Abstract
Background
Access to combination antiretroviral therapy (cART) is expanding in Latin America and the Caribbean (LAC). There is little information in this region regarding incidence of and factors associated with regimen failure and regimen change.
Methods
Antiretroviral-naïve adults starting cART from 2000-2014 at sites in seven countries throughout LAC were included. Cumulative incidence of virologic failure and major regimen change were estimated with death considered a competing event.
Findings
14,027 cART initiators (60% male, median age 37 years, median CD4 156 cells/mm3, median HIV-RNA 5·0 log10 copies/mL, and 28% with clinical AIDS) were followed for a median of 3·9 years. 1,719 patients presented virologic failure and 1,955 had a major regimen change. Excluding GHESKIO-Haiti (which did not regularly measure HIV-RNA), cumulative incidence of virologic failure was 7·8%, 19·2%, and 25·8% at one, three, and five years after cART initiation, respectively; cumulative incidence of major regimen change was 5·9%, 12·7%, and 18·2%. Incidence of major regimen change at GHESKIO-Haiti at five years was 10·7%. Virologic failure was associated with younger age (adjusted hazard ratio[aHR]=2·03 for 20 vs. 40 years; 95% confidence interval[CI] 1·68-2·44), infection through injection-drug use (IDU) (aHR=1·60; 95%CI 1·02-2·52), initiation in earlier calendar years (aHR=1·28 for 2002 vs. 2006; 95%CI 1·13-1·46), and starting with a boosted protease inhibitor (aHR=1·17 vs. non-nucleoside reverse transcriptase inhibitor; 95%CI 1·00-1·64).
Interpretation
Incidence of virologic failure was generally lower than in North America/Europe. Our results suggest the need to design strategies to reduce failure and major regimen change among younger patients and those with a history of IDU.
Funding
US National Institutes of Health: U01 AI069923.
Introduction
Combined antiretroviral therapy (cART) has markedly reduced morbidity and mortality of patients living with HIV/AIDS.1 Improved access to cART has resulted in a sustained increase in the number of people receiving cART in the last ten years reaching 12·9 million people at the end of 2013.2 Among resource-limited settings (RLS), Latin America and the Caribbean (LAC) have the highest rates of cART coverage, achieving more than 70% coverage of those in need in 2012, with 800,000 patients on cART at the end of 20133. National HIV programs in Brazil, Argentina, Colombia and Venezuela were established relatively early in the epidemic, and generally individualized provision of cART. This resulted in high coverage and also a very high number of different first-line regimens, a situation still observed in the region.
In 2013, the World Health Organization (WHO) updated its guidelines to promote earlier treatment initiation and enhanced patient monitoring.4 In LAC, the criteria for cART initiation vary among countries and have changed over time, with some countries now offering early treatment to all HIV-positive adults.5-11 However, the CD4 criterion for treatment initiation was below 350 cells/mm3 during much of 2000s. National clinical guidelines recommend measuring CD4 and HIV-1 viral load (VL) every three to six months in patients starting cART, and then every three to six months once VL suppression has been achieved.7,9-11 The number of VL determinations increased from a median of 1·2 per year in 2010 to 1·8 in 2012 in LAC, but with significant differences between countries.3 In 2011, 77% of adults on cART were receiving first-line regimens and 21% second-line regimens.
There is increasing evidence that the immunologic and virologic responses to treatment in RLS can equal those in high-income settings.12 However, switching to second-line regimens is less common in RLS, probably due to the cost of second-line drugs and lower frequency of VL monitoring.13,14 Therefore, reliable estimates of the incidence of first-line failure and major regimen change among individuals starting cART may help HIV programs to estimate the need for second-line drugs. In addition, the identification of factors associated with cART failure and regimen change may inform the development of preventive interventions aimed at improving durability of the first regimen. In this study, we analyzed data from the largest cohort of HIV-positive patients in LAC to estimate the cumulative incidence of failure and major change of initial cART and to study potentially relevant demographic and clinical factors.
Methods
Study design and Data sources
The Caribbean, Central and South America Network (CCASAnet) includes seven adult HIV clinical sites in seven countries (Argentina, Brazil, Chile, Haiti, Honduras, Mexico and Peru) within the International epidemiologic Databases to Evaluate AIDS (IeDEA).15 Clinical data were collected at each site, de-identified, and sent to the CCASAnet Data Coordinating Center at Vanderbilt University, Nashville, TN, USA (VDCC), for data harmonization and processing. The VDCC checked data for internal consistency and missingness, and the VDCC performed periodic quality assessment of data collection and validation through on-site data audits. When necessary, data were re-abstracted and re-entered.
Sites contributing data to this study were: Centro Médico Huésped, Buenos Aires, Argentina (CMH-Argentina); Instituto de Pesquisa Clinica Evandro Chagas, Fundação Oswaldo Cruz, Rio de Janeiro, Brazil (FC-Brazil); Fundación Arriarán, Santiago, Chile (FA-Chile); Le Groupe Haïtien d?Etude du Sarcome de Kaposi et des Infections Opportunistes, Port-au-Prince, Haiti (GHESKIO-Haiti); Instituto Hondureño de Seguridad Social and Hospital Escuela, Tegucigalpa, Honduras (IHSS/HE-Honduras); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City, Mexico (INCMNSZ-Mexico); and Instituto de Medicina Tropical Alexander von Humboldt, Lima, Perú (IMTAvH-Peru).
Institutional ethics review boards from all sites and Vanderbilt approved the project, waiving the requirement for individual patient informed consent except at IMTAvH-Peru where informed consent was obtained.
Antiretroviral-naïve adults (≥18 years) initiating their first cART on or after January 1, 2000 were included in this study. Database closing varied by site; latest dates of cART initiation were November 2013 (CMH-Argentina), October 2013 (FC-Brazil), December 2013 (FA-Chile), October 2011 (GHESKIO-Haiti), January 2014 (IHSS/HE-Honduras and INCMNSZ-Mexico), and February 2014 (IMTAvH-Peru).
Outcomes
Primary outcomes were time from cART initiation until virologic failure, major regimen modification, and a composite endpoint of the first of virologic failure or major regimen modification. The composite endpoint was used to try to capture the overall need for second line regimens due to either virologic failure and/or major regimen change Virologic failure was defined as one of the following: 1) VL never dropped below 400 copies/mL after 6 months of therapy, 2) VL dropped below 400 copies/mL but then there were two consecutive values >400 copies/mL, 3) VL dropped below 400 copies/mL but then there was a single measurement >1000 copies/mL. The third criterion was included because it reflects clinical practice in much of the region; the sensitivity of results was investigated by removing the third criterion. The cutoff of 400 copies/mL was chosen because it was the VL detection limit for many of the sites over much of the study period. A major regimen modification required starting a „second-line regimen?, defined as a boosted protease inhibitor (PI)-based regimen; if a patient?s first cART regimen included a boosted PI, then a second-line regimen required switching the PI. VL was not available for patients from GHESKIO-Haiti, so they were not included in the virologic failure and composite endpoint analyses.
