Abstract
Eukaryotic cell-free protein synthesis (CFPS) is limited by the dependence on costly high-energy phosphate compounds and exogenous enzymes to power protein synthesis (e.g., creatine phosphate and creatine kinase, CrP/CrK). Here, we report the ability to use glucose as a secondary energy substrate to regenerate ATP in a Saccharomyces cerevisiae crude extract CFPS platform. We observed synthesis of 3.64±0.35 μg mL−1 active luciferase in batch reactions with 16mM glucose and 25mM phosphate, resulting in a 16% increase in relative protein yield (μg protein/$ reagents) compared to the CrP/CrK system. Our demonstration provides the foundation for development of cost-effective eukaryotic CFPS platforms.
Keywords: Cell-free biology, cell-free protein synthesis, in vitro transcription and translation, protein expression, Saccharomyces cerevisiae, natural energy metabolism
Introduction
Cell-free protein synthesis (CFPS) is an emerging field that allows for the production of proteins without intact cells (Carlson et al., 2012; Hodgman and Jewett, 2012). Crude cell lysates, or extracts, are employed instead. Supplying chemical energy (in the form of ATP) for the aminoacylation of tRNAs and peptide bond formation has been a grand challenge for CFPS development (Carlson et al., 2012). Historically, high-energy phosphate bond donors; such as phosphoenolpyruvate (PEP), creatine phosphate (CrP) (Figure 1A), and acetyl phosphate have been used (Brödel et al., 2013; Carlson et al., 2012; Hodgman and Jewett, 2013; Kim and Swartz, 2001; Ryabova et al., 1995; Takai et al., 2010). In these cases, ATP regeneration requires the addition of pyruvate kinase, creatine kinase, or acetate kinase, respectively, or the endogenous presence of these enzymes in the cell extract. Unfortunately, rapid production of phosphate from these high-energy compounds has been shown to be inhibitory to CFPS (e.g., E. coli (Kim and Swartz, 2000) and yeast (Schoborg et al., 2014)). Furthermore, batch reactions using these secondary energy substrates typically provide only a brief burst of ATP. In addition, phosphorylated energy compounds are costly, which limits industrial applications (Calhoun and Swartz, 2005a, g; Swartz, 2006). To address these limitations, new cost-effective secondary energy regeneration systems are sought.
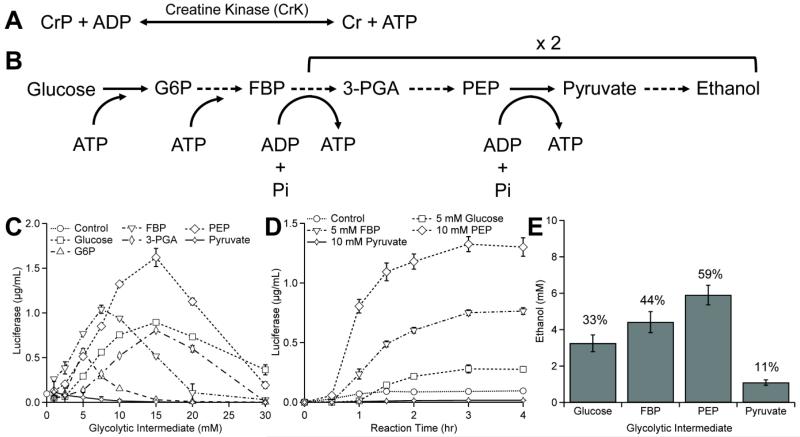
Figure 1. Glycolysis is active in yeast crude extract CFPS.
(A) Schematic of creatine phosphate (CrP)/creatine kinase (CrK) energy regeneration system. (B) Proposed glycolytic energy regeneration system in yeast crude extracts. (C) To assess the possibility of using glycolytic intermediates to fuel CFPS, six glycolytic intermediates (fructose 1,6-bisphosphate (FBP), phosphoenolpyruvate (PEP), glucose, 3-phosphglyceric acid (3-PGA), pyruvate, and glucose 6-phosphate (G6P)) were added as the sole secondary energy substrate to different yeast CFPS reactions in concentrations ranging from 0 mM to 30 mM and compared to a control composed of no secondary energy substrate (circle). Of the non-phosphorylated secondary energy substrates assessed, glucose is the highest yielding for yeast CFPS. (D) Time course reactions of active luciferase for several glycolytic intermediates for equivalent of 30 mM total carbon (e.g., 5 mM glucose or 10 mM PEP) and (E) HPLC analysis of ethanol production after 4-hour incubation for reactions performed in panel D. The numbers above each column denote the percentage of theoretical conversion of each secondary energy substrate to ethanol. Values shown are means with error bars representing the standard deviation of at least three independent experiments.
