Abstract
Objective
Day-to-day anatomical variations complicate bladder cancer radiotherapy treatment. This work quantifies the impact on target coverage and irradiated normal tissue volume for different adaptive strategies.
Methods
20 patients were retrospectively planned using different three-dimensional conformal radiotherapy treatment strategies for whole-bladder carcinoma: (i) “conventional” treatment used isotropic expansion of the clinical target volume (CTV) by 15 mm to the planning target volume (PTV) for daily treatment; (ii) “plan of the day” used daily volumetric on-treatment imaging [cone beam CT (CBCT)] to select from four available plans with varying superior PTV margins; (iii) “composite” strategies used on-treatment CBCTs from Fractions 1–3 to inform a composite CTV and adapted PTV (5- and 10-mm margins for composite 1 and composite 2, respectively) for subsequent treatment. Target coverage was evaluated from available CBCTs (the first three fractions then the minimum weekly thereafter), and the reduction in the irradiated volume (i.e. within the 95% isodose) was quantified.
Results
Plan of the day improved target coverage (i.e. all of the bladder within the 95% isodose throughout the treatment) relative to conventional treatment (p=0.10), while no such benefit was observed with composite 2. Target coverage was reduced with composite 1 relative to conventional treatment. The mean irradiated volume was reduced by 17.2%, 35.0% and 14.6% relative to conventional treatment, for plan of the day, composite 1 and composite 2, respectively (p<0.01 in all cases). No parameters predictive of large changes in bladder volume later in the treatment were identified.
Conclusions
Adaptive techniques can maintain or improve target coverage while allowing for reduced irradiated volume and possibly reduced toxicity. The plan-of-the-day technique appeared to provide the optimal balance between target coverage and normal tissue sparing.
Advances in knowledge
This study suggests that plan-of-the-day techniques will provide optimal outcomes for adaptive bladder radiotherapy.
Muscle-invasive bladder cancer affects over 3000 new patients every year in the UK [1]. Although patients can undergo radical cystectomy, bladder preservation offers comparable outcomes with the added advantage of the patient retaining their own bladder [2]. This means that there is increasing interest in bladder preservation. Patients are treated with maximal transurethral resection of the bladder, neoadjuvant chemotherapy and radiotherapy [3]. Use of concurrent radiosensitisers further improves outcomes [4,5]. With combined modality treatment, local control rates of 70–80% can be achieved. Although this is promising, there is scope for improvement. Higher radiotherapy doses may offer increased local control, but are limited by normal tissue toxicity, with some patients experiencing significant urinary and bowel toxicity [6].
Radiotherapy for bladder cancer in the UK involves irradiation of the entire bladder, with a generous margin to account for variations in bladder position, shape and size. However, this is likely to be a suboptimal approach, leading to unnecessarily high doses to normal tissue where bladder volume remains small, while failing to achieve target coverage for patients who encounter increasing bladder volume throughout treatment [7]. Henry et al [8] found that 26% of bladder patients monitored using cone beam CT (CBCT) required replanning owing to increasing bladder volume (53%), decreasing bladder volume (38%) and decreasing rectal volume (9%). The authors recommended development of adaptive radiotherapy protocols for these patients. Strategies aimed at reducing these variations by coaching patients to achieve consistent bladder volumes through drinking protocols have generally met with limited success, despite good patient compliance [9].
Daily variations throughout treatment make bladder radiotherapy technically challenging and, with the incidence of bowel toxicity, mean that adaptive strategies could be beneficial. Burridge et al [10] retrospectively investigated the potential of a “plan-of-the-day” approach to this problem, which involved generating three plans based on the bladder volume seen on the radiotherapy planning (RTP) scan with variable superior expansion margins (5, 10 and 15 mm) but uniformity in other directions (15 mm). Based on CBCT images acquired throughout treatment (days 1–5 and weekly thereafter), the optimal plan was selected for treatment. The study demonstrated an average small bowel sparing of 31 cm3 (maximum 76 cm3) compared with non-adaptive techniques.
