Abstract
Objective
To compare radiation exposure and image quality of oncological staging multidetector CT (MDCT) examinations of the chest, abdomen and pelvis with and without iterative reconstruction (IR).
Methods
40 patients with known malignancy underwent staging CT examinations at two time points. Both CT scans were performed on the same scanner (SOMATOM® Definition Flash, Siemens Healthcare, Forchheim, Germany). For the baseline scan, the tube current–time product was set to 250 mAs [image reconstruction: filtered back projection (FBP)] and for the follow-up scan to 150 mAs [reconstruction: iterative reconstruction (IR)]. Effective radiation doses were estimated based on dose–length products for both baseline and follow-up. Noise measurements in defined regions were compared for FBP and IR. Images were also subjectively evaluated for image quality by three radiologists with different levels of experience.
Results
Dose reduction was 44.4±8.2% for reduced-dose CT scans with IR compared with baseline with FBP. Image noise was not significantly different between images reconstructed with FBP and IR. The subjective quality of standard-dose FBP images and reduced-dose iteratively reconstructed CT images were identical.
Conclusion
Our results show the dose-reducing potential of IR of CT image data in oncological patients.
Advances in knowledge
The algorithm tested in the present scientific study allows a >45% dose reduction at maintained image quality.
With the advent of more effective first- and second-line cancer treatments, long-term survival of patients with malignant diseases has increased [1]. For example, the overall 5-year survival rate for non-Hodgkin's lymphoma in the USA has increased from 48% to almost 69% over the past decades, and 10-year survival rates have also improved substantially over the same time [2].
In a clinical setting, CT is used extensively in cancer diagnosis, staging, evaluation of response to treatment and active surveillance for cancer recurrence. As a consequence, patients receive multiple CT examinations during the course of their disease. Over the past 25 years, CT usage has increased 12-fold in the UK and 20-fold in the USA [3,4]. Overall, the mean effective dose in the USA from all medical X-ray procedures has increased 7-fold over this period [5], with the result that the major proportion of radiation exposure to the population nowadays comes from medical imaging, especially CT imaging. Recent reports on radiation-induced malignancy have sparked new concern and discussion in the medical community as well as among the general public [6-8]. In brief, there is reasonable epidemiological evidence that effective organ doses below 100 mSv result in a very small but non-negligible increase in cancer risk [9-11].
Therefore, CT imaging within a population of younger patients with primary oncological diseases remains a challenge. High image quality is needed for accurate tumour staging to define treatment strategies and to determine prognosis. At the same time, radiation exposure has to be minimised.
The dose associated with a CT scan of the abdomen and pelvis has decreased by a factor of two to three since the 1980s owing to a number of technical innovations [12]. Several recent dose reduction techniques are gaining widespread use. One of the most important technical features is automated exposure control (AEC), which adapts the tube current output and, consequently, the radiation dose to the diameter and attenuation of the patient [13]. Organ-based tube current modulation (TCM) is a new method that has recently been introduced for brain and body imaging [14]. The underlying technique of organ-based TCM reduces the X-ray tube current while the unit is rotating around the patient [15]. These technical features have been successfully implemented to reduce radiation exposure while maintaining image quality [16-18]. Regarding image reconstruction, CT scanners mostly use standard filtered back projection (FBP) methods in which excellent spatial resolution can be achieved, but at the cost of increased image noise.
With regard to image reconstruction and post-processing, a recently developed technology has the potential to further reduce radiation exposure. Iterative reconstruction (IR) techniques have demonstrated the potential to reduce radiation dose while maintaining high image quality [19-22] relative to the currently used FBP techniques. In contrast to FBP, IR enables a decoupling of spatial resolution and image noise. In IR, a correction loop is introduced into the image reconstruction process, called “theoretical iterative reconstruction”. The system creates a synthetic image, computes projections from the image, compares the original projection data and updates the image based on the difference between the calculated and the actual projections, which is very time consuming. IRIS (iterative reconstruction in image space; Siemens Healthcare, Forchheim, Germany) was developed as a method to translate a given number of IR loops into the image space rather than the raw data space, hence avoiding multiple time-consuming iterations of back projection. Information obtained by FBP as an initial image build is used to transform the measured value of each pixel to a new estimate of pixel values. These pixel values are subsequently compared with the ideal Hounsfield unit (HU) number that an image noise model predicts. This process is repeated in successive iterations until the final estimation and ideal pixel values ultimately converge. Using this technique, IRIS is able to selectively identify and subtract image noise, hence avoiding the time-consuming traditional reprojection [15,23].
