Abstract
An overall decrease of HIV prevalence is now observed in several key Asian countries due to effective prevention programs. The decrease in HIV prevalence and incidence may further improve with the scale-up of combination prevention interventions. The implementation of future prevention trials then faces important challenges. The opportunity to identify heterosexual populations at high risk such as female sex workers may rapidly wane. With unabating HIV epidemics among men who have sex with men (MSM) and transgender (TG) populations, an effective vaccine would likely be the only option to turn the epidemic. It is more likely that efficacy trials will occur among MSM and TG because their higher HIV incidence permits smaller and less costly trials. The constantly evolving patterns of HIV-1 diversity in the region suggest close monitoring of the molecular HIV epidemic in potential target populations for HIV vaccine efficacy trials. CRF01_AE remains predominant in southeast Asian countries and MSM populations in China. This relatively steady pattern is conducive to regional efficacy trials, and as efficacy warrants, to regional licensure. While vaccines inducing nonneutralizing antibodies have promise against HIV acquisition, vaccines designed to induce broadly neutralizing antibodies and cell-mediated immune responses of greater breadth and depth in the mucosal compartments should be considered for testing in MSM and TG. The rationale and design of efficacy trials of combination prevention modalities such as HIV vaccine and preexposure prophylaxis (PrEP) remain hypothetical, require high adherence to PrEP, are more costly, and present new regulatory challenges. The prioritization of prevention interventions should be driven by the HIV epidemic and decided by the country-specific health and regulatory authorities. Modeling the impact and cost–benefit may help this decision process.
Introduction
In 2012, there were 4.3 billion people living in Asia, about 60% of the global population, 2.9 billion people in the economically productive age band between 15 and 64 years and 750 million young women and men aged 15 to 24 years.1 The Asia Pacific region is economically dynamic.1 Trade and tourism in Asia are thriving and are expected to expand over the next decade. The further development of roads, communication, and other major infrastructure promises to intensify the interchange between China, India, and southeast Asia (SEA), and the emergence of Myanmar after long isolation will generate new economic activity. These elements may influence the evolution of the HIV epidemic patterns, HIV prevention programs, and, consequently, the feasibility and design of prevention trials in Asia. Despite its early prominence in the epidemic (Thailand and Cambodia), HIV-1 prevalence remains under 1% of the population in most countries.
The HIV epidemic in Asia remains concentrated in nature, affecting mostly higher risk subgroups, including men who have sex with men (MSM) and transgender women (TG), people who inject drugs (PWID), and female sex workers (FSW).2 These epidemiological features have considerable implications for the implementation of prevention intervention trials and further implementation of prevention policies in the region.
The data presented in this review were retrieved from several sources: 180 regional or country-specific articles listed on PubMed, 10 UNAIDS and WHO reports, and 7 country-specific reports from national health authorities. The HIV molecular epidemiology data were retrieved from published articles and from our original work. Specifically, the partial and full genome of HIV-1 sequences obtained from the Los Alamos National Laboratory HIV database (www.hiv.lanl.gov), from the U.S. Military HIV Research Program (MHRP), and from HIV-1 subtyping data from MHRP (unpublished data) were used to compile circulating subtype distributions by country from 2000 to 2007 and from 2008 to 2014. Although part of the Russian Federation is geographically located in Asia, most of the HIV epidemic is located in the eastern part of Russia.3 We therefore did not include the Russian Federation in our review.
We describe the current features of the HIV epidemic in high-risk populations of countries already engaged in prevention trials including HIV vaccine, preexposure prophylaxis (PrEP), and treatment as prevention (TasP) and discuss the implications for prevention trial design and implementation.
HIV Epidemic in Asia
Regional patterns and most at-risk populations
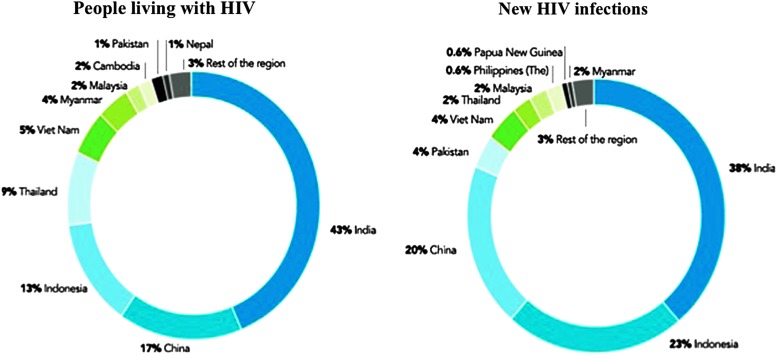
According to UNAIDS, at the end of 2013, in Asia and the Pacific there were 4.8 million people living with HIV, with China, India, Indonesia, Myanmar, Thailand, and Vietnam accounting for more than 90% of the people living with HIV in the region. Low national prevalence masks higher HIV prevalence and incidence rates in certain key populations with mixed pictures between and within countries. Figure 1 shows the percentage of people living with HIV and of new HIV infections by country.3 New infections declined by 58% in Myanmar, by 46% in Thailand, by 43% in Vietnam, and by 19% in India. New epidemics are emerging in the Philippines where, between 2001 and 2012, new HIV infections more than doubled, and in Indonesia with a 48% increase. The number of AIDS-related deaths in Asia fell by 37% between 2005 and 2013. For infected persons with access to antiretroviral therapy (ART), HIV infection has become a manageable chronic condition. Under the 2010 World Health Organization (WHO) guidelines, the overall treatment coverage is approximately 30% in Asia and the Pacific.4
FIG. 1.
HIV epidemic in Asia and the Pacific, 2013. Proportion by country of people living with HIV and of new HIV infections. UNAIDS Gap Report 2014.3
New HIV infections are concentrated among key populations at higher risk, more difficult to reach due to stigma and legal barriers. These key populations include PWID, FSW and their clients, MSM, and TG,5–8 and are mostly concentrated in major cities. In a recent meta-analysis of the HIV mode of transmission model (based on published data and UNAIDS reports from generalized and concentrated epidemics from 29 countries between 2003 and 2012), the estimated annual fraction of new HIV infections (FNI) among FSW remained low, with little variability by region and epidemic type despite variability in sexual behavior. In India and Thailand for example, the FNI among FSW was 2% and 4%, respectively. In contrast, the FNI among MSM was higher than in FSW, varied with countries, and increased with MSM population size.9
In a systematic review in low- and middle-income countries, the burden of HIV infection was disproportionately high among FSW in Asia and the Pacific, with a 29-fold increase in odds of living with HIV compared with women of reproductive age.10 As observed with other key populations, some geographic areas exhibit a higher HIV prevalence, e.g., in 2012, 22.5% in Hanoi,10 25% in Jayawijaya, Indonesia,11 and 15% in Pathein, Myanmar.12 In 2007, street-based FSW in Thailand had an especially high HIV prevalence (22.7%), 10 times higher than among venue-based FSW (2.5%).13 Table 1 shows available HIV prevalence and incidence figures among key populations in selected countries.
Table 1.
HIV Prevalence and Incidence in Key Populations in Thailand, China, Myanmar, and India
| HIV prevalence | HIV incidence | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| MSM | TG | PWID | FSW | MSM | TG | PWID | FSW | References | |
| Thailand | |||||||||
| Nationwide | 5.5–28.3% (2003–2007) | 12.5% (2000–2011) | 11.9% (2007–2011) | 8.2% (2006–2008) | 6,8,10,75 | ||||
| Bangkok | 30.8% (2007) | 23.6% (2013) | 22.7% street based; 2.5% venue based (2007) |
6.4% (2005) 1.95% (2008–2009) |
0.68% (2010) | 6,13,143,175 | |||
| 21.3% (2011) |
7.7% (2007) 5.9% (2011) |
7 | |||||||
|
2.7% (60% were MSM) 2006–2007 |
23 | ||||||||
| Chiang Mai | 16.9% (2007) | 10.9% (2013) | 75,175 | ||||||
| 16.5–6.9% in bisexuals (2011) | 9.30% (2011) | 8.2% (2011) | 75 | ||||||
| Bangkok, Ubon, Ratchathani, Lampang, Mahasarakam | 6.3% (2014) | Nittaya Phanuphak, unpublished data | |||||||
| Pattaya | 7.4% (2014) | 5% (2014) | 0.83% (2014) | Merlin Robb, personal communication | |||||
| Myanmar | |||||||||
| Nationwide | 7.8% (2011) | 18.4% (2008) 9.4% (2012) | 12,77 | ||||||
| Yangon | 5% (2011) | ||||||||
| Mandalay | 9% (2011) | ||||||||
| Pathein | 15% (2012) | 10,12 | |||||||
| China | |||||||||
| Nationwide | 7.4% (2.3–11.4) (2009) | 84 | |||||||
| 6% (2010) | 9% (2010) | 0.36% (2010) | 0.57% (2010) | 0.02% (2010) | 81 | ||||
| 4.9–18% (2008–2009) | 85 | ||||||||
| Beijing | 7.8% (2010) | 1.7% (2010) | 96 | ||||||
| Study in 16 cities | 5.3% (2009) | 94 | |||||||
| India | |||||||||
| Nationwide | 4.4% (2011) | 7.1% (2011) | 2.7% (2011) | 12 | |||||
| Maharashtra | 19% (2012) | 15 | |||||||
| Study in 12 cities | 7% (2012–2013) | 0.87% (2012–2013) | 105 | ||||||
| Study in 15 cities | 18.1% (2013) | 2.9% (2013) | 100 | ||||||
This table focuses on the most recent available data in selected countries and key populations where prevention intervention efficacy trials might be considered. Empty cells correspond to no data available. Data in italics correspond to potential target populations suitable for HIV vaccine trials.
