Abstract
BACKGROUND & AIMS
IL-10 deficient mice develop TH1/TH17-mediated colitis and IL-10-producing regulatory T cells suppress colitis, implicating IL-10 in maintaining mucosal homeostasis. We transferred germ-free (GF) or specific pathogen free (SPF) CD4+ cells from wild-type (wt) or IL-10−/− (ko) mice into wt or IL-10 ko Rag2−/− recipients to assess the relative importance of immunoregulatory IL-10 derived from T cells vs. antigen presenting cells (APC).
METHODS
CD4+ cells from either GF or SPF IL-10 ko or wt mice were injected into wt Rag2−/− or IL-10 ko Rag2−/− recipients. After 6-8 weeks we evaluated inflammation, spontaneous colonic cytokine secretion, T-bet, and TGF-β mRNA expression. Co-cultured APC-CD4+ cells were assayed for cytokines and FoxP3 and TGF-β/Smad signaling.
RESULTS
CD4+ cells from either GF or SPF IL-10 ko or wt mice induced more severe colitis and increased mucosal proinflammatory cytokines in IL-10 ko Rag2−/− than in wt Rag2−/− recipients. Either ko or wt CD4+ cells co-cultured with bacterial-pulsed IL-10 ko APC produced more IFN-γ, IL-12/23p40 and IL-17 than the same T cells cultured with wt APC. CD11b-positive APC were required for these effects. Blocking IL-10 receptors enhanced IFN-γ and IL-12/23p40 production while exogenous IL-10 suppressed these cytokines. IL-10-producing APC induced TGF-β-mediated retinoic acid-dependent differentiation of FoxP3+ Treg cells, while in vitro and in vivo blockade of the retinoic acid receptor reduced proportions of FoxP3+ cells.
CONCLUSIONS
IL-10 produced by APC is a key regulator of homeostatic T cell responses to commensal bacteria.
Keywords: APC, CD4+ cells, colitis, homeostasis
Inflammatory bowel diseases (IBD), including Crohn's disease and ulcerative colitis, are T cell-mediated inflammatory disorders induced by aggressive mucosal CD4+ cell responses to commensal enteric bacteria (1-3). Interleukin-10 (IL-10), in combination with transforming growth factor β (TGF-β), is a key inhibitor of effector T cell activation and mediator of mucosal homeostasis (1;4;5). IL-10 and IL-10 receptor beta (IL-10RB) deficient mice spontaneously develop TH1/TH17-mediated colitis when normal microbiota are present (6;7), whereas recombinant IL-10 delivered by parenteral injection or genetically engineered enteric bacteria prevents onset of experimental enterocolitis (8-10) and improves epithelial barrier properties (11). Furthermore, polymorphisms of either the IL-10 or IL-10R genes are associated with ulcerative colitis (12) and early onset pediatric Crohn's disease (13). Mutations in IL-10R A and B associated with severe, early onset fistulizing Crohn's disease mediate loss of functional IL-10 signaling and STAT3 (signal transducer and activator of transcription 3) phosphorylation (13). Thus, IL-10 has a crucial role in maintaining intestinal homeostasis (4).
IL-10 is produced by T cells, certain B cells, macrophages, dendritic cells (DC) and keratinocytes. This cytokine's immunosuppressive and anti-inflammatory activities are mediated through STAT3 (14) and act directly on macrophages and dendritic cells to inhibit IL-12 secretion and downregulate the expression of MHC Class II and costimulatory molecules (4;15-17). In addition, IL-10 directly affects T cell effector function by inhibiting T cell cytokine secretion and proliferation (18-20).
Multiple studies implicate regulatory CD4+ T cell subsets producing IL-10 in mucosal homeostasis (21-25). In vivo neutralization of IL-10 or transfer of IL-10−/− CD45RBlow CD4+ cells prevented inhibition of colitis by regulatory cells in the CD4+CD45RBhi/low T cell cotransfer SCID mouse model (21), however IL-10 deficient CD25+CD4+ cells, while less effective than IL-10 sufficient cells, nevertheless partially reverse colitis in the T cell transfer model (22). Selective deletion of IL-10 in CD4+ cells induces colitis (23) and ablation of IL-10 in FoxP3-expressing T cells also generates mild colitis (24). Furthermore, IL-10-secreting CD4+ T regulatory cells recognizing colonic bacterial antigens prevent colitis induced by bacterial antigen-specific CD4+ cells (25). IL-10 derived from cells other than T cells may be of importance in immunity to certain pathogens and regulation of colitis (26-28). However, the relative functional role of IL-10 produced by antigen presenting cells (APC) vs. T lymphocytes in mucosal immunoregulation remains uncertain.
We performed an in depth analysis of the innate and acquired immune response in IL-10 normal (wt) Rag2−/− or IL-10 deficient (ko) Rag2−/− recipients of IL-10 wt or IL-10 ko CD4+ cells. We assessed functional activities of IL-10 derived from T cells vs. APC in suppressing pathogenic TH1/TH17 immune responses to antigens of commensal intestinal microbiota in vivo and in vitro. Our results suggest that production of IL-10 by APC is a key determinant in preventing onset of bacterial antigen-driven CD4+ cell TH1/TH17-mediated chronic experimental colitis. Our studies mechanistically extend the recent observation that myeloid cell production of IL-10 maintains in vivo expression of FoxP3 during intestinal inflammation and prevents colitis in the CD45RBhigh CD4+ cell transfer model (29).
Materials and Methods
Mice
IL-10 ko mice (129S6/SvEv background) and Rag2−/− mice (129S6/SvEv background) (Taconic Farms, Germantown, NY) were crossed to obtain IL-10 ko/Rag2−/− double-deficient mice, which lack T and B cells and IL-10 production. GF mice were derived and maintained in the UNC National Gnotobiotic Rodent Resource Center.
Transfer of CD4+ cells and treatment of recipient mice
SPF IL-10 ko Rag-2−/− and IL-10 wt Rag2−/− mice were injected intraperitoneally with 5 × 105 CD4+ cells from spleens of either GF or SPF IL-10 wt or IL-10 ko donors. In a separate experiment, recipients of SPF IL-10 wt CD4+ T cells were given 100 μg of LE540 (Wako, Japan) or vehicle (1:1 DMSO plus soybean oil) by gavage 2 days before T cell transfer then every other day for the two week duration of the experiment.
Analysis of inflammation - see supplementary material.
Cell preparation, purification and culture - see supplementary material.
Cytokine measurements
To detect production of IFN-γ, IL-12/23p40, IL-10 or IL-17, ELISAs were performed in triplicate using R&D Systems products. See supplementary material.
