Abstract
Youth football players may incur hundreds of repetitive head impacts (RHI) in one season. Our recent research suggests that exposure to RHI during a critical neurodevelopmental period prior to age 12 may lead to greater later-life mood, behavioral, and cognitive impairments. Here, we examine the relationship between age of first exposure (AFE) to RHI through tackle football and later-life corpus callosum (CC) microstructure using magnetic resonance diffusion tensor imaging (DTI). Forty retired National Football League (NFL) players, ages 40–65, were matched by age and divided into two groups based on their AFE to tackle football: before age 12 or at age 12 or older. Participants underwent DTI on a 3 Tesla Siemens (TIM-Verio) magnet. The whole CC and five subregions were defined and seeded using deterministic tractography. Dependent measures were fractional anisotropy (FA), trace, axial diffusivity, and radial diffusivity. Results showed that former NFL players in the AFE <12 group had significantly lower FA in anterior three CC regions and higher radial diffusivity in the most anterior CC region than those in the AFE ≥12 group. This is the first study to find a relationship between AFE to RHI and later-life CC microstructure. These results suggest that incurring RHI during critical periods of CC development may disrupt neurodevelopmental processes, including myelination, resulting in altered CC microstructure.
Key words: : age at first exposure, American football, corpus callosum, diffusion tensor imaging, repetitive head impacts
Introduction
Traumatic brain injury (TBI) in youth sports is a growing public health concern given the millions of youth athletes participating annually in the United States alone.1 In addition to concussive injuries, recent evidence indicates that sustaining repetitive subconcussive head impacts through sports participation may result in long-term consequences, including behavioral symptoms,2,3 cognitive impairment,4 brain structure alterations,5 and neurodegenerative diseases, such as chronic traumatic encephalopathy (CTE).6–10 Neuroimaging and electrophysiological studies have identified structural and functional abnormalities in former contact sport athletes many years after they stopped playing.11–15 Tackle football players ages 7–12 may experience hundreds of repetitive head impacts (RHI), concussive and/or subconcussive, over the course of one season, several of which may exceed forces of 80 g.16,17 Our previous research suggests that incurring RHI during critical periods of neurodevelopment in childhood may lead to later-life mood, behavioral, and cognitive impairment.18,19 However, the impact of RHI incurred during youth on later-life brain structure has not yet been systematically examined.
A previous theory proposed that, due to its increased plasticity, recovery from concussions in the developing brain would be superior to that of the adult brain.20 More recent evidence suggests that children and adolescents are more likely to endure prolonged symptom recovery21–23 and are more vulnerable to poor outcomes.24,25 Moreover, neuroimaging studies show persistent structural and functional changes in the brain following mild TBI in children.26–28 Windows of vulnerability to brain trauma may be associated with critical periods of brain development occurring throughout childhood and adolescence.29–31 One such critical period occurs between ages 10–12 in males.32–38 Amygdalar and hippocampal volumes, as well as cortical thickness in several brain regions, reach peak levels during this time,37–40 with synaptic pruning beginning shortly thereafter to enhance efficient information processing.41,42 Peaks in the rate of myelination29,31 and cerebral blood flow,33 as well as significant improvements in network organization,43 also occur between ages 10–12, potentially making the brain more susceptible to functional and structural alterations following RHI.
Diffusion tensor imaging (DTI) is an advanced magnetic resonance imaging (MRI) technique that provides insight into the brain's white matter microstructure by measuring the magnitude and direction of the movement of water molecules.44 Within white matter, water molecules tend to diffuse along a course parallel to fiber tracts. The directionality of this diffusion is commonly measured using fractional anisotropy (FA).45 Higher FA values denote greater diffusion along one direction, as is observed in well-organized tissues.45 Trace is the sum of diffusion in all directions.44, 45 In poorly-organized tissues, the multi-directional movement of water molecules can occur with little resistance, resulting in high trace values.45 Other common diffusion measures include axial (AD) and radial (RD) diffusivity, which are thought to measure axonal and myelin pathology, respectively.46
Altered diffusivity is frequently observed following mild TBI (mTBI).47. Recent research using DTI,5,48–51 as well as other imaging modalities,52–55 also revealed altered brain structure and connectivity following prolonged exposure to RHI. Further, several studies report altered diffusivity following just one season of football48,49,51 and ice hockey50 play, when comparing preseason and post-season DTI measures. Bazarian and colleagues49 identified decreased FA and increased mean diffusivity values that persisted for at least 6 months post-season. Moreover, Koerte and colleagues5 compared elite adult soccer players with no history of concussion (i.e., only subconcussive blows to the head) to competitive swimmers and observed higher RD and AD in several brain regions. Findings from these studies provide further support for the notion that despite the lack of concussive symptoms, incurring repeated subconcussive head impacts is not without consequences.
The corpus callosum (CC) is the largest commissural fiber tract in the brain. It is particularly vulnerable to diffuse axonal injury, with head impacts due to the density and orientation of fibers, the position of the dural reflections creating barriers to brain movement, and increased shear strain on the tract when external acceleration forces are applied.56,57 The greatest shear strain occurs in the genu (anterior CC) and splenium (posterior CC),57 and these regions are frequently damaged in TBI.26,56–60 Studies also report CC microstructural damage following prolonged exposure to RHI in football,49 hockey,50 and soccer players.5 Further, several neuroimaging studies in children demonstrate disrupted CC development following TBI of varying severity.26,60–63 Key aspects of CC development, including high rates of myelination and axonal growth, occur between ages 8–12.34,64–66 However, the relationship between RHI experienced during this critical neurodevelopmental period and later-life CC microstructure has not been examined.
The purpose of this study was to examine the relationship between the age of first exposure (AFE) to RHI through tackle football and later-life CC microstructural alterations using DTI. We examined diffusion measures in two groups of former National Football League (NFL) players: those who started playing tackle football before age 12 (AFE <12) and those who started at age 12 or older (AFE ≥12). Twelve was chosen as the cut-off age based on the neurodevelopmental literature described above32–38 and previous work from our group.18,19 We hypothesized that the AFE <12 group would have altered diffusivity, particularly in the genu and splenium, compared with the AFE ≥12 group.
Methods
This research is part of Diagnosing and Evaluating Traumatic Encephalopathy using Clinical Tests (DETECT), an ongoing study with a primary goal of developing methods for diagnosing CTE during life. DETECT includes former NFL players and a control group. For this study, only former NFL players were included. DETECT study procedures are described elsewhere.19 The Boston University Medical Center Institutional Review Board approved all study procedures, and all neuroimaging procedures were approved by the Partners Institutional Review Board. Prior to participation, all participants provided written informed consent.