Estimates based on country information indicate that the regional population of MSM and TG that is at risk for HIV infection ranges from 10.5 to 27 million. TG women are 50 times more likely to acquire HIV than adult males or females of reproductive age. For example, 18% of surveyed male sex workers in Indonesia and Thailand tested HIV positive, 31% of TG sex workers in Jakarta, and 19% in Maharashtra.14
An estimated 3–4 million people living in Asia are PWID. In Indonesia and the Philippines, injecting drug use has been a significant factor in the spread of HIV. In 2012, HIV prevalence among PWID was 36.4% in Indonesia,14 13.6% in the Philippines,15 with an explosive epidemic in Cebu (53.8%), and 11.6% in Vietnam,16 with considerable geographic variations within countries. In contrast to previous figures on HIV prevalence in PWID,17,18 harm reduction programs have demonstrated a dramatic and beneficial impact on the epidemic in these populations.19
Little is known about the relative contribution of acute phase to HIV transmission in Asia. Individuals with acute HIV infection have an 8- to 26-fold greater risk for transmitting HIV compared to those with chronic infection because of their high viral load20–22 while they may remain undiagnosed by routine antibody tests.23 Phylogenetic clustering of new HIV infections in MSM supports acute HIV infection as one of the main drivers of ongoing HIV transmission in MSM in rising and ongoing HIV epidemics.24–26
HIV-1 molecular epidemiology
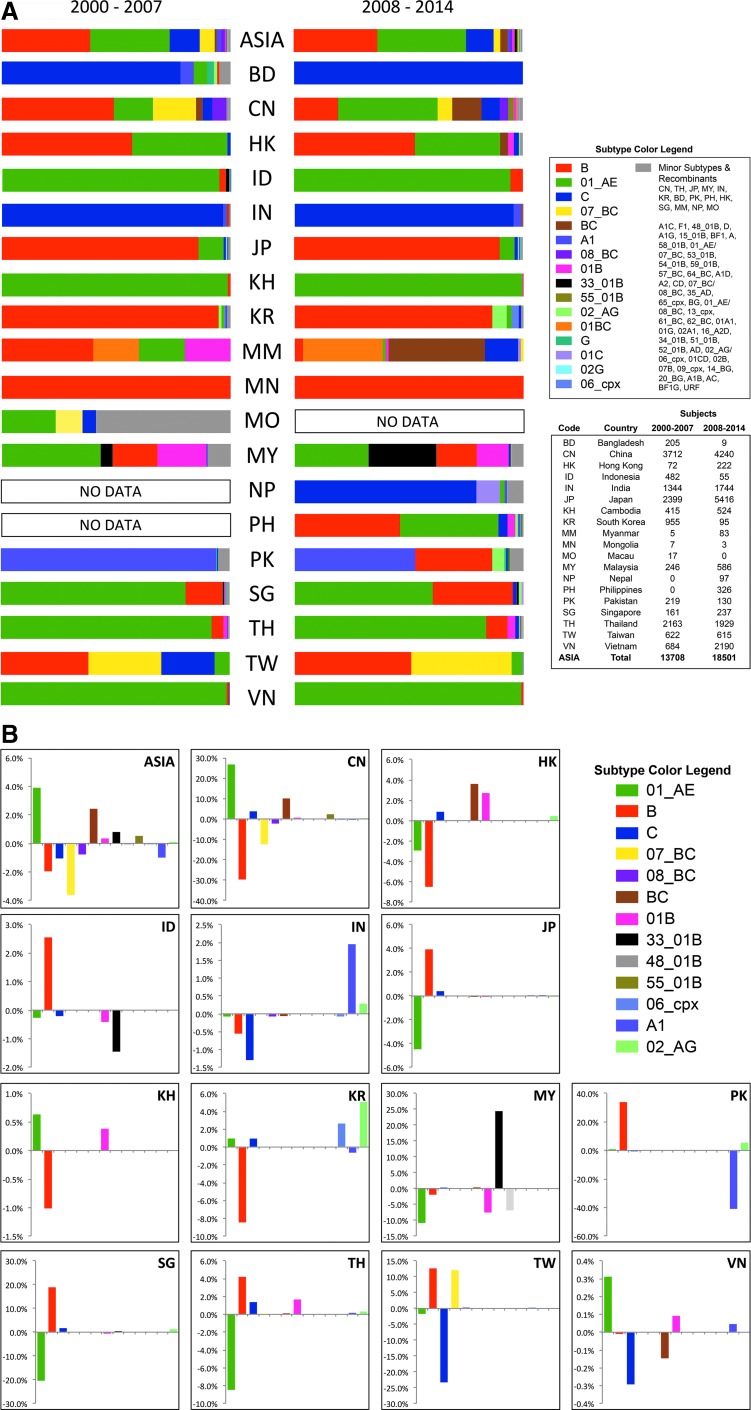
The geographic and temporal distribution of circulating HIV-1 subtypes by country from 2000 to 2007 and from 2008 to 2014 is shown in Fig. 2A. The relative percentage changes in HIV-1 subtype prevalence for selected countries is shown in Fig. 2B. CRF01_AE remains predominant in southeast Asia with a growing presence in China and an increasing number of recombinant forms containing CRF01_AE, B, and C subtypes.27–29 CRF01_AE dominates in Thailand (developed below), Cambodia,30 Indonesia,31 Laos, Myanmar,27 and Vietnam.32,33 In Malaysia, cocirculation of CRF01_AE and subtype B34 has resulted in the emergence of CRF33_01B in approximately 20% of HIV-1-infected individuals,35 now also described in Indonesia.36
FIG. 2.
HIV-1 molecular epidemiology in Asia. Partial and full genome of HIV-1 sequences obtained from the Los Alamos National Laboratory HIV database (www.hiv.lanl.gov) and the U.S. Military HIV Research Program (MHRP); HIV-1 subtyping data from the MHRP (personal communication, unpublished data) were used to compile circulating subtype distributions by country. (A) Geographic and temporal distribution of circulating HIV-1 subtypes by country for 2000–2007 and 2008–2014. (B) Relative percentage changes in HIV-1 subtype prevalence for selected countries and subtypes from 2000–2007 to 2008–2014. Bangladesh, Myanmar, Mongolia, Macau, Nepal, and Philippines are omitted due to insufficient data.
In Thailand, among 390 volunteers who were deferred from enrollment in the RV144 HIV vaccine trial due to preexisting HIV-1 infection molecular analyses showed the following subtype distribution: CRF01_AE: 91.7%, subtype B: 3.5%, B/CRF01_AE recombinants: 4.3%, and dual infections: 0.5%. CRF01_AE strains were 31% more diverse than those from the 1990s Thai epidemic that informed vaccine immunogen design, which represents a clear example of viral evolution that occurred between immunogen design and subsequent efficacy trial.37 The impact on vaccine efficacy is unknown. While sequence diversity of HIV remains a challenge for HIV vaccine development, the rate of diversification can present a problem given the timelines for efficacy testing.
In China, in a comprehensive mapping of HIV genotypes in various risk groups using samples collected between 2006 and 2008, CRF07_BC (35.5%), CRF01_AE (27.6%), CRF08_BC (20.1%), and Chinese/Thai subtype B or B′ (9.6%) were the four main HIV-1 strains. CRF07_BC and CRF08_BC were the primary drivers of infection among PWID. Recently, however, MSM is the risk group associated with the most rapid increases in HIV infections within China, and studies note HIV-1 strains of different subtypes and CRFs.38–42 In cities in northern China CRF01_AE infection in MSM is more common than subtype B,24,43–45 with similar trends in Beijing, Fujian, Guizho, Guandong, Guanxi, Hebei, Hong Kong, Hunan, Jiangsu, Jianxi, Shanghai, and Yunnan. CRF07_BC has recently been identified in young MSM and in some cities exceeds the prevalence of CRF01_AE infection (unpublished data).
The HIV epidemic among MSM is expanding to Japan and illustrates the ongoing mixing of CRF01_AE and subtype B lineages circulating among MSM populations in east Asia.46–48
Western Yunnan, bordering with Myanmar, is a known hotspot of recombination with the identification of CRF07_BC49,50 and CRF08_BC.51,52 In 2006, CRF01_AE was found (40.5%, mostly acquired by sexual transmission) at the Yunnan–Myanmar border.53 Recent data on HIV subtypes circulating in Myanmar are limited to the China–Myanmar border, mostly in PWID populations.54–56 A breakdown of HIV-1 in Yunnan includes CRF07_BC (18.9%), CRF08_BC (39.1%), and CRF01_AE (22.4%)57; pol sequences in newly diagnosed HIV-infected individuals from Dehong county, Yunnan, showed that subtype C accounted for 43.1%, URF for 18.4%, CRF01_AE for 17.7%, B for 10.7%, CRF08_BC for 8.4%, and CRF07_BC for 1.7%58; near-full-genome sequencing results show that many new CRFs have been generated in this region.59–62
In India, subtype C is the predominant circulating subtype,63–66 irrespective of the route of transmission,67,68 and has been reported as one of the parental strains of CRF07_BC and CRF08_BC in China.69 URFs of subtype C with B′ are increasing, especially in the northeastern states.27,70
Thailand
The Thai HIV epidemic is a very mature one. At its peak in 1991 Thailand had approximately 168,000 new adult HIV infections per year.71 Among other campaigns, the 100% condom program addressing the client–sex worker relationship and the reduction in the number of partners have brought a dramatic reduction in incidence, down to 8,135 new infections per year in 2013.72,73 The declining trend in HIV transmission rates despite an ever-growing prevalence indicates prevention success correlated with the national HIV/AIDS program.74
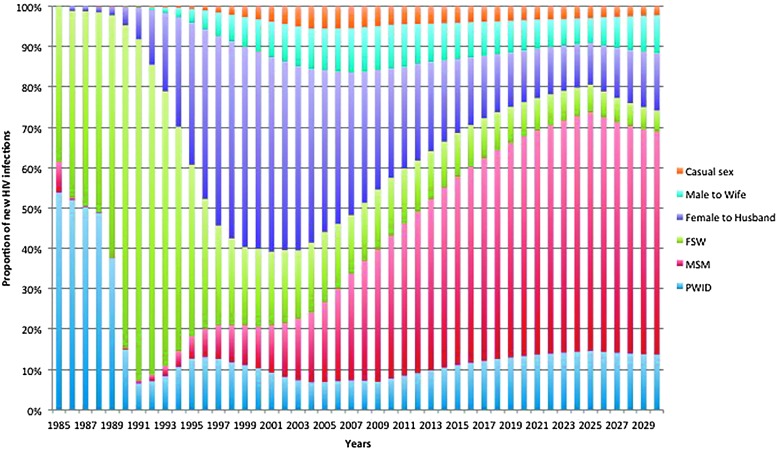
In 2007, HIV prevalence among MSM was 30.7% in Bangkok and 16.9% in Chiang Mai, essentially unchanged since 2005.6 The HIV prevalence found in subsequent studies ranged from 5.5% to 28.3% with an incidence rate of 8.2% in Chiang Mai6,75 and 6% in Bangkok between 2006 and 2008.7 The HIV prevalence in transgender women was estimated at 12.5% (5.1–19.9%) for the period 2000–2011.8 Another study conducted in Bangkok between 2008 and 2009 showed a much lower HIV incidence (1.95 per 100 person-years) among MSM recruited at the Thai Red Cross Anonymous Clinic through routine HIV counseling and testing activities.76 In 2013, 64% of new HIV infections occurred among key populations, 42% among MSM, while only 12% for FSW and clients and 10% for PWID.71 In a recent cohort study conducted in Pattaya, HIV incidence was 7.4 and 5.5 per 100 person-years among MSM and TG sex workers, respectively (Merlin Robb, personal communication). An ongoing “Test and Treat” cohort among MSM and TG in Bangkok, Ubon Ratchathani, Lampang, and Mahasarakam found a preliminary HIV incidence of 6.3% (Nittaya Phanuphak, unpublished data). The national projections show that MSM will remain the dominant key population for new HIV infections over the next 15 years (Fig. 3).71
FIG. 3.