Clinical stage prior to first cART initiation, or up to 30 days thereafter, was categorized as AIDS or not AIDS; clinical AIDS was defined as CDC stage C, WHO stage IV, or a specification of AIDS at first visit. CD4 at cART initiation was the CD4 count closest to cART initiation but no more than 180 days before or 7 days after. VL at cART initiation was the measurement closest to cART initiation but no more than 180 days before; any VL measurement after cART initiation was not included.
Statistical Analysis
The cumulative incidence after cART initiation (time 0) of the primary outcomes was estimated treating death as a competing event. Patients lost to follow-up, defined as no information available for the patient within the year prior to the database closing date, were censored at their last visit. In secondary virologic failure and composite endpoint analyses, patients with a gap of more than one year between VL measurements were censored at the date of the measurement preceding the gap. One year was chosen to avoid excessive censoring, because national guidelines for most of the sites recommend viral load monitoring every six months for patients on stable cART.
Risk factors for each of the endpoints were assessed using Cox proportional hazards models. All models, both unadjusted and adjusted, were stratified by CCASAnet site (i.e., the underlying hazard function was allowed to vary across sites).16,17 Primary models did not include data from GHESKIO-Haiti. The primary adjusted models included the following variables chosen a priori based on clinical relevance and availability: sex, probable route of infection (heterosexual, men who have sex with men [MSM], injection drug use [IDU], or other), first cART type (non-nucleoside reverse transcriptase inhibitor [NNRTI], PI, or Other), nucleoside reverse transcriptase inhibitor (NRTI) in first cART (zidovudine, stavudine, tenofovir, or Other), calendar year, age, CD4 count, VL, and clinical AIDS at cART initiation. CD4 was square root transformed and VL was log10 transformed. No interactions were included in the models. The adjusted analyses used multiple imputations with five replications to account for missing data; secondary analyses limited to only patients with complete data were performed and are shown in the Supplementary Material. Age, CD4, calendar year, and VL were included in the models using restricted cubic splines with four knots to relax linearity assumptions; the number of knots was pre-specified in an attempt to find a balance between flexibility and over-fitting.17 Non-linearity was examined with likelihood ratio tests. The proportional hazards assumption was examined using correlation with time tests of the scaled Schoenfeld residuals.18 In the imputed models, there was some evidence that the hazards were not proportional particularly according to year of cART initiation (p<0·05), but upon visualization, the violations did not seem extreme, and so for simplicity, Cox models remained unchanged. The Supplementary Material contains estimates based on models that relaxed the proportional hazards assumption by stratifying on year of cART initiation and type of first cART; these results are similar. Secondary analyses of predictors of major regimen modifications that included the site from GHESKIO-Haiti did not include VL and probable route of infection, as these variables were not available. All analyses were performed using R Statistical Software, Version 3.1.1; analysis scripts are available at http://biostat.mc.vanderbilt.edu/ArchivedAnalyses.
Role of the funding source
The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The Principles of Collaboration under which the CCASAnet multi-national collaboration was founded and the regulatory requirements of the different countries' IRBs require the submission and approval of a project concept sheet by the CCASAnet executive committee and the principal investigators at participating sites. All datasets provided by CCASAnet are de-identified according to HIPAA Safe Harbor guidelines. CCASAnet promotes the signing of a Data Use Agreement before HIV clinical data can be released. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
A total of 14,027 cART initiators met inclusion criteria (841 from CMH-Argentina, 1764 from FC-Brazil, 978 from FA-Chile, 6434 from GHESKIO-Haiti, 940 from IHSS/HE-Honduras, 792 from INCMNSZ-Mexico, and 2278 from IMTAvH-Peru). The median follow-up was 3·9 years (interquartile range [IQR] 2·0-6·5).
Table 1 shows characteristics of patients at cART initiation both by site and combined across sites and several site characteristics. More than two-thirds of patients were male at all sites except GHESKIO-Haiti and IHSS/HE-Honduras (44% and 55% respectively). The median age at cART initiation was 37 years. The majority of patients were in advanced stages of disease with a median CD4 of 156 cells/mm3 (IQR 61 to 253), 28% had a previous clinical AIDS diagnosis (ranging from 9% in FC-Brazil to 54% in INCMNSZ-Mexico), and a median VL of 5 log10 copies/mL (IQR 4·4-5·4). For sites performing VL monitoring, the median frequency of measurements was 2·7 per year, ranging from 0·5 in IHSS/HE-Honduras to 3·9 in CMH-Argentina. VL at cART initiation was not available for 24% of patients in IHSS/HE-Honduras, whereas this proportion was less than 5% for the other sites. The vast majority of patients at all sites started an NNRTI-based cART. Zidovudine was part of the nucleoside backbone in 70% of initial regimens (ranging from 26% in INCMNSZ-Mexico to 79% in IHSS/HE-Honduras), stavudine in 9% (ranging from 4% in FC-Brazil to 18% in IHSS/HE-Honduras), and tenofovir in 18% (ranging from 2% in in IHSS/HE-Honduras to 61% in INCMNSZ-Mexico).
Table 1.
Patient and site characteristics at combination antiretroviral (cART) initiation.
