Abstract
Greater inhibitory activity against Escherichia coli and levels of human β defensin (HBD)-2 in genital tract secretions predicted HIV acquisition in women in the HPTN 035 trial. We investigated whether higher levels of E. coli inhibitory activity and antimicrobial peptides in cervicovaginal lavage (CVL) samples predicted HIV acquisition in women in the CAPRISA 002 Acute Infection Study. E. coli inhibitory activity and antimicrobial peptides were quantified in CVL from a subset of CAPRISA 002 participants who did not seroconvert (n=39) and from seroconverting women prior to infection (n=17) and during acute infection (n=11). Women who acquired HIV had significantly greater preinfection CVL E. coli inhibitory activity (p=0.01) and HBD-1 levels (p=0.02) compared to women who remained uninfected. Preinfection E. coli inhibitory activity remained significantly associated with seroconversion following adjustment for the presence of bacterial vaginosis (OR 1.45; 95% CI 1.07, 1.97). Partial least squares discriminant analysis confirmed that preinfection CVL E. coli inhibitory activity, together with higher CVL concentrations of HBD-1 and secretory leukocyte protease inhibitor, distinguished seroconverters from nonseroconverters with 67% calibration accuracy. CVL concentrations of human neutrophil peptides (HNP) 1–3 increased significantly with acute infection (p=0.001) and correlated with plasma viral set point (r=0.66, p=0.03). E. coli inhibitory activity in genital tract secretions could provide a biomarker of HIV risk. The correlation between HNP 1–3 and viral set point merits further investigation of the relationship between mucosal inflammation during early HIV infection and disease progression.
Introduction
The ability of female genital secretions (FGS) to inhibit Escherichia coli growth in vitro may reflect contributions from molecules secreted by epithelial and immune cells, such as defensins and secretory leukocyte protease inhibitor (SLPI), and molecules secreted by the genital tract microbiota.1–5 However, the clinical relevance of E. coli inhibitory activity and the in vivo role of antimicrobial peptides in preventing genital tract infections and HIV are not well defined. Antimicrobial peptides such as defensins and cathelicidins may paradoxically increase the risk of HIV acquisition, transmission, and disease progression by promoting mucosal inflammation and target cell recruitment to the genital mucosa.6 Higher FGS concentrations of human neutrophil peptides (HNP) 1–3 and the cathelicidin LL-37 were independently associated with increased risk of HIV acquisition in a cohort of female sex workers in Kenya; it was hypothesized that higher concentrations of HNP 1–3 and LL-37 potentiated mucosal inflammation and target cell recruitment.7
In a substudy of the HPTN 035 trial, in which women at risk for HIV seroconversion were randomized to receive one of two topical HIV preexposure prophylaxis products (BufferGel or PRO 2000), placebo gel, or no gel, detectable human β defensin (HBD)-2 and higher E. coli inhibitory activity in FGS were associated with increased risk of HIV seroconversion [HBD-2 odds ratio (OR) 2.39; 95% (CI) 1.29, 4.41 and E. coli OR 1.22; 95% CI 1.04, 1.43].8 However, samples were available from only eight seroconverting women from the HPTN 035 trial substudy, and plasma viral set point data were not available to assess the relationship between E. coli inhibitory activity, antimicrobial peptides, and HIV progression.
The CAPRISA 002 Acute Infection Study, a prospective, observational study of uninfected women at high risk for HIV infection in KwaZulu-Natal, South Africa, reported that FGS inflammatory cytokine concentrations during acute infection were associated with higher viral set point and faster CD4+ T cell depletion at 12 months postinfection.9–11 While these studies suggested that genital inflammatory cytokine markers predicted more rapid HIV disease progression, they did not investigate antimicrobial peptide profiles or E. coli inhibitory activity. The objective of this study was to investigate the relationship between HIV acquisition risk and FGS E. coli inhibitory activity and antimicrobial peptides among a subset of women who participated in the CAPRISA 002 Acute Infection Study. Furthermore, we investigated whether FGS E. coli inhibitory activity and antimicrobial peptide concentrations were associated with plasma viral load set point, a correlate of rate of HIV disease progression.
