SUMMARY
The metabolite 2-oxoglutarate (also known as α-ketoglutarate, 2-ketoglutaric acid, or oxoglutaric acid) lies at the intersection between the carbon and nitrogen metabolic pathways. This compound is a key intermediate of one of the most fundamental biochemical pathways in carbon metabolism, the tricarboxylic acid (TCA) cycle. In addition, 2-oxoglutarate also acts as the major carbon skeleton for nitrogen-assimilatory reactions. Experimental data support the conclusion that intracellular levels of 2-oxoglutarate fluctuate according to nitrogen and carbon availability. This review summarizes how nature has capitalized on the ability of 2-oxoglutarate to reflect cellular nutritional status through evolution of a variety of 2-oxoglutarate-sensing regulatory proteins. The number of metabolic pathways known to be regulated by 2-oxoglutarate levels has increased significantly in recent years. The signaling properties of 2-oxoglutarate are highlighted by the fact that this metabolite regulates the synthesis of the well-established master signaling molecule, cyclic AMP (cAMP), in Escherichia coli.
INTRODUCTION
In order to cope with fluctuations in the availability of nutrients, organisms have developed a plethora of signal transduction systems to sense the prevailing nutritional status and generate the appropriate metabolic response. The two major nutrients needed by living organisms are carbon and nitrogen. Despite extensive knowledge on how both carbon and nitrogen metabolic pathways are regulated, one of the most fundamental questions that remain to be answered is how organisms, particularly microbes, coordinate nitrogen and carbon metabolism in order to maximize nutrient utilization and cell growth. An ideal signaling metabolite to coordinate the regulation of nitrogen and carbon metabolism should lie at the intersection between these two metabolic pathways. It has long been recognized that the tricarboxylic acid (TCA) cycle intermediate 2-oxoglutarate (2-OG) could, in theory, fulfill such a regulatory role, as 2-OG is used as the major carbon skeleton in nitrogen-assimilatory reactions (1). However, only recently have experimental data confirmed that 2-OG acts as a master regulatory metabolite.
This review aims to summarize recent advances in understanding the biological role of 2-OG as both a metabolic intermediate and an important signaling molecule. We focus primarily on Escherichia coli because the physiology and systems biology of metabolic control have been studied intensively in this model organism. In the first part of this review, we describe the roles of 2-OG as a TCA cycle intermediate and as the carbon skeleton for nitrogen assimilation reactions. This is followed by a review of methods and challenges of estimating 2-OG levels and a description of the major 2-OG-sensing proteins in prokaryotes. We then review the novel interplay between the 2-OG and cyclic AMP (cAMP) levels in E. coli. Finally, we briefly summarize the emerging role of 2-OG as a master regulator in eukaryotes and discuss the major points and challenges in 2-OG signaling that remain to be tackled.
METABOLIC ROLES OF 2-OG IN ENERGY PRODUCTION AND NITROGEN ASSIMILATION
When E. coli uses glucose, fatty acids, or some amino acids as a carbon source under aerobic conditions, these molecules are oxidized to acetyl-coenzyme A (acetyl-CoA), which is fed into the TCA cycle (Fig. 1). The acetyl group from acetyl-CoA is oxidized to two CO2 molecules, and the energy derived from these reactions is conserved either in reduced electron carriers or in ATP. The TCA cycle also carries out important anabolic functions; for instance, 2-oxoglutarate and oxaloacetate act as precursors for the synthesis of amino acids and nucleotides (see below). It is also notable that alternative TCA cycles operate in some prokaryotes. These alternative pathways are likely to occur in organisms that were previously thought to have incomplete TCA cycles due to the lack of 2-OG dehydrogenase (2–4).
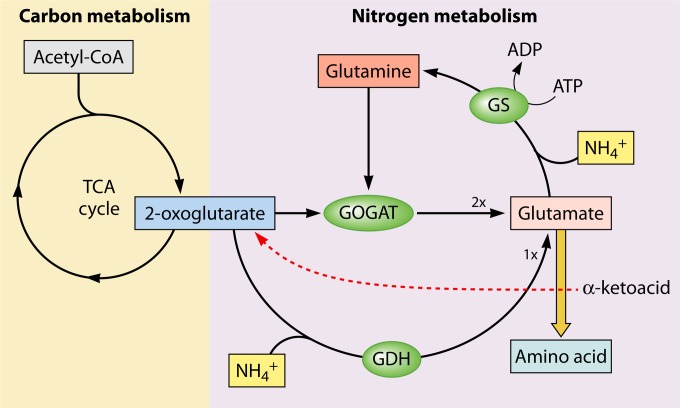
FIG 1.
Schematic representation of the TCA cycle and ammonium-assimilatory reactions in E. coli. Note that the GDH and GOGAT reactions produce one and two glutamate molecules, respectively (denoted by “1×” and “2×”). (Adapted from data in reference 139.)
Ammonium is the preferred nitrogen source for E. coli under aerobic conditions, as it supports high growth rates; this is also the case for many other microorganisms (5). Ammonium is assimilated into glutamine and glutamate; these two amino acids act as intracellular nitrogen donors to produce other nitrogen-containing organic compounds through transamination and transamidation reactions (5, 6). The synthesis of glutamine in E. coli and other bacteria occurs through glutamine synthetase (GS); this enzyme produces glutamine from ammonium and glutamate, in a reaction driven by the hydrolysis of one ATP molecule per one NH4+ ion assimilated (Fig. 1). In E. coli, the productive synthesis of glutamate from ammonium can occur by the following two different pathways: (i) combining the glutamine produced by GS with 2-OG, through the action of the enzyme glutamate synthase (GOGAT), resulting in the production of two glutamates (Fig. 1); and (ii) directly assimilating ammonium into 2-OG by the action of glutamate dehydrogenase (GDH), producing one glutamate (Fig. 1). The GDH pathway has been presumed to be used when ammonium is abundant, as its Km for ammonium is over 1 mM (7), whereas GS has a lower Km for ammonium (∼0.1 mM) (8). However, kinetic flux profiling experiments showed that even with high ammonium availability (10 mM), the GS-GOGAT pathway is the major ammonium-assimilatory route in E. coli (9). Another difference between the GS-GOGAT and GDH pathways is their respective Km values for 2-OG, which are ∼0.24 mM for GOGAT (10) and ∼0.64 mM for GDH (7, 11). As discussed below, 2-OG levels can fluctuate to levels below 0.1 mM; therefore, the GS-GOGAT pathway may be predominant under 2-OG deprivation conditions. Note that in either the GDH or GS-GOGAT pathway, the carbon receptor of the nitrogen atom is the TCA cycle intermediate 2-OG (Fig. 1). Therefore, 2-OG is at the intersection between carbon and nitrogen metabolism, connecting the catabolic function of the TCA cycle with the anabolic function of the nitrogen assimilation reactions.
FLUCTUATIONS IN THE 2-OG POOL IN RESPONSE TO NITROGEN AVAILABILITY
The determination of absolute metabolite concentrations within living cells is challenging. Many metabolites, such as 2-OG, turn over very quickly and may leak or degrade during extraction; hence, the sampling process must be quick to avoid perturbations in metabolite levels and to maintain metabolite stability (12–14). Furthermore, conversion of measured metabolite levels to molar concentrations within the cell is complicated by a number of factors, e.g., the internal cell volume may fluctuate (15), and it is hard to predict how the measured values correlate to free metabolites, as a fraction of metabolites are likely to be bound to proteins. Despite these challenges, many studies have determined the levels of 2-OG in E. coli cell extracts by applying either coupled enzymatic assays (16–18), mass spectrometry (9, 19–21), or high-pressure liquid chromatography (HPLC) methods (13). More recently, engineered fluorescent protein probes, or biosensors, have been developed to allow monitoring of 2-OG levels in cell extracts or directly inside living cells (22–24).
Because 2-OG acts as the carbon skeleton for the nitrogen assimilation reactions by either the GS-GOGAT or GDH pathway (Fig. 1), it is expected that the level of this metabolite will fluctuate according to the availability of ammonium. Indeed, pioneer studies demonstrated that the 2-OG concentration increases upon ammonium limitation under steady-state growth conditions (16, 17). Follow-up studies analyzed the dynamics of the 2-OG levels upon rapid shifts in ammonium concentration. In one case, the 2-OG level decreased from ∼1.4 mM under nitrogen starvation conditions to ∼0.3 mM just 1 min after the addition of 200 μM NH4Cl to the cell culture, returning to the initial 2-OG level after 15 min (18). In another study, the 2-OG level decreased from ∼12 mM under nitrogen starvation conditions to ∼0.6 mM 3 min after the addition of 10 mM NH4Cl (9). Conversely, E. coli cultivated with a high ammonium level (10 mM NH4Cl) exhibited an increase in intracellular 2-OG, from ∼0.5 mM to ∼2.5 mM, 15 s after ammonium was removed from the medium (13). These experiments with E. coli showed that there is an inverse correlation between ammonium availability and 2-OG accumulation. The same correlation applies to organisms such as archaea (25), cyanobacteria (26, 27), and the yeast Saccharomyces cerevisiae (19). Although, as mentioned above, precise quantitation of metabolite levels in living cells is challenging, these studies indicate that 2-OG is well suited to act as a metabolic signal of small and transient fluctuations in ammonium availability in a wide range of microbes. As pointed out below, the relationship between 2-OG levels and the nitrogen status has allowed the evolution of a diverse range of 2-OG sensor proteins that coordinate nitrogen and carbon metabolism in many prokaryotes.
It is worth mentioning that 87% of the 2-OG drained from the TCA cycle during ammonium assimilation by either GDH or GOGAT (Fig. 1) is estimated to be recycled by transamination (Fig. 1, red line) (5). The remaining 13% should be replenished by anaplerotic reactions (see below). Hence, the decrease in 2-OG that occurs when N-starved E. coli receives an ammonium shock should be transient, returning to initial levels after ammonium consumption, and indeed this is what is observed experimentally (18). Rapid reestablishment of 2-OG levels after ammonium consumption may be facilitated by increased transamination (Fig. 1, red line), particularly through the enzyme aspartate aminotransferase, with the equilibrium driven toward the forward reaction (net aspartate production) by low levels of 2-OG (9). Despite the partial replenishment of the 2-OG pool after transamination, productive de novo synthesis of 2-OG must occur to replenish the 2-OG that was consumed during nitrogen assimilation but was not recycled. Given the cyclic nature of the TCA cycle, in principle, the carbon skeleton of any of the TCA cycle intermediates can be transformed into 2-OG. Reactions that fill the TCA cycle with carbon are known as anaplerotic reactions. In E. coli, anaplerosis is accomplished by phosphoenolpyruvate (PEP) carboxylase, which converts PEP to the TCA cycle intermediate oxaloacetate. E. coli can also replenish TCA cycle intermediates by using the glyoxylate shunt. However, the activity of the glyoxylate shunt is regulated by carbon catabolic repression (CCR) (28), and also by reversible phosphorylation of isocitrate dehydrogenase (29).
Apart from the de novo synthesis of 2-OG, E. coli can take up 2-OG from the medium by using a constitutively expressed 2-OG/H+ symport permease system encoded by kgtP (30). Direct uptake from the medium has been confirmed using an in vivo 2-OG biosensor (22, 31). However, 2-OG seems to leak from E. coli cells under certain culture conditions (13). Interestingly, in Xanthomonas oryzae, the kgtP-encoded transporter plays an important role as a plant virulence effector, since it is secreted by the type III secretion system to the plant cell membrane, where it may be involved in 2-OG acquisition from host rice cells (32).
FLUCTUATIONS IN THE 2-OG POOL IN RESPONSE TO CARBON AVAILABILITY
There is increasing evidence that intracellular 2-OG levels are sensitive to variations in the carbon supply in E. coli. E. coli cells cultured in glycerol have an intracellular 2-OG concentration of ∼0.5 mM as determined by HPLC. When these cells were washed with medium without glycerol, the intracellular 2-OG level dropped to ∼0.3 mM within 15 s (13). In a separate study, the intracellular concentration of 2-OG (as measured by HPLC) was 0.35 mM for E. coli incubated in carbon-free M9 medium, and this increased to 2.6 mM 2-OG 30 min after the addition of 10 mM glucose to the medium (22). Reductions in intracellular 2-OG levels when E. coli is starved for carbon have also been detected by mass spectrometry (14, 19, 33). These data have been corroborated by studies using intracellular 2-OG biosensors. Zhang and colleagues developed an in vivo 2-OG sensor based on fluorescence resonance energy transfer (FRET) by inserting the 2-OG binding domain of the Azotobacter vinelandii NifA protein (see “The Nitrogenase Transcriptional Regulator Protein NifA”) between the FRET pair yellow fluorescent protein (YFP)-cyan fluorescent protein (CFP) (22, 31). This biosensor enabled monitoring of fluctuations in 2-OG levels in real time in response to variations in the carbon supply. These experiments showed that there is a quick and significant increase in the 2-OG level when glucose is added to carbon-starved cultures of E. coli (22, 31). In summary, evidence is building to support the notion that intracellular 2-OG levels may be a good indicator of the carbon supply in E. coli. This hypothesis is further supported by recent findings demonstrating that the 2-OG level coordinates multiple carbon-dedicated pathways, such as fatty acid production and carbohydrate uptake systems (see the following sections).
PROTEINS THAT ACT AS 2-OG SENSORS
Given the key role played by 2-OG in central carbon and nitrogen metabolism and the ability of this metabolite to reflect the balance between the nitrogen and carbon nutritional statuses, it is not surprising that organisms have evolved a variety of proteins that are able to sense 2-OG levels. The best-described proteins that act as 2-OG sensors in prokaryotes are reviewed in this section.
The PII Protein Family
Probably the most ancient sensors of 2-OG are proteins belonging to the PII family. The PII regulatory protein was initially identified through classical biochemical studies of the regulation of glutamine synthetase in E. coli and was the first protein to be recognized as a 2-OG sensor (34). PII proteins are well conserved and widely distributed in bacteria, in archaea, and in the chloroplasts of plants (35). Since this protein family was recently reviewed comprehensively (36–38), in this review we briefly describe the PII proteins and then focus on the regulation of acetyl-CoA carboxylase (ACC) by PII.
