Abstract
Traumatic brain injury (TBI) is a major public health issue exacting a substantial personal and economic burden globally. With the advent of “big data” approaches to understanding complex systems, there is the potential to greatly accelerate knowledge about mechanisms of injury and how to detect and modify them to improve patient outcomes. High quality, well-defined data are critical to the success of bioinformatics platforms, and a data dictionary of “common data elements” (CDEs), as well as “unique data elements” has been created for clinical TBI research. There is no data dictionary, however, for preclinical TBI research despite similar opportunities to accelerate knowledge. To address this gap, a committee of experts was tasked with creating a defined set of data elements to further collaboration across laboratories and enable the merging of data for meta-analysis. The CDEs were subdivided into a Core module for data elements relevant to most, if not all, studies, and Injury-Model-Specific modules for non-generalizable data elements. The purpose of this article is to provide both an overview of TBI models and the CDEs pertinent to these models to facilitate a common language for preclinical TBI research.
Key words: : common data elements, data dictionary, pre-clinical TBI models, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is now recognized as a major health issue that affects more than 3.5 million persons each year.1 The impact of TBI on the public includes the personal burden endured by survivors and their families and a substantial economic toll.2 Further, TBI may also be a risk factor for the later development of neurodegenerative disorders, including Alzheimer disease.3–13 Despite this huge encumbrance on society, no treatment has been shown to be efficacious despite multiple phase III clinical trials.14–17 While the reasons for these failures are complex, the inability to translate therapeutic efficacy observed in animal TBI models to clinical studies has been a major point of criticism and reflection.18
Cautionary tales regarding terminology and interpretation of experimental models include the designation of “severe TBI” for injured animals that are able to ambulate, eat, and groom within hours of TBI, unlike severe TBI in humans. Another major limitation of animal models of TBI is the apparent inability to compare data between laboratories, in part because of overt and subtle differences in injury parameters and outcome measures. Indeed, it is well known that small modifications to an injury device can have dramatic effects on outcome, yet there has not been a means to calibrate interpretation of different data sets between laboratories.
Nonetheless, the ability to compare data is of obvious importance in developing treatment strategies for TBI using preclinical models. Given that there are hundreds of drugs and biologics that demonstrate efficacy in animal models of TBI, an effective way to compare their effect sizes on a global outcome measure is critical for selecting the most promising therapeutics to use in clinical trials.
The National Institute of Neurological Disorders and Stroke (NINDS) has spearheaded the development of standardized definitions for basic units of data, or “common data elements (CDEs),”19 for clinical research in several neurological disorders, including TBI.20 Following on the success of the clinical CDEs, a committee of experts was tasked with developing a matrix of CDEs for preclinical TBI models.
Methods
Development and structure of pre-clinical CDEs for TBI
To address the widely heterogeneous aspects of human TBI, numerous animal TBI models have been developed over several decades. In particular, diverse variations in the species, sex, genetic backgrounds, injury biomechanics, neurobehavioral and neuropathological outcomes of models have emerged. Moreover, an array of iterative modifications of established models by individual laboratories adds further complexity.
To reasonably permit data comparison with respect to outcome, CDEs will need to address both “Core” data elements relevant to all studies, such as species, age and sex, as well as those specific to established individual models and their modifications and outcomes. To achieve this goal, a working group was established that comprised 11 experts with experience across a range of preclinical TBI models. Multiple iterative working group meetings and teleconferences were held to draft an overall structure of the CDEs. In addition, individual experts were tasked with identifying CDEs specific to each model.
The proposed CDEs were sent out for review to the larger TBI research community using the NINDS TBI Research listserv (https://list.nih.gov/cgi-bin/wa.exe?A0=TBI) to provide an opportunity for feedback and further improvements. The suggestions were reviewed, and many were incorporated into the matrix.
Results
Thus far, 167 data elements have been defined for preclinical TBI research. The data elements are organized around 10 modules, including a module of Core CDEs (Table 1) and 9 other modules relevant to specific injury models (outlined below) (Tables 2–6). Note that the full definitions, permissible values, and references are available on the NINDS TBI research website (http://www.ninds.nih.gov/research/tbi/index.htm).
Table 1.
Module 1: Core Common Data Elements for Pre-Clinical Traumatic Brain Injury Research
| Animal characteristics | Animal history | Assessments and outcomes | Injury model characteristics |
|---|---|---|---|
| Species Birth date Age Age group Sex Animal vendor Strain/genetic modifications Weight measurement |
Pre-injury subject housing Pre-injury conditions Pre-injury surgical procedures Injury group Injury date and time Anesthetic type Anesthetic route Anesthesia duration Analgesia type Injury severity Number of injury exposures Interval between injuries Post-injury surgical procedures Post-injury conditions Post-injury subject housing Treatment group Treatment onset Drug treatment route Treatment or therapy type Treatment control Treatment dose Survival time Euthanasia date and time Euthanasia type |
Outcome timing Assessment date and time Acute neurological assessment Apnea indicator Apnea duration Righting response time Toe pinch response Acute physiological assessments Brain imaging type Chronic physiologic assessments Memory/retention tests Learning/acquisition tests Sensory/motor tests Anxiety tests Social interaction tests Body weight change Histopathology |
Injury model characteristics External cause modeled Injury model Device manufacturer Device manufacturer other text Animal stabilization method Impact location side Impact location cortical region Impact location coordinates |
Table 2.
Modules 2–5
| Module 2. Weight drop injury relevant data elements | ||
|---|---|---|
| Invasive surgery Surface material Surgical procedure for cranial opening Craniotomy size Impactor/projectile mass Impactor/projectile material Impactor tip/projectile shape Impactor tip rigidity |
Weight drop height Weight drop guidance Weight drop characteristics Impactor velocity Contact surface type Contact surface area Impactor dwell time |
Impactor retraction WD-specific pre-injury surgical procedures WD-specific post-injury surgical procedures |
| Module 3. Fluid percussion injury relevant data elements | ||
|---|---|---|
| Surgical procedure for cranial opening Craniotomy size Connector angle Connector tube Connector tube length |
Connector tube material Port distal diameter Cement Transducer manufacturer |
Cap characteristics Peak pressure pulse Pressure wave duration |
| Module 4. Controlled cortical injury relevant data elements | ||
|---|---|---|
| Invasive surgery Surgical procedure for cranial opening Craniotomy size Impactor angle Impactor angle measurement |
Impactor tip/projectile shape Impactor tip rigidity Impactor depth setting |
Impactor dwell time Impactor velocity Surface material |
| Module 5. Projectile concussive impact model relevant data elements | ||
|---|---|---|
| Projectile driver mechanism Impactor/projectile material Impact distance Projectile velocity Helmet |
Impactor/projectile mass Impactor tip/projectile shape Peak pressure sensor film Contact surface type Contact surface area |
Contact pressure PCI-specific pre-injury surgical procedures PCI-specific post-injury surgical procedures |
WD, weight drop; PCI, projectile concussive impact.
Table 3.
Module 6
| Module 6. Blast-induced neurotrauma relevant data elements | ||
|---|---|---|
| Blast induced delivery device Pressure wave type Detonation type Detonation material quantity Driver gas Pressure wave medium Distance from detonation Blast tube or column area Blast tube length Shock tube driven section length Membrane thickness Membrane burst method Membrane burst pressure Tube end configuration Placement relative to shock tube |
Distance between animal and tube Animal orientation to blast wave Overpressure peak Overpressure rise time Overpressure wave duration Impulse Reflective wave overpressure Blast wind pressure Pressure sensor orientation Pressure sensor type Pressure sensor sampling frequency Incident pressure time history Body exposure Protective shielding location Protective shielding type |
Reflective surfaces Primary blast effects Secondary blast effects type Secondary blast effects specifications Tertiary blast effects Tertiary blast effects specifications Quaternary blast effects Systemic injury Extracranial injuries BIN-specific pre-injury surgical procedures BIN-specific post-injury surgical procedures |
BIN, blast-induced neurotrauma.
Table 4.
Module 7
| Module 7. Penetrating ballistic-like brain injury relevant data elements | ||
|---|---|---|
| Surgical procedure for cranial opening Craniotomy size PBBI probe PBBI orientation Balloon inflation diameter Balloon inflation volume Balloon life span Brain cavity volume |
Impactor tip/projectile shape Impactor tip rigidity Impactor depth setting Connector tube length Connector tube material Port distal diameter Cement |
Cap characteristics Peak pressure pulse Pressure wave duration PBBI-specific pre-injury surgical procedures PBBI-specific post-injury surgical procedures |
PBBI, penetrating ballistic-like brain injury.
Table 5.
