Abstract
Objective
To compare the influence of different iodinated contrast media with several dilutions on plaque attenuation in an ex vivo coronary model studied by multislice CT coronary angiography.
Methods
In six ex vivo left anterior descending coronary arteries immersed in oil, CT (slices/collimation 64×0.625 mm, temporal resolution 210 ms, pitch 0.2) was performed after intracoronary injection of a saline solution, and solutions of a dimeric isosmolar contrast medium (Iodixanol 320 mgI ml−1) and a monomeric high-iodinated contrast medium (Iomeprol 400 mgI ml−1) with dilutions of 1/80 (low concentration), 1/50 (medium concentration), 1/40 (high concentration) and 1/20 (very high concentration). Two radiologists drew regions of interest in the lumen and in calcified and non-calcified plaques for each solution. 29 cross-sections with non-calcified plaques and 32 cross-sections with calcified plaques were evaluated.
Results
Both contrast media showed different attenuation values within lumen and plaque (p<0.0001). The correlation between lumen and non-calcified plaque values was good (Iodixanol r=0.793, Iomeprol r=0.647). Clustered medium- and high-concentration solutions showed similar plaque attenuation values, signal-to-noise ratios (SNRs) (non-calcified plaque: medium solution SNR 31.3±15 vs 31.4±20, high solution SNR 39.4±17 vs 37.4±22; calcified plaque: medium solution SNR 305.2±133 vs 298.8±132, high solution SNR 323.9±138 vs 293±123) and derived contrast-to-noise ratios (p>0.05).
Conclusion
Differently iodinated contrast media have a similar influence on plaque attenuation profiles.
Advances in knowledge
Since iodine load affects coronary plaque attenuation linearly, different contrast media may be equally employed for coronary atherosclerotic plaque imaging.
Invasive conventional coronary angiography is considered the standard method for the evaluation of vascular lumen and stenoses of coronary artery disease (CAD). However, it is a technique that does not allow direct assessment of the coronary artery wall and plaque composition, which is considered to be a pivotal determinant of acute coronary events [1-3]. Intravascular ultrasound (IVUS) allows accurate qualitative and quantitative assessment of coronary atherosclerotic plaques [4]. However, IVUS is an invasive and expensive technique. CT coronary angiography (CTCA) is now widely implemented in the clinical routine [5], especially in patients at intermediate pretest probability of CAD [6]. Several studies comparing CTCA with IVUS have demonstrated the high potential of CTCA for coronary plaque imaging purposes [7-9]. It has been shown that CTCA has the potential to detect atherosclerotic plaques [10,11], to quantify their volumes [12], to characterise their composition (based on the X-ray attenuating properties of each structure [13]), and to assess positive remodelling of walls [14]. CTCA may non-invasively provide information on the total plaque burden (calcified and non-calcified components) for individual patients with suspected CAD [15].
High-iodinated contrast media (CM) are recommended for optimal coronary contrast intensity of CTCA [16]. However, several technical parameters, including CM influence, and intrinsic limitations may affect the attenuation measurements of coronary plaques [17-22]. The aim of our study was to compare two different CM with several dilutions of iodine load in the setting of coronary plaque assessment in an ex vivo model by means of CTCA.
Methods and materials
Specimens
Six ex vivo left anterior descending (LAD) coronary arteries were obtained at autopsy from six patients (five males and one female, mean age 52.7±21 years). Three (two males and one female) died of non-cardiovascular diseases, and three (all males) died of ischaemic heart disease. The Institutional Review Board approved the study protocol.
The specimens were prepared and examined separately. The excision was performed at least 1 cm proximally to the bifurcation of the circumflex artery for a length of at least 4 cm. Each coronary artery was fitted with two cannulas fixed with surgical thread in the proximal [in the left main (LM)] and distal ends (in the LAD). The circumflex artery and other collateral branches had been closed at their ends with thread. The specimens were flushed with saline solution.
Only the LAD was used because of its major length and diameter, higher prevalence of atherosclerosis and technical reasons (ability of the operator to fix the surgical thread in the LM without damaging the proximal LAD).
