Abstract
Increased afferent excitability has been proposed as an important pathophysiology of interstitial cystitis/bladder pain syndrome (IC/BPS) and overactive bladder (OAB). In this study, we investigated whether herpes simplex virus (HSV) vectors encoding poreless TRPV1, in which the segment in C terminus of TRPV1 receptor is deleted, suppress bladder overactivity and pain behavior using a rat model of chemical cystitis. Replication-defective HSV vectors encoding poreless TRPV1 were injected into the bladder wall of adult female Sprague-Dawley rats. Additionally, recombinant HSV virus (vHG) vectors were injected as control. Cystometry (CMG) under urethane anesthesia was performed 1 week after viral injection to evaluate bladder overactivity induced by resiniferatoxin (RTx, a TRPV1 agonist). RTx-induced nociceptive behavior such as licking (lower abdominal licking) and freezing (motionless head-turning) was observed 2 weeks after viral injection. GFP expression in L4/L6/S1 dorsal root ganglia and the bladder as well as c-Fos-positive cells in the L6 spinal cord dorsal horn were also evaluated 2 weeks after viral injection. In CMG, the poreless TRPV1 vector-treated group showed a significantly smaller reduction in intercontraction intervals and voided volume after RTx infusion than the vHG-treated control group. The number of the RTx-induced freezing events was significantly decreased in the poreless TRPV1 group than in the vHG group, whereas there was no significant difference of the number of RTx-induced licking events between groups. The number of c-Fos-positive cells in the DCM and SPN regions of the L6 spinal dorsal horn was significantly smaller in the poreless TRPV1 group than in the vHG group. Our results indicated that HSV vector-mediated gene delivery of poreless TRPV1 had a therapeutic effect on TRPV1-mediated bladder overactivity and pain behavior. Thus, the HSV vector-mediated gene therapy targeting TRPV1 receptors could be a novel modality for the treatment of OAB and/or hypersensitive bladder disorders such as IC/BPS.
Introduction
Interstitial cystitis/bladder pain syndrome (IC/BPS) is a chronic disease in which patients suffer from pelvic pain, urinary frequency, urgency, and other symptoms.1,2 There have been numerous reports attempting to detail source of IC/BPS pathogenesis,3–6 but the complete etiology remains unclear. Therefore, effective management of IC/BPS has not been established yet, and IC/BPS symptoms still result in a poor quality of life with sleep disruption, depression, and sexual dysfunction.7–9
In addition, increased excitability of bladder afferent pathways has been proposed as a potential pathophysiological mechanism not only of hypersensitive bladder disorders such as IC/BPS,10–13 but also of overactive bladder (OAB).14 It has also been reported that the transient receptor potential vanilloid-1 (TRPV1) receptor, predominantly expressed in C-fiber afferent pathways, greatly contributes to afferent sensitization in chronic pain conditions.15,16 However, the clinical application of TRPV1 antagonists for chronic pain has been hampered due in part to their adverse events (AEs), such as hyperthermia and/or impaired noxious heat sensation.17 Hence, the development of local therapies that can target TRPV1 receptors expressed in the affected organs and their afferent pathways without inducing systemic AEs would be useful for the treatment of OAB and chronic pelvic pain conditions, including IC/BPS.
The TRPV1 receptor is a nonselective cation channel that is activated by vanilloids, protons, and heat. The activated channel causes calcium influx, which results in cellular hyperexcitability. The TRPV1 receptor consists of six transmembrane domains with a pore region between the fifth and sixth domains, and intracellular N- and C-terminal tails. Garcia-Sanz et al. reported that the C-terminus of the TRPV1 receptor is a tetramer formed by the assembly of four identical subunits around a central aqueous pore.18 In their article, a TRP-like domain comprising 684Glu-721Arg was identified as a molecular determinant of the tetramerization of the individual receptor subunits. Additionally, mutant TRPV1 (referred to as poreless TRPV1), where 684Glu-721Arg was deleted, failed to respond to capsaicin.