Modules 8 and 9
| Module 8. Intracranial hemorrhage and subdural/subarachnoid hemorrhage relevant data elements | ||
|---|---|---|
| Hemorrhage cause Hemorrhage intended compartment Hemorrhage intended side Hemorrhage actual location |
Hemorrhage actual side Hemorrhage volume Injection material Injection duration Peak intracranial pressure |
ICH-specific pre-injury surgical procedures ICH-specific post-injury surgical procedures |
| Module 9. Increased intracranial pressure model relevant data elements | ||
|---|---|---|
| Intracranial pressure elevation-specific surgical procedures |
Increased pressure maneuver duration Anatomic location of ICP measurement |
Peak ICP ICP specific pre-injury surgical procedures ICH-specific post-injury surgical procedures |
ICH, intracranial hemorrhage; ICP, intracranial pressure.
Table 6.
Module 10
| Module 10. Porcine rotational acceleration model relevant data elements | ||
|---|---|---|
| Rotation plane Rotational motion duration |
Peak angular velocity Peak angular acceleration |
Peak angular deceleration Angular motion range |
Within structured forms, adapted from the Federal Interagency TBI Research (FITBIR) Informatics System data dictionary (https://fitbir.nih.gov), each named data element has a detailed description and is linked to its relevant classification and domain (e.g., Core and Animal Characteristics). In addition, each data element has permissible values, whether these are alphanumeric or text entries (e.g., male or female) or numeric values (e.g., velocity of impact: 0–10 m/sec). The structured forms include appropriate guidelines for data entry and references from the published literature (http://www.ninds.nih.gov/research/tbi/index.htm).
The level of detail to be captured using the CDEs was determined through an iterative process by working group members, under the dictum that data elements sufficient to influence the results of the study should be incorporated while minimizing the data entry burden to investigators where possible.
Core CDEs (Module 1)
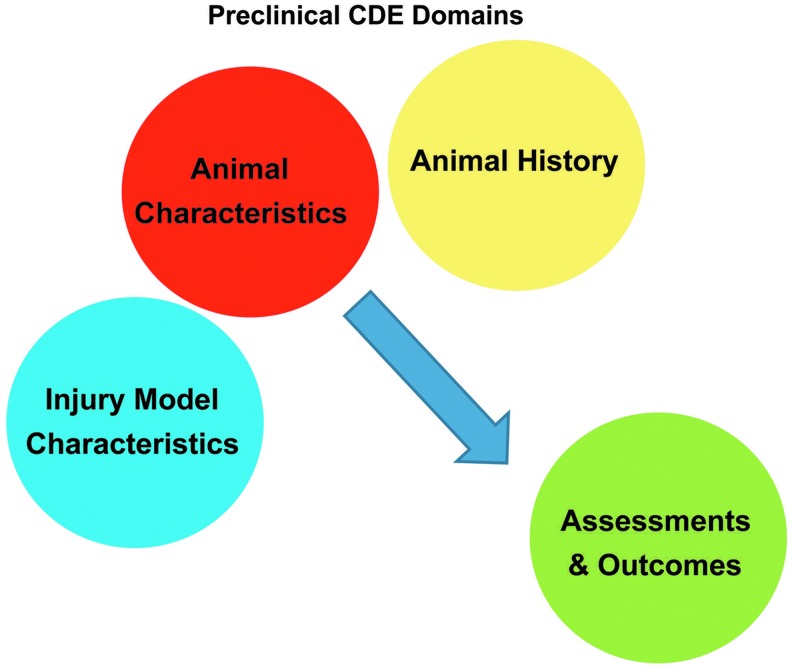
The first module is composed of Core CDEs because of their broad applicability to many preclinical studies. There are 57 Core CDEs, which are divided into four domains including (1) the animal characteristics, (2) injury model characteristics, (3) the animal history, and (4) assessments and outcome measures (Table 1, Figure 1). While some Core CDEs provide essential information that should be included in all preclinical research studies (e.g., age and species), others should be used as needed (e.g., brain imaging and acute physiological assessments). When a study is collecting these types of data, use of the Core CDEs is highly recommended to ensure that data will be collected in a standardized manner and will enable meta-analysis in the future.
FIG. 1.
The preclinical common data elements (CDEs) are organized around four domains: Animal Characteristics; Animal History (including treatments); Injury Model Characteristics; and Assessments and Outcomes. These domains describe factors and outcomes relevant to preclinical therapy development for traumatic brain injury. Color image is available online at www.liebertpub.com/neu
Modules of specific TBI animal models
Historically, experimental TBI models were categorized broadly as “focal,” or “diffuse.” Focal models included those that induce cerebral contusions, edema, and hematomas. In contrast, diffuse models displayed pathological features comprising more widespread vascular injury, ischemia, general brain swelling, and diffuse axonal injury (DAI). This stark distinction, however, is falling out of use because it is now recognized that few focal models actually induce exclusively localized pathology. In addition, the variation in the character and extent of pathologies between models of diffuse TBI models are too great to be captured under one heading. Instead, more recent descriptions of TBI models address key pathological features and/or injury severity, with the caveat that many other changes may also be present.
The goal for the development of preclinical TBI CDEs is to start with the most widely used models established in the literature (Tables 2–6). These models and some of the common variations in their execution are discussed below.
Head/brain impact models (Modules 2-5)
In the clinical setting, “focal TBI” is used to describe a spectrum of pathologies regardless of the biomechanical nature of injury. This includes intracerebral and intracranial hemorrhage, as well as one of the most common pathologies across the injury severity spectrum, cortical contusion. In contrast, the vast majority of laboratory models of focal TBI represent pathologies resulting from a blow to the head. Indeed, virtually all focal TBI models are more specifically cortical contusion models with or without more widespread neuropathology. Numerous species have been used to model cerebral contusion including cats,21 sheep,22 ferrets,23 non-human primates,24 pigs,25–30 and rodents.31–40 Mice and rats, however, have been, by far, the most widely used species primarily for reasons of convenience and economic viability.31–40
Currently, four general techniques are used to apply impact forces directly to the brain or skull of the animal and induce focal brain injury in rodents: weight drop,31–34 fluid percussion,37–39 controlled cortical impact,35,36 and projectile impact.40 The parameters of these models are designed to produce dynamic deformation of brain tissue over a target duration of approximately 10–50 msec, but it can be longer in some cases.41
As the name implies, weight drop models use weights that are dropped freely or through a guiding apparatus to generate an impact either on the closed cranium, a metal plate fixed to the cranium, or through a craniectomy directly on the dura. The widely recognized Marmarou model of impact acceleration in rats has been described as resulting in diffuse brain injury.42 In this model, a weight is dropped onto a plate fixed to the rat's cranium. While previous weight-drop models described the head as being fixed or positioned on a hard surface,34,43 in this adaptation, however, the head was not fixed and allowed to rotate downward. It has been suggested that this motion, in combination with the impact, results in overt widespread damage to axons.42 Nevertheless, there has been debate as to whether the axonal injury occurs as a result of the acceleration or of skull deformation. In addition to the issue of head stabilization, the surface material on which the animal is positioned can influence outcome (e.g., foam vs. rigid surface), as well as the impounder shape, material, and height from which it is dropped (Table 2).
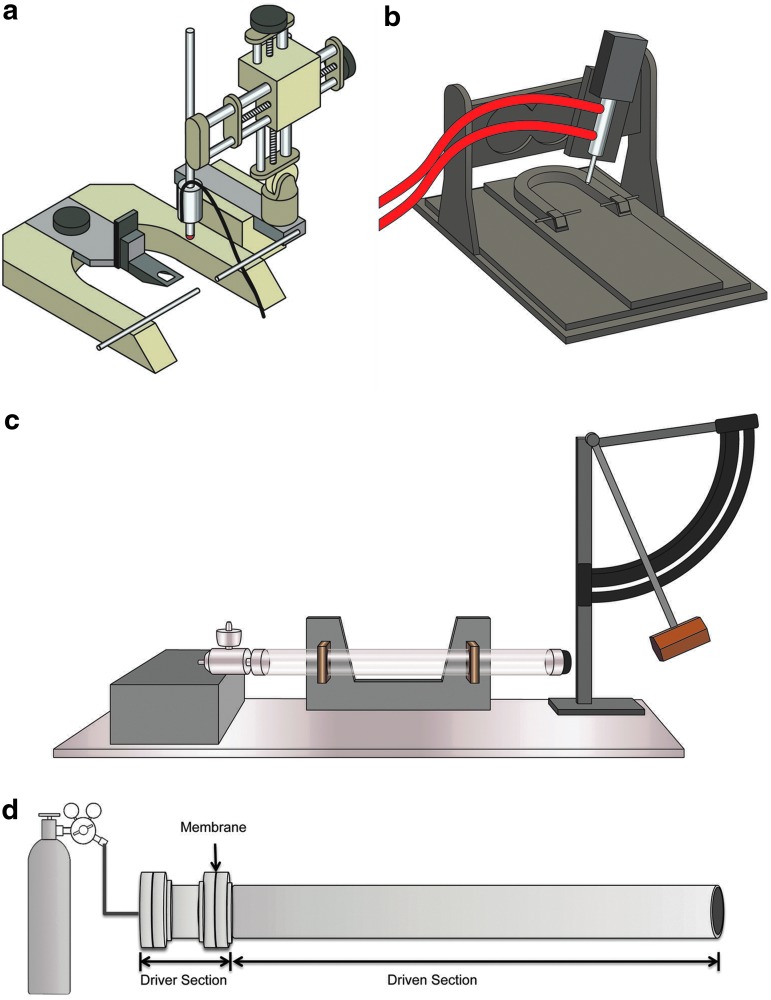
Fluid percussion (FP) models of brain injury use rapid injection of fluid through a sealed hollow post into the closed or open cranial cavity (Fig. 2c). The diameter and length of the fluid-filled tube are known variables with regard to injury level, in addition to the craniectomy size and shape through which the fluid pulse is injected. Moreover, simply the manufacturer of the fluid percussion device may result in high variation in the nature and extent of injury (Table 2).