Proportion of new HIV infections by mode of transmission, Thailand, 1985–2030. Thailand Working Group on HIV/AIDS Projection.71
Myanmar
Data on the HIV-1 epidemic in Myanmar remain scarce and of questionable representativeness. No HIV incidence data are available. In 2011, over 60% of new HIV infections occurred among FSW and their clients, PWID, and MSM. It is estimated that by the year 2015, these groups will constitute over 70% of new infections. According to the 2011 HIV sentinel serosurveillance, HIV prevalence rates among FSW dropped from 18.4% in 2008 to 9.4% in 2012.77 It is estimated that there are 40,000 to 80,000 FSW in Myanmar. The number of MSM is estimated at 240,000, mostly in Yangon and Mandalay, with an overall HIV prevalence of 7.8% (Mandalay 9%, Yangon 5%), with more than 81% having used a condom at last sex.12
Importantly, the current HIV epidemic figures in FSW may offer a unique, and perhaps the last, opportunity to access high-risk heterosexual populations for an HIV vaccine efficacy trial in SEA. However, this window of opportunity may quickly narrow as increased access to prevention services improve.78
China
Figure 4 shows the geographic distribution of cumulative HIV/AIDS cases at the end of 2011.79 Several HIV epidemic clusters have been described, illustrating the diversity of the epidemic.80 Zhang et al.81 used the public health data system of the Chinese Center for Disease Control and Prevention (China CDC) to characterize trends and prevalence for HIV from all 31 provinces. At the end of 2011, 780,000 people were living with HIV/AIDS in China, with 48,000 new infections. The routes of infection were predominately through heterosexual (52.2%) and homosexual (29.4%) transmission and through injecting drug use (18.0%). The HIV epidemic among PWID is decreasing in all regions outside southwest China and has stabilized at a high level in northwest China. Comparatively, HIV prevalence in FSW is much lower and stabilized at low levels in all regions except in the southwest. In 2010, the national HIV prevalence was 9.08% in PWID and 0.36% in FSW, with low incidence in both populations (0.57% and 0.02%, respectively).81
FIG. 4.
Geographic distribution of cumulative HIV/AIDS cases in China. China AIDS Response Progress Report, Ministry of Health of the People's Republic of China, UNAIDS, 2012.79
In contrast, HIV prevalence among Chinese MSM increased rapidly in all Chinese regions in the past decade and disproportionally affected southwest China where overlapping bisexual, commercial, and drug use behaviors are commonly observed.82 HIV prevalence in MSM increased from 1.77% in 2000 to 5.98% in 2010, with a national incidence of 0.98% in 2010.81 Several other studies confirm this trend.83 In a meta-analysis, the pooled HIV prevalence among MSM increased from 0.6% in 2003 to 7.4% in 2009 with a yearly increase of 1.1%. The pooled regional HIV prevalence ranged from 2.3% in east China to 11.4% in southwest China. The proportion of MSM in the annually reported HIV cases increased from 12% in 2007 to 33% in 2009.84 In 2008–2009, the overall HIV prevalence in 61 cities was 4.9%, with considerable heterogeneity between provinces. The highest prevalence was up to 18% in southwestern provinces.85
In the absence of interventions, HIV will spread very quickly in the MSM population86 with an estimated reproductive ratio of 3.9.87 Risk reduction interventions on HIV knowledge, attitudes, and behaviors were effective in reducing risk behaviors and improving knowledge and attitudes among Chinese MSM, but were not associated with a change in HIV prevalence.88 Approximately 25% of Chinese MSM are married,89 and 30% of these individuals have sex with a steady female partner, but with a low rate of condom use,90 constituting a dangerous bridge of HIV transmission from high-risk groups to the general population.91,92
Aggressive prevention interventions deployed by China over the past decade were unable to stem the HIV epidemic in MSM.93,94 A 4-fold increase in testing rates may prevent 42,000 new HIV infections among all at-risk groups in China over 5 years.95 Even enhanced levels of HIV testing and linkage to care including ART will not interrupt the expansion of the epidemic. Promoting condom use remains a crucial component of combination interventions in MSM.96
India
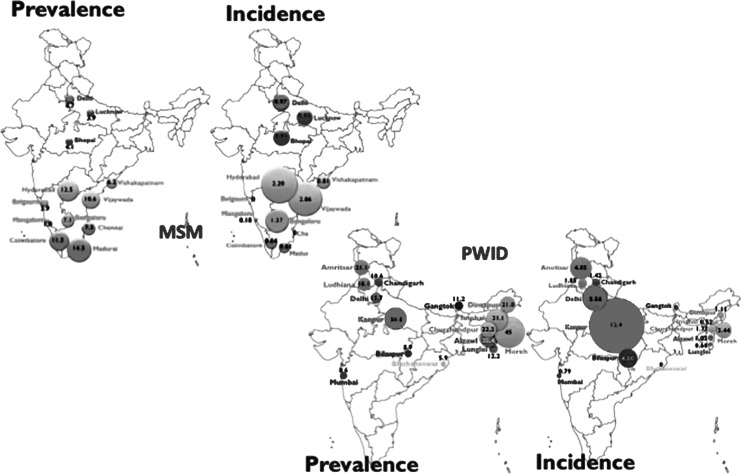
In 2011, the national adult HIV prevalence in India was 0.27% with a total number of people living with HIV/AIDS estimated at around 2.09 million, 39% among women, and 116,000 new infections, representing an overall reduction of 57% since 2000. Four states of south India (Andhra Pradesh, Karnataka, Maharashtra, and Tamil Nadu) account for 53% of all the HIV-infected population. India continues to portray a concentrated epidemic. The HIV prevalence among high-risk groups, i.e., FSW, PWID, MSM, and TG, is about 20 times higher than the general population (Fig. 5). The highest HIV prevalence is observed in PWID (7.1%), MSM (4.4%), FSW (2.7%), and STI clinic attendees (2.5%).97–99 A recent study conducted in 2013 among PWID from 15 cities showed that estimated HIV prevalence and incidence were 18.1% and 2.9 per 100 person-years, respectively.100 Sex work continues to be the most important source of HIV infection in India due to the large number of clients of sex workers (in particular single male migrants) who further transmit HIV infection to the general population.101,102
FIG. 5.
HIV prevalence and incidence in men who have sex with men (MSM) and people who inject drugs (PWID), India, 2012–2013. The MSM study was conducted in 12 cities105 and the PWID study was conducted in 15 cities.100
MSM remain hidden because anal intercourse is criminalized and marriage is socially required, with the current estimated HIV prevalence among MSM ranging between 7% and 16.5%.103 HIV prevalence was characterized among MSM in eight cities from Tamil Nadu where 34% were married and 40% self-identified as homosexual. HIV prevalence was 9%, and was higher among married men, 14%.104 A recent study (2012–2013) conducted among MSM from 12 cities across India confirms a relatively high HIV prevalence of 7% and a low HIV incidence of 0.87% (0–2.2%).105 MSM who reported injection drug use were 6.7 times more likely to be HIV infected. In another study in MSM who attended voluntary counseling and testing in Mumbai, HIV prevalence was 12.5%.106 Interestingly, heterosexual anal sex was reported by 11.9% of FSW in four high prevalence states in India.107 Meta-analysis of studies from developed countries has shown that the probability of HIV transmission is higher per act of receptive anal sex (1.7%) as compared to penovaginal sex (0.8%).108,109
Prevention Trials and Breakthrough Results
HIV vaccine clinical development
Table 2 provides the prevention efficacy trials conducted in Asia between 1995 and 2010. Considerable efforts at HIV vaccine clinical development have been deployed in the region since the mid-1990s.110 The first Phase III trial in a middle-income country (Vax003) tested a bivalent recombinant gp120 B/E (MN and A244 CRF01_AE) in alum (AIDSVAX B/E) in PWID in Bangkok, Thailand.111 The vaccine showed no efficacy against HIV acquisition or progression to disease.
Table 2.
HIV-1 Prevention Efficacy Trials Conducted in Asia Between 1995 and 2010
| Study | Years of implementation | Vaccines/drugs | Phase | Volunteers' risk | Location | HIV incidence per 100 person years | Result | References |
|---|---|---|---|---|---|---|---|---|
| Vax003 | 1995–1998 | AIDSVAX B/E gp120 in alum | III | People who inject drugs | Bangkok, Thailand | 3.4% | No efficacy | 111 |
| RV144 | 2003–2009 | ALVAC-HIV vCP1521 and AIDSVAX B/E rgp120 in alum | III | Community risk | Rayong and Chonburi provinces, Thailand | 0.28% | 31.2% efficacy at 42 months, 60% at 12 months against HIV acquisition; no effect on plasma viral load and CD4 count | 112–115 |
| iPrEx | 2007–2009 | Daily oral emtricitabineand tenofovir disoproxil fumarate | III | Men who have sex with men | Chiang Mai, Thailand (and 5 other countries)a | 3.9% overall | 44% in reduction of HIV incidence (global) | 144 |
| PrEP | 2005–2010 | Same as above | III | People who inject drugs | Bangkok, Thailand | 0.68% | 48.9% in reduction of HIV incidence | 143 |
| HPTN 052 (TasP) | 2007–2010 | Various combinations of antiretroviral drugsb | III | HIV-1 serodiscordant couples | Thailand, India (and 7 other countries)c | 1.2% overall | Relative reduction of 96% of linked HIV-1 transmissions after early ART initiation | 161,162 |
| TasP | 2003–2011 | Antiretroviral therapy | Retrospective observational cohort of HIV-1 serodiscordant couples | China | 2.6% | Relative reduction of 26% of HIV-1 transmission | 170 |
Peru, Ecuador, South Africa, Brazil, United States.
Combination of lamivudine and zidovudine (Combivir), efavirenz, atazanavir, nevirapine, tenofovir, lamivudine, zidovudine, didanosine, stavudine, combination of lopinavir and ritonavir (Kaletra and Aluvia), ritonavir, and combination of emtricitabine and tenofovir (Truvada).
Botswana, Kenya, Malawi, South Africa, Zimbabwe, Brazil, United States.
ALVAC-HIV (vCP1521), recombinant canarypox vector expressing Gag and protease subtype B (LAI) and env gp120 CRF01_AE (TH023) linked to the transmembrane-anchoring portion of subtype B gp41 (LAI) genes; AIDSVAX B/E, bivalent recombinant gp120 proteins subtype B (MN) and CRF01-AE (A244); iPrEx and PrEP, preexposure prophylaxis; TasP, treatment as prevention.
A community-based Phase III trial (RV144) provided the first evidence that an HIV-1 vaccine might prevent HIV infection.112,113 The prime-boost vaccine regimen consisted of a recombinant canarypox vector, ALVAC-HIV (vCP1521) prime and AIDSVAX B/E boost. The vaccine regimen was safe and generally well tolerated.114 The modified intent-to-treat analysis showed 31.2% efficacy after 42 months of follow-up. There was no effect on early postinfection HIV-1 RNA viral load or CD4+ T cell count or on clinical outcomes in longer-term follow-up. In a post-hoc analysis, vaccine efficacy was significantly higher (60%) at 12 months postvaccination, suggesting an early, but nondurable, vaccine effect.115 Although RV144 was not powered to assess the interaction of vaccine efficacy and risk behavior, post-hoc analysis suggested greater benefit in low-risk individuals.