Gene therapy, using various vectors such as herpes simplex virus (HSV) or adenovirus, has been investigated for the treatment of various pain conditions.19,20 Our previous studies demonstrated that replication-defective HSV vector-mediated gene therapy was a safe and effective modality of the treatment in various cystitis-model rats.21,22 Also, we employed the replication-defective HSV vectors encoding poreless TRPV1 in a previous study where poreless TRPV1 functioned as a modulator of the TRPV1 receptors in vitro as well as in mice with capsaicin-induced thermal hyperalgesia.20,23 Thus, in this study, we investigated the effect of replication-defective HSV vector-mediated gene therapy using expression of poreless TRPV1 on bladder overactivity and nociceptive behavior in a rat model of chemically induced cystitis in order to clarify whether the poreless TRPV1 gene therapy effectively suppresses TRPV1-receptor-mediated afferent activation in the bladder.
Materials and Methods
Vectors
We engineered the recombinant HSV virus (vHG) that has a deletion of the essential immediately early (IE) genes, ICP4 and ICP27, as well as the TAATGARAT elements within the promoters of IE genes, ICP22 and ICP 47, rendering their expression as early genes dependent on the expression of ICP4 and ICP27 from the complementing cell line used to propagate the vectors. In addition, the vHP vector contains two copies of poreless TRPV1 gene driven by the strong HCMV promoter that were inserted into the ICP4 loci of the vHG control vector replacing the enhanced green fluorescent protein (eGFP) cassettes (Fig. 1). The poreless TRPV1 mutant was constructed by deleting 684Glu-721Arg from the wild-type TRPV1 channels as previously described.18 The vectors were produced in the 7b ICP4–ICP27-expressing cell line and purified as previously described.24
Figure 1.
Herpes simplex virus (HSV) vectors construct. The (A) vHG and (B) poreless vectors (vHP) have a deletion of the essential immediately early (IE) genes, ICP4 and ICP27, as well as IE regulatory elements within the promoters of IE genes, ICP22 and ICP47, making their expression dependent on ICP4 and ICP27, and thus they are expressed as early genes only within the complementing cell line used to propagate the vectors. In the genome of the vHG, an HCMV immediate early promoter driving enhanced green fluorescent protein (EGFP) was inserted into both ICP4 loci, while in the poreless TRPV1 genome an HCMV promoter driving poreless TRPV1 was inserted.
Viral Vector Administration
Nine-week-old female Sprague-Dawley rats (Hilltop Labs, Scottdale, PA) were used according to the experimental protocol approved by the University of Pittsburgh Institutional Animal Care and Use Committee (IACUC). A lower abdominal incision was performed under pentobarbital (30 mg/kg) anesthesia, and viral suspension (30 μl, total of 1.1 × 108 plaque forming units) of vHP or vHG was injected at six sites (5 μl per site) of the bladder wall around the bladder base using a 30-gauge Hamilton syringe. Also, in order to evaluate the effects of the vHG control vector on bladder physiology, the sham-treated group of rats was administered with 30 μl of saline (5 μl per site) into the bladder wall.
Cystometry
A polyethylene-50 (PE50) catheter was inserted into the bladder through the dome and implanted under isoflurane anesthesia 5 days after viral vector administration. Two days after the insertion, continuous cystometry was performed to examine if the effects of poreless TRPV1 gene delivery are seen 1 week after the vHP vector treatment. After urethane (1.2 g/kg) was intraperitoneally administered, a PE50 catheter was connected to a pressure transducer and an infusion pump through a 3-way stopcock. Saline was continuously infused into the bladder at a rate of 0.04 ml/min. After baseline bladder activity was established, 1 μM of resiniferatoxin (RTx; Sigma Aldrich, St. Louis, MO), a TRPV1 agonist, was infused at a rate of 0.04 ml/min to induce bladder overactivity. RTx was dissolved in 10% ethanol, 10% Tween-80, and 80% saline, and diluted to the final concentration before use. The results were analyzed with Chart 5 software (AD Instruments, Milford, MA). Cystometric parameters such as basal pressure (BP), micturition threshold (MT), peak pressure (PP), intercontraction intervals (ICI), voided volume (VV), residual volume (RV), and voiding efficiency (VE) were evaluated before and after RTx infusion. The RTx-induced reduction rate of ICI or VV was calculated with an equation: [(ICI or VV during saline infusion − ICI or VV during RTx infusion)/ICI or VV during saline infusion, respectively].