Materials and Methods
Study subjects
This study was approved by the University of KwaZulu-Natal and the University of Cape Town Ethics Committees and by the Albert Einstein College of Medicine Institutional Review Board. A subset of women who participated in the CAPRISA 002 Acute Infection Study who had available CVL samples was included in this study.9 CAPRISA 002 participants attended monthly follow up visits for a maximum of 24 months. CVL (10 ml normal saline wash) was collected at enrollment into CAPRISA 002 and following HIV acquisition at the earliest time point when HIV infection was diagnosed.9 Time of HIV infection in the CAPRISA 002 Acute Infection Study was defined as the midpoint between the last negative HIV antibody test and the first positive antibody test or as 14 days prior to the first detectable plasma HIV-1 RNA on the same day as a negative HIV antibody test. Plasma viral load set point was defined as the moving average viral load closest to 52 (47–55) weeks postinfection.12
The primary objective of this study was to compare CVL E. coli inhibitory activity and antimicrobial peptide concentrations between preinfection samples from women who acquired HIV and samples from women who remained seronegative. The number of available samples was insufficient to allow for matching of participants based on clinical variables. CVL samples were available from 17 seroconverting women at a median of 41 weeks preinfection [interquartile range (IQR) 16.5–69] and from 39 nonseroconverting women within 4 months of enrollment. Nonseroconverting women remained HIV negative for the full duration (24 months) of follow-up. A secondary objective was to evaluate CVL E. coli inhibitory activity and antimicrobial peptide concentrations during acute infection and to identify potential associations between these variables and plasma viral load set point. CVL samples were available from 11 of the 17 seroconverting women at the time of acute infection [median 5 (IQR 5–7) weeks postinfection].
Study participants were screened for sexually transmitted infections (STI) as previously described.13 Bacterial vaginosis (BV) was defined as a Nugent score greater than seven. Herpes simplex virus (HSV)-1,2 DNA was detected from vaginal swabs by real-time multiplex polymerase chain reaction (M-PCR).14 CVL HIV-1 RNA was quantified using Nuclisens Easyq HIV-1 (version 1.2) (bioMerieux, Inc., Durham, NC). Plasma HIV-1 RNA was quantified using COBAS Amplicor HIV-1 Monitor version 1.5 or COBAS Ampliprep/COBAS TaqMan 48 Analyser (Roche Diagnostics, Branchburg, NJ), and CD4+ T cell counts (cells/μl) were quantified from blood by FACSCalibur (BD, Franklin Lakes, NJ).
Evaluation of CVL E. coli inhibitory activity and soluble immune mediators
Inhibitory activity against E. coli was quantified from CVL using a colony-forming unit (cfu) reduction assay.1 E. coli (ATCC strain 438227) was grown overnight to a concentration of approximately 109 cfu/ml. Bacteria (3 μl) were combined with CVL (27 μl) or control buffer. The mixture was incubated at 37°C for 2 h and then diluted in buffer to yield 800–1,000 colonies on control plates before plating on agar enriched with trypticase soy broth. Plates were incubated overnight and colonies were counted with ImageQuant TL v2005. All CVL samples were tested in duplicate. The percent inhibition of E. coli was calculated relative to the number of colonies formed on control plates.
Protein concentration from CVL was assessed by MicroBCA assay (Thermo Fisher Scientified, Rockford, IL). HBD-1, -2, and -3 (Alpha Diagnostics, San Antonio, TX), HNP 1–3 (HyCult Biotechnology, Uden, The Netherlands), and SLPI (R & D Systems, Minneapolis, MN) were quantified by ELISA.
Statistical analyses
Analyses were performed using GraphPad Prism Version 5 (GraphPad Software, San Diego, CA) and SAS Version 9.2 (SAS Inc., Cary, NC). Concentrations of protein, defensins, and SLPI were log-transformed to reduce skewness. Continuous variables were compared between nonseroconverters and seroconverters by t-test. Continuous variables were then compared between preinfection and acute time points for seroconverting women by the Wilcoxon signed-rank test. Categorical variables were analyzed by chi-squared or Fisher's exact tests. Paired categorical data were analyzed by McNemar's tests. Spearman's correlation coefficients were calculated to assess potential associations between continuous variables. All tests for statistical significance were two-sided, and p values<0.05 were considered significant. Logistic regression models were used to assess the effects of variables identified in univariate analysis on risk of seroconversion.