Real-time PCR - see supplementary material
Western blot analysis
Wild type CD4+ cells and IL-10 ko or IL-10 wt APC were mixed, stimulated with CBL (10μg/ml) in the presence or absence of TGF-β1, and phosphorylated Smad3 was evaluated as described in supplementary material.
Flow cytometry – see supplementary material
Statistical analysis
We used Prism 5 software (GraphPad, San Diego, CA) to compare means between two groups with two-tailed, unpaired Student's t tests; comparisons of means from multiple groups were analyzed with one-way ANOVA and Bonferroni post test. P-values lower than 0.05 were considered significant.
Results
Production of IL-10 by both non-T cells and by CD4+ cells determines susceptibility to chronic colitis
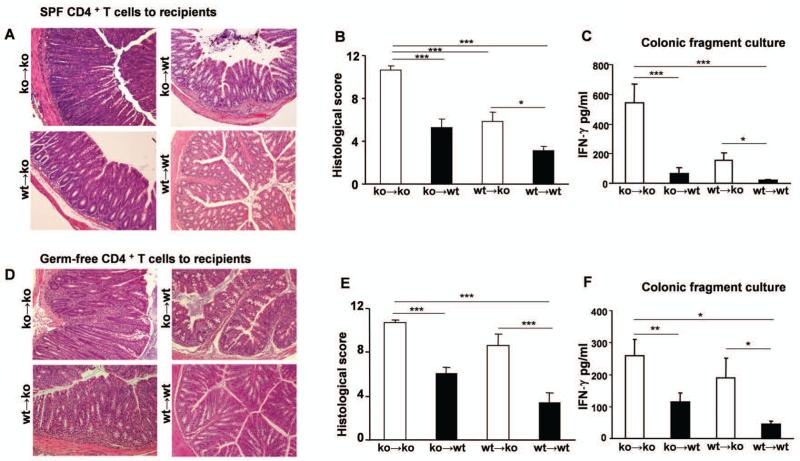
To directly assess the in vivo contribution of IL-10 derived from CD4+ cells vs. non-T cells in the development of colitis, we transferred CD4+ cells from SPF IL-10 ko mice with active colitis or from normal wt IL-10-producing donor mice into IL-10 ko Rag2−/− or IL-10 wt Rag2−/− recipients. IL-10 ko CD4+ cell–reconstituted IL-10 ko recipients (ko→ko) displayed severe colitis compared with IL-10 wt recipients of IL-10 ko CD4+ cells (ko→wt) (Fig. 1A). Clinically, IL-10 ko recipient mice exhibited severe chronic diarrhea. Histologic features of colitis in IL-10 ko recipients appeared typical of that in IL-10 ko mice with robust lamina propria and submucosal infiltration of mononuclear cells, crypt abscesses, marked crypt hyperplasia, and near total goblet cell depletion (Fig 1A). Some mice developed mucosal ulcerations. Blinded histologic inflammatory scores confirmed more severe inflammation in IL-10 ko vs IL-10 wt recipients (p<0.001) that received IL-10 ko CD4+ cells (Fig 1B). IL-10 ko recipient mice reconstituted with CD4+ cells from normal IL-10 producing wt mice developed moderate intestinal inflammation (wt→ko), whereas IL-10 wt mice reconstituted with IL-10 wt CD4+ cells did not develop colitis (wt→wt). Spontaneous IFN-γ production by colonic explants was significantly higher in IL-10 ko recipients reconstituted with ko or wt CD4+ cells compared with IL-10 wt recipient mice (Fig 1C). Similarly, colonic IL-12/23p40 production was elevated in IL-10 ko recipients reconstituted with CD4+ cells compared with IL-10 wt recipients (data not shown). These results demonstrate a crucial protective role for IL-10 production by the non-T cell population in Rag2−/− recipient mice and indicate that IL-10 secreting regulatory CD4+ cells are not sufficient to prevent colitis in the absence of IL-10 production by non-T cells in the recipient mice. Expression of T-bet, a transcription factor that regulates adaptive andinnate proinflammatory immune responses and is required for IFN-γ production was higher in the colon of IL-10 ko mice after transfer of either IL-10 ko or IL-10 wt CD4+ cells, with lower levels of T-bet detected in the colon of IL-10 wt recipient mice (Supplementary Figure 1).
Figure 1. Colonic inflammation in IL-10 wt Rag2−/− or IL-10 ko Rag2−/− recipients reconstituted with either SPF or GF IL-10 ko or wt CD4+ cells.
(A) Representative H&E stained sections of the distal colons of recipient mice 6-8 wk after transfer of SPF CD4+ cells (20× magnification). (B) Blinded histologic scores in the large intestine and (C) spontaneous secretion of IFN-γ by colonic fragments of recipients of SPF CD4+ cells. (D) Representative H&E stained sections of the distal colons of recipient mice 6-8 wk after transfer of GF CD4+ cells. (E) Blinded histologic scores and (F) IFN-γ secretion by colonic fragments of recipients of GF CD4+ cells. Results show mean ± SEM, pooled from three separate experiments, with (ko→ko, n=14; ko→wt, n=12; wt→ko, n=11; wt→wt, n=11) for recipients of SPF CD4+ cells and (ko→ko, n=7; ko→wt, n=12; wt→ko, n=11; wt→wt, n=14) for recipients of GF CD4+ cells. *p< 0.05, **p<0.01 & ***p<0.001.
Transfer of CD4+ cells from germ-free donors induces colitis in SPF IL-10-deficient recipients
To examine whether CD4+ cells that have not been stimulated by in vivo exposure to bacterial components develop into pathogenic effector cells that mediate disease in SPF IL-10 ko recipients, we transferred CD4+ cells from GF IL-10 ko mice, which have no colitis or clonal expansion of bacterial antigen-responsive CD4+ cells (31). Adoptive transfer of CD4+ cells from GF IL-10 ko mice induced severe colitis in SPF IL-10 ko recipients that was comparable to that seen after transfer of activated CD4+ cells from SPF IL-10 ko mice (compare Figs 1B and E). Likewise, reconstitution of IL-10 wt recipients with CD4+ cells from GF IL-10 ko mice induced mild to moderate inflammation (Fig 1E). Transfer of GF IL-10 wt CD4+ cells did not induce inflammation in IL-10 wt recipients but induced moderate-severe colitis in IL-10 ko hosts (Fig 1E). Spontaneous IFN-γ production by colonic explants was higher in IL-10 ko recipients reconstituted with GF IL-10 ko or GF IL-10 wt CD4+ cells compared with IL-10 wt − recipient mice (Fig 1F). These results demonstrate that CD4+ cells from both IL-10 ko and IL-10 wt GF mice can be programmed by IL-10 deficient APC to induce colitis in the presence of enteric bacterial antigens.