Adaptive techniques are complicated by intrafractional bladder filling: Lotz et al [11] demonstrated that bladder filling rates varied significantly in healthy volunteers, although flow rates for individuals were consistent. To investigate an adaptive plan-of-the-day strategy, Murthy et al [12] acquired megavoltage CT images before and after each treatment fraction, finding that >16% of patients no longer had their bladder contained within the required region at the end of treatment. Studies often account for this effect with an additional 2- to 3-mm margin for intrafractional expansion, although customised approaches have been investigated [13].
Alternative adaptive strategies can be broadly classed as “composite” plan approaches. These involve acquisition of several images of patient anatomy on successive days, from which a composite clinical target volume (CTV) [and planning target volume (PTV)] is determined as the union of CTVs observed on each scan. Pos et al [14] used this approach to define a composite CTV based on the observed position on CT scans for the first five fractions, subsequently expanding 10 mm isotropically to a composite PTV. This allowed a 40% reduction in overall irradiated volume with minimal compromise to target coverage.
The current work aims to expand on previous studies by making a direct quantitative comparison between different adaptive approaches for whole-bladder radiotherapy within the same patient cohort. Unlike earlier studies, a patient-specific comparison of the appropriateness of each technique will be provided to investigate whether the optimal adaptive strategy varies for particular patients, and whether the optimal strategy could be determined and adopted early in treatment. It was impractical to investigate all of the above approaches owing to subtle variations between investigators and so the focus is on specific examples of each broad approach.
Methods and materials
20 consecutive whole-bladder radiotherapy patients were entered into this retrospective planning study. Each patient had previously been treated using a conventional four-field three-dimensional conformal plan with 52.5 Gy in 20 fractions over 4 weeks on Elekta Synergy® linear accelerators equipped with CBCT (Elekta AB, Stockholm, Sweden). Patients were asked to empty their bladder before the RTP scan and prior to each treatment fraction and were treated using an offline set-up protocol that quantified systematic set-up errors of bony anatomy based on CBCT scans from the first three fractions, with consistency checks weekly thereafter. Each patient, therefore, had 5–9 CBCT scans, which were loaded into the Pinnacle3 treatment planning system (Philips Medical Systems, Andover, MA) for delineation and dose calculation.
Plan simulation
Plans were retrospectively simulated for different treatment techniques. CTV delineation was of the whole bladder based on the RTP, and International Commission on Radiation Units dose coverage constraints were observed. The techniques investigated were as follows.
Conventional
The CTV was expanded 15 mm isotropically to the PTV. The patient was treated each day according to the offline protocol described above.
Plan of the day
Four plans were created for each patient based on different PTV expansion margins from the CTV observed on the RTP scan: superior margins of 5, 10, 15 and 20 mm; 15 mm in all other directions. Images were aligned with translational shifts to bony anatomy based on daily CBCT. The most appropriate of the four treatment plans was chosen each day for treatment based on ensuring target coverage with minimal irradiation to tissue outside the PTV.
Composite
Patients were planned and initially treated according to the conventional technique for three fractions. The first three CBCTs were used to generate a composite CTV (union of CTVs from each scan), which was expanded isotropically to the composite PTV by either 5- or 10-mm margins (to composite 1 and composite 2, respectively). Simulations were evaluated using both daily online and the above offline set-up protocols.
Technique evaluation
CBCTs acquired for each patient were assessed to determine target coverage, irradiated volume and, where appropriate, plan selection throughout treatment. To account for variations in clinical implementation, the details of this procedure varied as follows.
Conventional
The CTV outlined on each available CBCT was assessed to ensure coverage within 2 mm inside the 95% isodose line (using the dose distribution calculated on the RTP scan). The 2-mm margin accounts for intrafractional bladder filling, as used by Burridge et al [10].
The irradiated volume was defined as the volume within the 95% isodose as indicated on the original plan. Because the same plan was delivered throughout, this was representative of the overall irradiated volume.
Plan of the day
For each CBCT scan, the bladder was delineated and the appropriate plan of the day (i.e. that which ensured target coverage within a 2-mm margin while restricting irradiated volume) was selected. If no plans provided adequate coverage, the plan achieving an optimal balance between the target coverage and the normal tissue sparing was selected [i.e. the largest plan if the compromised coverage was in the superior extent, or (if the coverage compromise was in any other direction) the plan which resulted in minimal normal tissue exposure].