Within the last decade, the E. coli CFPS platform has been able to activate natural metabolism within the lysate to fuel highly active CFPS from non-phosphorylated energy substrates and avoid costly substrates by replacing PEP with glucose (Calhoun and Swartz, 2005g; Jewett et al., 2008; Swartz, 2006). Mainly enabled by advances from Swartz and colleagues, glucose drives CFPS with a much lower cost and generates more ATP per secondary energy substrate molecule (Calhoun and Swartz, 2005g; Jewett et al., 2008; Swartz, 2006). For example, glucose has a 2:1 molar ratio of secondary energy metabolite to ATP (Figure 1B), compared to 1:1 ratio for both CrP and PEP (Kim et al., 2007a). As an extension of the pioneering works above, many groups have turned to use of slowly metabolized glucose polymers to fuel E. coli based CFPS, including starch (Kim et al., 2011), maltodextrin (Caschera and Noireaux, 2015; Wang and Zhang, 2009), and maltose (Caschera and Noireaux, 2014).
While E. coli based CFPS systems have been developed from non-phosphorylated energy substrates, making possible many new applications in industrial biotechnology and rapid prototyping (Bujara et al., 2010; Chappell et al., 2015; Karig et al., 2012; Shin and Noireaux, 2012; Sun et al., 2014; Takahashi et al., 2014; Yin et al., 2012; Zawada et al., 2011), most eukaryotic CFPS platforms have been limited to use of high-energy phosphate secondary energy substrates. This includes, for example, a yeast-based CFPS system we developed that leverages creatine phosphate and creatine phosphokinase (CrP/CrK) to power protein synthesis (Choudhury et al., 2014; Gan and Jewett, 2014; Hodgman and Jewett, 2013; Schoborg et al., 2014). Here, we sought to assess the possibility to activate glycolysis in crude cell extracts of yeast to regenerate cofactors and energy to provide the support system necessary to fuel highly active protein synthesis. The ability to use glucose to fuel CFPS is not only important for CFPS applications, but also can expand the impact of cell-free synthetic biology by joining a rapidly growing number of reports highlighting the ability to co-activate multiple biochemical systems in an integrated cell-free platform (Calhoun and Swartz, 2005a, g; Caschera and Noireaux, 2014, 2015; Fritz et al., 2015; Fritz and Jewett, 2014; Jewett et al., 2008; Jewett et al., 2013; Jewett and Swartz, 2004a, b). We demonstrate that it is indeed possible to power yeast CFPS reactions with glucose, and several non-phosphorylated energy sources, and have reached synthesis yields of 1.05±0.12 μg mL−1 active luciferase with 16 mM glucose. After demonstrating synthesis of luciferase from glucose as the sole secondary energy substrate, we optimized our glucose energy system with the addition of cyclic AMP (cAMP) and exogenous phosphate, reaching batch yields of 3.64±0.35 μg mL−1 active luciferase. To the best of our knowledge, our work is the first example of powering a eukaryotic CFPS reaction from the native glycolytic pathway. This opens the way to development of cost-effective eukaryotic CFPS platforms from multiple host organisms for a variety of applications.
Materials and Methods
Yeast extract preparation, CFPS reactions, and luciferase quantification were performed as previously described (Choudhury et al., 2014; Hodgman and Jewett, 2013; Schoborg et al., 2014), with the exception the energy regeneration system (CrP/CrK) was replaced with glycolytic intermediates. The concentration of magnesium glutamate (Mg(Glu)2) added to CFPS reactions was optimized for each extract, as CFPS yields are known to be sensitive to magnesium (Hodgman and Jewett, 2013) (e.g., Supplemental Figure 1A). We tested glucose, glucose-6-phosphate (G6P), 3-phosphoglyceric acid (3-PGA), phosphoenolpyruvate (PEP), fructose-1,6-bisphosphate (FBP), and pyruvate in concentrations ranging from 0 – 30 mM. We also tested CFPS reactions containing glucose in concentrations ranging from 0 – 25 mM glucose in combination with the CrP/CrK energy regeneration system. When denoted, 0.15 mM cAMP and phosphate (in the form of potassium phosphate, pH 7.4) were included in the reaction mixture. In reactions containing potassium phosphate, the overall potassium concentration is balanced by reducing the concentration of potassium glutamate. Reaction conditions can be found in Supplemental Table 1. HPLC analysis of ethanol was performed as previously described (Choudhury et al., 2014). Nucleotide analysis was performed as previously described (Schoborg et al., 2014) except the gradient for buffer B was adjusted to: 0 min, 0%; 10 min, 30%; 50 min, 80%; 55 min, 100%; 60 min, end.