IFN-γ, IL-17 and IL-12/23p40 responses are potentiated in IL-10 ko Rag2−/− recipients regardless of IL-10 production by transferred CD4+ cells
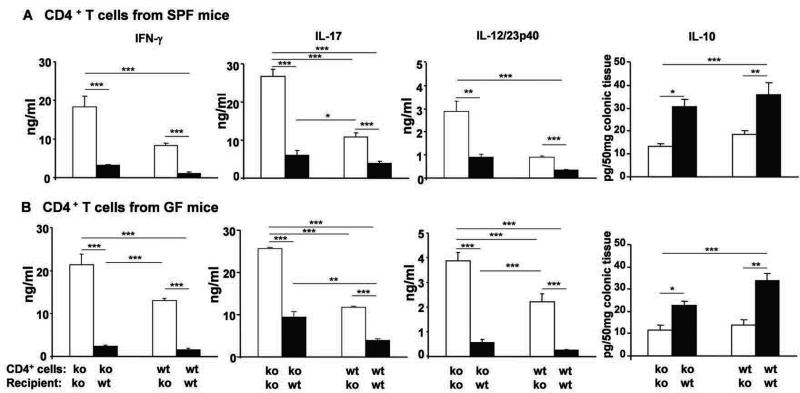
To further confirm differences in pathogenic cytokine production in IL-10 normal and deficient recipients after adoptive transfer, we examined in vitro cytokine responses to physiologic luminal cecal bacterial stimulation. Unfractionated MLN cells from recipient mice were stimulated in vitro with cecal bacterial lysate (CBL) and levels of IFN-γ, IL-17, and IL-12/23 p40 were measured. MLN cells collected from IL-10 ko recipients reconstituted with either SPF (Fig 2A) or GF (Fig 2B) IL-10 ko CD4+ cells produced markedly higher levels of IFN-γ, IL-17 and IL-12/23p40 following stimulation by CBL than did cells from IL-10 wt recipients. Similarly, levels of these proinflammatory cytokines were higher after CBL stimulation of MLN cells from IL-10 ko recipients of SPF or GF IL-10 wt CD4+ cells compared to cells from IL-10 wt recipients. These results indicate that non-T cell-derived IL-10 inhibits development of pathogenic T cell responses and promotes immunologic tolerance to luminal bacterial components in vivo, with some additional contribution by IL-10 producing CD4+ cells. Supporting this concept, ex vivo IL-10 levels were highest in supernatants of colonic fragment cultures from IL-10 wt recipients, with very little production by IL-10 ko recipients of IL-10 wt CD4+ cells compared with IL-10 ko recipients of IL-10 ko CD4+ cells. This suggests that IL-10 wt CD4+ cells in IL-10 ko recipients produced very little IL-10 in ex vivo cultures, although these cells produced some IL-10 in IL-10 wt recipients.
Figure 2. Cytokines produced by MLN cells after transfer of SPF or GF IL-10 ko or IL-10 wt CD4+ T cells.
Six-eight weeks after transfer of either SPF (A) or GF (B) CD4+ cells, unseparated MLN cells from IL-10 ko Rag2−/− or IL-10 wt Rag2−/− recipients were stimulated with CBL for 72 hours and IFN-γ, IL-17 and IL-12/23p40 secretion were measured. Spontaneous IL-10 by colonic fragments was evaluated. Results show mean ± SEM (number of mice/group same as Figure 1). *p< 0.05, **p<0.01, ***p<0.001.
Kinetic analysis of inflammation and proinflammatory cytokine production in ko→ko and ko→wt mice
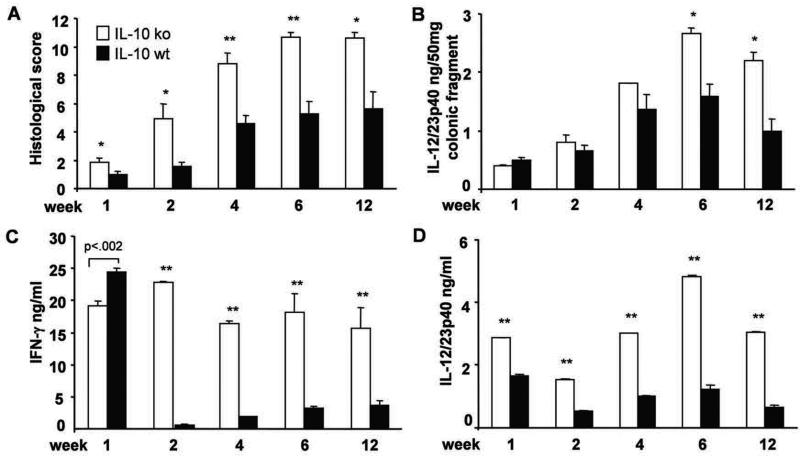
We next investigated the time course of colitis and pathogenic cytokine production after transfer of CD4+ cells from SPF IL-10 ko mice with colitis to IL-10 ko or IL-10 wt recipients. Histologic scores peaked 6 weeks after CD4+ cell transfer and were consistently higher in ko→ko relative to ko→wt mice at all times investigated (Fig 3A). Spontaneous secretion of IL-12/23p40 in overnight cultures of colonic tissues indicated greater innate immune cell activation in IL-10 ko compared to IL-10 wt recipients with statistically significant differences observed 6 weeks after transfer (Fig 3B). In vitro stimulation of MLN cells with CBL showed rapid activation of acquired and innate immune responses in IL-10 ko recipients, with near maximum IFN-γ secretion 1 week after CD4+ cell transfer (Fig 3C). Of interest, IFN-γ production 1 week after transfer of activated IL-10 ko CD4+ cells to IL-10 wt (ko→wt) mice was slightly higher than in IL-10 ko recipients (ko→ko), but rapidly decreased to consistently lower levels 2-12 weeks after transfer (Fig 3C). Production of CBL stimulated IL-12/23 p40 followed a similar time course (Fig 3D). These findings suggest that activated CBL-responsive CD4+ cells are rapidly down-regulated by IL-10-secreting APC, while lack of immunologic tolerance to commensal bacterial components persists in the absence of IL-10.
Figure 3. Time course of histological evaluation of colitis and proinflammatory cytokine production in recipient mice.
(A) Histologic score, (B) spontaneous IL-12/23p40 in colonic cultures, and amounts of (C) IFN-γ and (D) IL-12/23p40 produced by unfractionated MLN cells stimulated with CBL (10 μg/ml) after 3 day culture in vitro for mice evaluated 1, 2, 4, 6 and 12 weeks after transfer of SPF IL-10 ko CD4+ T cells to IL-10 ko Rag2−/− or IL-10 wt Rag2−/− recipients. ** p< 0.001 and *p<0.05 or p value shown above the bar vs. IL-10 wt Rag2−/− recipients. Results show mean ± SEM, 6-8 mice per group at each time point.