Contrast material
Two CM with different iodine concentration (Visipaque, Iodixanol 320 mgI ml−1; GE Healthcare, Cork, Ireland vs Iomeron, Iomeprol 400 mgI ml−1; Bracco, Milan, Italy) were compared in our study. Several dilutions of CM/saline in a fixed volume of 10 ml for each solution were employed to simulate in vivo conditions. The following solutions were used: a pure saline solution (1/∞; no CM was diluted) to resemble baseline conditions; four dilutions of dimeric isosmolar iodinated CM (Iodixanol 320 mgI ml−1): 1/80 (defined as low concentration solution, iodine load 40 mgI in a 10 ml volume), 1/50 (defined as medium concentration solution, iodine load 64 mgI), 1/40 (defined as high concentration solution, iodine load 80 mgI) and 1/20 (defined as very high concentration solution, iodine load 160 mgI); four dilutions of monomeric high-iodinated CM (Iomeprol 400 mgI ml−1): 1/80 (defined as low concentration solution, iodine load 50 mgI), 1/50 (defined as medium concentration solution, iodine load 80 mgI), 1/40 (defined as high concentration solution, iodine load 100 mgI) and 1/20 (defined as very high concentration solution, iodine load 200 mgI). Non-diluted CM have not been employed as CM are diluted in the bloodstream during in vivo administration [17,18].
Experimental setting
A plastic box was filled with vegetable oil to simulate epicardial fat. Prior to positioning the specimen in the oil, saline was injected through the sheaths to wash out the air bubbles in the lumen. Once the specimen had sunk into the oil, the solution was injected through the sheaths using a 10-ml syringe from the proximal end of the specimen. The same procedure was used for all the solutions.
Scan parameters
A CT scan (Brilliance 64, Philips Medical Systems, Cleveland, OH) had been initially performed to determine attenuation values (Hounsfield unit; HU) of all solutions in the syringes. Then, a CT scan was performed following the intracoronary injection of every solution for each vessel separately (i.e. nine scans). The specimens were injected in this order with the following solutions: pure saline solution; low, medium, high and very high concentration solutions of Iodixanol; low, medium, high and very high concentration solutions of Iomeprol. After each scan, the specimens were thoroughly flushed with saline to remove the traces of the former CM solution before the injection of the subsequent solution.
All scans were performed with the following parameters: slices/collimation 64×0.625 mm, rotation time 420 ms (effective temporal resolution, with 180° half-rotation reconstruction algorithm, 210 ms), feed per rotation 11.9 mm s−1 (pitch 0.2), 120 kV, 500 mAs, effective slice thickness 0.8 mm, reconstruction increment 0.4 mm, head-to-feet scan, field of view (FOV) 100 mm, convolution filter medium smooth (B). An artificial electrocardiogram signal was applied for all scans.
The specimen was kept in the same longitudinal position for all investigations (head-LM-to feet-LAD) to obtain direct cross-section images. All acquired data were stored in a dedicated workstation (Extended Brilliance™ Workspace, v.3.0.1.3200, Philips Medical Systems).
CTCA data analysis
Two radiologists with 5 years of experience in cardiac CT performed orthogonal views of all specimens for each CM solution in consensus. The operator loaded the data sets for each solution of the same specimen into a workstation screen, scrolling the data sets in parallel with standard soft-tissue window settings (window width 700 HU; window centre 140 HU). The operator drew the regions of interest (ROIs, minimum size 0.2 mm2) in the lumen of the vessel (defined as lumen), in the oil surrounding the specimen (defined as surrounding) and, if plaques were identified, in the soft-tissue components (defined as non-calcified plaque) and in the calcification within the coronary artery wall (defined as calcified plaque).
29 cross-sections with non-calcified plaques and 32 cross-sections with plaques containing gross calcifications were identified. For each ROI the mean and the standard deviation of the attenuation value were collected. The standard deviation HU values of the surrounding air included in the scan were also collected for the estimation of image noise.
Statistical analysis
The attenuation values were presented as means and standard deviations. The signal-to-noise ratio (SNR) was calculated as the mean attenuation value of the ROI/standard deviation of the air attenuation value. SNRs are presented and plotted as means and standard deviation. The contrast-to-noise ratio (CNR) was calculated as (mean attenuation A – mean attenuation B)/image noise (defined as the standard deviation of the air attenuation value). CNRs of lumen–non-calcified plaque, calcified plaque–lumen and calcified plaque–non-calcified plaque were calculated at the same level and plotted as the average for each solution. Confidence intervals were calculated at 95% using the critical value from the t-distribution.
Statistical evaluation was performed with dedicated software (SPSS® 11.5; SPSS Inc., Chicago, IL). First, the attenuation measurements of solutions clustered per CM type and structure (i.e. lumen, non-calcified and calcified plaque, and surrounding) were compared with a one-way analysis of variance (ANOVA) test. Separate ANOVA models for the two types of CM and for each structure were provided. A p-value of <0.0001 was considered for statistical significance in multiple comparisons. Then, clustered measurements of lumen and plaque (i.e. calcified and not calcified) attenuation values at the same level were correlated with Pearson's test for each CM and plotted. The absolute attenuation values, SNRs and CNRs of lumen, calcified and non-calcified plaque with paired CM solutions (i.e. Iodixanol vs Iomeprol) were compared with Student's t-test (p<0.05). Solutions with corresponding iodine load (high concentration Iodixanol vs medium concentration Iomeprol) were also compared (p<0.05).