FIG. 2.
Examples of common devices used to induce experimental traumatic brain injury that are modified in various ways that influence outcome. (a) Illustration of a controlled cortical impact (CCI) device that delivers stereotactic guidance for impact placement and uses electromagnetic force. (b) Illustration of a CCI device that relies on delivering an impact via a pneumatically controlled piston. The nature of the injury is modifiable by various factors including the impact velocity and geometry, the size, shape, and material of the impounder. (c) Illustration of a fluid percussion device. Known variables that influence histopathological and clinical outcome include the diameter and length of the fluid-filled tube, the craniectomy size and shape to which the fluid pulse is injected. (d) Representative shock tube assembly. Dimensions of the device vary dramatically, ranging from centimeters to tens of meters in length. A membrane/diaphragm (e.g., Mylar) is inserted between the driver section and driven section. Compressed air/gas fills the driver section to a pressure that ruptures the membrane inducing a characteristic blast shock wave that travels through the driven section. Test animals or materials are placed either inside or outside the driven section. Images courtesy of Dr. C. Edward Dixon and Mr. Michael Farmer (a–c), and Dr. Douglas H.Smith (d). Color image is available online at www.liebertpub.com/neu
Controlled cortical impact (CCI) is a rigid indentation method that typically uses a pneumatic, electronic, or spring-driven impactor to deform the brain through a craniectomy, at a pre-specified velocity and depth, with the dura open or intact (Fig. 2 a,b). More recently, various groups have used CCI directly onto the closed skull in attempts to model more mild and diffuse forms of TBI, often using repetitive injury paradigms.44–49 In addition to modifiable aspects of the model such as impact velocity and geometry, the size, shape, and material of the impounder can result in significant changes to the injury (Table 2).
Similar to FP, the size and location of the craniectomy alone can dramatically change injury severity, even when the same impounder is used. Moreover, for various impact models, the bone flap that is removed for injury is often not replaced. In contrast, other groups have opted for a craniotomy, where the original bone flap is affixed back in place or cranioplasty performed using synthetic material to reseal the skull. It is important to note that unless sealed, creating an opening in the skull may influence intracranial pressure (ICP) by acting as a decompressive craniectomy post-trauma, which can potentially affect outcome.50,51
A more recently published model, referred to as projectile concussive impact (PCI), relies on closed head impact via a projectile launched via the rapid sublimation of dry ice40 or compressed nitrogen.52 While the nature of the projectile is critical to the injury, other important variables include the location of impact, surface pressure at contact, the projectile's trajectory, velocity, and the presence or absence of a helmet (Table 2).
Animal models of blast-induced TBI (Module 6)
The incidence of blast-induced TBI has risen markedly in recent military engagements.53–56 Blast exposures are often complex events and may induce multiple types of TBI by direct impact, including penetrating injuries and rapid acceleration-deceleration injuries from being thrown or struck by objects, or from exposure to the primary blast wave itself. Thermal or chemical insults can also play a role.57,58
The role of “pure” or primary blast injury caused by the propagation of rapid pressure waves remains unclear, however. Specifically, the relative contribution of primary blast versus inertial forces in closed-head TBI is currently debated both clinically and experimentally.59–61 This lack of clinical information is a major limitation when attempting to generate appropriate models and underscores the need for the use of CDEs in an immature research area where causal mechanisms of injury are uncertain. Nonetheless, in attempts to simulate field conditions, animal models of blast TBI have directly used explosive material or experimental shock tubes to approximate blast conditions.
Direct explosive models have used a range of high explosives, with exposure being “open-field” absent walls/obstructions (e.g., 360-degree radius), “closed-field” within a defined space, and/or within “complex environments” consisting of partial walls/obstructions and vehicle surrogates. Various species have been examined including rodents,62,63 non-human primates,64 and pigs.65,66 To complement these efforts, in-laboratory blast testing has been performed using shock tubes, which are typically cylindrical tubes where rats,60,67–76 mice,77,78 and ferrets79 have been exposed to blast-like pressure wave propagation driven by compressed gas (e.g., air, nitrogen, helium) (Fig. 2d). Other studies have used explosive charge-driven shock tubes.80,81
To date, there are not standardized shock tube paradigms (e.g., gas vs. chemical explosives, tube design), species, location of the specimen, or use of body shielding and head mobility, maximum (peak) overpressure peak or overpressure duration; and all of these factors may greatly alter the nature of the injury, which again speak to the critical need for the use of CDEs in an emerging area of research.
Differences in the implementation of blast paradigms may, in part, explain the variations in reporting of thresholds and pathologies between laboratories. Indeed, perhaps because of the recent development of various models and the lack of clinical and neuropathological descriptions of blast-TBI, these models are conceivably the most varied in experimental TBI, and therefore also have the largest number of model-specific CDEs (Table 3).
Penetrating ballistic-like brain injury (PBBI) model (Module 7)
While closed head injuries are the most common type of injury in the civilian population, penetrating injuries from firearms remain a substantial cause of morbidity and mortality, particularly in young adults in the United States.82,83 In addition, penetrating injuries are significantly more prevalent in the military versus civilian population.84
Experimental models of penetrating brain injury are not widely studied, however. While several stab injury models have been reported,85–88 these fail to recapitulate the biomechanics of common penetrating injuries clinically, such as those associated with firearms. In contrast, PBBI89–91 was designed to simulate both bullet trajectory and the resultant cavitation from energy dissipation from a bullet round in the brain parenchyma. This model uses a probe with a rapidly inflatable tip. The size of the probe, magnitude and rate of inflation, as well as location can all influence the pathological nature of the injury (Table 4).
Intracranial hemorrhage (ICH) and subdural/subarachnoid hemorrhage (SDH/SAH) relevant data elements (Module 8) and ICP models (Module 9)
Acute intracranial hematoma is an extremely common consequence of TBI. In particular, acute subdural hematoma frequently results from tearing of the bridging or cortical veins, while acute epidural hematomas most commonly occur secondary to rupture of the middle meningeal artery or from bone bleeding. Despite the relative frequency of these pathologies, acute intracerebral hemorrhage is poorly studied when compared with other trauma-induced brain pathologies, perhaps as a result of their primary management being neurosurgical evacuation. Nonetheless, several models have been reported that largely depend on the introduction of autologous blood to the subdural or epidural space in rodents or larger animals.92–97 One group simulated the compressive effects of epidural hematoma in dogs using an inflatable balloon within the epidural space.98
Models of raised ICP have also been developed by balloon inflation within the subdural, epidural space or the lateral ventricle99–105 or via infusion of artificial cerebrospinal fluid or other fluid in to the cerebral ventricles or cisterna magna.106–110 Various aspects such as the location, nature, volume, and rate of the fluid injection or balloon inflation are important with regard to interpretation and comparability. Similarly, the magnitude of ICP attained as well as how and where it is measured is critical (Table 5).
Head rotational acceleration models (Module 10)
Rotational acceleration of the brain can be triggered by translational forces impacting the head inducing rotation, in the absence of head impact when the head is allowed to move freely during a sudden deceleration during which the body is restrained, or by pure rotation via head coupling to a rotational acceleration device. Head rotational acceleration causes various brain regions to undergo differential shear, tensile, and compressive forces that cause tissue deformation at high strain rates.111 The amount of shear strain is related not only to the amount of rotational acceleration, but also to the presence of intracranial dural compartments (e.g., falx, tentorium cerebri) and direction of motion. These inertial forces are responsible for DAI,112 one of the most common and important pathological features of TBI.113–116 Notably, while referred to as “diffuse” clinically, traumatic injury to axons is perhaps more accurately described as multifocal, preferentially involving midline white matter tracts such as the corpus callosum, internal capsules, brainstem, and cerebellar peduncles.113,115,117
Few models of DAI in gyrencephalic animals have been characterized, although these models are considered increasingly valuable because of their high clinical relevance to mild TBI or concussion. Their lack of widespread use in part reflects the difficulty of developing a model system that replicates the dynamics of diffuse injury, such as the inertial loading conditions produced in automotive crashes or at the moment of head impact.118
Because of the large effect of brain mass on angular acceleration, acceleration force magnitudes must be very large to compensate for the small brain volumes of most experimental animals and create the same mechanical loading of brain tissue that occurs in human TBI.111, 119–122 Indeed, only two animal models have been shown to replicate the key clinical features of DAI. These “inertial” injury models were initially characterized in non-human primates, using non-impact head rotational acceleration to produce coma in association with diffuse axonal damage.123 Non-human primates were originally chosen for this experimental model because of their large brain mass, which allows for mechanical devices to produce the magnitude of deceleration needed to create the development of high strain between regions of tissue.