Nociceptive Behavior
We previously reported that intravesical RTx administration induced two types of nociceptive behavior, licking (lower abdominal licking) and freezing (motionless head-turning to the lower abdomen), and that licking behavior is predominantly induced by urethral pain sensation carried through the pudendal nerve, whereas freezing behavior is related to pelvic nerve-mediated bladder pain.25,26 In order to examine if the effects of vHP treatment last more than 1 week, nociceptive behavior was observed 2 weeks after viral inoculation, as we previously reported.26 In brief, the rats were kept in a metabolic cage for at least 2 hr before behavioral observation. After 3 μM of RTx in a volume of 0.3 ml was administrated intravesically through a temporary indwelling urethral catheter, and kept for 1 min, each rat was returned to the metabolic cage. Thereafter, licking and freezing events were scored for 15 min periods divided into 5 sec intervals. When licking or freezing events occurred during a 5 sec interval, it was scored as one positive event.
GFP Expression in the Bladder and Dorsal Root Ganglia
Two hours after nociceptive behavior observation, which was performed 2 weeks after HSV vector inoculation, the rats were intracardially perfused with cold heparinized saline, followed with 4% paraformaldehyde (PFA; J.T. Baker, Phillipsburg, NJ) under isoflurane anesthesia. Then, the bladder, L4/L6/S1 dorsal root ganglia (DRG), and the L6 spinal cord were removed. Sections of the DRG and bladder tissues were used for GFP observation, while the L6 spinal cord was subjected to c-Fos IHC staining. The bladder and DRG tissues were postfixed overnight with 4% PFA in 0.1 M phosphate buffered saline (PBS; Gibco, Grand Island, NY) at 4°C. Thereafter, they were incubated in 20% sucrose in PBS for 48 hr at 4°C for cryoprotection, then embedded into OCT compound (Sakura Finetek USA, Inc., Torrance, CA) with 20% sucrose (2:1), and rapidly frozen. The frozen tissue sections were cut at a thickness of 12 μm (transverse sections) and thaw-mounted onto microscope slides (Fisher Scientific, Pittsburgh, PA). GFP expression in the bladder and the L4/L6/S1 DRG was evaluated using a fluorescent microscope. In addition, GFP expression in the L6 DRG of the nontreated control rats was observed as well.
c-Fos Staining in L6 Spinal Cord Dorsal Horn
It has been reported that an increase in c-Fos protein in the spinal neurons can be detected within a few hours following the bladder distension or chemical irritation.27–29 Birder and de Groat reported that c-Fos expression was most likely to be observed in the L6 spinal cord dorsal horn 2 hr after RTx administration into the bladder.30 The frozen spinal cord was cut into 40 μm sections. Every third serial section was incubated in 0.3% hydrogen peroxide (0.01 M PBS, Triton X100, and hydrogen peroxide; Sigma Aldrich) for 10 min. They were incubated for 48 hr at 4°C with primary antibody (rabbit anti-c-Fos, 1:10,000; Abcam, Cambridge, MA) in PBS containing 2% goat serum and 0.1% Triton X100. Thereafter, they were incubated in biotinylated second antibody (goat antirabbit IgG, 1:600; Abcam) and in avidin–biotin complex reagent (Vector Laboratories, Burlingame, CA), each for 2 hr at room temperature. c-Fos proteins were then visualized by diaminobenzidine and nickel ammonium sulfate with hydrogen peroxide (DAB; Vector Laboratories). c-Fos-positive cells were counted in four spinal cord regions of medial dorsal horn (MDH), lateral dorsal horn (LDH), dorsal commissure (DCM), and sacral parasympathetic nucleus (SPN).30
Statistical Analysis
Data were analyzed using statistical program R commander (version 2.8.1; the Comprehensive R Archive Network). All data values were expressed as mean ± SEM. A statistical comparison of differences was performed using Student's t-test. p-Values less than 0.05 were considered to be statistically significant.
Results
Comparison of Sham and vHG Control Vector treatments
In cystometry and behavioral testing, there were no significant differences in baseline cystometric parameters or RTx-induced pain behavior between sham and vHG control vector-treated groups (n = 6/group) (data not shown). Thus, further data analyses were performed in vHP and vHG vector-injected groups.
