Abstract
Significance: Oral wounds can lead to significant pain and discomfort as well as affect overall general health due to poor diet and inadequate nutrition. Besides many biological and pharmaceutical methods being investigated, there is growing interest in exploring various biophysical devices that utilize electric, magnetic, ultrasound, pressure, and light energy.
Recent Advances: Significant insight into mechanisms of these biophysical devices could provide a clear rationale for their clinical use. Preclinical studies are essential precursors in determining physiological mechanisms and elucidation of causal pathways. This will lead to development of safe and effective therapeutic protocols for clinical wound management.
Critical Issues: Identification of precise events initiated by biophysical devices, specifically photobiomodulation—the major focus of this review, offers promising avenues in improving oral wound management. The primary phase responses initiated by the interventions that distinctly contribute to the therapeutic response must be clearly delineated from secondary phase responses. The latter events are a consequence of the wound healing process and must not be confused with causal mechanisms.
Future Direction: Clinical adoption of these biophysical devices needs robust and efficacious protocols that can be developed by well-designed preclinical and clinical studies. Elucidation of the precise molecular mechanisms of these biophysical approaches could determine optimization of their applications for predictive oral wound care.

Praveen Arany, DDS, PhD
Scope and Significance
Failure or undue prolongation of any of the wound healing phases could be a potential threat to the human body. Data from the United States indicate that chronic wounds affect around 6.5 million patients with an estimated US$25 billion annual expenditure on their treatment.1 This situation is expected to grow rapidly worldwide due to an increasing aging population as well as a sharp rise in the incidence of metabolic diseases such as diabetes and obesity that profoundly affect wound healing. Oral wounds do not usually have a protracted time course, but oral care products are a US$10 billion industry that focuses on prevention of disease and damage to hard and soft tissues in the oral cavity. While wound healing in oral soft tissues has many similarities with dermal healing, it also has some unique aspects, such as the moist (saliva) environment, parakeratinized mucosa, lack of dermal appendages (hair follicles, sebaceous glands), rapid turnover of oral epithelia, polymicrobial niche with complex biofilms, and a relatively vigorous, cyclic mechanical (chewing, swallowing, or speaking) environment. A wide range of developmental anomalies (e.g., cleft lip or palate, ankyloglossia), infections (e.g., candida, herpes, caries, periodontal disease), and immune (e.g., lichen planus, recurrent aphthous ulcers) and traumatic etiopathologies could lead to wounds in the oral cavity.
Translational Relevance
Nonhealing oral wounds can result in significant morbidity due to restriction of diet and routine oral functions and, infrequently, lead to patient mortality. Any modality that could aid in wound closure by promoting epithelial cell migration, fibroblast proliferation, synthesis of extracellular matrix, and wound contraction or by promoting neutrophil and macrophage migration and function could have significant clinical benefits. Conventional approaches have utilized pharmaceuticals and biological agents successfully, but all of them have inherent limitations that could be potentially addressed with biophysical interventions outlined in this review.
Clinical Relevance
Given the unique aspects of oral healing and rigorous pathophysiological demand from the native tissues, wounds in the oral cavity can have a dramatic effect on the overall well being of the individual. Clinical dentistry has effectively focused on comprehensive hard tissue management using restorative (dentures, fillings) approaches. Soft tissue management has largely been limited to addressing the underlying infective or injurious stimuli. Use of tissue grafts and artificial matrices has been extensively tried, but are largely prohibitive due to cost or anatomical site. The hard tissue components of oral healing include bone (alveolar—bone around tooth socket and jaws) and teeth (predominantly dentin and cementum). Novel biophysical approaches that promote inherent healing and regenerative capacity of oral and dental tissues are very attractive due to their nonconsumable nature (unlike drugs and biological), ease of access to oral wounds, and efficacy of promoting the endogenous healing process that would reduce frequent patient visits and reduce cost of overall therapy.
Background
The process of wound healing is predominantly similar at all anatomical sites with four major overlapping phases. The first phase consists of clotting and coagulation; the second phase involves inflammation at the wounded site; the third phase consists of migration, proliferation, angiogenesis, phagocytosis, and matrix synthesis. This is followed by the final, fourth maturation phase that involves tissue remodeling and reorganization (Fig. 1). Oral wound healing has similar phases, but appears to follow a more rapid course and healing with minimal scar formation compared with wounds at other sites (e.g., skin).2 Both of these unique aspects of oral wound healing are attributed to the presence of excellent blood supply in the head and neck region, antibacterial and prohealing properties of saliva, as well as a more rapid turnover of the mucosal keratinocytes (epithelium).
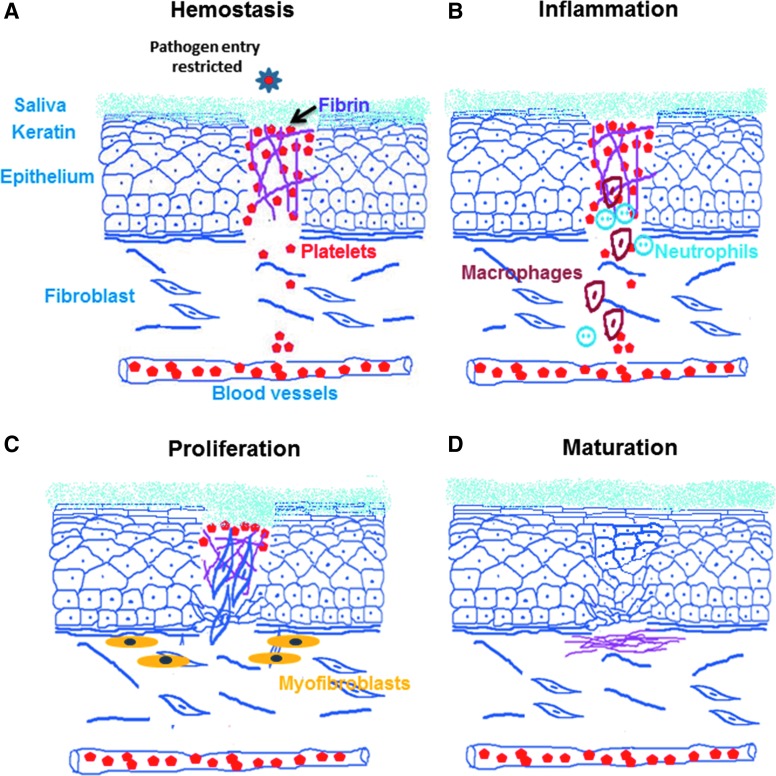
Figure 1.
Different stages of wound healing. (A) Hemostasis: wound closure starts with the first phase of clotting involving formation of immediate platelet plug, followed by initiation of the coagulation cascade. Oral wounds have a rich vascular supply and the salivary proteins which aid in forming a temporary hemostatic plug. (B) Inflammation: the second phase involves migration of acute (neutrophils) and eventually chronic inflammatory (monocytes–macrophages and lymphocytes) cells into the wound area. Moist oral mucosa possesses both innate (neutrophils and macrophages) and adaptive (immunoglobulins) immunities, which quickly resolve inflammation. This is well supported by chemical and enzymatic actions of salivary constituents. (C) Proliferation: the third phase consists of migration and proliferation of keratinocytes, endothelial cells, and fibroblasts that complete closure of wound. Proliferation and activation of fibroblasts to myofibroblasts hastens wound closure. (D) Maturation: the final fourth phase involves remodeling and reorganization that can be partial (scarring) or complete (regeneration).
Classical surgical approaches, such as distraction osteogenesis, tissue expansion for flap harvest, and negative pressure therapy, rely on externally applied mechanical forces that induce cell proliferation and angiogenesis. These responses occur at the clinical (millimeters to centimeters range) scale. Micromechanical responses to biophysical interventions appear to operate along similar principles, although at the molecular scale (microns to nanometers range) (Fig. 2).3 However, therapeutic dose regimens for latter devices are poorly defined as yet and have been relegated, unfortunately, as complementary or adjunctive interventions.