Results
We sought to fuel yeast CFPS by activating glycolysis and central metabolism with non-phosphorylated energy substrates. We expect this metabolism to be active given the fact that Eduard Büchner discovered in 1897 that yeast extract could convert sugar to ethanol and carbon dioxide (Buchner and Rapp, 1897). Initially, we screened for the ability of six different glycolytic intermediates to fuel combined transcription and translation in 15μL batch CFPS reactions for 4 h at 21°C (Figure 1C). The six intermediates included fructose 1,6-bisphosphate (FBP), phosphoenolpyruvate (PEP), glucose, 3-phosphglyceric acid (3-PGA), pyruvate, and glucose 6-phosphate (G6P) at concentrations ranging from 0-30mM. The CFPS reaction was programmed to synthesize luciferase as a model reporter protein and combined transcription and translation was enabled by the use of the Ω cap-independent translation initiation leader sequence (Gan and Jewett, 2014). Strikingly, our results demonstrated that it is indeed possible to activate yeast CFPS reactions from glycolytic intermediates upstream of pyruvate, reaching 1.04±0.45 and 1.62±0.10 μg mL−1 when powering the reaction with fructose 1,6-bisphosphate (FBP) and PEP, respectively. Of the six glycolytic intermediates, only pyruvate was unable to function as a secondary energy source (Figure 1C). The inability of pyruvate to power CFPS was expected due to the lack of ATP regenerating power of pyruvate alone in fermentation metabolic processes.
In order to more carefully understand the system dynamics, we subsequently performed time course CFPS reactions with the three highest-yielding intermediates (FBP, glucose, and PEP). This revealed that the choice of glycolytic intermediate impacted the rate of protein synthesis but not the reaction duration; in all cases protein synthesis had terminated after 4 hours (Figure 1D). Negative control reactions performed with pyruvate or no secondary energy substrate produced little to no luciferase (Figure 1D). The carbon from the glycolytic intermediates is expected to produce ethanol through fermentation, as has been shown in previous works (Buchner and Rapp, 1897; Khattak et al., 2014). Thus, we measured ethanol production to confirm glycolysis was active for each carbon source. As expected, we found that ethanol is synthesized when glucose, FBP, and PEP are able to power protein synthesis (Figure 1E). Ethanol is also produced in the presence of pyruvate, but no protein is synthesized due to limited ATP availability as described above (Figure 1E).
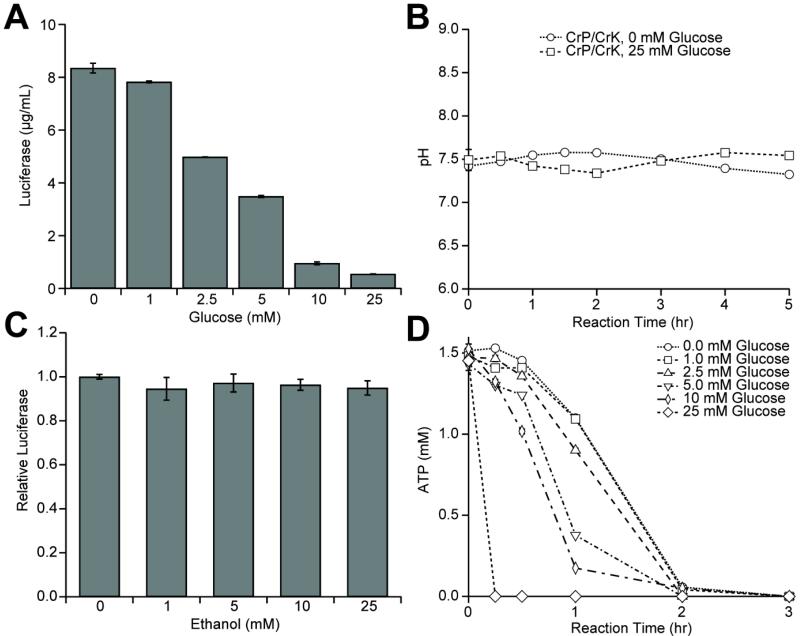
With the goal of increasing protein synthesis yields, we next tested a dual system, in which glucose is used in combination with CrP/CrK. Previously, such a system was demonstrated by Kim et al. to enhance yields in an E. coli CFPS platform (Kim et al., 2007b). Unexpectedly, we found that the addition of glucose to the CrP/CrK system severely inhibits CFPS, with 10 mM glucose addition resulting in an 89% reduction in protein synthesis (Figure 2A). We reasoned that this could result from a decrease in pH, as seen previously in E. coli CFPS platforms powered by glucose, or a toxicity effect from ethanol accumulation (Calhoun and Swartz, 2005a). However, we observed no change in pH during the course of the reaction (Figure 2B), and showed that ethanol is not toxic in our reactions at concentrations of up to 25 mM (Figure 2C), which far exceeded the expected ethanol produced (Figure 1E). Historically, nonproductive energy consumption has been identified as one of the primary reasons for early termination of CFPS. Thus, we used quantitative HPLC analysis to track the ATP pool over time. Nucleotide analysis revealed that the decrease in protein synthesis yields when glucose is added to the reaction is due to rapid ATP consumption. For example, in the presence of 25 mM glucose, ATP is fully consumed within the first 15 minutes of reaction (Figure 2D), constraining the ability to produce protein.
Figure 2. Yeast CFPS CrP/CrK plus glucose dual system for energy regeneration does not improve CFPS yields.
(A) 0 to 25 mM glucose was added to CFPS reactions containing 25 mM creatine phosphate (CrP) and 0.27 mg/mL creatine kinase (CrK). Increasing the starting glucose concentration decreases luciferase yields. (B) The pH of CFPS reactions containing 25 mM CrP, 0.27 mg/mL CrK, and either 0 mM or 25 mM glucose was measured at regular intervals. (C) To assess possible ethanol inhibition, various concentrations of ethanol, ranging from 0 mM to 25 mM, were added to CFPS reactions. Active luciferase yields are reported relative to the 0 mM ethanol condition, showing that inhibition was not observed. (D) The concentration of ATP was measured at intervals during CFPS reactions including 25 mM CrP, 0.27 mg/mL CrK, and 0 to 25 mM glucose. ATP is rapidly depleted as the starting glucose concentration is increased. Values shown in A-C are means with error bars representing the standard deviation of at least three independent experiments. Data from panel D traces are individual measurements.
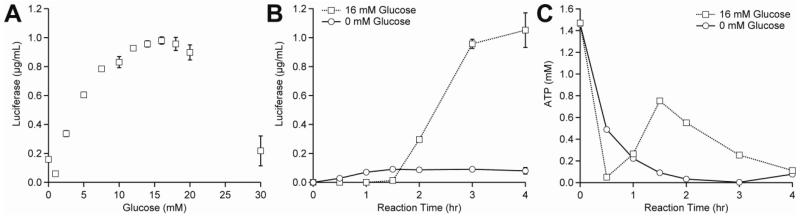
Given the inability to activate a dual energy regeneration system, we returned to the glucose-only system, and determined through an initial optimization that 16 mM glucose is the optimal substrate concentration (Figure 3A). We subsequently carried out a series of additional optimization experiments to try to increase CFPS. We explored the effects of reaction temperature, magnesium glutamate (Mg(Glu)2) concentration, potassium glutamate concentration, spermidine concentration and additives such as cyclic AMP (cAMP) (Supplemental Table 2). Despite a rigorous search, we only observed that addition of cAMP increased yields, suggesting that our original conditions for yeast CFPS captured a maximum. The addition of 0.15 mM cAMP increased our yields 1.5-fold, bringing our yields to approximately 1 μg mL−1 (Supplemental Figure 1B). The kinetics of protein synthesis follows an interesting trajectory when using glucose and cAMP. Specifically, protein synthesis is delayed when using glucose as the energy source (Figure 3B), which we attribute to ATP availability. ATP is rapidly consumed in the first 30 minutes of the reaction, but more than 50% is regenerated after 90 minutes (Figure 3C).
Figure 3. Optimizing yeast CFPS reaction conditions with glucose as a secondary energy substrate.