| N | CMH-Argentina |
FC-
Brazil |
FA-
Chile |
GHESKIO-Haiti |
IHHS/HE-
Honduras |
INCMNSZ- Mexico | IMTAvH-Peru | Combined | |
|---|---|---|---|---|---|---|---|---|---|
| N=841 | N=1764 | N=978 | N=6434 | N=940 | N=792 | N=2278 | N=14027 | ||
| Sex | 14027 | ||||||||
| Male | 71% ( 599) | 70% ( 1242) | 88% ( 864) | 44% (2833) | 55% ( 521) | 88% ( 697) | 71% (1618) | 60% (8374) | |
| Age at first cART | 14027 | 38 (32-46) | 36 (30-45) | 36 (31-43) | 38 (32-46) | 36 (30-43) | 34 (28-41) | 34 (28-42) | 37 (30-44) |
| Probable route of infection | 14027 | ||||||||
| Heterosexual | 28% ( 237) | 45% ( 793) | 28% ( 269) | 0% ( 0) | 60% ( 568) | 28% ( 221) | 64% (1448) | 25% (3536) | |
| MSM | 15% ( 126) | 37% ( 648) | 72% ( 700) | 0% ( 0) | 7% ( 62) | 67% ( 530) | 36% ( 809) | 20% (2875) | |
| IDU | 2% ( 16) | 1% ( 15) | 0% ( 4) | 0% ( 0) | 0% ( 1) | 1% ( 8) | 0% ( 0) | 0% ( 44) | |
| Other | 1% ( 7) | 1% ( 15) | 0% ( 3) | 0% ( 0) | 0% ( 3) | 1% ( 11) | 1% ( 19) | 0% ( 58) | |
| Unknown | 54% ( 455) | 17% ( 293) | 0% ( 2) | 100% (6434 | 33% ( 306) | 3% ( 22) | 0% ( 2) | 54% (7514) | |
| Clinical AIDS at first cART | 12621 | 11% ( 57) | 9% ( 136) | 45% ( 280) | 24% (1518) | 38% ( 352) | 54% ( 377) | 45% ( 847) | 28% (3567) |
| Missing | 37% (314) | 13% (226) | 37% (359) | 0% (8) | 3% (24) | 12% (97) | 17% (378) | 10% (1406) | |
| Baseline CD4, cells/mm3 | 12357 | 211 (119-281) | 223 (88-324) | 170 (65-232) | 154 (63-240) | 115 (58-195) | 152 (50-273) | 114 (46-240) | 156 (61-253) |
| Missing | 20% (169) | 15%(259) | 32% (313) | 6% (409) | 18% (170) | 19% (148) | 9% (202) | 12% (1670) | |
| Baseline log10 VL, copies/mL | 5069 | 4.6 (4·1-5·2) | 4·8 (4·2-5·3) | 5·0 (4·5-5·5) | -- | 5·0 (4·5-5·0) | 4·9 (4·7-5·1) | 5·2 (4·7-5·5) | 5·0 (4·4-5·4) |
| Missing | 25% (209) | 27% (471) | 32% (309) | 100% (6434) | 85% (797) | 19% (153) | 26% (585) | 64% (8958) | |
| Initial cART | 14027 | ||||||||
| NNRTI | 78% ( 653) | 72% (1274) | 87% ( 849) | 96% ( 6171) | 96% ( 899) | 82% ( 647) | 95% (2158) | 90% (12651) | |
| BOOSTED PI | 16% ( 133) | 24% ( 427) | 5% ( 47) | 3% ( 190) | 2% ( 18) | 16% ( 123) | 5% ( 111) | 7% ( 1049) | |
| 3 NRTI | 3% ( 28) | 0% ( 5) | 1% ( 6) | 1% ( 64) | 0% ( 1) | 1% ( 6) | 0% ( 2) | 1% ( 112) | |
| PI | 3% ( 25) | 3% ( 55) | 7% ( 70) | 0% ( 9) | 2% ( 22) | 2% ( 14) | 0% ( 6) | 1% ( 201) | |
| OTHER | 0% ( 2) | 0% ( 3) | 1% ( 6) | 0% ( 0) | 0% ( 0) | 0% ( 2) | 0% ( 1) | 0% ( 14) | |
| NRTIs in initial cART | 14027 | ||||||||
| ZDV | 73% ( 613) | 56% ( 995) | 77% ( 756) | 74% ( 4755) | 79% ( 745) | 26% ( 206) | 77% (1747) | 70% ( 9817) | |
| d4T | 10% ( 88) | 4% ( 71) | 8% ( 82) | 8% ( 518) | 18% ( 166) | 5% ( 42) | 11% ( 241) | 9% ( 1208) | |
| TDF | 5% ( 41) | 38% ( 665) | 3% ( 31) | 18% ( 1138) | 2% ( 22) | 61% ( 486) | 5% ( 109) | 18% ( 2492) | |
| Other | 12% ( 99) | 2% ( 33) | 11% ( 109) | 0% ( 23) | 1% ( 7) | 7% ( 58) | 8% ( 181) | 4% ( 510) | |
| Years of follow-up | 14207 | 4·8 (2·4-7·5) | 3·1 (1·4-5·7) | 7·9 (4·6-10·3) | 3·8 (2·3-5·9) | 4·7 (1·7-7·6) | 4·2 (1·2-6·7) | 3·2 (1·3-5·8) | 3·9 (2·0-6·5) |
| Number of VL measurements/year | 14207 | 4·1 (2·8-9·2) | 4·0 (2·5-10·2) | 2·5 (1·8-6·5) | 0 (0-0) | 1·0 (0·7-1·7) | 3·3 (2·7-6·0) | 2·5 (2·0-4·4) | 0·8 (0-3·1)* |
N is the number of non-missing values. Categorical variables are reported as percentages (count). Continuous variables are reported as medians (interquartile range). CMH-Argentina: Centro Médico Huésped, Buenos Aires, Argentina; FC- Brazill: Instituto de Pesquisa Clinica Evandro Chagas, Fundação Oswaldo Cruz, Rio de Janeiro, Brazil; FA-Chile: Fundaciόn Arriarán, Santiago, Chile; GHESKIO-Haiti: Le Groupe Haïtien d’Etude du Sarcome de Kaposi et des Infections Opportunistes, Port-au-Prince, Haiti; IHSS/HE-Honduras: Instituto Hondureño de Seguridad Social and Hospital Escuela, Tegucigalpa, Honduras; INCMNSZ-Mexico: Instituto Nacional de Ciencias Médicas y Nutriciόn Salvador Zubirán, Mexico City, Mexico; IMTAvH-Peru: Instituto de Medicina Tropical Alexander von Humboldt, Lima, Perú; MSM: men who have sex with men; IDU: injection drug use; NNRTI: non-nucleoside reverse transcriptase inhibitor; PI: protease inhibitor; NRTI: nucleoside reverse transcriptase inhibitor; ZDV: zidovudine; d4T: stavudine; TDF: tenofovir.
Median (interquartile range) for number of VL measurements/year excluding GHESKIO-Haiti was 2·9 (2·0-6·0).
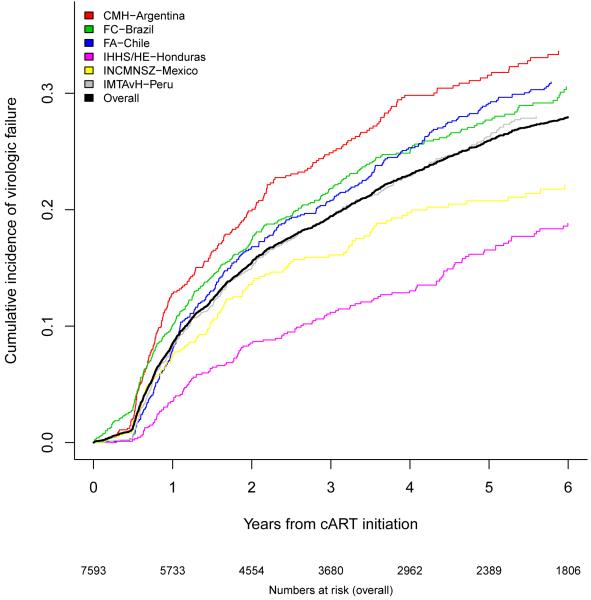
Figure 1A shows the cumulative incidence of virologic failure for the 6 Latin American sites (excluding GHESKIO-Haiti): 7·8% one year after cART initiation (95% confidence interval [CI] 7·2-8·5%), 19·2% three years after cART initiation (95% CI 18·2-20·2%), and 25·8% five years after cART initiation (95% CI 24·6-27·0%). Estimates were highest for CMH-Argentina, and slightly lower for FC-Brazil and FA-Chile. IMTAvH-Peru and INCMNSZ-Mexico were lower, and patients in IHSS/HE-Honduras were least likely to present virologic failure.
