Abstract
We investigated the Th1 protective and regulatory T and B cell (Treg and Breg) responses to pH1N1 monovalent influenza vaccine (IIV1) in HIV-infected pregnant women on combination antiretroviral therapy (cART). Peripheral blood mononuclear cells (PBMCs) from 52 study participants were cryopreserved before and after vaccination and analyzed by flow cytometry. pH1N1-specific Th1, Treg, and Breg responses were measured in PBMCs after in vitro stimulation with pH1N1 and control antigen. The cohort analysis did not detect changes in pH1N1-Th1, Treg, or Breg subsets postvaccination. However, individual analyses distinguished subjects who mounted vigorous Th1 responses postvaccination from others who did not. Postvaccination, high pH1N1-Th1 correlated with high pH1N1-Treg and Breg responses, suggesting that low influenza effector responses did not result from excessive vaccine-induced immune regulation. High postvaccination pH1N1-Th1 responses correlated with baseline high PHA- and pH1N1-IFN-γ ELISpot and circulating CD4+CD39+% and CD8+CD39+% Treg, with low CD8+ cell numbers and CD19+FOXP3+% Breg, but not with CD4+ cell numbers or HIV viral load. These data highlight the heterogeneity of T cell responses to vaccines in HIV-infected individuals on cART. Predictors of robust Th1 responses to IIV include CD8+ cell numbers, T cell functionality, and circulating Breg and Treg.
Introduction
Influenza infections are frequent and have increased morbidity in HIV-infected children and adults, including pregnant women, which underscores the importance of vaccine-conferred protection. Multiple studies showed poor antibody responses to influenza vaccines in HIV-infected individuals.1–4 However, studies also showed the efficacy of trivalent inactivated seasonal influenza vaccines (IIV3) in HIV-infected adults including pregnant women.3,4
This implies that antibody titers against influenza measured by hemagglutination inhibition (HAI) may not be a good surrogate of protection in HIV-infected individuals. HAI titers ≥1:40 were shown to decrease the incidence of influenza disease in immune-competent young adults by 50%, and the ability of IIVs to generate HAI titers ≥40 has been used as a benchmark to predict vaccine efficacy and to ensure FDA approval of new IIV products. Although a majority of studies conducted in immune-competent IIV recipients supports the value of the HAI ≥40 standard as a predictor of protection, recent studies have disputed the validity of this HAI standard both in immune-competent children and adults.5–7 Some potential explanations for the apparent lack of surrogacy of HAI titers in HIV-infected individuals include the following: (1) HAI-measured antibodies may not constitute a mechanistic surrogate of protection and their ability to predict protection against influenza is highly dependent on host factors, such as immunologic competency or age,8 and (2) HIV-infected individuals may be highly heterogeneous with respect to immune responses due to varying degrees of immune deficiency that may not be totally reflected by CD4 cell numbers, HIV plasma RNA levels, or use of combination antiretroviral therapy (cART).
P1086 was a study of the International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) network that investigated the safety and immunogenicity of two double doses of the pandemic H1N1 (pH1N1) IIV1 in HIV-infected pregnant women on cART. The primary analysis of P1086 showed that the immunization regimen was safe, but the immunogenicity measured by HAI titers was lower compared with historical immune-competent adult vaccinees.9 In a subsequent study of the B and T cell responses to pH1N1 in P1086 participants, we found that only pH1N1 HAI titers and IgG memory B cells increased after vaccination, whereas interferon gamma (IFN-γ) ELISpot-measured effector T cells (Teff) decreased, and IgA memory B cells and granzyme B (GrB) Teff did not significantly change from prevaccination to postvaccination.10 Surprisingly, however, the B and T cell responses to pH1N1 vaccine in P1086 participants were generally positively correlated despite their median trajectories over time going in different directions. This observation led us to hypothesize that some important interrelationships in immune responses to pH1N1 IIV1 may be identified by correlation analyses, even in cases in which summary statistics did not indicate significant or consistent changes in group medians.
In this exploratory study, we expanded the immunogenicity analysis of the pH1N1 IIV1 in a subset of HIV-infected pregnant women from P1086 by evaluating pH1N1-specific Th1 and cytotoxic responses and regulatory responses to vaccination. Th1 and cytotoxic cell-mediated immunity (CMI) is generally protective against viral infections and both human and animal studies showed its protective role against influenza.11–19
Participants and Methods
Study design
HIV-infected women 18 to 39 years of age, 14 to 34 weeks gestation, and on antiretroviral therapy, who consented to this study as per local IRB stipulations, received two 30 μg doses of unadjuvanted, inactivated pH1N1 vaccine, 21 to 28 days apart, at 31 U.S. IMPAACT sites, as previously described.9 Serum, plasma, and peripheral blood mononuclear cells (PBMCs) were collected and cryopreserved at entry before administration of the first dose of vaccine, before administration of the second dose (21 to 28 days postdose 1), and 10 to 14 days postdose 2. PBMCs were collected at entry and after each vaccination on the first half of participants enrolled at sites that were certified to cryopreserve PBMCs through the Immunology Quality Assurance program of the Division of AIDS of the National Institute of Allergy and Infectious Diseases. Here we report on the baseline and postdose 1 CMI responses.