Participants
Inclusion criteria for former NFL players in DETECT are: male; age 40–69; a minimum of 12 total years of organized football participation; and two years of play in the NFL. Additionally, participants must report a worsening of cognitive, behavioral, and mood symptoms for at least the last 6 months that is self-perceived, reported by others, or for which they have received treatment from a doctor. These symptoms may include difficulties with memory, planning and organization, impulsivity, violence, depression, anxiety, and/or apathy. As reported previously (with a sample that was nearly identical to the present sample),19 there were significant group differences in performance on select neuropsychological tests, with the AFE <12 group performing more poorly than the AFE ≥12 group; however, the mean performance of both groups was within 1.5 SD below demographically-corrected norms. Exclusion criteria are MRI and lumbar puncture contraindications or history of other diagnosed neurologic disease. Of the 74 former NFL players who had participated in DETECT at the time of the study, three did not have imaging data acquired. An additional five cases were excluded due to motion artifact, leaving 66 former NFL players eligible for this study.
Current age differed significantly between AFE groups when all subjects were included (AFE <12, n = 30, mean = 50.3 years, SD = 6.6; AFE ≥12, n = 36, mean = 57.4 years, SD = 7.4; p < 0.001). Because of this age difference and the resulting possibility of differences in style of football played in different chronological eras, we selected age-matched pairs for subsequent analyses. That is, one subject in the AFE <12 group was randomly paired, a priori, with another subject of the same age from the AFE ≥12 group if any existed. Twenty-six subjects (AFE <12 = 10 subjects; AFE ≥12 = 16 subjects) could not be matched within 2 years of age with a participant in the other AFE group, and, therefore, these subjects were not included in the analysis. The remaining 40 subjects were matched within 2 years of age, with 20 subjects in each AFE group (age at scan range = 40–65). Because of the potential impact of current age on CC integrity, it was determined that focusing on age-matched pairs was of greater methodological importance than including a larger sample size with large between-group age differences. Moreover, using age-matched pairs in this study reduced the standard error of the mixed-effects regression estimates, which increases the power for detecting clinically significant estimates.
Head impact exposure variables
AFE to tackle football was treated as a dichotomous variable and used to divide subjects into two groups: AFE <12 and AFE ≥12. Not surprisingly, duration of football play, defined as the total number of years, differed between AFE groups and was therefore used as a covariate. Duration was treated as a continuous variable.
MRI acquisition
Diffusion weighted images (DWI) were acquired on a 3T MR scanner (TIM Verio; Siemens Healthcare, Erlangen, Germany) with a 32 channel head coil. An echo planar imaging DWI sequence was used with the following parameters: repetition time, 11,700 msec; echo time, 85 msec; field of view, 256 mm; 128 × 128 matrix; 2.0 mm slice thickness; and parallel imaging using GRAPPA with acceleration factor 3. Seventy-three slices were acquired using 87 diffusion directions organized in multiple b-value shells, consisting of 64 diffusion-weighted images with a b-value of 900 sec/mm2, 10 images with a b-value of 400 sec/mm2, six images with a b-value of 100 sec/mm2, and seven images with b-value of 0 sec/mm2 used as baseline images.
Post-processing of diffusion tensor imaging data
Affine registration of the DWI to the baseline image was performed to remove intrascan misalignments due to eddy currents and head motion (FSL 4.1; FMRIB Software Library, the Oxford Centre for Functional MRI of the Brain, Oxford, UK). Additionally, an automated evaluation of DWI images for motion artifact was conducted using in-house software and resulted in the elimination of four cases. A visual inspection of all 87 components of each DWI also was performed and resulted in the elimination of one additional case due to motion artifact. Further, this quality check revealed dropped signals in less than five of 87 diffusion directions in six cases (AFE <12 = 1 case; AFE ≥12 = 5 cases). These six cases were included in the study. However, to eliminate possible influences of these signals, we excluded the diffusion directions and gradient information using in-house software. One direction was removed in two cases, two directions were removed in three cases, and three directions were removed in one case. A corrected DWI-file with the gradients eliminated was obtained for each of the respective participants.
Corpus callosum region of interest and tractography
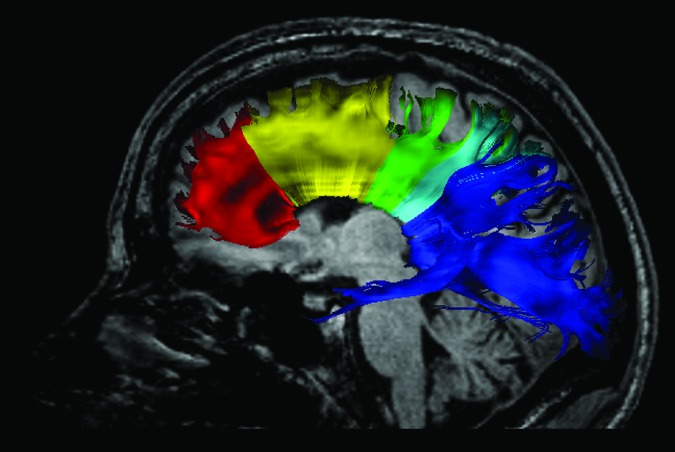
The whole CC was defined manually on the midsagittal slice, with one slice to each side of the midsagittal slice on the color-oriented FA map (n = 3 slices) using 3D Slicer software package version 4.3.1 (www.slicer.org; Surgical Planning Laboratory, Brigham and Women's Hospital, Boston, MA). The whole CC label map was mathematically subdivided into five subregions, as described by Hofer and Frahm67 (Fig. 1). The five subregions contain commissural fibers of prefrontal (region I), premotor and supplementary motor (region II), primary motor (region III), sensory (region IV), and parietal, temporal and occipital cortical (region V) areas.67
FIG. 1.
Tractography of the corpus callosum. The corpus callosum was subdivided into five regions containing commissural fibers of prefrontal (region I), premotor and supplementary motor (region II), primary motor (region III), sensory (region IV), and parietal, temporal and occipital cortical (region V) areas. Tracts were obtained using deterministic (streamline) tractography to trace fiber paths through the regions of interest. Color image is available online at www.liebertpub.com/neu
Seeding of fiber tracts through the whole CC and CC subregions was conducted in 3D Slicer using a deterministic (streamline) tractography approach, which uses the principal diffusion direction in each voxel to obtain fiber trajectories, with stopping criteria of FA lower than 0.15.68 To ensure that only CC fibers were included, exclusion regions of interest (ROIs) were used in the axial plane at the levels of the superior thalamus and rostral midbrain in order to eliminate corticothalamic fibers and corticospinal and corticobulbar fibers, respectively. Further, to ensure the accuracy of the CC subregion fiber extraction, regions not being examined were excluded (i.e., when fibers were extracted from region I, regions II-V were considered exclusion ROIs). Each tractography output was then inspected and if present, fibers clearly representing non-callosal tracts were manually removed in 3D Slicer. Fibers from the cingulum bundle and inferior longitudinal fasciculus were most frequently manually removed, while uncinate fasciculus and fornix fibers were manually removed in fewer cases. Mean FA, trace, AD, and RD were extracted for each CC tract. All of these procedures were carried out blind to AFE group membership and age.