IL-10 production by APC inhibits bacterial antigen-stimulated CD4+ TH1 and TH17 cell responses in vitro
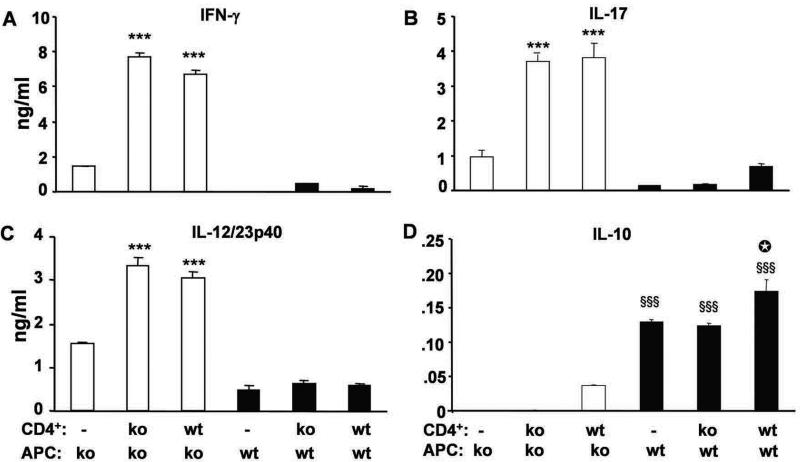
To further investigate the relative roles of IL-10 produced by APC vs. T lymphocytes in inflammatory responses, we performed APC-CD4+ cell co-cultures in vitro. CBL-pulsed IL-10 ko Rag2−/− APC co-cultured with either IL-10 wt or ko splenic CD4+ lymphocytes stimulated high amounts of IFN-γ, IL-17 and IL-12/23p40 secretion (Fig 4), whereas CD4+ cells stimulated with CBL-pulsed IL-10 wt Rag2−/− APC secreted much lower amounts of these cytokines. No significant differences were seen with CD4+ cells from IL-10 wt or IL-10 ko mice. These data, showing a dominant effect on bacterial antigen-stimulated TH1 and TH17 responses by CBL-pulsed APC, further confirm our in vivo results, which indicated that non-T cells determine responses of CD4+ cells after adoptive transfer.
Figure 4. In vitro cytokine production.
Splenic CD4+ T cells from SPF IL-10 ko or IL-10 wt mice were co-cultured with CBL-pulsed APC from IL-10 ko Rag2−/− or IL-10 wt Rag2−/− mice for 72 hours and (A) IFN-γ (B) IL-17, (C) IL-12/23p40 (D) IL-10 concentrations were measured. Representative results (one of three separate experiments) are shown as mean ± SEM of triplicate culture supernatants. ***p<0.001 vs. co-cultures containing IL-10 wt APC. §§§p<0.001 vs co-cultures containing IL-10 ko APC. □p<0.001 vs IL-10 wt APC alone or in co-culture with IL-10 ko CD4+ cells.
Because both APC and CD4+ cells produce IL-10, we designed experiments to evaluate the relative amounts of IL-10 produced by APC and by T cells. IL-10 wt CD4+ cells co-cultured with CBL-pulsed IL-10 ko APC secreted low levels of IL-10 (Fig 4D). CBL-pulsed IL-10 wt APC cultured alone (without T cells) secreted higher amounts of IL-10. We observed a small incremental increases in IL-10 secretion in co-cultures containing IL-10 wt CD4+ cells compared to IL-10 ko CD4+ cells stimulated with CBL-pulsed IL-10 wt APC. These results indicate that APC from normal IL-10-replete hosts are the primary source of immunosuppressive IL-10 in response to physiologic intestinal bacterial stimulation, with a small contribution from IL-10-secreting CD4+ cells.
We further investigated the effects of IL-10 production in vitro by either blocking the IL-10 receptor or by adding exogenous IL-10 to APC during pulsing with cecal bacterial lysate (Supplementary Figures 2 and 3). Our results indicate that suppressive activity of IL-10 signaling acts primarily in APC.
CD11b+ IL-10 wt APC regulate bacterial antigen-stimulated TH1 and TH17 responses
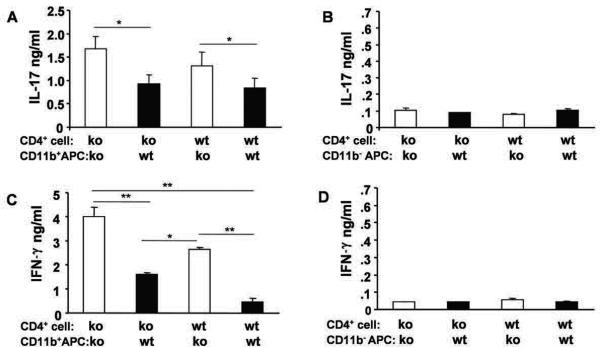
To determine which type of APC regulated CBL-induced TH1 and TH17 responses, we separated APC from IL-10 ko and wt Rag2−/− mice into CD11b-enriched or CD11b-depleted populations. Lower amounts of IL-17 and IFN-γ were produced in co-cultures containing CD11b-enriched wt APC compared to CD11b-enriched IL-10 ko APC co-cultures (Fig. 5A and 5C), suggesting that IL-10 secreting CD11b+ cells regulate CBL-induced cytokine proinflammatory production. As demonstrated in Fig. 5B and 5D, CD11b-depleted APC had little if any ability to activate IL-17 or IFN-γ producing CD4+ cells.
Figure 5. CD11b-enriched APC regulate cytokine production.
APC from IL-10 ko Rag2−/− or IL-10 wt Rag2−/− mice were separated into CD11b-enriched (CD11b+) or CD11b-depleted (CD11b−) populations by positive or negative magnetic bead sorting, respectively. Co-cultures contain either IL-10 ko or IL-10 wt splenic CD4+ T cells and CBL-pulsed APC. (A, B) IL-17 or (C, D) IFN-γ was measured in supernatants collected 72 hours after co-culture initiation. Representative results (one of three separate experiments) are shown as mean ± SEM of triplicate culture supernatants. *p< 0.05, **p<0.01.