Partial least squares discriminant analysis (PLSDA) was used to evaluate the antimicrobial peptide and E. coli inhibitory activity profiles that best distinguished between seroconverters and nonseroconverters.15,16 PLSDA utilizes covariance to identify linear combinations of independent variables (latent variables) that best differentiate between groups of interest. Every participant sample was assigned a score, which was then visualized in the latent variable space (score plots). Latent variable loadings (loadings plots) were then used to identify multivariate combinations of individual variables associated with seroconversion. To reduce the risk of overfitting, only markers with a variable importance projection (VIP) score of >1 were included. All data were normalized with mean centering and variance scaling. Cross-validation was performed by iteratively excluding subsets of data in groups of four to five during model calibration, then using excluded data samples to test model predictions. Orthogonal signal correction was used to improve model interpretability.
Results
Description of study subjects
The women who contributed samples to this study were all urban, Zulu-speaking women from KwaZulu-Natal, South Africa. Demographic details of the full cohort were previously published.9 The prevalence of BV by Nugent's score was similarly high between seroconverters at the preinfection time point (n=17) and nonseroconverters (n=39) (64.7% vs. 61.5%; p=0.82) (Table 1). The median Nugent scores [7 (IQR 5–8) vs. 8 (IQR 3–9); p=0.84] and the proportions of women utilizing injectable long-acting hormonal contraception (29.4% vs. 25.6%, respectively; p=0.75) were also similar between seroconverters and nonseroconverters (Table 1). None of the women reported use of oral contraceptive pills. The proportions of women with HSV-2 seropositivity, chlamydia, gonorrhea, or trichomoniasis did not differ significantly between preinfection seroconverters and nonseroconverters (Table 1). HSV DNA was not detected in preinfection seroconverter vulvovaginal swabs but was detected in four swabs from nonseroconverters (p=0.30).
Table 1.
Distribution of Subject Characteristics and Logistic Regression Estimate for HIV Acquisition Risk
| Subject characteristics1 | Seroconverters (n=17) | Nonseroconverters (n=39) | p value |
|---|---|---|---|
| Injectable hormonal contraceptive use, number positive (%) | 5 (29.4) | 10 (25.6) | 0.75 |
| Nugent score (median, IQR) | 7 (5–8) | 8 (3–9) | 0.84 |
| Bacterial vaginosis,2 number positive (%) | 11 (64.7) | 24 (61.5) | 0.82 |
| Herpes simplex virus-2 IgG, number positive (%) | 15 (88.2) | 34 (87.2) | 1.0 |
| Chlamydia trachomatis NAAT, number positive (%) | 1 (5.9) | 3 (7.7) | 1.0 |
| Neisseria gonorrhoeae NAAT, number positive (%) | 0 (0) | 2 (5.1) | 1.00 |
| Triahamonas vaginalis NAAT, number positive (%) | 4 (23.5) | 8 (20.5) | 1.00 |
| % Inhibition of Escherichia coli (mean±SD) | 54.44±27.99 (n=16) | 34.11±17.92 (n=38) | 0.01 |
| Total protein3 (mean±SD) | 2.39±0.33 | 2.33±0.34 (n=38) | 0.56 |
| SLPI3 (mean±SD) | 5.4±0.63 | 5.17±0.54 | 0.16 |
| HNP 1–33 (mean±SD) | 4.29±0.76 | 4.19±0.59 | 0.58 |
| HBD-13 (mean±SD) | 3.39±0.4 | 3.1±0.42 | 0.02 |
| HBD-23 (mean±SD) | 3.48±0.95 | 3.72±0.6 | 0.25 |
| HBD-33 (mean±SD) | 2.93±0.49 | 2.85±0.46 | 0.56 |
| Logistic regression estimate for increased risk of HIV acquisition | |||
|---|---|---|---|
| Variable/effect | Odds ratio | 95% confidence interval | p value |
| Bacterial vaginosis4 | 1.5 | 0.36–6.38 | 0.58 |
| % Inhibition of E. coli5 | 1.45 | 1.07–1.97 | 0.02 |
| HBD-16 | 4.43 | 0.82–23.83 | 0.08 |
Baseline characteristics of seroconverting subjects (prior to infection) and nonseroconverting subjects.