RV144 provided an opportunity to perform a correlates of risk analysis,116 and a case-control study showed that IgG antibodies to the scaffolded V1V2 region of gp120 Env correlated with a decreased risk of infection while the presence of IgA antibodies to Env was directly associated with infection risk.117–119 There was no enhancement of HIV-1 infection risk when compared to the placebo group. A subsequent analysis showed that RV144 antibodies to subtype A, C, and CRF01_AE gp70 V1V2 scaffolded proteins also correlated inversely with risk,120 suggesting that the RV144 regimen might protect against HIV strains heterologous (A and C) to the vaccine components.
The Env IgG/IgA ratio significantly correlated with increased risk of infection (decreased vaccine efficacy).121 In the presence of low vaccine-elicited IgA responses, both antibody-dependent cellular cytotoxicity (ADCC) and neutralizing antibody (Nab) responses correlated with decreased risk of infection. ADCC responses were predominantly directed to the C1 conformational region of gp120.122 IgA antibodies elicited by RV144 could block anti-C1 IgG-mediated ADCC.121 It is suggested that nonneutralizing antibodies mediating ADCC are directed against viral entry epitopes including the CD4-inducible A32 epitope of a highly conserved region of gp120 at the gp120–gp41 interface,123 and there is evidence of synergy between the anti-C1 and anti-V2 activities, with increased anti-V1V2 binding in the presence of vaccine-induced anti-C1 antibody.124 IgGs to linear epitopes in the V2 and V3 regions of gp120 are part of a complex interplay of immune responses that contributed to protection in RV144.125 These correlates were supported by corresponding V2 mutations in a "sieve" analysis of breakthrough viruses suggesting that genetic mutations away from V2 sequences found in the vaccine (positions 169K and 181I) were associated with altered efficacy.126
Follow-up trials are continuing127 to explore systemic and mucosal immune responses elicited by late boosts (7–8 years post) administered to RV144 vaccine recipients, the value of additional boosts at 12, 15, and 18 months to the RV144 regimen, the immune responses in mucosal compartments and memory B cells, and new adjuvant formulations.
One of the main objectives for future vaccines is to counter HIV-1 variability, between subtypes and intrasubtype, as diversity within geographically circumscribed epidemics increases with time.128 Whether various envelope immunogens eliciting V2 antibodies are functionally strain-specific, region-specific, or universal in a cross-clade manner and universal correlates of risk in populations with various modes of transmission remains to be demonstrated in future efficacy trials.
Other vaccine approaches including immunogens derived from conserved sequences or mosaics expressed by various vectors (Ad26, MVA) have recently been reviewed.127,129 Our current understanding of the immune correlates of protection suggests that no specific vaccine approach should be privileged over the other and that all reasonable vaccine approaches deserve to be pursued.130,131 At least for the pox-protein prime-boost strategy, the analysis of the correlates of risk raises hypotheses that might be used to down-select products in advance of efficacy testing.
In China, a plasmid DNA and a replication-competent TianTan vaccinia HIV vaccine vector expressing HIV-1 CN54 CRF07_B′/C genes132 is now in Phase II. Preliminary results suggest that both vaccines are safe and immunogenic.133 An efficacy trial in MSM populations might be envisaged with possibly an envelope protein boost.
India conducted three Phase I trials testing HIV-1 subtype C-derived vaccine candidates.134–137 HIV vaccine clinical development came to a halt and shifted to designing immunogens able to induce HIV-specific broadly neutralizing antibodies (www.iavi.org). The prioritization of trial-related financial and healthcare provisions, including access to an efficacious vaccine posttrial, among MSM in India indicates the importance of trials providing such services, as well as the value of formative research in identifying key concerns among participating communities in resource-limited settings.138
Preexposure prophylaxis (PrEP) and microbicides
A meta-analysis of PrEP randomized controlled trials in high-risk populations defined as MSM, PWID, and HIV-discordant heterosexual couples demonstrated an overall risk reduction of 47%.139 Early PrEP trials in Cambodia failed to launch due to ethical, political, and logistical concerns raised by civil society leaders during 2004,140–142 and initiation of the Bangkok Tenofovir PrEP study was delayed for several years.143 Two clinical trials of oral PrEP for HIV-negative individuals conducted in Thailand showed a reduction in HIV acquisition in MSM144 and in PWID143 (Table 2). Daily oral Truvada was approved by the U.S. FDA for PrEP in 2012145 and the WHO146–148 and CDC149,150 recommended PrEP for certain populations.
Results of the PROUD study and of the on-demand (before and after sexual intercourse) “Ipergay” PrEP study showed a relative reduction of 86% in the incidence of HIV acquisition among MSM.151,152 The results of the Ipergay trial also raise hope that costs of a PrEP strategy among MSM could be considerably reduced. PrEP is still considered an individual prevention intervention for HIV-negative individuals at a time at which governments struggle to sustain funding for HIV treatment programs,153 although the increasing HIV incidence among MSM is an argument to include PrEP for HIV prevention for this particular group.5,7 High-risk individuals in China154–156 and Thailand157,158 have signaled their acceptance of PrEP if offered. PrEP demonstration projects and effectiveness trials are ongoing in India and Thailand. Moreover, PrEP and male circumcision were recently recommended (although not reimbursed by national coverage scheme) in high-risk groups such as MSM and were included in the 2014 Thailand National Guidelines on HIV/AIDS Treatment and Prevention.159
Treatment as Prevention (TasP)
The introduction of potent combination ART in 1996 and the public health approach to HIV treatment in resource-limited settings in 2002 have changed the course of the HIV epidemic.160 HPTN 052,161,162 conducted in 13 sites in nine countries including India and Thailand, demonstrated that earlier ART reduces the risk of heterosexual HIV transmission by 96%163 in discordant couples and confirmed results of observational and ecological studies.164–169 These results prompted a retrospective study of treated and untreated discordant couples in China,170 which showed a relative reduction of 26% of HIV-1-linked transmission (Table 2).
In India, early ART was cost effective over a 5-year period and very cost effective over a lifetime ($1,800 and $530 per life-year saved, respectively).171 A recent modeling study in India suggests that in the presence of existing condom-based interventions, existing ART programs could avert 11–28% of the remaining HIV infections in FSW between 2014 and 2024.172 Several studies of TasP are ongoing in India, Vietnam, Thailand, China, and Indonesia. In China, a 10-fold increase in ART could decrease the number of HIV-related deaths by 58% and the number of new infections by 25% by 2015.95 In Thailand, an additional $100 million was invested in high-impact interventions focusing on key populations, and an estimated 20,000 people will escape HIV infection, 22,000 deaths will be averted, and $300 million in projected savings and labor productivity gains will be accrued.173
Implications for HIV Prevention Trials
HIV prevention trials in community-risk populations may be difficult in Asia.127,174 Despite undeniable gaps in HIV prevention and in access to care and treatment of key populations,14 an overall decrease of HIV prevalence is observed in the region due to effective prevention programs. Furthermore, with treatment programs starting at higher CD4 thresholds, HIV incidence may significantly decrease in all populations. The implementation of prevention trials then faces important challenges.
Although HIV prevalence still remains high in some cities (23.6% in Bangkok, 10.9% in Chiang Mai),175 the success of harm reduction programs has yielded a decreasing HIV incidence in PWID.19,176 HIV vaccine efficacy trials in PWID are unlikely to proceed as illustrated in the HPTN 058 trial conducted in Xinjiang and Guangxi Provinces, China, and Chiang Mai, Thailand where only 7 of 1,157 HIV-uninfected PWID acquired HIV over a 2-year follow-up.177,178 In Project Accept (HPTN 043) conducted in Africa and 14 sites in Thailand, Thailand was excluded from the analysis because of an unexpectedly low HIV prevalence.179 In addition, a high proportion of new circulating recombinant forms is now found in PWID, as illustrated by CRF01_AE/B′/C recombinants (42.6%) in northern Myanmar at the border with China54–56 and India.65 Taken together the burden of multiple transmitted/founder variants in PWID180 and rates of HIV superinfection181 represent major challenges for any HIV vaccine and would complicate trials in areas with higher proportions of CRF/URFs, such as China, India, and Myanmar.
Efficacy trials that target MSM and TG populations with current HIV incidence in these groups offer the benefit of being smaller and less costly. However, the recent results of the PROUD and Ipergay PrEP studies showing a reduction of 86% in the incidence of HIV acquisition along with the impact of TasP among MSM populations suggest that the expansion of these prevention strategies may rapidly lead to low HIV incidence among MSM populations. Circumcision rates among MSM remain low (<20%) in countries such as Thailand76 or China.182,183 Although a recent study in India suggests that circumcised MSM who predominantly take the receptive role in anal intercourse may be at a lower risk of HIV infection,184 evidence that circumcision reduces HIV among MSM remains weak and inconsistent.185,186 Despite the willingness to undergo circumcision among MSM populations,187,188 male circumcision is not promoted as a priority in Asian countries with concentrated epidemics and has not been recommended for MSM unless they also engage in vaginal sex.148 It is therefore unlikely that male circumcision will significantly contribute to reducing the HIV incidence among MSM populations. A lower HIV incidence may make HIV vaccine trials in MSM populations infeasible.
The decision will be made on a country-by-country basis after careful analysis of the most recent epidemiological data and of the use of PrEP. It has also been argued that vaccine protection might be easier to achieve in high-risk populations with predominantly heterosexual transmission such as those found in Africa,108,174 in contrast to MSM populations with rectal transmission, with the highest risk mode of sexual HIV transmission at 1:20–1:300 infections per exposure.189 This adds to the complexity of the decision as to which HIV vaccine strategy makes best scientific sense for efficacy testing among MSM populations.
The evolving patterns of HIV-1 diversity in the region illustrate the need to closely monitor the molecular HIV epidemic in potential target populations for HIV vaccine efficacy trials.28,29 CRF01_AE remains predominant in southeast Asian countries and MSM populations in China and may provide a rationale for regional efficacy trials, for example, Thailand and China.110 However, bivalent or trivalent subtype-specific vaccines targeting predominant circulating forms, such as CRF01_AE, subtype B, and, potentially, C seem more appropriate.128 Other vaccine approaches such as prime-boost regimens using DNA and vectors (Ad26, MVA) expressing mosaic antigens190,191 or conserved sequences must also be seriously considered as global HIV vaccine strategies.192–195
The rationale and design of efficacy trials of combination prevention modalities such as HIV vaccine and PrEP have recently been explored.196,197 Such trials are complex, and the clinical and regulatory challenges remain hypothetical. Ultimately the prioritization of prevention interventions should be driven by the HIV epidemic and decided by the country-specific health and regulatory authorities. Modeling the impact and cost–benefits of combined interventions may help this decision process.
Conclusions
An overall decrease in HIV prevalence has been observed in several key Asian countries due to effective prevention programs. The decrease in HIV prevalence and HIV incidence may hopefully further improve with the scale-up of combination prevention interventions. The implementation of prevention trials then faces important challenges. The opportunity to identify heterosexual populations at high risk may rapidly wane. With unabating HIV epidemics among MSM and TG, an effective vaccine would likely be the only option to turn the epidemic. It is therefore more likely that efficacy trials will occur in the MSM and TG populations because their higher HIV incidence would allow smaller and less costly trials. The evolving patterns of HIV-1 diversity in the region suggest the need for close monitoring of the molecular HIV epidemic in potential target populations. However, CRF01_AE remains predominant in southeast Asian countries and among MSM populations in China. This would be conducive to regional efficacy trials, and as efficacy warrants, to regional licensure. The rationale and design of efficacy trials of combination prevention modalities such as HIV vaccine and PrEP remain for the moment hypothetical and would be more costly and challenging for the regulatory approval process to licensure. The prioritization of prevention interventions should be driven by the HIV epidemic and decided by the country-specific health and regulatory authorities. Modeling the impact and cost–benefit interventions may help this decision process.