IL-10 producing APC induce regulatory T cell differentiation in a TGF-β and retinoic acid-dependent manner
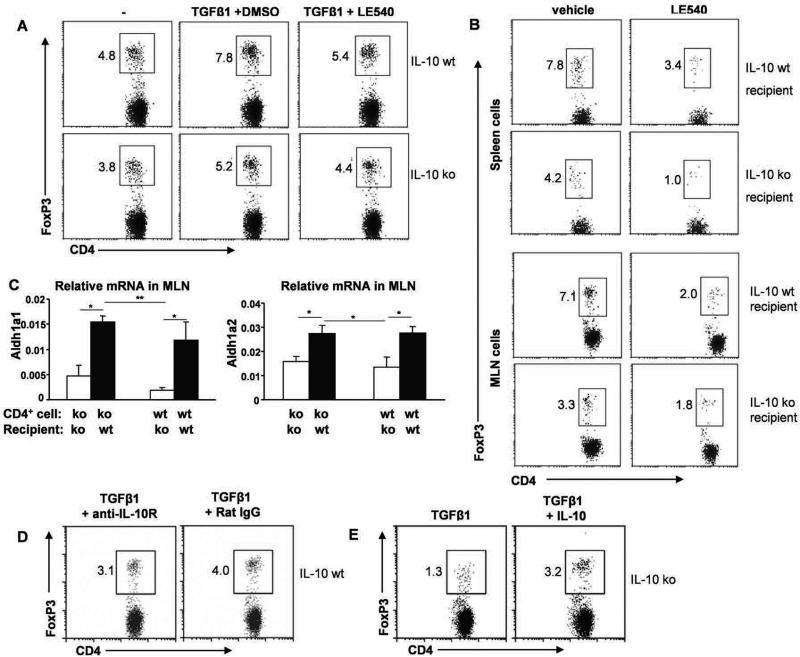
To evaluate the influence of IL-10 producing APC on FoxP3+ Treg cell development, we stimulated IL-10 wt CD4+ cells with CBL-pulsed APC derived from either IL-10 wt or ko Rag2−/− mice with or without TGF-β. After 4 days, a greater porportion of CD4+ cells co-cultured with CBL-pulsed IL-10 wt APC vs IL-10 ko APC expressed FoxP3 (Fig 6A). To investigate mechanisms by which IL-10-producing APC efficiently generate FoxP3+ Treg cells, we examined the role of the vitamin A metabolite retinoic acid (RA). A synthetic retinoic acid receptor antagonist LE540 added to APC–CD4+ cell co-cultures reduced the number of TGF-β-dependent FoxP3+ Treg cells induced by IL-10-producing APC from 7.8% to 5.4% while IL-10 ko APC were less efficient (5.2% to 4.4%) (Fig 6A). These results indicate that both IL-10 and retinoic acid contribute to TGF-β-mediated generation of inducible or expandable FoxP3+ Treg cells by CBL-pulsed APC. To further investigate the retinoic acid receptor pathway in combination with IL-10 in regulating Foxp3+ Treg population in vivo, cells from LE540-treated mice were evaluated. Fewer FoxP3+ cells were seen in both spleens and MLN of LE540-treated compared to the vehicle-treated control recipients (Fig. 6B). Expression of Aldh1a1 and Aldh1a2, which encode dehydrogenases that convert vitamin A to RA, was monitored by real-time PCR in MLN from IL-10 ko or wt recipient mice after CD4+ cell transfer. Significant upregulation of Aldh1a1 and Aldh1a2 mRNA was observed in MLN of IL-10 wt compared to IL-10 ko recipient mice 6 weeks after transfer of either IL-10 ko or IL-10 wt CD4+ cells (Fig 6C). These results suggest that IL-10 produced by APC stimulates expression of isoforms of this RA enzyme.
Figure 6. Induction of FoxP3+ Treg cells by IL-10 wt Rag2−/− or IL-10 ko Rag2−/− APC.
(A) Intracellular FoxP3 expressed by IL-10 wt CD4+ cells co-cultured with IL-10 wt or IL-10 ko Rag2−/− APC for 4 days in the presence or absence of TGF-β1 plus LE540 or vehicle (DMSO). (B) Intracellular FoxP3 in CD4+ cells from recipients treated in vivo with LE540 or vehicle (DMSO in soybean oil). (C) Expression of Aldh1a1 and Aldh1a2 mRNA relative to β-actin in MLN from IL-10 ko Rag2−/− or IL-10 wt Rag2−/− recipient mice. Representative results (one of two separate experiments) are shown as mean ± SEM of triplicate qPCR. *p< 0.05, **p<0.01. (D) FoxP3 expression by IL-10 wt CD4+ cells co-cultured with IL-10 wt APC in the presence of TGF-β1(1 ng/ml) and anti-IL-10R or isotype control (30 μg/ml). (E) FoxP3 expression by IL-10 wt CD4+ cells co-cultured with IL-10 ko APC in the presence of TGF-β1 (1 ng/ml) with or without recombinant IL-10 (500 pg/ml) for 4 days. Representative results (one of three experiments) are shown for D and E.
We further investigated the ability of endogenous IL-10 from APC to induce FoxP3 generation by adding anti-IL-10R antibody to IL-10 wt CD4+ cells co-cultured with IL-10 wt Rag2−/− APC and TGF-β1. In cultures containing TGF-β1 plus anti-IL-10R, fewer CD4+ cells expressed FoxP3 compared to CD4+ cells cultured with TGF-β plus rat IgG isotype control (Fig 6D). Conversely, TGF-β1-stimulated Foxp3 expression was higher in IL-10 wt CD4+ cells co-cultured with IL-10 ko APC in the presence of exogenous IL-10 compared to cultures that did not contain added IL-10 (Fig 6E). These results indicate that IL-10-producing APC stimulated by commensal bacterial components promote the generation and/or expansion of FoxP3-expressing Treg cells in the presence of TGF-β.
IL-10 producing APC induce TGF-β1-mediated Smad signaling
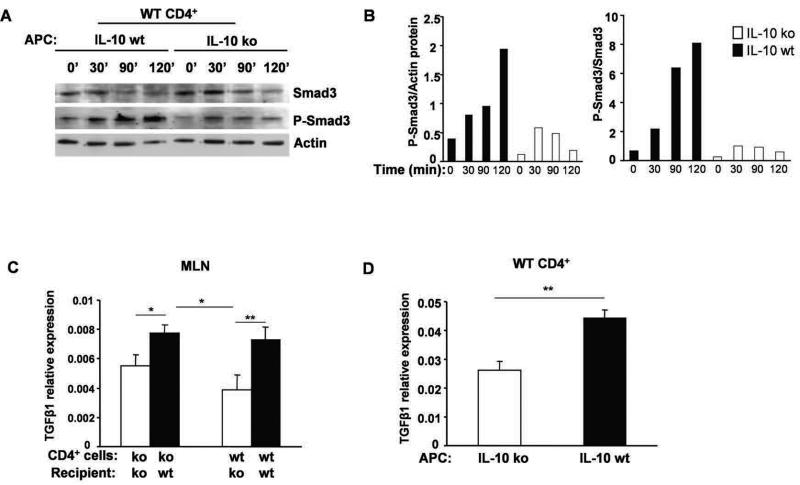
To investigate a possible role for APC-derived IL-10 in TGF-β-mediated Smad signaling, IL-10 wt or ko APC were pulsed with CBL and co-cultured with IL-10 wt CD4+ cells with or without TGF-β1. Following TGF-β1 stimulation, Smad3 was progressively phosphorylated in IL-10 wt CD4+ cells co-cultured with APC from IL-10 wt mice over 2 hours but only transiently phosphorylated to a lesser degree in CD4+ cells co-cultured with APC from IL-10 ko mice (Fig 7A and B).