Statistical analysis
Due to the need to include covariates in the analysis, a multi-variate mixed-effects linear regression model was used to determine the effect of AFE to tackle football on all DTI measures. This model included duration of play and body mass index (BMI) as covariates. BMI has been shown to be negatively correlated with the integrity of the corpus callosum.69 The model also adjusted for correlations within the age-matched pairs and between DTI measures from the same subject to account for possible inflation of type I error. All analyses were conducted using SAS 9.3.
Results
Participant demographics and athletic history
Demographic information, athletic history, and other health-related factors for the age-matched groups are described in Table 1. AFE across the 40 participants ranged from age 6 to age 17 (median = 11.5 years old). Duration of football play (AFE <12, mean = 20.3 years, SD = 3.4; AFE ≥12, mean = 18.1 years, SD = 3.1; p = 0.039) and BMI (AFE <12, mean = 30.4, SD = 3.0; AFE ≥12, mean = 33.7, SD = ,4.7; p < 0.013) differed significantly between these two age-matched groups.
Table 1.
Demographic and Athletic Information
| Mean (SD) | ||||
|---|---|---|---|---|
| AFE <12 years(n = 20) | AFE ≥12 years(n = 20) | T Value | p Value | |
| Age at scan (years) | 52.2 (6.5) | 52.5 (6.2) | −0.150 | 0.882 |
| Age of first exposure to tackle football (years) | 9.1 (1.4) | 14.1 (1.4) | −11.313 | < 0.001 |
| Education (years) | 16.7 (1.1) | 16.3 (0.9) | 1.292 | 0.204 |
| Duration of football play (years) | 20.3 (3.4) | 18.1 (3.1) | 2.140 | 0.039 |
| Duration of play in the NFL (years) | 7.4 (2.4) | 9.1 (2.8) | −1.993 | 0.053 |
| *Total number of concussions, median (IQR) | 45 (179.5) | 40 (285.3) | 395 | 0.697a |
| Body mass index | 30.4 (3.0) | 33.7 (4.7) | −2.623 | 0.013 |
| Race, African American, n (%) | 6 (30.0) | 11 (55.0) | 2.558− | 0.11b |
| Primary position group, n (%) | 10.921− | 0.053b | ||
| Offensive line, n (%) | 1 (2.5) | 9 (22.5) | ||
| Running back, n (%) | 1 (2.5) | 1 (2.5) | ||
| Tight end, n (%) | 1 (2.5) | 1 (2.5) | ||
| Defensive line, n (%) | 3 (7.5) | 4 (10.0) | ||
| Linebacker, n (%) | 7 (17.5) | 3 (7.5) | ||
| Defensive back, n (%) | 7 (17.5) | 2 (5.0) | ||
| Played other contact sport, n (%) | 6 (30.0) | 8 (40.0) | 0.440 | 0.507b |
| Use of performance enhancing drugs, n (%) | 4 (21.1) | 2 (11.8) | 0.662c | |
| Significant use of alcohol, n (%) | 11 (55.0) | 12 (60.0) | 0.102 | 0.749b |
| Significant use of illicit drugs, n (%) | 12 (60.0) | 11 (55.0) | 0.102 | 0.749b |
| Hypertension, n (%) | 11 (55.0) | 9 (45.0) | 0.400 | 0.527b |
| High cholesterol, n (%) | 8 (42.1) | 12 (60.0) | 1.249 | 0.264b |
| Heart disease, n (%) | 1 (5.3) | 1 (5.6) | 1.000c | |
| Diabetes, n (%) | 1 (5.0) | 3 (15.8) | 0.342c | |
After being given a modern definition of concussion.77
Wilcoxon signed rank test.
Chi-square test.
Fisher's exact test.
SD, standard deviation; AFE, age of first exposure; NFL, National Football League; IQR interquartile range.
AFE group comparison
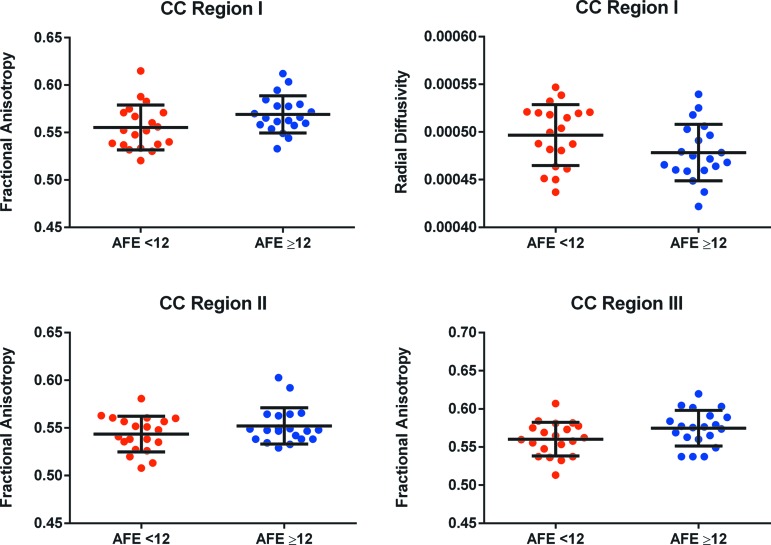
Results from the mixed-effects linear model investigating between-group differences are shown in Table 2. Duration of play and BMI were not significant predictors of any measures. After adjustment for duration of play and BMI,69 the AFE <12 group displayed significantly lower FA in the anterior CC regions (I, II, and III) and higher RD in region I, compared with the AFE ≥12 group (Fig. 2). AD and trace did not differ significantly between groups.
Table 2.