Figure 1A.
Cumulative incidence of virologic failure by site.
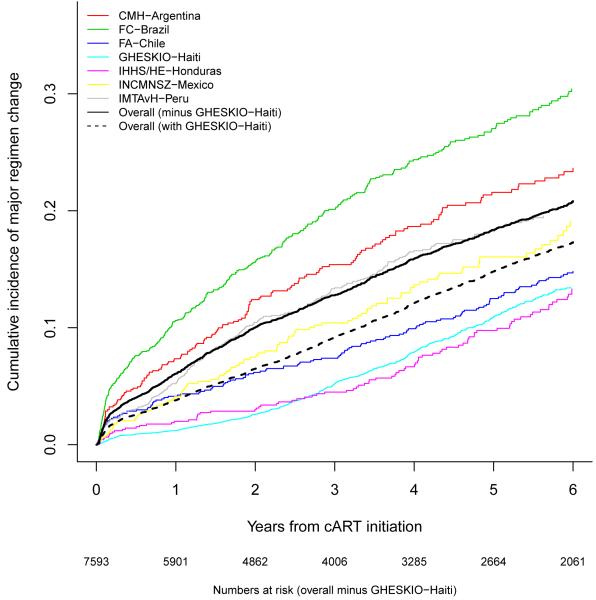
The cumulative incidence of a major regimen change by study site is shown in Figure 1B. The cumulative incidence of a major regimen change across all sites excluding GHESKIO-Haiti was 5·9% after one year of cART (95% CI 5·3-6·4%), 12·7% after three years (95% CI 11·9-13·5%), and 18·2% after five years (95% CI 17·2-19·2%). The cumulative incidence of a major regimen change across all sites including GHESKIO-Haiti was 3·7% after one year of cART (95% CI 3·4-4·0%), 9·0% after three years (95% CI 8·5-9·5%), and 14·6% after five years (95% CI 13·9-15·3%).
Figure 1B.
Cumulative incidence of major regimen change by site.
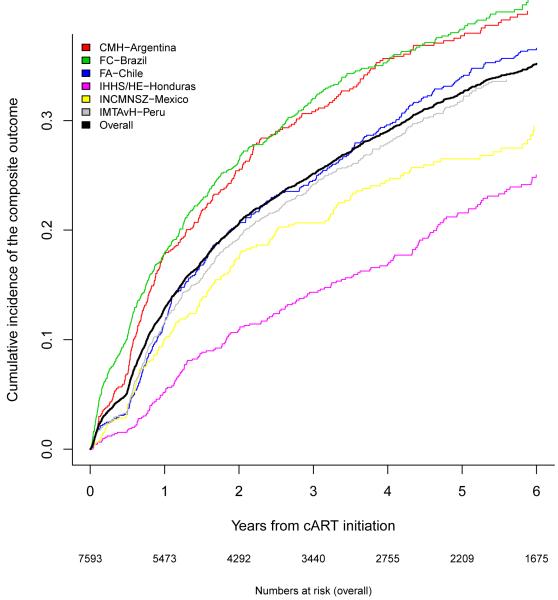
The cumulative incidence of the composite endpoint, virologic failure or major regimen change, by study site, excluding GHESKIO-Haiti, is shown in Figure 1C. The cumulative incidence of the composite endpoint across all six sites was 12·1% after one year of cART (95% CI 11·3-12·9%), 24·9% after three years (95% CI 23·9-26%), and 32·4% after five years (95% CI 31·1-33·6%).
Figure 1C.
Cumulative incidence of virologic failure or major regimen change by site.
Table 2 reports the association between patient characteristics at cART initiation and virologic failure combined across sites. Younger patients were more likely to fail (adjusted hazard ratio [aHR]=2·03 for 20 vs. 40 years; 95% CI 1·68-2·44; p<0·001). Compared with those whose probable route of infection was heterosexual sex, MSM were less likely to fail (aHR=0·73; 95% CI 0·64-0·83), whereas those with injection drug use history were more likely to fail (aHR=1·60; 95% CI 1·02-2·52). Patients starting cART in earlier calendar years were also more likely to fail (aHR=1·28 for 2002 vs. 2006; 95% CI 1·13-1·46). Compared with NNRTI-based regimens, boosted PI-based regimens were not significantly associated with increased virologic failure (aHR=1·17 [95% CI 1·00-1·36] whereas other regimens were more likely to fail (1·33 [1·07-1·64]). Compared with patients starting a zidovudine-containing regimen, those starting with tenofovir were less likely to fail (aHR=0·71, 95% CI 0·58-0·87).
Table 2.
Adjusted hazard ratios (HR), confidence intervals (CI), and p-values (P) for virologic failure, major regimen change, and the composite endpoint of the first of virologic failure or major regimen change for 6 Latin American sites (Haiti not included).