Results
The attenuation values of every solution obtained in a 10-ml syringe after dilution were 13±2.1 HU for saline; 122±6.7 HU and 142.5±8.5 HU for low concentration solutions (1/80) of Iodixanol and Iomeprol, respectively; 200.6±6.5 HU and 230.2±7.8 HU for medium concentration solutions (1/50) of Iodixanol and Iomeprol, respectively; 259.7±6.2 HU and 339.6±7 HU for high concentration solutions (1/40) of Iodixanol and Iomeprol, respectively; 473.7±7.3 HU and 559.8±8.2 HU for very high concentration solutions (1/20) of Iodixanol and Iomeprol, respectively.
The standard deviation attenuation values of the surrounding air were 1.8 HU (low), 1.8 HU (medium), 1.8 HU (high) and 2 HU (very high) for Iodixanol solutions, and 1.8 HU (low), 1.8 HU (medium), 1.9 HU (high) and 2.3 HU (very high) for Iomeprol solutions.
A total of 1809 attenuation measurements were performed in consensus. 70 sections of lumen and surrounding oil, 29 sections with non-calcified plaques (in total 261 measurements with all solutions), and 32 sections with calcified plaques (in total 288 measurements with all solutions) were assessed.
Baseline mean attenuation values obtained with saline solution were 37.9±24.4 HU for lumen (SNR 20±12.8), −121.6±8.4 HU for surrounding oil (SNR −64±4.4), 497.5±256.3 HU for calcified plaque (SNR 261.9±134.9) and 19±22.1 HU for non-calcified plaque (SNR 10±11.7).
Iodixanol solutions presented significantly different attenuation values within lumen, non-calcified plaque (both p<0.0001) and calcified plaque (p=0.00017); attenuation values of surrounding oil (p=0.52348) were not significantly different. Iomeprol solutions presented significantly different attenuation values within lumen, non-calcified plaque and calcified plaque (p<0.0001); attenuation values of surrounding oil (p=0.09148) were not significantly different.
Mean attenuation values and SNRs with CM paired solutions are summarised in Table 1.
Table 1. Summary of the mean attenuation values (Hounsfield unit; HU) and signal-to-noise ratio (SNR) values measured in each structure with every contrast media solution (p<0.05).
| Iodixanol |
Iomeprol |
Iodixanol |
Iomeprol |
||||||
| Structure | Solution | Mean HU±SD | Mean HU±SD | Δ | p-value | SNR±SD | SNR±SD | Δ | p-value |
| Lumen | Low | 124.3±48.8 | 152.3±41.9 | −28 | <0.05 | 69.1±27.1 | 84.6±23.3 | −15.5 | <0.05 |
| Medium | 183±53.7 | 197.7±53.4 | −14.7 | 0.106 | 101.7±29.8 | 109.8±29.7 | −8.1 | 0.106 | |
| High | 204.5±55.4 | 231.5±66.4 | −27 | <0.05 | 113.6±30.8 | 121.9±35 | −8.3 | 0.139 | |
| Very high | 332.2±91.5 | 436.6±117.4 | −104.4 | <0.05 | 166.1±45.8 | 189.8±51.1 | −23.7 | <0.05 | |
| Non-calcified plaque | Low | 34.3±22.1 | 47.8±27.3 | −13.5 | <0.05 | 19.1±12.3 | 26.5±15.2 | −7.4 | <0.05 |
| Medium | 56.3±27 | 56.5±37.2 | −0.2 | 0.980 | 31.3±15 | 31.4±20.7 | −0.1 | 0.980 | |
| High | 70.9±31.6 | 71.1±41.9 | −0.2 | 0.981 | 39.4±17.5 | 37.4±22.1 | +2 | 0.710 | |
| Very high | 112.5±43.2 | 121.5±59 | −9 | 0.511 | 56.3±21.6 | 52.8±25.7 | +3.5 | 0.582 | |
| Calcified plaque | Low | 542.1±244.5 | 516.4±281.6 | +25.7 | 0.697 | 301.2±135.8 | 286.9±156.4 | +14.3 | 0.697 |
| Medium | 549.4±241 | 537.8±238.6 | +11.6 | 0.847 | 305.2±133.9 | 298.8±132.6 | +6.4 | 0.847 | |
| High | 583±249.8 | 556.7±234.6 | +26.3 | 0.665 | 323.9±138.8 | 293±123.5 | +30.9 | 0.350 | |
| Very high | 662.7±247.3 | 694.6±231.7 | −31.9 | 0.596 | 331.4±123.6 | 302±100.8 | +29.4 | 0.301 | |
| Surrounding | Low | −120±8.8 | −117.8±5.6 | −2.2 | 0.076 | −66.7±4.9 | −65.4±3.1 | −1.3 | 0.076 |
| Medium | −119.7±4.9 | −117±6.7 | −2.7 | <0.05 | −66.5±2.7 | −65±3.7 | −1.5 | <0.05 | |
| High | −120.6±6.2 | −117.7±5.8 | −2.9 | <0.05 | −67±3.5 | −61.9±3 | −5.1 | <0.05 | |
| Very high | −120.1±8.5 | −116.5±5.6 | −3.6 | <0.05 | −60.1±4.2 | −50.7±2.4 | −9.4 | <0.05 |
SD, standard deviation.