Mixed Effects Linear Regression Results Comparing Age of First Exposure (AFE) Groups
| AFE <12 years (n = 20) | AFE ≥12 years (n = 20) | Adjusted estimated difference | |||||
|---|---|---|---|---|---|---|---|
| Adjusted mean | Standard error | Adjusted mean | Standard error | (AFE ≥12 - AFE <12) | Standard error | p Value | |
| Whole CC | |||||||
| FA | 0.5667 | 0.02211 | 0.5756 | 0.02279 | 0.00888 | 0.00480 | 0.066 |
| Trace | 0.002618 | 0.000128 | 0.002609 | 0.000132 | −0.000009 | 0.00004 | 0.817 |
| AD | 0.001504 | 0.000056 | 0.001509 | 0.000058 | 0.000004 | 0.00002 | 0.799 |
| RD | 0.000551 | 0.000040 | 0.000544 | 0.000042 | −0.000007 | 0.00001 | 0.549 |
| Region I | |||||||
| FA | 0.5568 | 0.02229 | 0.5723 | 0.02296 | 0.01549 | 0.00620 | 0.013 |
| Trace | 0.002537 | 0.000127 | 0.002490 | 0.000131 | −0.00005 | 0.00003 | 0.164 |
| AD | 0.001442 | 0.000056 | 0.001435 | 0.000058 | −0.000007 | 0.00002 | 0.642 |
| RD | 0.000542 | 0.000040 | 0.000521 | 0.000041 | −0.00002 | 0.00001 | 0.048 |
| Region II | |||||||
| FA | 0.5451 | 0.02215 | 0.5553 | 0.02283 | 0.01020 | 0.00515 | 0.049 |
| Trace | 0.002541 | 0.000127 | 0.002518 | 0.000131 | −0.00002 | 0.00003 | 0.487 |
| AD | 0.001436 | 0.000056 | 0.001434 | 0.000058 | −0.000001 | 0.00002 | 0.947 |
| RD | 0.000547 | 0.000040 | 0.000535 | 0.000041 | −0.00001 | 0.00001 | 0.222 |
| Region III | |||||||
| FA | 0.5618 | 0.02210 | 0.5778 | 0.02278 | 0.01602 | 0.00466 | < 0.001 |
| Trace | 0.002497 | 0.000126 | 0.002478 | 0.000130 | −0.00002 | 0.00002 | 0.445 |
| AD | 0.001437 | 0.000055 | 0.001446 | 0.000057 | 0.000010 | 0.00001 | 0.433 |
| RD | 0.000524 | 0.000040 | 0.000509 | 0.000041 | −0.00001 | 0.00001 | 0.052 |
| Region IV | |||||||
| FA | 0.5366 | 0.02276 | 0.5510 | 0.02342 | 0.01441 | 0.00901 | 0.112 |
| Trace | 0.002607 | 0.000128 | 0.002621 | 0.000132 | 0.000014 | 0.00004 | 0.739 |
| AD | 0.001456 | 0.000056 | 0.001481 | 0.000058 | 0.000024 | 0.00002 | 0.167 |
| RD | 0.000569 | 0.000041 | 0.000564 | 0.000042 | −0.000006 | 0.00002 | 0.703 |
| Region V | |||||||
| FA | 0.5895 | 0.02298 | 0.5958 | 0.02364 | 0.006353 | 0.01008 | 0.529 |
| Trace | 0.002774 | 0.000135 | 0.002767 | 0.000138 | −0.000007 | 0.00007 | 0.9182 |
| AD | 0.001618 | 0.000058 | 0.001621 | 0.000059 | 0.000003 | 0.00003 | 0.9051 |
| RD | 0.000572 | 0.000043 | 0.000567 | 0.000044 | −0.000006 | 0.00002 | 0.8169 |
Adjusted for duration (years) of football and body mass index.
FA, fractional anisotropy; CC, corpus callosum; AD, axial diffusivity; RD, radial diffusivity.
FIG. 2.
Scatter plots illustrating fractional anisotropy (FA) in corpus callosum regions I, II, and III, and radial diffusivity (RD) in region I. Those with an age of first exposure to tackle football prior to age 12 had significantly lower FA in Regions I, II, and III, and higher RD in Region I, than those who began playing football at age 12 or later. Error bars signify one standard deviation from the mean. Color image is available online at www.liebertpub.com/neu
Discussion
This study is the first to evaluate the relationship between the age a retired NFL player began playing tackle football and later-life CC white matter microstructure. We observed significantly lower FA (regions I, II, and III) and higher RD (region I) in the anterior CC in former NFL players who began playing tackle football prior to age 12, compared with those who began playing tackle football at age 12 or older. Although preliminary, these results suggest that incurring RHI through tackle football play during a critical period of anterior CC growth before age 12 may disrupt developmental processes, possibly resulting in lasting alterations in anterior CC white matter microstructure.
The results of this study extend our previous research showing greater later-life mood, behavioral impairment, and cognitive impairment in retired NFL players with exposure to RHI through tackle football prior to age 12.18,19 Bourlas and colleagues18 studied former high school, college, and professional football players and found that the AFE <12 group self-reported greater executive dysfunction, apathy, and depression than the AFE ≥12 group. Further, the AFE <12 group had approximately three times greater odds of having later-life clinically-meaningful depression and executive dysfunction. Stamm and colleagues19 found that former NFL players in an AFE <12 group performed significantly worse on objective measures of executive functioning, memory, and estimated verbal intelligence than those in the AFE ≥12 group. The results of the present study further support the vulnerability of the developing brain to RHI prior to age 12 and for the first time, show a relationship between AFE to RHI and later-life white matter microstructure alterations.
Callosal anatomy and neurodevelopment may at least partially explain the findings of this study. FA increases rapidly in the CC prior to age 12, and this rise is thought to be driven by a decrease in RD associated with increased myelination.34,65,66,70 The genu and splenium reach 90% of peak FA by age 11,34 followed by a much slower increase in FA until peak levels are reached in the early 20s.34,64,65 Snook and colleagues66 showed a greater slope of increase in FA in the genu than in the splenium between ages 8–12, suggesting greater anterior CC development during this time. The genu and anterior midbody of the CC contain small-diameter, lightly-myelinated fibers, and the genu has the highest proportion of unmyelinated fibers in the adult CC.71 These fiber types are preferentially vulnerable to damage and have limited ability to recover following TBI.72,73 It is possible that anterior callosal neuroanatomy, combined with incomplete and rapid myelination between ages 8–12, may predispose the anterior CC to detrimental effects of RHI experienced during this critical neurodevelopmental period. The reduced RD observed in the AFE <12 group in this study suggests that RHI may disrupt the normal myelination process in childhood, possibly leading to a reduced peak level of myelination in the adult brain. However, further research beginning in children is needed to better understand the long-term consequences that incurring RHI has on the developing brain.
Although the AFE <12 group played football for approximately 2 years longer than those in the AFE ≥12 group, duration of football play was not a significant predictor of white matter microstructural outcome in this study. Previous studies examining the effect of duration, as a proxy for overall exposure, on brain structure and function has been mixed. One study found an association between duration of play and presence and severity of CTE neuropathology,8 while other studies found no effect of this variable on later-life mood, behavior, and cognitive functioning18,19 or diffusivity measures.5 More research is needed to elucidate the relationship between duration of football and later-life brain structure, function, and neurodegeneration, and on the interaction effects of total duration of play and initial age of play on later life changes.