(A) The optimal starting concentration of glucose was determined via addition of 0-30 mM of glucose to CFPS reactions containing 0.15 mM cAMP. The optimum was observed at 16 mM glucose. (B) Luciferase and (C) ATP concentrations were measured at regular intervals over time in CFPS reactions containing 16 mM glucose or 0 mM glucose. Values shown are means with error bars representing the standard deviation of at least three independent experiments.
With the ability to fuel CFPS by glycolysis at hand, we next investigated the use of slowly metabolized carbon polymers to slow the initial consumption of ATP. We demonstrated that soluble starch can fuel CFPS, though at much lower yields than the glucose system, reaching only ~0.3μg mL−1 with 1.4% (w/v) starch (Supplemental Figures 2A and 2B). Using starch did not reduce initial consumption of ATP, with only 0.2 mM left after 30 minutes of the reaction (Supplemental Figure 2C). Our data suggest that ATP regeneration limits the use of starch when compared to glucose alone. Specifically, the regeneration of ATP when using starch is lower than with 16 mM glucose, leading to a lower protein yield. Supplying α-Glucosidase and amyloglucosidase enzymes did not improve protein synthesis yields, suggesting the activity of our crude lysates is sufficient to metabolize starch (Supplemental Figure 2D).
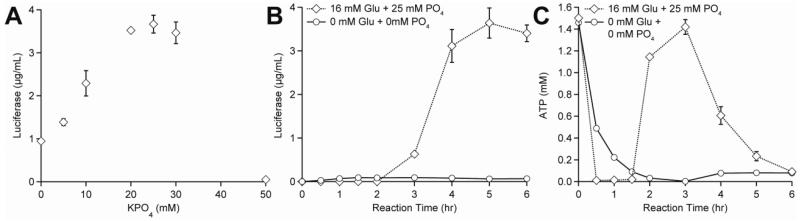
Although we demonstrated proof of principle with starch as an energy substrate, yields remained higher with the glucose energy regeneration system. Therefore, we returned to the glucose system to search for parameters that could increase the level of luciferase synthesized. Previously, Calhoun and Swartz showed that the use of non-phosphorylated energy substrates can result in phosphate limitation during energy regeneration. They observed that the addition of 10 mM inorganic phosphate provided a 3-fold increase in CFPS yields compared to their glucose-driven E. coli CFPS system alone (Calhoun and Swartz, 2005a). Building off this advance, we evaluated the addition of 0-50 mM inorganic phosphate in the form of potassium phosphate to our glucose-driven yeast CFPS system, while keeping the total potassium concentration constant (i.e., addition of potassium phosphate was balanced by adjusting the concentration of potassium glutamate) (Figure 4A). With the addition of 25 mM inorganic phosphate, CFPS yields increased almost 3.5-fold, reaching 3.64±0.35 μg mL−1 (Figure 4A). Figure 4B shows luciferase accumulation over time.
Figure 4. CFPS reactions with glucose are phosphate-limited: Increasing phosphate concentration increases protein yields and prolongs the CFPS reaction.
(A) The optimal amount of exogenous phosphate was determined via addition of 0-50 mM of phosphate to CFPS reactions containing 16 mM glucose. (B) Luciferase and (C) ATP concentration were measured at regular intervals in CFPS reactions containing 16 mM glucose and 25 mM phosphate or 0 mM glucose + 0 mM phosphate. Values shown are means with error bars representing the standard deviation of at least three independent experiments.
As reported for the glucose and starch systems, protein production appears to be linked to ATP availability, which can be described by Atkinson’s adenylate Energy Charge (E.C.) calculation (Atkinson, 1968) (Supplemental Figure 3A). In vivo studies have shown energy is limiting in systems with an E.C. less than 0.8 (Chapman et al., 1971). In reactions containing glucose and phosphate, we observed that ATP is rapidly consumed within the first 30 minutes of the reaction, but now almost 100% is regenerated after 3 hours (Figure 4C), enabling protein synthesis to extend to 5 h (Figure 4B). The observed ATP regeneration coincides exactly with initiation of protein synthesis and the point at which E.C. rises above 0.8, between 2 – 3 hours (Supplemental Figure 3B). Based on our observations from adenylate energy charge calculations, we propose that this trend in ATP concentration is observed due to the activation of glucose metabolism. At the start of the reaction, ATP is consumed in the pay-in phase of glycolysis while glucose is metabolized. After all available glucose has been consumed, ATP is regenerated by glucose metabolism and accumulates until sufficient ATP is available for protein synthesis.