Acknowledgments
The preparation of this article was supported in part by an Interagency Agreement Y1-AI-2642-12 between the U.S. Army Medical Research and Materiel Command (USAMRMC) and the National Institutes of Allergy and Infectious Diseases. In addition, this work was supported by a cooperative agreement (W81XWH-07-2-0067) between the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., and the U.S. Department of Defense (DOD).
The opinions herein are those of the authors and should not be construed as official or representing the views of the U.S. Department of Defense or Department of the Army and of the World Health Organization.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.United Nations Economic and Social Commission for Asia and the Pacific: Statistical Year Book for Asia and the Pacific. Bangkok, Thailand, 2013 [Google Scholar]
- 2.Brown T. and Peerapatanapokin W: The Asian Epidemic Model: A process model for exploring HIV policy and programme alternatives in Asia. Sex Transm Infect 2004;80(Suppl 1):i19– 24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.UNAIDS: The Gap Report. Geneva, Switzerland, 2014 [Google Scholar]
- 4.World Health Organization: The global health sector strategy on HIV/AIDS 2011– 2015: An interim review of progress. Geneva, Switzerland, 2014 [Google Scholar]
- 5.Beyrer C, Baral SD, van Griensven F, et al. : Global epidemiology of HIV infection in men who have sex with men. Lancet 2012;380(9839):367–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Griensven F. and de Lind van Wijngaarden JW: A review of the epidemiology of HIV infection and prevention responses among MSM in Asia. AIDS 2010;24(Suppl 3):S30–40 [DOI] [PubMed] [Google Scholar]
- 7.van Griensven F, Thienkrua W, McNicholl J, et al. : Evidence of an explosive epidemic of HIV infection in a cohort of men who have sex with men in Thailand. AIDS 2013;27(5):825–832 [DOI] [PubMed] [Google Scholar]
- 8.Baral SD, Poteat T, Stromdahl S, Wirtz AL, et al. : Worldwide burden of HIV in transgender women: A systematic review and meta-analysis. Lancet Infect Dis 2013;13(3):214–222 [DOI] [PubMed] [Google Scholar]
- 9.Shubber Z, Mishra S, Vesga JF, and Boily MC: The HIV Modes of Transmission model: A systematic review of its findings and adherence to guidelines. J Int AIDS Soc 2014;17:18928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baral S, Beyrer C, Muessig K, et al. : Burden of HIV among female sex workers in low-income and middle-income countries: A systematic review and meta-analysis. Lancet Infect Dis 2012;12(7):538–549 [DOI] [PubMed] [Google Scholar]
- 11.Ministry of Health of Indonesia: Integrated Biological and Behavioral Survey (IBBS) 2011. Jakarta, Indonesia, 2011 [Google Scholar]
- 12.National AIDS Programme: HIV Sentinel Sero-surveillance Survey Report, 2012. Nay Pyi Taw, Myanmar, 2013 [Google Scholar]
- 13.Manopaiboon C, Prybylski D, Subhachaturas W, et al. : Unexpectedly high HIV prevalence among female sex workers in Bangkok, Thailand in a respondent-driven sampling survey. Int J STD AIDS 2013;24(1):34–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.UNAIDS: HIV in Asia and the Pacific—UNAIDS report 2013. Bangkok, Thailand, 2013 [Google Scholar]
- 15.National AIDS Council: Philippines Global AIDS Response Progress Report. Manila, Philippines, 2012 [Google Scholar]
- 16.Viet Nam Administration for AIDS Control: HIV Sentinel Surveillance PLus among High Risk Groups in Vietnam 2012. Hanoi, Viet Nam, 2013 [Google Scholar]
- 17.Srikantiah P, Ghidinelli M, Bachani D, et al. : Scale-up of national antiretroviral therapy programs: Progress and challenges in the Asia Pacific region. AIDS 2010;24(Suppl 3):S62–71 [DOI] [PubMed] [Google Scholar]
- 18.Bergenstrom AM. and Abdul-Quader AS: Injection drug use, HIV and the current response in selected low-income and middle-income countries. AIDS 2010;24(Suppl 3):S20–29 [DOI] [PubMed] [Google Scholar]
- 19.Dutta A, Wirtz AL, Baral S, et al. : Key harm reduction interventions and their impact on the reduction of risky behavior and HIV incidence among people who inject drugs in low-income and middle-income countries. Curr Opin HIV AIDS 2012;7(4):362–368 [DOI] [PubMed] [Google Scholar]
- 20.Hollingsworth TD, Anderson RM, and Fraser C: HIV-1 transmission, by stage of infection. J Infect Dis 2008;198(5):687–693 [DOI] [PubMed] [Google Scholar]
- 21.Han X, Xu J, Chu Z, et al. : Screening acute HIV infections among Chinese men who have sex with men from voluntary counseling & testing centers. PLoS One 2011;6(12):e28792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen MS, Shaw GM, McMichael AJ, and Haynes BF: Acute HIV-1 infection. N Engl J Med 2011;364(20):1943–1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ananworanich J, Phanuphak N, de Souza M, et al. : Incidence and characterization of acute HIV-1 infection in a high-risk Thai population. J Acquir Immune Defic Syndr 2008;49(2):151–155 [DOI] [PubMed] [Google Scholar]
- 24.Ye J, Xin R, Yu S, et al. : Phylogenetic and temporal dynamics of human immunodeficiency virus type 1 CRF01_AE in China. PLoS One 2013;8(1):e54238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fisher M, Pao D, Brown AE, et al. : Determinants of HIV-1 transmission in men who have sex with men: A combined clinical, epidemiological and phylogenetic approach. AIDS 2010;24(11):1739–1747 [DOI] [PubMed] [Google Scholar]
- 26.Audelin AM, Cowan SA, Obel N, et al. : Phylogenetics of the Danish HIV epidemic: The role of very late presenters in sustaining the epidemic. J Acquir Immune Defic Syndr 2013;62(1):102–108 [DOI] [PubMed] [Google Scholar]
- 27.Hemelaar J, Gouws E, Ghys PD, and Osmanov S: Global trends in molecular epidemiology of HIV-1 during 2000–2007. AIDS 2011;25(5):679–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hemelaar J: The origin and diversity of the HIV-1 pandemic. Trends Mol Med 2012;18(3):182–192 [DOI] [PubMed] [Google Scholar]
- 29.Aldrich C. and Hemelaar J: Global HIV-1 diversity surveillance. Trends Mol Med 2012;18(12):691–694 [DOI] [PubMed] [Google Scholar]
- 30.Zolfo M, Schapiro JM, Phan V, et al. : Genotypic impact of prolonged detectable HIV type 1 RNA viral load after HAART failure in a CRF01_AE-infected cohort. AIDS Res Hum Retroviruses 2011;27(7):727–735 [DOI] [PubMed] [Google Scholar]
- 31.Merati TP, Ryan CE, Spelmen T, et al. : CRF01_AE dominates the HIV-1 epidemic in Indonesia. Sex Health 2012;9(5):414–421 [DOI] [PubMed] [Google Scholar]
- 32.Nouhin J, Donchai T, Hoang KT, et al. : Natural polymorphisms of HIV-1 CRF01_AE integrase coding region in ARV-naive individuals in Cambodia, Thailand and Vietnam: An ANRS AC12 working group study. Infect Genet Evol 2011;11(1):38–43 [DOI] [PubMed] [Google Scholar]
- 33.Lazaro E, Tram LT, Bellecave P, et al. : Molecular characterization of HIV-1 CRF01_AE in Mekong Delta, Vietnam, and impact of T-cell epitope mutations on HLA recognition (ANRS 12159). PLoS One 2011;6(10):e26244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oyomopito RA, Li PC, Sungkanuparph S, et al. : Evaluating immunologic response and clinical deterioration in treatment-naive patients initiating first-line therapies infected with HIV-1 CRF01_AE and subtype B. J Acquir Immune Defic Syndr 2013;62(3):293–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lau KA, Wang B, Miranda-Saksena M, et al. : Evidence for possible biological advantages of the newly emerging HIV-1 circulating recombinant form from Malaysia—CRF33_01B in comparison to its progenitors—CRF01_AE and subtype B. Curr HIV Res 2010;8(3):259–271 [DOI] [PubMed] [Google Scholar]
- 36.Sahbandar IN, Takahashi K, Djoerban Z, et al. : Current HIV type 1 molecular epidemiology profile and identification of unique recombinant forms in Jakarta, Indonesia. AIDS Res Hum Retroviruses 2009;25(7):637–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kijak GH, Tovanabutra S, Rerks-Ngarm S, et al. : Molecular evolution of the HIV-1 Thai epidemic between the time of RV144 immunogen selection to the execution of the vaccine efficacy trial. J Virol 2013;87(13):7265–7281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang W, Xu J, Jiang S, et al. : The dynamic face of HIV-1 subtypes among men who have sex with men in Beijing, China. Curr HIV Res 2011;9(2):136–139 [DOI] [PubMed] [Google Scholar]
- 39.Guo H, Wei JF, Yang H, et al. : Rapidly increasing prevalence of HIV and syphilis and HIV-1 subtype characterization among men who have sex with men in Jiangsu, China. Sex Transm Dis 2009;36(2):120–125 [DOI] [PubMed] [Google Scholar]
- 40.Wang W, Jiang S, Li S, et al. : Identification of subtype B, multiple circulating recombinant forms and unique recombinants of HIV type 1 in an MSM cohort in China. AIDS Res Hum Retroviruses 2008;24(10):1245–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao J, Cai W, Zheng C, et al. : Origin and outbreak of HIV-1 CRF55_01B among MSM in Shenzhen, China. J Acquir Immune Defic Syndr 2014;66(3):e65–67 [DOI] [PubMed] [Google Scholar]
- 42.Han X, An M, Zhang W, et al. :. Genome sequences of a novel HIV-1 circulating recombinant form CR F55_01B, identified in China. Genome Announc 2013;1(1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feng Y, He X, Hsi JH, et al. : The rapidly expanding CRF01_AE epidemic in China is driven by multiple lineages of HIV-1 viruses introduced in the 1990s. AIDS 2013;27(11):1793–1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.An M, Han X, Xu J, et al. : Reconstituting the epidemic history of HIV strain CRF01_AE among men who have sex with men (MSM) in Liaoning, northeastern China: Implications for the expanding epidemic among MSM in China. J Virol 2012;86(22):12402–12406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He X, Xing H, Ruan Y, et al. : A comprehensive mapping of HIV-1 genotypes in various risk groups and regions across China based on a nationwide molecular epidemiologic survey. PLoS One 2012;7(10):e47289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kondo M, Lemey P, Sano T, et al. : Emergence in Japan of an HIV-1 variant associated with transmission among men who have sex with men (MSM) in China: First indication of the International Dissemination of the Chinese MSM lineage. J Virol 2013;87(10):5351–5361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang W, Meng Z, Zhou M, et al. : Near full-length sequence analysis of two new HIV type 1 unique (CRF01_AE/B) recombinant forms among men who have sex with men in China. AIDS Res Hum Retroviruses 2012;28(4):411–417 [DOI] [PubMed] [Google Scholar]
- 48.Wu J, Meng Z, Xu J, et al. : New emerging recombinant HIV-1 strains and close transmission linkage of HIV-1 strains in the Chinese MSM population indicate a new epidemic risk. PLoS One 2013;8(1):e54322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shao Y, Zhao F, Yang W, et al. : The identification of recombinant HIV-1 strains in IDUs in southwest and northwest China. Chinese J Exp Clin Virol 1999;13:109. [PubMed] [Google Scholar]