The segmentation method used in this study may have contributed to the lack of differences between groups in the posterior CC regions.67 Region V represents posterior callosal fibers connecting temporal, parietal, and occipital regions. However, the temporal and parietal fibers coursing through this CC region are smaller and lightly myelinated, while the CC fibers connecting occipital regions are larger and highly myelinated.71 These fibers may be differentially affected by RHI. Combining both fiber types in one region may have reduced the ability to observe posterior differences between AFE groups in this study. Future research should consider investigating the effects of AFE to RHI in CC fibers connecting temporal, parietal, and occipital fibers separately. Additionally, differing developmental trajectories of anterior and posterior CC regions also may contribute to the lack of diffusivity differences between AFE groups in posterior CC regions.
There are several limitations to this study that should be taken into account. First, the generalizability of these results may not extend to other groups. For example, the biomechanics and amount of RHI experienced by former NFL players may differ from that of athletes in other high-risk sports, such as soccer and ice hockey. Additionally, developmental trajectories37–39 and outcomes74 following mTBI differ between males and females; therefore, these results also may not apply to females exposed to RHI during youth. Second, it is not known whether continued exposure to RHI in adolescence and adulthood influences the brain's ability to recover following childhood exposure to RHI. Future studies should investigate individuals whose highest levels of football played were college and high school, as well as individuals with an AFE <12 but who stopped incurring RHI after age 12. Third, establishment of causality between AFE to RHI and altered anterior callosal diffusivity cannot be made due to the cross-sectional nature of the study design. Future studies should, therefore, utilize a longitudinal design beginning with younger, current athletes.
Fourth, the results of this study should not be interpreted as concluding that incurring RHI at or after age 12 is without consequences to CC integrity. AFE could be one of several factors, including other aspects of head impact or injury exposure, genetics, and other health-related issues, that may influence later-life outcome following RHI. Fifth, although using age-matched pairs was appropriate for this study, it resulted in a reduced sample size, which is an important limitation of this research. Lastly, although CC pathology has been reported in CTE, the results of this study do not suggest that the participants have or will develop this neurodegenerative disease. Pathological processes resulting from disrupted white matter development may differ from the tauopathy-based neurodegeneration of CTE. More research is needed to determine whether or not incurring RHI during critical neurodevelopmental periods is a risk factor for the development of CTE.
Increased awareness of the acute and long-term consequences of repeated concussive and subconcussive head trauma has resulted in policy and rule changes in multiple sports at all levels of play, as well as legislation intended to protect youth and adolescent athletes.16,75 However, replication of our results is necessary before using these findings as rationale to implement significant rule or policy changes. Further, it has been suggested that a recent decline in youth sport participation may be attributed, in part, to concerns of parents and guardians about brain trauma.76 More investigation into later-life outcomes from exposure to RHI in childhood is necessary to address these concerns, to increase safety in youth sports, and to allow youth athletes to take advantage of the enormous benefits of sports participation without the possibility of long-term consequences.
In conclusion, this study found that former NFL players who started playing tackle football prior to age 12 had lower FA and greater RD in anterior CC regions, compared with those who started playing football at age 12 or older. Exposure to RHI during a critical period of neurodevelopment may disrupt normal axonal maturation and myelination, leading to permanently altered white matter microstructure. More research is needed to understand the impact of RHI incurred in childhood on later-life brain structure and function.
Acknowledgments
The authors extend their appreciation to the study participants who make this work possible.
This study was supported by the National Institutes of Health (R01 NS 078337; F31 NS 081957 [J.M.S.]; P30 AG13846; UL1-TR000157, P41 EB015902 [O.P.]; T32MH019733 [C.M.B.]), and participant travel was funded by gifts from JetBlue Airlines, the National Football League (NFL), and the NFL Players Association. This study was also partly supported by the Else Kröner-Fresenius Foundation, Germany (I.K., M.M.), and by a VA Merit Award (M.E.S., M.C., L.L., A.Z.).
Author Disclosure Statement
RAS is a paid consultant to Quest Diagnostics, Amarantus Bioscience, and Adelphi Values. He also serves as an expert advisor to attorneys for cases pertaining to the long-term consequences of repetitive brain trauma. He receives royalties from Psychological Assessment Resources for the publication of neuropsychological tests. For all other authors, no competing financial interests exist.
References
- 1.National Council of Youth Sports. (2008). Report on Trends and Participation in Organized Youth Sports. Available at: www.ncys.org/pdfs/2008/2008-ncys-market-research-report.pdf Accessed August31, 2015
- 2.Guskiewicz K.M., Marshall S.W., Bailes J., McCrea M., Harding H.P., Jr., Matthews A., Mihalik J.R., and Cantu R.C. (2007). Recurrent concussion and risk of depression in retired professional football players. Med. Sci. Sports Exerc. 39, 903–909 [DOI] [PubMed] [Google Scholar]
- 3.Seichepine D.R., Stamm J.M., Daneshvar D.H., Riley D.O., Baugh C.M., Gavett B.E., Tripodis Y., Martin B., Chaisson C., McKee A.C., Cantu R.C., Nowinski C.J., and Stern R.A. (2013). Profile of self-reported problems with executive functioning in college and professional football players. J. Neurotrauma 30, 1299–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guskiewicz K.M., Marshall S.W., Bailes J., McCrea M., Cantu R.C., Randolph C., and Jordan B.D. (2005). Association between recurrent concussion and late-life cognitive impairment in retired professional football players. Neurosurgery 57, 719–726 [DOI] [PubMed] [Google Scholar]