More recently, a porcine model of rotational acceleration brain injury has been developed, using young adult miniature swine,111,124 which have a brain mass of approximately 70–100 g, comparable to that of the non-human primates. In addition, neonatal and pediatric domestic swine models have been developed.125,126 Peak coronal plane rotational accelerations were found to range from 0.6–1.7×105 rad/sec2. Rotational acceleration at these parameters was sufficient to consistently produce axonal injury throughout the white matter, particularly subcortically.
The complex biomechanics involved in this model are vital to clinical and neuropathological outcomes. Specifically, attaining the relevant peak rotational accelerations and velocities, as well as the maximal duration of rotation are critical in replicating human pathology (Table 6). Moreover, studies using this model demonstrated that the plane of head rotational acceleration in reference to the brainstem is important in determining the induction and duration of loss of consciousness after injury.127.128
Implementation
The preclinical CDEs are currently accessible via the NINDS TBI Research website (http://www.ninds.nih.gov/research/tbi/index.htm) and in the future will be accessible via the FITBIR Informatics System, currently operational for clinical TBI research (https://fitbir.nih.gov/). The FITBIR Informatics System was developed as a web-based platform designed to permit cross-site meta-analysis and data comparisons and sharing of clinical research data within the TBI research community. The preclinical CDEs will provide standardized definitions or a “data dictionary” for the data submitted by preclinical TBI investigators. In addition, if investigators use the Protocol and Form Research Management System (ProFoRMS), a web-based data collection/research tool that permits real time electronic data collection (as is normally done in individual notebooks), data will be automatically uploaded into FITBIR, thus limiting the workload for investigators.
Another major advantage of the system is that once specific forms are published, standardized and vetted sets of data elements, e.g., for a specific experimental model, will be available to the wider research community to use. To ensure high quality data, FITBIR has quality control measures that reject data that are outside of permissible values. In the future, it is anticipated that FITBIR will also have links to analytical tools to facilitate data analysis.
Discussion
Goals and utility of pre-clinical CDEs for TBI
The preclinical CDEs aim to capture sufficient detail to identify likely sources of variability that in the past have confounded cross-comparison between studies. Notably, as described above, many of these variables are often subtle and inadequately described in published articles. Incorporating this detail into a readily accessible and searchable database will open avenues of cross-comparison between data sets not previously possible and will potentially accelerate the advancement of preclinical TBI research. Such widespread data sharing will not only foster collaboration but will also provide an important platform to address specific scientific questions using existing data sets and meta-analyses. Mapping of preclinical CDEs to existing clinical CDEs may have important utility for translation.
Notably, to permit standardization, established CDEs require stability. As new models are generated and existing models modified, however, the addition of new CDEs (in the form of new modules, as well additional unique data elements) will be incorporated. As such, it is envisioned that the CDEs will be a “living document” with flexibility to update in a dynamic fashion.
Having a centralized and accessible database, such as FITBIR, would also be advantageous not only with regard to study comparison, but also may serve to standardize and increase the rigor of future data collection. Specifically, FITBIR has a tool (ProFORMS) that makes it possible for investigators to create electronic forms that automatically load the data into the database. The creation of ProFoRMS for preclinical research will provide a useful resource that promotes standardized data collection across groups and may be particularly helpful to new investigators. Reference values and existing data sets will also serve as a resource for validation of models in new laboratories. Study design can be aided by searching data for appropriate outcome measures, e.g., behavioral testing at specific time points post-injury. Finally, a potentially important outcome of data submission in the context of CDEs will be the inclusion of studies with negative findings, which are often not submitted or accepted for publication.129,130 This reporting is a much-needed resource that will allow investigators to avoid unnecessary duplication of studies and the associated waste of resources.
Lastly, while there are few established preclinical CDEs for any disorder, the spinal cord injury (SCI) research community has also undertaken steps toward the identification of key information needed for preclinical research studies and standardization of data elements.131 Although TBI and SCI produce uniquely different types of neurotrauma, there are many common mechanisms of injury, and ways to integrate the TBI and SCI preclinical CDEs should be explored in the future. There is much to learn about the feasibility and utility of preclinical CDEs, but it is hoped that they will facilitate data sharing and collaboration within and across preclinical and clinical research fields and ultimately lead to biomarker discovery and effective therapies for TBI.
Acknowledgments
We would like to acknowledge the U.S. Department of Defense and the National Institutes of Health who contributed funding for The Federal Interagency Traumatic Brain Injury Research (FITBIR) Informatics System.
Author Disclosure Statement
The views expressed are those of the authors and do not necessarily reflect those of the agencies or institutions with which they are affiliated, including the United States Department of Health and Human Services. This work is not an official document, guidance, or policy of the United States government, nor should any official endorsement be inferred. No competing or conflicting financial interests exist.
S.T. Ahlers: The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the U.S. Government. I am a military service member (or employee of the U.S. Government). This work was prepared as part of my official duties. Title 17 U.S.C. § 105 provides that copyright protection under this title is not available for any work of the U.S. Government. Title 17 U.S.C. § 101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person's official duties.
F.C. Tortella: Material has been reviewed by the Walter Reed Army Institute of Research. There is no objection to its presentation and/or publication. The opinions or assertions contained herein are the private views of the authors and are not to be construed as official, or as reflecting true views of Department of the Army or Department of Defense.
References
- 1.Coronado V.G., McGuire L.C., Sarmiento K., Bell J., Lionbarger M.R., Jones C.D., Geller A.I., Khoury N., and Xu L. (2012). Trends in traumatic brain injury in the U.S. and the public health response: 1995–2009. J Safety Res 43, 299–307 [DOI] [PubMed] [Google Scholar]
- 2.Corso P., Finkelstein E., Miller T., Fiebelkorn I., and Zaloshnja E. (2006). Incidence and lifetime costs of injuries in the United States. Inj. Prev. 12, 212–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Molgaard C.A., Stanford E.P., Morton D.J., Ryden L.A., Schubert K.R., and Golbeck A.L. (1990). Epidemiology of head trauma and neurocognitive impairment in a multi-ethnic population. Neuroepidemiology 9, 233–242 [DOI] [PubMed] [Google Scholar]