Typical PII proteins are homotrimers that form a barrel-like structure; each subunit carries a long and solvent-exposed loop, called the T loop, which is vital for PII protein function. The three clefts formed between the PII subunits can be occupied competitively by either ATP or ADP, suggesting that PII proteins can potentially act as energy sensors in vivo (39). Each PII trimer can also bind up to three 2-OG molecules; the 2-OG binding sites are formed between PII subunits and require the previous binding of MgATP (Fig. 2) (40). Binding of MgATP, ADP, or MgATP plus 2-OG alters the PII structure and the ability of PII to bind and modulate the activity of a variety of target proteins (37, 41). Some PII proteins, like E. coli GlnB, show strong negative cooperativity for the occupation of the three 2-OG binding sites when MgATP is saturating. The first 2-OG molecule binds with a Kd (dissociation constant) in the low micromolar range, and consecutive occupations of the second and third 2-OG binding sites exhibit Kds that are 1 to 2 orders of magnitude higher (42, 43). This behavior makes PII an ideal biological sensor of 2-OG with the ability to respond to a wide range of 2-OG concentrations (44). However, it should be noted that cooperative binding of 2-OG to PII proteins is not a universal feature, since in some cases calorimetric titrations fit only a “one-set-of-sites” binding model (41, 45), perhaps reflecting a physiological requirement to respond to a more restricted range of 2-OG concentrations.
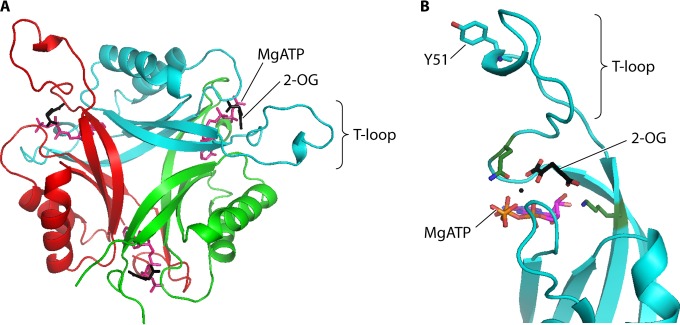
FIG 2.
PII protein structure. (A) PII trimer. The binding sites for MgATP (magenta) and 2-OG (black) at the lateral clefts between each subunit are indicated. (B) Closer view of the 2-OG binding site. 2-OG interacts with the side chains of conserved lysine (K58) and glutamine (Q39) residues (green sticks) and also with MgATP. 2-OG binding affects the PII T-loop structure, thereby altering the affinity between PII and its binding protein targets. In proteobacteria, the structure of the T loop is also influenced by reversible uridylylation at the conserved tyrosine residue indicated (Y51). The figure was prepared using Pymol and PDB entry 3MHY.
Another interesting characteristic of PII is its potential to integrate the 2-OG signal with energy sensing. Given that MgATP and ADP bind to PII competitively and that 2-OG binding requires preoccupancy with MgATP, an increased apparent affinity for 2-OG is observed at high ATP/ADP ratios (43, 46, 47). This interplay between the ATP/ADP ratio and 2-OG binding is further complicated by the fact that some PII members apparently function as an ATPase whose activity is regulated by 2-OG levels (48).
The PII protein structure can be affected further by reversible covalent modification at a conserved tyrosine residue located at the top of each of its three T loops (Fig. 2). In proteobacteria, PII proteins are found in uridylylated forms under nitrogen-limiting conditions and are rapidly deuridylylated under conditions of nitrogen excess (37, 49). In most cases studied so far, the nitrogen signaling molecule glutamine controls PII uridylylation through allosteric regulation of the bifunctional uridylyl transferase/uridylyl-removing enzyme GlnD (50). Covalent modification by GlnD is modulated by binding of the ligands ATP, ADP, and 2-OG to PII (43, 47, 51, 52).
Regulation of acetyl-CoA carboxylase by PII and 2-OG.
Since PII is able to sense important metabolites, it is not surprising that PII proteins control the activity of a vast range of target proteins (37, 53). In E. coli, the archetypical function of PII is the regulation of nitrogen assimilation. It fulfils this function by controlling the activity of the ammonium transporter AmtB (54) and the expression and activity of the glutamine synthetase enzyme (50, 55). More recently, it was also observed that E. coli GlnB can modulate the activity of an enzyme dedicated to the carbon metabolism, i.e., acetyl-CoA carboxylase (ACC) (56).
ACC catalyzes the first and committed step in fatty acid biosynthesis, the carboxylation of acetyl-CoA to generate malonyl-CoA. ACC is divided into the following three functional modules: the biotin carboxylase (BC), the carboxyltransferase (CT), and the biotin carboxyl carrier protein (BCCP) (57). BC catalyzes the first half-reaction, the carboxylation of a biotin group attached to BCCP, using bicarbonate and ATP as substrates. The second half-reaction is catalyzed by CT, which transfers the carboxyl from carboxyl-biotin to acetyl-CoA to generate malonyl-CoA (57, 58). Typical ACC enzymes can be divided into two classes. Those found in most eukaryotes have all three functional modules within a single polypeptide. On the other hand, in most prokaryotes and in the chloroplasts of most plants, ACC is formed by combining different polypeptides, corresponding to the BC, CT, and BCCP functional modules (58).
Searches for novel targets of PII in the Arabidopsis thaliana chloroplast and in the bacterium Azospirillum brasilense both led to the identification of BCCP as a binding partner of PII (59, 60). This interaction was further observed in E. coli by use of purified proteins. The detection of the BCCP-PII interaction in distantly related organisms supports the hypothesis that BCCP is an ancient binding partner of PII (60). The functional role of the BCCP-PII interaction is also conserved. Measurements of ACC activity using A. thaliana chloroplast extracts or using purified E. coli ACC came to the same conclusion: PII binding to BCCP leads to ACC inhibition by reducing the ACC turnover rate (Fig. 3). The BCCP-PII interaction and ACC inhibition are relieved by 2-OG in a dose-dependent manner (56). Hence, PII acts as a 2-oxoglutarate-sensitive dissociable regulatory subunit of ACC (Fig. 3). In vivo studies of plants have corroborated these observations. A knockout mutation in the gene encoding PII increased the accumulation of fatty acids in A. thaliana seeds (61). These studies support a model in which 2-OG levels act as a conserved switch to regulate ACC activity, and thus fatty acid biosynthesis, in bacteria and plants.
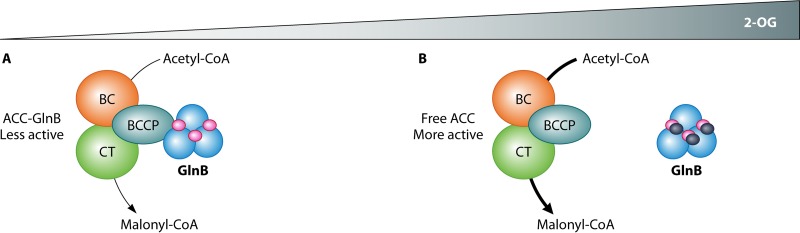
FIG 3.
GlnB acts as a 2-OG-sensitive dissociable regulatory subunit of acetyl-CoA carboxylase (ACC). (A) With low 2-OG levels, GlnB is bound to 3 MgATP molecules (magenta) and interacts with the BCCP component of ACC, turning down the enzyme activity. (B) When the 2-OG concentration increases, the three 2-OG binding sites of GlnB are occupied (dark blue circles), resulting in a GlnB T-loop structure that abrogates interaction with and inhibition of ACC.
The Nitrogenase Transcriptional Regulator Protein NifA
PII proteins are involved in the regulation of nitrogen fixation in both archaea and bacteria, affecting the transcription of nitrogen fixation genes (nif genes) and/or nitrogenase activity (36, 53, 62). In proteobacteria, PII influences nif gene expression by controlling the activity of a transcriptional activator named NifA in response to the level of fixed nitrogen. The N status can thus be conveyed to NifA via the uridylylation state of PII proteins and by the capacity of these signal transduction proteins to sense 2-OG levels as described above. The potential for PII proteins to sense the adenylate energy charge (ATP/ADP ratio) may also be important in this context, as nitrogen fixation is a very energy-intensive process.
In some diazotrophs, the activity of NifA is regulated by stoichiometric interaction with a partner regulatory protein, NifL, which senses the redox status and inhibits NifA when the oxygen concentration is unfavorable for nitrogen fixation (63–65). The PII protein GlnK modulates the interaction between NifL and NifA in response to the N status (66). In the model diazotroph Azotobacter vinelandii, deuridylylated GlnK interacts with NifL under conditions with high glutamine levels, resulting in the formation of a GlnK-NifL-NifA ternary complex in which NifA is inactivated under conditions of N excess (45, 67). In contrast, with low glutamine levels, uridylylated GlnK does not interact with NifL, consequently allowing NifA activity under N-limiting conditions (Fig. 4). Unlike the E. coli PII proteins, A. vinelandii GlnK does not exhibit strong negative cooperativity with respect to the binding of 2-OG. Surprisingly, the interaction between nonmodified GlnK and NifL is maintained even at a high 2-OG concentration (2 mM) in vitro, suggesting that this target interaction is regulated primarily by the uridylylation state of GlnK (45).
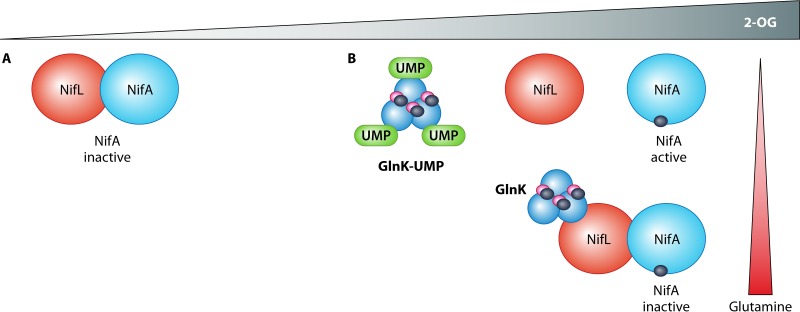
FIG 4.
Involvement of 2-OG and glutamine in the regulation of NifA activity by NifL and GlnK. (A) At low-2-OG concentrations, NifL forms a complex with NifA and NifA activity is inhibited. (B) At high concentrations, 2-OG (dark blue circles) binds to NifA to prevent inhibition by NifL. At low glutamine concentrations, GlnK is uridylylated and unable to interact with NifL. At high glutamine concentrations, GlnK is deuridylylated, and the nonmodified form of GlnK interacts with NifL, resulting in the formation of a ternary GlnK-NifL-NifA complex. This complex is formed even when GlnK is saturated with MgATP (magenta circles) and 2-OG (dark blue circles).
Most NifA proteins contain an N-terminal GAF domain, which apparently regulates the catalytic activity and/or the DNA binding functions of the activator. In the case of A. vinelandii NifA, the GAF domain regulates the interaction with NifL in response to the concentration of 2-OG, which binds directly to the GAF domain, with a dissociation constant of ∼60 μM (68). When NifL is absent, binding of 2-OG to the GAF domain of the isolated NifA protein does not influence its activity. However, both the oxidized and reduced forms of NifL inhibit NifA activity in vitro in the absence of 2-OG (68, 69) (Fig. 4). Further support for a physiological role for 2-OG comes from the observation that a substitution in the GAF domain which prevents 2-OG binding to NifA obviates escape from inhibition by NifL (70).
The ability of 2-OG to prevent the interaction between NifL and NifA is completely overridden when either the PAS domain of NifL becomes oxidized or GlnK is deuridylylated. Hence, 2-OG is effective as an allosteric regulator only under reducing, N-limiting conditions. This may explain why the GlnK-NifL interaction is relatively insensitive to 2-OG, as this will permit maintenance of the inhibitory GlnK-NifL-NifA ternary complex even at high 2-OG concentrations (45). Overall, the function of 2-OG in this regulatory system is to antagonize inhibition by NifL under conditions that are favorable for nitrogen fixation (Fig. 4). This would potentially enable sensing of the carbon status by NifA in order to ensure that the high carbon (energy) demand for nitrogen fixation can be met. However, it is also possible that 2-OG sensing by NifA provides a backup mechanism for sensing the nitrogen status.
In diazotrophic bacteria that do not contain NifL, the response to the fixed nitrogen status is mediated by direct interaction of PII proteins with the GAF domain of NifA. In some organisms, for example, A. brasilense, Herbaspirillum seropedicae, and Rhodospirillum rubrum, the uridylylated form of the PII protein is apparently required to prevent intramolecular repression of NifA activity by the GAF domain under nitrogen-limiting conditions (71–73). In contrast, in Rhodobacter capsulatus and Azorhizobium caulinodans, PII proteins are required to inactivate NifA under conditions of nitrogen excess (74, 75). However, the role of 2-OG in these interactions is not well understood.
The NtcA Transcriptional Regulator in Cyanobacteria
In cyanobacteria, some of the functions of the PII protein in regulating nitrogen assimilation have been taken over by NtcA, a global transcriptional regulator belonging to the Crp-Fnr family (76–78) that senses the N status. As noted above, in cyanobacteria, high 2-OG levels are indicative of nitrogen deficiency (26). 2-OG binds to NtcA and acts as an allosteric effector by increasing the affinity of NtcA for specific DNA binding targets, resulting in transcriptional activation in vitro (79–81). Canonical NtcA binding sites are palindromic and, in promoters subject to activation, are frequently centered at position ∼−41.5 with respect to the transcriptional start site (77, 82), similar to class II promoters activated by Crp and Fnr (83). However, unlike Crp, NtcA can also act as a repressor.
Crystal structures of NtcA in both the apo and 2-OG-bound forms reveal, as expected, that NtcA has a fold similar to that of the cAMP-binding protein Crp and that the NtcA subunits form a tight dimer in the asymmetric unit (84, 85). Both proteins contain an effector binding domain (EBD) adopting a β-barrel motif connected to a DNA binding domain via a long α-helix (the C-helix). The positioning of the ligand in the EBD is similar in both proteins, and they even share some binding residues (84, 85). However, the modes of allosteric activation by the ligands appear to be different. In the case of Crp, the binding of cAMP to the EBD causes an extension of the C-helices, resulting in rotation and appropriate positioning of the DNA recognition helices to enable stable DNA binding (86, 87). In contrast, in NtcA signal transmission, 2-OG binding to the EBD results in a relatively small change in the conformation of the bridging C-helices, forming a tightly coiled coil, which shortens the distance between the two recognition helices in the DBD to adopt a more favorable DNA binding conformation (Fig. 5). This may explain why binding of 2-OG to NtcA results in a relatively small enhancement in the affinity for DNA, compared with a large change in affinity brought about by the binding of cAMP to Crp (85, 88). Structural characterization of the 2-OG binding site in NtcA has been valuable for designing chemical derivatives of 2-OG that mimic its signaling function in vivo (89).