- 5.Koerte I.K., Ertl-Wagner B., Reiser M., Zafonte R., and Shenton M.E. (2012). White matter integrity in the brains of professional soccer players without a symptomatic concussion. JAMA 308, 1859–1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKee A.C., Cantu R.C., Nowinski C.J., Hedley-Whyte E.T., Gavett B.E., Budson A.E., Santini V.E., Lee H.S., Kubilus C.A., and Stern R.A. (2009). Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J. Neuropathol. Exp. Neurol. 68, 709–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKee A.C., Gavett B.E., Stern R.A., Nowinski C.J., Cantu R.C., Kowall N.W., Perl D.P., Hedley-Whyte E.T., Price B., Sullivan C., Morin P., Lee H.S., Kubilus C.A., Daneshvar D.H., Wulff M., and Budson A.E. (2010). TDP-43 proteinopathy and motor neuron disease in chronic traumatic encephalopathy. J. Neuropathol. Exp. Neurol. 69, 918–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKee A.C., Stern R.A., Nowinski C.J., Stein T.D., Alvarez V.E., Daneshvar D.H., Lee H.S., Wojtowicz S.M., Hall G., Baugh C.M., Riley D.O., Kubilus C.A., Cormier K.A., Jacobs M.A., Martin B.R., Abraham C.R., Ikezu T., Reichard R.R., Wolozin B.L., Budson A.E., Goldstein L.E., Kowall N.W., and Cantu R.C. (2013). The spectrum of disease in chronic traumatic encephalopathy. Brain 136, 43–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stern R.A., Daneshvar D.H., Baugh C.M., Seichepine D.R., Montenigro P.H., Riley D.O., Fritts N.G., Stamm J.M., Robbins C.A., McHale L., Simkin I., Stein T.D., Alvarez V.E., Goldstein L.E., Budson A.E., Kowall N.W., Nowinski C.J., Cantu R.C., and McKee A.C. (2013). Clinical presentation of chronic traumatic encephalopathy. Neurology 81, 1122–1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montenigro P.H., Baugh C.M., Daneshvar D.H., Mez J., Budson A.E., Au R., Katz D., Cantu R.C., and Stern R.A. (2014). Clinical subtypes of chronic traumatic encephalopathy: literature review and proposed research diagnostic criteria for traumatic encephalopathy syndrome. Alzheimers Res. Ther. 6, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hampshire A., Macdonald A., and Owen A.M. (2013). Hypoconnectivity and hyperfrontality in retired American football players. Sci. Rep. 3, 2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hart J., Jr., Kraut M.A., Womack K.B., Strain J., Didehbani N., Bartz E., Conover H., Mansinghani S., Lu H., and Cullum C.M. (2013). Neuroimaging of cognitive dysfunction and depression in aging retired National Football League players: a cross-sectional study. JAMA Neurol. 70, 326–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strain J., Didehbani N., Cullum C.M., Mansinghani S., Conover H., Kraut M.A., Hart J., Jr., and Womack K.B. (2013). Depressive symptoms and white matter dysfunction in retired NFL players with concussion history. Neurology 81, 25–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Beaumont L., Theoret H., Mongeon D., Messier J., Leclerc S., Tremblay S., Ellemberg D., and Lassonde M. (2009). Brain function decline in healthy retired athletes who sustained their last sports concussion in early adulthood. Brain 132, 695–708 [DOI] [PubMed] [Google Scholar]
- 15.Tremblay S., De Beaumont L., Henry L.C., Boulanger Y., Evans A.C., Bourgouin P., Poirier J., Theoret H., and Lassonde M. (2013). Sports concussions and aging: a neuroimaging investigation. Cereb. Cortex 23, 1159–1166 [DOI] [PubMed] [Google Scholar]
- 16.Cobb B.R., Urban J.E., Davenport E.M., Rowson S., Duma S.M., Maldjian J.A., Whitlow C.T., Powers A.K., and Stitzel J.D. (2013). Head impact exposure in youth football: elementary school ages 9–12 years and the effect of practice structure. Ann. Biomed. Eng. 41, 2463–2473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daniel R.W., Rowson S., and Duma S.M. (2012). Head impact exposure in youth football. Ann. Biomed. Eng. 40, 976–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bourlas A.P., Stamm J.M., Baugh C.M., Daneshvar D.H., Breaud A.H., Robbins C.A., Rile D.O., Martin B.M., McClean M.D., Au R., Gioia G., Ozonoff A., McKee A.C., Nowinski C.J., Cantu R.C., Tripodis Y., and Stern R.A. (2014). Relationship between age of first exposure to tackle football and later-life mood, behavior, and cognition. Brain. Inj. 28, 517–87824758283 [Google Scholar]
- 19.Stamm J.M., Bourlas A.P., Baugh C.M., Fritts N.G., Daneshvar D., Martin B.M., McClean M.D., Tripodis Y., and Stern R.A. (2015). Age of first exposure to football and later-life cognitive impairment in former NFL players. Neurology 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schneider G.E. (1979). Is it really better to have your brain lesion early? A revision of the “Kennard principle.” Neuropsychologia 17, 557–583 [DOI] [PubMed] [Google Scholar]
- 21.Zuckerman S.L., Lee Y.M., Odom M.J., Solomon G.S., Forbes J.A., and Sills A.K. (2012). Recovery from sports-related concussion: Days to return to neurocognitive baseline in adolescents versus young adults. Surg. Neurol. Int. 3, 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moser R.S., Schatz P., and Jordan B.D. (2005). Prolonged effects of concussion in high school athletes. Neurosurgery 57, 300–306 [DOI] [PubMed] [Google Scholar]
- 23.Sim A., Terryberry-Spohr L., and Wilson K.R. (2008). Prolonged recovery of memory functioning after mild traumatic brain injury in adolescent athletes. J. Neurosurg. 108, 511–516 [DOI] [PubMed] [Google Scholar]
- 24.Giza C.C., Griesbach G.S., and Hovda D.A. (2005). Experience-dependent behavioral plasticity is disturbed following traumatic injury to the immature brain. Behav. Brain Res. 157, 11–22 [DOI] [PubMed] [Google Scholar]
- 25.Hessen E., Nestvold K., and Anderson V. (2007). Neuropsychological function 23 years after mild traumatic brain injury: a comparison of outcome after paediatric and adult head injuries. Brain Inj. 21, 963–979 [DOI] [PubMed] [Google Scholar]
- 26.Ewing-Cobbs L., Prasad M.R., Swank P., Kramer L., Cox C.S., Jr., Fletcher J.M., Barnes M., Zhang X., and Hasan K.M. (2008). Arrested development and disrupted callosal microstructure following pediatric traumatic brain injury: relation to neurobehavioral outcomes. Neuroimage 42, 1305–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beauchamp M.H., Ditchfield M., Maller J.J., Catroppa C., Godfrey C., Rosenfeld J.V., Kean M.J. and Anderson V.A. (2011). Hippocampus, amygdala and global brain changes 10 years after childhood traumatic brain injury. Int. J. Dev. Neurosci. 29, 137–143 [DOI] [PubMed] [Google Scholar]