Figure 7. Smad3 and P-Smad3 protein and TGF-β1 mRNA expression.
APC from IL-10 wt Rag2−/− and IL-10 ko Rag2−/− mice co-cultured with IL-10 wt CD4+ cells were stimulated with TGF-β1 in the presence of CBL (10 μg/ml). Cells were harvested at times shown and Western blot analysis visualized Smad3 and P-Smad3. (A) Representative blots from two experiments are shown. (B) Densitometric analysis of the Western blot shown in (A) p-Smad3/actin left panel, p-Smad3/total Smad3 right panel. (C) TGF-β1 mRNA expression in MLN from IL-10 ko Rag2−/− or IL-10 wt Rag2−/− recipient mice reconstituted with IL-10 ko or IL-10 wt CD4+ cells. Representative results (one of three experiments) are shown as mean ± SEM of triplicate qPCR. (D) TGF-β1 mRNA expression in co-cultured IL-10 wt CD4+ cells with CBL-pulsed APC from either IL-10 ko Rag2−/− or IL-10 wt Rag2−/− mice. Representative results (one of two separate experiments) are shown as mean ± SEM of triplicate qPCR.
TGF-β is known to play a key role in controlling proliferation, differentiation, and function of numerous immune and non-immune cells and to interact with IL-10 (5). To determine if IL-10 from APC influenced TGF-β expression in this transfer model, TGF-β1 mRNA in MLN of recipient mice was evaluated. The levels of mRNA expression of TGF-β in the MLN of IL-10 wt recipients were higher than those in IL-10 ko recipients after 6 weeks irrespective of whether wt or ko CD4+ cells were transferred (Fig 7C). In parallel studies using in vitro APC-CD4+ cell co-cultures, higher levels of TGF-β1 mRNA were detected in APC from IL-10 wt mice compared to IL-10 ko mice co-cultured with IL-10 wt CD4+ cells (Fig 7D). These results indicate that IL-10 produced by APC promotes both TGF-β expression and signaling.
Discussion
We demonstrate that IL-10 production by APC is a key mediator of mucosal homeostasis. Lack of IL-10 in recipient T cell-deficient mice resulted in unopposed activation of bacterial antigen-driven immune responses and onset of aggressive colitis following transfer of either IL-10 sufficient or deficient CD4+ cells. Prior in vivo bacterial antigen-mediated activation and clonal expansion was not required, since CD4+ cells from GF mice also induced colitis. In vitro APC-CD4+ cell co-cultures documented the key role of IL-10 secretion by APC in suppressing commensal bacterial antigen-driven IFN-γ and IL-17 production. Furthermore, we document interactions between APC-produced IL-10, TGF-β and retinoic acid in expanding CD4+ FoxP3+ cells.
Our results are consistent with several previous observations that non-T cell sources of IL-10 attenuate experimental colitis and other T cell-mediated inflammatory responses and also induce regulatory T cells (26;29;32-34). IL-10 produced by APC suppressed antigen-specific T cell responses in transgenic mice expressing IL-10 directed by the MHC Class II promoter (32), similar to the findings that constitutive expression of IL-10 in macrophages suppressed differentiation of antigen-activated naïve T cells to become effector cells and attenuated experimental autoimmune uveitis to a greater degree than did IL-10-transgenic T cell lines (34). Murai et al used a similar strategy to ours, transferring CD4+CD45RBhigh T cells into IL-10 deficient Rag1−/− recipient mice (29) to demonstrate that IL-10 produced by CD11b+ myeloid cells was required to maintain FoxP3 expression and inhibitory Treg activity during inflammation. Gregori et al. reported that a subset of IL-10-secreting human dendritic cells, DC-10 cells, induce TR1 cells via a IL-10- dependent ILT4/HLA-G signaling pathway (35). In preliminary studies, Nguyen et al demonstrated that Wiskott-Aldrich Syndrome (WASP) deficiency of innate immune cells was associated with reduced IL-10 production, defective Treg generation and induction of colitis by transfer of normal CD4+ cells (36). However, other investigations support the contrasting view that regulatory T cells are the requisite source of IL-10 that prevents and treats experimental colitis (21-24;37) and that induction of IL-10-secreting regulatory T cells (Tr1 and FoxP3- Treg) does not require IL-10 from other sources (38). Conditional deletion of IL-10 in CD4+ cells induces severe colitis similar to that in mice with global deletion of IL-10 (23) and selective deletion of IL-10 under control of the FoxP3 promoter results in colitis, although this is less aggressive than seen in IL-10−/− mice (24). The latter result and the less than complete protection of co-transferred IL-10 deficient CD4+CD25+ with IL-10-producing CD4+CD45RBhigh cells compared with total loss of protection with anti-IL-10R neutralizing antibody (22) suggest that non-Treg cell production of IL-10 contributes to protection against colitis. Darrasse-Jeze et al propose a feedback regulatory loop between DC and Treg cells dependent on Flt-c-induced DC proliferation, MHC II interaction with Treg and Treg secretion of IL-10, which decreases dendritic cell numbers (39). In a schistosomiasis model, IL-10 produced by both innate and CD25+ regulatory T cells prolongs host survival (40). Likewise, our in vivo transfer studies demonstrate combined inhibitory activities of IL-10 produced by CD4+ cells and by non-T/non-B cells, since we observed intermediate colitis scores in wt→ko and in ko→wt mice. In vitro, we observed more striking differences in proinflammatory cytokine production by CBL-stimulated IL-10 ko and IL-10 wt APC-CD4+ cell co-cultures, regardless of the source of CD4+ T cells (IL-10 deficient or replete), suggesting a more dominant inhibitory effect of IL-10 secreted by APC than by CD4+ cells. These results support and extend our previous studies that demonstrated synergistic stimulation of APC-derived IL-10 by CBL plus IFN-γ (41). In addition, we and others previously demonstrated that non-T cells regulate susceptibility to immune-mediated colitis in the HLA-B27 transgenic rat model (42;43).