Cystometry
During saline infusion, there was no significant difference in ICI or VV between vHP and vHG vector-injected groups. RTx infusion induced a substantial reduction in ICI and VV in the vHG control vector-injected group, whereas there was a relatively moderate reduction in the vHP group. The reduction rates of ICI and VV after RTx infusion were significantly smaller in the vHP group than in the vHG group (55 ± 3% vs. 68 ± 4%, p = 0.03, and 51 ± 5% vs. 65 ± 3%, p = 0.03, respectively; n = 6/group) (Fig. 2). There were no significant differences in other cystometric parameters such as BP, MT, PP, or RV between the two groups.
Figure 2.
Cystometry under urethane anesthesia in control vHG and poreless vHP groups. Representative traces of cystometry (A) and the effect of resiniferatoxin (RTx), a TRPV1 agonist, on intercontraction interval (ICI) (B) and on voided volume (C). (A) Saline was continuously infused into the bladder at a rate of 0.04 ml/min. After baseline bladder activity was established, 1 μM of RTx was infused at a rate of 0.04 ml/min. (B) The reduction rate of ICI is significantly smaller in the vHP group than in the vHG group (68 ± 4% vs. 55 ± 3%, p = 0.03). The reduction rate was calculated with an equation: (ICI during saline infusion − ICI during RTx infusion)/ICI during saline infusion. (C) The reduction rate of voided volume is significantly smaller in the vHP group than in the vHG group (65 ± 3% vs. 51 ± 5%, p = 0.03). The reduction rate was calculated with an equation: (voided volume during saline infusion − voided volume during RTx infusion)/voided volume during saline infusion.
Nociceptive Behavior
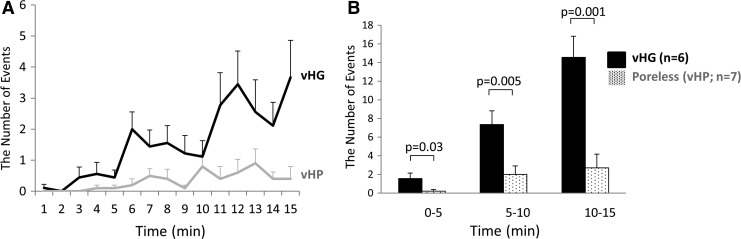
As found in our previous study, the highest number of licking events was observed in the early phase and gradually decreased over time during the 15 min observation period. However, freezing events were increased gradually over time and observed at the highest level in the late phase.21,22 There was no significant difference in licking behavior events between the two groups (Fig. 3). On the other hand, the number of freezing events was significantly decreased in the vHP group (n = 7) compared with the vHG group (n = 6) during each of 5 min periods of the entire observation time (Fig. 4.)
Figure 3.
Resiniferatoxin (RTx)-induced licking behavior. RTx (3 μM) was administered into the bladder through a temporary indwelling urethral catheter and kept there for 1 min. The number of licking events was counted for a 15 min period with 5 sec intervals. (A) Time-course changes in the number of licking behavior events. (B) Comparison of licking behavior events between vHG and poreless vHP groups. The 15 min observation time was divided into three periods: early (0–5 min), middle (5–10 min), and late (10–15 min). There was no significant (n.s.) difference in the licking events between two groups.
Figure 4.
Resiniferatoxin (RTx)-induced freezing behavior. RTx (3 μM) was administered into the bladder through a temporary indwelling urethral catheter and kept for 1 min. The number of freezing events was counted for 15 min. (A) Time-course changes in the number of freezing behavior events. (B) Comparison of freezing behavior events between vHG and poreless vHP groups. The 15 min observation time was divided into three periods: early (0–5 min), middle (5–10 min), and late (10–15 min). The poreless vHP-injected group showed the significantly lower number of freezing events in all of three 5 min periods than the vHG-injected group.
GFP Expression in the Bladder and S1/L6 DRG
In order to identify the HSV vector-transduced cells, we examined GFP expression from the control vector that expresses GFP from the strong CMV promoter. GFP-positive cells were observed under a fluorescent microscope in the bladder muscle layer and the S1/L6 DRG, where bladder afferent neurons originate. However, GFP expression was not seen either in the L4 DRG of vHG-injected group of rats or in the L6/S1 DRG of nontreated normal rats (Fig. 5)
Figure 5.