Flow cytometry
PBMCs were frozen, stored, shipped, and thawed as per the IMPAACT version of the HIV/AIDS Network Coordination protocol (www.hanc.info/labs/Pages/SOPs.aspx). After overnight rest, PBMCs with viability ≥70% and viable recovery ≥50% were resuspended at 106 PBMC/ml in RPMI 1640 supplemented with antibiotics and 10% human AB blood group serum and were stimulated for 48 h with 2 TCID50/cell of A/California/7/2009 Pandemic X-179A H1N1 Influenza virus or medium control in the presence of 1 μg/ml of each of anti-CD28 (BD Biosciences; L293) and anti-CD49d (BD Biosciences; B7651) monoclonal antibodies (mAbs). Brefeldin A (Sigma-Aldrich) was added to a final concentration of 10 μg/ml for the last 12–15 h of the incubation. After washing and counting, PBMCs were surface stained using the following conjugated mAbs: anti-CD3- PECy7, anti- CD8-APC-AF 750, and anti-CD19-PECy5, and then fixed and permeabilized with Cytofix/Cytoperm (BD Biosciences) and stained with anti-IL-10-APC, anti-FOXP3-FITC, anti-TGF-β-PE (Cederlane; TB21), anti-MIP1β-PE (BD Biosciences; D21-1351), anti-TNF-α-PerCP Cy5.5 (Biolegend; MAb11), anti-Perforin-APC (Biolegend; dG9), and anti-IL-2-FITC (BD Biosciences; 5344.111). Total T and B cells and subpopulations were counted on Guava easyCyte 8HT (Millipore) and analyzed with FlowJo (Treestar). Subsets were expressed as a percentage of the parent CD3+CD4+, CD3+CD8+, or CD3−CD19+ cell population.
Statistical methods
This was an exploratory substudy for which no sample size calculations were performed. Baseline characteristics were summarized using descriptive measures. Changes in pH1N1-specific effector, memory, and regulatory T cell subsets from baseline to postimmunization were assessed using the Wilcoxon matched pairs signed-rank tests. The final flow cytometric analyses were restricted to samples with ≥100 events in the CD4+, CD8+, or CD19+ anchor gates. A sensitivity analysis showed that the inclusion of samples with <100 events in the anchor gates would not have changed the results. Spearman correlation analyses were performed to assess the strength of associations and test their statistical significance. All analyses were performed using SAS Version 9.2 (SAS Institute Inc.) and graphs were produced using the R software.
Results
Characteristics of the study population
This study used PBMCs from 52 subjects enrolled in the P1086 parent study who completed vaccination before delivery and had cryopreserved PBMCs stored before and after vaccination. At enrollment, women had a mean age of 27 years, and medians of 33% CD4+ T cells and 1.9 log10 HIV RNA copies/ml of plasma, all of which were similar to the baseline measures of the total population in the parent study (Table 1).
Table 1.
Demographics and HIV Disease Characteristics
| Characteristic | All | Substudy |
|---|---|---|
| Number of subjects | 119 | 52 |
| Race | ||
| Black | 71 (60%) | 32 (62%) |
| Ethnicity | ||
| Latino | 41 (34%) | 21 (40%) |
| Age (years) | ||
| Median | 29 | 27 |
| Interquartile range (IQR) | (22, 32) | (23, 31) |
| Gestational age (weeks) | ||
| Median | 25 | 27 |
| IQR | (20, 29) | (21, 30) |
| Receiving ARVs | 119 (100%) | 52 (100%) |
| Type of ARV regimen | ||
| HAART | 112 (94%) | 46 (88%) |
| Other | 7 (6%) | 6 (12%) |
| CD4 percent | ||
| Median | 32 | 33 |
| IQR | (23, 39) | (27, 41) |
| CD4 count (cells/mm3) | ||
| Median | 481 | 496 |
| IQR | (350, 647) | (388, 635) |
| CD8 percent | ||
| Median | 46 | 44 |
| IQR | (38, 52) | (38, 51) |
| CD8 count (cells/mm3) | ||
| Median | 686 | 643 |
| IQR | (529, 893) | (527, 851) |
| N | N = 109 | N = 49 |
| log10 RNA count | ||
| Mediana | 1.9 | 1.9 |
| IQR | (1.7, 2.6) | (1.7, 2.6) |
The lower limit of detection varied among subjects depending on the assay used at the clinical research site; RNA values below the limit of detection were replaced with the lower detection limit of the assay.
Th1 and cytotoxic CMI responses to pH1N1 monovalent vaccine measured by flow cytometry
pH1N1-specific Th1 responses were characterized by the expression of interleukin-2 (IL-2) and tumor necrosis factor-alpha (TNF-α), and cytotoxic responses by expression of perforin in pH1N1-stimulated PBMCs after subtraction of mock-stimulated controls. pH1N1-specific MIP1β expression, which is an integral component of both Th1 and cytotoxic responses, was also measured on stimulated CD4+ and CD8+ T cells. In aggregate, there were no significant changes from baseline to postdose 1 in the pH1N1-specific T cell immunity (Table 2 and Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/aid). However, further analyses showed that Th1 CMI responses to vaccination were highly correlated (Table 3). This level of coordination of the protective CMI responses suggested that the differences among subjects represented a true difference in their ability to respond to the vaccine as opposed to a random finding.
Table 2.