In our kinetic analysis of adoptive transfer recipients, IFN-γ production by bacterial lysate-stimulated MLN CD4+ cells was quite high one week after transfer in ko→wt mice, but was rapidly downregulated by 2 weeks, consistent with suppressive activities of IL-10 produced by non-lymphoid cells in the Rag2−/− recipients. Mechanistic in vitro studies indicated that IL-10-producing APC induced TGF-β-stimulated FoxP3 expressing CD4+ Treg more efficiently than did IL-10 ko APC, facilitated SMAD signaling after TGF-β exposure, and augmented expression of TGF-β in APC-CD4+ cell co-cultures. In vivo confirmation of the latter results were provided by greater TGF-β expression in MLN of wt recipients compared with IL-10 ko mice after CD4+ cell transfer. Other investigators have demonstrated interactions between IL-10 and TGF-β to coordinately regulate homeostatic mucosal immune responses. IL-10 has been shown to facilitate expression of TGF-β-secreting Treg cells (5) and maintain FoxP3 expression and regulatory activity in an inflammatory milieu (29). We demonstrated that induction of TGF-β-stimulated FoxP3+ CD4+ Treg cells by CBL-activated IL-10-secreting APC was dependent on RA in vitro and in vivo. Furthermore, expression of Aldh1a1 and Aldh1a2, which convert vitamin A to RA, were upregulated in IL-10 wt mice compared with IL-10 ko recipients after CD4+ T cell transfer from either IL-10 ko or wt mice. RA function is complex, however, since RA reciprocally regulates FoxP3 and IL-10 in CD4+ Treg following activation of dendritic cells by TLR 9 ligation (44).
In conclusion, the observation that IL-10 production by APC is a key factor in inhibiting bacterial antigen responsive CD4+ TH1 and TH17 effector cells that cause colitis can focus therapeutic efforts to stimulate this pathway. These efforts must consider, however, that a small subset of IBD patients may be refractory to exogenous or endogenous IL-10 due to loss of IL-10 signaling (13), comparable to that seen in HLA-B27 transgenic rats (45).
Supplementary Material
Acknowledgements
The authors thank Susie May for expert secretarial support, Lisa Holt, Yoshiyuki Mishima, and Guoling Luo for technical assistance, and Joseph Galanko, Biostatistics Core of the Center for Gastrointestinal Biology and Disease.
These studies were funded by UPHS grants R01 DK 053347, P40 RR018603 (National Gnotobiotic Rodent Resource Center), P30 DK034987 (Center for Gastrointestinal Biology and Disease), and the Crohn's and Colitis Foundation of America.
Abbreviations
- APC
antigen presenting cells
- CBL
cecal bacterial lysate
- FoxP3
forkhead box P3
- GF
germ-free
- IFN
interferon
- IL
interleukin
- ko
knockout
- MLN
mesenteric lymph nodes
- RA
retinoic acid
- Rag2
recombination activating gene 2
- SPF
specific pathogen free
- TGF
transforming growth factor
- TH
T helper cell
- Treg
T regulatory cell
- wt
wild type
Footnotes
The authors have no conflicts of interest.
Author Involvement:
Bo Liu, DVM, Ph.D.: Acquisition of data, analysis and interpretation of data, drafting the manuscript.
Susan L. Tonkonogy, Ph.D.: Analysis and interpretation of data, critical revision of the manuscript for important intellectual content.
R. Balfour Sartor, M.D.: Study concept and design, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, obtained funding.
Preliminary results were reported in abstract form at the 2007 Annual Meeting of the American Gastroenterological Association (Digestive Disease Week)
REFERENCES
- 1.Xavier RJ, Podolsky DK. Unraveling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 2.Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134:577–594. doi: 10.1053/j.gastro.2007.11.059. [DOI] [PubMed] [Google Scholar]
- 3.Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066–2078. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maynard CL, Weaver CT. Diversity in the contribution of interleukin-10 to T-cell-mediated immune regulation. Immunol Rev. 2008;226:219–233. doi: 10.1111/j.1600-065X.2008.00711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuss IJ, Boirivant M, Lacy B, et al. The interrelated roles of TGF-beta and IL-10 in the regulation of experimental colitis. J.Immunol. 2002;168:900–908. doi: 10.4049/jimmunol.168.2.900. [DOI] [PubMed] [Google Scholar]
- 6.Kuhn R, Lohler J, Rennick D, et al. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 7.Spencer SD, Di Marco F, Hooley J, et al. The orphan receptor CRF2-4 is an essential subunit of the interleukin 10 receptor. J.Exp.Med. 1998;187:571–578. doi: 10.1084/jem.187.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berg DJ, Davidson N, Kuhn R, et al. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J.Clin.Invest. 1996;98:1010–1020. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herfarth HH, Mohanty SP, Rath HC, et al. Interleukin 10 suppresses experimental chronic, granulomatous inflammation induced by bacterial cell wall polymers. Gut. 1996;39:836–845. doi: 10.1136/gut.39.6.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steidler L, Hans W, Schotte L, et al. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science. 2000;289:1352–1355. doi: 10.1126/science.289.5483.1352. [DOI] [PubMed] [Google Scholar]
- 11.Madsen KL, Lewis SA, Tavernini MM, et al. Interleukin 10 prevents cytokine-induced disruption of T84 monolayer barrier integrity and limits chloride secretion. Gastroenterology. 1997;113:151–159. doi: 10.1016/s0016-5085(97)70090-8. [DOI] [PubMed] [Google Scholar]
- 12.Franke A, Balschun T, Karlsen TH, et al. Sequence variants in IL10, ARPC2 and multiple other loci contribute to ulcerative colitis susceptibility. Nat Genet. 2008;40:1319–1323. doi: 10.1038/ng.221. [DOI] [PubMed] [Google Scholar]
- 13.Glocker EO, Kotlarz D, Boztug K, et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med. 2009;361:2033–2045. doi: 10.1056/NEJMoa0907206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams L, Bradley L, Smith A, et al. Signal transducer and activator of transcription 3 is the dominant mediator of the anti-inflammatory effects of IL-10 in human macrophages. J Immunol. 2004;172:567–576. doi: 10.4049/jimmunol.172.1.567. [DOI] [PubMed] [Google Scholar]
- 15.de Waal M, Haanen J, Spits H, et al. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J.Exp.Med. 1991;174:915–924. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fiorentino DF, Zlotnik A, Mosmann TR, et al. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–3822. [PubMed] [Google Scholar]