- 28.Sinopoli K.J., Chen J.K., Wells G., Fait P., Ptito A., Taha T., and Keightley M. (2014). Imaging “brain strain” in youth athletes with mild traumatic brain injury during dual-task performance. J. Neurotrauma 31, 1843–1859 [DOI] [PubMed] [Google Scholar]
- 29.Andersen S.L. and Teicher M.H. (2008). Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 31, 183–191 [DOI] [PubMed] [Google Scholar]
- 30.Bardin J. (2012). Neurodevelopment: unlocking the brain. Nature 487, 24–26 [DOI] [PubMed] [Google Scholar]
- 31.Anderson V., Spencer-Smith M., and Wood A. (2011). Do children really recover better? Neurobehavioural plasticity after early brain insult. Brain 134, 2197–2221 [DOI] [PubMed] [Google Scholar]
- 32.Chugani H.T., Phelps M.E., and Mazziotta J.C. (1987). Positron emission tomography study of human brain functional development. Ann. Neurol. 22, 487–497 [DOI] [PubMed] [Google Scholar]
- 33.Epstein H.T. (1999). Stages of increased cerebral blood flow accompany stages of rapid brain growth. Brain Dev. 21, 535–539 [DOI] [PubMed] [Google Scholar]
- 34.Lebel C., Walker L., Leemans A., Phillips L., and Beaulieu C. (2008). Microstructural maturation of the human brain from childhood to adulthood. Neuroimage 40, 1044–1055 [DOI] [PubMed] [Google Scholar]
- 35.Shaw P., Kabani N.J., Lerch J.P., Eckstrand K., Lenroot R., Gogtay N., Greenstein D., Clasen L., Evans A., Rapoport J.L., Giedd J.N., and Wise S.P. (2008). Neurodevelopmental trajectories of the human cerebral cortex. J. Neurosci. 28, 3586–3594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lenroot R.K., Schmitt J.E., Ordaz S.J., Wallace G.L., Neale M.C., Lerch J.P., Kendler K.S., Evans A.C., and Giedd J.N. (2009). Differences in genetic and environmental influences on the human cerebral cortex associated with development during childhood and adolescence. Hum. Brain Mapp. 30, 163–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giedd J.N., Blumenthal J., Jeffries N.O., Castellanos F.X., Liu H., Zijdenbos A., Paus T., Evans A.C., and Rapoport J.L. (1999). Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci. 2, 861–863 [DOI] [PubMed] [Google Scholar]
- 38.Uematsu A., Matsui M., Tanaka C., Takahashi T., Noguchi K., Suzuki M., and Nishijo H. (2012). Developmental trajectories of amygdala and hippocampus from infancy to early adulthood in healthy individuals. PloS One 7, e46970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lenroot R.K. and Giedd J.N. (2006). Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci. Biobehav. Rev. 30, 718–729 [DOI] [PubMed] [Google Scholar]
- 40.Grieve S.M., Korgaonkar M.S., Clark C.R., and Williams L.M. (2011). Regional heterogeneity in limbic maturational changes: evidence from integrating cortical thickness, volumetric and diffusion tensor imaging measures. Neuroimage 55, 868–879 [DOI] [PubMed] [Google Scholar]
- 41.Kaller C.P., Heinze K., Mader I., Unterrainer J.M., Rahm B., Weiller C., and Kostering L. (2012). Linking planning performance and gray matter density in mid-dorsolateral prefrontal cortex: moderating effects of age and sex. Neuroimage 63, 1454–1463 [DOI] [PubMed] [Google Scholar]
- 42.Kelly A.M., Di Martino A., Uddin L.Q., Shehzad Z., Gee D.G., Reiss P.T., Margulies D.S., Castellanos F.X., and Milham M.P. (2009). Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cereb. Cortex 19, 640–657 [DOI] [PubMed] [Google Scholar]
- 43.Chen Z., Liu M., Gross D.W., and Beaulieu C. (2013). Graph theoretical analysis of developmental patterns of the white matter network. Front Hum Neurosci 7, 716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pierpaoli C., Jezzard P., Basser P.J., Barnett A., and Di Chiro G. (1996). Diffusion tensor MR imaging of the human brain. Radiology 201, 637–648 [DOI] [PubMed] [Google Scholar]
- 45.Basser P.J. and Pierpaoli C. (1996). Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J. Magn. Reson. B 111, 209–219 [DOI] [PubMed] [Google Scholar]
- 46.Song S.K., Sun S.W., Ramsbottom M.J., Chang C., Russell J., and Cross A.H. (2002). Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage 17, 1429–1436 [DOI] [PubMed] [Google Scholar]
- 47.Shenton M.E., Hamoda H.M., Schneiderman J.S., Bouix S., Pasternak O., Rathi Y., Vu M.A., Purohit M.P., Helmer K., Koerte I., Lin A.P., Westin C.F., Kikinis R., Kubicki M., Stern R.A., and Zafonte R. (2012). A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav. 6, 137–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bazarian J.J., Zhu T., Blyth B., Borrino A., and Zhong J. (2012). Subject-specific changes in brain white matter on diffusion tensor imaging after sports-related concussion. Magn. Reson. Imaging 30, 171–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bazarian J.J., Zhu T., Zhong J., Janigro D., Rozen E., Roberts A., Javien H., Merchant-Borna K., Abar B., and Blackman E.G. (2014). Persistent, long-term cerebral white matter changes after sports-related repetitive head impacts. PloS One 9, e94734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koerte I.K., Kaufmann D., Hartl E., Bouix S., Pasternak O., Kubicki M., Rauscher A., Li D.K., Dadachanji S.B., Taunton J.A., Forwell L.A., Johnson A.M., Echlin P.S., and Shenton M.E. (2012). A prospective study of physician-observed concussion during a varsity university hockey season: white matter integrity in ice hockey players. Part 3 of 4. Neurosurg. Focus 33, E3: 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Davenport E.M., Whitlow C.T., Urban J.E., Espeland M.A., Jung Y., Rosenbaum D.A., Gioia G.A., Powers A.K., Stitzel J.D., and Maldjian J.A. (2014). Abnormal white matter integrity related to head impact exposure in a season of high school varsity football. J. Neurotrauma 31, 1617–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Breedlove E.L., Robinson M., Talavage T.M., Morigaki K.E., Yoruk U., O'Keefe K., King J., Leverenz L.J., Gilger J.W., and Nauman E.A. (2012). Biomechanical correlates of symptomatic and asymptomatic neurophysiological impairment in high school football. J. Biomech. 45, 1265–1272 [DOI] [PubMed] [Google Scholar]