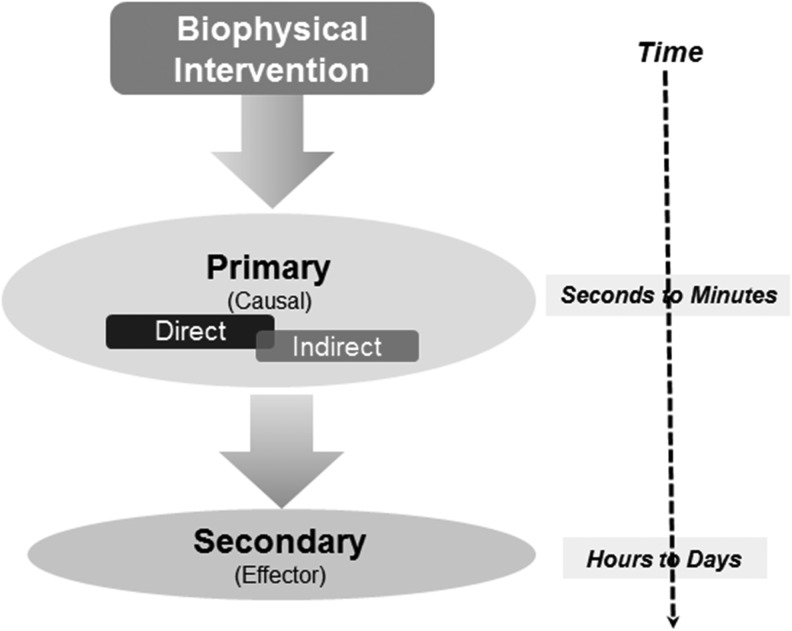
Figure 2.
Strategy of dissecting mechanistic events following interventions. Wound healing progression over time has discrete biological responses that include immediate events (primary—direct and indirect) and subsequent (secondary phase) events. The former represent a causal relationship with the biophysical interventions, while the latter are effector pathways that are a sequela of the wound healing process.
When assessing molecular mechanisms, it is important to distinguish the primary causal pathways that are directly modulated by the interventional therapies. The secondary, downstream effector pathways are a natural consequence of the biological healing–regenerative response (Fig. 2). The primary phase involves causal factor(s) that sense and transduce the biophysical intervention into a biochemical or biological response. These events are necessary (lack of which prevents occurrence of therapeutic benefit) and sufficient, by themselves, to generate the biological response. Some reported examples of these causal events include generation of highly reactive, transient biochemical intermediates, changes in cellular ionic gradients, changes in cell polarity, and changes in physical conformation of a biological factor, among others. The secondary phase involves the effector mechanisms that are sequelae of the routine, or improved biological responses, following the therapeutic interventions. These events are clearly necessary, but are not sufficient (cannot by themselves initiate therapeutic response). It would be prudent to note that there may often be factors initiated (primary phase) by the intervention that are also involved in the subsequent effector (secondary phase) responses. While investigating the mechanisms of biophysical interventions, care should be taken to focus on identifying the critical pivotal factor(s) (causal events) that will aid in gaining mechanistic insights.4 A useful yardstick often employed in the distinction of causal versus effector mechanisms is the temporal (time-dependent) separation following an intervention. However, this is largely restrictive due to current technical limitations of assessing rapid biological events, both at temporal and spatial length scales. Significant improvements in technologies and instrumentation, such as super-resolution microscopy, in vivo imaging, spectroscopy, and single-cell analyses, among many others, are allowing deciphering of molecular mechanisms at previously unavailable biological scales.
Novel Biophysical Approaches for Wound Healing
To promote wound healing phases, several biophysical therapies have been utilized that employ different basic principles. Two of the biophysical techniques, microcurrent and electromagnetic fields, are based on the premise that differences in electrical potential along the different layers of the skin or mucosa are determined by the asymmetric expression of sodium and potassium ion pumps. This is termed as transepithelial potential (TEP) with the stratum corneum (outermost epithelial layer) being electronegative, while subepithelial layers are electropositive. Wound healing and tissue regeneration are driven by a closed-loop self-repair system that uses signals (electrical) to initiate repair following injury.5 These biophysical interventions appear to modulate the disrupted endogenous electromagnetic fields and aid in reestablishment of TEP.6 The other three biophysical therapies, namely ultrasound (US), pressure, and light therapies, have been demonstrated to have clinical benefits and their molecular mechanisms appear to involve both biophysical and biochemical perturbations. However, their precise primary biological targets remain to be fully elucidated.
Microcurrent
Microcurrent energy has been used for wound care and is also known by terms such as microcurrent electrical stimulation, microamp therapy, microamperage electrical stimulation, or low-intensity electrical stimulation. Upon injury, the injured tissue has a lower level of ATP due to a disruption of the sodium–potassium pump, leading to increased epithelial electrical resistance and lower cellular capacitance, resulting in altered TEP. Microcurrent energy reverses the cellular capacitance by application of an extrinsic electrical stimulus that promotes reestablishment of the TEP, facilitating wound healing. Microcurrent energy has been shown to induce polarized signaling of epidermal growth factor (EGF), integrins, and phosphoinositide 3 kinase/phosphatase and tensin homolog (PI3K/PTEN),7 leading to biological responses, such as increase in cell division,8 secretion of growth factors,9 ATP synthesis,9–11 and promotion of wound reepithelialization. Moreover, protocols utilizing mono- and biphasic pulsed currents, usually in the microampere, applied to wounds and periulcer skin with spherical electrodes positioned on the wound have demonstrated positive results consistently (Fig. 3A).12,13 Commercial acceptance of this technology is already evident with various products currently available in the market, such as patterned metallic patches and bandages as well as metallic nanoparticle-based therapies. Among several applications, microcurrent electrical nerve stimulation has been shown to reduce pain in temporomandibular disorder patients compared with occlusal splint therapy alone.14 There has been little effort to directly assess efficacy of microcurrents in oral soft tissue wounds, but a distinct application of electrical energy to activate enamel (outermost, calcified tooth structure) mineralization (EAER) has been recently suggested, but few details are available as yet.
Figure 3.
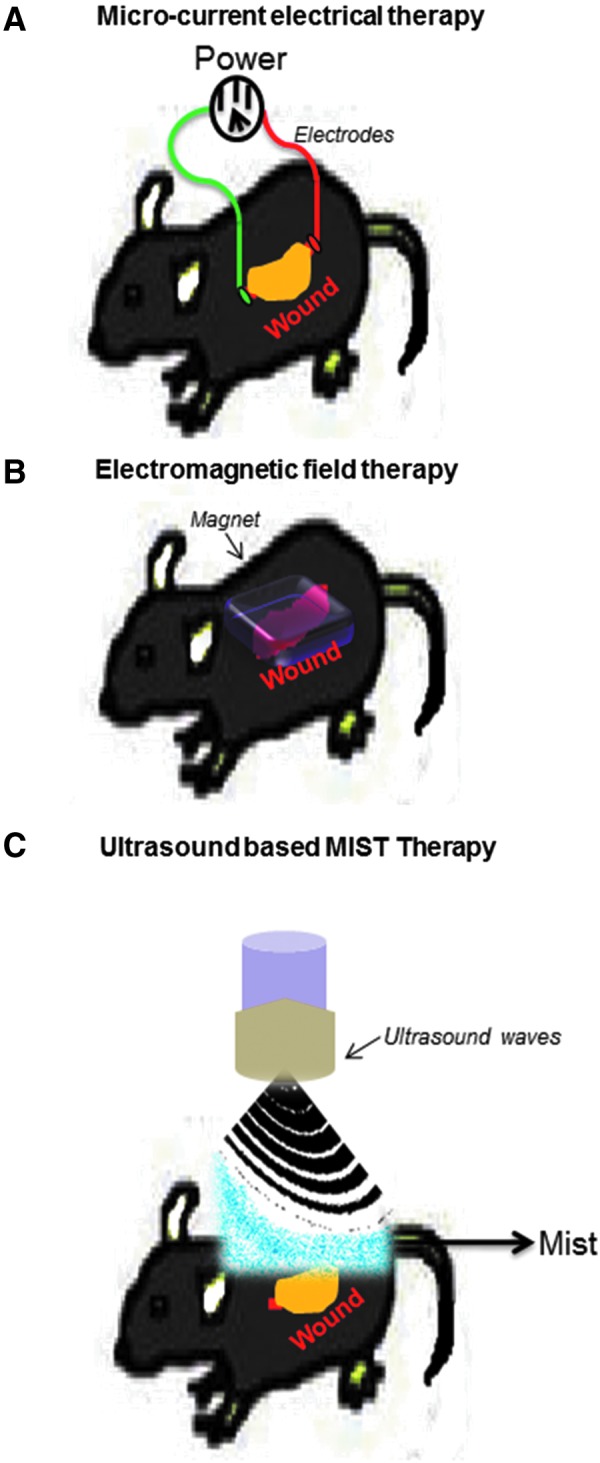
Biophysical energies in current wound management. (A) Microcurrent: outline showing application of microcurrent energy using electrodes positioned on opposite sides of a wound in mice. (B) Pulsed electromagnetic fields (PEMFs): outline of induction of PEMFs on wounds in mice to promote healing. (C) Ultrasound: outline showing MIST™ therapy that uses low-frequency ultrasound delivered along with saline mist to the wound bed in mice.