Graser et al [24] showed that IR is able to maintain image quality of CT brain scans while radiation exposure is reduced at the same time. However, in body imaging to date there is little evidence to show the potential of IR to reduce dose; to the best of our knowledge, no study exists comparing FBP with IR in one patient population imaged at different time points.
We therefore compared radiation dose and image quality of CT scans of the chest, abdomen and pelvis using two different CT protocols in the same patients with oncological diseases on the same CT scanner: standard-dose contrast-enhanced multidetector CT (MDCT) reconstructed with FBP (sdFBP) at baseline and reduced-dose contrast-enhanced CT reconstructed with IRIS (rdIR) at follow-up.
Methods and materials
Patient population
Because patients were scheduled for routine CT examinations and a significant dose reduction was expected based on the tube current–time product reduction for the follow-up scans, ethical approval from the Institutional Review Board was not required for this study. A research fellow retrospectively identified adult patients aged over 18 years who were referred for staging examinations of the chest, abdomen and pelvis by the department of oncology between March and October 2010 (n=1225). We selected those patients for evaluation (n=40) who had been scanned twice on the same scanner (SOMATOM® Definition FLASH, Siemens Healthcare): at baseline before, and at follow-up after an IR algorithm (IRIS) became available (average time interval between the paired CT examinations 8.0±0.3 months). The study population consisted of 21 males and 19 females: average age 60±13 years; range 23–87 years. For each patient, weight and height were recorded for calculation of body mass indices (BMIs), and no significant BMI reduction between both CT scans was found (24.0±0.5 kg m–2 for the first scan vs 23.7±0.5 kg m–2 during the second scan, p=0.7)
MDCT scanning technique
All CT scans were performed on a dual-source CT scanner (SOMATOM Definition FLASH) using a 128×0.6 mm collimation, a pitch of 1.0 and 120 kVp tube voltage using online dose modulation (CARE Dose4D, Siemens Healthcare). The gantry rotation time was 0.5 s and the reconstructed slice thickness/increment was 5/5 mm and 1/0.75 mm. 5 mm reconstructed slice thickness represents a standard value as required by most guidelines for criteria-based reading, e.g. Response Evaluation Criteria in Solid Tumours (RECIST). Therefore, we routinely reconstruct 5 mm axial and coronal slices in our oncological patients. No other slice thickness was used for evaluation in the present study. Acquiring data at thin collimation, e.g. 128×0.6 mm, represents the standard of care in modern MDCT in order to allow for isotropic voxel sizes and high-quality multiplannar reconstructions. For reconstruction, common B30f and I30f filters were used (named “I”30f because it is used for IR). All CT scans covered the chest, abdomen and pelvis and were obtained 70 s after intravenous injection of 1.9 ml kg−1 body weight of a non-ionic contrast agent (Iomeron® 400, Bracco Diagnostics, Milan, Italy).
At baseline, standard dose CT scans were obtained at 120 kVp and a reference tube current–time product of 250 mAs with online dose modulation; image data were reconstructed using standard-dose FBP (sdFBP). Dose reduction for follow-up CT scans was achieved by significantly lowering the tube current–time product from 250 to 150 mAs while all other parameters were kept constant. We chose these settings because the first pre-clinical studies had shown that a 40% reduction of the tube current–time product is feasible when image data are reconstructed with IR. The IR algorithm used in this study (IRIS) was installed on our dual-source CT scanner in January 2010.
Radiation exposure
The volumetric CT dose indices (CTDIvol) for sdFBP and for rdIR were retrieved from the picture archiving and communication system (PACS; Syngo Imaging 2010, Siemens Healthcare). For the CT scanner used in this study, the accuracy of the manufacturer's displayed CTDIvol was tested as part of our daily routine quality control. Based on the recorded dose–length product (DLP), the effective dose in millisieverts was estimated using a conversion factor of 0.0015 [25] for both sdFBP and rdIR scans.