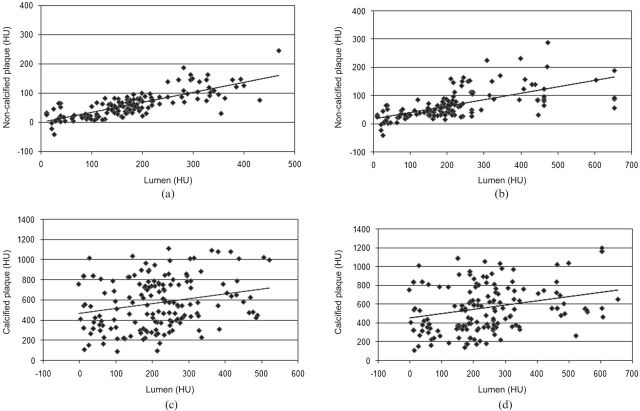
After clustering the paired attenuation values of lumen and coronary plaque (non-calcified and calcified) at the same level in all the solutions and with each CM, the correlation between the attenuation values of lumen and non-calcified plaque was good for both CM (Iodixanol r=0.793, Iomeprol r=0.647); a low correlation was found between the attenuation values of lumen and calcified plaque for both CM (Iodixanol r=0.239, Iomeprol 0.275) (Figure 1).
Figure 1.
Lumen attenuation vs plaque attenuation. All the attenuation values (Hounsfield unit; HU) measured within the lumen and the plaque (non-calcified and calcified) are plotted. The distribution is clearly linear between lumen and non-calcified plaque for Iodixanol (r=0.793) (a) and Iomeprol (r=0.647) (b). Correlation is lower between lumen and calcified plaque for Iodixanol (r=0.239) (c) and Iomeprol (r=0.275) (d).
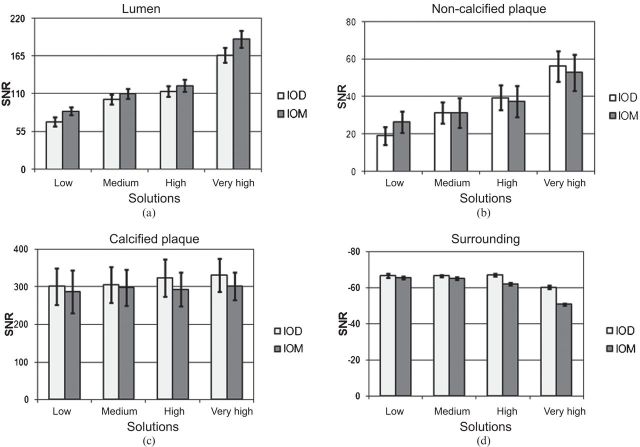
The SNR values of paired CM solutions clustered per structure have a common trend with increasing concentration (Figure 2) (p>0.05, apart from low and very high solutions for lumen, and low solutions for non-calcified plaque; Table 1).
Figure 2.
Signal-to-noise ratio (SNR) values of paired contrast media (CM) solutions clustered per structure: (a) lumen; (b) non-calcified plaque; (c) calcified plaque; (d) surrounding oil. The SNR values appear to have a common trend with increasing CM concentration. Bars indicate confidence intervals at 95%. IOD, Iodixanol; IOM, Iomeprol.
The CNR values (lumen–non-calcified plaque, calcified plaque–lumen, calcified plaque–non-calcified plaque ratios) of paired CM solutions also have a common trend (p>0.05, apart from very high solutions for lumen–non-calcified plaque ratio) (Figure 3).
Figure 3.