FIG 5.
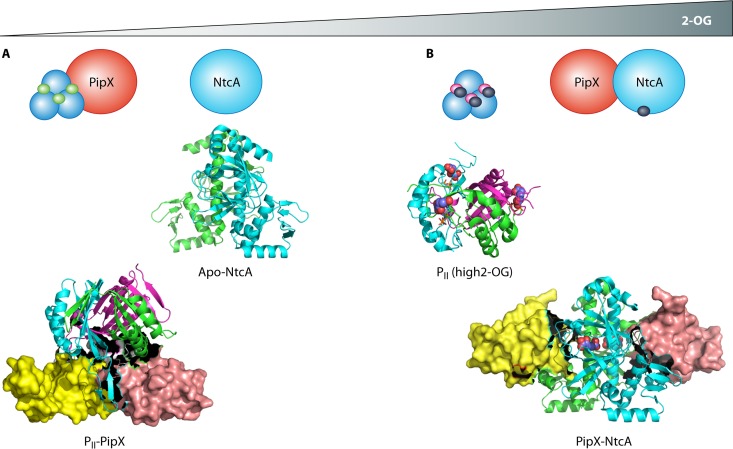
Role of 2-OG in partner switching by PipX and coactivation of NtcA. (A) At low 2-OG concentrations, PipX binds to PII and NtcA is primarily in the apo form (not bound to 2-OG). Binding of ADP to PII (green circles) also favors formation of the PII-PipX complex. (B) At high concentrations of 2-OG, PII is saturated with 2-OG (dark blue circles) and ATP (magenta circles), disfavoring its interaction with PipX. Conversely, the binding of 2-OG to NtcA favors its interaction with PipX, resulting in an enhancement of transcriptional activation. Structures of protein complexes and unbound proteins are shown beneath the schematic. PipX is shown as a surface structure, and 2-OG is shown as blue and red spheres. Binding of 2-OG to NtcA results in realignment of the recognition helices to increase its affinity for DNA. Binding of 2-OG to PII retracts the extended T loop, inhibiting complex formation with PipX. The PDB codes for the structures are as follows: 2XG8 (for PII-PipX; note that this complex consists of trimeric PII bound to a trimer of PipX), 3LA7 (apo-NtcA), 2XUL (PII with high 2-OG level), and 2XKO (PipX-NtcA, which comprises a dimer of PipX bound to a dimer of NtcA).
The rather simplistic model in which the binding of 2-OG to NtcA influences its affinity for DNA and, consequently, transcriptional activation has been complicated by the discovery of the protein PipX as the binding partner of NtcA. PipX acts as a coactivator of NtcA and links PII-mediated signaling with NtcA-regulated gene expression via a partner-swapping mechanism (90). PipX was originally identified as a PII-interacting protein (91) but was subsequently shown to interact with NtcA, in addition to PII, in a 2-OG-dependent manner (90). Whereas the PipX-NtcA complex is stabilized at high 2-OG levels, the interaction between PipX and PII is impaired by the presence of 2-OG and ATP (Fig. 5). This leads to a model in which PipX is bound to PII under conditions of nitrogen sufficiency (low 2-OG) and coactivates NtcA under nitrogen-deficient conditions (high 2-OG) (90) (Fig. 5). Intriguingly, the 2-OG-controlled partner swapping of PipX between NtcA and PII is modulated by subtle fluctuations in ADP levels, which favor formation of the PII-PipX complex at high ADP concentrations. This results in a turning down of the 2-OG signal by ADP so that PII can efficiently compete with NtcA for PipX and deactivate this transcription factor in the presence of 2-OG (92). This highly sophisticated partner-switching mechanism results in fine-tuning of NtcA-dependent gene expression (77, 93). The crystal structure of the PipX-NtcA complex contains two monomers of PipX interacting with each subunit of the 2-OG-bound NtcA dimer. Domain interactions in the activated form of NtcA are stabilized by the binding of PipX, which suggests that PipX drives the equilibrium toward the active conformation of NtcA (84, 94). In agreement with this, biochemical studies revealed that PipX increases the affinity of NtcA for promoters and the effective affinity of NtcA for 2-OG (88). Modeling of the PipX-NtcA complex on DNA suggests that PipX may also enhance NtcA-dependent transcription through interaction with the C-terminal domain of the α-subunit of RNA polymerase (84).
The Archaeal Transcriptional Repressor NrpR
In the archaeon Methanococcus maripaludis, genes required for nitrogen assimilation under nitrogen-deficient conditions are subject to regulation by the transcription factor NrpR. This regulatory protein represses the expression of the nitrogen fixation (nif) and glutamine synthetase (glnA) genes by binding to operator sequences when fixed nitrogen is abundant (95). The affinity of NrpR for the operator sites is lowered under nitrogen deficiency conditions due to increased 2-OG levels (25, 96). Homologues of M. maripaludis NrpR are widely distributed in the Euryarchaeota and also found in a few bacterial species. However, the domain architecture of these NrpR homologues is not conserved, and they can be classified into three different groups (97). In M. maripaludis, NrpR has an N-terminal helix-turn-helix (HTH) DNA binding domain fused to two consecutive NrpR domains (Fig. 6). Other organisms, such as Archaeoglobus fulgidus, encode an NrpR consisting of an N-terminal HTH linked to a single NrpR-like domain. A third class is found in organisms, such as Methanosarcina mazei, which encode two different NrpR polypeptides, one similar to the second group (HTH fused to a single NrpR domain; NrpR1) and a second polypeptide carrying a single NrpR domain without a DNA binding motif, named NrpR2 (97).
FIG 6.
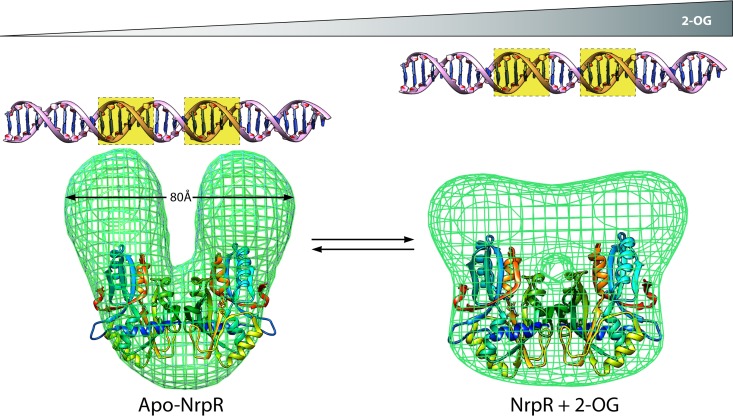
Effect of 2-OG on NrpR structure and DNA binding activity. The crystal structure of the NRD2 domain of NrpR (PDB code 4NEX; rainbow ribbon) is shown, fitted into the electron microscopy electron density maps of full-length M. maripaludis NrpR (green mesh) in both the apo form (EMD2221; left) and the 2-OG-bound form (EMD2222; right). In the apo state, the two HTH DNA binding domains of NrpR (not shown) are presumed to be positioned in the correct orientation and distance to enable binding of NrpR to two adjacent major grooves (highlighted with yellow dotted squares) in the operator sequence (modeled as B-form DNA and shown above the protein density). The conformational change induced by the binding of 2-OG converts NrpR from a U-shaped molecule to a trapezium-shaped particle in which the two DNA binding domains are too far apart to recognize the operator sequence, and consequently, transcription of nitrogen assimilation genes is derepressed.
Genome-wide expression analysis of an M. mazei NrpR1 knockout strain showed elevated transcription of 27 genes under nitrogen sufficiency conditions, including nifH, glnA1, and glnK1 (98). The M. mazei NrpR1 and NrpR2 proteins physically interact and bind as a complex to the nifH and glnK1 promoters in the presence of low 2-OG levels (99). The NrpR2 polypeptide also interacts with the general transcriptional factors (TBP and TFB) in the presence of low 2-OG levels. Hence, the NrpR1-NrpR2 complex binds to operator sequences when the 2-OG concentration is low, through both the NrpR1 DNA-binding HTH motif and interactions between NrpR2 and the general transcriptional factors; this allows repression of nitrogen assimilation genes under nitrogen-sufficient conditions (99).
The structure of one of the NrpR-like domains of the M. maripaludis NrpR protein has been solved. This domain forms a dimer in which each monomer has a cleft predicted to bind 2-OG, as suggested by molecular docking analysis (100). The putative NrpR 2-OG binding site is conserved among other 2-OG binding proteins. It was observed that Asn, Tyr, Ser, and Arg residues are commonly found in 2-OG binding sites (100). A model structure of the full-length NrpR protein of M. maripaludis was fitted into the electron microscopy electron density maps obtained in the presence or absence of 2-OG. The data suggest that 2-OG induces a major conformational change in NrpR which separates the two HTH motifs in the NrpR dimer, with the consequence that the HTH motifs are then too far away to allow stable interaction with the operator sites on DNA (100) (Fig. 6).
The Carbohydrate PTS
The carbohydrate phosphotransferase system (PTS) is present in a number of bacterial species and is responsible for the uptake of hexoses (PTS sugars), including glucose, mannose, and glucosamine; this system can also transport bi- and trisaccharides. The PTS also plays a number of regulatory roles that are thought to be the primary function of this system (101). Since the PTS has been studied extensively and comprehensively reviewed elsewhere (102, 103), in this review we only briefly describe the overall features of the PTS, focusing primarily on its regulation by 2-OG in E. coli.
The PTS acts as a phosphorylation cascade coupling the transport of carbohydrates to their phosphorylation. The basic components of the PTS are similar in all species studied (102). In E. coli, the phosphorelay begins in the cytosol with the EI protein, which catalyzes its autophosphorylation using the glycolysis intermediate phosphoenolpyruvate (PEP) as a phosphoryl donor (104). The EI phosphoryl group is then transferred to another cytosolic protein, HPr. The cytosolic EI and HPr proteins act as common phosphoryl donors to a group of membrane-associated transporters, the EII components, which are specific for different carbohydrates (105). Typical EII components consist of an integral membrane domain, which facilitates sugar transport, together with associated cytoplasmic domains that can be carried by separate polypeptides (102). For instance, the glucose-specific EII complex in E. coli consists of the cytosolic EIIAGlc component (encoded by crr) and the integral membrane component EIICBGlc (encoded by ptsG) (105). The phosphoryl group is transferred from HPr to EIIAGlc and then relayed to EIICBGlc, where it is then transferred to the incoming glucose (Fig. 7).
FIG 7.
Regulation of AC activity by 2-OG levels. (A) When glucose is unavailable, phosphorylated EIIA (EIIA-P) results in accumulated activated AC, cAMP production, and the use of alternative carbon sources. Under these conditions, 2-OG levels are low, allowing EI autophosphorylation and active phosphorelay to EIIA. (B) In the presence of glucose, EIIA is mainly dephosphorylated (denoted by the pale P signal) due to phosphotransfer to the incoming glucose. High glucose increases 2-OG levels, thereby inhibiting EI autophosphorylation and phosphorelay to EIIA. The accumulation of unphosphorylated EIIA inhibits AC activity and cAMP production. AC activity is further inhibited by 2-OG, by an unknown mechanism. (C) Nitrogen levels may also affect cAMP production. In a situation where E. coli is using glucose and a poor nitrogen source and is suddenly presented with a good nitrogen source (i.e., ammonium shock conditions), a sudden increase in the assimilation of ammonia into amino acids (denoted by a large red arrow) will drain carbon skeletons (and 2-OG), augmenting the demand for carbon and energy for protein biosynthesis. This reduction in 2-OG levels is expected to activate the PTS phosphorelay, thereby augmenting the concentration of EIIA-P and partially activating AC. cAMP will activate carbon catabolic pathways, thereby fulfilling the increased carbon and energy demand.
Components of the PTS, particularly EIIA, also act as signaling modules controlling the activity of a number of downstream targets. For example, when glucose is available, its transport results in net dephosphorylation of the PTS proteins (Fig. 7B). Unphosphorylated EIIAGlc directly binds to a number of transporters for nonpreferred carbon sources (non-PTS sugars), inhibiting their activity and thereby ensuring that the genes required for their catabolism are not induced, in a mechanism known as inducer exclusion (102, 103). Unphosphorylated EIIBCGlc also sequesters the transcriptional repressor Mlc to the cell membrane, affecting the expression of genes involved in carbohydrate metabolism. On the other hand, when glucose is limiting, the PTS proteins are mainly observed to be phosphorylated. The phosphorylated form of EIIAGlc activates adenylate cyclase (AC), resulting in the accumulation of cAMP, which binds to the catabolic repressor protein (CRP), affecting the expression of a vast array of genes, in a regulatory mechanism known as carbon catabolite repression (CCR).
Even though this model for regulation of AC is widely accepted, other unknown factors are believed to participate in the control of AC activity, as supported by in vitro studies (106; for a review, see reference 107). The presence of additional regulatory factors is further supported by the observation that non-PTS carbon sources and low nitrogen availability tend to reduce cAMP production. As noted below, recent data support a model in which 2-OG is a key player in controlling AC activity, and this may explain, at least in part, some of the “unidentified signaling elements.”
Regulation of carbohydrate PTS phosphorylation by 2-OG.
Doucette et al. observed that glucose and d-mannitol uptake in E. coli is inhibited by nitrogen limitation. Using metabolomic approaches together with mutant analysis, they concluded that the inhibition of PTS carbohydrate uptake was caused by the accumulation of 2-OG that occurs under nitrogen limitation conditions (108). In vitro analysis with purified proteins demonstrated that the inhibition of carbohydrate uptake was caused by the inhibition of EI autophosphorylation in the presence of 2-OG. These authors noted that 2-OG directly influences EI activity in a noncompetitive fashion, suggesting that PEP and 2-OG have different binding sites on EI (108). Direct binding of 2-OG to the C-terminal region of E. coli EI has been confirmed by nuclear magnetic resonance (NMR), with a reported Kd of ∼2.2 mM (109). However, in contrast to the data reported by Doucette et al., the NMR analysis indicated that PEP and 2-OG compete for the same binding site on EI. Indeed, molecular docking models indicate that 2-OG can occupy the PEP binding site on the EI C-terminal domain (109, 110).
These studies imply that the status of EI phosphorylation, and thus of all subsequent proteins in the PTS cascade, is controlled by the 2-OG/PEP ratio. Hence, 2-OG has the potential to influence not only PTS carbohydrate transport but also the PTS signaling functions. These include inducer exclusion, regulation of AC activity and consequent cAMP accumulation, and CCR (Fig. 7). Indeed, pioneer studies showed that when 2-OG is added to E. coli cultivated in the presence of glycerol, there is a decrease in cAMP synthesis (111). A similar effect was observed by Rabinowitz and coworkers, who noted a reduction in the rate of accumulation of cAMP when a membrane-permeating 2-OG analog (dimethyl-ketoglutarate) was added to E. coli using glycerol as a carbon source (108).