Image quality analysis
A research fellow who was not involved in data analysis obtained patient-specific quantitative noise measurements for all 40 sdFBP and 40 rdIR CT scans. The signal (HU) and image noise [HU standard deviation (SD)] were measured by placing circular regions of interest (ROIs; 150–200 mm2) on four defined regions: liver parenchyma, aorta, paraspinal musculature and retroperitoneal fat. To obtain reliable measurements for liver parenchyma, four ROIs were positioned in different segments, carefully avoiding large hepatic vessels. These regions were first evaluated separately, then summarised and the arithmetic mean over the four values was averaged. Background noise measurements were based on recorded SDs in a region of interest placed outside the anterior abdominal wall at the same level (extrathoracic air) and within the lung (thoracic air). These data were used to calculate HU differences. In addition, the contrast-to-noise ratio (CNR) in the aorta (and liver, subsequently) was measured using the following formula: (SDliver (aorta) – SDparaspinal musculature)/SDretroperitoneal fat. These values were again compared for sdFBP and rdIR.
The subjective image quality of the two different CT scans (sdFBP and rdIR) was almost identical and suitable for diagnostic analysis. We therefore decided to visually assess whether any given CT image was reconstructed with FBP or IR. A research fellow not involved in the image reading selected representative images from all CT examinations and loaded them onto the PACS. Blinded side-by-side comparisons of sdFBP and rdIR CT images of the same patients were performed. The images were displayed in a random fashion on the two monitors of the PACS workstation (calibrated conforming to the digital imaging and communications in medicine calibration and quality assurance tested every day). Three radiologists with 1, 7 and 12 years of experience in body CT were asked to decide whether images were reconstructed with or without IR. All data sets were displayed at three standard window/level settings: soft-tissue, lung and bone windows (level/width approximately 40/400, 700/2000 and 400/1600 HU, respectively). The CT images displayed at soft-tissue windows were shown at three different body levels: (1) the aortic arch, (2) the porta hepatis and (3) the acetabula. Images were also shown in a bone window (400/1600 HU) at level (3) and using lung window (700/2000 HU) settings at level (1). The research fellow recorded in how many cases the radiologists were able to correctly identify the reconstruction algorithm used for each data set.
Statistical analysis
The patients' age and gender, CNRs, differences in HU values, reviewers' gradings for image quality and dose values were analysed using either a paired t-test or a two-way analysis of variance (SigmaStat, v. 3.5, Systat Software GmbH, Erkrath, Germany). Concerning qualitative analysis, the percentage of correctly identified CT images acquired with IR was calculated. A p-value of ≤0.05 was considered statistically significant.
Results
Radiation exposure
In the patient population, we measured an average BMI of 24.0±0.5 kg m–2 (range 19.6–30.9 kg m–2) at baseline and 23.7±0.5 kg m–2 at follow-up (p=0.7). Dose reduction was 44.4±8.2% for rdIR scans compared with sdFBP scans (Table 1). For sdFBP scans, the average effective dose was 12.0±0.5 mSv compared with 6.7±0.2 mSv for rdIR scans (Table 1) (p<0.05). CT scans acquired with sdFBP averaged a CTDIvol of 12.1±0.5 mGy compared with 6.9±0.2 mGy for rdIR (Table 1) (p<0.05). For sdFBP CT scans, the average DLP was 801.5±33.0 mGy cm compared with 447.8±13.9 mGy cm for rdRI (Table 1).
Table 1. Radiation exposure.
| Reconstruction method | Effective dose (mSv) | CTDIvol (mGy) | DLP (mGy cm) |
| sdFBP | 12.0±0.5 | 12.1±0.5 | 801.5±33.0 |
| rdIR | 6.7±0.2a | 6.9±0.2a | 447.8±13.9a |
CTDIvol, CT dose index (represents the average absorbed radiation dose over the x, y and z directions); DLP, dose–length product; rdIR, reduced-dose contrast-enhanced CT reconstructed with iterative reconstruction in image space; sdFBP, standard-dose contrast-enhanced multidetector CT reconstructed with filtered back projection.
ap<0.001 (paired t-test).