- 4.Mortimer J.A., French L.R., Hutton J.T., and Schuman L.M. (1985). Head injury as a risk factor for Alzheimer's disease. Neurology 35, 264–267 [DOI] [PubMed] [Google Scholar]
- 5.Mortimer J.A., van Duijn C.M., Chandra V., Fratiglioni L., Graves A.B., Heyman A., Jorm A.F., Kokmen E., Kondo K., Rocca W.A., and et al. (1991). Head trauma as a risk factor for Alzheimer's disease: a collaborative re-analysis of case-control studies. EURODEM Risk Factors Research Group. Int. J. Epidemiol. 20, Suppl 2, S28–S35 [DOI] [PubMed] [Google Scholar]
- 6.Graves A.B., White E., Koepsell T.D., Reifler B.V., van Belle G., Larson E.B., and Raskind M. (1990). The association between head trauma and Alzheimer's disease. Am. J. Epidemiol. 131, 491–501 [DOI] [PubMed] [Google Scholar]
- 7.O'Meara E.S., Kukull W.A., Sheppard L., Bowen J.D., McCormick W.C., Teri L., Pfanschmidt M., Thompson J.D., Schellenberg G.D., and Larson E.B. (1997). Head injury and risk of Alzheimer's disease by apolipoprotein E genotype. Am. J. Epidemiol. 146, 373–384 [DOI] [PubMed] [Google Scholar]
- 8.Salib E., and Hillier V. (1997). Head injury and the risk of Alzheimer's disease: a case control study. Int. J. Geriatr. Psychiatry 12, 363–368 [DOI] [PubMed] [Google Scholar]
- 9.Guo Z., Cupples L.A., Kurz A., Auerbach S.H., Volicer L., Chui H., Green R.C., Sadovnick A.D., Duara R., DeCarli C., Johnson K., Go R.C., Growdon J.H., Haines J.L., Kukull W.A., and Farrer L.A. (2000). Head injury and the risk of AD in the MIRAGE study. Neurology 54, 1316–1323 [DOI] [PubMed] [Google Scholar]
- 10.Schofield P.W., Tang M., Marder K., Bell K., Dooneief G., Chun M., Sano M., Stern Y., and Mayeux R. (1997). Alzheimer's disease after remote head injury: an incidence study. J. Neurol. Neurosurg. Psychiatry 62, 119–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Plassman B.L., Havlik R.J., Steffens D.C., Helms M.J., Newman T.N., Drosdick D., Phillips C., Gau B.A., Welsh-Bohmer K.A., Burke J.R., Guralnik J.M., and Breitner J.C. (2000). Documented head injury in early adulthood and risk of Alzheimer's disease and other dementias. Neurology 55, 1158–1166 [DOI] [PubMed] [Google Scholar]
- 12.Fleminger S., Oliver D.L., Lovestone S., Rabe-Hesketh S. and Giora A. (2003). Head injury as a risk factor for Alzheimer's disease: the evidence 10 years on; a partial replication. J. Neurol. Neurosurg. Psychiatry 74, 857–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson V.E., Stewart W., and Smith D.H. (2012). Widespread tau and amyloid‐beta pathology many years after a single traumatic brain injury in humans. Brain Pathol. 22, 142–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Menon D.K. (2009). Unique challenges in clinical trials in traumatic brain injury. Crit. Care Med. 37, Suppl 1, S129–S135 [DOI] [PubMed] [Google Scholar]
- 15.Narayan R.K., Michel M.E., Ansell B., Baethmann A., Biegon A., Bracken M.B., Bullock M.R., Choi S.C., Clifton G.L., Contant C.F., Coplin W.M., Dietrich W.D., Ghajar J., Grady S.M., Grossman R.G., Hall E.D., Heetderks W., Hovda D.A., Jallo J., Katz R.L., Knoller N., Kochanek P.M., Maas A.I., Majde J., Marion D.W., Marmarou A., Marshall L.F., McIntosh T.K., Miller E., Mohberg N., Muizelaar J.P., Pitts L.H., Quinn P., Riesenfeld G., Robertson C.S., Strauss K.I., Teasdale G., Temkin N., Tuma R., Wade C., Walker M.D., Weinrich M., Whyte J., Wilberger J., Young A.B., and Yurkewicz L. (2002). Clinical trials in head injury. J. Neurotrauma 19, 503–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McConeghy K.W., Hatton J., Hughes L., and Cook A.M. (2012). A review of neuroprotection pharmacology and therapies in patients with acute traumatic brain injury. CNS Drugs 26, 613–636 [DOI] [PubMed] [Google Scholar]
- 17.Lu J., Gary K.W., Neimeier J.P., Ward J., and Lapane K.L. (2012). Randomized controlled trials in adult traumatic brain injury. Brain Inj. 26, 1523–1548 [DOI] [PubMed] [Google Scholar]
- 18.Marklund N., and Hillered L. (2011). Animal modelling of traumatic brain injury in preclinical drug development: where do we go from here? Br. J. Pharmacol. 164, 1207–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Institute of Neurological Disorders and Stroke. NINDS Common Data Elements: Traumatic Brain Injury CDE Standards. Available at: http://www.commondataelements.ninds.nih.gov/TBI.aspx#tab%20=%20Data_Standards&tab=Data_Standards Accessed November2014
- 20.Hicks R., Giacino J., Harrison-Felix C., Manley G., Valadka A., and Wilde E.A. (2013). Progress in developing common data elements for traumatic brain injury research: version two—the end of the beginning. J. Neurotrauma 30, 1852–1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sullivan H.G., Martinez J., Becker D.P., Miller J.D., Griffith R., and Wist A.O. (1976). Fluid-percussion model of mechanical brain injury in the cat. J. Neurosurg. 45, 521–534 [PubMed] [Google Scholar]
- 22.Grimmelt A.C., Eitzen S., Balakhadze I., Fischer B., Wolfer J., Schiffbauer H., Gorji A., and Greiner C. (2011). Closed traumatic brain injury model in sheep mimicking high-velocity, closed head trauma in humans. Cent. Eur. Neurosurg. 72, 120–126 [DOI] [PubMed] [Google Scholar]
- 23.Lighthall J.W. (1988). Controlled cortical impact: a new experimental brain injury model. J. Neurotrauma 5, 1–15 [DOI] [PubMed] [Google Scholar]
- 24.Ommaya A.K., Hirsch A.E., Flamm E.S., and Mahone R.H. (1966). Cerebral concussion in the monkey: an experimental model. Science 153, 211–212 [DOI] [PubMed] [Google Scholar]
- 25.Manley G.T., Rosenthal G., Lam M., Morabito D., Yan D., Derugin N., Bollen A., Knudson M.M., and Panter S.S. (2006). Controlled cortical impact in swine: pathophysiology and biomechanics. J. Neurotrauma 23, 128–139 [DOI] [PubMed] [Google Scholar]
- 26.Alessandri B., Heimann A., Filippi R., Kopacz L., and Kempski O. (2003). Moderate controlled cortical contusion in pigs: effects on multi-parametric neuromonitoring and clinical relevance. J. Neurotrauma 20, 1293–1305 [DOI] [PubMed] [Google Scholar]
- 27.Zhang J., Groff R.F., IV, Chen X.H., Browne K.D., Huang J., Schwartz E.D., Meaney D.F., Johnson V.E., Stein S.C., Rojkjaer R., and Smith D.H. (2008). Hemostatic and neuroprotective effects of human recombinant activated factor VII therapy after traumatic brain injury in pigs. Exp. Neurol. 210, 645–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Durham S.R., Raghupathi R., Helfaer M.A., Marwaha S., and Duhaime A.C. (2000). Age-related differences in acute physiologic response to focal traumatic brain injury in piglets. Pediatr. Neurosurg. 33, 76–82 [DOI] [PubMed] [Google Scholar]
- 29.Grate L.L., Golden J.A., Hoopes P.J., Hunter J.V., and Duhaime A.C. (2003). Traumatic brain injury in piglets of different ages: techniques for lesion analysis using histology and magnetic resonance imaging. J. Neurosci. Methods 123, 201–206 [DOI] [PubMed] [Google Scholar]
- 30.Missios S., Harris B.T., Dodge C.P., Simoni M.K., Costine B.A., Lee Y.L., Quebada P.B., Hillier S.C., Adams L.B., and Duhaime A.C. (2009). Scaled cortical impact in immature swine: effect of age and gender on lesion volume. J. Neurotrauma 26, 1943–1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nilsson B., Ponten U., and Voigt G. (1977). Experimental head injury in the rat. Part 1: Mechanics, pathophysiology, and morphology in an impact acceleration trauma model. J. Neurosurg. 47, 241–251 [DOI] [PubMed] [Google Scholar]
- 32.Nilsson B., and Nordstrom C.H. (1977). Experimental head injury in the rat. Part 3: Cerebral blood flow and oxygen consumption after concussive impact acceleration. J. Neurosurg. 47, 262–273 [DOI] [PubMed] [Google Scholar]
- 33.Feeney D.M., Boyeson M.G., Linn R.T., Murray H.M., and Dail W.G. (1981). Responses to cortical injury: I. Methodology and local effects of contusions in the rat. Brain Res. 211, 67–77 [DOI] [PubMed] [Google Scholar]