Strikingly, however, 2-OG also appears to participate in an additional signaling loop, in which 2-OG (and other α-keto acids, such as oxaloacetate) influences AC activity in the absence of a functional PTS. By utilizing E. coli strains in which elevated transport of 2-OG was achieved through upregulation of the kgtP transporter, Hwa and coworkers demonstrated that the addition of 2-OG causes transient repression of lacZ expression and reduces the level of cAMP (112). This corresponds to the classical phenomenon of CCR mediated by CRP-cAMP. Since the transient decrease in cAMP production mediated by 2-OG was completely independent of the PTSGlu system, it appears that this represents a novel signaling loop providing hierarchical control of CCR, in which 2-OG either directly regulates AC or interacts with a 2-OG-responsive regulatory partner of AC (112) (Fig. 7B).
Overall, these data now establish that 2-OG is a master regulator that not only influences the activities of the PTS but also controls CCR via a PTS-independent route (112, 113). 2-OG therefore plays a pivotal role in coordinating carbon and nitrogen metabolism in this system, since, as mentioned above, the level of 2-OG is inversely correlated with nitrogen availability. As a consequence, the quality of the nitrogen source influences the accumulation of cAMP (e.g., see Fig. 4D in the work of Doucette et al. [108]) (Fig. 7C).
The Regulatory PTS (PTSNtr)
Apart from the canonical sugar-related PTS, some bacteria encode another system, named PTSNtr, which is not involved in carbohydrate uptake and apparently is dedicated to regulatory functions (101, 114, 115). In E. coli, the PTSNtr comprises EINtr, encoded by ptsP; NPr, the product of ptsO; and EIIANtr, encoded by ptsN. These proteins are paralogs of the sugar PTS components EI, HPr, and EII, respectively. EIIANtr can be phosphorylated in vitro by adding PEP, EINtr, and NPr (114). Hence, the phosphorelay cascade in PTSNtr is analogous to that in the canonical sugar PTS (Fig. 8). In fact, there is evidence for cross talk between the canonical sugar PTS and the PTSNtr (114, 116). However, notable differences between the two systems include the following: first, the presence of a regulatory N-terminal GAF domain in E. coli EINtr (similar to that found in NifA), and second, the absence of an identified final acceptor of the phosphoryl group from EIIANtr. These findings support the hypothesis that PTSNtr has a regulatory function.
FIG 8.
Hypothetical relationship between nitrogen status, phosphorylation of EIIANtr, and nitrogen metabolism. Ammonium limitation increases the intracellular 2-OG/glutamine ratio, enhancing EINtr phosphorylation and phosphorelay to EIIANtr, which accumulates in the phosphorylated form. P-EIIANtr does not inhibit Trk activity, resulting in an accumulation of intracellular K+ and thereby favoring the binding of σS to RNA polymerase and the expression of genes involved in the general starvation response. This scenario is likely to occur under conditions of high extracellular K+, where Trk is active and Kdp expression is repressed.
The genes encoding the PTSNtr components, ptsN and ptsO, were first identified in Klebsiella pneumoniae and in E. coli, adjacent to the rpoN gene, which encodes the alternative RNA polymerase sigma factor σ54, required for transcription of nitrogen assimilation genes (114, 117–119). Similar genomic contexts are found in other proteobacteria (102), suggesting that PTSNtr could be involved in the regulation of nitrogen metabolism by controlling σ54 activity (117). However, the effect of a ptsN knockout on the activity of σ54-dependent promoters is at most modest (114, 117, 120). Furthermore, deletion of genes encoding the PTSNtr caused no growth phenotype or defect in nitrogen utilization. Some of the phenotypes attributed to ptsN mutations in early studies were traced to an ilvG frameshift mutation present in some E. coli strains (114, 121–123).
Regulation of PTSNtr phosphorylation by 2-OG.
Recent studies by Seok and colleagues support a link between PTSNtr and nitrogen regulation. Using an in vitro-reconstituted system, they demonstrated that 2-OG and glutamine (signals of the carbon and nitrogen statuses) reciprocally regulate the phosphorylation state of PTSNtr. Glutamine inhibits EINtr autophosphorylation, whereas 2-OG stimulates it, which is consistent with the accumulation of unphosphorylated EIIANtr under ammonium-sufficient conditions in vivo (124). The binding sites for glutamine and 2-OG were mapped to the GAF domain of EINtr (124). However, glutamine, not 2-OG, was found to bind the GAF domain of EINtr from Sinorhizobium meliloti (125). Hence, glutamine sensing might be a primary function of EINtr. Although these studies help to explain how nitrogen signals control the PTSNtr phosphorelay, a direct connection between the PTSNtr phosphorylation status and nitrogen regulation remains to be determined. A variety of different cellular processes are controlled directly or indirectly by PTSNtr in various organisms (103, 115), but none of these are directly related to nitrogen metabolic pathways.
In E. coli, the best-characterized function of PTSNtr involves regulation of the expression and activity of K+ transporters (126, 127), a function also conserved in Rhizobium leguminosarum (128, 129). In E. coli, unphosphorylated EIIANtr has two effects on K+ accumulation: first, it binds to the low-affinity K+ transporter Trk and inhibits the accumulation of high intracellular K+ levels (126), and second, it activates transcription of the high-affinity K+ transporter KdpFABC through direct interaction with the potassium sensor kinase KdpD (127) (Fig. 8). Trk and Kdp are believed to be the major routes of K+ uptake in E. coli, with Km values for K+ of 1.5 mM and 10 μM, respectively (130, 131). It was speculated that PTSNtr would function to regulate intracellular K+ according to the carbon supply. When a good carbon source is available, EIIANtr would be dephosphorylated (due to cross talk with the canonical sugar PTS), resulting in increased Kdp expression with a consequent accumulation of K+. Intracellular K+ would, in turn, act as an allosteric activator of metabolic enzymes (127).
Although the studies described above point to a possible role for 2-OG in communicating the carbon status to the PTSNtr, the observation that glutamine binds to the GAF domain of EINtr suggests that this system is responsive to the nitrogen status (124, 125). In the following paragraph, we discuss a potential hypothesis in which the PTSNtr may signal nitrogen availability via regulation of potassium transport.
Transcriptome analysis revealed that the expression of many genes is affected by a ptsN deletion, with the most dramatic effect being upregulation of the σS regulon (132). It was concluded that K+ regulates the selectivity of RNA polymerase toward σ70 or σS such that, with high K+ levels, RNA polymerase preferentially binds σS (132). Induction of the σS (RpoS) regulon by E. coli enables acclimatization to nutrient starvation, including nitrogen deprivation (133–136). The signaling mechanism for σS regulon induction under nitrogen starvation conditions remains elusive (136), although RpoS activity is indirectly regulated by the Ntr regulatory system (137). Therefore, it is tempting to speculate that the PTSNtr may signal nitrogen starvation to activate the σS starvation response. With a low ammonium supply (high 2-OG and low glutamine levels), phosphorylated EIIANtr would accumulate, avoiding inhibition of Trk activity and resulting in increased intracellular K+ levels, thereby favoring an interaction between σS and RNA polymerase (Fig. 8). Such a scenario is likely to occur when extracellular K+ levels are relatively high, when Trk is active and Kdp expression is repressed (Fig. 8). The activity of RpoS is also affected by EIIANtr in A. vinelandii. The accumulation of unphosphorylated EIIANtr reduced the expression of the RpoS-dependent gene arpR (138). Although the connection between RpoS activity and EIIANtr has not been established in A. vinelandii, these data fit with the model suggested in Fig. 8. Accumulation of unphosphorylated EIIANtr would downregulate Trk activity, reducing the intracellular K+ level and, thereby, RpoS activity.
Other, Less-Characterized Prokaryotic 2-OG Sensor Proteins
The Bacillus subtilis transcriptional regulator GltC.
The expression of the B. subtilis gltAB genes, encoding the GOGAT enzyme (Fig. 1), is regulated by two transcription factors, GltC and TnrA (139). GltC is a member of the LysR family, whose DNA binding activity is typically affected by interaction with small molecules. It was observed that GltC activates gltAB transcription, which is enhanced in the presence of 2-OG and reduced in the presence of glutamate. These data support a model in which GltC acts as a sensor of the 2-OG/glutamate ratio (140).
The cyanobacterial transcriptional regulator CcmR.
Efficient carbon fixation through RubisCO in phototrophic cyanobacteria relies on the ability to concentrate inorganic carbon (Ci) near the RubisCO active site. High-affinity Ci transporter systems are induced upon Ci limitation. In Synechocystis sp., a LysR transcription factor, namely, CcmR, acts a repressor of some Ci-concentrating mechanisms (141). The DNA binding activity of CcmR is enhanced in the presence of 2-OG, NADP+, 2-phosphoglycolate, or ribulose bisphosphate (142).
The two-component 2-OG signaling system KguS/KguR in uropathogenic E. coli.
In a search for novel two-component signaling systems specifically present in uropathogenic E. coli, Cai and coworkers identified a novel regulatory pair, KguS/KguR, which is presumably responsive to 2-OG (143). Expression of the KguS/KguR pair is induced upon oxygen limitation, and as long as 2-OG is present, this activates the transcription of genes located on a pathogenicity island which are involved in the utilization of extracellular 2-OG as a carbon source under anaerobic conditions (143).
2-OG SIGNALING IN EUKARYOTES
As mentioned previously, nitrogen starvation leads to increased 2-OG levels in the unicellular eukaryote S. cerevisiae (19). Increasing evidence supports the view that 2-OG also acts as a conserved starvation signaling molecule in Metazoa (144). When the nematode Caenorhabditis elegans experiences starvation, 2-OG levels increase (144), and similar increases in 2-OG levels were observed in humans subjected to prolonged or acute exercise (145, 146). In animals, either starvation or exercise is expected to activate amino acid catabolism in order to provide carbon skeletons for anaplerosis, which could explain the increased 2-OG levels observed under these circumstances (144).
The addition of 2-OG to the culture medium increased the life span of C. elegans (144). In order to identify the sensor protein responsible for this effect, the drug affinity responsive target stability (DARTS) technique, which relies on increased resistance to proteolysis when a protein interacts with a small molecule (147), was used to detect 2-OG binding proteins. This analysis identified the β-subunit of the mitochondrial ATP synthase as the 2-OG binding target in both human cell lines and C. elegans. The binding of 2-OG to ATP synthase reduced the activity of the enzyme and mitochondrial oxygen consumption (144). These data support the view that 2-OG acts as an important signaling molecule in Metazoa, and this proposition is further supported by the identification of 2-OG as a ligand for a human G-coupled receptor, named either GPR99 or OXGR1 (148). This receptor is mostly expressed in the kidney and may regulate acid-base balance (149).
The importance of 2-OG as a signaling molecule has similarly long been recognized in algae and plants (150, 151), supported by the presence of chloroplast-located PII signal transduction proteins in these organisms. Indeed, both fatty acid and arginine biosynthesis pathways are regulated by 2-OG in A. thaliana, by controlling the interaction between PII and the acetyl-CoA carboxylase and the N-acetyl-glutamate kinase (NAGK) enzymes, respectively (59, 152, 153). Surprisingly, however, a recent study revealed that the structural features of PII proteins from members of the Brassicaceae, such as A. thaliana, are atypical compared with those found in most of the plant kingdom. In contrast to A. thaliana, green plant and algal PII proteins contain a plant-specific C-terminal extension that forms a novel loop in the structure, termed the Q loop, which confers a low-affinity glutamine binding site (154). In vitro studies with Chlamydomonas reinhardtii PII demonstrated that it binds NAGK in a glutamine-dependent manner, in addition to the 2-OG-regulated interaction. A recombinant form of A. thaliana PII containing a functional Q loop from C. reinhardtii exhibited glutamine dependency in the NAGK interaction, confirming that the plant-specific C-terminal extension mediates the glutamine response. These observations suggest that glutamine sensing is a conserved feature of PII signaling in most plant chloroplasts (154).
Fluctuations in 2-OG levels may affect the activity of enzymes that use 2-OG as the substrate. Enzymes belonging to the family of 2-OG-dependent dioxygenases (2-OGDO) are particularly worthy of mention in this context, since they are widespread in nature and have diverse metabolic functions, including regulation of secondary metabolism in plants (151) and the catalysis of both DNA and histone demethylation in animals (155). There is increasing evidence that 2-OG levels can influence the epigenetic landscape through regulation of chromatin structure and histone modifications, thus providing a link between cellular metabolism and cell proliferation and differentiation (156).
CONCLUDING REMARKS
The coordination of microbial metabolic pathways is mediated by fluctuations in the concentrations of key metabolites, which enable allosteric transfer of this information to sensor proteins, thereby generating the appropriated metabolic response (157). Examples of metabolites that act as key players in microbial signal transduction are glutamine, cAMP, and ppGpp. Each of these metabolites is dedicated to the transfer of information upon the availability of a specific class of nutrient, namely, nitrogen, carbon, and amino acids, respectively (157). The ability of these metabolites to cross-regulate unrelated metabolic pathways seems to be relatively minor. For example, in addition to its huge effect on carbon metabolism (102), cAMP influences nitrogen metabolism by regulating glnA and glnHPQ expression in E. coli via CRP (158, 159).
A signaling metabolite that is well suited to fulfill a broader signaling function and to coordinate both carbon and nitrogen metabolism is 2-OG. This compound is a key intermediate of the TCA cycle and is used as a carbon skeleton for nitrogen assimilation (Fig. 1). As such, 2-OG reflects the carbon and nitrogen balance (1, 157), a signaling characteristic which seems to be conserved throughout nature. Not surprisingly, the list of regulatory proteins and metabolic pathways controlled by 2-OG has expanded exponentially and now includes nitrogen- and carbon-dedicated metabolic pathways (Fig. 9). In prokaryotes, 2-OG regulates several transcriptional factors and PII protein activity (Fig. 9). The recent inclusion of PTSGlu, PTSNtr, and adenylate cyclase as targets of 2-OG has extended the regulatory repertoire of this molecule to an unprecedented level, raising 2-OG above cAMP in the signal transduction hierarchy (Fig. 9).
FIG 9.
