrylowicz CM, Oostra BA, Williamson R, Levin M. A mutation in the interferon-gamma-receptor gene and susceptibility to mycobacterial infection. N Engl J Med. 1996;335:1941–9. doi: 10.1056/NEJM199612263352602. [DOI] [PubMed] [Google Scholar]
- 2.Jouanguy E, Altare F, Lamhamedi S, Revy P, Emile JF, Newport M, Levin M, Blanche S, Seboun E, Fischer A, Casanova JL. Interferon-gamma-receptor deficiency in an infant with fatal bacille Calmette-Guérin infection. N Engl J Med. 1996;335:1956–61. doi: 10.1056/NEJM199612263352604. [DOI] [PubMed] [Google Scholar]
- 3.Bustamante J, Boisson-Dupuis S, Abel L, Casanova JL. Mendelian susceptibility to mycobacterial disease: genetic, immunological, and clinical features of inborn errors of IFN-y immunity. Semin Immunol. 2014;26:454–70. doi: 10.1016/j.smim.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tesi B, Sieni E, Neves C, Romano F, Cetica V, Cordeiro A, Chiang S, Schlums H, Galli L, Avenali S, Tondo A, Canessa C, Henter JI, Nordenskjöld M, Hsu AP, Holland SM, Neves JF, Azzari C, Bryceson YT. Hemophagocytic lymphohistiocytosis in 2 patients with underlying IFN-γ receptor deficiency. J Allergy Clin Immunol. 2015 doi: 10.1016/j.jaci.2014.11.030. [DOI] [PubMed] [Google Scholar]
- 5.Dorman SE, Picard C, Lammas D, Heyne K, van Dissel JT, Baretto R, Rosenzweig SD, Newport M, Levin M, Roesler J, Kumararatne D, Casanova JL, Holland SM. Clinical features of dominant and recessive interferon gamma receptor 1 deficiencies. Lancet. 2004;364:2113–21. doi: 10.1016/S0140-6736(04)17552-1. [DOI] [PubMed] [Google Scholar]
- 6.Bax HI, Freeman AF, Anderson VL, Vesterhus P, Laerum D, Pittaluga S, Wilson WH, Holland SM. B-cell lymphoma in a patient with complete interferon gamma receptor 1 deficiency. J Clin Immunol. 2013;33:1062–6. doi: 10.1007/s10875-013-9907-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noordzij JG, Hartwig NG, Verreck FA, De Bruin-Versteeg S, De Boer T, Van Dissel JT, De Groot R, Ottenhoff TH, Van Dongen JJ. Two patients with complete defects in interferon gamma receptor-dependent signaling. J Clin Immunol. 2007;27:490–96. doi: 10.1007/s10875-007-9097-8. [DOI] [PubMed] [Google Scholar]
- 8.Chantrain CF, Bruwier A, Brichard B, Largent V, Chapgier A, Feinberg J, Casanova JL, Stalens JP, Vermylen C. Successful hematopoietic stem cell transplantation in a child with active disseminated Mycobacterium fortuitum infection and interferon- gamma receptor 1 deficiency. Bone Marrow Transplant. 2006;38:75–6. doi: 10.1038/sj.bmt.1705399. [DOI] [PubMed] [Google Scholar]
- 9.Roesler J, Horwitz ME, Picard C, Bordigoni P, Davies G, Koscielniak E, Levin M, Veys P, Reuter U, Schulz A, Thiede C, Klingebiel T, Fischer A, Holland SM, Casanova JL, Friedrich W. Hematopoietic stem cell transplantation for complete IFN-gamma receptor 1 deficiency: a multi-institutional survey. J Pediatr. 2004;145:806–12. doi: 10.1016/j.jpeds.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 10.Horwitz ME, Uzel G, Linton GF, Miller JA, Brown MR, Malech HL, Holland SM. Persistent Mycobacterium avium infection following nonmyeloablative allogeneic peripheral blood stem cell transplantation for interferon-gamma receptor-1 deficiency. Blood. 2003;102:2692–4. doi: 10.1182/blood-2003-04-1268. [DOI] [PubMed] [Google Scholar]