- 34.Shapira Y., Shohami E., Sidi A., Soffer D., Freeman S., and Cotev S. (1988). Experimental closed head injury in rats: mechanical, pathophysiologic, and neurologic properties. Crit. Care Med. 16, 258–265 [DOI] [PubMed] [Google Scholar]
- 35.Dixon C.E., Clifton G.L., Lighthall J.W., Yaghmai A.A., and Hayes R.L. (1991). A controlled cortical impact model of traumatic brain injury in the rat. J. Neurosci. Methods 39, 253–262 [DOI] [PubMed] [Google Scholar]
- 36.Smith D.H., Soares H.D., Pierce J.S., Perlman K.G., Saatman K.E., Meaney D.F., Dixon C.E., and McIntosh T.K. (1995). A model of parasagittal controlled cortical impact in the mouse: cognitive and histopathologic effects. J. Neurotrauma 12, 169–178 [DOI] [PubMed] [Google Scholar]
- 37.Dixon C.E., Lyeth B.G., Povlishock J.T., Findling R.L., Hamm R.J., Marmarou A., Young H.F., and Hayes R.L. (1987). A fluid percussion model of experimental brain injury in the rat. J. Neurosurg. 67, 110–119 [DOI] [PubMed] [Google Scholar]
- 38.McIntosh T.K., Vink R., Noble L., Yamakami I., Fernyak S., Soares H., and Faden A.L. (1989). Traumatic brain injury in the rat: characterization of a lateral fluid-percussion model. Neuroscience 28, 233–244 [DOI] [PubMed] [Google Scholar]
- 39.McIntosh T.K., Noble L., Andrews B., and Faden A.I. (1987). Traumatic brain injury in the rat: characterization of a midline fluid-percussion model. Cent. Nerv. Syst. Trauma 4, 119–134 [DOI] [PubMed] [Google Scholar]
- 40.Chen Z., Leung L.Y., Mountney A., Liao Z., Yang W., Lu X.C., Dave J., Deng-Bryant Y., Wei G., Schmid K., Shear D.A., and Tortella F.C. (2012). A novel animal model of closed-head concussive-induced mild traumatic brain injury: development, implementation, and characterization. J. Neurotrauma 29, 268–280 [DOI] [PubMed] [Google Scholar]
- 41.Smith D.H., and Meaney D.F. (2000). Axonal damage in traumatic brain injury. The Neuroscientist 6, 483–495 [Google Scholar]
- 42.Marmarou A., Foda M.A., van den Brink W., Campbell J., Kita H., and Demetriadou K. (1994). A new model of diffuse brain injury in rats. Part I: Pathophysiology and biomechanics. J. Neurosurg. 80, 291–300 [DOI] [PubMed] [Google Scholar]
- 43.Chen Y., Constantini S., Trembovler V., Weinstock M., and Shohami E. (1996). An experimental model of closed head injury in mice: pathophysiology, histopathology, and cognitive deficits. J. Neurotrauma 13, 557–568 [DOI] [PubMed] [Google Scholar]
- 44.Mouzon B.C., Bachmeier C., Ferro A., Ojo J.O., Crynen G., Acker C.M., Davies P., Mullan M., Stewart W. and Crawford F. (2014). Chronic neuropathological and neurobehavioral changes in a repetitive mild traumatic brain injury model. Ann. Neurol. 75, 241–254 [DOI] [PubMed] [Google Scholar]
- 45.Bennett R.E., Mac Donald C.L., and Brody D.L. (2012). Diffusion tensor imaging detects axonal injury in a mouse model of repetitive closed-skull traumatic brain injury. Neurosci. Lett. 513, 160–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Creed J.A., DiLeonardi A.M., Fox D.P., Tessler A.R., and Raghupathi R. (2011). Concussive brain trauma in the mouse results in acute cognitive deficits and sustained impairment of axonal function. J. Neurotrauma 28, 547–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hylin M.J., Orsi S.A., Rozas N.S., Hill J.L., Zhao J., Redell J.B., Moore A.N., and Dash P.K. (2013). Repeated mild closed head injury impairs short-term visuospatial memory and complex learning. J. Neurotrauma 30, 716–726 [DOI] [PubMed] [Google Scholar]
- 48.Mouzon B., Chaytow H., Crynen G., Bachmeier C., Stewart J., Mullan M., Stewart W., and Crawford F. (2012). Repetitive mild traumatic brain injury in a mouse model produces learning and memory deficits accompanied by histological changes. J. Neurotrauma 29, 2761–2773 [DOI] [PubMed] [Google Scholar]
- 49.Shitaka Y., Tran H.T., Bennett R.E., Sanchez L., Levy M.A., Dikranian K., and Brody D.L. (2011). Repetitive closed-skull traumatic brain injury in mice causes persistent multifocal axonal injury and microglial reactivity. J. Neuropathol. Exp. Neurol. 70, 551–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zweckberger K., Eros C., Zimmermann R., Kim S.W., Engel D., and Plesnila N. (2006). Effect of early and delayed decompressive craniectomy on secondary brain damage after controlled cortical impact in mice. J. Neurotrauma 23, 1083–1093 [DOI] [PubMed] [Google Scholar]
- 51.Zweckberger K., Stoffel M., Baethmann A., and Plesnila N. (2003). Effect of decompression craniotomy on increase of contusion volume and functional outcome after controlled cortical impact in mice. J. Neurotrauma 20, 1307–1314 [DOI] [PubMed] [Google Scholar]
- 52.Leung L.Y., Larimore Z., Holmes L., Cartagena C., Mountney A., Deng-Bryant Y., Schmid K., Shear D. and Tortella F. (2014). The WRAIR projectile concussive impact model of mild traumatic brain injury: re-design, testing and preclinical validation. Ann. Biomed. Eng. 42, 1618–1630 [DOI] [PubMed] [Google Scholar]
- 53.Phillips Y.Y. and Richmond D.R. (1991). Primary blast injury and basic research: a brief history, in: Conventional Warfare: Ballistic, Blast, and Burn Injuries. Bellamy R.F., Zajtchuk R. (eds) [Google Scholar]
- 54.Hoge C.W., McGurk D., Thomas J.L., Cox A.L., Engel C.C., and Castro C.A. (2008). Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N. Engl. J. Med. 358, 453–463 [DOI] [PubMed] [Google Scholar]
- 55.Okie S. (2005). Traumatic brain injury in the war zone. N. Engl. J. Med. 352, 2043–2047 [DOI] [PubMed] [Google Scholar]
- 56.Warden D. (2006). Military TBI during the Iraq and Afghanistan wars. J. Head Trauma Rehabil. 21, 398–402 [DOI] [PubMed] [Google Scholar]
- 57.DePalma R.G., Burris D.G., Champion H.R., and Hodgson M.J. (2005). Blast injuries. N. Engl. J. Med. 352, 1335–1342 [DOI] [PubMed] [Google Scholar]
- 58.Moore D.F., Jérusalem A., Nyein M., Noels L., Jaffee M.S., and Radovitzky R.A. (2009). Computational biology—modeling of primary blast effects on the central nervous system. Neuroimage 47, T10–T20 [DOI] [PubMed] [Google Scholar]
- 59.Goldstein L.E., Fisher A.M., Tagge C.A., Zhang X.L., Velisek L., Sullivan J.A., Upreti C., Kracht J.M., Ericsson M., Wojnarowicz M.W., Goletiani C.J., Maglakelidze G.M., Casey N., Moncaster J.A., Minaeva O., Moir R.D., Nowinski C.J., Stern R.A., Cantu R.C., Geiling J., Blusztajn J.K., Wolozin B.L., Ikezu T., Stein T.D., Budson A.E., Kowall N.W., Chargin D., Sharon A., Saman S., Hall G.F., Moss W.C., Cleveland R.O., Tanzi R.E., Stanton P.K., and McKee A.C. (2012). Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci. Transl. Med. 4, 134ra160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garman R.H., Jenkins L.W., Switzer R.C., III, Bauman R.A., Tong L.C., Swauger P.V., Parks S., Ritzel D.V., Dixon C.E., Clark R., Bayir H., Kagan V., Jackson E., and Kochanek P.M. (2011). Blast exposure in rats with body shielding is characterized primarily by diffuse axonal injury. J. Neurotrauma 28, 947–959 [DOI] [PubMed] [Google Scholar]
- 61.Saljo A., Mayorga M., Bolouri H., Svensson B., and Hamberger A. (2011). Mechanisms and pathophysiology of the low-level blast brain injury in animal models. Neuroimage 54,Suppl 1, S83–S88 [DOI] [PubMed] [Google Scholar]
- 62.Kaur C., Singh J., Lim M.K., Ng B.L., Yap E.P., and Ling E.A. (1995). The response of neurons and microglia to blast injury in the rat brain. Neuropathol. Appl. Neurobiol. 21, 369–377 [DOI] [PubMed] [Google Scholar]
- 63.Kaur C., Singh J., Lim M.K., Ng B.L., Yap E.P., and Ling E.A. (1997). Ultrastructural changes of macroglial cells in the rat brain following an exposure to a non-penetrative blast. Ann. Acad. Med. Singapore 26, 27–29 [PubMed] [Google Scholar]
- 64.Lu J., Ng K.C., Ling G., Wu J., Poon D.J., Kan E.M., Tan M.H., Wu Y.J., Li P., Moochhala S., Yap E., Lee L.K., Teo M., Yeh I.B., Sergio D.M., Chua F., Kumar S.D., and Ling E.A. (2012). Effect of blast exposure on the brain structure and cognition in Macaca fascicularis. J. Neurotrauma 29, 1434–1454 [DOI] [PubMed] [Google Scholar]