Electromagnetic fields
Historically, magnets have been used for different medical therapies, including wound healing, with little information about its scientific basis.15,16 In 1957, Fukada and Yasuda from Japan demonstrated the piezoelectric effect in bone. They noted the ability of mechanical stress to generate polarization in the healing bone and application of an extrinsic electric field-generated strain within the tissues.17 A wide range of studies, both in the clinic and laboratory, have led to the use of electromagnetic fields (EMFs) in promotion of bone and, more recently, cartilage healing. Their utility in promoting soft tissue and skin healing is also being currently explored. The molecular pathways involved in EMFs have been outlined in a recent review that indicates that application of EMF induces expression of key growth factors in wounded tissues.18 EMF is used for clinical applications as either static or pulsed fields at low frequencies ranging from 1 to 3 mT (Fig. 3B). Application of a low-power, static magnetic field over an excisional wound has shown to improve wound healing,19 while pulsed electromagnetic fields (PEMFs) have been shown to promote neovascularization by inducing endothelial cell proliferation and lumenization through increased expression of fibroblast growth factor-2 (FGF-2), a known inducer of angiogenesis.20 In the head and neck region, PEMF therapy has been shown to have beneficial effects on healing of mandibular fractures treated with closed reduction,21 while its applications for oral soft tissue healing are yet to be investigated.
Ultrasound
Ultrasound energy is an oscillating, sound pressure wave with frequency ranging from 20 kilohertz to several gigahertz. A major difference from its popular use as a diagnostic modality is that therapeutic ultrasounds utilize higher intensities, either focused or unfocused beams, and rely on both thermal and mechanical interactions for their therapeutic benefits. The mechanical effects of US are termed acoustic streaming and cavitation. Acoustic streaming provides a direct driving force capable of displacing ions and small molecules within the interacting liquid media. Cavitation refers to formation of low-pressure vapor cavities due to rapid change in pressure within liquid environments that can implode, generating powerful shockwaves.22 Readers are referred to a recent review for more details of its various medical applications.23 Both high-frequency (1–4 MHz) and low-frequency (22.5–40 kHz) ultrasound have been shown to promote wound healing. The healing effect of high frequency appears to be largely dependent on heating (must reach a temperature of 40–45°C for at least 5 min), while the low frequency appears to be predominantly dependent on its mechanical effects.24 US therapy is noted to have distinct effects on specific phases of wound healing, such as release of histamine by degranulation of mast cells during the inflammatory phase, promoting resolution of the acute phase to aid healing,25 accelerating fibrinolysis,26 fibroblast recruitment and activation, as well as collagen secretion in the proliferative stage as well as angiogenesis,27 all contributing to promoting healing. A recent modification is the concurrent use of a saline mist with noncontact low frequencies termed MIST™ therapy (Celleration, Inc., Eden Prairie, MN) (Fig. 3C).28 This therapy has been shown to be a gentle, painless clinical protocol that is capable of stimulating cells at the wound bed to secrete more growth factors,29 increase collagen synthesis, reduce inflammation, and induce vasodilation.28 Although dentistry utilizes similar technologies routinely in debridement and disinfection of periodontal defects, there has been limited direct exploration of its utility in oral soft tissue healing.
Pressure (negative and positive pressure)
Accumulation of interstitial fluid within wounds can greatly impede the natural healing process. Application of pressure, if applied effectively, can facilitate reabsorption of fluid by veins and lymphatics, leading to improved wound healing. Use of negative pressure appears to be more effective compared with positive pressure (compression therapy). The biological response to these therapies has been attributed to multiple actions, such as removal of wound exudate, retaining a moist environment, reduction of edema and bacterial burden, physical approximate wound margins, and increased wound perfusion. These result in promoting leukocyte and fibroblast migration into the wounds, which, in turn, secrete growth factors for improving the healing milieu.30 It has been noted that negative pressure energy requires significant adjunctive clinical pain management, although the biological basis for this phenomenon is unclear.31 Clinical conditions, which are amenable to negative pressure therapies, include wounds with lymphatic damage, venous stasis ulcers, diabetic wounds, and wounds with fistulae. Given the complex mechanical and fluid (saliva) oral environment, there has been little effort at using either negative or positive pressure in oral wound management. A major technological advance that appears to be necessary is development of appropriate miniaturized devices for use within the oral cavity.
Photobiomodulation
There appears to be ample anecdotal scientific literature for the use of light in wound care from sterilization, desiccation, and promotion of healing. The use of phototherapy briefly gained notoriety in the early 1900s with Niel Finsen's work that used concentrated light radiation for treatment of lupus vulgaris and was awarded the 1903 Nobel Prize in physiology or medicine. However, the precise biological basis of these observations was unclear. Numerous studies since then have precisely characterized the biochemical pathways for vitamin D metabolism, the visual photoreception in the retina, and, more recently, nonvisual photoreceptive pathways that maintain circadian rhythm. The invention of the LASER (light amplification by stimulated emission of radiation) in 1960 led to initial health concerns over its electromagnetic nature. High-energy, ionizing electromagnetic radiations, such as gamma, X-ray, and ultraviolet, are well known to generate DNA damage and genotoxicity. However, high-power visible and infrared lasers are nonionizing and are very popular as surgical tools due to their precision and concurrent photocoagulation (bloodless surgical fields). Interestingly, Mester et al., in 1968, showed that laser treatments at low doses were able to stimulate hair growth and promote wound healing in mice, suggesting that lasers have biostimulatory responses.32,33
With the advancement of technology and increased knowledge about the beneficial effect of laser, both coherent (lasers) and noncoherent (light-emitting diode [LED]) light sources have been routinely used for therapy. Treatments using low-dose light therapies are known by various names, such as soft laser, cold laser, low-level light/laser therapy, biostimulation, or photobiomodulation (PBM). The latter term, PBM, appears to be the most suitable descriptor of the process and is defined as a form of phototherapy that utilizes nonionizing sources (including broadband light, LEDs, and lasers) in the visible and infrared spectrum that result in therapeutic benefits, such as alleviation of pain or inflammation, immunomodulation and promotion of wound healing, and tissue regeneration. PBM is a nonthermal process involving endogenous chromophores that elicit photophysical (linear and nonlinear effects) and photochemical events at various length scales, resulting in beneficial photobiological responses. Its clinical application is termed as PBM therapy. Low-dose laser responses have been noted to follow a biphasic response that is best described by the Arndt–Schulz law, where a weak stimulus is noted to improve a specific biological function and a stronger stimulus elevates it further. However, after its peak response is achieved, further increase of stimulus strength results in a negative response.34 The biological mechanisms contributing to these biphasic responses are yet unclear, but the following sections outline our current understanding of PBM therapy.
Molecular mechanisms of PBM
The effects of low-dose laser appear to involve both photochemical and photoacoustic (photomechanical) effects. The action spectra for biological responses have been noted to be within the visible to near-infrared range (400–1,000 nm) that is generally referred to as the optical window of PBM. While most studies previously have focused on the use of 660 and 810 nm, other studies with 400, 632, 680, 840, 904, 940, 980, and 1,064 nm have all demonstrated distinct therapeutic benefits. The redundancy noted with some of these wavelengths raises interesting questions about the identity and nature (modified biochemical states) of biological chromophores potentially contributing to the clinical responses noted.
PBM mechanisms can be broadly categorized into primary and secondary phases based on the time from intervention. Primary phase events are considered causal and are further subcategorized into direct events—that result from interaction of radiant energy with biological tissues—and the indirect events—that immediately follow former events. Both are transient in nature, critical (necessary and sufficient), and induce potent downstream biological responses that contribute to the secondary phase events. Given the limited repertoire of biological molecules and pathways, it is very likely that key biological molecules or pathways play critical roles in both primary and secondary phase events and care should be taken to distinguish between their roles.