- 50.Su L, Graf M, Zhang Y, et al. : Characterization of a virtually full-length human immunodeficiency virus type 1 genome of a prevalent intersubtype (C/B') recombinant strain in China. J Virol 2000;74(23):11367–11376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rodenburg CM, Li Y, Trask SA, et al. : Near full-length clones and reference sequences for subtype C isolates of HIV type 1 from three different continents. AIDS Res Hum Retroviruses 2001;17(2):161–168 [DOI] [PubMed] [Google Scholar]
- 52.Piyasirisilp S, McCutchan FE, Carr JK, et al. : A recent outbreak of human immunodeficiency virus type 1 infection in southern China was initiated by two highly homogeneous, geographically separated strains, circulating recombinant form AE and a novel BC recombinant. J Virol 2000;74(23):11286–11295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Y, Lu L, Ba L, et al. : Dominance of HIV-1 subtype CRF01_AE in sexually acquired cases leads to a new epidemic in Yunnan province of China. PLoS Med 2006;3(11):e443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Williams CT, Liu W, and Levy JA: Crossing over: Drug network characteristics and injection risk along the China-Myanmar border. AIDS Behav 2011;15(5):1011–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu J, Jia Y, Xu Q, et al. : Phylodynamics of HIV-1 unique recombinant forms in China-Myanmar border: Implication for HIV-1 transmission to Myanmar from Dehong, China. Infect Genet Evol 2012;12(8):1944–1948 [DOI] [PubMed] [Google Scholar]
- 56.Pang W, Zhang C, Duo L, et al. : Extensive and complex HIV-1 recombination between B', C and CRF01_AE among IDUs in south-east Asia. AIDS 2012;26(9):1121–1129 [DOI] [PubMed] [Google Scholar]
- 57.Su YZ, Ma YL, Jia MH, et al. : Update on diversity and distribution of HIV-1 subtypes in Yunnan province. Epidemiol Infect 2013;141(11):2418–2427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen M, Ma Y, Duan S, et al. : Genetic diversity and drug resistance among newly diagnosed and antiretroviral treatment-naive HIV-infected individuals in western Yunnan: A hot area of viral recombination in China. BMC Infect Dis 2012;12:382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wei H, Liu Y, Feng Y, et al. : Genome sequence of a novel HIV-1 circulating recombinant form (CRF57_BC) identified from Yunnan, China. AIDS Res Hum Retroviruses 2014;30(4):384–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wei H, His J, Feng Y, et al. : Identification of a novel HIV-1 circulating recombinant form (CRF62_BC) in western Yunnan of China. AIDS Res Hum Retroviruses 2014;30(4):380–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hsi J, Wei H, Xing H, et al. : Genome sequence of a novel HIV-1 circulating recombinant form (CRF64_BC) identified from Yunnan, China. AIDS Res Hum Retroviruses 2014;30(4):389–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Feng Y, Wei H, Hsi J, et al. : Identification of a novel HIV type 1 circulating recombinant form (CRF65_cpx) composed of CRF01_AE and subtypes B and C in western Yunnan, China. AIDS Res Hum Retroviruses 2014;30(6):598–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sahni AK, Prasad VV, and Seth P: Genomic diversity of human immunodeficiency virus type-1 in India. Int J STD AIDS 2002;13(2):115–118 [DOI] [PubMed] [Google Scholar]
- 64.Khan IF, Vajpayee M, Prasad VV, and Seth P: Genetic diversity of HIV type 1 subtype C env gene sequences from India. AIDS Res Hum Retroviruses 2007;23(7):934–940 [DOI] [PubMed] [Google Scholar]
- 65.Neogi U, Sood V, Banerjee S, et al. : Global HIV-1 molecular epidemiology with special reference to genetic analysis of HIV-1 subtypes circulating in North India: Functional and pathogenic implications of genetic variation. Indian J Exp Biol 2009;47(6):424–431 [PubMed] [Google Scholar]
- 66.Rodriguez MA, Ding M, Ratner D, et al. : High replication fitness and transmission efficiency of HIV-1 subtype C from India: Implications for subtype C predominance. Virology 2009;385(2):416–424 [DOI] [PubMed] [Google Scholar]
- 67.Neogi U, Sood V, Ronsard L, et al. : Genetic architecture of HIV-1 genes circulating in north India and their functional implications. Indian J Med Res 2011;134(6):769–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Neogi U, Sahoo PN, Arumugam K, et al. : Higher prevalence of predicted X4-tropic strains in perinatally infected older children with HIV-1 subtype C in India. J Acquir Immune Defic Syndr 2012;59(4):347–353 [DOI] [PubMed] [Google Scholar]
- 69.Tee KK, Pybus OG, Li XJ, et al. : Temporal and spatial dynamics of human immunodeficiency virus type 1 circulating recombinant forms 08_BC and 07_BC in Asia. J Virol 2008;82(18):9206–9215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Neogi U, Bontell I, Shet A, et al. : Molecular epidemiology of HIV-1 subtypes in India: Origin and evolutionary history of the predominant subtype C. PLoS One 2012;7(6):e39819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thailand Working Group on HIV/AIDS Projection: AIDS Epidemic Model–Projection for HIV/AIDS in Thailand. 2010–2030. Summary Report. Bangkok: Ministry of Public Health, Thailand, 2012 [Google Scholar]
- 72.Rojanapithayakorn W: The 100% condom use programme in Asia. Reprod Health Matters 2006;14(28):41–52 [DOI] [PubMed] [Google Scholar]
- 73.van Griensven F, Phanuphak N, and Srithanaviboonchai K: Biomedical HIV prevention research and epidemic control in Thailand: Two sides of the same coin. Sex Health 2014;11(2):180–199 [DOI] [PubMed] [Google Scholar]
- 74.Park LS, Siraprapasiri T, Peerapatanapokin W, et al. : HIV transmission rates in Thailand: Evidence of HIV prevention and transmission decline. J Acquir Immune Defic Syndr 2010;54(4):430–436 [DOI] [PubMed] [Google Scholar]
- 75.Chariyalertsak S, Kosachunhanan N, Saokhieo P, et al. : HIV incidence, risk factors, and motivation for biomedical intervention among gay, bisexual men, and transgender persons in Northern Thailand. PLoS One 2011;6(9):e24295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Phanuphak N, Colby D, Pinyakorn S, et al. : on behalf of the RV233/SEARCH008 Study Group: Low incidence of HIV infection in an anonymous HIV counselling and testing clinic cohort in Bangkok, Thailand despite high HIV prevalence and self-report of high-risk behaviour. J Viral Eradication 2015;1:89–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Swe LA. and Rashid A: HIV prevalence among the female sex workers in major cities in Myanmar and the risk behaviors associated with it. HIV/AIDS–Res Palliative Care 2013;5:223–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Williams B, Baker D, Buhler M, and Petrie C: Increase coverage of HIV and AIDS services in Myanmar. Confl Health 2008;2:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ministry of Health of the People's Republic of China: 2012 China AIDS Response Progress Report: UNAIDS, 2012 [Google Scholar]
- 80.Qian S, Guo W, Xing J, et al. : Diversity of HIV/AIDS epidemic in China: A result from hierarchical clustering analysis and spatial autocorrelation analysis. AIDS 2014;28(12):1805–1813 [DOI] [PubMed] [Google Scholar]
- 81.Zhang L, Chow EP, Jing J, et al. : HIV prevalence in China: Integration of surveillance data and a systematic review. Lancet Infect Dis 2013;13(11):955–963 [DOI] [PubMed] [Google Scholar]
- 82.Chow EP, Lau JT, Zhuang X, et al. : HIV prevalence trends, risky behaviours, and governmental and community responses to the epidemic among men who have sex with men in China. Biomed Res Int 2014;2014:607261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhong F, Liang B, Xu H, et al. : Increasing HIV and decreasing syphilis prevalence in a context of persistently high unprotected anal intercourse; six consecutive annual surveys among men who have sex with men in Guangzhou, China, 2008 to 2013. PLoS One 2014;9(7):e103136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mi G. and Wu Z: A review of studies on sero-sorting among HIV positive men who have sex with men. Chin J AIDS STD 2010;16:201–203 [Google Scholar]
- 85.Wu Z, Xu J, Liu E, et al. : HIV and syphilis prevalence among men who have sex with men: A cross-sectional survey of 61 cities in China. Clin Infect Dis 2013;57(2):298–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shang H, Xu J, Han X, et al. : HIV prevention: Bring safe sex to China. Nature 2012;485(7400):576–577 [DOI] [PubMed] [Google Scholar]
- 87.Lou J, Wu J, Chen L, et al. : A sex-role-preference model for HIV transmission among men who have sex with men in China. BMC Public Health 2009;9(Suppl 1):S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lu H, Liu Y, Dahiya K, et al. : Effectiveness of HIV risk reduction interventions among men who have sex with men in China: A systematic review and meta-analysis. PLoS One 2013;8(8):e72747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chow EP, Wilson DP, and Zhang L: What is the potential for bisexual men in China to act as a bridge of HIV transmission to the female population? Behavioural evidence from a systematic review and meta-analysis. BMC Infect Dis 2011;11:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.He Q, Peng WJ, Zhang JQ, et al. : Prevalence of unprotected anal intercourse and unprotected vaginal intercourse among HIV-positive men who have sex with men in China: A meta-analysis. Sex Transm Infect 2012;88(3):229–233 [DOI] [PubMed] [Google Scholar]
- 91.Yun K, Xu JJ, Reilly KH, et al. : Prevalence of bisexual behaviour among bridge population of men who have sex with men in China: A meta-analysis of observational studies. Sex Transm Infect 2011;87(7):563–570 [DOI] [PubMed] [Google Scholar]
- 92.Chow EP, Wilson DP, and Zhang L: Estimating HIV incidence among female partners of bisexual men in China. Int J Infect Dis 2012;16(5):e312–320 [DOI] [PubMed] [Google Scholar]
- 93.Ye S, Xiao Y, Jin C, et al. : Effectiveness of integrated HIV prevention interventions among Chinese men who have sex with men: Evaluation of a 16-city public health program. PLoS One 2012;7(12):e50873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li HM, Peng RR, Li J, et al. : HIV incidence among men who have sex with men in China: A meta-analysis of published studies. PLoS One 2011;6(8):e23431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang L, Gray RT, and Wilson DP: Modelling the epidemiological impact of scaling up HIV testing and antiretroviral treatment in China. Sex Health 2012;9(3):261–271 [DOI] [PubMed] [Google Scholar]
- 96.Lou J, Blevins M, Ruan Y, et al. : Modeling the impact on HIV incidence of combination prevention strategies among men who have sex with men in Beijing, China. PLoS One 2014;9(3):e90985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.National AIDS Control Organisation, Department of AIDS Control, Minsitry of Health & Family Welfare: Annual Report. New Delhi, India, 2012–2013 [Google Scholar]