Change in pH1N1-Specific Th1 and Cytotoxic T Cell Subsets from Baseline to Postdose 1 pH1N1 Vaccine
| Variable (%)a,b | Median (IQR) | p valuec |
|---|---|---|
| CD4+IL-2+ | −0.17 (−1.98, 2.02) | 0.93 |
| CD4+MIP1β+ | 0.53 (−2.05, 2.38) | 0.81 |
| CD4+MIP1β+IL-2+ | −0.09 (−0.46, 0.49) | 0.91 |
| CD4+PERF+ | −0.12 (−2.85, 2.21) | 0.79 |
| CD4+TNF-α+ | −0.56 (−2.27, 1.21) | 0.31 |
| CD4+PERF+TNF-α+ | −0.32 (−0.76, 0.79) | 0.69 |
| CD8+IL-2+ | 0.56 (−2.31, 1.94) | 0.85 |
| CD8+MIP1β+ | 0.58 (−2.60, 4.32) | 0.35 |
| CD8+MIP1β+IL-2+ | 0.14 (−0.79, 1.44) | 0.53 |
| CD8+PERF+ | −0.24 (−3.59, 3.93) | 0.65 |
| CD8+TNF-α+ | −0.69 (−2.79, 2.62) | 0.62 |
| CD8+PERF+TNF-α+ | −0.37 (−1.45, 1.66) | 0.77 |
N = 49.
Subsets, measured by flow cytometry, are expressed as a percentage of the parent CD4+ or CD8+ B cell population.
Wilcoxon matched pairs signed-rank tests.
Table 3.
Correlation of pH1N1-Specific T Cell Subset Changes from Baseline to Postdose 1 pH1N1 Vaccine
| ρc(p value) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CD4+ | CD8+ | ||||||||||
| T cell subset (%)a,b | MIP1β+ | MIP1β+IL-2+ | PERF+ | TNF-α+ | PERF+TNF-α+ | IL-2+ | MIP1β+ | MIP1β+IL-2+ | PERF+ | TNF-α+ | PERF+TNF-α+ |
| CD4+IL-2+ | 0.69 (<0.0001) | 0.76 (<0.0001) | 0.60 (<0.0001) | 0.61 (<0.0001) | 0.60 (<0.0001) | 0.38 (0.007) | 0.44 (0.001) | 0.38 (0.008) | 0.41 (0.003) | 0.34 (0.02) | 0.36 (0.01) |
| CD4+ MIP1β+ | 1 | 0.82 (<0.0001) | 0.55 (<0.0001) | 0.59 (<0.0001) | 0.58 (<0.0001) | 0.23 (0.11) | 0.58 (<0.0001) | 0.40 (0.004) | 0.40 (0.004) | 0.34 (0.02) | 0.34 (0.02) |
| CD4+ MIP1β+IL-2+ | — | 1 | 0.44 (0.002) | 0.48 (0.001) | 0.52 (0.0001) | 0.34 (0.02) | 0.54 (<0.0001) | 0.54 (<0.0001) | 0.45 (0.001) | 0.29 (0.04) | 0.43 (0.002) |
| CD4+ PERF+ | — | — | 1 | 0.51 (0.0002) | 0.79 (0.0001) | 0.17 (0.25) | 0.23 (0.11) | 0.18 (0.22) | 0.42 (0.003) | 0.35 (0.02) | 0.29 (0.04) |
| CD4+ TNF-α+ | — | — | — | 1 | 0.69 (<0.0001) | 0.08 (0.58) | 0.21 (0.16) | 0.10 (0.50) | 0.22 (0.12) | 0.40 (0.004) | 0.22 (0.14) |
| CD4+ PERF+ TNF-α+ | — | — | — | — | 1 | 0.04 (0.79) | 0.15 (0.30) | 0.11 (0.44) | 0.24 (0.10) | 0.25 (0.08) | 0.21 (0.15) |
| CD8+IL-2+ | — | — | — | — | — | 1 | 0.66 (<0.0001) | 0.84 (<0.0001) | 0.70 (<0.0001) | 0.40 (0.004) | 0.55 (<0.0001) |
| CD8+ MIP1β+ | — | — | — | — | — | — | 1 | 0.84 (<0.0001) | 0.69 (<0.0001) | 0.47 (0.001) | 0.58 (<0.0001) |
| CD8+ MIP1β+IL-2+ | — | — | — | — | — | — | — | 1 | 0.75 (<0.0001) | 0.45 (0.001) | 0.64 (<0.0001) |
| CD8+ PERF+ | — | — | — | — | — | — | — | — | 1 | 0.62 (<0.0001) | 0.76 (<0.0001) |
| CD8+ TNF-α+ | — | — | — | — | — | — | — | — | — | 1 | 0.76 (<0.0001) |
| CD8+ PERF+ TNF-α+ | — | — | — | — | — | — | — | — | — | — | 1 |
N = 49.
Subsets, measured by flow cytometry, are expressed as a percentage of the parent CD4+ or CD8+ T cell population.
Spearman correlation coefficients.
Bold represents the values that were significant at a 0.05 level. Italics indicates the values that were marginally significant.
Regulatory T cell responses to vaccination
To determine the effect that T cell regulation may play in the modulation of the protective immune response to the pH1N1 vaccine in HIV-infected pregnant women, we examined the changes from baseline to postdose 1 of multiple subsets of T and B regulatory subsets characterized by the expression of FoxP3, IL-10, and/or TGF-β (Table 4 and Supplementary Table S2). In aggregate, we did not find significant changes in pH1N1-specific regulatory T or B cell after pH1N1 vaccination except for a small decrease in the CD4+TGF-β+% (median difference = −0.99%; p = 0.02).
Table 4.