Because CBCT images were not available for all fractions, when calculating irradiated volume, the volume within the 95% isodose for each plan of the day was determined and a weighted mean was calculated (based on the proportion of CBCT scans requiring each plan).
Composite
The first three fractions were treated with the conventional technique, so CBCTs 1–3 were checked for target coverage in comparison with the original plan. Subsequently, all CBCTs, including those from the first three fractions, were evaluated in terms of target coverage based on the composite plan.
The irradiated volume was quantified using a weighted mean. For each patient, the conventional plan was delivered for 3 fractions, followed by the composite plan for the subsequent 17. When calculating the irradiated volume from the composite plan, all CBCT scans were assumed to be equally representative of the patient anatomy throughout the remainder of the treatment.
Statistical analysis
Analysis of variance was used to quantify the variation in bladder wall position of each coordinate axis. The statistical significance of improvements in target coverage was assessed using a paired sign test. The significance of reductions in irradiated volume relative to the conventional technique was determined using a two-tailed Wilcoxon matched-pairs signed-rank test with P≤0.05 regarded as statistically significant.
Results
Analysis of the variation in the bladder wall throughout treatment demonstrated that the variation in the superior and posterior axes was significantly greater than the others (p<0.01). Although anterior variation was greater than right, left and inferior, the difference was not statistically significant. Variable numbers of CBCT scans were available across the cohort but the mean was 7.1 scans (two patients had five scans, five had six, three had seven, nine had eight and one had nine).
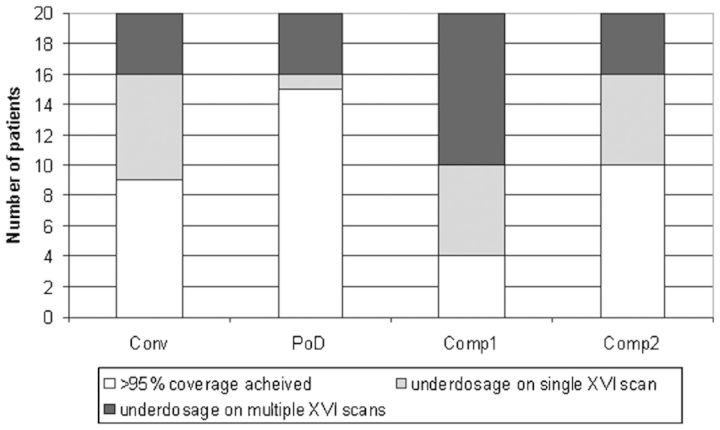
The incidence of acceptable target coverage for each technique is illustrated in Figure 1. The composite techniques appeared to offer no advantage over the conventional technique in terms of target coverage; indeed, the composite 1 approach had an adverse impact. The plan-of-the-day technique demonstrated increased target coverage but the improvement did not reach statistical significance (p=0.10). The impact of set-up error on target coverage was found to be minimal when the composite strategies were evaluated after simulation of daily online correction.
Figure 1.
Evaluation of target coverage for different adaptive techniques. Comp, composite; Conv, conventional; PoD, plan of the day; XVI, X-ray volumetric imaging.
Analysis of underdosed regions indicated that, whereas the conventional and plan-of-the-day techniques failed to achieve adequate coverage equally on the superior, anterior and posterior walls (the latter being more problematic for the plan-of-the-day technique, with 3/5 failures on the posterior wall), 5/10 failures of composite 2 were at the superior extent (the other 5 being split between compromises to anterior, posterior and lateral coverage). Two out of five of the failures to achieve target coverage using the plan-of-the-day technique were attributed to a very large rectum at RTP scan. The only patient in whom composite 2 achieved improved target coverage relative to plan of the day was one in whom coverage of the superior wall was compromised (Patient 14; Figure 2). This was because of a larger bladder volume than was anticipated being present throughout treatment but increasing for later fractions, meaning that the systematic change was corrected for sufficiently by composite 2 but not by the available plan-of-the-day plans.