HSV vector-mediated green fluorescent protein (GFP) expression in the bladder (A and B), L6 dorsal root ganglia (DRG) (C and D), and L4 DRG (E) from rats treated with vHG control vectors. The photomicrographs (B) and (D) show the magnified portions of (A) and (C) indicated by rectangular boxes, respectively. GFP-positive cells were observed in the bladder (A and B) and the S1/L6 DRG (C and D) in rats treated with vHG control vectors. There were GFP-positive cells neither in the L4 DRG in the rats treated with vHG vectors (E) nor in the L6 DRG in the nontreated rats (data not shown). Scale bars indicate 200 μm (A and C) and 100 μm (B, D, and E). Color images available online at www.liebertpub.com/hum
c-Fos Staining in the L6 Spinal Cord Dorsal Horn
The number of c-Fos-positive IHC cells within the DCM and SPN regions of the L6 spinal dorsal horn was significantly (p < 0.05) reduced in the vHP-injected group (7 ± 2 and 3 ± 0.9 per section, respectively, n = 7) than in the vHG-injected group (24 ± 3 and 9 ± 1 cells per section, respectively, n = 6). In contrast, no significant difference was seen in the number of c-Fos-positive cells in the MDH and LDH regions between the two groups of vector-injected rats (Fig. 6).
Figure 6.
c-Fos immunostaining in the L6 spinal cord dorsal horn. (A) The L6 spinal cord dorsal horn was divided into four regions: medial dorsal horn (MDH), lateral dorsal horn (LDH), dorsal commissure (DCM), and sacral parasympathetic nucleus (SPN). The vHG-injected group (B) had more c-Fos-positive cells (black arrows) than the poreless vHP-injected group (C.) (D) There were the significantly lower number of c-Fos-positive cells in the DCM and SPN regions in the poreless vHP-injected group than in the control vector vHG-injected group. Scale bars indicate 200 μm.
Discussion
The results of this study indicate that (1) HSV vectors injected into the bladder wall are transported into L6/S1 DRG neurons via bladder afferent nerve fibers as shown by HSV vector expressed GFP-positive cells being detected in the bladder and L6 DRG (Fig. 5) from HSV control vector vHG-injected rats; (2) in rats treated with the poreless TRPV1-encoding HSV vector vHP, bladder overactivity as measured by ICI and voided volume during intravesical RTx administration (Fig. 2) was reduced compared with vHG-treated control rats; (3) vHP-injected rats showed a decrease in freezing behavior (Fig. 4) induced by nociceptive stimuli in the bladder; and (4) vHP-injected rats showed a decrease in c-Fos expression in the L6 spinal cord dorsal horn after intravesical RTx administration (Fig. 6).
The positive GFP expression after vHG injection into the bladder wall was observed in L6/S1 DRG where bladder afferent neurons originate, but not in L4 DRG neurons, which do not contain bladder afferent neurons.14 These results indicate that the virus vectors, when injected into the bladder, can express reporter and therapeutic proteins specifically in the bladder and its afferent pathways, but not in afferent neurons innervating other organs.
In the cystometric analysis, the vHG-injected group of rats exhibited bladder overactivity after RTx instillation as evidenced by a statistically significant reduction in ICI and voided volume during RTx infusion compared with ICI and voided volume during saline infusion. Because RTx stimulates the TRPV1 receptor, which is predominantly expressed in C-fiber bladder afferent pathways,31 bladder overactivity induced by RTx is presumed to be elicited by activation of TRPV1-expressing C-fiber bladder afferents. The vHP-injected group also showed a reduction in ICI and voided volume during RTx infusion; however, the reduction rate was significantly smaller than that for the control vHG-injected group. According to a previous study, TRPV1 receptors possessing the poreless subunit do not respond to capsaicin stimulation because of a failure to form the pore channel.18 Thus, our results are likely to show that poreless TRPV1 receptors, which are transduced and expressed in bladder afferent pathways, can suppress TRPV1 activation in response to RTx because of a block in TRPV1 channel activation.