- 11.Reuter U, Roesler J, Thiede C, Schulz A, Classen CF, Oelschlagel U, Debatin KM, Friedrich W. Correction of complete interferon-gamma receptor 1 deficiency by bone marrow transplantation. Blood. 2002;100:4234–5. doi: 10.1182/blood-2002-02-0433. [DOI] [PubMed] [Google Scholar]
- 12.Moilanen P, Korppi M, Hovi L, Chapgier A, Feinberg J, Kong XF, Boisson-Dupuis S, Arola M, Casanova JL, Saarinen-Pihkala UM. Successful hematopoietic stem cell transplantation from an unrelated donor in a child with interferon gamma receptor deficiency. Pediatr Infect Dis J. 2009;28:658–60. doi: 10.1097/INF.0b013e318195092e. [DOI] [PubMed] [Google Scholar]
- 13.Delisle JS, Gaboury L, Bélanger MP, Tassé E, Yagita H, Perreault C. Graft-versus-host disease causes failure of donor hematopoiesis and lymphopoiesis in interferon-gamma receptor-deficient hosts. Blood. 2008;112:2111–9. doi: 10.1182/blood-2007-12-130534. [DOI] [PubMed] [Google Scholar]
- 14.Rottman M, Soudais C, Vogt G, Renia L, Emile JF, Decaluwe H, Gaillard JL, Casanova JL. IFN-gamma mediates the rejection of haematopoietic stem cells in IFN-gammaR1-deficient hosts. Plos Medicine. 2008;5:E26. doi: 10.1371/journal.pmed.0050026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sologuren I, Boisson-Dupuis S, Pestano J, Vincent QB, Fernández-Pérez L, Chapgier A, Cárdenes M, Feinberg J, García-Laorden MI, Picard C, Santiago E, Kong X, Jannière L, Colino E, Herrera-Ramos E, Francés A, Navarrete C, Blanche S, Faria E, Remiszewski P, Cordeiro A, Freeman A, Holland S, Abarca K, Valerón-Lemaur M, Gonçalo-Marques J, Silveira L, García-Castellano JM, Caminero J, Pérez-Arellano JL, Bustamante J, Abel L, Casanova JL, Rodríguez-Gallego C. Partial recessive IFN-gammaR1 deficiency: genetic, immunological and clinical features of 14 patients from 11 kindreds. Hum Mol Genet. 2011;20:1509–23. doi: 10.1093/hmg/ddr029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valente G, Ozmen L, Novelli F, Geuna M, Palestro G, Forni G, Garotta G. Distribution of interferon-gamma receptor in human tissues. Eur J Immunol. 1992;22:2403–12. doi: 10.1002/eji.1830220933. [DOI] [PubMed] [Google Scholar]
- 17.Camcioglu Y, Picard C, Lacoste V, Dupuis S, Akçakaya N, Cokura H, Kaner G, Demirkesen C, Plancoulaine S, Emile JF, Gessain A, Casanova JL. HHV-8-associated Kaposi sarcoma in a child with IFNgammaR1 deficiency. J Pediatr. 2004;144:519–23. doi: 10.1016/j.jpeds.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 18.Taramasso L, Boisson-Dupuis S, Garrè ML, Bondi E, Cama A, Nozza P, Morana G, Casanova JL, Marazzi MG. Pineal germinoma in a child with interferon-γ receptor 1 deficiency. case report and literature review. J Clin Immunol. 2014;34:922–7. doi: 10.1007/s10875-014-0098-0. [DOI] [PubMed] [Google Scholar]
- 19.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, von Reyn CF, Wallace RJ, Jr, Winthrop K. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases.; ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Disease Society of America. Am J Respir Crit Care Med. 2007;175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) The IFNGR1 genotypes (c.523delT and c.652del3, mutant alleles; wt, wild-type) of all family members are indicated. A black square and an arrow indicate the patient.