Figure 2.
Plan selection frequency using the plan-of-the-day technique. Data are expressed as percentages owing to the variable number of acquired scans. Adequate coverage was not always achieved in Patients 9, 13, 14, 18 and 20; XVI, X-ray volumetric imaging.
Frequency of plan selection for the plan-of-the-day technique is illustrated in Figure 2. It indicates that the plan-of-the-day 5 mm plan alone was sufficient for >80% of the treatment in 13/20 patients, implying a substantial reduction in irradiated volume for those patients relative to the conventional technique.
Figure 3 illustrates the variation in CTV volume (normalised to volume at RTP scan) throughout treatment for each patient, stratified by the success of the composite 2 technique in achieving target coverage. It is apparent that predicting the subsequent pattern of bladder volume variation from early CBCT scans is impractical because 5/10 patients who were ultimately underdosed maintained consistent bladder volumes below that of the RTP scan for early fractions. Bladder volume at RTP was also poorly predictive.
Figure 3.

Variation in bladder volume throughout treatment normalised to volume at the radiotherapy planning (RTP) scan. The cohort is split into those in whom the composite 2 technique achieved adequate coverage (a) and those in whom it was unsuccessful in the case of only one cone beam CT (CBCT) scan (b) or multiple CBCT scans (c). Lines represent patients failing as a direct result of increases in bladder volume late in treatment. CTV, clinical target volume; XVI, X-ray volumetric imaging.
The reduction in irradiated volume is illustrated in Figure 4, demonstrating tissue-sparing benefits from all adaptive techniques relative to the conventional technique. The large variation in irradiated volume for each of the techniques masks the systematic improvements owing to the adaptive techniques: the Wilcoxon matched-pairs signed-rank test revealed statistically significant reductions in irradiated volume (p<0.01 in all cases). The slight decrease in irradiated volume for the plan of the day relative to the composite 2 technique was not statistically significant.
Figure 4.
Irradiated volume (i.e. within the 95% isodose) comparison between different treatment strategies. Black squares represent mean values, error bars represent maximum and minimum values across the study cohort, grey regions represent mean±standard deviation. Percentage reductions of mean irradiated volume relative to the conventional technique are stated. Comp, composite; Conv, conventional; PoD, plan of the day.
Discussion
The current study demonstrates that adaptive strategies can maintain or improve dose coverage of the whole bladder relative to conventional techniques while significantly reducing the volume of irradiated bowel, potentially improving the therapeutic ratio. Large variations in bladder wall position, together with the unpredictability of increased bladder volume in the later stages of treatment, suggest a need for daily anatomical monitoring and flexibility in daily plan selection (particularly with regard to the superior margin) if optimal therapeutic improvement is to be achieved.
Observed variations in bladder wall position broadly agree with those observed in previous studies. The exception is the large movement of the posterior wall, which is not reported in previous studies and was not explained by review of the patient notes. A weak correlation was observed across this cohort between posterior wall variation and changing rectal volume, suggesting that this motion, and the resulting target coverage failures, might be reduced by efforts to maintain rectal volume (e.g. use of rectal balloons or daily enema). The limited lateral and inferior movement of the bladder wall suggests the possibility of reducing expansion margins in these directions, although the extent of any resulting improvement to the therapeutic ratio is not clear.
The flexibility in plan selection afforded by the plan-of-the-day adaptive strategy improves target coverage relative to the conventional technique, while significantly reducing the volume of irradiated bowel. It should be noted that the larger PTV plan (20-mm superior margin) was required for 5/20 patients (Figure 2; Patient 20 would have been treated with the larger volume to maximise target coverage) and represented the sole means by which the plan-of-the-day strategy increased target coverage compared with the conventional treatment technique. Burridge et al [10] did not include this larger volume and saw a corresponding increased rate in major underdosage (as defined in the current study) of 25%. Despite the increased planning time, it is therefore appropriate for any plan-of-the-day library to include a plan with a larger irradiated volume. Since the principal variation in irradiated volume occurs in the superior direction with this technique, it can be inferred that the observed reduction in irradiated volume would be in this direction and would therefore impact directly on the dose delivered to the bowel. Although difficult to quantify, it is therefore anticipated that the clinical impact on bowel toxicity provided by a given reduction in irradiated volume would be greater for the plan-of-the-day technique than for the composite techniques, from which the dosimetric impact of a given volumetric reduction would be spread more uniformly.