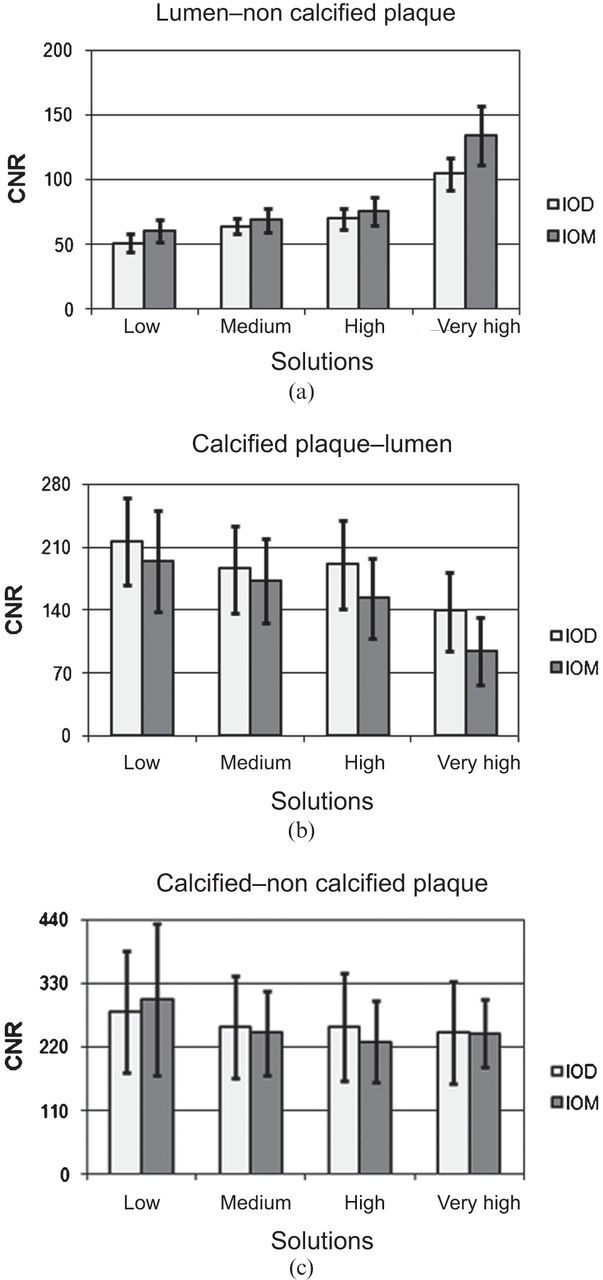
Contrast-to-noise ratio (CNR) values of paired contrast media solutions clustered per structure: (a) lumen–non-calcified plaque; (b) calcified plaque–lumen; (c) calcified plaque–non-calcified plaque. Bars indicate confidence intervals at 95%. IOD, Iodixanol; IOM, Iomeprol.
Attenuation values and SNRs of corresponding iodine load solutions (high concentration Iodixanol vs medium concentration Iomeprol=80 mgI) were compared and the results were not significantly different for lumen (p=0.4628), calcified (p=0.4620) and non-calcified plaque (p=0.1183); derived CNRs were also not significantly different (p>0.05).
Discussion
The ability of CTCA to detect coronary atherosclerotic plaques and even characterise their composition has achieved great interest [7-15]. However, it is controversial whether the absolute HU values are reliable measurements for determining the plaque composition and identifying the corresponding tissue. Becker et al [23] compared the atherosclerotic lesions of 11 human cadaver heart specimens detected by multislice CT (MSCT) with the histopathological findings; they concluded that CTCA could be a promising tool for the characterisation of coronary calcified (often fibrocalcified) and non-calcified plaques (HU density depending on the ratio of lipid and fibrous tissue component). However, coronary plaque imaging with CTCA is a challenging task because several parameters such as lumen attenuation, convolution filtering, spatial resolution and reader experience are reported to affect plaque attenuation measurements [17-22]. A phantom study revealed that intracoronary attenuation can significantly affect the measured plaque attenuation, to the extent that accurate plaque tissue differentiation may be influenced [17]. Cademartiri et al [18,24] in an ex vivo coronary specimen studied with 16-slice CT also demonstrated that the attenuation of plaques is significantly modified by intracoronary attenuation by using high-iodinated CM.
In our study, we compared the influence of two differently iodinated CM with several dilutions on plaque attenuation in an ex vivo coronary model, studied with the improved temporal resolution of 64-slice CT. Our study confirmed that non-calcified plaque attenuation values clustered for CM type are significantly different with various CM dilutions. A good correlation was found between the attenuation values of lumen and non-calcified plaque for both CM, even higher for Iodixanol than Iomeprol. A lower degree of correlation was found between the attenuation values of lumen and calcified plaque for both types of CM. SNRs and CNRs were calculated in order to provide absolute image quality parameters, since coronary plaque assessment is affected by reader variability [22]. Absolute attenuation values, SNRs and CNRs within plaques were not substantially different in clustered medium and high concentration solutions of CM, which are routinely achieved in CTCA [6,16].