Schematic representation of proteins and metabolic pathways whose activity is affected by 2-OG levels. The 2-OG level is influenced by the supply of carbon and nitrogen. In turn, intracellular fluctuations in 2-OG are perceived by various sensory proteins, including PII, the EI component, both PTSGlu and PTSNtr, and a range of transcriptional factors. In E. coli, 2-OG regulates adenylate cyclase (AC) through the action of PTSGlu and also through an as yet unidentified mechanism (question mark). In Metazoa, 2-OG levels directly affect the activity of ATP synthase.
Despite the emergence of 2-OG as a master signaling metabolite, a number of important questions remain to be answered. Although 2-OG is central to the interface between carbon and nitrogen metabolism, it is not always employed as the sole integrator of these signals. Indeed, under some conditions, this could be a dangerous physiological strategy, since the level of 2-OG might not always be representative of the carbon/nitrogen ratio. For example, under conditions of carbon starvation, a low concentration of 2-OG might not necessarily be indicative of nitrogen excess if the cell is also suffering from nitrogen starvation. The PII signal transduction proteins provide fail-safe integration of the carbon and nitrogen statuses through directly sensing the 2-OG concentration and also by sensing glutamine, either directly through the Q linker or indirectly via posttranslational modifications. Another well-suited module for integrating the carbon and nitrogen statuses is the E. coli PTSNtr, as its phosphorelay cascade is modulated by both 2-OG and glutamine (124). However, despite several reported functions of PTSNtr in different model bacteria, a direct connection between PTSNtr and regulation of a specific metabolic pathway remains elusive. Moreover, S. meliloti PTSNtr binds only glutamine, suggesting that in this case the primary function is to sense the nitrogen status.
Although 2-OG appears to play an overarching role in carbon sensing through modulating the level of cAMP, the question remains as to why there is apparent functional redundancy in the control circuitry. Since 2-OG can apparently bypass the PTSGlu to regulate adenylyl cyclase activity, why is there a need for a PTS-dependent route for controlling AC in response to 2-OG (Fig. 9)? The PTS-dependent route is dependent on relatively high concentrations of 2-OG, as the Kd for binding to the C-terminal domain of EI is ∼2 mM. It is possible that the primary function of this route is feedback inhibition of glucose transport and that the effect on AC activity is secondary. This is one case where 2-OG clearly acts to integrate signals of carbon and nitrogen regulation when, for example, a sudden addition of excess nitrogen results in the depletion of carbon skeletons, which is homeostatically compensated by increased glycolytic flux (see Fig. 7C in reference 108). In contrast, the PTS-independent route of inhibition of AC activity by 2-OG (112) explains why non-PTS carbon sources can influence CCR. However, a mechanistic understanding of this newly discovered role for 2-OG is lacking. We still do not know, for example, which protein is the direct target for 2-OG in this case and the affinity of its interaction with this target, let alone the mechanism whereby adenylyl cyclase activity is regulated.
Even more enigmatic is the physiological role of 2-OG (and glutamine) in controlling PTSNtr activity in E. coli. While this could maintain the linkage between nitrogen availability and σS activity via accumulation of intracellular potassium (Fig. 8), other functions are also plausible. One potential hypothesis is that PTSNtr controls nonspecific uptake of ammonium by the potassium transporters in response to nitrogen availability. Ammonium (NH4+) is the preferred nitrogen source for E. coli, and NH4+ and K+ have similar charges, ionic radii, and hydration shells (160). Hence, K+ and NH4+ transporters from various sources do allow significant amounts of these similar ions to permeate (160–165). In Saccharomyces cerevisiae, ammonium becomes toxic when cells are cultured with low K+ levels, and such toxicity is apparently caused by ammonium uptake via potassium transporters (163). When E. coli is cultured in media containing low potassium levels (which favors Kdp activity), the presence of high ammonium concentrations increases the rate of oxygen and glucose consumption by a wild-type strain but not an isogenic kdp null strain (161). These results were interpreted as nonspecific uptake of NH4+ by the Kdp transporter resulting in a transient increase in the intracellular ammonium concentration, followed by leakage of NH3 out of the cells via passive diffusion across the cell membrane (161). This would result in a Gibbs energy-wasting futile cycle, explaining the increase in cellular energy needs in the wild-type strain (166). Although ammonium toxicity has not been documented for E. coli, tight control of ammonium uptake is vital to restrict the energetic cost of futile cycling (167). Consequently, the activity of the ammonium transporter AmtB is tightly regulated in response to the external ammonium concentration, through the formation of a protein complex with the dedicated PII-like protein GlnK (54). Since nonspecific ammonium uptake by K+ transporters will result in unrestricted futile cycling, we favor the hypothesis that the PTSNtr may also participate in the control of nonspecific ammonium transport, particularly through the low-affinity K+ transporter Trk, which is likely to exhibit enhanced transport of noncognate ions. According to this scenario, nonphosphorylated EIIANtr would accumulate under conditions of ammonium excess, inhibiting Trk activity and enhancing the expression of Kdp, which is expected to be more efficient than Trk in discriminating between K+ and NH4+. However, there is little evidence at present to support this hypothesis.
Given the signaling properties of 2-OG, it is highly likely that other regulatory proteins responsive to 2-OG remain to be described. The deployment of the DARTS technique to successfully identify ATP synthase as a 2-OG binding target in eukaryotes (144) provides an elegant example of how this field is likely to move ahead in the future. There is already strong evidence to support the notion that 2-OG acts as a master regulatory metabolite in eukaryotes, a view already anticipated by the presence of PII proteins in algae and plants. The recent development of 2-OG-specific affinity columns (168) may help to identify novel 2-OG sensors and extend the number of metabolic pathways in which this exciting regulatory metabolite plays a role.
ACKNOWLEDGMENTS
L.F.H. acknowledges financial support from CNPq, INCT, and Fundação Araucária. R.D. acknowledges support from the Biotechnology and Biological Sciences Research Council (BBSRC).
We are grateful to Mike Merrick (John Innes Centre) and Karl Forchhammer (Tubingen University) for helpful comments and discussions.
Biographies

Luciano F. Huergo studied biology for his undergraduate degree at the Universidade Federal do Paraná, Brazil, and obtained his Ph.D. in biochemistry at the Universidade Federal do Paraná in 2006. He worked as a visiting scientist at John Innes Centre, United Kingdom (2005), and at the Institute for Glycomics at Griffith University, Australia (2013). From 2008 to 2015, he was a professor at the Universidade Federal do Paraná in Curitiba, Brazil. In 2015, he moved to the Setor Litoral of Universidade Federal do Paraná in Matinhos, Brazil, where he is currently establishing a new microbiology and molecular biology lab. The major focus of his current research is the biology of PII proteins and the regulation of bacterial metabolism.

Ray Dixon is Research Group Leader at the John Innes Centre in Norwich, United Kingdom. He studied microbiology for his undergraduate degree at the University of Reading and obtained a D.Phil. in microbial genetics at the University of Sussex in 1973. He remained at the Unit of Nitrogen Fixation, University of Sussex, to lead a research group focusing on the molecular biology of nitrogen fixation before moving to his current position in Norwich in 1995. The major aim of his current research is to understand how nitrogen fixation is regulated in response to the oxygen, carbon, and fixed nitrogen status.
REFERENCES
- 1.Commichau FM, Forchhammer K, Stulke J. 2006. Regulatory links between carbon and nitrogen metabolism. Curr Opin Microbiol 9:167–172. doi: 10.1016/j.mib.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Tian J, Bryk R, Shi S, Erdjument-Bromage H, Tempst P, Nathan C. 2005. Mycobacterium tuberculosis appears to lack alpha-ketoglutarate dehydrogenase and encodes pyruvate dehydrogenase in widely separated genes. Mol Microbiol 57:859–868. doi: 10.1111/j.1365-2958.2005.04741.x. [DOI] [PubMed] [Google Scholar]
- 3.Zhang S, Bryant DA. 2011. The tricarboxylic acid cycle in cyanobacteria. Science 334:1551–1553. doi: 10.1126/science.1210858. [DOI] [PubMed] [Google Scholar]
- 4.Xiong W, Brune D, Vermaas WF. 2014. The gamma-aminobutyric acid shunt contributes to closing the tricarboxylic acid cycle in Synechocystis sp. PCC 6803. Mol Microbiol 93:786–796. doi: 10.1111/mmi.12699. [DOI] [PubMed] [Google Scholar]
- 5.Reitzer L. 2003. Nitrogen assimilation and global regulation in Escherichia coli. Annu Rev Microbiol 57:155–176. doi: 10.1146/annurev.micro.57.030502.090820. [DOI] [PubMed] [Google Scholar]
- 6.Magasanik B. 1982. Genetic control in nitrogen assimilation in bacteria. Annu Rev Genet 16:135–168. doi: 10.1146/annurev.ge.16.120182.001031. [DOI] [PubMed] [Google Scholar]
- 7.Sharkey MA, Engel PC. 2008. Apparent negative co-operativity and substrate inhibition in overexpressed glutamate dehydrogenase from Escherichia coli. FEMS Microbiol Lett 281:132–139. doi: 10.1111/j.1574-6968.2008.01086.x. [DOI] [PubMed] [Google Scholar]
- 8.Alibhai M, Villafranca JJ. 1994. Kinetic and mutagenic studies of the role of the active site residues Asp-50 and Glu-327 of Escherichia coli glutamine synthetase. Biochemistry 33:682–686. doi: 10.1021/bi00169a008. [DOI] [PubMed] [Google Scholar]
- 9.Yuan J, Doucette CD, Fowler WU, Feng XJ, Piazza M, Rabitz HA, Wingreen NS, Rabinowitz JD. 2009. Metabolomics-driven quantitative analysis of ammonia assimilation in E. coli. Mol Syst Biol 5:302. doi: 10.1038/msb.2009.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mantsala P, Zalkin H. 1976. Glutamate synthase. Properties of the glutamine-dependent activity. J Biol Chem 251:3294–3299. [PubMed] [Google Scholar]
- 11.Veronese FM, Boccu E, Conventi L. 1975. Glutamate dehydrogenase from Escherichia coli: induction, purification and properties of the enzyme. Biochim Biophys Acta 377:217–228. doi: 10.1016/0005-2744(75)90304-6. [DOI] [PubMed] [Google Scholar]
- 12.Bennett BD, Yuan J, Kimball EH, Rabinowitz JD. 2008. Absolute quantitation of intracellular metabolite concentrations by an isotope ratio-based approach. Nat Protoc 3:1299–1311. doi: 10.1038/nprot.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan D, Lenz P, Hwa T. 2011. Overcoming fluctuation and leakage problems in the quantification of intracellular 2-oxoglutarate levels in Escherichia coli. Appl Environ Microbiol 77:6763–6771. doi: 10.1128/AEM.05257-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rabinowitz JD, Kimball E. 2007. Acidic acetonitrile for cellular metabolome extraction from Escherichia coli. Anal Chem 79:6167–6173. doi: 10.1021/ac070470c. [DOI] [PubMed] [Google Scholar]
- 15.Cayley S, Lewis BA, Guttman HJ, Record MT Jr. 1991. Characterization of the cytoplasm of Escherichia coli K-12 as a function of external osmolarity. Implications for protein-DNA interactions in vivo. J Mol Biol 222:281–300. [DOI] [PubMed] [Google Scholar]
- 16.Senior PJ. 1975. Regulation of nitrogen metabolism in Escherichia coli and Klebsiella aerogenes: studies with the continuous culture technique. J Bacteriol 123:407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reyes-Ramirez F, Little R, Dixon R. 2001. Role of Escherichia coli nitrogen regulatory genes in the nitrogen response of the Azotobacter vinelandii NifL-NifA complex. J Bacteriol 183:3076–3082. doi: 10.1128/JB.183.10.3076-3082.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Radchenko MV, Thornton J, Merrick M. 2010. Control of AmtB-GlnK complex formation by intracellular levels of ATP, ADP, and 2-oxoglutarate. J Biol Chem 285:31037–31045. doi: 10.1074/jbc.M110.153908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brauer MJ, Yuan J, Bennett BD, Lu W, Kimball E, Botstein D, Rabinowitz JD. 2006. Conservation of the metabolomic response to starvation across two divergent microbes. Proc Natl Acad Sci U S A 103:19302–19307. doi: 10.1073/pnas.0609508103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schumacher J, Behrends V, Pan Z, Brown DR, Heydenreich F, Lewis MR, Bennett MH, Razzaghi B, Komorowski M, Barahona M, Stumpf MP, Wigneshweraraj S, Bundy JG, Buck M. 2013. Nitrogen and carbon status are integrated at the transcriptional level by the nitrogen regulator NtrC in vivo. mBio 4:e00881-13. doi: 10.1128/mBio.00881-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zimmermann M, Sauer U, Zamboni N. 2014. Quantification and mass isotopomer profiling of alpha-keto acids in central carbon metabolism. Anal Chem 86:3232–3237. doi: 10.1021/ac500472c. [DOI] [PubMed] [Google Scholar]