Image quality analysis
Average liver parenchyma image noise was 10.3±0.2 HU for sdFBP and 10.0±0.2 HU for rdIR (p=0.324) (Figure 1). Image noise of retroperitoneal fat (9.8±0.3 HU for sdFBP and 9.5±0.3 HU for rdIR, p=0.319) and paraspinal musculature (10.4±0.3 HU for sdFBP and 9.9±0.3 HU for rdIR, p=0.170) was also not statistically significantly different. Image noise determined by measurements in intra- and extrathoracic air revealed no statistically significant difference between sdFBP and rdIR (intrathoracic: 6.0±0.1 HU for sdFBP and 6.0±0.1 HU for rdIR, p=0.924; extrathoracic: 6.7±0.1 HU for sdFBP and 7.1±0.2 HU for rdIR, p=0.172). Only for the aorta was a significant noise reduction found for rdIR (10.3±0.3 HU) compared with sdFBP (11.0±0.3 HU; p=0.014) (Figure 1).
Figure 1.
Image noise measurements in Hounsfield units. Image noise was measured within retroperitoneal fat, paraspinal musculature, liver and aorta. Background noise measurements were taken outside the anterior abdominal wall [extrathoracic air (air ex.)] and within the lungs [thoracic air (air in.)]. Dark-grey columns show standard-dose filtered back projection CT scans (sdFBP) and light grey columns show image noise of reduced-dose CT images with iterative reconstruction. IRIS, iterative reconstruction in image space.
The CNRs in the aorta and liver revealed no statistically significant differences between sdFBP (liver 5.7±0.6; aorta 9.0±0.5) and rdIR (liver 5.1±0.3; aorta 9.4±0.5, Table 2).
Table 2. Contrast-to-noise ratios.
| Region | sdFBP | rdIR |
| Liver | 5.7±0.6 | 5.1±0.3 n.s. |
| Aorta | 9.0±0.5 | 9.4±0.5 n.s. |
n.s, not significant; rdIR, reduced-dose contrast-enhanced CT reconstructed with iterative reconstruction in image space; sdFBP, standard-dose contrast-enhanced multidetector CT reconstructed with filtered back projection
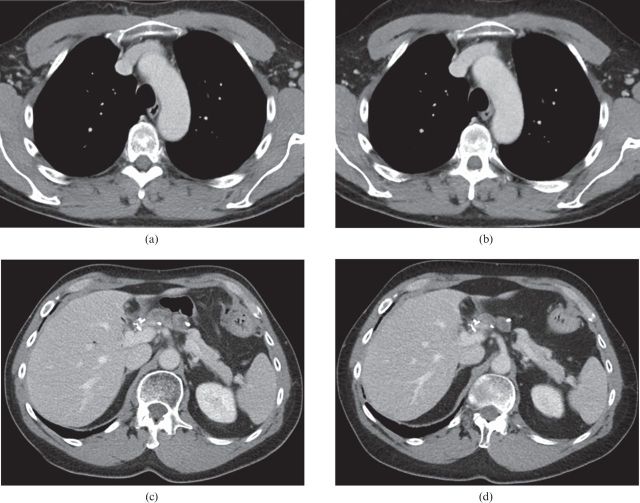
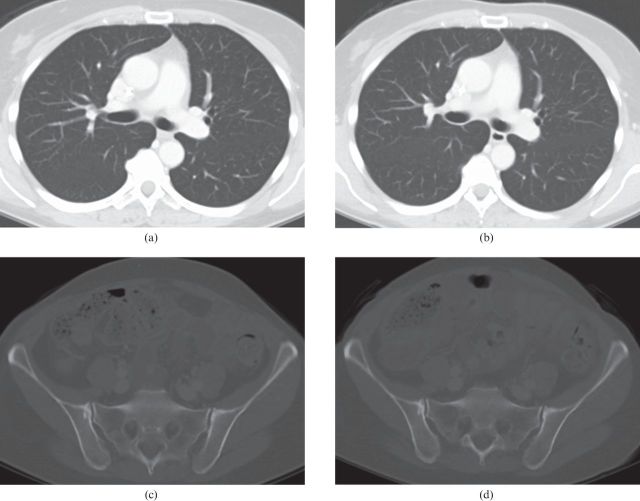
In 64.3% of all cases, IR was correctly recognised based on the visual impression of the images. There was no difference regarding correct identification of IR depending on the level of experience of the interpreting radiologists. In 60.5% of cases, the radiologist with the shortest CT experience (half a year) detected IR correctly. In 64.5% and 64.0% of cases, IR was correctly stated by radiologists with 7 and 15 years of experience, respectively. Images displayed at soft-tissue window settings (Figure 2a–d) were recognised as IR in the majority of cases: 93.0% for the mediastinum, 97.9% for the abdomen and 100% for the pelvis. In contrast to these results, all three readers had difficulty determining which image was iteratively reconstructed in lung or bone windows (Figure 3a–d). Concerning the lung window, in 86.7% of cases, none of the radiologists was able to decide which image had been iteratively constructed, and only 13.3% correct decisions were made. Similar results were found for the bone window: in 90% no decision for or against IR could be made and only in 10% was IR recognised correctly. In summary, IR was correctly recognised in soft-tissue window settings in 97.3% (false decisions were made in 1.9% and indifferent results were obtained in 0.8%). By contrast, for bone or lung windows, it was impossible to decide which image was iteratively reconstructed in 88.2% of cases, and in as few as 11.8% of instances IR was correctly identified (no wrong decisions were made).