It is known that freezing events are likely to be brought on by activating bladder afferents in the pelvic nerve,21,22,26 indicating that the freezing behavior in response to intravesical RTx-mediated nociceptive stimulation represents bladder pain as the result of TRPV1 receptor activation in bladder afferent pathways. In this study, the number of freezing behavior events was significantly decreased in the vHP-injected group. Thus, it is assumed that HSV vector-mediated expression of poreless TRPV1 in bladder afferent pathways blunted TRPV1-mediated activation in the bladder, thereby suppressing bladder pain sensation. In contrast, there was no significant difference in licking behavior events between the vHG control vector-injected and vHP poreless vector-injected groups. Because the licking behavior is induced by stimulation of the urethral afferents in the pudendal nerves,25,26 our results imply that the vectors inoculated into the bladder wall do not have an impact on the nervous system innervating other organs such as the urethra. Taken together with the result of the GFP expression, it can be concluded that gene therapy with replication-defective HSV vectors is an organ-specific treatment and effective to suppress bladder pain behavior induced by intravesical nociceptive stimuli.
We also performed c-Fos staining in the L6 spinal cord after RTx administration to investigate the nociceptive input from the bladder. In the vHG-injected group, the c-Fos-positive cells were observed in four regions of the spinal cord dorsal horn. Compared with the vHG-injected group, there were significantly fewer c-Fos-positive cells in the DCM and SPN regions in the vHP-injected rat group. Birder and de Groat previously reported that the majority of c-Fos-positive cells were observed in the DCM and SPN regions after bladder stimulation,30 which implies that c-Fos expression in these regions is associated with activation of bladder afferent nerves. Therefore, our results in the c-Fos expression study are likely to demonstrate further evidence showing that HSV vector-mediated poreless TRPV1 delivery can inhibit the activation of bladder afferent pathways as c-Fos activation is a marker of bladder afferent activation via a number of stimuli.
Various aspects of the natural biology of HSV are attractive when considering it as a gene therapy vector, especially for the treatment of diseases of the peripheral nervous system (PNS). As primary afferent neurons are the natural targets of HSV during wild-type virus infection of humans, it represents a major advantage over other vector systems as the target cells for PNS diseases that one is trying to transduce are the exact ones this virus natural infects. Moreover, the vector genome is large (>152 kb) and about a half is not essential for growth of the virus in culture for propagation; therefore, multiple32 or large transgenes33 can be accommodated. Another advantage is that HSV does not integrate into the host genome,34 and so insertional mutagenesis that could potentially be tumorigenic to the host is not a concern. Gene transfer-based delivery may also be used to provide local high levels of a gene product, while minimizing potential systemic side effects. Because the vectors used in this study were constructed by deleting immediate early (IE) genes, ICP4 and ICP27, as well as important elements within the promoters of IE genes, ICP22 and ICP47, they cannot replicate in the noncomplementing cells, which renders the vectors to be replication defective and dramatically less cytotoxic. In a phase I clinical study using a similar replication-defective HSV vector encoding preproenkephalin, no treatment-related AEs were reported in humans.35 Therefore, the replication-defective HSV vector encoding poreless TRPV1 could be a novel treatment for IC/BPS and/or OAB, in which afferent sensitization is one of the major pathophysiological conditions.
However, there are some limitations in the present study. First, this study aimed to confirm that the poreless TRPV1 gene delivery to the bladder and its afferent pathways can ameliorate TRPV1 receptor-mediated afferent activation in the bladder. Therefore, further studies are needed to evaluate whether the vHP vector treatment has similar effects on bladder hypersensitivity induced by other stimulations to evaluate the clinical utility of the poreless TRPV1 gene therapy. Second, this study evaluated the effects of vHP vector treatment for 1–2 weeks after viral inoculation; therefore, the duration and time course of viral infection or therapeutic effects are not known although our previous studies using HSV vectors encoding other genes showed that increased virus mRNA levels were detected at 4 weeks after viral injection22,36 and that the antinociceptive effect lasted up to 4 weeks using a neuropathic pain model.36 Further studies including time course evaluation are warranted so that we might eventually move forward to future translational studies.