As compared to the glucose only system, ATP regeneration is improved in the glucose/phosphate system, resulting in prolonged availability of a high concentration of ATP, which manifests in higher protein synthesis yields. This is the longest reported batch yeast CFPS reaction to date, to the best of our knowledge. In follow-up experiments, we confirmed that the optimal concentrations of cAMP remained the same in the glucose/phosphate energy system as in the glucose system (Supplemental Figure 4).
Conclusion
In summary, we have developed a new energy regeneration system for yeast CFPS that uses glucose and phosphate. This novel approach removes the need for an expensive phosphorylated secondary energy source and avoids inhibitory phosphate accumulation. To our knowledge, this is the first time that a eukaryotic-based CFPS system has been powered by natural energy metabolism of a non-phosphorylated energy substrate. Although our yields do not exceed those previously reported with yeast extract and the CrP/CrK system (Choudhury et al., 2014), we have increased the relative protein yield (μg protein/$ reagents) by 16% with our novel glucose/phosphate system (Supplemental Figure 5). Further optimization of this platform through host strain engineering, as has been done in E. coli–based systems (Calhoun and Swartz, 2005a; Hong et al., 2015), holds promise to result in a cost-effective eukaryotic CFPS platform for high throughput protein expression, synthetic biology, and proteomic and structural genomic applications. We anticipate that yeast CFPS will become a major player alongside other CFPS technologies in years to come.
Supplementary Material
Highlights.
We developed the first-ever eukaryotic CFPS platform powered by glucose.
Reactions containing glucose and inorganic phosphate prolong CFPS 1-2 hours.
We improved the relative protein yield (μg protein/$ reagents) of yeast CFPS by 16%.
Acknowledgements
We acknowledge Northwestern University and the DARPA Biomedicines on Demand program (N66001-13-C-4024) for support. M.C.J. is a Packard Fellow for Science and Engineering. J.C.S. is supported by the Northwestern Biotechnology Training Program (NIH T32GM008449) and the Clare Boothe Luce Graduate Fellowship. The authors thank Jennifer Kay for performing and developing HPLC methods.
Footnotes
Author Contributions. M.J.A., C.E.H., and M.C.J. conceived and supervised the study; M.J.A., J.C.S., C.E.H., and M.C.J. designed experiments; M.J.A. and J.C.S. performed experiments; M.J.A. and J.C.S. analyzed data; M.J.A., J.C.S., C.E.H. and M.C.J. wrote and revised the manuscript.
References
- Atkinson DE. The energy charge of the adenylate pool as a regulatory parameter. Interaction with feedback modifiers. Biochemistry. 1968;7:4030–4034. doi: 10.1021/bi00851a033. [DOI] [PubMed] [Google Scholar]
- Brödel AK, Sonnabend A, Roberts LO, Stech M, Wüstenhagen DA, Kubick S. IRES-Mediated Translation of Membrane Proteins and Glycoproteins in Eukaryotic Cell-Free Systems. PLoS ONE. 2013;8:e82234. doi: 10.1371/journal.pone.0082234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchner E, Rapp R. Alkoholische gährung ohne hefezellen. Berichte der deutschen chemischen Gesellschaft. 1897;30:2668–2678. [Google Scholar]
- Bujara M, Schumperli M, Billerbeck S, Heinemann M, Panke S. Exploiting cell-free systems: Implementation and debugging of a system of biotransformations. Biotechnol Bioeng. 2010;106:376–389. doi: 10.1002/bit.22666. [DOI] [PubMed] [Google Scholar]
- Calhoun KA, Swartz JR. An economical method for cell-free protein synthesis using glucose and nucleoside monophosphates. Biotechnology progress. 2005a;21:1146–1153. doi: 10.1021/bp050052y. [DOI] [PubMed] [Google Scholar]
- Calhoun KA, Swartz JR. Energizing cell-free protein synthesis with glucose metabolism. Biotechnology and bioengineering. 2005g;90:606–613. doi: 10.1002/bit.20449. [DOI] [PubMed] [Google Scholar]