Primary phase
The primary effects of PBM involve photochemical and photophysical events that occur within seconds to minutes of treatment. The key process involving conversion of irradiant energy requires a primary photoacceptor that usually has two parts, a chromophore (absorbs light) and a functional protein. These rapid photobiological responses would also be spatially limited due to their extremely reactive nature as well as diffusion limitations of compartmentalized biological (e.g., intra- vs. extracellular) systems. This makes elucidation of these primary events currently very challenging due to technical limitations in investigating biological systems. Nonetheless, attempts to further dissect the temporal sequence of responses suggest there are two distinct events within the primary phase response—direct and indirect events.
Primary direct events (sensors)
The first step is converting the physical form of light energy as it interacts with biological tissues. From our understanding of classical phototransduction visual pathway and spectroscopic techniques, there appear to be two distinct direct primary events. The first involves absorption of photons and transfer of energy to generate photochemical intermediates (Type I or II photoreactions). Absorption by a biological chromophore results in release of electrons through an oxidation–reduction (redox) reaction, generating reactive species that have a wide range of biological targets initiating potent, long-term biological responses.35,36 Photoacceptors in the visible spectrum are well characterized and could contribute to the observed clinical benefits from visible light treatments. However, the distinct benefits noted with near and mid-infrared light treatments do not have known photoacceptors within this spectrum, raising interesting questions if there are unique, yet undescribed, biological candidates. Alternatively, near infrared (NIR)-biological tissue interactions may involve a nonlinear optical phenomenon, generating higher harmonic effects that could subsequently utilize photoacceptors in the visible spectrum.37
The second group of interactions involves a physical perturbation of the molecular structure (photoacoustic–photomechanical). These interactions result in changes in conformation of a molecule that facilitates its physiological functions.38 Besides the direct effects of low-dose lasers on the conformational structure of biological molecules, the biochemical (reactive oxygen species [ROS]) events could also secondarily generate biologically significant conformational changes. Both these events essentially act as sensors that occur extremely rapidly, occurring within fractions of seconds. Some examples of well-characterized mammalian photoacceptors include seven dehydrocholesterol (vitamin D3 in skin), rhodopsin (rod cells in retina), photopsin (cones cells in retina), melanopsin (photosensitive ganglion cells in retina), cytochrome C oxidase (CCO) (mitochondria), flavins, porphyrins, and NADPH, among others. It should be noted that both of these photochemical and photoacoustic events in excessive amounts could result in detrimental damage to biological targets in a dose-dependent manner, perhaps accounting for the observed biphasic clinical effects noted with PBM therapy. However, there appear to be multiple regulatory steps in subsequent downstream events that can either amplify or dampen decisive biological responses, such as effective substrate concentrations, catalysts, inhibitors, or scavengers, among others. The precise molecular events involved in this initial key step in light–biological tissue interactions need careful exploration of these events and will be critical to our understanding of PBM clinical dosing and therapeutic responses.
Primary indirect events (transducers)
Generation of extremely transient biochemical intermediates or conformational activation of biological molecules immediately results in cascading biological reactions. These latter events can be categorized as the primary indirect effects or transducers, which encompass sequential events occurring over seconds to minutes. These events appear to be exquisitely coupled to the nature and location of the primary photoacceptor molecule as well as the pathophysiological context (disease/disorders). Some of the major primary indirect events noted with PBM therapy are summarized below.
Small molecules: Photoabsorption and transfer of electrons (redox reaction) to oxygen species result in formation of radicals collectively called ROS. Many of these ROS are well characterized, such as superoxide, hydrogen peroxide, and hydroxyl radicals. Transfer of energy to nitrogen leads to generation of nitric oxide (NO), which, in turn, can react with superoxide anion (O2−) produced by inflammatory cells, forming peroxynitrite (ONOO−), both of which have been suggested to contribute to PBM responses.39 Seminal work by Karu described one of the primary photoacceptors of visible light as CCO, a component of the mitochondrial respiratory chain.40 These investigators demonstrated that the absorption spectra obtained for different oxidation states of CCO correlated with the action spectra for the biological responses to light.41,42 It has also been observed that exposure of purified CCO enzyme to a helium–neon (633 nm) laser leads to increased oxidation and electron transfer.43 The normal physiological role of CCO is to maintain the proton motive force across the inner mitochondrial membrane. Photoabsorption by CCO results in disruption of the electron transfer process leading to increased ATP production.15 The ATP produced by the laser treatment has been shown to modulate a wide range of biological responses, including activation or synthesis of DNA, RNA, proteins, enzymes, and other cellular components needed to improve performance and repair or regenerate cells and tissues.
Another prominent effect of photoabsorption by CCO is dissociation of noncovalently bound ligand (NO) from its metal (Fe/Cu redox centers). CCO has two ligands, namely oxygen (covalent bond) and NO (coordinate bond), the latter being a competitive inhibitor for oxygen-driven respiration.44 NO binds to the reduced binuclear heme–copper (CuB/a3) center of CCO in a reversible and competitive manner. As coordinate bonding (CCO and NO) is much weaker than covalent bonding (CCO and O2), visible and NIR light are easily able to dissociate NO.45 One of the direct effects of NO released from CCO by the light results in vasodilatation and has been shown to involve cGMP-mediated activation of Ca-sensitive K (Kc) channels (Fig. 4).46 Broadly, the released NO has been shown to promote potent wound healing responses, such as in keratinocyte and tenocyte proliferation, endothelial migration and lumenization, macrophage function, angiogenesis in ischemic limb injuries, reduction of reperfusion injury, and vasodilation, and promote stem cell differentiation, among others.47–52
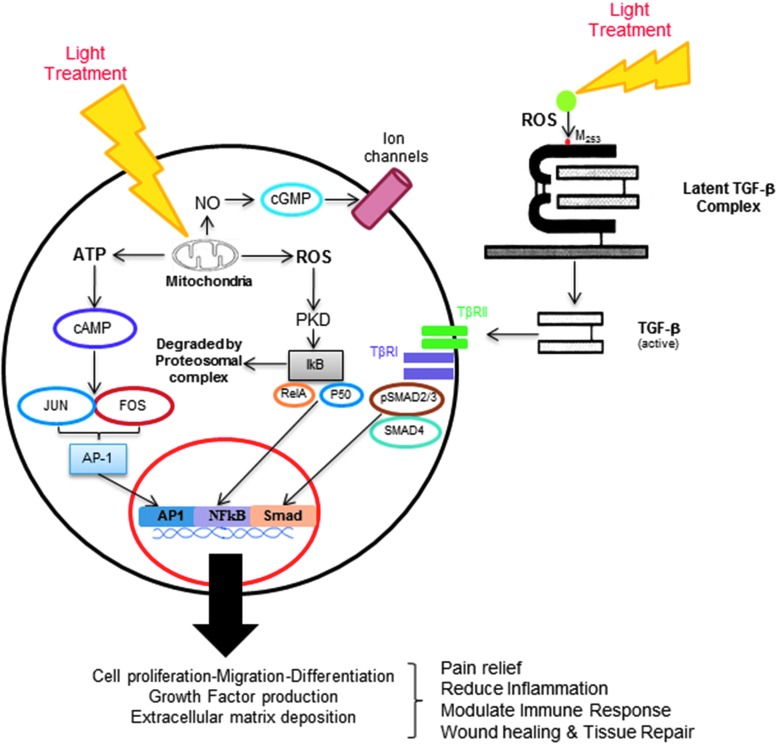
Figure 4.
Mechanisms of photobiomodulation (PBM). Interaction of light and biological tissues leads to generation of transient and extremely reactive chemical intermediates, reactive oxygen species (ROS), in both extracellular and intracellular compartments. These ROS can react rapidly with various components inducing potent cellular responses. Among the best characterized pathways, intracellular photoabsorption by cytochrome C oxidase disrupts mitochondrial function, resulting in increased ATP synthesis and nitric oxide (NO) release. A recently elucidated extracellular pathway noted generation of ROS-mediated activation of transforming growth factor-β1 (TGF-β1) following PBM therapy. Both intracellular and extracellular pathways induce specific signal transduction pathways that recruit transcription factors leading to a concerted gene expression contributing to therapeutic PBM effects on wound healing.