Conclusions
The replication-defective HSV vectors expressing poreless TRPV1 reduced TRPV1-mediated bladder overactivity and bladder afferent-specific pain behavior in rats. Therefore, poreless TRPV1 gene therapy could be a novel treatment modality for IC/BPS and/or OAB.
Acknowledgments
Our research was funded by NIH DK088836, P01 DK044935, and DOD W81XWH-12-1-0565.
Author Disclosure
No competing financial interests exist.
References
- 1.Chancellor MB, Yoshimura N. Treatment of interstitial cystitis. Urology 2004;63:85–92 [DOI] [PubMed] [Google Scholar]
- 2.Moutzouris D-A, Falagas ME. Interstitial cystitis: An unsolved enigma. Clin J Am Soc Nephrol 2009;4:1844–1857 [DOI] [PubMed] [Google Scholar]
- 3.Lilly JD, Parsons CL. Bladder surface glycosaminoglycans is a human epithelial permeability barrier. Surg Gynecol Obstet 1990;171:493–496 [PubMed] [Google Scholar]
- 4.Slobodov G, Feloney M, Gran C, et al. Abnormal expression of molecular markers for bladder impermeability and differentiation in the urothelium of patients with interstitial cystitis. J Urol 2004;171:1554–1558 [DOI] [PubMed] [Google Scholar]
- 5.Ochs RL, Stein TW, Jr., Peebles CL, et al. Autoantibodies in interstitial cystitis. J Urol 1994;151:587–592 [DOI] [PubMed] [Google Scholar]
- 6.Keay S, Zhang CO, Trifillis AL, et al. Urine autoantibodies in interstitial cystitis. J Urol 1997;157:1083–1087 [PubMed] [Google Scholar]
- 7.Gardella B, Porru D, Ferdeghini F, et al. Insight into urogynecologic features of women with interstitial cystitis/painful bladder syndrome. Eur Urol 2008;54:1145–1151 [DOI] [PubMed] [Google Scholar]
- 8.Nickel JC, Tripp DA, Pontari M, et al. Psychosocial phenotyping in women with interstitial cystitis/painful bladder syndrome: A case control study. J Urol 2010;183:167–172 [DOI] [PubMed] [Google Scholar]
- 9.Tripp DA, Nickel JC, Wong J, et al. Mapping of pain phenotypes in female patients with bladder pain syndrome/interstitial cystitis and controls. Eur Urol 2012;62:1188–1194 [DOI] [PubMed] [Google Scholar]
- 10.Hayashi Y, Takimoto K, Chancellor MB, et al. Bladder hyperactivity and increased excitability of bladder afferent neurons associated with reduced expression of Kv1.4 alpha-subunit in rats with cystitis. Am J Physiol Regul Integr Comp Physiol 2009;296:R1661–R1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshimura N, de Groat WC. Increased excitability of afferent neurons innervating rat urinary bladder after chronic bladder inflammation. J Neurosci 1999;19:4644–4653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshimura N, Oguchi T, Yokoyama H, et al. Bladder afferent hyperexcitability in bladder pain syndrome/interstitial cystitis. Int J Urol 2014;21 Suppl 1:18–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Homma Y. Hypersensitive bladder: A solution to confused terminology and ignorance concerning interstitial cystitis. Int J Urol 2014;21 Suppl 1:43–47 [DOI] [PubMed] [Google Scholar]
- 14.de Groat WC, Griffiths D, Yoshimura N. Neural control of the lower urinary tract. Compr Physiol 2015;5:327–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charrua A, Cruz CD, Cruz F, Avelino A. Transient receptor potential vanilloid subfamily 1 is essential for the generation of noxious bladder input and bladder overactivity in cystitis. J Urol 2007;177:1537–1541 [DOI] [PubMed] [Google Scholar]
- 16.Homma Y, Nomiya A, Tagaya M, et al. Increased mRNA expression of genes involved in pronociceptive inflammatory reactions in bladder tissue of interstitial cystitis. J Urol 2013;190:1925–1931 [DOI] [PubMed] [Google Scholar]
- 17.Szallasi A, Sheta M. Targeting TRPV1 for pain relief: Limits, losers and laurels. Expert Opin Invest Drugs 2012;21:1351–1369 [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Sanz N, Fernandez-Carvajal A, Morenilla-Palao C, et al. Identification of a tetramerization domain in the C terminus of the vanilloid receptor. J Neurosci 2004;24:5307–5314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goss JR, Goins WF, Glorioso JC. Gene therapy applications for the treatment of neuropathic pain. Expert Rev Neurother 2007;7:487–506 [DOI] [PubMed] [Google Scholar]