Figure 2.
CT images displayed at soft-tissue window settings. CT images of thorax and abdomen obtained on the same patient. (a,c) Standard-dose filtered back projection images; (b,d) reduced-dose iteratively reconstructed images.
Figure 3.
CT images displayed at lung (for thorax) and bone (for pelvis) window. CT images of lung and pelvis on the same patient. (a,c) Standard-dose filtered back projection images; (b,d) reduced-dose iteratively reconstructed images.
Discussion
The results of our study show that IR can be used to maintain excellent image quality at significantly reduced radiation exposure values in oncological staging examinations. For the first time, we performed an intra-individual comparison of FBP and IR for examinations acquired at two time points within a defined patient cohort, thereby eliminating potential confounding factors that might influence image quality and noise.
The use of IR has previously been described for different types of CT examinations with applications for scanners from different companies [e.g. ASIR (adaptive statistical IR) by GE Healthcare or iDose by Philips].
Prakash et al [26] compared 98 CT scans of the chest reconstructed using ASIR with 54 CT scans that were performed using FBP. Despite a 27.6% dose reduction, ASIR images had less noise than FBP images. Moreover, the same authors reported that a new dedicated high-definition ASIR (ASIR HD) algorithm was superior to ASIR and FBP in 24 patients to assess subtle diffuse lung disease [27].
Graser et al [24] showed for non-contrast CT of the head that IRIS in combination with organ-based TCM is able to significantly improve image quality at reduced radiation exposure to the lenses, cerebrum, cerebellum and thyroid gland by 41.9%, 34.5%, 30.5% and 34.9%, respectively.
For the abdomen, the use of IR has been described in several patient studies. Prakash et al [28] compared an IR technique with FBP and determined a 25.1% dose reduction on top of a significant reduction in image noise. Moreover, Flicek et al [29] reported that in CT colonography, a true low-dose examination, the use of IR techniques allows reduction of the radiation dose by 50% without compromising image quality. For MDCT of the abdomen, Saraga et al [30] compared radiation doses of routine-dose CT with FBP and abdominal low-dose CT with IR in 53 patients. Despite reduced image sharpness on average and small patients, low-dose CT with IR had acceptable image quality similar to that of routine-dose CT with FBP [30]. In this study, different peak kilovoltages (140 kVp vs 120 kVp) and a variety of CT scanners and image reconstructions were used, thereby limiting comparability of effective dose values [30]. The authors reported a radiation dose reduction of 23–66% for abdominal low-dose CT using IR compared with routine-dose CT using FBP [30]. New phantom studies using IR even showed increased image quality and maintained diagnostic accuracy at a significantly reduced radiation dose (up to 50%) compared with FBP [31,32].