- 22.Zhang C, Wei ZH, Ye BC. 2013. Quantitative monitoring of 2-oxoglutarate in Escherichia coli cells by a fluorescence resonance energy transfer-based biosensor. Appl Microbiol Biotechnol 97:8307–8316. doi: 10.1007/s00253-013-5121-5. [DOI] [PubMed] [Google Scholar]
- 23.Chen HL, Bernard CS, Hubert P, My L, Zhang CC. 2013. Fluorescence resonance energy transfer based on interaction of PII and PipX proteins provides a robust and specific biosensor for 2-oxoglutarate, a central metabolite and a signaling molecule. FEBS J doi: 10.1111/j.1742-4658.2013.12702.x.24428626. [DOI] [PubMed] [Google Scholar]
- 24.Luddecke J, Forchhammer K. 2013. From PII signaling to metabolite sensing: a novel 2-oxoglutarate sensor that details PII-NAGK complex formation. PLoS One 8:e83181. doi: 10.1371/journal.pone.0083181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dodsworth JA, Cady NC, Leigh JA. 2005. 2-Oxoglutarate and the PII homologues NifI1 and NifI2 regulate nitrogenase activity in cell extracts of Methanococcus maripaludis. Mol Microbiol 56:1527–1538. doi: 10.1111/j.1365-2958.2005.04621.x. [DOI] [PubMed] [Google Scholar]
- 26.Muro-Pastor MI, Reyes JC, Florencio FJ. 2001. Cyanobacteria perceive nitrogen status by sensing intracellular 2-oxoglutarate levels. J Biol Chem 276:38320–38328. [DOI] [PubMed] [Google Scholar]
- 27.Laurent S, Chen H, Bedu S, Ziarelli F, Peng L, Zhang CC. 2005. Nonmetabolizable analogue of 2-oxoglutarate elicits heterocyst differentiation under repressive conditions in Anabaena sp. PCC 7120. Proc Natl Acad Sci U S A 102:9907–9912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sauer U, Eikmanns BJ. 2005. The PEP-pyruvate-oxaloacetate node as the switch point for carbon flux distribution in bacteria. FEMS Microbiol Rev 29:765–794. doi: 10.1016/j.femsre.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Cozzone AJ, El-Mansi M. 2005. Control of isocitrate dehydrogenase catalytic activity by protein phosphorylation in Escherichia coli. J Mol Microbiol Biotechnol 9:132–146. doi: 10.1159/000089642. [DOI] [PubMed] [Google Scholar]
- 30.Seol W, Shatkin AJ. 1991. Escherichia coli kgtP encodes an alpha-ketoglutarate transporter. Proc Natl Acad Sci U S A 88:3802–3806. doi: 10.1073/pnas.88.9.3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang C, Ye BC. 2014. A single fluorescent protein-based sensor for in vivo 2-oxogluatarate detection in cell. Biosens Bioelectron 54:15–19. doi: 10.1016/j.bios.2013.10.038. [DOI] [PubMed] [Google Scholar]
- 32.Guo W, Cai LL, Zou HS, Ma WX, Liu XL, Zou LF, Li YR, Chen XB, Chen GY. 2012. Ketoglutarate transport protein KgtP is secreted through the type III secretion system and contributes to virulence in Xanthomonas oryzae pv. oryzae. Appl Environ Microbiol 78:5672–5681. doi: 10.1128/AEM.07997-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chubukov V, Sauer U. 2014. Environmental dependence of stationary-phase metabolism in Bacillus subtilis and Escherichia coli. Appl Environ Microbiol 80:2901–2909. doi: 10.1128/AEM.00061-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adler SP, Purich D, Stadtman ER. 1975. Cascade control of Escherichia coli glutamine synthetase. Properties of the PII regulatory protein and the uridylyltransferase-uridylyl-removing enzyme. J Biol Chem 250:6264–6272. [PubMed] [Google Scholar]
- 35.Sant'Anna F, Trentini D, Weber SD, Cecagno R, da Silva SC, Schrank I. 2009. The PII superfamily revised: a novel group and evolutionary insights. J Mol Evol 68:322–336. doi: 10.1007/s00239-009-9209-6. [DOI] [PubMed] [Google Scholar]
- 36.Huergo LF, Pedrosa FO, Muller-Santos M, Chubatsu LS, Monteiro RA, Merrick M, Souza EM. 2012. PII signal transduction proteins: pivotal players in post-translational control of nitrogenase activity. Microbiology 158:176–190. doi: 10.1099/mic.0.049783-0. [DOI] [PubMed] [Google Scholar]
- 37.Huergo LF, Chandra G, Merrick M. 2013. P(II) signal transduction proteins: nitrogen regulation and beyond. FEMS Microbiol Rev 37:251–283. doi: 10.1111/j.1574-6976.2012.00351.x. [DOI] [PubMed] [Google Scholar]
- 38.Forchhammer K. 2010. The network of P(II) signalling protein interactions in unicellular cyanobacteria. Adv Exp Med Biol 675:71–90. doi: 10.1007/978-1-4419-1528-3_5. [DOI] [PubMed] [Google Scholar]
- 39.Jiang P, Ninfa AJ. 2009. Sensation and signaling of alpha-ketoglutarate and adenylylate energy charge by the Escherichia coli PII signal transduction protein require cooperation of the three ligand-binding sites within the PII trimer. Biochemistry 48:11522–11531. doi: 10.1021/bi9011594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Truan D, Huergo LF, Chubatsu LS, Merrick M, Li XD, Winkler FK. 2010. A new P(II) protein structure identifies the 2-oxoglutarate binding site. J Mol Biol 400:531–539. doi: 10.1016/j.jmb.2010.05.036. [DOI] [PubMed] [Google Scholar]
- 41.Truan D, Bjelic S, Li XD, Winkler FK. 2014. Structure and thermodynamics of effector molecule binding to the nitrogen signal transduction PII protein GlnZ from Azospirillum brasilense. J Mol Biol 426:2783–2799. doi: 10.1016/j.jmb.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 42.Jiang P, Peliska JA, Ninfa AJ. 1998. Enzymological characterization of the signal-transducing uridylyltransferase/uridylyl-removing enzyme (EC 2.7.7.59) of Escherichia coli and its interaction with the PII protein. Biochemistry 37:12782–12794. doi: 10.1021/bi980667m. [DOI] [PubMed] [Google Scholar]
- 43.Jiang P, Ninfa AJ. 2007. Escherichia coli PII signal transduction protein controlling nitrogen assimilation acts as a sensor of adenylate energy charge in vitro. Biochemistry 46:12979–12996. doi: 10.1021/bi701062t. [DOI] [PubMed] [Google Scholar]
- 44.Fokina O, Chellamuthu VR, Forchhammer K, Zeth K. 2010. Mechanism of 2-oxoglutarate signaling by the Synechococcus elongatus PII signal transduction protein. Proc Natl Acad Sci U S A 107:19760–19765. doi: 10.1073/pnas.1007653107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Little R, Colombo V, Leech A, Dixon R. 2002. Direct interaction of the NifL regulatory protein with the GlnK signal transducer enables the Azotobacter vinelandii NifL-NifA regulatory system to respond to conditions replete for nitrogen. J Biol Chem 277:15472–15481. doi: 10.1074/jbc.M112262200. [DOI] [PubMed] [Google Scholar]
- 46.da Rocha RA, Weschenfelder TA, de Castilhos F, de Souza EM, Huergo LF, Mitchell DA. 2013. Mathematical model of the binding of allosteric effectors to the Escherichia coli PII signal transduction protein GlnB. Biochemistry 52:2683–2693. doi: 10.1021/bi301659r. [DOI] [PubMed] [Google Scholar]
- 47.Gerhardt EC, Araujo LM, Ribeiro RR, Chubatsu LS, Scarduelli M, Rodrigues TE, Monteiro RA, Pedrosa FO, Souza EM, Huergo LF. 2012. Influence of the ADP/ATP ratio, 2-oxoglutarate and divalent ions on Azospirillum brasilense PII protein signalling. Microbiology 158:1656–1663. doi: 10.1099/mic.0.058446-0. [DOI] [PubMed] [Google Scholar]
- 48.Radchenko MV, Thornton J, Merrick M. 2013. PII signal transduction proteins are ATPases whose activity is regulated by 2-oxoglutarate. Proc Natl Acad Sci U S A 110:12948–12953. doi: 10.1073/pnas.1304386110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Merrick M. 2014. Post-translational modification of P II signal transduction proteins. Front Microbiol 5:763. doi: 10.3389/fmicb.2014.00763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jiang P, Peliska JA, Ninfa AJ. 1998. The regulation of Escherichia coli glutamine synthetase revisited: role of 2-ketoglutarate in the regulation of glutamine synthetase adenylylation state. Biochemistry 37:12802–12810. doi: 10.1021/bi980666u. [DOI] [PubMed] [Google Scholar]
- 51.Teixeira PF, Jonsson A, Frank M, Wang H, Nordlund S. 2008. Interaction of the signal transduction protein GlnJ with the cellular targets AmtB1, GlnE and GlnD in Rhodospirillum rubrum: dependence on manganese, 2-oxoglutarate and the ADP/ATP ratio. Microbiology 154:2336–2347. doi: 10.1099/mic.0.2008/017533-0. [DOI] [PubMed] [Google Scholar]
- 52.Bonatto AC, Souza EM, Oliveira MA, Monteiro RA, Chubatsu LS, Huergo LF, Pedrosa FO. 2012. Uridylylation of Herbaspirillum seropedicae GlnB and GlnK proteins is differentially affected by ATP, ADP and 2-oxoglutarate in vitro. Arch Microbiol 194:643–652. doi: 10.1007/s00203-012-0799-9. [DOI] [PubMed] [Google Scholar]
- 53.Leigh JA, Dodsworth JA. 2007. Nitrogen regulation in bacteria and archaea. Annu Rev Microbiol 61:349–377. doi: 10.1146/annurev.micro.61.080706.093409. [DOI] [PubMed] [Google Scholar]
- 54.Coutts G, Thomas G, Blakey D, Merrick M. 2002. Membrane sequestration of the signal transduction protein GlnK by the ammonium transporter AmtB. EMBO J 21:536–545. doi: 10.1093/emboj/21.4.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jiang P, Ninfa AJ. 1999. Regulation of autophosphorylation of Escherichia coli nitrogen regulator II by the PII signal transduction protein. J Bacteriol 181:1906–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gerhardt EC, Rodrigues TE, Muller-Santos M, Pedrosa FO, Souza EM, Forchhammer K, Huergo LF. 2015. The bacterial signal transduction protein GlnB regulates the committed step in fatty acid biosynthesis by acting as a dissociable regulatory subunit of acetyl-CoA carboxylase. Mol Microbiol 95:1025–1035. doi: 10.1111/mmi.12912. [DOI] [PubMed] [Google Scholar]
- 57.Cronan JE Jr, Waldrop GL. 2002. Multi-subunit acetyl-CoA carboxylases. Prog Lipid Res 41:407–435. doi: 10.1016/S0163-7827(02)00007-3. [DOI] [PubMed] [Google Scholar]
- 58.Tong L. 2013. Structure and function of biotin-dependent carboxylases. Cell Mol Life Sci 70:863–891. doi: 10.1007/s00018-012-1096-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Feria Bourrellier AB, Valot B, Guillot A, Ambard-Bretteville F, Vidal J, Hodges M. 2010. Chloroplast acetyl-CoA carboxylase activity is 2-oxoglutarate-regulated by interaction of PII with the biotin carboxyl carrier subunit. Proc Natl Acad Sci U S A 107:502–507. doi: 10.1073/pnas.0910097107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rodrigues TE, Gerhardt EC, Oliveira MA, Chubatsu LS, Pedrosa FO, Souza EM, Souza GA, Muller-Santos M, Huergo LF. 2014. Search for novel targets of the PII signal transduction protein in bacteria identifies the BCCP component of acetyl-CoA carboxylase as a PII binding partner. Mol Microbiol 91:751–761. doi: 10.1111/mmi.12493. [DOI] [PubMed] [Google Scholar]
- 61.Baud S, Feria Bourrellier AB, Azzopardi M, Berger A, Dechorgnat J, Daniel-Vedele F, Lepiniec L, Miquel M, Rochat C, Hodges M, Ferrario-Mery S. 2010. PII is induced by WRINKLED1 and fine-tunes fatty acid composition in seeds of Arabidopsis thaliana. Plant J 64:291–303. doi: 10.1111/j.1365-313X.2010.04332.x. [DOI] [PubMed] [Google Scholar]
- 62.Dixon R, Kahn D. 2004. Genetic regulation of biological nitrogen fixation. Nat Rev Microbiol 2:621–631. doi: 10.1038/nrmicro954. [DOI] [PubMed] [Google Scholar]
- 63.Martinez-Argudo I, Little R, Shearer N, Johnson P, Dixon R. 2004. The NifL-NifA system: a multidomain transcriptional regulatory complex that integrates environmental signals. J Bacteriol 186:601–610. doi: 10.1128/JB.186.3.601-610.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Slavny P, Little R, Salinas P, Clarke TA, Dixon R. 2010. Quaternary structure changes in a second Per-Arnt-Sim domain mediate intramolecular redox signal relay in the NifL regulatory protein. Mol Microbiol 75:61–75. doi: 10.1111/j.1365-2958.2009.06956.x. [DOI] [PubMed] [Google Scholar]
- 65.Little R, Salinas P, Slavny P, Clarke TA, Dixon R. 2011. Substitutions in the redox-sensing PAS domain of the NifL regulatory protein define an inter-subunit pathway for redox signal transmission. Mol Microbiol 82:222–235. doi: 10.1111/j.1365-2958.2011.07812.x. [DOI] [PubMed] [Google Scholar]
- 66.Rudnick P, Kunz C, Gunatilaka MK, Hines ER, Kennedy C. 2002. Role of GlnK in NifL-mediated regulation of NifA activity in Azotobacter vinelandii. J Bacteriol 184:812–820. doi: 10.1128/JB.184.3.812-820.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Little R, Reyes-Ramirez F, Zhang Y, van Heeswijk WC, Dixon R. 2000. Signal transduction to the Azotobacter vinelandii NifL-NifA regulatory system is influenced directly by interaction with 2-oxoglutarate and the PII regulatory protein. EMBO J 19:6041–6050. doi: 10.1093/emboj/19.22.6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Little R, Dixon R. 2003. The amino-terminal GAF domain of Azotobacter vinelandii NifA binds 2-oxoglutarate to resist inhibition by NifL under nitrogen-limiting conditions. J Biol Chem 278:28711–28718. doi: 10.1074/jbc.M301992200. [DOI] [PubMed] [Google Scholar]
- 69.Perry S, Shearer N, Little R, Dixon R. 2005. Mutational analysis of the nucleotide-binding domain of the anti-activator NifL. J Mol Biol 346:935–949. doi: 10.1016/j.jmb.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 70.Martinez-Argudo I, Little R, Dixon R. 2004. Role of the amino-terminal GAF domain of the NifA activator in controlling the response to the antiactivator protein NifL. Mol Microbiol 52:1731–1744. doi: 10.1111/j.1365-2958.2004.04089.x. [DOI] [PubMed] [Google Scholar]