Change of pH1N1-Specific Regulatory T and B Cell Subsets from Baseline to Postdose 1 pH1N1 Vaccine
| Variable (%)a,b | Median (IQR) | p valuec |
|---|---|---|
| CD4+FoxP3+ | 0.29 (−2.50, 1.59) | 0.97 |
| CD4+IL-10+ | 0.37 (−2.08, 1.78) | 0.87 |
| CD4+IL-10+FoxP3+ | −0.04 (−0.34, 0.18) | 0.40 |
| CD4+TGF-β+ | −0.99 (−3.83, 0.87) | 0.02 |
| CD4+TGF-β+FoxP3+ | −0.11 (−0.56, 0.27) | 0.14 |
| CD8+FoxP3+ | 0.51 (−2.59, 3.65) | 0.53 |
| CD8+IL-10+ | 0.55 (−2.23, 2.26) | 0.40 |
| CD8+IL-10+FoxP3+ | 0.12 (−0.40, 0.82) | 0.48 |
| CD8+TGF-β+ | −0.79 (−3.59, 1.21) | 0.18 |
| CD8+TGF-β+FoxP3+ | −0.15 (−0.55, 0.85) | 0.98 |
| CD19+FoxP3+ | 0.47 (−1.53, 2.54) | 0.46 |
| CD19+IL-10+ | −0.71 (−4.13, 2.70) | 0.47 |
| CD19+IL-10+FoxP3+ | −0.01 (−0.16, 0.33) | 0.49 |
| CD19+TGF-β+ | 0.08 (−3.66, 1.11) | 0.51 |
| CD19+TGF-β+FoxP3+ | 0.01 (−0.35, 0.24) | 0.73 |
N = 45
Subsets, measured by flow cytometry, are expressed as a percentage of the parent CD4+, CD8+, or CD19+ population.
Wilcoxon matched pairs signed-rank test.
Bold represents the values that were significant at a 0.05 level.
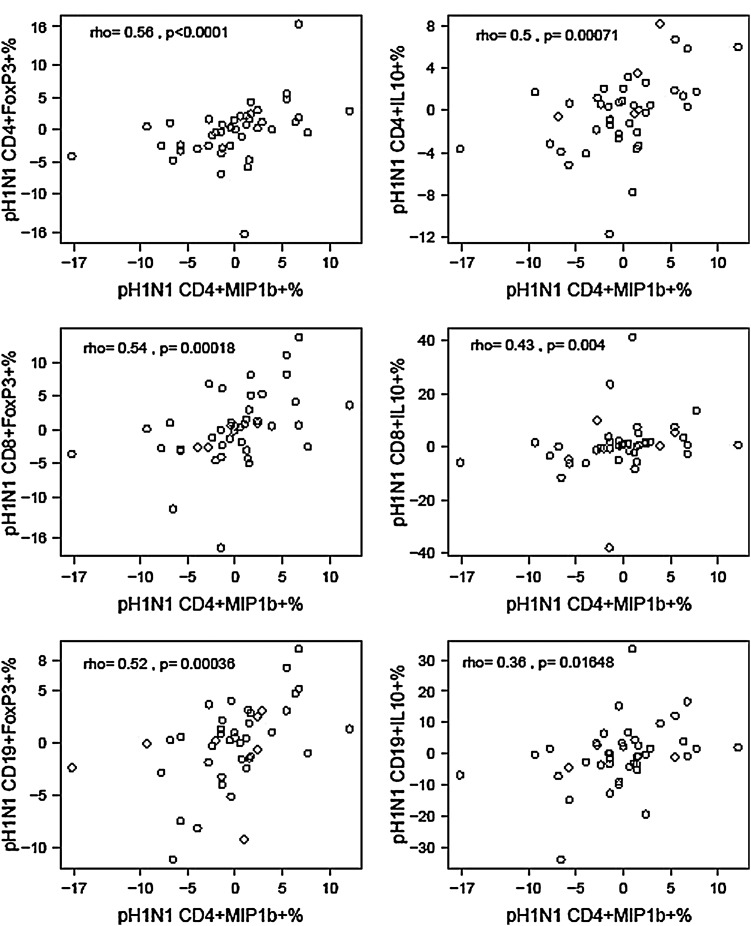
Further analyses of Treg responses revealed heterogeneity similar to that observed for the Th1 and cytotoxic T cell responses (data not shown). Surprisingly, however, when we examined the correlations between changes from baseline to postvaccination between pH1N1-specific Th1 and cytotoxic protective responses and regulatory responses (Fig. 1), we found significant positive correlations of Th1 and cytotoxic protective with regulatory responses to the vaccine, suggesting coordination instead of antagonism of these responses.
FIG. 1.
Correlations between change from baseline to postdose 1 in pH1N1-specific CD4+MIP1β+% and regulatory T cell subsets. Data were derived from peripheral blood mononuclear cells (PBMC) of 52 HIV-infected pregnant women collected before and 21–28 days after the administration of a double-dose of pH1N1 IIV1. Data points represent pH1N1-specific T cell subset frequencies at postvaccination after subtraction of prevaccination frequencies. The coefficients of correlations and p values measured with the Spearman correlation test shown on the graphs demonstrate highly significant associations between the changes in CD4+MIP1β+% (x axes), representing Th1 responses, in response to vaccination and selected Treg- and Breg-subset% changes (y axes) after vaccination.
Baseline characteristics associated with CMI responses to pH1N1 immunization
To determine the factors that may contribute to the protective T cell responses generated by influenza vaccination, we performed correlation analyses of the baseline immunologic, virologic, and select demographic characteristics with pH1N1-specific CD4+MIP1β+% increases after vaccination used as a general exemplification of protective responses (Table 5). The data showed that baseline CD4+ T cells and HIV plasma RNA load were not associated with pH1N1-specific CD4+MIP1β+ changes after vaccination. However, the increase in pH1N1-specific CMI after vaccination was associated with high baseline pH1N1- and PHA-IFN-γ spot-forming cells, high circulating CD4+CD39+% and CD8+CD39+% Treg of unknown specificity, and low circulating CD19+FOXP3+% Breg of unknown specificity.