Although the composite 1 approach provided inadequate target coverage owing to the smaller 5-mm margin, the composite 2 approach maintained target coverage relative to the conventional technique. The inherent assumption that bladder position on all subsequent days would be within a 10-mm margin of that of the first 3 fractions was correct for 10/20 patients and grossly incorrect on only 4/20. The high rate of minor underdosage (i.e. inadequate target coverage on only one CBCT scan) was found to be primarily because of superior extension of the bladder later in the treatment. Other possible modifications to the composite strategy not directly evaluated in this work may allow for improvements to these results. The use of a larger expansion margin (e.g. 15 mm), either isotropic or anisotropic, to the composite PTV would make the composite strategy less susceptible to both later increases in bladder volume and positional errors in all directions. However, regardless of the margin size used, this approach would still be subject to the same geometric misses encountered in early fractions (guaranteeing at least two major and four minor underdosages in the current cohort), while also increasing the irradiated volume and thereby negating much of the therapeutic advantage relative to the conventional non-adaptive strategy. Inclusion of the planning scan into the creation of the composite CTV would have an impact only on patients in whom the CTV delineated on the planning scan extended beyond those seen in the early fractions. In the current cohort, the target coverage results of this study would change for only one patient (being reclassified from a major to a minor underdosage), while 8/20 patients would see the composite CTV, and therefore the irradiated volume and resulting probability of normal tissue complications, increased.
Hybrid approaches such as that of Foroudi et al [15] (in which plans covering small, medium and large bladder volumes as identified on the first five CBCTs, with a 5-mm PTV expansion margin in each case, are available for subsequent daily selection) were not investigated as part of this work. In terms of the success of target coverage, in which the plan associated with the largest volume is of primary importance, they differ from the composite strategy evaluated above primarily by using more CBCT scans (i.e. the first five, rather than three) to generate the composite CTV on which the subsequent plans available for selection are based. Although this may improve target coverage relative to that observed from composite 2 in this study, such adaptive approaches are still inherently susceptible to increased bladder volume later in the treatment. An approach in which few assumptions are made regarding projected bladder volume for subsequent fractions would be more robust to observed volumetric changes than any strategy that requires early bladder volumes to be predictive of the rest of treatment, which are therefore reliant on successful implementation of drinking protocols, a questionable assumption in light of recent data [9]. Although it would be possible to ask patients to void their bladder if the volume was found to be in excess of that required, this may not be practical in busier centres and may have the disadvantage of requiring an additional concomitant dose from repeat imaging.
It has been suggested that the optimal solution to the problem of variable bladder volumes may be to select patients for a composite planning approach on the basis of consistent bladder volumes throughout the first week of treatment [16]. The results of the current study do not appear to be compatible with this approach, with large volumes being observed later in treatment for several patients. The optimal adaptive strategy for a particular patient cannot therefore be reliably predicted early in treatment, which reinforces the findings of Lalondrelle et al [13], in which no parameters predictive of bladder volume throughout treatment were found.
An optimal adaptive strategy would account for the subset of patients (5/20 in the current study) presenting with a much larger superior extension of the bladder relative to earlier fractions. This may be of particular concern for patients unable to easily follow local drinking/voiding protocols. Such patients do not appear to be predictable in advance and target coverage can only be assured for a majority of patients by maintaining the option of delivering treatment from a range of possible plans, from which the most appropriate can be selected each day.
Implementation of plan-of-the-day techniques requires thorough training of staff in image-guidance techniques and interpretation of anatomical information within CBCT scans [17], the reliability of which has been demonstrated [18]. Also, the inherent need for daily online imaging and analysis will have a negative impact on workflow efficiency, which may be problematic in a busy clinical environment.