- 53.Singh R., Meier T.B., Kuplicki R., Savitz J., Mukai I., Cavanagh L., Allen T., Teague T.K., Nerio C., Polanski D., and Bellgowan P.S. (2014). Relationship of collegiate football experience and concussion with hippocampal volume and cognitive outcomes. JAMA 311, 1883–1888 [DOI] [PubMed] [Google Scholar]
- 54.Talavage T.M., Nauman E.A., Breedlove E.L., Yoruk U., Dye A.E., Morigaki K.E., Feuer H., and Leverenz L.J. (2013). Functionally-detected cognitive impairment in high school football players without clinically-diagnosed concussion. J. Neurotrauma 31, 327–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abbas K., Shenk T.E., Poole V.N., Breedlove E.L., Leverenz L.J., Nauman E.A., Talavage T.M., and Robinson M.E. (2014). Alteration of default mode network in high school football athletes due to repetitive sub-concussive mTBI: a resting state fMRI study. Brain Connect. 5, 91–101 [DOI] [PubMed] [Google Scholar]
- 56.Aoki Y., Inokuchi R., Gunshin M., Yahagi N., and Suwa H. (2012). Diffusion tensor imaging studies of mild traumatic brain injury: a meta-analysis. J. Neurol. Neurosurg. Psychiatry 83, 870–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McAllister T.W., Ford J.C., Ji S., Beckwith J.G., Flashman L.A., Paulsen K., and Greenwald R.M. (2012). Maximum principal strain and strain rate associated with concussion diagnosis correlates with changes in corpus callosum white matter indices. Ann. Biomed. Eng. 40, 127–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kumar R., Gupta R.K., Husain M., Chaudhry C., Srivastava A., Saksena S., and Rathore R.K. (2009). Comparative evaluation of corpus callosum DTI metrics in acute mild and moderate traumatic brain injury: its correlation with neuropsychometric tests. Brain Inj. 23, 675–685 [DOI] [PubMed] [Google Scholar]
- 59.Kumar R., Saksena S., Husain M., Srivastava A., Rathore R.K., Agarwal S., and Gupta R.K. (2010). Serial changes in diffusion tensor imaging metrics of corpus callosum in moderate traumatic brain injury patients and their correlation with neuropsychometric tests: a 2-year follow-up study. J. Head Trauma Rehabil. 25, 31–42 [DOI] [PubMed] [Google Scholar]
- 60.Wu T.C., Wilde E.A., Bigler E.D., Li X., Merkley T.L., Yallampalli R., McCauley S.R., Schnelle K.P., Vasquez A.C., Chu Z., Hanten G., Hunter J.V., and Levin H.S. (2010). Longitudinal changes in the corpus callosum following pediatric traumatic brain injury. Dev. Neurosci. 32, 361–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Levin H.S., Benavidez D.A., Verger-Maestre K., Perachio N., Song J., Mendelsohn D.B., and Fletcher J.M. (2000). Reduction of corpus callosum growth after severe traumatic brain injury in children. Neurology 54, 647–653 [DOI] [PubMed] [Google Scholar]
- 62.Levin H.S., Wilde E.A., Chu Z., Yallampalli R., Hanten G.R., Li X., Chia J., Vasquez A.C., and Hunter J.V. (2008). Diffusion tensor imaging in relation to cognitive and functional outcome of traumatic brain injury in children. J. Head Trauma Rehabil. 23, 197–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wilde E.A., Chu Z., Bigler E.D., Hunter J.V., Fearing M.A., Hanten G., Newsome M.R., Scheibel R.S., Li X., and Levin H.S. (2006). Diffusion tensor imaging in the corpus callosum in children after moderate to severe traumatic brain injury. J. Neurotrauma 23, 1412–1426 [DOI] [PubMed] [Google Scholar]
- 64.Lebel C. and Beaulieu C. (2011). Longitudinal development of human brain wiring continues from childhood into adulthood. J. Neurosci. 31, 10937–10947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lebel C., Caverhill-Godkewitsch S., and Beaulieu C. (2010). Age-related regional variations of the corpus callosum identified by diffusion tensor tractography. Neuroimage 52, 20–31 [DOI] [PubMed] [Google Scholar]
- 66.Snook L., Paulson L.A., Roy D., Phillips L., and Beaulieu C. (2005). Diffusion tensor imaging of neurodevelopment in children and young adults. Neuroimage 26, 1164–1173 [DOI] [PubMed] [Google Scholar]
- 67.Hofer S. and Frahm J. (2006). Topography of the human corpus callosum revisited—comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage 32, 989–994 [DOI] [PubMed] [Google Scholar]
- 68.Mori S. and van Zijl P.C. (2002). Fiber tracking: principles and strategies - a technical review. N.M.R. Biomed. 15, 468–480 [DOI] [PubMed] [Google Scholar]
- 69.Xu J., Li Y., Lin H., Sinha R., and Potenza M.N. (2013). Body mass index correlates negatively with white matter integrity in the fornix and corpus callosum: a diffusion tensor imaging study. Hum. Brain Mapp. 34, 1044–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Song S.K., Yoshino J., Le T.Q., Lin S.J., Sun S.W., Cross A.H., and Armstrong R.C. (2005). Demyelination increases radial diffusivity in corpus callosum of mouse brain. NeuroImage 26, 132–140 [DOI] [PubMed] [Google Scholar]
- 71.Aboitiz F., Scheibel A.B., Fisher R.S., and Zaidel E. (1992). Fiber composition of the human corpus callosum. Brain Res. 598, 143–153 [DOI] [PubMed] [Google Scholar]
- 72.Reeves T.M., Phillips L.L., and Povlishock J.T. (2005). Myelinated and unmyelinated axons of the corpus callosum differ in vulnerability and functional recovery following traumatic brain injury. Exp. Neurol. 196, 126–137 [DOI] [PubMed] [Google Scholar]
- 73.Reeves T.M., Smith T.L., Williamson J.C., and Phillips L.L. (2012). Unmyelinated axons show selective rostrocaudal pathology in the corpus callosum after traumatic brain injury. J. Neuropathol. Exp. Neurol. 71, 198–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zuckerman S.L., Apple R.P., Odom M.J., Lee Y.M., Solomon G.S., and Sills A.K. (2014). Effect of sex on symptoms and return to baseline in sport-related concussion. J. Neurosurg. Pediatr. 13, 72–81 [DOI] [PubMed] [Google Scholar]
- 75.Baugh C.M., Kroshus E., Bourlas A.P., and Perry K.I. (2014). Requiring athletes to acknowledge receipt of concussion-related information and responsibility to report symptoms: a study of the prevalence, variation, and possible improvements. J. Law Med. Ethics 42, 297–313 [DOI] [PubMed] [Google Scholar]
- 76.Dahler D. (2014). Concussions among youth athletes getting a serious look. CBS News [Google Scholar]
- 77.Robbins C.A., Daneshvar D.H., Picano J.D., Gavett B.E., Baugh C.M., Riley D.O., Nowinski C.J., McKee A.C., Cantu R.C., and Stern R.A. (2014) Self-reported concussion history: impact of providing a definition of concussion. Open Access J. Sports. Med. 5, 99–103 [DOI] [PMC free article] [PubMed] [Google Scholar]