Kinases, enzymes, hormones, and growth factor: Among its many biological targets, PBM (632 nm)-generated ROS has been shown to activate Src that has potent effects on cell proliferation, attachment, migration, and survival.53 Src is a nonreceptor tyrosine kinase that can interact with a large number of biological pathways, including EGF, MAPK, FAK, and STAT3, among others.54 Using a combination of Src luciferase reporters and immunoblotting, the investigators noted that the ability of laser-generated ROS increased Src activity that could be abrogated by prior neutralization with ROS scavengers, catalase, or superoxide dismutase. Activation of Src closely correlated with the cell survival fraction in a laser dose-dependent manner. Another study noted that the ability of low-dose laser (810 nm) treatments increased acetylcholinesterase (AChE) activity in saline-suspended human erythrocytes without disrupting its membrane electrochemical potential.55 The biological functions of erythrocyte AChE appear to involve hemopoiesis and cell–cell interactions that mediate cell growth and differentiation.56,57 The clinical significance of increased AChE activity following PBM treatments is unclear, although it has been correlated in hereditary spherocytosis with lipid reorganization of RBC membranes.
Growth hormone is an anabolic peptide hormone that stimulates tissue growth, proliferation, regeneration, and rejuvenation. It has been noted to increase bone mineralization, muscle mass, gluconeogenesis, and lipolysis, and stimulate the immune system, among others. The somatotrophic cells of the anterior pituitary have some storage granules with both growth hormone and cytochrome oxidase within them. Treatment with LED (670 nm) was noted to increase growth hormone secretion in both their in vivo rat and in vitro pituitary gland cultures.58 The investigators noted that the released hormone appeared to be partly due to disaggregation of oligomeric isoforms. This suggests that PBM treatments could directly modulate hormone secretion from an endocrine gland.
Transforming growth factors-β (TGF-β) are a superfamily of multifunctional cytokines involved in cellular proliferation, differentiation, and physiological homeostasis.59 TGF-β1 is secreted as an inactive (latent) precursor consisting of a native (mature) TGF-β dimer noncovalently associated with latency-associated peptide (LAP), which forms the small latent complex (TGF-β–LAP). For the TGF-β pathway to be activated, it has to be dissociated from its latent complex, allowing the dimer to bind its surface receptor, TGF-βRII, which subsequently recruits and activates an intracellular receptor, TGF-βRI.60 TGF-β is known to be a potent chemoattractant for early inflammatory cells like neutrophils and monocytes/macrophages.61 However, the overall effect of TGF-β on the secondary inflammatory cells, specifically on T lymphocytes, is largely inhibitory, thereby inhibiting autoimmunity. TGF-β plays a prominent role in the proliferation, resolution, and remodeling of the wound tissue by promoting keratinocytes, endothelial, and fibroblast cell migration, as well as matrix synthesis.
We previously noted the ability of NIR-PBM therapy to promote oral wound healing in human oral extraction wounds.62 In this study, we noted that the laser wounds appeared to have increased TGF-β1 by immunohistochemistry compared with the nonlaser-treated control wound from the same patient. This observation suggested that there may be a direct effect of the laser treatment on latent TGF-β1 complexes, which are present in abundance from the degranulated platelets following hemostasis. This clinical observation was further explored in the laboratory using a wide range of biochemical, molecular, and transgenic approaches. Low-power laser treatments were noted to generate ROS that interacts with a specific amino acid residue on the latent TGF-β1, methionine 253 on LAP, resulting in a conformation change leading to its activation (Fig. 4).63 Using a dentin–pulp healing model, low-power laser treatments were noted to induce tertiary dentin, a normal sequela of tooth pulp wound healing. Furthermore, using conditional transgenic mice that specifically removed TGF-βRII from the dentin-forming cells (odontoblasts and dental stem cells), the key role of TGF-β1 in mediating this process was clearly outlined. Thus, activation of TGF-β1 by PBM therapy contributes to both oral mucosal and pulp–dentin wound healing. The ability of laser-generated ROS-activated TGF-β1 offers significant clinical utility and significant avenues for future investigations.
Transcription factors: ROS generation from light treatments can lead to modulation of antioxidants, protein modification, and induction of gene expression following cellular stress.64 These ROS-mediated responses are detected by numerous mechanisms within the cell, especially in the endoplasmic reticulum (ER) that detects redox-modified, misfolded, or aggregated proteins. In mild damage scenarios, the ER response involves either a reparative process that utilizes chaperones such as the heat shock proteins or degradation by the proteosomal pathway. In extreme cases, due to excessive damage, the ER response invokes cell death pathways involving autophagy and apoptosis. One example of ROS stress-mediated redox change in proteins is generation of cysteine-mediated disulfide linkages. Cysteine residues do not form disulfide bonds unless the intracellular redox balance is shifted toward oxidative stress. The formation of disulfide bonds generally leads to alteration of both conformation and activity of a number of enzymes, most notably of phosphatases. Inactivation of a specific phosphatase by the oxidative stress could result in prolonged activity of kinases within the cell that can result in persistent intracellular signal cascade and transcriptional changes.65
Cell stress responses are mediated by transcription factors initiated by changes in the cellular redox state. Among them, some prominent players include redox factor-1 (Ref-1)-dependent activator protein-1 (AP-1), Jun and Fos, nuclear factor kappa-B (NF-κB), p53, activating transcription factor/cAMP response element-binding protein (ATF/CREB), and hypoxia-inducible factor-1α (HIF-1α). It has been demonstrated that NF-κB can be activated in mouse embryonic fibroblasts by 810 or 980 nm laser treatments. Furthermore, it was observed that NF-κB activation was dependent on induction of ROS and this could be abrogated by laser treatments in the presence of antioxidants. These observations indicate that PBM modulates mitochondrial respiration and activates redox-sensitive NF-κB signaling through generation of ROS.36 Similarly, it has been shown that PBM (both 660 and 780 nm) improves the healing of skin flaps by enhancing the amount of new vessels formed in the tissue by modulating vascular endothelial growth factor (VEGF) secretion and HIF-1α expression in a dose-dependent manner.66 PBM has also been shown to rescue dendritic atrophy in the brain by upregulating brain-derived neurotrophic factor (BDNF) expression through induction of CREB.67 Overall, the controlled expression and activity of transcriptional factors in response to laser-generated cellular stress are capable of coordinating a broad range of beneficial responses.
Secondary phase
Once the primary phases of light–biological tissue interactions are initiated, they induce myriad, downstream, secondary effector pathways that involve transcriptional and translational responses. These secondary phase responses usually take hours to days and have a distinct long-term impact on functional recovery. These secondary responses in wound healing include induction of autocrine or paracrine signaling, cell migration, proliferation or differentiation, matrix synthesis, angiogenesis, and vascular remodeling, among others. PBM effects on wound healing have been shown to positively affect each of the four phases of wound healing. In the hemostatic phase, PBM has been noted to promote platelet aggregation and activation. In the inflammatory phase, it is known to promote proliferation and degranulation of mast cells.68 In the proliferative phase, it induces proliferation of cells (fibroblasts, keratinocytes, osteoblasts, and chondrocytes) as well as induces matrix synthesis.69 In the maturation phase, PBM therapy improves reorganization and remodeling of wounds, aiding in improved tensile strength and restoring functional architecture of repaired tissues.70
Various types of lasers have been used to promote oral wound healing of both hard and soft tissues.71,72 PBM has been noted to enhance epithelization and improve wound healing after gingivectomy and gingivoplasty operations.73 There are also numerous reports of improved healing following surgical use of high-power lasers compared with routine scalpel excisions.74 This can be potentially attributed to the low-power (PBM) zones around areas of high-power surgical laser use. The popular use of high doses (photothermal) or blue light sources (<400 nm) is based on their antimicrobial effects that ensure reduced pathogenic burden, promoting healing. This is akin to the more popular ultraviolet phototherapy or dye-enhanced, visible, or NIR light treatments termed photodynamic therapy. These techniques utilize either simple dyes (toluidine blue, psoralen, porphyrin derivatives) or immune agents (antibody tagged to metallic nanoparticles) that enhance photoabsorption and increase photodestructive effects. These efforts are particularly relevant for the oral cavity due to the presence of a large commensal microbiota that harbors opportunistic pathogenic strains. Investigators have largely pursued periodontal defects where a variety of protocols, especially with Nd:YAG and Er:YAG lasers, have used a combination of their antimicrobial and adjacent PBM effects to improve hard (bone) and soft tissue (periodontal ligament attachment, gingiva) healing. However, clinical results thus far have been equivocal and there remain more questions about the rigor and robustness of specific treatment protocols.