| Virologic Failure | Major Regimen Change | Composite | ||||
|---|---|---|---|---|---|---|
| HR 95% CI | P | HR 95% CI | P | HR 95% CI | P | |
|
|
||||||
| Male | 0·92 (0·81-1·04) | 0·16 | 0·71 (0·62-0·81) | < 0·0001 | 0·83 (0·75-0·93) | 0·0007 |
| Age (years) | < 0·0001 | < 0·0001 | < 0·0001 | |||
| 20 | 2·03 (1·68-2·44) | 1·88 (1·52-2·33) | 1·82 (1·55-2·15) | |||
| 30 | 1·20 (1·07-1·34) | 1·24 (1·10-1·41) | 1·20 (1·09-1·32) | |||
| 40 (ref) | 1·00 | 1·00 | 1·00 | |||
| 50 | 0·85 (0·78-0·92) | 0·87 (0·80-0·96) | 0·88 (0·82-0·94) | |||
| Probable route of infection | <0·0001 | 0·05 | <0·0001 | |||
| Heterosexual (ref) | 1·00 | 1·00 | 1·00 | |||
| MSM | 0·73 (0·64-0·83) | 0·83 (0·72-0·96) | 0·75 (0·67-0·84) | |||
| IDU | 1·60 (1·02-2·52) | 1·14 (0·64-2·04) | 1·35 (0·87-2·10) | |||
| Other | 0·88 (0·74-1·03) | 0·84 (0·70-1·02) | 0·85 (0·73-0·98) | |||
| Clinical AIDS at first cART | 1·13 (1·00-1·27) | 0·06 | 1·08 (0·94-1·24) | 0·25 | 1·08 (0·96-1·21) | 0·21 |
| CD4 at first cART, cells/mm3 | 0·96 | 0·07 | 0·96 | |||
| 50 | 1·00 (0·86-1·16) | 1·24 (1·04-1·48) | 1·03 (0·89-1·20) | |||
| 100 | 1·01 (0·88-1·17) | 1·19 (1·00-1·41) | 1·04 (0·89-1·21) | |||
| 200 | 1·00 (0·91-1·10) | 1·07 (0·96-1·20) | 1·01 (0·94-1·09) | |||
| 350 (ref) | 1·00 | 1·00 | 1·00 | |||
| HIV-1 VL (log10), copies/mL | 0·10 | 0·30 | 0·26 | |||
| 4 | 0·92 (0·78-1·08) | 0·94 (0·81-1·08) | 0·97 (0·86-1·09) | |||
| 5 (ref) | 1·00 | 1·00 | 1·00 | |||
| 6 | 1·18 (0·97-1·42) | 0·95 (0·80-1·14) | 1·12 (0·97-1·30) | |||
| Year of first cART | 0·002 | < 0·0001 | 0·009 | |||
| 2002 | 1·28 (1·13-1·46) | 0·86 (0·73-1·00) | 1·10 (0·98-1·24) | |||
| 2004 | 1·10 (1·04-1·15) | 0·90 (0·85-0·96) | 1·01 (0·99-1·06) | |||
| 2006 (ref) | 1·00 | 1·00 | 1·00 | |||
| 2008 | 1·01 (0·92-1·10) | 1·20 (1·08-1·33) | 1·11 (1·03-1·20) | |||
| 2010 | 1·05 (0·90-1·22) | 1·37 (1·15-1·64) | 1·24 (1·09-1·42) | |||
| First cART type | 0·009 | 0·01 | 0·04 | |||
| NNRTI (ref) | 1·00 | 1·00 | 1·00 | |||
| Boosted PI | 1·17 (1·00-1·36) | 0·91 (0·77-1·09) | 1·18 (1·03-1·35) | |||
| Other | 1·33 (1·07-1·64) | 0·63 (0·46-0·87) | 1·11 (0·91-1·36) | |||
| NRTIs in first cART | 0·0006 | 0·004 | <0·0001 | |||
| ZDV (ref) | 1·00 | 1·00 | 1·00 | |||
| d4T | 1·13 (0·97-1·33) | 1·19 (0·99-1·43) | 1·17 (1·02-1·35) | |||
| TDF | 0·71 (0·58-0·87) | 0·83 (0·68-1·02) | 0·72 (0·61-0·85) | |||
| Other | 1·18 (0·98-1·43) | 1·32 (1·06-1·64) | 1·22 (1·03-1·45) | |||
P-values for tests of non-linearity:
Virologic Failure: Overall: 0·007, age: 0·007, CD4: 0·88, HIV-1 VL: 0·34, year of first cART: 0·02.
Major Regimen Change: Overall: 0·26, age: 0·07, CD4 0·82, HIV-1 VL: 0·41, year of first cART: 0·27.
Composite: Overall: 0·01, age: 0·01, CD4: 0·88, HIV-1 VL: 0·60, year of first cART: 0·009.
MSM: men who have sex with men; IDU: injection drug use; cART: combination antiretroviral therapy; VL: viral load; NNRTI: non-nucleoside reverse transcriptase inhibitor; PI: protease inhibitor; NRTI: nucleoside reverse transcriptase inhibitor; ZDV: zidovudine; d4T: stavudine; TDF: tenofovir.
Table 2 also reports the association between patient characteristics and a major regimen change across the 6 Latin American sites. Males were less likely to have a major regimen change (aHR=0·71, 95% CI 0·62-0·81). Similar to the virologic failure analyses, younger age and a history of IDU were associated with a major regimen change. In contrast to the virologic failure analyses, earlier year of first cART was associated with a decreased risk of regimen change (aHR=0·86 for 2002 vs. 2006), and patients starting a first cART not classified as NNRTI-based tended to be less likely to have a major regimen change (aHR=0·91, 95% CI 0·77-1·09 for boosted PI-based regimens and 0·63, 95% CI 0·46-0·87 for other regimens). Similar to the virologic failure analysis, patients starting with stavudine were more likely to have a major regimen change whereas those starting with tenofovir were less likely (aHR 1·19 and 0·83, respectively). The association between patient characteristics and the composite endpoint of virologic failure or major regimen change is also shown in Table 2.
The association between predictors of interest and a major regimen change in GHESKIO-Haiti and comparison with the six Latin American sites is shown in Table 3. Predictors of major regimen change were remarkably similar between GHESKIO-Haiti and the Latin American sites: female sex, younger age, more recent calendar year, and lower CD4 at cART initiation were associated with a greater risk of a major regimen change in adjusted analyses. Patients starting a first cART not classified as NNRTI- or PI-based were less likely to have a major regimen change.
Table 3.
Adjusted hazard ratios (HR), confidence intervals (CI), and p-values (P) for major regimen change at GHESKIO-Haiti and for the six Latin American sites.
| GHESKIO-Haiti | Latin American Sites | |||
|---|---|---|---|---|
|
| ||||
| HR 95% CI | P | HR 95% CI | P | |
| Male | 0·73 (0·66-0·80) | < 0·0001 | 0·66 (0·58-0·74) | < 0·0001 |
| Age (years) | < 0·0001 | < 0·0001 | ||
| 20 | 1·90 (1·59-2·26) | 1·86 (1·51-2·31) | ||
| 30 | 1·28 (1·17-1·41) | 1·23 (1·09-1·40) | ||
| 40 (ref) | 1·00 | 1·00 | ||
| 50 | 0·90 (0·84-0·96) | 0·88 (0·81-0·97) | ||
| Clinical AIDS at first cART | 1·04 (0·92-1·17) | 0·54 | 1·10 (0·95-1·27) | 0·22 |
| CD4 at first cART, cells/mm3 | < 0·0001 | 0·02 | ||
| 50 | 1·47 (1·24-1·75) | 1·26 (1·04-1·52) | ||
| 100 | 1·35 (1·15-1·58) | 1·20 (0·99-1·44) | ||
| 200 | 1·11 (1·00-1·23) | 1·10 (0·99-1·21) | ||
| 350 (ref) | 1·00 | 1·00 | ||
| Year of first cART | < 0·0001 | < 0·0001 | ||
| 2002 | 0·90 (0·77-1·04) | 0·86 (0·73-1·00) | ||
| 2004 | 0·90 (0·85-0·96) | 0·90 (0·85-0·96) | ||
| 2006 (ref) | 1·00 | 1·00 | ||
| 2008 | 1·46 (1·33-1·60) | 1·20 (1·08-1·33) | ||
| 2010 | 1·88 (1·61-2·19) | 1·37 (1·15-1·63) | ||
| First cART type | 0·008 | 0·01 | ||
| NNRTI (ref) | 1·00 | 1·00 | ||
| Boosted PI | 0·86 (0·73-1·01) | 0·92 (0·77-1·10) | ||
| Other | 0·68 (0·51-0·91) | 0·62 (0·45-0·86) | ||
| NRTIs in first cART | 0·009 | 0·004 | ||
| ZDV (ref) | 1·00 | 1·00 | ||
| d4T | 1·14 (0·98-1·33) | 1·18 (0·98-1·42) | ||
| TDF | 0·91 (0·77-1·09) | 0·82 (0·67-1·01) | ||
| Other | 1·35 (1·09-1·67) | 1·32 (1·06-1·65) | ||
P-values for tests of non-linearity:
GHESKIO-Haiti: Overall: <0·0001, age: 0·004, CD4: 0·46, year of first cART: <0·0001.