- Carlson ED, Gan R, Hodgman CE, Jewett MC. Cell-free protein synthesis: applications come of age. Biotechnology advances. 2012;30:1185–1194. doi: 10.1016/j.biotechadv.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caschera F, Noireaux V. Synthesis of 2.3 mg/ml of protein with an all Escherichia coli cell-free transcription-translation system. Biochimie. 2014;99:162–168. doi: 10.1016/j.biochi.2013.11.025. [DOI] [PubMed] [Google Scholar]
- Caschera F, Noireaux V. A cost-effective polyphosphate-based metabolism fuels an all E. coli cell-free expression system. Metabolic engineering. 2015;27:29–37. doi: 10.1016/j.ymben.2014.10.007. [DOI] [PubMed] [Google Scholar]
- Chapman AG, Fall L, Atkinson DE. Adenylate energy charge in Escherichia coli during growth and starvation. J Bacteriol. 1971;108:1072–1086. doi: 10.1128/jb.108.3.1072-1086.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell J, Takahashi MK, Lucks JB. Creating small transcription activating RNAs. Nature chemical biology. 2015;11:214–220. doi: 10.1038/nchembio.1737. [DOI] [PubMed] [Google Scholar]
- Choudhury A, Hodgman CE, Anderson MJ, Jewett MC. Evaluating fermentation effects on cell growth and crude extract metabolic activity for improved yeast cell-free protein synthesis. Biochemical Engineering Journal. 2014;91:140–148. [Google Scholar]
- Fritz BR, Jamil OK, Jewett MC. Implications of macromolecular crowding and reducing conditions for in vitro ribosome construction. Nucleic acids research. 2015 doi: 10.1093/nar/gkv329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz BR, Jewett MC. The impact of transcriptional tuning on in vitro integrated rRNA transcription and ribosome construction. Nucleic acids research. 2014;42:6774–6785. doi: 10.1093/nar/gku307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan R, Jewett MC. A combined cell-free transcription-translation system from Saccharomyces cerevisiae for rapid and robust protein synthesis. Biotechnology journal. 2014;9:641–651. doi: 10.1002/biot.201300545. [DOI] [PubMed] [Google Scholar]
- Hodgman CE, Jewett MC. Cell-free synthetic biology: thinking outside the cell. Metabolic engineering. 2012;14:261–269. doi: 10.1016/j.ymben.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgman CE, Jewett MC. Optimized extract preparation methods and reaction conditions for improved yeast cell-free protein synthesis. Biotechnology and bioengineering. 2013;110:2643–2654. doi: 10.1002/bit.24942. [DOI] [PubMed] [Google Scholar]
- Hong SH, Kwon YC, Martin RW, Des Soye BJ, de Paz AM, Swonger KN, Ntai I, Kelleher NL, Jewett MC. Improving Cell-Free Protein Synthesis through Genome Engineering of Escherichia coli Lacking Release Factor 1. Chembiochem : a European journal of chemical biology. 2015;16:844–853. doi: 10.1002/cbic.201402708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewett MC, Calhoun KA, Voloshin A, Wuu JJ, Swartz JR. An integrated cell-free metabolic platform for protein production and synthetic biology. Molecular systems biology. 2008;4:220. doi: 10.1038/msb.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewett MC, Fritz BR, Timmerman LE, Church GM. In vitro integration of ribosomal RNA synthesis, ribosome assembly, and translation. Molecular systems biology. 2013;9:678. doi: 10.1038/msb.2013.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewett MC, Swartz JR. Mimicking the Escherichia coli cytoplasmic environment activates long-lived and efficient cell-free protein synthesis. Biotechnology and bioengineering. 2004a;86:19–26. doi: 10.1002/bit.20026. [DOI] [PubMed] [Google Scholar]
- Jewett MC, Swartz JR. Substrate replenishment extends protein synthesis with an in vitro translation system designed to mimic the cytoplasm. Biotechnology and bioengineering. 2004b;87:465–472. doi: 10.1002/bit.20139. [DOI] [PubMed] [Google Scholar]
- Karig DK, Iyer S, Simpson ML, Doktycz MJ. Expression optimization and synthetic gene networks in cell-free systems. Nucleic Acids Res. 2012;40:3763–3774. doi: 10.1093/nar/gkr1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khattak WA, Ul-Islam M, Ullah MW, Yu B, Khan S, Park JK. Yeast cell-free enzyme system for bio-ethanol production at elevated temperatures. Process Biochemistry. 2014;49:357–364. [Google Scholar]