For the present study, we performed a retrospective analysis of CT scans performed for staging of malignancy in patients who received both a standard-dose and a reduced-dose CT scan on the same CT scanner. Patients investigated on a different CT scanner were excluded from our study, and all CT scanning parameters were kept constant during both standard- and reduced-dose CT scans, including tube voltage, reconstruction slice thickness and acquisition time (70 s after injection of non-ionic contrast agent).
Reducing radiation exposure during oncological staging examinations is of great importance, especially in younger patients with malignant diseases; a reduction in dose, however, will only be accepted by radiologists if there is no decrease in diagnostic image quality. We arbitrarily chose a reduction in tube current–time product from 250 to 150 mAs based on our experience from a pilot study (unpublished data). At 150 mAs and 120 kVp, image quality would be limited by increased noise when images were reconstructed with FBP algorithms, and the goal of the present study was to show that image quality is maintained when IR algorithms are used. Our results underline that the subjective quality and acceptability of sdFBP and rdIR are identical. In the majority of cases, even well-trained and highly experienced radiologists were not able to decide which set of images were iteratively reconstructed. Based on these results, we can assume that both rdIR and sdFBP images are of similar diagnostic accuracy, and our increasing clinical experience underlines this statement. This is also confirmed by constant image noise between the two examinations: IR compensates for the reduction in radiation dose and maintains noise values at low and fully acceptable levels at a greater than 40% dose reduction. Based on these results, we now routinely apply IR techniques for body imaging, but also for head, coronary and run-off CT examinations.
With the latest-generation algorithms, raw data information (typically visualised in a so-called sinogram) can now be used in the iterative image reconstruction process. In addition to the well-established approach of IRIS, the data are now also reprojected in the raw data space, allowing to validate (or affirm) the images with the measured data (SAFIRE, Siemens Healthcare). The detected deviations are reconstructed using the weighted FBP (wFBP), yielding an updated image. Image noise can be further subtracted by correcting geometrical imperfections of the initial reconstruction. This also applies for potential artefacts that occur with any system using FBP. With this, it is possible to reduce radiation dose up to 60% for a wide range of applications and with high image quality.
We performed this study in order to determine image quality and noise of data sets reconstructed with IR compared with FBP. Based on our clinical experience, we did not expect any difference in the diagnostic accuracy of rdIR and sdFBP images. However, it is one of the limitations of our work that we did not address diagnostic accuracy of CT examinations in the present study, and further research will be needed to pursue this issue. Another potential limitation of our study is the small patient population and the lack of morbidly obese patients with a BMI over 35 kg m−2. Furthermore, we focused on the evaluation of oncological staging examinations as a frequently performed procedure; for most indications and in order to comply with standardised reporting recommendations, it is sufficient to reconstruct images at a slice thickness of 5 mm. Our results cannot be generalised to thin-slice reconstructions as in CT angiography or cardiac CT. Heilbron and Leipsic [33] reported submillisievert cardiac CT studies performed by the use of IR, thereby indicating that this type of algorithm might be successfully used in this setting. In a recent study, Gosling et al [34] stated that the dose in cardiac CT acquired with FBP (5.4 mSv) is similar to invasive diagnostic cardiac catheterisation (6.3 mSv), while IR allowed substantial reduction of the radiation dose (down to 2.5 mSv) for cardiac CT. Further research is needed to determine if the diagnostic accuracy for coronary CT angiography (CTA) is maintained in data sets reconstructed with IR.