Latin American sites: Overall: 0·35, age: 0·05, CD4: 0·84, year of first cART: 0·83.
cART: combination antiretroviral therapy; VL: viral load; NNRTI: non-nucleoside reverse transcriptase inhibitor; PI: protease inhibitor; NRTI: nucleoside reverse transcriptase inhibitor; ZDV: zidovudine; d4T: stavudine; TDF: tenofovir.
Sensitivity analyses considered incidences and risk factors for virologic failure and the composite endpoint for the six Latin American sites, using a stricter definition for virologic failure (one measurement >1000 copies was not sufficient to establish virologic failure in this sensitivity analysis). Although the incidence of virologic failure was lower, results were largely unchanged. These results are included in the supplemental material.
A secondary analysis censored viral load measurements with gaps of more than a year at the start of the gap. Incidence of virologic failure and major regimen change were similar to the primary analysis. Female sex, younger age, more recent calendar year and cART type were associated with a greater risk of failure or a major regimen change in adjusted analyses. These results are shown in the supplemental material.
Of the 1,719 patients who had virologic failure, 909 (52·8%) did not have a major regimen modification registered. Among the 909 patients who failed but who did not have a major regimen modification, 704 had VL data after the first failure, and of these, 379 were still failing. In contrast, of the 1,955 patients who had a major regimen change, only 810 (41·4%) had a previously documented virologic failure.
A total of 2176 (15·5%) of patients were lost to follow-up over the study period. LTFU was particularly high at the sites in Argentina (33%) and Honduras (25%). Patients LTFU were more likely to be younger than those who were alive at the end of follow-up – details are given in the Supplementary Material.
Discussion
This is the first study to evaluate cumulative incidences of virologic failure and major regimen change after first cART in seven Latin American countries. Incidences of virologic failure were low and comparable to those reported in middle- and low-income settings,19 and lower than those in Europe and North America.20,21 Incidences of major treatment modification were also low and lower compared to those in Europe and North America.22
We found that younger patients and those with history of IDU were more likely to fail cART, and were more likely to have a major regimen change. These associations may be attributable to increased levels of medication non-adherence also observed in other studies.23
Consistent with other studies, patients starting a NNRTI-based regimen had lower incidences of failure than those starting a boosted PI regimen probably influenced by adherence rates and toxicities.24,25 Compared with patients starting a zidovudine-containing regimen, those starting with stavudine were more likely to fail or switch regimens whereas those starting with tenofovir were less likely to fail or switch. These results may reflect non-adherence and poor tolerability due to toxicities; however, this study did not obtain information on medication adherence and reasons for switch of cART. The reason for major regimen change was likely different from virologic failure for many patients who changed cART. In any case, these patients required a major change to a second line regimen, which is an important event regardless of the reason for the change. Virologic failure was also associated with initiating treatment in earlier calendar years, which is consistent with trends seen in other studies.24,26,27 Improvements in efficacy and tolerability of antiretroviral agents, changes in clinical care such as increased clinician experience, and other unmeasured variables may be contributing to this trend. In contrast, incidence of major regimen change increased over time. This latter result may reflect increasing virologic monitoring and availability of second-line drugs. While the diversity of ARV regimens has changed over the 14-year period of the study, in an attempt to isolate associations, our analyses have controlled for study site, year of cART initiation and type of regimen in the first regimen.
We did not find the association between gender and failure described in other studies. However, major cART change was more likely among women. Women were reported to stop PI-based treatment on their own28 and to present toxicity more frequently than men, specifically stavudine associated toxicities,29 zidovudine induced anemia,30 and nevirapine induced rash.31
A proportion of patients with documented virologic failure were not prescribed a new regimen. This may be explained by adherence concerns not measured in our study or by limited availability of a new regimen. These patients represent a risk for transmission of HIV-1 strains with resistant mutations. Conversely, a significant proportion of patients who switched regimens did not meet virologic criteria for failure.
This analysis has several strengths and limitations. Strengths include the large number of patients with systematically collected data in a real-world clinic setting, in a region where HIV has been under-studied. An additional strength includes the quality and accuracy of the data: VL measurements and other key variables were audited at each site by external investigators. A major study limitation is the lack of data on adherence and toxicity or reasons for ART change. Many of the virologic failures may be due to poor adherence and many of the major regimen changes may be due to toxicities or other non-failure reasons, which we were unable to capture. Regimen changes in this study were defined and classified retrospectively Other variables may be associated with virologic failure and major regimen change that we were not able to include, for example social and behavioral factors In addition, our measure of virologic failure is a function of the frequency of VL measurement, which was highly variable between sites. Indeed, the site in Honduras had the lowest estimated rates of virologic failure and major regimen change, and the lowest frequency of VL measurements. HIV/AIDS programs with limited access to virologic monitoring tend to be those with limited access to second-line regimens. All analyses stratified by study site, but estimated common hazard ratios for predictor variables across sites; it is likely that some hazard ratios differed by site, and an alternative, albeit more complicated, analysis would be to obtain separate hazard ratios by site and then to pool results.32 Another limitation is the lack of data on use of dose-fixed-formulations that can improve adherence and were more available in recent years. The lower probability of failure in patients initiating tenofovir may be confounded by the use of tenofovir in coformulated pills, but the same confounding effect is also possible for zidovudine. Finally, a substantial number of patients in some sites were loss to follow-up, and it is unclear whether these patients were receiving care at other facilities or had abandoned cART altogether.