- 71.Chen S, Liu L, Zhou X, Elmerich C, Ji-Lun L. 2005. Functional analysis of the GAF domain of NifA in Azospirillum brasilense: effects of Tyr-Phe mutations on NifA and its interaction with GlnB. Mol Genet Genomics 273:415–422. doi: 10.1007/s00438-005-1146-5. [DOI] [PubMed] [Google Scholar]
- 72.Zou X, Zhu Y, Pohlmann EL, Li J, Zhang Y, Roberts GP. 2008. Identification and functional characterization of NifA variants that are independent of GlnB activation in the photosynthetic bacterium Rhodospirillum rubrum. Microbiology 154:2689–2699. doi: 10.1099/mic.0.2008/019406-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Oliveira MA, Aquino B, Bonatto AC, Huergo LF, Chubatsu LS, Pedrosa FO, Souza EM, Dixon R, Monteiro RA. 2012. Interaction of GlnK with the GAF domain of Herbaspirillum seropedicae NifA mediates NH4+-regulation. Biochimie 94:1041–1047. doi: 10.1016/j.biochi.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 74.Michel-Reydellet N, Kaminski PA. 1999. Azorhizobium caulinodans PII and GlnK proteins control nitrogen fixation and ammonia assimilation. J Bacteriol 181:2655–2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Drepper T, Gross S, Yakunin AF, Hallenbeck PC, Masepohl B, Klipp W. 2003. Role of GlnB and GlnK in ammonium control of both nitrogenase systems in the phototrophic bacterium Rhodobacter capsulatus. Microbiology 149:2203–2212. doi: 10.1099/mic.0.26235-0. [DOI] [PubMed] [Google Scholar]
- 76.Vega-Palas MA, Flores E, Herrero A. 1992. NtcA, a global nitrogen regulator from the cyanobacterium Synechococcus that belongs to the Crp family of bacterial regulators. Mol Microbiol 6:1853–1859. doi: 10.1111/j.1365-2958.1992.tb01357.x. [DOI] [PubMed] [Google Scholar]
- 77.Espinosa J, Rodriguez-Mateos F, Salinas P, Lanza VF, Dixon R, de la Cruz F, Contreras A. 2014. PipX, the coactivator of NtcA, is a global regulator in cyanobacteria. Proc Natl Acad Sci U S A 111:E2423–E2430. doi: 10.1073/pnas.1404097111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Picossi S, Flores E, Herrero A. 2014. ChIP analysis unravels an exceptionally wide distribution of DNA binding sites for the NtcA transcription factor in a heterocyst-forming cyanobacterium. BMC Genomics 15:22. doi: 10.1186/1471-2164-15-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tanigawa R, Shirokane M, Maeda SS, Omata T, Tanaka K, Takahashi H. 2002. Transcriptional activation of NtcA-dependent promoters of Synechococcus sp. PCC 7942 by 2-oxoglutarate in vitro. Proc Natl Acad Sci U S A 99:4251–4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vazquez-Bermudez MF, Herrero A, Flores E. 2002. 2-Oxoglutarate increases the binding affinity of the NtcA (nitrogen control) transcription factor for the Synechococcus glnA promoter. FEBS Lett 512:71–74. doi: 10.1016/S0014-5793(02)02219-6. [DOI] [PubMed] [Google Scholar]
- 81.Valladares A, Flores E, Herrero A. 2008. Transcription activation by NtcA and 2-oxoglutarate of three genes involved in heterocyst differentiation in the cyanobacterium Anabaena sp. strain PCC 7120. J Bacteriol 190:6126–6133. doi: 10.1128/JB.00787-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Herrero A, Muro-Pastor AM, Flores E. 2001. Nitrogen control in cyanobacteria. J Bacteriol 183:411–425. doi: 10.1128/JB.183.2.411-425.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee DJ, Minchin SD, Busby SJ. 2012. Activating transcription in bacteria. Annu Rev Microbiol 66:125–152. doi: 10.1146/annurev-micro-092611-150012. [DOI] [PubMed] [Google Scholar]
- 84.Llacer JL, Espinosa J, Castells MA, Contreras A, Forchhammer K, Rubio V. 2010. Structural basis for the regulation of NtcA-dependent transcription by proteins PipX and PII. Proc Natl Acad Sci U S A 107:15397–15402. doi: 10.1073/pnas.1007015107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhao MX, Jiang YL, He YX, Chen YF, Teng YB, Chen Y, Zhang CC, Zhou CZ. 2010. Structural basis for the allosteric control of the global transcription factor NtcA by the nitrogen starvation signal 2-oxoglutarate. Proc Natl Acad Sci U S A 107:12487–12492. doi: 10.1073/pnas.1001556107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Popovych N, Tzeng SR, Tonelli M, Ebright RH, Kalodimos CG. 2009. Structural basis for cAMP-mediated allosteric control of the catabolite activator protein. Proc Natl Acad Sci U S A 106:6927–6932. doi: 10.1073/pnas.0900595106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sharma H, Yu S, Kong J, Wang J, Steitz TA. 2009. Structure of apo-CAP reveals that large conformational changes are necessary for DNA binding. Proc Natl Acad Sci U S A 106:16604–16609. doi: 10.1073/pnas.0908380106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Forcada-Nadal A, Forchhammer K, Rubio V. 2014. SPR analysis of promoter binding of Synechocystis PCC6803 transcription factors NtcA and CRP suggests cross-talk and sheds light on regulation by effector molecules. FEBS Lett 588:2270–2276. doi: 10.1016/j.febslet.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 89.Liu X, Wang Y, Laurini E, Posocco P, Chen H, Ziarelli F, Janicki A, Qu F, Fermeglia M, Pricl S, Zhang CC, Peng L. 2013. Structural requirements of 2-oxoglutaric acid analogues to mimic its signaling function. Org Lett 15:4662–4665. doi: 10.1021/ol401914z. [DOI] [PubMed] [Google Scholar]
- 90.Espinosa J, Forchhammer K, Burillo S, Contreras A. 2006. Interaction network in cyanobacterial nitrogen regulation: PipX, a protein that interacts in a 2-oxoglutarate dependent manner with PII and NtcA. Mol Microbiol 61:457–469. doi: 10.1111/j.1365-2958.2006.05231.x. [DOI] [PubMed] [Google Scholar]
- 91.Burillo S, Luque I, Fuentes I, Contreras A. 2004. Interactions between the nitrogen signal transduction protein PII and N-acetyl glutamate kinase in organisms that perform oxygenic photosynthesis. J Bacteriol 186:3346–3354. doi: 10.1128/JB.186.11.3346-3354.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zeth K, Fokina O, Forchhammer K. 2014. Structural basis and target-specific modulation of ADP sensing by the Synechococcus elongatus PII signaling protein. J Biol Chem 289:8960–8972. doi: 10.1074/jbc.M113.536557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Espinosa J, Forchhammer K, Contreras A. 2007. Role of the Synechococcus PCC 7942 nitrogen regulator protein PipX in NtcA-controlled processes. Microbiology 153:711–718. doi: 10.1099/mic.0.2006/003574-0. [DOI] [PubMed] [Google Scholar]
- 94.Zhao MX, Jiang YL, Xu BY, Chen Y, Zhang CC, Zhou CZ. 2010. Crystal structure of the cyanobacterial signal transduction protein PII in complex with PipX. J Mol Biol 402:552–559. doi: 10.1016/j.jmb.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 95.Lie TJ, Leigh JA. 2003. A novel repressor of nif and glnA expression in the methanogenic archaeon Methanococcus maripaludis. Mol Microbiol 47:235–246. [DOI] [PubMed] [Google Scholar]
- 96.Lie TJ, Wood GE, Leigh JA. 2005. Regulation of nif expression in Methanococcus maripaludis: roles of the euryarchaeal repressor NrpR, 2-oxoglutarate, and two operators. J Biol Chem 280:5236–5241. doi: 10.1074/jbc.M411778200. [DOI] [PubMed] [Google Scholar]
- 97.Lie TJ, Dodsworth JA, Nickle DC, Leigh JA. 2007. Diverse homologues of the archaeal repressor NrpR function similarly in nitrogen regulation. FEMS Microbiol Lett 271:281–288. doi: 10.1111/j.1574-6968.2007.00726.x. [DOI] [PubMed] [Google Scholar]
- 98.Weidenbach K, Ehlers C, Kock J, Ehrenreich A, Schmitz RA. 2008. Insights into the NrpR regulon in Methanosarcina mazei Go1. Arch Microbiol 190:319–332. doi: 10.1007/s00203-008-0369-3. [DOI] [PubMed] [Google Scholar]
- 99.Weidenbach K, Ehlers C, Kock J, Schmitz RA. 2010. NrpRII mediates contacts between NrpRI and general transcription factors in the archaeon Methanosarcina mazei Go1. FEBS J 277:4398–4411. doi: 10.1111/j.1742-4658.2010.07821.x. [DOI] [PubMed] [Google Scholar]
- 100.Wisedchaisri G, Dranow DM, Lie TJ, Bonanno JB, Patskovsky Y, Ozyurt SA, Sauder JM, Almo SC, Wasserman SR, Burley SK, Leigh JA, Gonen T. 2010. Structural underpinnings of nitrogen regulation by the prototypical nitrogen-responsive transcriptional factor NrpR. Structure 18:1512–1521. doi: 10.1016/j.str.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cases I, Velazquez F, de Lorenzo V. 2007. The ancestral role of the phosphoenolpyruvate-carbohydrate phosphotransferase system (PTS) as exposed by comparative genomics. Res Microbiol 158:666–670. doi: 10.1016/j.resmic.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 102.Deutscher J, Francke C, Postma PW. 2006. How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria. Microbiol Mol Biol Rev 70:939–1031. doi: 10.1128/MMBR.00024-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Deutscher J, Ake FM, Derkaoui M, Zebre AC, Cao TN, Bouraoui H, Kentache T, Mokhtari A, Milohanic E, Joyet P. 2014. The bacterial phosphoenolpyruvate:carbohydrate phosphotransferase system: regulation by protein phosphorylation and phosphorylation-dependent protein-protein interactions. Microbiol Mol Biol Rev 78:231–256. doi: 10.1128/MMBR.00001-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Weigel N, Kukuruzinska MA, Nakazawa A, Waygood EB, Roseman S. 1982. Sugar transport by the bacterial phosphotransferase system. Phosphoryl transfer reactions catalyzed by enzyme I of Salmonella typhimurium. J Biol Chem 257:14477–14491. [PubMed] [Google Scholar]
- 105.Postma PW, Lengeler JW, Jacobson GR. 1993. Phosphoenolpyruvate:carbohydrate phosphotransferase systems in bacteria. Microbiol Rev 57:543–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Park YH, Lee BR, Seok YJ, Peterkofsky A. 2006. In vitro reconstitution of catabolite repression in Escherichia coli. J Biol Chem 281:6448–6454. doi: 10.1074/jbc.M512672200. [DOI] [PubMed] [Google Scholar]
- 107.Gorke B, Stulke J. 2008. Carbon catabolite repression in bacteria: many ways to make the most out of nutrients. Nat Rev Microbiol 6:613–624. doi: 10.1038/nrmicro1932. [DOI] [PubMed] [Google Scholar]
- 108.Doucette CD, Schwab DJ, Wingreen NS, Rabinowitz JD. 2011. Alpha-ketoglutarate coordinates carbon and nitrogen utilization via enzyme I inhibition. Nat Chem Biol 16:894–901. doi: 10.1038/nchembio.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Venditti V, Ghirlando R, Clore GM. 2013. Structural basis for enzyme I inhibition by alpha-ketoglutarate. ACS Chem Biol 8:1232–1240. doi: 10.1021/cb400027q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Venditti V, Tugarinov V, Schwieters CD, Grishaev A, Clore GM. 2015. Large interdomain rearrangement triggered by suppression of micro- to millisecond dynamics in bacterial enzyme I. Nat Commun 6:5960. doi: 10.1038/ncomms6960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Daniel J, Danchin A. 1986. 2-Ketoglutarate as a possible regulatory metabolite involved in cyclic AMP-dependent catabolite repression in Escherichia coli K12. Biochimie 68:303–310. doi: 10.1016/S0300-9084(86)80027-X. [DOI] [PubMed] [Google Scholar]
- 112.You C, Okano H, Hui S, Zhang Z, Kim M, Gunderson CW, Wang YP, Lenz P, Yan D, Hwa T. 2013. Coordination of bacterial proteome with metabolism by cyclic AMP signalling. Nature 500:301–306. doi: 10.1038/nature12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rabinowitz JD, Silhavy TJ. 2013. Systems biology: metabolite turns master regulator. Nature 500:283–284. doi: 10.1038/nature12544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Powell BS, Court DL, Inada T, Nakamura Y, Michotey V, Cui X, Reizer A, Saier MH, Reizer J. 1995. Novel proteins of the phosphotransferase system encoded within the rpoN operon of Escherichia coli. J Biol Chem 270:4822–4839. doi: 10.1074/jbc.270.9.4822. [DOI] [PubMed] [Google Scholar]
- 115.Pfluger-Grau K, Gorke B. 2010. Regulatory roles of the bacterial nitrogen-related phosphotransferase system. Trends Microbiol 18:205–214. doi: 10.1016/j.tim.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 116.Zimmer B, Hillmann A, Gorke B. 2008. Requirements for the phosphorylation of the Escherichia coli EIIANtr protein in vivo. FEMS Microbiol Lett 286:96–102. doi: 10.1111/j.1574-6968.2008.01262.x. [DOI] [PubMed] [Google Scholar]
- 117.Merrick MJ, Coppard JR. 1989. Mutations in genes downstream of the rpoN gene (encoding σ54) of Klebsiella pneumoniae affect expression from σ54-dependent promoters. Mol Microbiol 3:1765–1775. doi: 10.1111/j.1365-2958.1989.tb00162.x. [DOI] [PubMed] [Google Scholar]
- 118.Reizer J, Reizer A, Merrick MJ, Plunkett G III, Rose DJ, Saier MH Jr. 1996. Novel phosphotransferase-encoding genes revealed by analysis of the Escherichia coli genome: a chimeric gene encoding an enzyme I homologue that possesses a putative sensory transduction domain. Gene 181:103–108. doi: 10.1016/S0378-1119(96)00481-7. [DOI] [PubMed] [Google Scholar]
- 119.Begley GS, Jacobson GR. 1994. Overexpression, phosphorylation, and growth effects of ORF162, a Klebsiella pneumoniae protein that is encoded by a gene linked to rpoN, the gene encoding σ54. FEMS Microbiol Lett 119:389–394. [DOI] [PubMed] [Google Scholar]