In conclusion, our results show the potential of IR of CT image data in oncological patients: the algorithm tested in the present scientific study allows a greater than 45% dose reduction at maintained image quality. Further research is needed to determine the potential of IR in high-resolution MDCT applications, like coronary CTA; also, studies looking at diagnostic accuracies of IR and FBP images have to be carried out. In our experience, image quality of sdFBP and rdIR images is fully comparable. We therefore recommend the use of IR in oncological patients in order to exploit the dose reduction potential of this new technology.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277–300. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, et al. Cancer statistics, 2004. CA Cancer J Clin 2004;54:8–29. [DOI] [PubMed] [Google Scholar]
- 3.UK Department of Health. Department of Health Hospital Activity Statistics: Imaging and Radiodiagnostics. [Cited 12 February 2012]. Available from: http://www.performance.doh.gov.uk/hospitalactivity/data_requests/imaging_and_radiodiagnostics.htm. [Google Scholar]
- 4.Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med 2007;357:2277–84. [DOI] [PubMed] [Google Scholar]
- 5.Mettler FA. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology 2008;248:254–63. [DOI] [PubMed] [Google Scholar]
- 6.Einstein AJ. Medical imaging: the radiation issue. Nat Rev Cardiol 2009;6:436–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerber TC, Kantor B, McCollough CH. Radiation dose and safety in cardiac computed tomography. Cardiol Clin 2009;27:665–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Linton OW, Mettler FA., Jr National conference on dose reduction in CT, with an emphasis on pediatric patients. AJR Am J Roentgenol 2003;181:321–9. [DOI] [PubMed] [Google Scholar]
- 9.Preston DL, Ron E, Tokuoka S, Funamoto S, Nishi N, Soda M, et al. Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiat Res 2007;168:1–64 [DOI] [PubMed] [Google Scholar]
- 10.Muirhead CR, O'Hagan JA, Haylock RG, Phillipson MA, Willcock T, Berridge GL, et al. Mortality and cancer incidence following occupational radiation exposure: third analysis of the National Registry for Radiation Workers. Br J Cancer 2009;100:206–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cardis E, Vrijheid M, Blettner M, Gilbert E, Hakama M, Hill C, et al. The 15-Country Collaborative Study of Cancer Risk among Radiation Workers in the Nuclear Industry: estimates of radiation related cancer risks. Radiat Res 2007;167:396–416. [DOI] [PubMed] [Google Scholar]
- 12.McCollough CH. CT dose: how to measure, how to reduce. Health Phys 2008;95:508–17. [DOI] [PubMed] [Google Scholar]
- 13.Lee CH, Goo JM, Ye HJ, Ye SJ, Park CM, Chun EJ, et al. Radiation dose modulation techniques in the multidetector CT-era: from basics to practice. Radiographics 2008;28:1451–9. [DOI] [PubMed] [Google Scholar]
- 14.Vollmar SV. Reduction of dose to the female breast as a result of spectral optimisation for high-contrast thoracic CT imaging: a phantom study. Br J Radiol 2009;82:920–9. [DOI] [PubMed] [Google Scholar]
- 15.Flohr TG, Klotz E, Allmendinger T, Raupach R. Pushing the envelope: new computed tomography techniques for cardiothoracic imaging. J Thorac Imaging 2010;25:100–11. [DOI] [PubMed] [Google Scholar]
- 16.Funama Y, Awai K, Nakayama Y, Kakei K, Nagasue N, Shimamura M, et al. Radiation dose reduction without degradation of low-contrast detectability at abdominal multisection CT with a low-tube voltage technique: phantom study. Radiology 2005;237:905–10. [DOI] [PubMed] [Google Scholar]
- 17.Kalra MK, Maher MM, Blake MA, Lucey BC, Karau K, Toth TL, et al. Detection and characterization of lesions on low-radiation-dose abdominal CT images postprocessed with noise reduction filters. Radiology 2004;232:791–7. [DOI] [PubMed] [Google Scholar]
- 18.Kalra MK, Maher MM, Toth TL, Hamberg LM, Blake MA, Shepard JA, et al. Strategies for CT radiation dose optimization. Radiology 2004;230:619–28. [DOI] [PubMed] [Google Scholar]