- 98.Mishra RM, Dube M, Sahu D, et al. : Changing epidemiology of HIV in Mumbai: An application of the Asian epidemic model. Glob J Health Sci 2012;4(5):100–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Godbole S, Sane S, Kamble P, et al. : Predictors of bisexual behaviour among MSM attending intervention sites may help in prevention interventions for this bridge to the heterosexual epidemic in India: Data from HIV sentinel surveillance. PLoS One 2014;9(9):e107439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lucas GM, Solomon SS, Srikrishnan AK, et al. : High HIV burden among people who inject drugs in 15 Indian cities. AIDS 2015;29(5):619–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gangakhedkar RR, Bentley ME, Divekar AD, et al. : Spread of HIV infection in married monogamous women in India. JAMA 1997;278(23):2090–2092 [PubMed] [Google Scholar]
- 102.Newmann S, Sarin P, Kumarasamy N, et al. : Marriage, monogamy and HIV: A profile of HIV-infected women in south India. Int J STD AIDS 2000;11(4):250–253 [DOI] [PubMed] [Google Scholar]
- 103.Thomas B, Mimiaga MJ, Kumar S, et al. : HIV in Indian MSM: Reasons for a concentrated epidemic and strategies for prevention. Indian J Med Res 2011;134(6):920–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Solomon SS, Srikrishnan AK, Sifakis F, et al. : The emerging HIV epidemic among men who have sex with men in Tamil Nadu, India: Geographic diffusion and bisexual concurrency. AIDS Behav 2010;14(5):1001–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Solomon SS, Mehta SH, Srikrishnan AK, et al. : High HIV prevalence and incidence among MSM across 12 cities in India. AIDS 2015;29(6):723–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kumta S, Lurie M, Weitzen S, et al. : Bisexuality, sexual risk taking, and HIV prevalence among men who have sex with men accessing voluntary counseling and testing services in Mumbai, India. J Acquir Immune Defic Syndr 2010;53(2):227–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Alexander M, Mainkar M, Deshpande S, et al. : Heterosexual anal sex among female sex workers in high HIV prevalence states of India: Need for comprehensive intervention. PLoS One 2014;9(2):e88858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Boily MC, Baggaley RF, Wang L, et al. : Heterosexual risk of HIV-1 infection per sexual act: Systematic review and meta-analysis of observational studies. Lancet Infect Dis 2009;9(2):118–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Boily MC, Baggaley RF, and Masse B: The role of heterosexual anal intercourse for HIV transmission in developing countries: Are we ready to draw conclusions? Sex Transm Infect 2009;85(6):408–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nitayaphan S, Ngauy V, O'Connell R, and Excler JL: HIV epidemic in Asia: Optimizing and expanding vaccine development. Expert Rev Vaccines 2012;11(7):805–819 [DOI] [PubMed] [Google Scholar]
- 111.Pitisuttithum P, Gilbert P, Gurwith M, et al. : Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J Infect Dis 2006;194(12):1661–1671 [DOI] [PubMed] [Google Scholar]
- 112.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. : Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med 2009;361(23):2209–2220 [DOI] [PubMed] [Google Scholar]
- 113.Gilbert PB, Berger JO, Stablein D, et al. : Statistical interpretation of the RV144 HIV vaccine efficacy trial in Thailand: A case study for statistical issues in efficacy trials. J Infect Dis 2011;203(7):969–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pitisuttithum P, Rerks-Ngarm S, Bussaratid V, et al. : Safety and reactogenicity of canarypox ALVAC-HIV (vCP1521) and HIV-1 gp120 AIDSVAX B/E vaccination in an efficacy trial in Thailand. PLoS One 2011;6(12):e27837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Robb ML, Rerks-Ngarm S, Nitayaphan S, et al. : Risk behaviour and time as covariates for efficacy of the HIV vaccine regimen ALVAC-HIV (vCP1521) and AIDSVAX B/E: A post-hoc analysis of the Thai phase 3 efficacy trial RV 144. Lancet Infect Dis 2012;12(7):531–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Plotkin SA. and Gilbert PB: Nomenclature for immune correlates of protection after vaccination. Clin Infect Dis 2012;54(11):1615–1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Haynes BF, Gilbert PB, McElrath MJ, et al. : Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med 2012;366(14):1275–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Karasavvas N, Billings E, Rao M, et al. : The Thai Phase III HIV Type 1 Vaccine trial (RV144) regimen induces antibodies that target conserved regions within the V2 loop of gp120. AIDS Res Hum Retroviruses 2012;28(11):1444–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zolla-Pazner S, deCamp AC, Cardozo T, et al. : Analysis of V2 antibody responses induced in vaccinees in the ALVAC/AIDSVAX HIV-1 vaccine efficacy trial. PLoS One 2013;8(1):e53629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zolla-Pazner S, Decamp A, Gilbert PB, et al. : Vaccine-induced IgG antibodies to V1V2 regions of multiple HIV-1 subtypes correlate with decreased risk of HIV-1 infection. PLoS One 2014;9(2):e87572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tomaras GD, Ferrari G, Shen X, et al. : Vaccine-induced plasma IgA specific for the C1 region of the HIV-1 envelope blocks binding and effector function of IgG. Proc Natl Acad Sci USA 2013;110(22):9019–9024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ferrari G, Pollara J, Kozink D, et al. : An HIV-1 gp120 envelope human monoclonal antibody that recognizes a C1 conformational epitope mediates potent antibody-dependent cellular cytotoxicity (ADCC) activity and defines a common ADCC epitope in human HIV-1 serum. J Virol 2011;85(14):7029–7036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lewis GK, Guan Y, Kamin-Lewis R, et al. : Epitope target structures of Fc-mediated effector function during HIV-1 acquisition. Curr Opin HIV AIDS 2014;9(3):263–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pollara J, Bonsignori M, Moody MA, et al. : HIV-1 vaccine-induced C1 and V2 Env-specific antibodies synergize for increased antiviral activities. J Virol 2014;88(14):7715–7726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gottardo R, Bailer RT, Korber BT, et al. : Plasma IgG to linear epitopes in the V2 and V3 regions of HIV-1 gp120 correlate with a reduced risk of infection in the RV144 Vaccine Efficacy Trial. PLoS One 2013;8(9):e75665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rolland M, Edlefsen PT, Larsen BB, et al. : Increased HIV-1 vaccine efficacy against viruses with genetic signatures in Env V2. Nature 2012;490(7420):417–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Excler JL, Tomaras GD, and Russell ND: Novel directions in HIV-1 vaccines revealed from clinical trials. Curr Opin HIV AIDS 2013;8(5):421–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hraber P, Korber BT, Lapedes AS, et al. : Impact of clade, geography, and age of the epidemic on HIV-1 neutralization by antibodies. J Virol 2014;88(21):12623–12643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Excler JL, Robb ML, and Kim JH: HIV-1 vaccines: Challenges and new perspectives. Hum Vaccin Immunother 2014;10(6):1734–1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Excler JL, Ake J, Robb ML, et al. : Nonneutralizing functional antibodies: A new 'old' paradigm for HIV vaccines. Clin Vaccine Immunol 2014;21(8):1025–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zolla-Pazner S: A critical question for HIV vaccine development: Which antibodies to induce? Science 2014;345(6193):167–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liu L, Qiu C, Huang Y, et al. : Potent T cell responses induced by single DNA vaccine boosted with recombinant vaccinia vaccine. Virol Sin 2013;28(2):109–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shao Y, Liu Y, and Xu J: The safety and immunogenicity of HIV-1 vaccines based on DNA and replication competent vaccinia vector in Phase I clinical trial. AIDS Res Hum Retroviruses 2010;26:A-1–A-184 [Google Scholar]
- 134.Mehendale S, van Lunzen J, Clumeck N, et al. : A phase 1 study to evaluate the safety and immunogenicity of a recombinant HIV type 1 subtype C adeno-associated virus vaccine. AIDS Res Hum Retroviruses 2008;24(6):873–880 [DOI] [PubMed] [Google Scholar]
- 135.Mehendale S, Sahay S, Thakar M, et al. : Safety and immunogenicity of tgAAC09, a recombinant adeno-associated virus type 2 HIV-1 subtype C vaccine in India. Indian J Med Res 2010;132:168–175 [PubMed] [Google Scholar]
- 136.Ramanathan VD, Kumar M, Mahalingam J, et al. : A Phase 1 study to evaluate the safety and immunogenicity of a recombinant HIV type 1 subtype C-modified vaccinia Ankara virus vaccine candidate in Indian volunteers. AIDS Res Hum Retroviruses 2009;25(11):1107–1116 [DOI] [PubMed] [Google Scholar]
- 137.Mehendale S, Thakar M, Sahay S, et al. : Safety and immunogenicity of DNA and MVA HIV-1 subtype C vaccine prime-boost regimens: A phase I randomised trial in HIV-uninfected Indian volunteers. PLoS One 2013;8(2):e55831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Newman PA, Chakrapani V, Weaver J, et al. : Willingness to participate in HIV vaccine trials among men who have sex with men in Chennai and Mumbai, India. Vaccine 2014;32(44):5854–5861 [DOI] [PubMed] [Google Scholar]
- 139.Jiang J, Yang X, Ye L, et al. : Pre-exposure prophylaxis for the prevention of HIV infection in high risk populations: A meta-analysis of randomized controlled trials. PLoS One 2014;9(2):e87674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ahmad K: Trial of antiretroviral for HIV prevention on hold. Lancet Infect Dis 2004;4(10):597. [DOI] [PubMed] [Google Scholar]
- 141.Cohen J: Cambodia: Can a drug provide some protection? Science 2003;301(5640):1660–1661 [DOI] [PubMed] [Google Scholar]
- 142.Cohen J: AIDS research. Cambodian leader throws novel prevention trial into limbo. Science 2004;305(5687):1092. [DOI] [PubMed] [Google Scholar]
- 143.Choopanya K, Martin M, Suntharasamai P, et al. : Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2013;381(9883):2083–2090 [DOI] [PubMed] [Google Scholar]
- 144.Grant RM, Lama JR, Anderson PL, et al. : Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010;363(27):2587–2599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.U.S. Food and Drug Administration: www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm312210.htm Accessed June11, 2014
- 146.World Health Organization: Guidance on Pre-Exposure Oral Prophylaxis (PrEP) for Serodiscordant Couples, Men and Transgender Women Who Have Sex with Men at High Risk of HIV: Recommendations for Use in the Context of Demonstration Projects. Geneva, Switzerland, 2012 [PubMed] [Google Scholar]
- 147.World Health Organization: Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection (2013). Geneva, Switzerland, 2013 [PubMed] [Google Scholar]