In our comparison, differences are given by the changing iodine load of the CM solutions. That is confirmed by the fact that mean attenuation values as well as SNRs and CNRs of paired corresponding iodine load solutions (high concentration Iodixanol vs medium concentration Iomeprol: 80 mgI) were not significantly different (Figure 4). Different iodinated CM have a similar influence on plaque attenuation profiles with corresponding trends depending on the iodine load. Therefore, we demonstrated that different types of iodinated CM may be equally employed for plaque imaging purposes. Given CM influence, a lower intracoronary attenuation with a corresponding modification of the iodine delivery (ID) rate formula [IDr=gI s−1=flow (ml s−1)×concentration (gI ml−1)], depending on the CM employed, may allow a better in vivo assessment of coronary atherosclerotic plaques [25].
Figure 4.
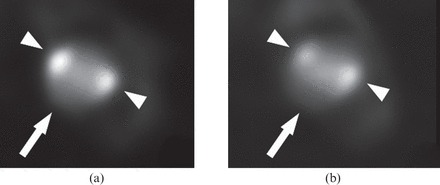
Example of an atherosclerotic plaque (arrowhead, calcified plaques; arrow, non-calcified plaque) CT cross-section studied with corresponding iodine load contrast media solutions (80 mgI). (a) High concentration Iodixanol solution. (b) Medium concentration Iomeprol solution.
Heavy calcifications may also affect the capability of the operator to assess non-calcified plaques [23,26]. On the other hand, the limited resolution of CTCA in association with the blooming effect of the CM in the lumen could hamper the identification of small calcifications [21]. According to our study, calcified plaques may also slightly enhance their attenuation with increasing CM solutions. CM protocols should take into account this emerging evidence and could be modulated on the basis of coronary calcium score.
There are several limitations to our study. The first one is the lack of histopathology examination and correlation; however, the aim of our study was not to compare pathology with MSCT, but to assess the influence of different CM on plaque attenuation measurements. In this context, Leschka et al [27] recently provided a reliable plaque characterisation with dual-source CT compared with histopathology. Furthermore, we did not investigate the influence of ROI dimensions [20] and image reconstruction parameters, such as convolution filtering and slice thickness [19,28]. No reader variability was assessed [22]; however, the two readers in our study had 5 years of experience in cardiac CT. The readers assessed coronary plaque in consensus to improve their performance; conversely, the consensus reading is likely to have led to an underestimation of the variation in measurements. In vivo applications should also test the chemical structure and viscosity of different CM, which may have an influence on attenuation profiles. Finally, we did not investigate the potential impact of blood attenuation, since it was preferred to use pure saline in the mixture to facilitate the effective removal of CM solutions after each injection [18]. The possible influence of the passage of CM through the specimens should have been minimised by saline flushing.
In the future, higher spatial resolution of new CT scanners [29], novel automated software [30] and revolutionary contrast agents [31] may allow improved clinical coronary plaque imaging. The advent of radiation dose-saving CT scanners [32] could also promote the in vivo assessment of the treatment effect on coronary plaques [33].
Conclusion
In conclusion, different iodinated CM have a similar influence on plaque attenuation profiles and may be equally employed for plaque imaging purposes. Therefore, appropriate CM protocols should be tested and enforced in vivo to provide improved coronary atherosclerotic plaque imaging.