Table 5.
Correlation Between pH1N1-Specific CD4+MIP1β+% Changes from Baseline to Postdose 1 pH1N1 Vaccine and Baseline Characteristics with Potential Predicting Value
| Characteristica | ρb | p value | N |
|---|---|---|---|
| Race, black | −0.002 | 0.99 | 46 |
| Ethnicity, latino | 0.14 | 0.35 | 47 |
| Type ARV regimen, HAART | 0.01 | 0.95 | 49 |
| pH1N1 HAI titers ≥40 | −0.04 | 0.79 | 49 |
| Age (years) | 0.09 | 0.52 | 49 |
| CD4 count (cells/mm3) | −0.03 | 0.86 | 49 |
| CD4 % | 0.12 | 0.42 | 49 |
| CD8 count (cells/mm3) | −0.29 | 0.05 | 46 |
| CD8 % | −0.22 | 0.13 | 49 |
| log10 HIV-RNA (cp/ml) | −0.12 | 0.41 | 49 |
| pH1N1 HAI titers | 0.02 | 0.92 | 49 |
| pH1N1 IFN-γ SFC | 0.35 | 0.02 | 44 |
| pH1N1 granzyme B SFC | 0.08 | 0.62 | 41 |
| PHA IFN-γ SFC | 0.28 | 0.06 | 44 |
| CD4+CD39+% | 0.37 | 0.03 | 36 |
| CD4+HLADR+CD38+% | 0.12 | 0.50 | 36 |
| CD4+TGF-β+% | 0.18 | 0.28 | 36 |
| CD8+CD39+% | 0.38 | 0.02 | 36 |
| CD8+HLADR+CD38+% | 0.17 | 0.32 | 36 |
| CD8+TGF-β+% | 0.16 | 0.34 | 36 |
| CD4+IL-10+% | 0.27 | 0.10 | 37 |
| CD4+FOXP3+% | −0.22 | 0.19 | 37 |
| CD4+CD25+FOXP3+% | −0.21 | 0.21 | 37 |
| CD8+IL-10+% | 0.13 | 0.46 | 37 |
| CD8+FOXP3+% | −0.19 | 0.27 | 37 |
| CD8+CD25+FOXP3+% | −0.14 | 0.41 | 37 |
| CD19+IL-10+% | 0.18 | 0.28 | 36 |
| CD19+FOXP3+% | −0.36 | 0.03 | 36 |
| CD19+CD25+FOXP3+% | −0.11 | 0.53 | 36 |
| CD19+CD25+% | 0.06 | 0.72 | 36 |
Subsets, measured by flow cytometry, are expressed as a percentage of the parent CD4+, CD8+, or CD19+ population.
Spearman correlation coefficients.
Bold represents the values that were significant at a 0.05 level.
Italics indicates the values that were marginally significant.
SFC, spot-forming cells.
Discussion
This study showed that HIV-infected pregnant women had highly heterogeneous Th1 and cytotoxic T cell responses to pH1N1 demonstrated by the wide interquartile differences, such that significant group changes from baseline were not observed. However, correlation analyses indicated that this heterogeneity did not merely represent random variation, since these analyses revealed patterns of interrelationships across the effector T cell data. The CMI response to influenza vaccines is important because it is essential for protective antibody responses11–13 and it generates cytotoxic T cells that clear virally infected cells.14–19 The strongest predictors of protective Th1 and cytotoxic CMI responses to pH1N1 IIV1 were high baseline IFN-γ ELISpot results after in vitro PBMC stimulation with pH1N1 or PHA. The lack of specificity of the baseline IFN-γ ELISpot results that predicted a good specific CMI response to pH1N1 vaccination suggested that they functioned as a measure of the overall ability of the host to mount CMI responses. The corollary of this observation is that nonspecific high ELISpot responses in HIV-infected individuals may predict their ability to mount specific CMI to vaccines and/or infections in general.
We found a marginal association of high pH1N1-CMI responses with low CD8+ T cells, which is in agreement with previous reports,20 but we did not find any associations with other traditional markers of HIV disease such as CD4+ cell numbers or plasma HIV load. It is important to note that all women in this study received cART and a majority had high CD4+ cell numbers and <1,000 HIV RNA c/ml of plasma, which leaves open the possibility that subjects with a wider range of values on these variables may exhibit an association of CD4+ cell numbers and plasma HIV load with CMI responses to vaccination. It is also important to mention that in our previous studies we showed a positive association of entry CD4+ T cells with the magnitude of antibody responses to this vaccine and a negative association of HIV viral load with pH1N1-specific IFN-γ ELISpot responses after vaccination.