The anti-inflammatory effects of PBM therapy have been well documented with direct effects on both pro- and anti-inflammatory factors, such as IL-1, IL-8, Cox1, and 2, among others.75,76 The inflammatory phase plays a key role in clearing debris and promoting migration of keratinocytes and fibroblasts for healing to progress. The ability of PBM to modulate inflammation and subsequent immune microenvironment could contribute synergistically to its therapeutic benefits. The effects of PBM on promoting wound healing have focused on induction of cytokines and growth factor expression. PBM has been shown to induce several growth factors, including bFGF, FGF-1, FGF-2, PIGF, NGF, IGF-1, HGF, SCF, TGF-β, and VEGF, over longer time periods (hours to days).77 Many of these factors are capable of directly affecting key players in wound healing components, such as keratinocytes, fibroblast proliferation and migration, wound contraction, inflammatory cells (neutrophils, monocyte–macrophage), neovascularization, matrix synthesis, and remodeling.78–80 For example, Medrado et al. used a 670 nm gallium–aluminum–arsenide (GaAlAs) diode laser to study cutaneous wound healing in rats. They noted a clear reduction in edema and inflammation along with increased myofibroblasts and collagen fibers in the laser-treated wounds after 3 days compared with control wounds.81 The laser-treated cutaneous wound demonstrated increased desmin and alpha-smooth muscle actin-positive fusiform cells that corresponded with greatest reduction in wound size, indicating a potent effect of laser treatments on wound contraction. There are numerous reports on the positive effects of various lasers in Refs.,82,83 but there are also several reports with negative or no effects.84 While a similar device and dosimetry were apparently used, lack of details on precise parameters makes it difficult to interpret these negative results.85,86 This emphasizes the need to further understand PBM mechanisms that will aid in optimizing effective clinical protocols.
Take-Home Message.
• Various biophysical devices can promote wound healing.
• Specific molecular mechanisms vary between each modality.
• The time-dependent biological responses to specific intervention may offer insights into their molecular mechanisms.
• Photobiomodulation is increasingly popular for wound care and specifically suitable for oral wound care. While some of its mechanisms are well worked out, others are being actively explored.
Future Directions
The field of wound care management needs innovative new approaches. The biophysical interventions outlined in this review provide exciting new potential avenues. Oral wounds could be specifically more amenable to PBM due to their superficial nature and easy accessibility. Clinical efficacy of many of these forms of energies has been distinctly demonstrated in laboratory and animal models, as well as in human studies. Future adoption of these technologies will be based on a better understanding of their mechanisms that will aid in mainstream adoption for wound care.
Summary
This review focuses on novel biophysical energies to promote wound healing. Among all the biophysical energies to promote wound healing discussed in this review, PBM therapy appears to have been well investigated compared with the other biophysical approaches and is particularly more suitable to the oral wound environment. The use of these biophysical devices offers significant utility for clinical management, and exciting new advances offer innovative opportunities to develop safe and effective oral wound management regimens.
Abbreviations and Acronyms
- AChE
acetylcholinesterase
- AP-1
activator protein-1
- ATF/CREB
activating transcription factor/cAMP response element-binding protein
- BDNF
brain-derived neurotrophic factor
- bFGF
basic fibroblast growth factor
- CCO
cytochrome C oxidase
- EAER
electrically accelerated and enhanced remineralization
- EGF
epidermal growth factor
- EMF
electromagnetic field
- ER
endoplasmic reticulum
- FGF
fibroblast growth factor
- HGF
hepatocyte growth factor
- HIF-1α
hypoxia-inducible factor-1α
- IGF
insulin-like growth factor
- KGF
keratinocyte growth factor
- LAP
latency-associated peptide
- LED
light-emitting diode
- NF-κB
nuclear factor kappa-B
- NGF
nerve growth factor
- NIR
near infrared
- NO
nitric oxide
- PBM
photobiomodulation
- PEMF
pulsed electromagnetic field
- PIGF
placental growth factor
- PI3K/PTEN
phosphoinositide 3 kinase/phosphatase and tensin homolog
- ROS
reactive oxygen species
- Ref-1
redox factor-1
- SCF
stem cell factor
- TEP
transepithelial potential
- TGF-β
transforming growth factor-β
- US
ultrasound
- VEGF
vascular endothelial growth factor
Acknowledgment and Funding Sources
This work was supported by the Intramural Research Program of the National Institute of Dental and Craniofacial Research, National Institutes of Health.
Author Disclosure and Ghostwriting
The authors declare they have no conflicts of interests and have contributed directly to this manuscript.
About the Authors
Imran Khan, PhD, MSc, is a postdoctoral fellow at National Institute of Dental and Craniofacial Research (NIDCR), National Institutes of Health (NIH), Bethesda. He received his PhD from Indian Institute of Science, Bangalore, India, where he worked on the role of TGF-β in oral submucous fibrosis. His current research focus is on the use of light therapy for tissue repair and regeneration. Praveen R. Arany, DDS, PhD, is an assistant clinical investigator and chief at the Cell Regulation and Control Section, NIDCR, NIH, Bethesda. His group works on the molecular mechanism and clinical translation of PBM therapy in wound healing and tissue regeneration applications in medicine and dentistry.
References
- 1.Sen CK, Gordillo GM, Roy S, et al. . Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen 2009;17:763–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hakkinen L, Uitto VJ, Larjava H. Cell biology of gingival wound healing. Periodontol 2000 2000;24:127–152 [PubMed] [Google Scholar]
- 3.Lancerotto L, Orgill DP. Mechanoregulation of angiogenesis in wound healing. Adv Wound Care (New Rochelle) 2014;3:626–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arany PR, Mooney DJ. At the edge of translation—materials to program cells for directed differentiation. Oral Dis 2011;17:241–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levin M. Bioelectric mechanisms in regeneration: unique aspects and future perspectives. Semin Cell Dev Biol 2009;20:543–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nuccitelli R. A role for endogenous electric fields in wound healing. Curr Top Dev Biol 2003;58:1–26 [DOI] [PubMed] [Google Scholar]
- 7.Zhao M, Song B, Pu J, et al. . Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature 2006;442:457–460 [DOI] [PubMed] [Google Scholar]
- 8.Bayat M, Asgari-Moghadam Z, Maroufi M, Rezaie FS, Rakhshan M. Experimental wound healing using microamperage electrical stimulation in rabbits. J Rehabil Res Dev 2006;43:219–226 [DOI] [PubMed] [Google Scholar]
- 9.Cheng N, Van Hoof H, Bockx E, et al. . The effects of electric currents on ATP generation, protein synthesis, and membrane transport of rat skin. Clin Orthop Relat Res 1982;264–272 [PubMed] [Google Scholar]
- 10.Lee BY, Al-Waili N, Stubbs D, et al. . Ultra-low microcurrent in the management of diabetes mellitus, hypertension and chronic wounds: report of twelve cases and discussion of mechanism of action. Int J Med Sci 2009;7:29–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Das AM, Harris DA. Control of mitochondrial ATP synthase in heart cells: inactive to active transitions caused by beating or positive inotropic agents. Cardiovasc Res 1990;24:411–417 [DOI] [PubMed] [Google Scholar]