Diagnosis by Nugent's score.
Quantified from cervicovaginal lavage fluid and expressed as log10 (μg/ml) units (total protein) or as log10 (pg/ml) units (SLPI, HNP 1–3, HBD 1–3).
Presence or absence of bacterial vaginosis by Nugent's score.
Estimated increased risk of HIV acquisition for every 10% increase in bactericidal E. coli activity.
Estimated increased risk of HIV acquisition for every log10 unit increase in concentration of HBD-1.
IQR, interquartile range; NAAT, nucleic acid amplification test; SD, standard deviation; SLPI, secretory leukocyte protease inhibitor; HNP, human neutrophil peptides; HBD, human β defensin.
For the 11 seroconverting women who had CVL available at both the preinfection and acute infection time points, the median Nugent score was eight for both time points (p=0.22), and the proportions of women with BV did not differ significantly (72.7% vs. 90.9 %, respectively; p=0.50). One woman was diagnosed with chlamydia preinfection, two women were diagnosed with trichomoniasis preinfection, and no women were diagnosed with chlamydia or trichomoniasis at the acute infection time point (p=1.0 for chlamydia, p=0.47 for trichomoniasis). Although no women at the preinfection time point had gonorrhea, five women had gonorrhea during their acute infection visit (p=0.04). Ten of the 11 women were HSV-2 seropositive at the preinfection time point, and all 11 women were HSV-2 seropositive by the acute infection time point (p=1.0); no women had detectable HSV shedding. The median plasma HIV-1 viral load at acute infection was 360,000 copies/ml (IQR 41,600–621,000), and the median CD4+ T cell count was 435 cells/μl (IQR 201–974). The median plasma viral load set point at 52 weeks postinfection was 56,033 copies/ml (IQR 3,162–195,133). In CVL, HIV-1 RNA was detected in 4/10 (40%) acutely infected samples (one sample was not available for testing) with a median viral load of 1,110 copies/ml (IQR 199.8–1,614).
Preinfection E. coli inhibitory activity was higher in seroconverters than nonseroconverters and was associated with HIV acquisition risk
Preinfection mean CVL E. coli inhibitory activity was significantly higher in seroconverters compared to nonseroconverters [54.4%±standard deviation (SD) 28.0 vs. 34.1%±17.9; p=0.01] (Table 1). In addition, mean CVL concentrations of HBD-1 were significantly higher in seroconverters compared to nonseroconverters [3.4 log10 (pg/ml) units±SD 0.4 vs. 3.1±0.4; p=0.02]. There were no significant differences in CVL concentrations of protein, HBD-2, HBD-3, HNP 1–3, or SLPI between seroconverters and nonseroconverters.
In a logistic regression model that adjusted for the presence of BV, the odds of HIV acquisition increased 1.45-fold for every 10% increase in E. coli inhibitory activity (OR 1.45; 95% CI 1.07, 1.97; p=0.02) (Table 1). Each log10 unit increase in CVL HBD-1 concentration was associated with a 4.43 times greater risk of HIV acquisition (OR 4.43; 95% CI 0.82, 23.83; p=0.08); however, this result was of borderline statistical significance.
Higher CVL E. coli inhibitory activity, HBD-1, and SLPI were associated with HIV seroconversion by PLSDA
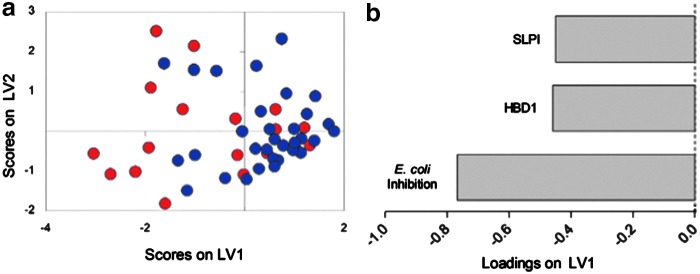
PLSDA was used to determine which combinations of preinfection variables were most useful for classifying women as potential HIV seroconverters or nonseroconverters. CVL E. coli inhibitory activity, together with concentrations of SLPI and HBD-1, classified women as those who subsequently seroconverted versus those who did not with 67% calibration accuracy and 64% cross-validation accuracy (Fig. 1a). The first two latent variables in this model captured 86% of the variance in the data set. Examination of the loadings plot indicated that latent variable 1 (LV1) best differentiated the preinfection group from those who remained uninfected, and correspondingly the seroconverter (negative scores on LV1; Fig. 1a) profile was associated with elevated CVL E. coli inhibitory activity, SLPI, and HBD-1 (all negatively loaded on LV1, Fig. 1b). The combined model including CVL E. coli inhibitory activity, SLPI, and HBD-1 performed slightly better in classifying women as seroconverters or nonseroconverters than a model that used E. coli inhibition alone (32% calibration error for combined vs. 39% calibration error for E. coli inhibition alone, data not shown).