- 65.Bauman R.A., Ling G.S., Tong L., Januszkiewicz A., Agoston D., Delanerolle N., Kim J., Ritzel D., Bell R., Ecklund J.M., Armonda R., Bandak F., and Parks S. (2009). An introductory characterization of a combat-casualty-care relevant swine model of closed head injury resulting from exposure to explosive blast. J. Neurotrauma 26, 841–860 [DOI] [PubMed] [Google Scholar]
- 66.de Lanerolle N.C., Bandak F., Kang D., Li A.Y., Du F., Swauger P., Parks S., Ling G., and Kim J.H. (2011). Characteristics of an explosive blast-induced brain injury in an experimental model. J. Neuropathol. Exp. Neurol. 70, 1046–1057 [DOI] [PubMed] [Google Scholar]
- 67.Saljo A., Bao F., Hamberger A., Haglid K.G., and Hansson H.A. (2001). Exposure to short-lasting impulse noise causes microglial and astroglial cell activation in the adult rat brain. Pathophysiology 8, 105–111 [DOI] [PubMed] [Google Scholar]
- 68.Saljo A., Bao F., Jingshan S., Hamberger A., Hansson H.A., and Haglid K.G. (2002). Exposure to short-lasting impulse noise causes neuronal c-Jun expression and induction of apoptosis in the adult rat brain. J. Neurotrauma 19, 985–991 [DOI] [PubMed] [Google Scholar]
- 69.Saljo A., Bao F., Shi J., Hamberger A., Hansson H.A., and Haglid K.G. (2002). Expression of c-Fos and c-Myc and deposition of beta-APP in neurons in the adult rat brain as a result of exposure to short-lasting impulse noise. J. Neurotrauma 19, 379–385 [DOI] [PubMed] [Google Scholar]
- 70.Cernak I., Wang Z., Jiang J., Bian X., and Savic J. (2001). Cognitive deficits following blast injury-induced neurotrauma: possible involvement of nitric oxide. Brain Inj. 15, 593–612 [DOI] [PubMed] [Google Scholar]
- 71.Cernak I., Wang Z., Jiang J., Bian X., and Savic J. (2001). Ultrastructural and functional characteristics of blast injury-induced neurotrauma. J. Trauma 50, 695–706 [DOI] [PubMed] [Google Scholar]
- 72.Long J.B., Bentley T.L., Wessner K.A., Cerone C., Sweeney S., and Bauman R.A. (2009). Blast overpressure in rats: recreating a battlefield injury in the laboratory. J. Neurotrauma 26, 827–840 [DOI] [PubMed] [Google Scholar]
- 73.Readnower R.D., Chavko M., Adeeb S., Conroy M.D., Pauly J.R., McCarron R.M., and Sullivan P.G. (2010). Increase in blood-brain barrier permeability, oxidative stress, and activated microglia in a rat model of blast-induced traumatic brain injury. J. Neurosci. Res. 88, 3530–3539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Saljo A., Bolouri H., Mayorga M., Svensson B., and Hamberger A. (2010). Low-level blast raises intracranial pressure and impairs cognitive function in rats: prophylaxis with processed cereal feed. J. Neurotrauma 27, 383–389 [DOI] [PubMed] [Google Scholar]
- 75.Park E., Gottlieb J.J., Cheung B., Shek P.N., and Baker A.J. (2011). A model of low-level primary blast brain trauma results in cytoskeletal proteolysis and chronic functional impairment in the absence of lung barotrauma. J. Neurotrauma 28, 343–357 [DOI] [PubMed] [Google Scholar]
- 76.Cullen D.K., Browne K.D., Xu Y., Adeeb S., Wolf J.A., McCarron R.M., Yang S., Chavko M., and Smith D.H. (2011). Blast-induced color change in photonic crystals corresponds with brain pathology. J. Neurotrauma 28, 2307–2318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Koliatsos V.E., Cernak I., Xu L., Song Y., Savonenko A., Crain B.J., Eberhart C.G., Frangakis C.E., Melnikova T., Kim H., and Lee D. (2011). A mouse model of blast injury to brain: initial pathological, neuropathological, and behavioral characterization. J. Neuropathol. Exp. Neurol. 70, 399–416 [DOI] [PubMed] [Google Scholar]
- 78.Cernak I., Merkle A.C., Koliatsos V.E., Bilik J.M., Luong Q.T., Mahota T.M., Xu L., Slack N., Windle D., and Ahmed F.A. (2011). The pathobiology of blast injuries and blast-induced neurotrauma as identified using a new experimental model of injury in mice. Neurobiol. Dis. 41, 538–551 [DOI] [PubMed] [Google Scholar]
- 79.Rafaels K.A., Bass C.R., Panzer M.B., Salzar R.S., Woods W.A., Feldman S.H., Walilko T., Kent R.W., Capehart B.P., Foster J.B., Derkunt B., and Toman A. (2012). Brain injury risk from primary blast. J. Trauma Acute Care Surg. 73, 895–901 [DOI] [PubMed] [Google Scholar]
- 80.Saljo A., Bao F., Haglid K.G., and Hansson H.A. (2000). Blast exposure causes redistribution of phosphorylated neurofilament subunits in neurons of the adult rat brain. J. Neurotrauma 17, 719–726 [DOI] [PubMed] [Google Scholar]
- 81.Reneer D.V., Hisel R.D., Hoffman J.M., Kryscio R.J., Lusk B.T., and Geddes J.W. (2011). A multi-mode shock tube for investigation of blast-induced traumatic brain injury. J. Neurotrauma 28, 95–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Coronado V.G., Xu L., Basavaraju S.V., McGuire L.C., Wald M.M., Faul M.D., Guzman B.R., and Hemphill J.D.; Centers for Disease Control and Prevention (CDC). (2011). Surveillance for traumatic brain injury-related deaths—United States, 1997–2007. MMWR Surveill. Summ. 60, 1–32 [PubMed] [Google Scholar]
- 83.Asemota A.O., George B.P., Bowman S.M., Haider A.H., and Schneider E.B. (2013). Causes and trends in traumatic brain injury for United States adolescents. J. Neurotrauma 30, 67–75 [DOI] [PubMed] [Google Scholar]
- 84.Sapsford W. (2003). Penetrating brain injury in military conflict: does it merit more research? J. R. Army Med. Corps 149, 5–14 [DOI] [PubMed] [Google Scholar]
- 85.Loncarevic-Vasiljkovic N., Pesic V., Tanic N., Milanovic D., Popic J., Kanazir S., and Ruzdijic S. (2009). Changes in markers of neuronal and glial plasticity after cortical injury induced by food restriction. Exp. Neurol. 220, 198–206 [DOI] [PubMed] [Google Scholar]
- 86.Nishihara T., Ochi M., Sugimoto K., Takahashi H., Yano H., Kumon Y., Ohnishi T., and Tanaka J. (2011). Subcutaneous injection containing IL-3 and GM-CSF ameliorates stab wound-induced brain injury in rats. Exp. Neurol. 229, 507–516 [DOI] [PubMed] [Google Scholar]
- 87.DeKosky S.T., Styren S.D., O'Malley M.E., Goss J.R., Kochanek P., Marion D., Evans C.H., and Robbins P.D. (1996). Interleukin-1 receptor antagonist suppresses neurotrophin response in injured rat brain. Ann. Neurol. 39, 123–127 [DOI] [PubMed] [Google Scholar]
- 88.Mueller C.A., Schluesener H.J., Fauser U., Conrad S., and Schwab J.M. (2007). Lesional expression of the endogenous angiogenesis inhibitor endostatin/collagen XVIII following traumatic brain injury (TBI). Exp. Neurol. 208, 228–237 [DOI] [PubMed] [Google Scholar]
- 89.Williams A.J., Hartings J.A., Lu X.C., Rolli M.L., Dave J.R., and Tortella F.C. (2005). Characterization of a new rat model of penetrating ballistic brain injury. J. Neurotrauma 22, 313–331 [DOI] [PubMed] [Google Scholar]
- 90.Williams A.J., Hartings J.A., Lu X.C., Rolli M.L., and Tortella F.C. (2006). Penetrating ballistic-like brain injury in the rat: differential time courses of hemorrhage, cell death, inflammation, and remote degeneration. J. Neurotrauma 23, 1828–1846 [DOI] [PubMed] [Google Scholar]
- 91.Williams A.J., Ling G.S., and Tortella F.C. (2006). Severity level and injury track determine outcome following a penetrating ballistic-like brain injury in the rat. Neurosci. Lett. 408, 183–188 [DOI] [PubMed] [Google Scholar]
- 92.Miller J.D., Bullock R., Graham D.I., Chen M.H., and Teasdale G.M. (1990). Ischemic brain damage in a model of acute subdural hematoma. Neurosurgery 27, 433–439 [DOI] [PubMed] [Google Scholar]
- 93.Balikci M., Koc K., Anik I., Anik Y., Cekmen M.B., Yazir Y., Ceylan S., and Ceylan S. (2008). Biochemical effects of experimental epidural hematoma on brain parenchyma of rats. Neurol. Res. 30, 450–456 [DOI] [PubMed] [Google Scholar]
- 94.Ganz J.C., and Zwetnow N.N. (1988). Analysis of the dynamics of experimental epidural bleeding in swine. Acta Neurochir. (Wien) 95, 72–81 [DOI] [PubMed] [Google Scholar]