- 120.Ninfa AJ. 2011. Unnecessary signaling: poorly named? J Bacteriol 193:4571–4573. doi: 10.1128/JB.05682-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Reaves ML, Rabinowitz JD. 2011. Characteristic phenotypes associated with ptsN-null mutants in Escherichia coli K-12 are absent in strains with functional ilvG. J Bacteriol 193:4576–4581. doi: 10.1128/JB.00325-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lawther RP, Calhoun DH, Adams CW, Hauser CA, Gray J, Hatfield GW. 1981. Molecular basis of valine resistance in Escherichia coli K-12. Proc Natl Acad Sci U S A 78:922–925. doi: 10.1073/pnas.78.2.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Soupene E, van Heeswijk WC, Plumbridge J, Stewart V, Bertenthal D, Lee H, Prasad G, Paliy O, Charernnoppakul P, Kustu S. 2003. Physiological studies of Escherichia coli strain MG1655: growth defects and apparent cross-regulation of gene expression. J Bacteriol 185:5611–5626. doi: 10.1128/JB.185.18.5611-5626.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lee CR, Park YH, Kim M, Kim YR, Park S, Peterkofsky A, Seok YJ. 2013. Reciprocal regulation of the autophosphorylation of enzyme INtr by glutamine and alpha-ketoglutarate in Escherichia coli. Mol Microbiol 88:473–485. doi: 10.1111/mmi.12196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Goodwin RA, Gage DJ. 2014. Biochemical characterization of a nitrogen-type phosphotransferase system reveals that enzyme EI(Ntr) integrates carbon and nitrogen signaling in Sinorhizobium meliloti. J Bacteriol 196:1901–1907. doi: 10.1128/JB.01489-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lee CR, Cho SH, Yoon MJ, Peterkofsky A, Seok YJ. 2007. Escherichia coli enzyme IIANtr regulates the K+ transporter TrkA. Proc Natl Acad Sci U S A 104:4124–4129. doi: 10.1073/pnas.0609897104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Luttmann D, Heermann R, Zimmer B, Hillmann A, Rampp IS, Jung K, Gorke B. 2009. Stimulation of the potassium sensor KdpD kinase activity by interaction with the phosphotransferase protein IIA(Ntr) in Escherichia coli. Mol Microbiol 72:978–994. doi: 10.1111/j.1365-2958.2009.06704.x. [DOI] [PubMed] [Google Scholar]
- 128.Prell J, Mulley G, Haufe F, White JP, Williams A, Karunakaran R, Downie JA, Poole PS. 2012. The PTS(Ntr) system globally regulates ATP-dependent transporters in Rhizobium leguminosarum. Mol Microbiol 84:117–129. doi: 10.1111/j.1365-2958.2012.08014.x. [DOI] [PubMed] [Google Scholar]
- 129.Untiet V, Karunakaran R, Kramer M, Poole P, Priefer U, Prell J. 2013. ABC transport is inactivated by the PTS(Ntr) under potassium limitation in Rhizobium leguminosarum 3841. PLoS One 8:e64682. doi: 10.1371/journal.pone.0064682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Siebers A, Altendorf K. 1988. The K+-translocating Kdp-ATPase from Escherichia coli. Purification, enzymatic properties and production of complex- and subunit-specific antisera. Eur J Biochem 178:131–140. [DOI] [PubMed] [Google Scholar]
- 131.Rhoads DB, Waters FB, Epstein W. 1976. Cation transport in Escherichia coli. VIII. Potassium transport mutants. J Gen Physiol 67:325–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lee CR, Cho SH, Kim HJ, Kim M, Peterkofsky A, Seok YJ. 2010. Potassium mediates Escherichia coli enzyme IIA(Ntr)-dependent regulation of sigma factor selectivity. Mol Microbiol 78:1468–1483. doi: 10.1111/j.1365-2958.2010.07419.x. [DOI] [PubMed] [Google Scholar]
- 133.Gyaneshwar P, Paliy O, McAuliffe J, Jones A, Jordan MI, Kustu S. 2005. Lessons from Escherichia coli genes similarly regulated in response to nitrogen and sulfur limitation. Proc Natl Acad Sci U S A 102:3453–3458. doi: 10.1073/pnas.0500141102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gyaneshwar P, Paliy O, McAuliffe J, Popham DL, Jordan MI, Kustu S. 2005. Sulfur and nitrogen limitation in Escherichia coli K-12: specific homeostatic responses. J Bacteriol 187:1074–1090. doi: 10.1128/JB.187.3.1074-1090.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mandel MJ, Silhavy TJ. 2005. Starvation for different nutrients in Escherichia coli results in differential modulation of RpoS levels and stability. J Bacteriol 187:434–442. doi: 10.1128/JB.187.2.434-442.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Peterson CN, Mandel MJ, Silhavy TJ. 2005. Escherichia coli starvation diets: essential nutrients weigh in distinctly. J Bacteriol 187:7549–7553. doi: 10.1128/JB.187.22.7549-7553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zafar MA, Carabetta VJ, Mandel MJ, Silhavy TJ. 2014. Transcriptional occlusion caused by overlapping promoters. Proc Natl Acad Sci U S A 111:1557–1561. doi: 10.1073/pnas.1323413111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Muriel-Millan LF, Moreno S, Romero Y, Bedoya-Perez LP, Castaneda M, Segura D, Espin G. 2015. The unphosphorylated EIIA(Ntr) protein represses the synthesis of alkylresorcinols in Azotobacter vinelandii. PLoS One 10:e0117184. doi: 10.1371/journal.pone.0117184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gunka K, Commichau FM. 2012. Control of glutamate homeostasis in Bacillus subtilis: a complex interplay between ammonium assimilation, glutamate biosynthesis and degradation. Mol Microbiol 85:213–224. doi: 10.1111/j.1365-2958.2012.08105.x. [DOI] [PubMed] [Google Scholar]
- 140.Picossi S, Belitsky BR, Sonenshein AL. 2007. Molecular mechanism of the regulation of Bacillus subtilis gltAB expression by GltC. J Mol Biol 365:1298–1313. doi: 10.1016/j.jmb.2006.10.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Woodger FJ, Bryant DA, Price GD. 2007. Transcriptional regulation of the CO2-concentrating mechanism in a euryhaline, coastal marine cyanobacterium, Synechococcus sp. strain PCC 7002: role of NdhR/CcmR. J Bacteriol 189:3335–3347. doi: 10.1128/JB.01745-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Daley SM, Kappell AD, Carrick MJ, Burnap RL. 2012. Regulation of the cyanobacterial CO2-concentrating mechanism involves internal sensing of NADP+ and alpha-ketoglutarate levels by transcription factor CcmR. PLoS One 7:e41286. doi: 10.1371/journal.pone.0041286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cai W, Wannemuehler Y, Dell'anna G, Nicholson B, Barbieri NL, Kariyawasam S, Feng Y, Logue CM, Nolan LK, Li G. 2013. A novel two-component signaling system facilitates uropathogenic Escherichia coli's ability to exploit abundant host metabolites. PLoS Pathog 9:e1003428. doi: 10.1371/journal.ppat.1003428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chin RM, Fu X, Pai MY, Vergnes L, Hwang H, Deng G, Diep S, Lomenick B, Meli VS, Monsalve GC, Hu E, Whelan SA, Wang JX, Jung G, Solis GM, Fazlollahi F, Kaweeteerawat C, Quach A, Nili M, Krall AS, Godwin HA, Chang HR, Faull KF, Guo F, Jiang M, Trauger SA, Saghatelian A, Braas D, Christofk HR, Clarke CF, Teitell MA, Petrascheck M, Reue K, Jung ME, Frand AR, Huang J. 2014. The metabolite alpha-ketoglutarate extends lifespan by inhibiting ATP synthase and TOR. Nature 510:397–401. doi: 10.1038/nature13264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lewis GD, Farrell L, Wood MJ, Martinovic M, Arany Z, Rowe GC, Souza A, Cheng S, McCabe EL, Yang E, Shi X, Deo R, Roth FP, Asnani A, Rhee EP, Systrom DM, Semigran MJ, Vasan RS, Carr SA, Wang TJ, Sabatine MS, Clish CB, Gerszten RE. 2010. Metabolic signatures of exercise in human plasma. Sci Transl Med 2:33ra37. doi: 10.1126/scitranslmed.3001006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Brugnara L, Vinaixa M, Murillo S, Samino S, Rodriguez MA, Beltran A, Lerin C, Davison G, Correig X, Novials A. 2012. Metabolomics approach for analyzing the effects of exercise in subjects with type 1 diabetes mellitus. PLoS One 7:e40600. doi: 10.1371/journal.pone.0040600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lomenick B, Hao R, Jonai N, Chin RM, Aghajan M, Warburton S, Wang J, Wu RP, Gomez F, Loo JA, Wohlschlegel JA, Vondriska TM, Pelletier J, Herschman HR, Clardy J, Clarke CF, Huang J. 2009. Target identification using drug affinity responsive target stability (DARTS). Proc Natl Acad Sci U S A 106:21984–21989. doi: 10.1073/pnas.0910040106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.He W, Miao FJ, Lin DC, Schwandner RT, Wang Z, Gao J, Chen JL, Tian H, Ling L. 2004. Citric acid cycle intermediates as ligands for orphan G-protein-coupled receptors. Nature 429:188–193. doi: 10.1038/nature02488. [DOI] [PubMed] [Google Scholar]
- 149.Tokonami N, Morla L, Centeno G, Mordasini D, Ramakrishnan SK, Nikolaeva S, Wagner CA, Bonny O, Houillier P, Doucet A, Firsov D. 2013. α-Ketoglutarate regulates acid-base balance through an intrarenal paracrine mechanism. J Clin Invest 123:3166–3171. doi: 10.1172/JCI67562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hodges M. 2002. Enzyme redundancy and the importance of 2-oxoglutarate in plant ammonium assimilation. J Exp Bot 53:905–916. doi: 10.1093/jexbot/53.370.905. [DOI] [PubMed] [Google Scholar]
- 151.Araujo WL, Martins AO, Fernie AR, Tohge T. 2014. 2-Oxoglutarate: linking TCA cycle function with amino acid, glucosinolate, flavonoid, alkaloid, and gibberellin biosynthesis. Front Plant Sci 5:552. doi: 10.3389/fpls.2014.00552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Feria Bourrellier AB, Ferrario-Mery S, Vidal J, Hodges M. 2009. Metabolite regulation of the interaction between Arabidopsis thaliana PII and N-acetyl-l-glutamate kinase. Biochem Biophys Res Commun 387:700–704. doi: 10.1016/j.bbrc.2009.07.088. [DOI] [PubMed] [Google Scholar]
- 153.Ferrario-Mery S, Besin E, Pichon O, Meyer C, Hodges M. 2006. The regulatory PII protein controls arginine biosynthesis in Arabidopsis. FEBS Lett 580:2015–2020. doi: 10.1016/j.febslet.2006.02.075. [DOI] [PubMed] [Google Scholar]
- 154.Chellamuthu VR, Ermilova E, Lapina T, Luddecke J, Minaeva E, Herrmann C, Hartmann MD, Forchhammer K. 2014. A widespread glutamine-sensing mechanism in the plant kingdom. Cell 159:1188–1199. doi: 10.1016/j.cell.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 155.Salminen A, Kaarniranta K, Hiltunen M, Kauppinen A. 2014. Krebs cycle dysfunction shapes epigenetic landscape of chromatin: novel insights into mitochondrial regulation of aging process. Cell Signal 26:1598–1603. doi: 10.1016/j.cellsig.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 156.Carey BW, Finley LW, Cross JR, Allis CD, Thompson CB. 2015. Intracellular alpha-ketoglutarate maintains the pluripotency of embryonic stem cells. Nature 518:413–416. doi: 10.1038/nature13981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chubukov V, Gerosa L, Kochanowski K, Sauer U. 2014. Coordination of microbial metabolism. Nat Rev Microbiol 12:327–340. doi: 10.1038/nrmicro3238. [DOI] [PubMed] [Google Scholar]
- 158.Tian ZX, Li QS, Buck M, Kolb A, Wang YP. 2001. The CRP-cAMP complex and downregulation of the glnAp2 promoter provides a novel regulatory linkage between carbon metabolism and nitrogen assimilation in Escherichia coli. Mol Microbiol 41:911–924. [DOI] [PubMed] [Google Scholar]
- 159.Mao XJ, Huo YX, Buck M, Kolb A, Wang YP. 2007. Interplay between CRP-cAMP and PII-Ntr systems forms novel regulatory network between carbon metabolism and nitrogen assimilation in Escherichia coli. Nucleic Acids Res 35:1432–1440. doi: 10.1093/nar/gkl1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Howitt S, Udvardi M. 2000. Structure, function and regulation of ammonium transporters in plants. Biochim Biophys Acta 1465:152–170. doi: 10.1016/S0005-2736(00)00136-X. [DOI] [PubMed] [Google Scholar]
- 161.Buurman ET, Teixeira de Mattos MJ, Neijssel OM. 1991. Futile cycling of ammonium ions via the high affinity potassium uptake system (Kdp) of Escherichia coli. Arch Microbiol 155:391–395. [DOI] [PubMed] [Google Scholar]
- 162.Wei Y, Southworth TW, Kloster H, Ito M, Guffanti AA, Moir A, Krulwich TA. 2003. Mutational loss of a K+ and NH4+ transporter affects the growth and endospore formation of alkaliphilic Bacillus pseudofirmus OF4. J Bacteriol 185:5133–5147. doi: 10.1128/JB.185.17.5133-5147.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hess DC, Lu W, Rabinowitz JD, Botstein D. 2006. Ammonium toxicity and potassium limitation in yeast. PLoS Biol 4:e351. doi: 10.1371/journal.pbio.0040351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Coskun D, Britto DT, Li M, Oh S, Kronzucker HJ. 2013. Capacity and plasticity of potassium channels and high-affinity transporters in roots of barley and Arabidopsis. Plant Physiol 162:496–511. doi: 10.1104/pp.113.215913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.ten Hoopen F, Cuin TA, Pedas P, Hegelund JN, Shabala S, Schjoerring JK, Jahn TP. 2010. Competition between uptake of ammonium and potassium in barley and Arabidopsis roots: molecular mechanisms and physiological consequences. J Exp Bot 61:2303–2315. doi: 10.1093/jxb/erq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kleiner D. 1993. NH4+ transport systems, p 379–396. In Bakker EP. (ed), Alkali cation transport systems in prokaryotes. CRC Press, Boca Raton, FL. [Google Scholar]
- 167.Boogerd FC, Ma H, Bruggeman FJ, van Heeswijk WC, Garcia-Contreras R, Molenaar D, Krab K, Westerhoff HV. 2011. AmtB-mediated NH3 transport in prokaryotes must be active and as a consequence regulation of transport by GlnK is mandatory to limit futile cycling of NH4+/NH3. FEBS Lett 585:23–28. doi: 10.1016/j.febslet.2010.11.055. [DOI] [PubMed] [Google Scholar]
- 168.Wang Y, Assaf Z, Liu X, Ziarelli F, Latifi A, Lamrabet O, Quelever G, Qu F, Zhang CC, Peng L. 2014. A “click” chemistry constructed affinity system for 2-oxoglutaric acid receptors and binding proteins. Org Biomol Chem 12:6470–6475. doi: 10.1039/C4OB01005A. [DOI] [PubMed] [Google Scholar]