FIG. 1.
Scores and loadings plots for partial least squares discriminant analysis (PLSDA) model. (a) A PLSDA model using cervicovaginal lavage (CVL) E. coli inhibition and concentrations of human β defensin (HBD)-1 and secretory leukocyte protease inhibitor (SLPI) in samples prior to HIV infection classified women as seroconverters or nonseroconverters with 67% accuracy (red: women who acquired HIV; blue: women who remained uninfected). (b) Latent variable 1 separated women who acquired HIV from those who remained uninfected. E. coli inhibition, HBD-1, and SLPI were all negatively loaded on LV1, suggesting they were positively associated with HIV acquisition.
Acute HIV infection was associated with increased human neutrophil peptides but decreased SLPI concentrations in CVL
Mean CVL HNP 1–3 levels increased significantly from preinfection to acute infection [4.2 log10 (pg/ml) units±SD 0.9 vs. 5.3±0.7; p=0.001] (Fig. 2), while mean concentrations of SLPI in CVL were significantly lower during acute infection compared to preinfection [4.8 log10 (pg/ml) units±SD 0.76 vs. 5.5±0.54; p=0.02]. Mean CVL E. coli inhibitory activity increased from preinfection to acute infection, but this increase did not reach statistical significance (56.1%±SD 35.6 vs. 74.6%±10.1; p=0.13). Mean Nugent scores and levels of protein, HBD-2, and HBD-1 did not differ significantly between preinfection and acute infection (Fig. 2). Samples were not available for HBD-3 quantification in the acute infection group. Five women in the acute infection group had gonorrhea at the time of sample collection. However, CVL mean E. coli inhibition and concentrations of protein, SLPI, and defensins did not differ significantly between acute infection samples collected from women with and without gonorrhea (p>0.05 for all, data not shown).
FIG. 2.

Comparison of Nugent scores, cervicovaginal lavage (CVL) E. coli inhibitory activity, and CVL concentrations of secretory leukocyte protease inhibitor (SLPI), human neutrophil peptides (HNP) 1–3, human β defensin (HBD)-2, HBD-1, and protein from 11 seroconverting women preinfection and during acute infection. CVL E. coli inhibitory activity was unavailable for one woman at the preinfection time point.
E. coli inhibitory activity and antimicrobial peptide concentrations were elevated in acute infection CVL samples with detectable HIV-1 RNA
Mean E. coli inhibitory activity (82.25%±SD 6.24 vs. 68.43±5.38; p=0.003) and concentrations of HBD-1 (4.28±SD 0.33 vs. 3.33±SD 0.33; p=0.002) and HBD-2 (4.18±SD 0.36 vs. 2.92±SD 0.36; p<0.001) were significantly higher in the four acute infection CVL samples with detectable HIV-1 RNA compared to the six samples without detectable HIV-1 RNA (Table 2). Mean concentrations of HNP 1–3 and SLPI were also higher in CVL samples with detectable HIV-1 RNA, although these differences were not significant.
Table 2.