- 17.Ding L, Linsley PS, Huang LY, et al. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. J Immunol. 1993;151:1224–1234. [PubMed] [Google Scholar]
- 18.de Waal Malefyt R, Yssel H, de Vries JE. Direct effects of IL-10 on subsets of human CD4+ T cell clones and resting T cells. Specific inhibition of IL-2 production and proliferation. J Immunol. 1993;150:4754–4765. [PubMed] [Google Scholar]
- 19.Schandene L, Alonso-Vega C, Willems F, et al. B7/CD28-dependent IL-5 production by human resting T cells is inhibited by IL-10. J Immunol. 1994;152:4368–4374. [PubMed] [Google Scholar]
- 20.Taga K, Mostowski H, Tosato G. Human interleukin-10 can directly inhibit T-cell growth. Blood. 1993;81:2964–2971. [PubMed] [Google Scholar]
- 21.Asseman C, Mauze S, Leach MW, et al. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J.Exp.Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uhlig HH, Coombes J, Mottet C, et al. Characterization of Foxp3+CD4+CD25+ and IL-10-secreting CD4+CD25+ T cells during cure of colitis. J Immunol. 2006;177:5852–5860. doi: 10.4049/jimmunol.177.9.5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roers A, Siewe L, Strittmatter E, et al. T cell-specific inactivation of the interleukin 10 gene in mice results in enhanced T cell responses but normal innate responses to lipopolysaccharide or skin irritation. J Exp Med. 2004;200:1289–1297. doi: 10.1084/jem.20041789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubtsov YP, Rasmussen JP, Chi EY, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 25.Cong Y, Weaver CT, Lazenby A, et al. Bacterial-reactive T regulatory cells inhibit pathogenic immune responses to the enteric flora. J.Immunol. 2002;169:6112–6119. doi: 10.4049/jimmunol.169.11.6112. [DOI] [PubMed] [Google Scholar]
- 26.Mizoguchi A, Mizoguchi E, Takedatsu H, et al. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002;16:219–230. doi: 10.1016/s1074-7613(02)00274-1. [DOI] [PubMed] [Google Scholar]
- 27.Kane MM, Mosser DM. The role of IL-10 in promoting disease progression in leishmaniasis. J Immunol. 2001;166:1141–1147. doi: 10.4049/jimmunol.166.2.1141. [DOI] [PubMed] [Google Scholar]
- 28.Lang R, Rutschman RL, Greaves DR, et al. Autocrine deactivation of macrophages in transgenic mice constitutively overexpressing IL-10 under control of the human CD68 promoter. J Immunol. 2002;168:3402–3411. doi: 10.4049/jimmunol.168.7.3402. [DOI] [PubMed] [Google Scholar]
- 29.Murai M, Turovskaya O, Kim G, et al. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat Immunol. 2009;10:1178–1184. doi: 10.1038/ni.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Veltkamp C, Tonkonogy SL, de Jong YP, et al. Continuous stimulation by normal luminal bacteria is essential for the development and perpetuation of colitis in Tg(epsilon26) mice. Gastroenterology. 2001;120:900–913. doi: 10.1053/gast.2001.22547. [DOI] [PubMed] [Google Scholar]
- 31.Sellon RK, Tonkonogy S, Schultz M, et al. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun. 1998;66:5224–5231. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Groux H, Cottrez F, Rouleau M, et al. A transgenic model to analyze the immunoregulatory role of IL-10 secreted by antigen-presenting cells. J Immunol. 1999;162:1723–1729. [PubMed] [Google Scholar]
- 33.De Winter H, Elewaut D, Turovskaya O, et al. Regulation of mucosal immune responses by recombinant interleukin 10 produced by intestinal epithelial cells in mice. Gastroenterology. 2002;122:1829–1841. doi: 10.1053/gast.2002.33655. [DOI] [PubMed] [Google Scholar]
- 34.Agarwal RK, Horai R, Viley AM, et al. Abrogation of anti-retinal autoimmunity in IL-10 transgenic mice due to reduced T cell priming and inhibition of disease effector mechanisms. J Immunol. 2008;180:5423–5429. doi: 10.4049/jimmunol.180.8.5423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gregori S, Tomasoni D, Pacciani V, et al. Differentiation of type 1 T regulatory (Tr1) cells by tolerogenic DC-10 requires the IL-10-dependent ILT4/HLA-G pathway. Blood. 2010;116:935–44. doi: 10.1182/blood-2009-07-234872. [DOI] [PubMed] [Google Scholar]
- 36.Nguyen DD, Boden EK, Maillard MH, Nagler CR, Snapper SB. Abnormal innate immune cells can induce colitogenicity in normal adaptive immune cells. Gastroenterology. 2008;134(4 Suppl 1):A43. [Google Scholar]
- 37.Hagenbaugh A, Sharma S, Dubinett SM, et al. Altered immune responses in interleukin 10 transgenic mice. J Exp Med. 1997;185:2101–2110. doi: 10.1084/jem.185.12.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maynard CL, Harrington LE, Janowski KM, et al. Regulatory T cells expressing interleukin 10 develop from Foxp3+ and Foxp3-precursor cells in the absence of interleukin 10. Nat Immunol. 2007;8:931–941. doi: 10.1038/ni1504. [DOI] [PubMed] [Google Scholar]
- 39.Darrasse-Jeze G, Deroubaix S, Mouquet H, et al. Feedback control of regulatory T cell homeostasis by dendritic cells in vivo. J Exp Med. 2009;206:1853–1862. doi: 10.1084/jem.20090746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hesse M, Piccirillo CA, elkaid Y, et al. The pathogenesis of schistosomiasis is controlled by cooperating IL-10-producing innate effector and regulatory T cells. J Immunol. 2004;172:3157–3166. doi: 10.4049/jimmunol.172.5.3157. [DOI] [PubMed] [Google Scholar]
- 41.Albright CA, Sartor RB, Tonkonogy SL. Endogenous antigen presenting cell-derived IL-10 inhibits T lymphocyte responses to commensal enteric bacteria. Immunol Lett. 2009;123:77–87. doi: 10.1016/j.imlet.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoentjen F, Tonkonogy SL, Liu B, et al. Adoptive transfer of nontransgenic mesenteric lymph node cells induces colitis in athymic HLA-B27 transgenic nude rats. Clin Exp.Immunol. 2006;143:474–483. doi: 10.1111/j.1365-2249.2006.03013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Breban M, Hammer RE, Richardson JA, et al. Transfer of the inflammatory disease of HLA-B27 transgenic rats by bone marrow engraftment. J.Exp.Med. 1993;178:1607–1616. doi: 10.1084/jem.178.5.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maynard CL, Hatton RD, Helms WS, et al. Contrasting roles for all-trans retinoic acid in TGF-beta-mediated induction of Foxp3 and IL-10 genes in developing regulatory T cells. J Exp Med. 2009;206:343–357. doi: 10.1084/jem.20080950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qian BF, Tonkonogy SL, Sartor RB. Reduced responsiveness of HLA-B27 transgenic rat cells to TGF-β and IL-10-mediated regulation of IFN-γ production. Inflamm Bowel Dis. 2008;14:921–930. doi: 10.1002/ibd.20415. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.