- Kim D-M, Swartz JR. Prolonging cell-free protein synthesis by selective reagent additions. Biotechnol Prog. 2000;16:385–390. doi: 10.1021/bp000031y. [DOI] [PubMed] [Google Scholar]
- Kim DM, Swartz JR. Regeneration of adenosine triphosphate from glycolytic intermediates for cell-free protein synthesis. Biotechnology and bioengineering. 2001;74:309–316. [PubMed] [Google Scholar]
- Kim H-C, Kim T-W, Kim D-M. Prolonged production of proteins in a cell-free protein synthesis system using polymeric carbohydrates as an energy source. Process Biochem. 2011;46:1366–1369. [Google Scholar]
- Kim T-W, Keum J-W, Oh I-S, Choi C-Y, Kim H-C, Kim D-M. An economical and highly productive cell-free protein synthesis system utilizing fructose-1,6-bisphosphate as an energy source. J Biotechnol. 2007a;130:389–393. doi: 10.1016/j.jbiotec.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Kim T-W, Oh I-S, Keum J-W, Kwon Y-C, Byun J-Y, Lee K-H, Choi C-Y, Kim D-M. Prolonged cell-free protein synthesis using dual energy sources: Combined use of creatine phosphate and glucose for the efficient supply of ATP and retarded accumulation of phosphate. Biotechnol Bioeng. 2007b;97:1510–1515. doi: 10.1002/bit.21337. [DOI] [PubMed] [Google Scholar]
- Ryabova LA, Vinokurov LM, Shekhovtsova EA, Alakhov YB, Spirin AS. Acetyl phosphate as an energy source for bacterial cell-free translation systems. Analytical biochemistry. 1995;226:184–186. doi: 10.1006/abio.1995.1208. [DOI] [PubMed] [Google Scholar]
- Schoborg JA, Hodgman CE, Anderson MJ, Jewett MC. Substrate replenishment and byproduct removal improve yeast cell-free protein synthesis. Biotechnology journal. 2014;9:630–640. doi: 10.1002/biot.201300383. [DOI] [PubMed] [Google Scholar]
- Shin J, Noireaux V. An E. coli cell-free expression toolbox: application to synthetic gene circuits and artificial cells. ACS synthetic biology. 2012;1:29–41. doi: 10.1021/sb200016s. [DOI] [PubMed] [Google Scholar]
- Sun ZZ, Yeung E, Hayes CA, Noireaux V, Murray RM. Linear DNA for Rapid Prototyping of Synthetic Biological Circuits in an Escherichia coli Based TX-TL Cell-Free System. ACS Synth. Biol. 2014;3:387–397. doi: 10.1021/sb400131a. [DOI] [PubMed] [Google Scholar]
- Swartz J. Developing cell-free biology for industrial applications. Journal of industrial microbiology & biotechnology. 2006;33:476–485. doi: 10.1007/s10295-006-0127-y. [DOI] [PubMed] [Google Scholar]
- Takahashi MK, Chappell J, Hayes CA, Sun ZZ, Kim J, Singhal V, Spring KJ, Al-Khabouri S, Fall CP, Noireaux V, Murray RM, Lucks JB. Rapidly Characterizing the Fast Dynamics of RNA Genetic Circuitry with Cell-Free Transcription-Translation (TX-TL) Systems. ACS synthetic biology. 2014 doi: 10.1021/sb400206c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai K, Sawasaki T, Endo Y. Practical cell-free protein synthesis system using purified wheat embryos. Nat Protoc. 2010;5:227–238. doi: 10.1038/nprot.2009.207. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang YHP. Cell-free protein synthesis energized by slowly-metabolized maltodextrin. BMC Biotechnol. 2009;9:58. doi: 10.1186/1472-6750-9-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin G, Garces ED, Yang J, Zhang J, Tran C, Steiner AR, Roos C, Bajad S, Hudak S, Penta K, Zawada J, Pollitt S, Murray CJ. Aglycosylated antibodies and antibody fragments produced in a scalable in vitro transcription-translation system. mAbs. 2012;4:217–225. doi: 10.4161/mabs.4.2.19202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawada JF, Yin G, Steiner AR, Yang J, Naresh A, Roy SM, Gold DS, Heinsohn HG, Murray CJ. Microscale to manufacturing scale-up of cell-free cytokine production--a new approach for shortening protein production development timelines. Biotechnology and bioengineering. 2011;108:1570–1578. doi: 10.1002/bit.23103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.