To determine if T cell regulation played a role in the heterogeneity of the CMI responses to pH1N1 vaccine, we investigated the relationship of pH1N1-specific Th1 and cytotoxic T cell responses to the vaccine with circulating, nonspecific Treg and Breg at baseline and with pH1N1-specific Treg and Breg after vaccination. pH1N1-specific effector T cell responses to vaccination correlated with high pH1N1-specific Treg and Breg after vaccination, suggesting that Treg and Breg were generated through a feedback mechanism as previously proposed.21 High CMI responses to pH1N1 vaccine also correlated with low circulating CD19+FOXP3+% Breg at baseline, albeit not with the better characterized CD19+IL-10+% Breg subset. Nevertheless, the data suggest that a high nonspecific B cell regulatory environment may reduce CMI responses to vaccination similarly to the effect of Breg on HIV-1-specific T cell responses of infected individuals.22 In contrast, high pH1N1-specific CMI responses after vaccination correlated with high baseline circulating CD4+CD39+% and CD8+CD39+% Treg, which was surprising in view of their presumed regulatory activity. CD4+CD39+ Treg are increased in HIV-infected individuals.23–25
Although CD39+ Treg have not been shown to correlate with opportunistic infections or any AIDS or non-AIDS adverse events, they have been presumed to contribute to the immune suppression that characterizes HIV infection through diminution of IL-2 synthesis.24 CD39 is an ectoenzyme that converts ATP to ADP/AMP. CD39 acts in concert with CD73, which converts ATP, ADP, and AMP to adenosine, which binds to the adenosine receptor and activates the T cell inhibitory pathway. Interestingly, CD73+ Treg have been associated with decreased T cell activation, inflammation, and general immune preservation, while CD39+ Treg have been associated with HIV immune suppression.26–28
In summary, the effect of CD39+ Treg in the context of HIV infection is controversial and needs to be further investigated. Our data also need to be confirmed and mechanistically explained in future studies. As new Treg counteractive treatment modalities are being developed,29–31 it is important to continue to study the relationship of Treg and Breg with CMI responses to vaccination, which may provide a framework to understand the potential for increasing responses to vaccines in HIV-infected individuals by manipulation of their Treg and Breg populations.
This study had some limitations, including the investigation of a small subset of CMI responses and Treg and Breg. In addition, we did not have a control group of HIV-uninfected pregnant women to definitively show that the heterogeneity of the responses was HIV specific. In line with the exploratory nature of the study, we did not adjust the analyses for multiple comparisons in order to capture all possible signals, and the results presented here need to be confirmed by additional studies.
Our findings are highly significant for the medical practice, because health care providers frequently use CD4+ cell numbers and HIV load to gauge the optimal time for vaccine administration to HIV-infected individuals. Our data indicate that in HIV-infected pregnant women on cART, the predictive value of these traditional markers is reduced for CMI responses. In contrast, nonspecific CMI responses had a strong predictive value for T cell vaccine immunogenicity. If confirmed in other groups of HIV-infected individuals, this observation may suggest the value of assessing CMI function (by ELISpot or other cytokine production) as an indicator of optimal timing for vaccination.
Supplementary Material
Acknowledgments
The authors appreciate the contributions of the women who participated in this study and the assistance of research personnel at the study sites.
Additional members of the P1086 Protocol Team: George Siberry, MD, MPH, Pediatric Adolescent and Maternal AIDS Branch, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Maryland; Judi Miller, RN, BSN, Division of AIDS, National Institute of Allergy and Infectious Diseases, Bethesda, Maryland; Elizabeth Petzold, PhD, Social & Scientific Systems, Silver Spring, Maryland; Wende Levy, RN, MS, Social & Scientific Systems, Silver Spring, Maryland; Barbara Heckman, BS, Frontier Science and Technology Research Foundation, Buffalo, New York; Ruth Ebiasah, PharmD, MS, RPh, Division of AIDS, National Institute of Allergy and Infectious Diseases, Bethesda, MD; Paul Palumbo, MD, Dartmouth-Hitchcock Medical Center, Lebanon, New Hampshire; Joan Dragavon, MLM, University of Washington, Seattle, Washington; Lori Donelson, RN, BSN, CCRC, Westat, Rockville, Maryland; Andrea Jurgrau, MSRN, CPNP, Columbia-Presbyterian Medical Center, New York, New York; David Garry, DO, Jacobi Medical Center, Bronx, New York.