Identifying patients failing first-line treatment is a major challenge in cART programs in RLS. Patients are often not switched to a second-line regimen in a timely manner, if ever, and risk of mortality is increased among patients who fail first-line ART.33 In settings where VL measurement is not routinely available, patients are switched to second-line cART regimens according to WHO clinical and immunologic criteria, which lack both sensitivity and specificity and are associated with unacceptable treatment failure misclassification.34 Additionally, the development of NNRTI-associated resistance mutations resulting from virologic failure has been associated with infrequent VL monitoring.35 Therefore, increasing the availability of VL monitoring is crucial to improve treatment outcomes and minimize transmitted resistance.
This study estimates the incidence of initial cART failure, thereby providing data that may be used to inform programs in LAC of the need for second-line ART. Approximately one-quarter of patients starting cART may require changing to a second-line regimen within five years. In order to maximize the benefits of cART programs, specific support to young individuals and those with a history of drug use should be provided, viral load monitoring should be expanded, and more modern first-line regimens should be adopted.
Panel
Evidence before
Access to combination antiretroviral therapy (cART) is expanding in Latin America and the Caribbean. We searched PubMed using the following search terms: (“antiretroviral therapy” OR “antiretroviral treatment”) AND (“virologic failure” OR “treatment failure”) AND (“Latin America” OR “Caribbean”) in March 2014. There was little information in this region regarding incidence of and factors associated with regimen failure and regimen change.
Added value
This study assessed virologic failure and regimen change in 14,027 cART initiators who were followed for a median of 3·9 years. Data were collected systematically in a real-world clinic setting, VL measurements and other key variables were audited at each site by external investigators. Virologic failure was associated with younger age, infection through injection-drug use, initiation in earlier calendar years and starting with a boosted protease inhibitor.
Implications of all available evidence
Approximately one-quarter of patients may require changing to a second-line regimen within five years. To maximize cART benefits, specific support to young individuals and those with a history of drug use should be provided, viral load monitoring should be expanded and more modern treatment regimens should be adopted.
Supplementary Material
Acknowledgments
We gratefully acknowledge all patients, caregivers, and data managers involved in the CCASAnet cohorts.
Funding
This work was supported by the National Institute of Allergy and Infectious Diseases, US National Institutes of Health, as part of the International Epidemiologic Databases to Evaluate AIDS (IeDEA): U01 AI069923.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
We declare that we have no conflicts of interest.
References
- 1.Palella FJ, Jr., Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998 Mar 26;338(13):853–60. doi: 10.1056/NEJM199803263381301. PubMed PMID: 9516219. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization Global Update on the Health Sector Response to HIV. 2014 Accessed 09/01/2015. Available from: http://apps.who.int/iris/bitstream/10665/128494/1/9789241507585_eng.pdf.
- 3.Pan American Health Organization Antiretroviral Treatment in the Spotlight: A Public Health Analysis in Latin America and the Caribbean. 2013 Accessed 09/01/2015. Available from: http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&gid=23710&Itemid.
- 4.World Health Organization Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. 2013 Accessed 09/01/2015. Available from: http://www.who.int/hiv/pub/guidelines/arv2013/en/ [PubMed]
- 5.Ministerio de Salud Dirección de Sida y ETS. Guía para el manejo de los pacientes adultos con infección por VIH. 2013 Accessed 09/01/2015. Available from: http://www.msal.gov.ar/images/stories/bes/graficos/0000000109cnt-2013-05_guia-manejo-pacientes-adultos.pdf.
- 6.Ministério da Saúde Protocolo clínico e diretrizes terapêuticas para manejo da infecção pelo hiv em adultos. 2013 Accessed 12/01/2015. Available from: http://www.aids.gov.br/sites/default/files/anexos/publicacao/2013/55308/protocolo_13_3_2014_pdf_28003.pdf.
- 7.Ministerio de Salud Guía Clínica AUGE. Sindrome de Inmunodeficiencia adquirida VIH/SIDA. 2013 [Google Scholar]
- 8.Secretaria de Salud Consejo Nacional para la Prevencion y Control del Sida (CONASIDA). Guía de manejo antirretroviral de las personas con VIH. México, (5a) 2012 Accessed 15/12/2014. Available from: http://www.censida.salud.gob.mx/descargas/atencion/GUIA_ARV_2012.pdf.
- 9.Ministerio de Salud Norma Técnica para el Tratamiento Antirretroviral de Gran Actividad – TARGA en Adultos Infectados por el Virus de la Inmunodeficiencia Humana. NT No 004-MINSA/DGSP-V.02. Lima, Perú. 2005 Accessed 15/12/2014. Available from: http://www2.paho.org/hq/dmdocuments/2010/Peru-ADULTOS-2005.pdf.
- 10.Ministère de la santé publique et de la population (mspp) Manuel de normes de prise en charge clinique et thérapeutique des adultes et adolescents vivant avec le vih. 2008.
- 11.Secretaría de Salud de Honduras, Subsecretaría de Riesgos Poblacionales, Dirección General de Promoción de la Salud, Departamento ITS/VIH/SIDA Manual de atención integral al adulto y adolescente con VIH. 2013.
- 12.Ivers LC, Kendrick D, Doucette K. Efficacy of antiretroviral therapy programs in resource-poor settings: a meta-analysis of the published literature. Clin Infect Dis. 2005 Jul 15;41(2):217–24. doi: 10.1086/431199. PubMed PMID: 15983918. [DOI] [PubMed] [Google Scholar]
- 13.Keiser O, Orrell C, Egger M, et al. Public-health and individual approaches to antiretroviral therapy: township South Africa and Switzerland compared. PLoS Med. 2008 Jul 8;5(7):e148. doi: 10.1371/journal.pmed.0050148. PubMed PMID: 18613745. Pubmed Central PMCID: 2443185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pujades-Rodriguez M, O'Brien D, Humblet P, Calmy A. Second-line antiretroviral therapy in resource-limited settings: the experience of Medecins Sans Frontieres. AIDS. 2008 Jul 11;22(11):1305–12. doi: 10.1097/QAD.0b013e3282fa75b9. PubMed PMID: 18580610. [DOI] [PubMed] [Google Scholar]
- 15.McGowan CC, Cahn P, Gotuzzo E, et al. Cohort Profile: Caribbean, Central and South America Network for HIV research (CCASAnet) collaboration within the International Epidemiologic Databases to Evaluate AIDS (IeDEA) programme. Int J Epidemiol. 2007 Oct;36(5):969–76. doi: 10.1093/ije/dym073. PubMed PMID: 17846055. [DOI] [PubMed] [Google Scholar]
- 16.RL KJaP . The Statistical Analysis of Failure Time Data. John Wiley & Sons, Hoboken; New Jersey: 2002. pp. 118–19. [Google Scholar]
- 17.Harrell FE J . Regression Modeling Strategies, with applications to linear models, logistic regression, and survival analysis. Springer-Verlag; New York, NY: 2001. [Google Scholar]
- 18.T. GPaT Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–26. [Google Scholar]
- 19.Renaud-Théry Françoise, Duncombe Chris, Kerr Stephen, Thierry Sigrid, Perriëns Joseph. Adult antiretroviral therapy in resource limited settings: a systematic review of first-line failure and attrition rates Accessed 09/01/2015. Available from: http://www.who.int/hiv/topics/treatment/First_Line_ART_failure_RLS_metanalysis.pdf.