- 12.Houghton PE. Clinical trials involving biphasic pulsed current, microcurrent, and/or low-intensity direct current. Adv Wound Care (New Rochelle) 2014;3:166–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee BY, Wendell K, Al-Waili N, Butler G. Ultra-low microcurrent therapy: a novel approach for treatment of chronic resistant wounds. Adv Ther 2007;24:1202–1209 [DOI] [PubMed] [Google Scholar]
- 14.Zuim PR, Garcia AR, Turcio KH, Hamata MM. Evaluation of microcurrent electrical nerve stimulation (MENS) effectiveness on muscle pain in temporomandibular disorders patients. J Appl Oral Sci 2006;14:61–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szor JK, Topp R. Use of magnet therapy to heal an abdominal wound: a case study. Ostomy Wound Manage 1998;44:24–29 [PubMed] [Google Scholar]
- 16.Man D, Man B, Plosker H. The influence of permanent magnetic field therapy on wound healing in suction lipectomy patients: a double-blind study. Plast Reconstr Surg 1999;104:2261–2266; discussion 2267–2268 [DOI] [PubMed] [Google Scholar]
- 17.Fukada E, Yasuda I. On the piezoelectric effect of bone. J Phys Soc Japan 1957;12:1158–1162 [Google Scholar]
- 18.Costin GE, Birlea SA, Norris DA. Trends in wound repair: cellular and molecular basis of regenerative therapy using electromagnetic fields. Curr Mol Med 2012;12:14–26 [DOI] [PubMed] [Google Scholar]
- 19.Henry SL, Concannon MJ, Yee GJ. The effect of magnetic fields on wound healing: experimental study and review of the literature. Eplasty 2008;8:e40. [PMC free article] [PubMed] [Google Scholar]
- 20.Tepper OM, Callaghan MJ, Chang EI, et al. . Electromagnetic fields increase in vitro and in vivo angiogenesis through endothelial release of FGF-2. FASEB J 2004;18:1231–1233 [DOI] [PubMed] [Google Scholar]
- 21.Abdelrahim A, Hassanein HR, Dahaba M. Effect of pulsed electromagnetic field on healing of mandibular fracture: a preliminary clinical study. J Oral Maxillofac Surg 2011;69:1708–1717 [DOI] [PubMed] [Google Scholar]
- 22.Wu J, Nyborg WL. Ultrasound, cavitation bubbles and their interaction with cells. Adv Drug Deliv Rev 2008;60:1103–1116 [DOI] [PubMed] [Google Scholar]
- 23.Miller DL, Smith NB, Bailey MR, et al. . Overview of therapeutic ultrasound applications and safety considerations. J Ultrasound Med 2012;31:623–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uhlemann C, Heinig B, Wollina U. Therapeutic ultrasound in lower extremity wound management. Int J Low Extrem Wounds 2003;2:152–157 [DOI] [PubMed] [Google Scholar]
- 25.Young SR, Dyson M. Effect of therapeutic ultrasound on the healing of full-thickness excised skin lesions. Ultrasonics 1990;28:175–180 [DOI] [PubMed] [Google Scholar]
- 26.Harpaz D, Chen X, Francis CW, Marder VJ, Meltzer RS. Ultrasound enhancement of thrombolysis and reperfusion in vitro. J Am Coll Cardiol 1993;21:1507–1511 [DOI] [PubMed] [Google Scholar]
- 27.Young SR, Dyson M. The effect of therapeutic ultrasound on angiogenesis. Ultrasound Med Biol 1990;16:261–269 [DOI] [PubMed] [Google Scholar]
- 28.Kavros SJ, Liedl DA, Boon AJ, Miller JL, Hobbs JA, Andrews KL. Expedited wound healing with noncontact, low-frequency ultrasound therapy in chronic wounds: a retrospective analysis. Adv Skin Wound Care 2008;21:416–423 [DOI] [PubMed] [Google Scholar]
- 29.Lai J, Pittelkow MR. Physiological effects of ultrasound mist on fibroblasts. Int J Dermatol 2007;46:587–593 [DOI] [PubMed] [Google Scholar]
- 30.Miller MS, Lowery CA. Negative pressure wound therapy: “a rose by any other name”. Ostomy Wound Manage 2005;51:44–46, 48–49 [PubMed] [Google Scholar]
- 31.Waldie K. Pain associated with negative pressure wound therapy. Br J Nurs 2013;22:S15–S16, S18–S21 [DOI] [PubMed] [Google Scholar]
- 32.Tibbs MK. Wound healing following radiation therapy: a review. Radiother Oncol 1997;42:99–106 [DOI] [PubMed] [Google Scholar]
- 33.Mester E, Szende B, Gartner P. [The effect of laser beams on the growth of hair in mice]. Radiobiol Radiother (Berl) 1968;9:621–626 [PubMed] [Google Scholar]
- 34.Huang YY, Chen AC, Carroll JD, Hamblin MR. Biphasic dose response in low level light therapy. Dose Response 2009;7:358–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arany PR. Photobiomodulation: poised from the fringes. Photomed Laser Surg 2012;30:507–509 [DOI] [PubMed] [Google Scholar]
- 36.Chen AC, Arany PR, Huang YY, et al. . Low-level laser therapy activates NF-kB via generation of reactive oxygen species in mouse embryonic fibroblasts. PLoS One 2011;6:e22453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palczewska G, Vinberg F, Stremplewski P, et al. . Human infrared vision is triggered by two-photon chromophore isomerization. Proc Natl Acad Sci U S A 2014;111:E5445–E5454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liebert AD, Bicknell BT, Adams RD. Protein conformational modulation by photons: a mechanism for laser treatment effects. Med Hypotheses 2014;82:275–281 [DOI] [PubMed] [Google Scholar]
- 39.Assis L, Moretti AI, Abrahao TB, et al. . Low-level laser therapy (808 nm) reduces inflammatory response and oxidative stress in rat tibialis anterior muscle after cryolesion. Lasers Surg Med 2012;44:726–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karu T. Laser biostimulation: a photobiological phenomenon. J Photochem Photobiol B 1989;3:638–640 [DOI] [PubMed] [Google Scholar]
- 41.Karu TI, Afanas'eva NI. [Cytochrome c oxidase as the primary photoacceptor upon laser exposure of cultured cells to visible and near IR-range light]. Dokl Akad Nauk 1995;342:693–695 [PubMed] [Google Scholar]
- 42.Wong-Riley MT, Liang HL, Eells JT, et al. . Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: role of cytochrome c oxidase. J Biol Chem 2005;280:4761–4771 [DOI] [PubMed] [Google Scholar]
- 43.Pastore D, Greco M, Passarella S. Specific helium-neon laser sensitivity of the purified cytochrome c oxidase. Int J Radiat Biol 2000;76:863–870 [DOI] [PubMed] [Google Scholar]
- 44.Brown GC. Regulation of mitochondrial respiration by nitric oxide inhibition of cytochrome c oxidase. Biochim Biophys Acta 2001;1504:46–57 [DOI] [PubMed] [Google Scholar]
- 45.Karu TI, Pyatibrat LV, Afanasyeva NI. Cellular effects of low power laser therapy can be mediated by nitric oxide. Lasers Surg Med 2005;36:307–314 [DOI] [PubMed] [Google Scholar]
- 46.Archer SL, Huang JM, Hampl V, Nelson DP, Shultz PJ, Weir EK. Nitric oxide and cGMP cause vasorelaxation by activation of a charybdotoxin-sensitive K channel by cGMP-dependent protein kinase. Proc Natl Acad Sci U S A 1994;91:7583–7587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lohr NL, Keszler A, Pratt P, Bienengraber M, Warltier DC, Hogg N. Enhancement of nitric oxide release from nitrosyl hemoglobin and nitrosyl myoglobin by red/near infrared radiation: potential role in cardioprotection. J Mol Cell Cardiol 2009;47:256–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ahn KB, Kang SS, Park OJ, Kim TI. Irradiation by gallium-aluminum-arsenate diode laser enhances the induction of nitric oxide by Porphyromonas gingivalis in RAW 264.7 cells. J Periodontol 2014;85:1259–1265 [DOI] [PubMed] [Google Scholar]
- 49.Tsai WC, Cheng JW, Chen JL, et al. . Low-level laser irradiation stimulates tenocyte proliferation in association with increased NO synthesis and upregulation of PCNA and cyclins. Lasers Med Sci 2014;29:1377–1384 [DOI] [PubMed] [Google Scholar]