The methodology used in this study was intended to allow robust comparison of different adaptive techniques, given the limitations of the available data. There are a number of flaws in the methodology which are outlined as follows:
Owing to the limited data available for this study, more robust composite approaches such as using the first five fractions to form the composite CTV were not evaluated. However, re-analysis of the data used to inform this study to assume that the fourth and fifth imaged fractions occurred in the first week (and so are used to inform a five-scan composite CTV) would have affected the target coverage results (Figure 1) for the composite 2 strategy in two patients: one patient would have had a major underdosage reclassified as a minor underdosage, while a minor underdosage in a second patient would have been corrected. So, although a composite strategy based on scans from the first five fractions would have improved target coverage relative to that shown in Figure 1, the rate of geometric miss would still have been higher than using a plan-of-the-day strategy, owing to its susceptibility to cause increases in bladder volume. It should also be noted that this estimate would be likely to overpredict the success of the composite strategy owing to the increased undersampling of later scans that were not used to inform the composite CTV.
The acquired CBCT scans were assumed to be representative of the entire treatment. This may underestimate the occurrence of outliers, particularly on more rigid techniques (i.e. composite strategies), possibly leading to the observed target coverage being unrealistically high. However, such errors would not have an impact on the resulting conclusions and this approach has been used in numerous similar studies.
More customised composite approaches, such as adaptive–predictive organ localisation (A-POLO), were not analysed in this study because the requisite patient data were unavailable. A-POLO relies on a series of three pre-treatment CT scans acquired over 30 min that are intended to reliably indicate a consistent pattern of bladder filling for that patient over the course of a hypofractionated treatment (36 Gy or 30 Gy in six or five fractions, respectively). Three plans are then generated based on isotropic 15-mm PTV expansion margins for PTV0, PTV15 and PTV30. The study found that the technique improved on the existing non-adaptive method but that approximately 13% of fractions would still be underdosed had this technique been applied. However, it is difficult to directly compare these results with the current study because a necessarily more conservative assumption of intrafractional motion (5 mm) was used when assessing coverage [13].
Although this study would ideally have analysed the reduction in exposed bowel volume, the difficulties in calculating the accumulated dose in mobile, non-rigid organs at risk such as the bowel means that the reduced dose to the body volume was used as a surrogate instead; specifically, the comparative volume of tissue contained within the 95% isodose for the different strategies as delivered throughout the treatment. Any error inherent to this approach would be expected to apply equally for all evaluated techniques and would therefore not be expected to have a significant impact on comparative outcomes.
Owing to the lack of accurate means of accumulating the dose received by different regions of the bladder throughout treatment, it has not been possible for this work to quantify the true dosimetric extent and projected clinical impact of the observed compromises to target coverage. An attempt has been made to address this concern by distinguishing “major” and “minor” geometric misses. The former might be expected to lead to a significantly increased risk of local failure if multiple instances of dosimetric compromise occur in the same malignant region of the bladder (in the current study this appeared to be the case in three out of four and four out of four instances of major underdosage for the plan-of-the-day and composite 2 strategies, respectively).
Conclusions
Dosimetric advantages relative to current conventional techniques for a range of adaptive strategies for whole-bladder radiotherapy have been demonstrated. Owing to the limited success of drinking protocols and the widely observed variations in daily anatomy, adaptive strategies that assume predictability in bladder volume throughout treatment will probably be more susceptible to geometric miss and target underdosage for a proportion of patients. Of the techniques evaluated in this work, which can be routinely implemented with standard equipment, the plan-of-the-day technique appears to provide the optimal balance between target coverage and normal tissue sparing.