Participating sites and site personnel: University of Miami Pediatric/Perinatal HIV/AIDS CRS (Amanda Cotter, MD; Gwendolyn B. Scott, MD; Erika Lopez-Bertiery, MD; Safia Khan, MD); University of Puerto Rico Pediatric HIV/AIDS Research Program CRS (Irma L. Febo, MD; Carmen D. Zorrilla, MD; Vivian Tamayo-Agrait, MD; Ruth Santos, RN, MPH); University of Southern California, LA NICHD CRS (Alice Stek, MD; Michael Neely, MD; LaShonda Spencer, MD; Andrea Kovacs, MD); Washington Hospital Center NICHD CRS (Sara Parker, MD; Patricia Tanjutco, MD; Vanessa Emmanuel, BA; Liv Thulin); Texas Children's Hospital CRS (Shelly Buschur, RN, CNM; Mary Paul, MD; Filiz Seeborg, MD; Kathy Pitts, PhD); Chicago Children's CRS (Jessica Shore, RN; Sarah Sutton, MD); UCSD Maternal, Child, and Adolescent HIV CRS (Stephen A. Spector, MD; Andrew Hull, MD; Mary Caffery, RN, MSN; Jean Manning, RN, BSN); DUMC Pediatric CRS (Margaret Donnelly, PA; Mary Jo Hassett, RN; Elizabeth Livingston, MD; Julieta Giner, RN); Rush University Cook County Hospital, Chicago NICHD CRS (Mariam Aziz, MD; Latania Logan, MD; Julie Schmidt, MD; Helen Cejtin, MD); The Children's Hospital of Philadelphia IMPAACT CRS (Samuel Parry, MD; Rita Leite, MD); University of South Florida, Tampa NICHD CRS (Karen L. Bruder, MD; Gail Lewis, RN; Patricia Emmanuel, MD; Tampa General Hospital); San Juan City Hospital PR NICHD CRS (Elvia Perez, MPH, MA; Rodrigo Diaz, MD; Dalila Guzman, RPh; Midnela Acevedo-Flores, MD); South Florida CDC, Ft. Lauderdale NICHD CRS; Johns Hopkins University, Baltimore NCHD CRS (Allison Agwu, MD, ScM; Todd Noletto, MPH; Jennifer Chang, BS; Andi Weiss, PharmD); Tulane University, New Orleans NICHD CRS (Chi Dola, MD; Thomas Alchediak, MD; Yvette Luster, RN; Sheila Bradford, RN); Bronx-Lebanon Hospital IMPAACT CRS (Jenny Gutierrez, MD; Mahboobullah Mirza Baig, MD; Stefan Hagmann, MD; Murli Purswani, MD); NJ Medical School CRS (Arlene D. Bardeguez, MD, MPH; Charmane Calilap-Bernardo, RN; Linda Bettica, RN); Columbia IMPAACT CRS (Andrea Jurgrau, CNP; Gina Silva, RN; Alice Higgins, RN; Marc Foca, MD); Metropolitan Hospital NICHD CRS (Mahrukh Bamji, MD; Santa Paul, MD; Siobhan Riley, MPH; Deepali Jain, MD); Children's Hospital of Boston NICHD CRS (Sandra K. Burchett, MD, MS; Ruth Tuomala, MD; Arlene Buck, RN; Catherine Kneut, RN, CPNP); University of Colorado, Denver NICHD CRS (Jennifer Dunn, FNP-C; Paul Harding, MS; Kay Kinzie, FNP-C; Jenna Wallace, MSW; Supported by NIH/NCATS Colorado CTSI Grant Number UL1 TR000154); St. Jude/UTHSC CRS (L. Jill Utech, RN, MSN, CCRP; Edwin Thorpe, Jr., MD; Nina Sublette, RN, FNP, PhD; Pam Finnie, MSN); WNE Maternal Pediatric Adolescent AIDS CRS; UCLA-Los Angeles/Brazil AIDS Consortium (LABAC) CRS (Jaime G. Deville, MD; Karin Nielsen-Saines, MD; Nicole Falgout, RN; Joseph Geffen); Boston Medical Center Pediatric HIV Program NICHD CRS; Jacobi Medical Center Bronx NICHD CRS; Seattle Children's Hospital CRS; SUNY Stony Brook NICHD CRS (Denise Ferraro, FNP; Erin Infanzon; Michele Kelly, NP; Jennifer Griffin, CNM); Miller Children's Hospital Long Beach, CA NICHD CRS (Audra Deveikis, MD; Janielle Jackson-Alvarez, RN; Tempe K. Chen, MD; Jagmohan S. Batra, MD); University of Florida College of Medicine, Jacksonville NICHD CRS (Mobeen Rathore, MD; Ayesha Mirza, MD; Nizar Maraqa, MD; Kathleen Thoma, MA, CCRP); University of California, San Francisco NICHD CRS (Diane Wara, MD; Nicole Tilton, PNP; Mica Muscat, PNP).
Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) [U01 AI068632], the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Institute of Mental Health (NIMH) [AI068632]. Support of the sites was provided by the National Institute of Allergy and Infectious Diseases (NIAID) and the NICHD International and Domestic Pediatric and Maternal HIV Clinical Trials Network funded by NICHD (contract number N01-DK-9-001/HHSN267200800001C). The laboratory work was supported by N01HD33162 (97-07) and the statistical work by the Statistical and Data Analysis Center at Harvard School of Public Health under the National Institute of Allergy and Infectious Diseases cooperative agreement #5 U01 AI41110 with the Pediatric AIDS Clinical Trials Group (PACTG) and #1 U01 AI068616 with the IMPAACT Group. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the NIH, the United States Government, or the U.S. Department of State.