- 20.Goss JR, Gold MS, Glorioso JC. HSV vector-mediated modification of primary nociceptor afferents: An approach to inhibit chronic pain. Gene Ther 2009;16:493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Funahashi Y, Oguchi T, Goins WF, et al. Herpes simplex virus vector mediated gene therapy of tumor necrosis factor-alpha blockade for bladder overactivity and nociception in rats. J Urol 2013;189:366–373 [DOI] [PubMed] [Google Scholar]
- 22.Yokoyama H, Oguchi T, Goins WF, et al. Effects of herpes simplex virus vector-mediated enkephalin gene therapy on bladder overactivity and nociception. Hum Gene Ther 2013;24:170–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Srinivasan R, Huang S, Chaudhry S, et al. An HSV vector system for selection of ligand-gated ion channel modulators. Nat Methods 2007;4:733–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goins WF, Huang S, Cohen JB, Glorioso JC. Engineering HSV-1 vectors for gene therapy. Methods Mol Biol 2014;1144:63–79 [DOI] [PubMed] [Google Scholar]
- 25.Lecci A, Giuliani S, Lazzeri M, et al. The behavioral response induced by intravesical instillation of capsaicin rats is mediated by pudendal urethral sensory fibers. Life Sci 1994;55:429–436 [DOI] [PubMed] [Google Scholar]
- 26.Saitoh C, Chancellor MB, de Groat WC, Yoshimura N. Effects of intravesical instillation of resiniferatoxin on bladder function and nociceptive behavior in freely moving, conscious rats. J Urol 2008;179:359–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hunt SP, Pini A, Evan G. Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature 1987;328:632–634 [DOI] [PubMed] [Google Scholar]
- 28.Menetrey D, Gannon A, Levine JD, Basbaum AI. Expression of c-fos protein in interneurons and projection neurons of the rat spinal cord in response to noxious somatic, articular, and visceral stimulation. J Comp Neurol 1989;285:177–195 [DOI] [PubMed] [Google Scholar]
- 29.Bullitt E. Expression of c-fos-like protein as a marker for neuronal activity following noxious stimulation in the rat. J Comp Neurol 1990;296:517–530 [DOI] [PubMed] [Google Scholar]
- 30.Birder LA, de Groat WC. Increased c-fos expression in spinal neurons after irritation of the lower urinary tract in the rat. J Neurosci 1992;12:4878–4889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoshimura N, Erdman SL, Snider MW, de Groat WC. Effects of spinal cord injury on neurofilament immunoreactivity and capsaicin sensitivity in rat dorsal root ganglion neurons innervating the urinary bladder. Neuroscience 1998;83:633–643 [DOI] [PubMed] [Google Scholar]
- 32.Krisky DM, Marconi PC, Oligino TJ, et al. Development of herpes simplex virus replication-defective multigene vectors for combination gene therapy applications. Gene Ther 1998;5:1517–1530 [DOI] [PubMed] [Google Scholar]
- 33.Akkaraju GR, Huard J, Hoffman EP, et al. Herpes simplex virus vector-mediated dystrophin gene transfer and expression in MDX mouse skeletal muscle. J Gene Med 1999;1:280–289 [DOI] [PubMed] [Google Scholar]
- 34.Mellerick DM, Fraser NW. Physical state of the latent herpes simplex virus genome in a mouse model system: Evidence suggesting an episomal state. Virology 1987;158:265–275 [DOI] [PubMed] [Google Scholar]
- 35.Fink DJ, Wechuck J, Mata M, et al. Gene therapy for pain: Results of a phase I clinical trial. Ann Neurol 2011;70:207–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hao S, Mata M, Glorioso JC, Fink DJ. HSV-mediated expression of interleukin-4 in dorsal root ganglion neurons reduces neuropathic pain. Mol Pain. 2006;2:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]