References
- 1.United Kingdom Cancer Research. Cancer-stats report—bladder cancer. London, UK: Cancer Research UK; 2008. [Google Scholar]
- 2.Cowan RA, McBain CA, Ryder WD, Wylie JP, Logue JP, Turner SL, et al. Radiotherapy for muscle-invasive carcinoma of the bladder: results of a randomized trial comparing conventional whole bladder with dose-escalated partial bladder radiotherapy. Int J Radiat Oncol Biol Phys 2004;59:197–207. [DOI] [PubMed] [Google Scholar]
- 3.Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data. Eur Urol 2005;48:202–6. [DOI] [PubMed] [Google Scholar]
- 4.James ND, Hussain SA, Hall E, Jenkins P, Tremlett J, Rawlings C, et al. Radiotherapy with or without chemotherapy in muscle-invasive bladder cancer. N Engl J Med 2012;366:1477–88. [DOI] [PubMed] [Google Scholar]
- 5.Hoskin PJ, Rojas AM, Bentzen SM, Saunders MI. Radiotherapy with concurrent carbogen and nicotinamide in bladder carcinoma. J Clin Oncol 2010;28:4912–18. [DOI] [PubMed] [Google Scholar]
- 6.Majewski W, Tarnawski R. Acute and late toxicity in radical radiotherapy for bladder cancer. Clin Oncol (R Coll Radiol) 2009;21:598–609. [DOI] [PubMed] [Google Scholar]
- 7.Button MR, Staffurth JN. Clinical application of image-guided radiotherapy in bladder and prostate cancer. Clin Oncol 2010;22:698–706. [DOI] [PubMed] [Google Scholar]
- 8.Henry A, Summers H, Woolley A. Evaluating the need for adaptive radiotherapy for conformal bladder radiotherapy. Radiother Oncol 2010;96:S28. [Google Scholar]
- 9.Ockwell C, Hawkins M, Convery H, Thomas K, Smith E, Burke K, et al. Evaluation of bladder volumes following coaching of patients receiving radical radiotherapy to the rectum. Radiother Oncol 2010;96:S29. [Google Scholar]
- 10.Burridge N, Amer A, Marchant TE, Sykes J, Stratford J, Henry A, et al. Online adaptive radiotherapy of the bladder: small bowel irradiated-volume reduction. Int J Radiat Oncol Biol Phys 2006;66:892–7. [DOI] [PubMed] [Google Scholar]
- 11.Lotz HT, Pos F, Hulshof M, van Herk M, Lebesque JV, Duppen JC, et al. Tumour motion and deformation during external radiotherapy of bladder cancer. Int J Radiat Oncol Biol Phys 2006;64:1551–8. [DOI] [PubMed] [Google Scholar]
- 12.Murthy V, Master Z, Adurkar P, Mallick I, Mahantshetty U, Bakshi G, et al. ‘Plan of the day’ adaptive radiotherapy for bladder cancer using helical tomotherapy. Radiother Oncol 2011;99:55–60. [DOI] [PubMed] [Google Scholar]
- 13.Lalondrelle S, Huddart R, Warren-Oseni K, Hansen VN, McNair H, Thomas K, et al. Adaptive-predictive organ localization using cone-beam computed tomography for improved accuracy in external beam radiotherapy for bladder cancer. Int J Radiat Oncol Biol Phys 2011;79:705–12. [DOI] [PubMed] [Google Scholar]
- 14.Pos FJ, Hulshof M, Lebesque J, Lotz H, van Tienhoven G, Moonen L, et al. Adaptive radiotherapy for invasive bladder cancer: a feasibility study. Int J Radiat Oncol Biol Phys 2001;81:765–71. [DOI] [PubMed] [Google Scholar]
- 15.Foroudi F, Wong J, Kron T, Rolfo A, Haworth A, Roxby P, et al. Online adaptive radiotherapy for muscle-invasive bladder cancer: results of a pilot study. Int J Radiat Oncol Biol Phys 2011;81:765–71. [DOI] [PubMed] [Google Scholar]
- 16.Lalondrelle S, Huddart R. Improving radiotherapy for bladder cancer: an opportunity to integrate new technologies. Clin Oncol (R Coll Radiol) 2009;21:380–4. [DOI] [PubMed] [Google Scholar]
- 17.Taylor H, Lalondrelle S, McDonald F. Optimising radiation treatment in the pelvic region. Radiother Oncol 2010;96:S27. [Google Scholar]
- 18.Foroudi F, Haworth A, Pangehel A, Wong J, Roxby P, Duchesne G, et al. Inter-observer variability of clinical target volume delineation for bladder cancer using CT and cone beam CT. J Med Im Radiat Oncol 2009;53:100–6. [DOI] [PubMed] [Google Scholar]