- 95.Sasaki M., and Dunn L. (2001). A model of acute subdural hematoma in the mouse. J. Neurotrauma 18, 1241–1246 [DOI] [PubMed] [Google Scholar]
- 96.Wang D., Jiang R., Liu L., Dong J.F., and Zhang J.N. (2010). Membrane neovascularization and drainage of subdural hematoma in a rat model. J. Neurotrauma 27, 1489–1498 [DOI] [PubMed] [Google Scholar]
- 97.Tsuchida E., and Bullock R. (1995). The effect of the glycine site-specific N-methyl-D-aspartate antagonist ACEA1021 on ischemic brain damage caused by acute subdural hematoma in the rat. J. Neurotrauma 12, 279–288 [DOI] [PubMed] [Google Scholar]
- 98.Ebmeyer U., Safar P., Radovsky A., Obrist W., Alexander H., and Pomeranz S. (1998). Moderate hypothermia for 48 hours after temporary epidural brain compression injury in a canine outcome model. J. Neurotrauma 15, 323–336 [DOI] [PubMed] [Google Scholar]
- 99.Ryan J.B., Hicks M., Cropper J.R., Garlick S.R., Kesteven S.H., Wilson M.K., Feneley M.P., and Macdonald P.S. (2003). Functional evidence of reversible ischemic injury immediately after the sympathetic storm associated with experimental brain death. J. Heart Lung Transplant. 22, 922–928 [DOI] [PubMed] [Google Scholar]
- 100.Janda M., Bajorat J., Simanski O., Noldge-Schomburg G., Hofmockel R., and Schutze M. (2012). A surgical technique for a terminal intracranial hypertension model in pigs. Lab. Anim. 46, 258–260 [DOI] [PubMed] [Google Scholar]
- 101.Daley M.L., Pasupathy H., Griffith M., Robertson J.T., and Leffler C.W. (1995). Detection of loss of cerebral vascular tone by correlation of arterial and intracranial pressure signals. IEEE Trans. Biomed. Eng. 42, 420–424 [DOI] [PubMed] [Google Scholar]
- 102.Fitch W., McDowall D.G., Keaney N.P., and Pickerodt V.W. (1977). Systemic vascular responses to increased intracranial pressure. 2. The ‘Cushing’ response in the presence of intracranial space-occupying lesions: systemic and cerebral haemodynamic studies in the dog and the baboon. J. Neurol. Neurosurg. Psychiatry 40, 843–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Johnston I.H., Rowan J.O., Harper A.M., and Jennett W.B. (1973). Raised intracranial pressure and cerebral blood flow. 2. Supratentorial and infratentorial mass lesions in primates. J. Neurol. Neurosurg. Psychiatry 36, 161–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nitta M., Tsutsui T., Ueda Y., Ladds A., and Symon L. (1990). The effects of an extradural expanding lesion on regional intracranial pressure, blood flow, somatosensory conduction and brain herniation: an experimental study in baboons. Acta Neurochir. (Wien.) 104, 30–37 [DOI] [PubMed] [Google Scholar]
- 105.Shalit M.N., and Cotev S. (1974). Interrelationship between blood pressure and regional cerebral blood flow in experimental intracranial hypertension. J. Neurosurg. 40, 594–602 [DOI] [PubMed] [Google Scholar]
- 106.Barbiro-Micahely E., and Mayevsky A. (2001). Multiparametric monitoring of brain under elevated intracranial pressure in a rat model. J. Neurotrauma 18, 711–725 [DOI] [PubMed] [Google Scholar]
- 107.Botel C., and Brinker T. (1994). Measurement of the dynamics of the cerebrospinal fluid system in the rat. J. Exp. Anim. Sci. 36, 78–83 [PubMed] [Google Scholar]
- 108.Hauerberg J., and Juhler M. (1994). Cerebral blood flow autoregulation in acute intracranial hypertension. J. Cereb. Blood Flow Metab. 14, 519–525 [DOI] [PubMed] [Google Scholar]
- 109.Malkinson T.J., Cooper K.E., and Veale W.L. (1985). Induced changes in intracranial pressure in the anesthetized rat and rabbit. Brain Res. Bull. 15, 321–328 [DOI] [PubMed] [Google Scholar]
- 110.Tsai M.L., and Wang B.H. (1988). Effects of propranolol on the cardiovascular responses to intracranial pressure elevation in rats. Chin. J. Physiol. 31, 113–124 [PubMed] [Google Scholar]
- 111.Meaney D.F., Smith D.H., Shreiber D.I., Bain A.C., Miller R.T., Ross D.T,. and Gennarelli T.A. (1995). Biomechanical analysis of experimental diffuse axonal injury. J. Neurotrauma 12, 689–694 [DOI] [PubMed] [Google Scholar]
- 112.Graham D.I., and Lantos P.L. (2002). Greenfield's Neuropathology. 7th ed. Arnold: London [Google Scholar]
- 113.Adams J.H., Graham D.I., Murray L.S., and Scott G. (1982). Diffuse axonal injury due to nonmissile head injury in humans: an analysis of 45 cases. Ann. Neurol. 12, 557–563 [DOI] [PubMed] [Google Scholar]
- 114.Adams J.H., Doyle D., Ford I., Gennarelli T.A., Graham D.I., and McLellan D.R. (1989). Diffuse axonal injury in head injury: definition, diagnosis and grading. Histopathology 15, 49–59 [DOI] [PubMed] [Google Scholar]
- 115.Adams J.H., Graham D.I., Gennarelli T.A., and Maxwell W.L. (1991). Diffuse axonal injury in non-missile head injury. J. Neurol. Neurosurg. Psychiatry 54, 481–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Graham D.I., Adams J.H., Nicoll J.A., Maxwell W.L., and Gennarelli T.A. (1995). The nature, distribution and causes of traumatic brain injury. Brain Pathol. 5, 397–406 [DOI] [PubMed] [Google Scholar]
- 117.Johnson V.E., Stewart W., and Smith D.H. (2013). Axonal pathology in traumatic brain injury. Exp. Neurol. 246, 35–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Smith D.H., Meaney D.F., Shull W.H. (2003). Diffuse axonal injury in head trauma. J. Head Trauma Rehab 18, 307–316 [DOI] [PubMed] [Google Scholar]
- 119.Margulies S.S., Thibault L.E., and Gennarelli T.A. (1990). Physical model simulations of brain injury in the primate. J. Biomech. 23, 823–836 [DOI] [PubMed] [Google Scholar]
- 120.Holbourn A.H. (1943). Mechanics of head injury. Lancet 242, 438–441 [Google Scholar]
- 121.Holbourn A.H. (1945). Mechanics of brain injuries. Brit. Med. Bull. 3, 147–148 [Google Scholar]
- 122.Thibault L., Gennarelli TA, Margulies SS, et al. (1990). The Strain dependent pathophysiological consequences of inertial loading on central nervous system tissue. Proceedings of the International Conference on the Biomechanics of Impact Lyon, France, pp. 191–202 [Google Scholar]
- 123.Gennarelli T.A., Thibault L.E., Adams J.H., Graham D.I., Thompson C.J., and Marcincin R.P. (1982). Diffuse axonal injury and traumatic coma in the primate. Ann. Neurol. 12, 564–574 [DOI] [PubMed] [Google Scholar]
- 124.Ross D.T., Meaney D.F., Sabol M.K., Smith D.H., and Gennarelli T.A. (1994). Distribution of forebrain diffuse axonal injury following inertial closed head injury in miniature swine. Exp. Neurol. 126, 291–299 [DOI] [PubMed] [Google Scholar]
- 125.Ibrahim N.G., Ralston J., Smith C., and Margulies S.S. (2010). Physiological and pathological responses to head rotations in toddler piglets. J. Neurotrauma 27, 1021–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Raghupathi R., and Margulies S.S. (2002). Traumatic axonal injury after closed head injury in the neonatal pig. J. Neurotrauma 19, 843–853 [DOI] [PubMed] [Google Scholar]
- 127.Browne K.D., Chen X.H., Meaney D.F., and Smith D.H. (2011). Mild traumatic brain injury and diffuse axonal injury in swine. J. Neurotrauma 28, 1747–1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Smith D.H., Nonaka M., Miller R., Leoni M., Chen X.H., Alsop D., and Meaney D.F. (2000). Immediate coma following inertial brain injury dependent on axonal damage in the brainstem. J. Neurosurg. 93, 315–322 [DOI] [PubMed] [Google Scholar]
- 129.Knight J. (2003). Negative results: Null and void. Nature 422, 554–555 [DOI] [PubMed] [Google Scholar]
- 130.Dirnagl U., and Lauritzen M. (2010). Fighting publication bias: introducing the Negative Results section. J. Cereb. Blood Flow Metab. 30, 1263–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lemmon V.P., Ferguson A.R., Popovich P.G., Xu X.M., Snow D.M., Igarashi M., Beattie C.E., and Bixby J.L.; MAS Consortium. (2014). Minimum information about a spinal cord injury experiment: a proposed reporting standard for spinal cord injury experiments. J. Neurotrauma 31, 1354–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]