- 148.World Health Organization: Consolidated Guidelines on HIV Prevention, Diagnosis, Treatment and Care for Key Populations. Geneva, Switzerland, 2014 [PubMed] [Google Scholar]
- 149.Centers for Disease Control and Prevention: Interim guidance for clinicians considering the use of preexposure prophylaxis for the prevention of HIV infection in heterosexually active adults. MMWR Morb Mortal Wkly Rep 2012;61(31):586–589 [PubMed] [Google Scholar]
- 150.Centers for Disease Control and Prevention: Update to Interim Guidance for Preexposure Prophylaxis (PrEP) for the Prevention of HIV Infection: PrEP for injecting drug users. MMWR Morb Mortal Wkly Rep 2013;62(23):463–465 [PMC free article] [PubMed] [Google Scholar]
- 151.McCormack S. PROUD Study Group: Pragmatic Open-Label Randomised Trial of Preexposure Prophylaxis: The PROUD Study. CROI 2015. Seattle, WA [Google Scholar]
- 152.Molina JM CC, Spire B, Pialoux G, et al. : for the ANRS Ipergay Study Group: On Demand PrEP with Oral TDF/FTC in MSM: Results of the ANRS Ipergay Trial. CROI 2015. Seattle, WA [Google Scholar]
- 153.Patton C. and Kim HJ: The cost of science: Knowledge and ethics in the HIV pre-exposure prophylaxis trials. J Bioeth Inq 2012;9(3):295–310 [DOI] [PubMed] [Google Scholar]
- 154.Zhou F, Gao L, Li S, et al. : Willingness to accept HIV pre-exposure prophylaxis among Chinese men who have sex with men. PLoS One 2012;7(3):e32329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhang Y, Peng B, She Y, et al. : Attitudes toward HIV pre-exposure prophylaxis among men who have sex with men in western China. AIDS Patient Care STDS 2013;27(3):137–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ye L, Wei S, Zou Y, et al. : HIV pre-exposure prophylaxis interest among female sex workers in Guangxi, China. PLoS One 2014;9(1):e86200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wheelock A, Eisingerich AB, Ananworanich J, et al. : Are Thai MSM willing to take PrEP for HIV prevention? An analysis of attitudes, preferences and acceptance. PLoS One 2013;8(1):e54288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Grant RM, Anderson PL, McMahan V, et al. : Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: A cohort study. Lancet Infect Dis 2014;14(9):820–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Department of Disease Control, Thai Ministry of Public Health: Thailand National Guidelines on HIV/AIDS Treatment and Prevention. www.thaiaidssociety.org/images/PDF/hiv_guideline_2557.pdf Accessed February10, 2015
- 160.Hammer SM: Antiretroviral treatment as prevention. N Engl J Med 2011;365(6):561–562 [DOI] [PubMed] [Google Scholar]
- 161.Cohen MS, Chen YQ, McCauley M, et al. : Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011;365(6):493–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Grinsztejn B, Hosseinipour MC, Ribaudo HJ, et al. : Effects of early versus delayed initiation of antiretroviral treatment on clinical outcomes of HIV-1 infection: Results from the phase 3 HPTN 052 randomised controlled trial. Lancet Infect Dis 2014;14(4):281–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cohen J: Breakthrough of the year. HIV treatment as prevention. Science 2011;334(6063):1628. [DOI] [PubMed] [Google Scholar]
- 164.Anglemyer A, Horvath T, and Rutherford G: Antiretroviral therapy for prevention of HIV transmission in HIV-discordant couples. JAMA 2013;310(15):1619–1620 [DOI] [PubMed] [Google Scholar]
- 165.Granich R, Crowley S, Vitoria M, et al. : Highly active antiretroviral treatment as prevention of HIV transmission: Review of scientific evidence and update. Curr Opin HIV AIDS 2010;5(4):298–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Granich R, Gupta S, Suthar AB, et al. : Antiretroviral therapy in prevention of HIV and TB: Update on current research efforts. Curr HIV Res 2011;9(6):446–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kahn JG, Marseille EA, Bennett R, et al. : Cost-effectiveness of antiretroviral therapy for prevention. Curr HIV Res 2011;9(6):405–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gupta S, Granich R, Suthar AB, et al. : Global policy review of antiretroviral therapy eligibility criteria for treatment and prevention of HIV and tuberculosis in adults, pregnant women, and serodiscordant couples. J Acquir Immune Defic Syndr 2013;62(3):e87–97 [DOI] [PubMed] [Google Scholar]
- 169.Mayer K, Gazzard B, Zuniga JM, et al. : Controlling the HIV epidemic with antiretrovirals: IAPAC consensus statement on treatment as prevention and preexposure prophylaxis. J Int Assoc Provid AIDS Care 2013;12(3):208–216 [DOI] [PubMed] [Google Scholar]
- 170.Jia Z, Mao Y, Zhang F, et al. : Antiretroviral therapy to prevent HIV transmission in serodiscordant couples in China (2003–11): A national observational cohort study. Lancet 2013;382(9899):1195–1203 [DOI] [PubMed] [Google Scholar]
- 171.Walensky RP, Ross EL, Kumarasamy N, et al. : Cost-effectiveness of HIV treatment as prevention in serodiscordant couples. N Engl J Med 2013;369(18):1715–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mishra S, Mountain E, Pickles M, et al. : Exploring the population-level impact of antiretroviral treatment: The influence of baseline intervention context. AIDS 2014;28(Suppl 1):S61–72 [DOI] [PubMed] [Google Scholar]
- 173.UNAIDS: Smart Investments. Geneva, Switzerland, 2013 [Google Scholar]
- 174.Gouws E, Cuchi P, and the International Collaboration on Estimating HIVIbMoT: Focusing the HIV response through estimating the major modes of HIV transmission: A multi-country analysis. Sex Transm Infect 2012;88(Suppl 2):i76–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Prybylski D, Manopaiboon C, Visavakum P, et al. : Diverse HIV epidemics among people who inject drugs in Thailand: Evidence from respondent-driven sampling surveys in Bangkok and Chiang Mai. Drug Alcohol Depend 2015;148:126–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sharma M, Oppenheimer E, Saidel T, et al. : A situation update on HIV epidemics among people who inject drugs and national responses in South-East Asia Region. AIDS 2009;23(11):1405–1413 [DOI] [PubMed] [Google Scholar]
- 177.Lucas GM, Beauchamp G, Aramrattana A, et al. : Short-term safety of buprenorphine/naloxone in HIV-seronegative opioid-dependent Chinese and Thai drug injectors enrolled in HIV Prevention Trials Network 058. Int J Drug Policy 2012;23(2):162–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Metzger DS, Donnell D, Celentano DD, et al. : Expanding substance use treatment options for HIV prevention with buprenorphine-naloxone: HIV Prevention Trials Network 058. J Acquir Immune Defic Syndr 2015;68(5):554–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Coates TJ, Kulich M, Celentano DD, et al. : Effect of community-based voluntary counselling and testing on HIV incidence and social and behavioural outcomes (NIMH Project Accept; HPTN 043): A cluster-randomised trial. Lancet Glob Health 2014;2(5):e267–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bar KJ, Li H, Chamberland A, et al. : Wide variation in the multiplicity of HIV-1 infection among injection drug users. J Virol 2010;84(12):6241–6247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Redd AD, Quinn TC, and Tobian AA: Frequency and implications of HIV superinfection. Lancet Infect Dis 2013;13(7):622–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zeng Y, Zhang L, Li T, et al. : Risk factors for HIV/syphilis infection and male circumcision practices and preferences among men who have sex with men in China. Biomed Res Int 2014;2014:498987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zhou C, Raymond HF, Ding X, et al. : Anal sex role, circumcision status, and HIV infection among men who have sex with men in Chongqing, China. Arch Sex Behav 2013;42(7):1275–1283 [DOI] [PubMed] [Google Scholar]
- 184.Schneider JA, Michaels S, Gandham SR, et al. : A protective effect of circumcision among receptive male sex partners of Indian men who have sex with men. AIDS Behav 2012;16(2):350–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Templeton DJ, Millett GA, and Grulich AE: Male circumcision to reduce the risk of HIV and sexually transmitted infections among men who have sex with men. Curr Opin Infect Dis 2010;23(1):45–52 [DOI] [PubMed] [Google Scholar]
- 186.Wiysonge CS, Kongnyuy EJ, Shey M, et al. : Male circumcision for prevention of homosexual acquisition of HIV in men. Cochrane Database Syst Rev 2011;(6):CD007496. [DOI] [PubMed] [Google Scholar]
- 187.Tieu HV, Phanuphak N, Ananworanich J, et al. : Acceptability of male circumcision for the prevention of HIV among high-risk heterosexual men in Thailand. Sex Transm Dis 2010;37(6):352–355 [DOI] [PubMed] [Google Scholar]
- 188.Lau JT, Zhang J, Yan H, et al. : Acceptability of circumcision as a means of HIV prevention among men who have sex with men in China. AIDS Care 2011;23(11):1472–1482 [DOI] [PubMed] [Google Scholar]
- 189.Shaw GM. and Hunter E: HIV transmission. Cold Spring Harb Perspect Med 2012;2(11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Barouch DH, Liu J, Li H, et al. : Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature 2012;482(7383):89–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Barouch DH, Stephenson KE, Borducchi EN, et al. : Protective efficacy of a global HIV-1 mosaic vaccine against heterologous SHIV challenges in rhesus monkeys. Cell 2013;155(3):531–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Letourneau S, Im EJ, Mashishi T, et al. : Design and pre-clinical evaluation of a universal HIV-1 vaccine. PLoS One 2007;2(10):e984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Koopman G, Beenhakker N, Nieuwenhuis I, et al. : DNA/long peptide vaccination against conserved regions of SIV induces partial protection against SIVmac251 challenge. AIDS 2013;27(18):2841–2851 [DOI] [PubMed] [Google Scholar]
- 194.Hayton EJ, Rose A, Ibrahimsa U, et al. : Safety and tolerability of conserved region vaccines vectored by plasmid DNA, simian adenovirus and modified vaccinia virus ankara administered to human immunodeficiency virus type 1-uninfected adults in a randomized, single-blind phase I trial. PLoS One 2014;9(7):e101591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Borthwick N, Ahmed T, Ondondo B, et al. : Vaccine-elicited human T cells recognizing conserved protein regions inhibit HIV-1. Mol Ther 2014;22(2):464–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Excler JL, Rida W, Priddy F, et al. : AIDS vaccines and preexposure prophylaxis: Is synergy possible? AIDS Res Hum Retroviruses 2011;27(6):669–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Janes H, Gilbert P, Buchbinder S, et al. : In pursuit of an HIV vaccine:D efficacy trials in the context of partially effective nonvaccine prevention modalities. AIDS Res Hum Retroviruses 2013;29(11):1513–1523 [DOI] [PMC free article] [PubMed] [Google Scholar]