References
- 1.Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol 2000;20:1262–75. [DOI] [PubMed] [Google Scholar]
- 2.Fuster V, Badimon L, Badimon JJ, Chesebro JH. The pathogenesis of coronary artery disease and the acute coronary syndromes (1). N Engl J Med 1992;326:242–50. [DOI] [PubMed] [Google Scholar]
- 3.Fuster V, Badimon L, Badimon JJ, Chesebro JH. The pathogenesis of coronary artery disease and the acute coronary syndromes (2). N Engl J Med 1992;326:310–18. [DOI] [PubMed] [Google Scholar]
- 4.Mintz GS, Nissen SE, Anderson WD, Bailey SR, Erbel R, Fitzgerald PJ, et al. American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS). A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol 2001;37:1478–92. [DOI] [PubMed] [Google Scholar]
- 5.Cademartiri F, Maffei E, Notarangelo F, Ugo F, Palumbo A, Lina D, et al. 64-slice computed tomography coronary angiography: diagnostic accuracy in the real world. Radiol Med 2008;113:163–80. [DOI] [PubMed] [Google Scholar]
- 6.Schroeder S, Achenbach S, Bengel F, Burgstahler C, Cademartiri F, de Feyter P, et al. Cardiac computed tomography: indications, applications, limitations, and training requirements: report of a Writing Group deployed by the Working Group Nuclear Cardiology and Cardiac CT of the European Society of Cardiology and the European Council of Nuclear Cardiology. Eur Heart J 2008;29:531–56. [DOI] [PubMed] [Google Scholar]
- 7.Kopp AF, Schroeder S, Baumbach A, Kuettner A, Georg C, Ohnesorge B, et al. Non-invasive characterisation of coronary lesion morphology and composition by multislice CT: first results in comparison with intracoronary ultrasound. Eur Radiol 2001;11:1607–11. [DOI] [PubMed] [Google Scholar]
- 8.Schroeder S, Kopp AF, Baumbach A, Meisner C, Kuettner A, Georg C, et al. Noninvasive detection and evaluation of atherosclerotic coronary plaques with multislice computed tomography. J Am Coll Cardiol 2001;37:1430–5. [DOI] [PubMed] [Google Scholar]
- 9.Leber AW, Knez A, Becker A, Becker C, von Ziegler F, Nikolaou K, et al. Accuracy of multidetector spiral computed tomography in identifying and differentiating the composition of coronary atherosclerotic plaques: a comparative study with intracoronary ultrasound. J Am Coll Cardiol 2004;43:1241–7. [DOI] [PubMed] [Google Scholar]
- 10.Achenbach S, Moselewski F, Ropers D, Ferencik M, Hoffmann U, MacNeill B, et al. Detection of calcified and noncalcified coronary atherosclerotic plaque by contrast-enhanced, submillimeter multidetector spiral computed tomography: a segment-based comparison with intravascular ultrasound. Circulation 2004;109:14–17. [DOI] [PubMed] [Google Scholar]
- 11.Leber AW, Knez A, von Ziegler F, Becker A, Nikolaou K, Paul S, et al. Quantification of obstructive and nonobstructive coronary lesions by 64-slice computed tomography: a comparative study with quantitative coronary angiography and intravascular ultrasound. J Am Coll Cardiol 2005;46:147–54. [DOI] [PubMed] [Google Scholar]
- 12.Leber AW, Becker A, Knez A, von Ziegler F, Sirol M, Nikolaou K, et al. Accuracy of 64-slice computed tomography to classify and quantify plaque volumes in the proximal coronary system: a comparative study using intravascular ultrasound. J Am Coll Cardiol 2006;47:672–7. [DOI] [PubMed] [Google Scholar]
- 13.Leber AW, Knez A, White CW, Becker A, von Ziegler F, Muehling O, et al. Composition of coronary atherosclerotic plaques in patients with acute myocardial infarction and stable angina pectoris determined by contrast-enhanced multislice computed tomography. Am J Cardiol 2003;91:714–18. [DOI] [PubMed] [Google Scholar]
- 14.Achenbach S, Ropers D, Hoffmann U, MacNeill B, Baum U, Pohle K, et al. Assessment of coronary remodeling in stenotic and nonstenotic coronary atherosclerotic lesions by multidetector spiral computed tomography. J Am Coll Cardiol 2004;43:842–7. [DOI] [PubMed] [Google Scholar]
- 15.Mollet NR, Cademartiri F, Nieman K, Saia F, Lemos PA, McFadden EP, et al. Noninvasive assessment of coronary plaque burden using multislice computed tomography. Am J Cardiol 2005;95:1165–9. [DOI] [PubMed] [Google Scholar]
- 16.Cademartiri F, Mollet NR, Lemos PA, Saia F, Midiri M, de Feyter PJ, et al. Higher intracoronary attenuation improves diagnostic accuracy in MDCT coronary angiography. AJR Am J Roentgenol 2006;187:W430–3. [DOI] [PubMed] [Google Scholar]