Antimicrobial Peptide Concentrations in Cervicovaginal Lavage Fluid Samples with Detectable or Nondetectable HIV RNA During Acute HIV Infection
| Detectable HIV RNA (n=4) | Nondetectable HIV RNA (n=6) | p value | |
|---|---|---|---|
| CVL HIV RNA viral load (copies/ml), median (IQR) | 1,110 (199.8–1,624) | ||
| % Inhibition of E. coli (mean±SD) | 85.25±6.24 | 68.43±5.38 | 0.003 |
| Total protein1 (mean±SD) | 2.69±0.18 | 2.15±0.73 | 0.18 |
| SLPI1 (mean±SD) | 5.37±0.20 | 4.5±0.79 | 0.06 |
| HNP 1–31 (mean±SD) | 5.74±0.19 | 5.06±0.84 | 0.15 |
| HBD-11 (mean±SD) | 4.28±0.33 | 3.33±0.36 | 0.002 |
| HBD-21 (mean±SD) | 4.18±0.36 (n=3) | 2.92±0.36 | <0.001 |
Quantified from CVL fluid and expressed as log10 (μg/ml) units (total protein) or as log10 (pg/ml) units (SLPI, HNP 1–3, HBD 1–3).
SLPI, secretory leukocyte protease inhibitor; HNP, human neutrophil peptides; HBD, human β defensin.
CVL defensin concentrations during acute infection correlated with E. coli inhibitory activity and plasma viral load set point
CVL E. coli inhibitory activity at acute infection correlated positively with CVL concentrations of protein (r=0.71, p=0.02), HBD-1 (r=0.61, p=0.05), and HBD-2 (r=0.91, p=0.001). A trend toward a positive correlation between CVL E. coli inhibitory activity at acute infection and CVL concentrations of HNP 1–3 (r=0.49, p=0.13) and SLPI (r=0.57, p=0.07) was observed. Of note, significant correlations between CVL E. coli inhibitory activity and antimicrobial peptides were not detected in samples from nonseroconverting women or in seroconverting women at the preinfection time point. Acute infection CVL concentrations of HNP 1–3 correlated positively and significantly with viral set point (r=0.66, p=0.03).
Discussion
In this study of women enrolled in the CAPRISA 002 study, higher E. coli inhibitory activity and HBD-1 in CVL were associated with increased risk of HIV acquisition. These results are consistent with the findings of a substudy of HPTN 035, in which higher levels of E. coli inhibitory activity and HBD-2 in FGS were associated with a higher risk of HIV acquisition among eight seroconverting women, and indicate that E. coli inhibitory activity may provide a biomarker of HIV acquisition risk.8
The precise mediators of E. coli inhibitory activity in FGS have not yet been elucidated. It is likely that the relative contributions from vaginal microbiota and host-derived soluble immune mediators at the genital mucosa differ between distinct populations of women. In a prior study of 99 U.S. women who did not have an STI or BV and who were at low risk for HIV acquisition, E. coli inhibitory activity did not correlate with CVL concentrations of defensins and other host-derived molecules.1 Instead, proteomic studies from five participants with CVL inhibition >90% showed that this activity was associated with surface proteins specific to Lactobacillus crispatus and L. jensenii.1 Vaginal microbiota data are not yet available for the women included in our study. However, the high Nugent scores among participants in this study, coupled with the observed correlations between defensins and E. coli inhibitory activity during acute HIV infection, suggest that among women with a paucity of lactobacilli, E. coli inhibitory activity may represent defensin influx and mucosal inflammation. The observed associations between defensins, CVL E. coli inhibitory activity, and HIV seroconversion merit further investigation in prospective studies that utilize protein mass spectrometry of FGS to identify the precise mediators that confer HIV risk.