Mark J. Abzug, Sharon A. Nachman, and Myron J. Levin contributed equally to this work.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Flynn PM, Nachman S, Muresan P, et al. : Safety and immunogenicity of 2009 pandemic H1N1 influenza vaccination in perinatally HIV-1-infected children, adolescents, and young adults. J Infect Dis 2012;206:421–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinberg A, Song LY, Walker R, et al. : Anti-influenza serum and mucosal antibody responses after administration of live attenuated or inactivated influenza vaccines to HIV-infected children. J Acquir Immune Defic Syndr 2010;55:189–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Madhi SA, Maskew M, Koen A, et al. : Trivalent inactivated influenza vaccine in African adults infected with human immunodeficient virus: Double blind, randomized clinical trial of efficacy, immunogenicity, and safety. Clin Infect Dis 2011;52:128–137 [DOI] [PubMed] [Google Scholar]
- 4.Madhi SA, Kuwanda L, Venter M, and Violari A: Prospective cohort study comparing seasonal and H1N1(2009) pandemic influenza virus illnesses in HIV-infected children during 2009. Pediatr Infect Dis J 2014;33:174–176 [DOI] [PubMed] [Google Scholar]
- 5.Black S, Nicolay U, Vesikari T, et al. : Hemagglutination inhibition antibody titers as a correlate of protection for inactivated influenza vaccines in children. Pediatr Infect Dis J 2011,30:1081–1085 [DOI] [PubMed] [Google Scholar]
- 6.Tsang TK, Cauchemez S, Perera RA, et al. : Association between antibody titers and protection against influenza virus infection within households. J Infect Dis 2014;210:684–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohmit SE, Petrie JG, Cross RT, et al. : Influenza hemagglutination-inhibition antibody titer as a correlate of vaccine-induced protection. J Infect Dis 2011;204:1879–1885 [DOI] [PubMed] [Google Scholar]
- 8.McElhaney JE, Ewen C, Zhou X, et al. : Granzyme B: Correlates with protection and enhanced CTL response to influenza vaccination in older adults. Vaccine 2009;27:2418–2425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abzug MJ, Nachman SA, Muresan P, et al. : Safety and immunogenicity of 2009 pH1N1 vaccination in HIV-infected pregnant women. Clin Infect Dis 2013;56:1488–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weinberg A, Muresan P, Richardson KM, et al. : Determinants of vaccine immunogenicity in HIV-infected pregnant women: Analysis of B and T cell responses to pandemic H1N1 monovalent vaccine. PLoS One 2015;10:e0122431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pedersen GK, Madhun AS, Breakwell L, et al. : T-helper 1 cells elicited by H5N1 vaccination predict seroprotection. J Infect Dis 2012;206:158–166 [DOI] [PubMed] [Google Scholar]
- 12.Eto D, Lao C, DiToro D, et al. : IL-21 and IL-6 are critical for different aspects of B cell immunity and redundantly induce optimal follicular helper CD4 T cell (Tfh) differentiation. PLoS One 2011;6:e17739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nayak JL, Fitzgerald TF, Richards KA, et al. : CD4+ T-cell expansion predicts neutralizing antibody responses to monovalent, inactivated 2009 pandemic influenza A (H1N1) virus subtype H1N1 vaccine. J Infect Dis 2013;207:297–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belshe RB, Gruber WC, Mendelman PM, et al. : Correlates of immune protection induced by live, attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine. J Infect Dis 2000;181:1133–1137 [DOI] [PubMed] [Google Scholar]
- 15.Teijaro JR, Verhoeven D, Page CA, et al. : Memory CD4 T cells direct protective responses to influenza virus in the lungs through helper-independent mechanisms. J Virol 2010;84:9217–9226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agrati C, Gioia C, Lalle E, et al. : Association of profoundly impaired immune competence in H1N1v-infected patients with a severe or fatal clinical course. J Infect Dis 2010;202:681–689 [DOI] [PubMed] [Google Scholar]
- 17.Guo H, Santiago F, Lambert K, et al. : T cell-mediated protection against lethal 2009 pandemic H1N1 influenza virus infection in a mouse model. J Virol 2011;85:448–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hemann EA, Kang SM, and Legge KL: Protective CD8 T cell-mediated immunity against influenza A virus infection following influenza virus-like particle vaccination. J Immunol 2013;191:2486–2494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilkinson TM, Li CK, Chui CS, et al. : Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat Med 2012;18:274–280 [DOI] [PubMed] [Google Scholar]
- 20.Fabbiani M, Sidella L, Corbi M, et al. : HIV-infected patients show impaired cellular immune response to influenza vaccination compared to healthy subjects. Vaccine 2013;31:2914–2918 [DOI] [PubMed] [Google Scholar]
- 21.Baeyens A, Saadoun D, Billiard F, et al. : Effector T cells boost regulatory T cell expansion by IL-2, TNF, OX40, and plasmacytoid dendritic cells depending on the immune context. J Immunol 2015;194:999–1010 [DOI] [PubMed] [Google Scholar]
- 22.Liu J, Zhan W, Kim CJ, et al. : IL-10-producing B cells are induced early in HIV-1 infection and suppress HIV-1-specific T cell responses. PloS One 2014;9:e89236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Presicce P, Orsborn K, King E, et al. : Frequency of circulating regulatory T cells increases during chronic HIV infection and is largely controlled by highly active antiretroviral therapy. PLoS One 2011;6:e28118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jenabian MA, Seddiki N, Yatim A, et al. : Regulatory T cells negatively affect IL-2 production of effector T cells through CD39/adenosine pathway in HIV infection. PLoS Path 2013;9:e1003319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tenorio AR, Martinson J, Pollard D, et al. : The relationship of T-regulatory cell subsets to disease stage, immune activation, and pathogen-specific immunity in HIV infection. J Acquir Immune Defic Syndr 2008;48:577–580 [DOI] [PubMed] [Google Scholar]
- 26.Carriere M, Lacabaratz C, Kok A, et al. : HIV "elite controllers" are characterized by a high frequency of memory CD8+ CD73+ T cells involved in the antigen-specific CD8+ T-cell response. J Infect Dis 2014;209:1321–1330 [DOI] [PubMed] [Google Scholar]
- 27.Moreno-Fernandez ME, Rueda CM, Rusie LK, and Chougnet CA: Regulatory T cells control HIV replication in activated T cells through a cAMP-dependent mechanism. Blood 2011;117:5372–5380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schuler PJ, Macatangay BJ, Saze Z, et al. : CD4(+)CD73(+) T cells are associated with lower T-cell activation and C reactive protein levels and are depleted in HIV-1 infection regardless of viral suppression. AIDS 2013;27:1545–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brahmer JR, Tykodi SS, Chow LQ, et al. : Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455–2465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamid O, Robert C, Daud A, et al. : Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 2013;369:134–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Topalian SL, Hodi FS, Brahmer JR, et al. : Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443–2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.