- 20.Ledergerber B, Egger M, Opravil M, et al. Clinical progression and virological failure on highly active antiretroviral therapy in HIV-1 patients: a prospective cohort study. Swiss HIV Cohort Study. Lancet. 1999 Mar 13;353(9156):863–8. doi: 10.1016/s0140-6736(99)01122-8. PubMed PMID: 10093977. [DOI] [PubMed] [Google Scholar]
- 21.Erb P, Battegay M, Zimmerli W, Rickenbach M, Egger M. Effect of antiretroviral therapy on viral load, CD4 cell count, and progression to acquired immunodeficiency syndrome in a community human immunodeficiency virus-infected cohort. Swiss HIV Cohort Study. Arch Intern Med. 2000 Apr 24;160(8):1134–40. doi: 10.1001/archinte.160.8.1134. PubMed PMID: 10789606. [DOI] [PubMed] [Google Scholar]
- 22.Abgrall S, Ingle SM, May MT, et al. Durability of first ART regimen and risk factors for modification, interruption or death in HIV-positive patients starting ART in Europe and North America 2002-2009. AIDS. 2013 Mar 13;27(5):803–13. doi: 10.1097/QAD.0b013e32835cb997. PubMed PMID: 23719350. [DOI] [PubMed] [Google Scholar]
- 23.Nolan S, Milloy MJ, Zhang R, et al. Adherence and plasma HIV RNA response to antiretroviral therapy among HIV-seropositive injection drug users in a Canadian setting. AIDS Care. 2011 Aug;23(8):980–7. doi: 10.1080/09540121.2010.543882. PubMed PMID: 21480010. [DOI] [PubMed] [Google Scholar]
- 24.Martin DA, Luz PM, Lake JE, et al. Improved virologic outcomes over time for HIV-infected patients on antiretroviral therapy in a cohort from Rio de Janeiro, 1997-2011. BMC Infect Dis. 2014;14:322. doi: 10.1186/1471-2334-14-322. PubMed PMID: 24919778. Pubmed Central PMCID: 4067376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imaz A, Llibre JM, Navarro J, et al. Effectiveness of efavirenz compared with ritonavir-boosted protease-inhibitor-based regimens as initial therapy for patients with plasma HIV-1 RNA above 100,000 copies/ml. Antivir Ther. 2014;19(6):569–77. doi: 10.3851/IMP2736. PubMed PMID: 24458091. [DOI] [PubMed] [Google Scholar]
- 26.Lampe FC, Gatell JM, Staszewski S, et al. Changes over time in risk of initial virological failure of combination antiretroviral therapy: a multicohort analysis, 1996 to 2002. Arch Intern Med. 2006 Mar 13;166(5):521–8. doi: 10.1001/archinte.166.5.521. PubMed PMID: 16534038. [DOI] [PubMed] [Google Scholar]
- 27.Delaugerre C, Ghosn J, Lacombe JM, et al. Significant Reduction in HIV Virologic Failure During a 15-Year Period in a Setting With Free Healthcare Access. Clin Infect Dis. 2014 Oct 23; doi: 10.1093/cid/ciu834. PubMed PMID: 25344539. [DOI] [PubMed] [Google Scholar]
- 28.Menzaghi B, Ricci E, Vichi F, et al. Gender differences in HIV infection: is there a problem? Analysis from the SCOLTA cohorts. Biomed Pharmacother. 2014 Apr;68(3):385–90. doi: 10.1016/j.biopha.2014.01.007. PubMed PMID: 24613008. [DOI] [PubMed] [Google Scholar]
- 29.Menezes CN, Maskew M, Sanne I, Crowther NJ, Raal FJ. A longitudinal study of stavudine-associated toxicities in a large cohort of South African HIV infected subjects. BMC Infect Dis. 2011;11:244. doi: 10.1186/1471-2334-11-244. PubMed PMID: 21923929. Pubmed Central PMCID: 3189398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tedaldi EM, Absalon J, Thomas AJ, Shlay JC, van den Berg-Wolf M. Ethnicity, race, and gender. Differences in serious adverse events among participants in an antiretroviral initiation trial: results of CPCRA 058 (FIRST Study) J Acquir Immune Defic Syndr. 47(4):441–8. doi: 10.1097/QAI.0b013e3181609da8. PubMed PMID: 18176329. [DOI] [PubMed] [Google Scholar]
- 31.Bottaro EG, Huberman MJ, Iannella Mdel C, et al. Nevirapine-associated toxicity in clinical practice in Buenos Aires, Argentina. J Int Assoc Physicians AIDS Care (Chic) 2010 Sep-Oct;9(5):306–12. doi: 10.1177/1545109710376250. PubMed PMID: 20923955. [DOI] [PubMed] [Google Scholar]
- 32.Giganti MJ, Luz PM, Caro-Vega Y, et al. A Comparison of Seven Cox Regression-Based Models to Account for Heterogeneity Across Multiple HIV Treatment Cohorts in Latin America and the Caribbean. AIDS Res Hum Retroviruses. 2015 May;31(5):496–503. doi: 10.1089/aid.2014.0241. PubMed PMID: 25647087. Pubmed Central PMCID: 4426314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pujades-Rodriguez M, Balkan S, Arnould L, et al. Treatment failure and mortality factors in patients receiving second-line HIV therapy in resource-limited countries. JAMA. 2010 Jul 21;304(3):303–12. doi: 10.1001/jama.2010.980. PubMed PMID: 20639564. [DOI] [PubMed] [Google Scholar]
- 34.Westley BP, DeLong AK, Tray CS, et al. Prediction of treatment failure using 2010 World Health Organization Guidelines is associated with high misclassification rates and drug resistance among HIV-infected Cambodian children. Clin Infect Dis. 2012 Aug;55(3):432–40. doi: 10.1093/cid/cis433. PubMed PMID: 22539664. Pubmed Central PMCID: 3491779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gupta RK, Hill A, Sawyer AW, et al. Virological monitoring and resistance to first- line highly active antiretroviral therapy in adults infected with HIV-1 treated under WHO guidelines: a systematic review and meta-analysis. Lancet Infect Dis. 2009 Jul;9(7):409–17. doi: 10.1016/S1473-3099(09)70136-7. PubMed PMID: 19555900. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.