- 19.Elbakri IA, Fessler JA. Statistical image reconstruction for polyenergetic x-ray computed tomography. IEEE Trans Med Imaging 2002;21:89–99. [DOI] [PubMed] [Google Scholar]
- 20.Fessler JA, Ficaro EP, Clinthorne NH, Lange K. Grouped-coordinate ascent algorithms for penalized-likelihood transmission image reconstruction. IEEE Trans Med Imaging 1997;16:166–75. [DOI] [PubMed] [Google Scholar]
- 21.Lasio GM, Whiting BR, Williamson JF. Statistical reconstruction for x-ray computed tomography using energy-integrating detectors. Phys Med Biol 2007;52:2247–66. [DOI] [PubMed] [Google Scholar]
- 22.Nuyts J, De Man B, Dupont P, Defrise M, Suetens P, Mortelmans L. Iterative reconstruction for helical CT: a simulation study. Phys Med Biol 1998;43:729–37. [DOI] [PubMed] [Google Scholar]
- 23.Thibault JB, Sauer KD, Bouman CA, Hsieh J. A three-dimensional statistical approach to improved image quality for multislice helical CT. Med Phys 2007;34:4526–44. [DOI] [PubMed] [Google Scholar]
- 24.Graser A, Becker HC, Augart D, Karpitschka M, Johnson T, Reiser M. Imaging applications, software and post-processing (9 presentations). SS 1611 - Monday, March 7, 10: 30–12:00/Room N/O/CME: 1.50 h/ECR 2010. [Google Scholar]
- 25.American AssociationofPhysicistsinMedicine. The measurement, reporting, and management of radiation dose in CT: report of AAPM Task Group of the Diagnostic Imaging Council CT Committee. AAPM report no. 96. College Park, MD: American Association of Physicists in Medicine; 2008. [Google Scholar]
- 26.Prakash P, Kalra MK, Digumarthy SR, Hsieh J, Pien H, Singh S, et al. Radiation dose reduction with chest computed tomography using adaptive statistical iterative reconstruction technique: initial experience. J Comput Assist Tomogr 2010;34:40–5. [DOI] [PubMed] [Google Scholar]
- 27.Prakash P, Kalra MK, Ackman JB, Digumarthy SR, Hsieh J, Do S, et al. Diffuse lung disease: CT of the chest with adaptive statistical iterative reconstruction technique. Radiology 2010;256:261–9. [DOI] [PubMed] [Google Scholar]
- 28.Prakash P, Kalra MK, Kambadakone AK, Pien H, Hsieh J, Blake MA, et al. Reducing abdominal CT radiation dose with adaptive statistical iterative reconstruction technique. Invest Radiol 2010;45:202–10. [DOI] [PubMed] [Google Scholar]
- 29.Flicek KT, Hara AK, Silva AC, Wu Q, Peter MB, Johnson CD. Reducing the radiation dose for CT colonography using adaptive statistical iterative reconstruction: A pilot study. AJR Am J Roentgenol 2010;195:126–31. [DOI] [PubMed] [Google Scholar]
- 30.Sagara Y, Hara AK, Pavlicek W, Silva AC, Paden RG, Wu Q. Abdominal CT: comparison of low-dose CT with adaptive statistical iterative reconstruction and routine-dose CT with filtered back projection in 53 patients. AJR Am J Roentgenol 2010;195:713–19. [DOI] [PubMed] [Google Scholar]
- 31.Husarik DB, Marin D, Samei E, Richard S, Chen B, Jaffe TA, et al. Radiation dose reduction in abdominal computer tomography during the late hepatic arterial phase using a model-based iterative reconstruction algorithm: how low can we go? Invest Radiol 2012;47:468–74. [DOI] [PubMed] [Google Scholar]
- 32.Schindera ST, Diedrichsen L, Müller HC, Rusch O, Marin D, Schmidt B, et al. Iterative reconstruction algorithm for abdominal multidetector CT at different tube voltages: assessment of diagnostic accuracy, image quality, and radiation dose in a phantom study. Radiology 2011;260:454–62. [DOI] [PubMed] [Google Scholar]
- 33.Heilbron BG, Leipsic J. Submillisievert coronary computed tomography angiography using adaptive statistical iterative reconstruction - a new reality. Can J Cardiol 2010;26:35–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gosling O, Loader R, Venables P, Roobottom C, Rowles N, Bellenger N, et al. A comparison of radiation doses between state-of-the-art multislice CT coronary angiography with iterative reconstruction, multislice CT coronary angiography with standard filtered back-projection and invasive diagnostic coronary angiography. Heart 2010;96:922–6. [DOI] [PubMed] [Google Scholar]