- 50.Mitchell UH, Mack GL. Low-level laser treatment with near-infrared light increases venous nitric oxide levels acutely: a single-blind, randomized clinical trial of efficacy. Am J Phys Med Rehabil 2013;92:151–156 [DOI] [PubMed] [Google Scholar]
- 51.Plass CA, Loew HG, Podesser BK, Prusa AM. Light-induced vasodilation of coronary arteries and its possible clinical implication. Ann Thorac Surg 2012;93:1181–1186 [DOI] [PubMed] [Google Scholar]
- 52.Lohr NL, Ninomiya JT, Warltier DC, Weihrauch D. Far red/near infrared light treatment promotes femoral artery collateralization in the ischemic hindlimb. J Mol Cell Cardiol 2013;62:36–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang J, Xing D, Gao X. Low-power laser irradiation activates Src tyrosine kinase through reactive oxygen species-mediated signaling pathway. J Cell Physiol 2008;217:518–528 [DOI] [PubMed] [Google Scholar]
- 54.Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol 1997;13:513–609 [DOI] [PubMed] [Google Scholar]
- 55.Kujawa J, Zavodnik L, Zavodnik I, Bryszewska M. Low-intensity near-infrared laser radiation-induced changes of acetylcholinesterase activity of human erythrocytes. J Clin Laser Med Surg 2003;21:351–355 [DOI] [PubMed] [Google Scholar]
- 56.Lawson AA, Barr RD. Acetylcholinesterase in red blood cells. Am J Hematol 1987;26:101–112 [DOI] [PubMed] [Google Scholar]
- 57.Kujawa J, Pasternak K, Zavodnik I, Irzmanski R, Wrobel D, Bryszewska M. The effect of near-infrared MLS laser radiation on cell membrane structure and radical generation. Lasers Med Sci 2014;29:1663–1668 [DOI] [PubMed] [Google Scholar]
- 58.Hymer WC, Welsch J, Buchmann E, Risius M, Whelan HT. Modulation of rat pituitary growth hormone by 670 nm light. Growth Horm IGF Res 2009;19:274–279 [DOI] [PubMed] [Google Scholar]
- 59.Massague J, Blain SW, Lo RS. TGFbeta signaling in growth control, cancer, and heritable disorders. Cell 2000;103:295–309 [DOI] [PubMed] [Google Scholar]
- 60.Keski-Oja J, Koli K, von Melchner H. TGF-beta activation by traction? Trends Cell Biol 2004;14:657–659 [DOI] [PubMed] [Google Scholar]
- 61.Li MO, Flavell RA. TGF-beta: a master of all T cell trades. Cell 2008;134:392–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arany PR, Nayak RS, Hallikerimath S, Limaye AM, Kale AD, Kondaiah P. Activation of latent TGF-beta1 by low-power laser in vitro correlates with increased TGF-beta1 levels in laser-enhanced oral wound healing. Wound Repair Regen 2007;15:866–874 [DOI] [PubMed] [Google Scholar]
- 63.Jobling MF, Mott JD, Finnegan MT, et al. . Isoform-specific activation of latent transforming growth factor beta (LTGF-beta) by reactive oxygen species. Radiat Res 2006;166:839–848 [DOI] [PubMed] [Google Scholar]
- 64.Liu H, Colavitti R, Rovira II, Finkel T. Redox-dependent transcriptional regulation. Circ Res 2005;97:967–974 [DOI] [PubMed] [Google Scholar]
- 65.Denu JM, Tanner KG. Specific and reversible inactivation of protein tyrosine phosphatases by hydrogen peroxide: evidence for a sulfenic acid intermediate and implications for redox regulation. Biochemistry 1998;37:5633–5642 [DOI] [PubMed] [Google Scholar]
- 66.Cury V, Moretti AI, Assis L, et al. . Low level laser therapy increases angiogenesis in a model of ischemic skin flap in rats mediated by VEGF, HIF-1alpha and MMP-2. J Photochem Photobiol B 2013;125:164–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Meng C, He Z, Xing D. Low-level laser therapy rescues dendrite atrophy via upregulating BDNF expression: implications for Alzheimer's disease. J Neurosci 2013;33:13505–13517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fathabadie FF, Bayat M, Amini A, Bayat M, Rezaie F. Effects of pulsed infra-red low level-laser irradiation on mast cells number and degranulation in open skin wound healing of healthy and streptozotocin-induced diabetic rats. J Cosmet Laser Ther 2013;15:294–304 [DOI] [PubMed] [Google Scholar]
- 69.AlGhamdi KM, Kumar A, Moussa NA. Low-level laser therapy: a useful technique for enhancing the proliferation of various cultured cells. Lasers Med Sci 2012;27:237–249 [DOI] [PubMed] [Google Scholar]
- 70.Enwemeka CS, Parker JC, Dowdy DS, Harkness EE, Sanford LE, Woodruff LD. The efficacy of low-power lasers in tissue repair and pain control: a meta-analysis study. Photomed Laser Surg 2004;22:323–329 [DOI] [PubMed] [Google Scholar]
- 71.Sun G, Tuner J. Low-level laser therapy in dentistry. Dent Clin North Am 2004;48:1061–1076, viii [DOI] [PubMed] [Google Scholar]
- 72.Walsh LJ. The current status of low level laser therapy in dentistry. Part 1. Soft tissue applications. Aust Dent J 1997;42:247–254 [DOI] [PubMed] [Google Scholar]
- 73.Ozcelik O, Cenk Haytac M, Kunin A, Seydaoglu G. Improved wound healing by low-level laser irradiation after gingivectomy operations: a controlled clinical pilot study. J Clin Periodontol 2008;35:250–254 [DOI] [PubMed] [Google Scholar]
- 74.Tamarit-Borras M, Delgado-Molina E, Berini-Aytes L, Gay-Escoda C. Removal of hyperplastic lesions of the oral cavity. A retrospective study of 128 cases. Med Oral Patol Oral Cir Bucal 2005;10:151–162 [PubMed] [Google Scholar]
- 75.Pallotta RC, Bjordal JM, Frigo L, et al. . Infrared (810-nm) low-level laser therapy on rat experimental knee inflammation. Lasers Med Sci 2012;27:71–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Oliveira MC, Jr., Greiffo FR, Rigonato-Oliveira NC, et al. . Low level laser therapy reduces acute lung inflammation in a model of pulmonary and extrapulmonary LPS-induced ARDS. J Photochem Photobiol B 2014;134:57–63 [DOI] [PubMed] [Google Scholar]
- 77.Peplow PV, Baxter GD. Gene expression and release of growth factors during delayed wound healing: a review of studies in diabetic animals and possible combined laser phototherapy and growth factor treatment to enhance healing. Photomed Laser Surg 2012;30:617–636 [DOI] [PubMed] [Google Scholar]
- 78.Avci P, Gupta A, Sadasivam M, et al. . Low-level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring. Semin Cutan Med Surg 2013;32:41–52 [PMC free article] [PubMed] [Google Scholar]
- 79.Posten W, Wrone DA, Dover JS, Arndt KA, Silapunt S, Alam M. Low-level laser therapy for wound healing: mechanism and efficacy. Dermatol Surg 2005;31:334–340 [DOI] [PubMed] [Google Scholar]
- 80.Desmet KD, Paz DA, Corry JJ, et al. . Clinical and experimental applications of NIR-LED photobiomodulation. Photomed Laser Surg 2006;24:121–128 [DOI] [PubMed] [Google Scholar]
- 81.Medrado AR, Pugliese LS, Reis SR, Andrade ZA. Influence of low level laser therapy on wound healing and its biological action upon myofibroblasts. Lasers Surg Med 2003;32:239–244 [DOI] [PubMed] [Google Scholar]
- 82.Mester E, Korenyi-Both A, Spiry T, Scher A, Tisza S. Stimulation of wound healing by means of laser rays. (Clinical and electron microscopical study). Acta Chir Acad Sci Hung 1973;14:347–356 [PubMed] [Google Scholar]
- 83.Schindl A, Schindl M, Schindl L, Jurecka W, Honigsmann H, Breier F. Increased dermal angiogenesis after low-intensity laser therapy for a chronic radiation ulcer determined by a video measuring system. J Am Acad Dermatol 1999;40:481–484 [DOI] [PubMed] [Google Scholar]
- 84.Lundeberg T, Malm M. Low-power HeNe laser treatment of venous leg ulcers. Ann Plast Surg 1991;27:537–539 [DOI] [PubMed] [Google Scholar]
- 85.Peplow PV, Chung TY, Baxter GD. Laser photobiomodulation of proliferation of cells in culture: a review of human and animal studies. Photomed Laser Surg 2010;28 Suppl 1:S3–S40 [DOI] [PubMed] [Google Scholar]
- 86.Peplow PV, Chung TY, Baxter GD. Laser photobiomodulation of wound healing: a review of experimental studies in mouse and rat animal models. Photomed Laser Surg 2010;28:291–325 [DOI] [PubMed] [Google Scholar]