- 17.Schroeder S, Flohr T, Kopp AF, Meisner C, Kuettner A, Herdeg C, et al. Accuracy of density measurements within plaques located in artificial coronary arteries by X-ray multislice CT: results of a phantom study. J Comput Assist Tomogr 2001;25:900–6. [DOI] [PubMed] [Google Scholar]
- 18.Cademartiri F, Mollet NR, Runza G, Bruining N, Hamers R, Somers P, et al. Influence of intracoronary attenuation on coronary plaque measurements using multislice computed tomography: observations in an ex vivo model of coronary computed tomography angiography. Eur Radiol 2005;15:1426–31. [DOI] [PubMed] [Google Scholar]
- 19.Cademartiri F, La Grutta L, Runza G, Palumbo A, Maffei E, Mollet NR, et al. Influence of convolution filtering on coronary plaque attenuation values: observations in an ex vivo model of multislice computed tomography coronary angiography. Eur Radiol 2007;17:1842–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horiguchi J, Fujioka C, Kiguchi M, Shen Y, Althoff CE, Yamamoto H, et al. Soft and intermediate plaques in coronary arteries: how accurately can we measure CT attenuation using 64-MDCT? AJR Am J Roentgenol 2007;189:981–8. [DOI] [PubMed] [Google Scholar]
- 21.van derGiessen AG, Gijsen FJ, Wentzel JJ, Jairam PM, van Walsum T, Neefjes LA, et al. Small coronary calcifications are not detectable by 64-slice contrast enhanced computed tomography. Int J Cardiovasc Imaging 2011;27:143–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saur SC, Alkadhi H, Stolzmann P, Baumüller S, Leschka S, Scheffel H, et al. Effect of reader experience on variability, evaluation time and accuracy of coronary plaque detection with computed tomography coronary angiography. Eur Radiol 2010;20:1599–606. [DOI] [PubMed] [Google Scholar]
- 23.Becker CR, Nikolaou K, Muders M, Babaryka G, Crispin A, Schoepf UJ, et al. Ex vivo coronary atherosclerotic plaque characterization with multi-detector-row CT. Eur Radiol 2003;13:2094–8. [DOI] [PubMed] [Google Scholar]
- 24.Cademartiri F, Maffei E, Palumbo AA, Malagò R, La Grutta L, Meiijboom WB, et al. Influence of intra-coronary enhancement on diagnostic accuracy with 64-slice CT coronary angiography. Eur Radiol 2008;18:576–83. [DOI] [PubMed] [Google Scholar]
- 25.Brodoefel H, Burgstahler C, Sabir A, Yam CS, Khosa F, Claussen CD, et al. Coronary plaque quantification by voxel analysis: dual-source MDCT angiography versus intravascular sonography. AJR Am J Roentgenol 2009;192:W84–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Weert TT, Ouhlous M, Zondervan PE, Hendriks JM, Dippel DW, van Sambeek MR, et al. In vitro characterization of atherosclerotic carotid plaque with multidetector computed tomography and histopathological correlation. Eur Radiol 2005;15:1906–14. [DOI] [PubMed] [Google Scholar]
- 27.Leschka S, Seitun S, Dettmer M, Baumüller S, Stolzmann P, Goetti R, et al. Ex vivo evaluation of coronary atherosclerotic plaques: characterization with dual-source CT in comparison with histopathology. J Cardiovasc Comput Tomogr 2010;4:301–8. [DOI] [PubMed] [Google Scholar]
- 28.Achenbach S, Boehmer K, Pflederer T, Ropers D, Seltmann M, Lell M, et al. Influence of slice thickness and reconstruction kernel on the computed tomographic attenuation of coronary atherosclerotic plaque. J Cardiovasc Comput Tomogr 2010;4:110–15. [DOI] [PubMed] [Google Scholar]
- 29.Korosoglou G, Mueller D, Lehrke S, Steen H, Hosch W, Heye T, et al. Quantitative assessment of stenosis severity and atherosclerotic plaque composition using 256-slice computed tomography. Eur Radiol 2010;20:1841–50. [DOI] [PubMed] [Google Scholar]
- 30.Dey D, Schepis T, Marwan M, Slomka PJ, Berman DS, Achenbach S. Automated three-dimensional quantification of noncalcified coronary plaque from coronary CT angiography: comparison with intravascular US. Radiology 2010;257:516–22. [DOI] [PubMed] [Google Scholar]
- 31.Cormode DP, Roessl E, Thran A, Skajaa T, Gordon RE, Schlomka JP, et al. Atherosclerotic plaque composition: analysis with multicolor CT and targeted gold nanoparticles. Radiology 2010;256:774–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lell M, Marwan M, Schepis T, Pflederer T, Anders K, Flohr T, et al. Prospectively ECG-triggered high-pitch spiral acquisition for coronary CT angiography using dual source CT: technique and initial experience. Eur Radiol 2009;19:2576–83. [DOI] [PubMed] [Google Scholar]
- 33.Hoffmann H, Frieler K, Schlattmann P, Hamm B, Dewey M. Influence of statin treatment on coronary atherosclerosis visualised using multidetector computed tomography. Eur Radiol 2010;20:2824–33. [DOI] [PubMed] [Google Scholar]