We also used PLSDA to identify relationships between CVL E. coli inhibitory activity and antimicrobial peptides that were most associated with HIV seroconversion and found that seroconversion was most associated with a multivariate profile that included higher CVL E. coli inhibition, HBD-1, and SLPI. Moreover, we observed a trend toward higher SLPI concentrations in acute infection CVL samples with detectable HIV-1 RNA compared to samples without detectable HIV-1 RNA. Taken alone, the weak association between higher CVL concentrations of SLPI and HIV seroconversion and detectable HIV-1 RNA is somewhat surprising. SLPI exerts activity against HIV in vitro,17 and prior studies have identified associations between lower SLPI concentrations and HIV seroconversion risk in larger populations.18 It is possible that higher levels of HIV-1 RNA in the genital tract may induce epithelial cell expression of SLPI, and the association between higher SLPI levels preinfection and HIV seroconversion risk could represent HIV-1-induced expression of SLPI by epithelial cells in the setting of repeated HIV exposure from sexual partners.19
Although defensins have potent anti-HIV activity ex vivo,20,21 we identified higher HBD-1 concentrations in preinfection seroconverters compared to nonseroconverters, and acute infection CVL samples with detectable HIV-1 RNA had significantly higher concentrations of HBD-1 and -2 relative to samples without detectable HIV-1 RNA. HBD-2 expression may be induced by inflammatory stimuli17,22 and by HIV-1 Tat activation of NF-κB-dependent pathways.23 However, HBD-1 is thought to be expressed constitutively, and its association with increased risk of HIV acquisition requires further investigation. Our finding of higher genital tract HNP 1–3 levels at acute infection could reflect recruitment of degranulating neutrophils by IL-8, MIP-3α, and other proinflammatory mediators24 that may promote activation of NF-κB and recruitment of HIV target cells.6,25 The correlation between CVL HNP 1–3 and plasma viral set point suggests that concentrations of defensins at the genital mucosa during acute HIV infection could impact long-term HIV progression and underscores the relationship between mucosal inflammatory cytokine profiles and viral set point described in prior CAPRISA 002 studies.10,11
The limitations of this small study are that we do not have detailed information about the vaginal microbiota, and white blood cell quantification was not available from samples for which Nugent scores were performed. There are several important variables, such as sexual behaviors, intravaginal practices, phase of menstrual cycle, and genital tract pathogens (other than those for which participants were tested) that may have impacted our results. Moreover, the dilutional effect of CVL may have limited our ability to detect molecules present in low concentrations. Despite these limitations, this study describes a significant association between E. coli inhibitory activity and HIV acquisition risk. This inexpensive and noninvasive assay may provide a feasible biomarker of HIV seroconversion risk in populations of women with a higher prevalence of subclinical or clinical BV, and the combination of this biomarker with concentrations of SLPI and defensins may increase predictive value. If validated in larger studies, this assay may be useful to identify women most in need of prevention strategies and to screen potential subjects for HIV prevention studies. Identifying the host and microbial mediators that contribute to this activity could also inform the development of effective HIV preexposure prophylaxis strategies.
Acknowledgments
This work was supported by Public Health Service Grants K23AI089271 and AI051519. The CAPRISA 002 Acute Infection Study was part of the Comprehensive International Program of Research on AIDS (CIPRA) of the National Institute of Allergy and Infectious Disease (NIAID), National Institutes of Health (NIH) Grant AI51794 and received support from the South African Department of Science and Technology and the National Research Foundation Grant 67385. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Kalyoussef S, Nieves E, Dinerman E, et al. : Lactobacillus proteins are associated with the bactericidal activity against E. coli of female genital tract secretions. PloS One 2012;7(11):e49506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Madan RP, Carpenter C, Fiedler T, et al. : Altered biomarkers of mucosal immunity and reduced vaginal Lactobacillus concentrations in sexually active female adolescents. PloS One 2012;7(7):e40415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valore EV, Wiley DJ, and Ganz T: Reversible deficiency of antimicrobial polypeptides in bacterial vaginosis. Infect Immun 2006;74(10):5693–5702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghartey JP, Smith BC, Chen Z, et al. : Lactobacillus crispatus dominant vaginal microbiome is associated with inhibitory activity of female genital tract secretions against Escherichia coli. PloS One 2014;9(5):e96659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghartey JP, Carpenter C, Gialanella P, et al. : Association of bactericidal activity of genital tract secretions with Escherichia coli colonization in pregnancy. Am J Obstet Gynecol 2012;207(4):e291–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haase AT: Targeting early infection to prevent HIV-1 mucosal transmission. Nature 2010;464(7286):217–223 [DOI] [PubMed] [Google Scholar]
- 7.Levinson P, Kaul R, Kimani J, et al. : Levels of innate immune factors in genital fluids: Association of alpha defensins and LL-37 with genital infections and increased HIV acquisition. AIDS 2009;23(3):309–317 [DOI] [PubMed] [Google Scholar]
- 8.Dezzutti CS, Richardson BA, Marrazzo JM, et al. : Mucosal Escherichia coli bactericidal activity and immune mediators are associated with HIV-1 seroconversion in women participating in the HPTN 035 trial. J Infect Dis 2012;206(12):1931–1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Loggerenberg F, Mlisana K, Williamson C, et al. : Establishing a cohort at high risk of HIV infection in South Africa: Challenges and experiences of the CAPRISA 002 acute infection study. PloS One 2008;3(4):e1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberts L, Passmore JA, Mlisana K, et al. : Genital tract inflammation during early HIV-1 infection predicts higher plasma viral load set point in women. J Infect Dis 2012;205(2):194–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bebell LM, Passmore JA, Williamson C, et al. : Relationship between levels of inflammatory cytokines in the genital tract and CD4+ cell counts in women with acute HIV-1 infection. J Infect Dis 2008;198(5):710–714 [DOI] [PubMed] [Google Scholar]
- 12.Roberts L, Passmore JA, Williamson C, et al. : Plasma cytokine levels during acute HIV-1 infection predict HIV disease progression. AIDS 2010;24(6):819–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mlisana K, Naicker N, Werner L, et al. : Symptomatic vaginal discharge is a poor predictor of sexually transmitted infections and genital tract inflammation in high-risk women in South Africa. J Infect Dis 2012;206(1):6–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewis DA, Muller E, Steele L, et al. : Prevalence and associations of genital ulcer and urethral pathogens in men presenting with genital ulcer syndrome to primary health care clinics in South Africa. Sex Transmit Dis 2012;39(11):880–885 [DOI] [PubMed] [Google Scholar]
- 15.Lau KS, Cortez-Retamozo V, Philips SR, et al. : Multi-scale in vivo systems analysis reveals the influence of immune cells on TNF-alpha-induced apoptosis in the intestinal epithelium. PLoS Biol 2012;10(9):e1001393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lau KS, Juchheim AM, Cavaliere KR, et al. : In vivo systems analysis identifies spatial and temporal aspects of the modulation of TNF-alpha-induced apoptosis and proliferation by MAPKs. Sci Signal 2011;4(165):ra16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cole AM. and Cole AL: Antimicrobial polypeptides are key anti-HIV-1 effector molecules of cervicovaginal host defense. Am J Reprod Immunol 2008;59(1):27–34 [DOI] [PubMed] [Google Scholar]
- 18.Morrison C, Fichorova RN, Mauck C, et al. : Cervical inflammation and immunity associated with hormonal contraception, pregnancy, and HIV-1 seroconversion. J Acquir Immune Defic Syndr 2014;66(2):109–117 [DOI] [PubMed] [Google Scholar]
- 19.Jana NK, Gray LR, and Shugars DC: Human immunodeficiency virus type 1 stimulates the expression and production of secretory leukocyte protease inhibitor (SLPI) in oral epithelial cells: A role for SLPI in innate mucosal immunity. J Virol 2005;79(10):6432–6440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghosh M, Fahey JV, Shen Z, et al. : Anti-HIV activity in cervical-vaginal secretions from HIV-positive and -negative women correlate with innate antimicrobial levels and IgG antibodies. PloS One 2010;5(6):e11366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martellini JA, Cole AL, Venkataraman N, et al. : Cationic polypeptides contribute to the anti-HIV-1 activity of human seminal plasma. FASEB J 2009;23(10):3609–3618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ganz T, Selsted ME, Szklarek D, et al. : Defensins. Natural peptide antibiotics of human neutrophils. J Clin Invest 1985;76(4):1427–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ju SM, Goh AR, Kwon DJ, et al. : Extracellular HIV-1 Tat induces human beta-defensin-2 production via NF-kappaB/AP-1 dependent pathways in human B cells. Mol Cells 2012;33(4):335–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cole AM: Innate host defense of human vaginal and cervical mucosae. Curr Top Microbiol Immunol 2006;306:199–230 [PubMed] [Google Scholar]
- 25.Hiscott J, Kwon H, and Genin P: Hostile takeovers: Viral appropriation of the NF-kappaB pathway. J Clin Invest 2001;107(2):143–151 [DOI] [PMC free article] [PubMed] [Google Scholar]