Abstract
Objective
Pulmonary tuberculosis (PTB) is often associated with human immunodeficiency virus (HIV) in South Africa. Bronchial artery embolisation (BAE) is a specialised, expensive and risky procedure. The aim of this study was to investigate the impact of coinfection with HIV and PTB on the success of BAE.
Methods
A retrospective cross-sectional study of sequential BAE procedures during 2006 and 2007 was performed. Rates of procedural and clinical outcome, reasons for failures and the impact of cluster of differentiation cell type 4 (CD4) level on failure were investigated. Patients were included if they presented with massive or life-threatening haemoptysis with a diagnosis of previous or active PTB and their HIV status was known, for the first two attempts at BAE only.
Results
The study population consisted of 74 patients who were HIV positive and 33 who were HIV negative. Statistically, procedural success did not imply a clinically successful outcome, and HIV status and CD4 level did not correlate significantly with procedural success. Statistically, no technical reason had an impact on the success of the procedure when correlated with HIV status. The detection of lymphadenopathy was noted in 19.1% of patients who were HIV positive and in 42.4% of patients who were HIV negative, and was the only feature of significance.
Conclusion
Coinfection with HIV does not have an impact on the success of BAE in patients with active PTB or with the sequelae of PTB who present with massive or life-threatening haemoptysis. Technical success does not imply clinical success, regardless of HIV status. Improvement in technique locally may improve outcome.
Advances in knowledge
PTB coinfection with HIV should not affect the decision to consider BAE.
The rate of pulmonary tuberculosis (PTB) in the province of KwaZulu Natal (KZN), South Africa, is among the highest in the country and is often associated with human immunodeficiency virus (HIV) coinfection. New PTB infection cases in 2007 approximated 40%, with a coinfection rate of 14.8% [1]. The cure rate of new smear-positive cases of PTB for KZN was 52.9% and was the lowest in the country during 2007 [2]. The possible causes of massive haemoptysis in the Western world are extensive but the most common aetiology in sub-Saharan Africa is secondary to PTB or the sequela of previous PTB infections such as bronchiectasis [3].
The role of bronchial artery embolisation (BAE) in the management of haemoptysis has been extensively investigated internationally [4-15] and locally [16]. Regardless of the aetiology, BAE is at best a palliative procedure [3,17] to staunch the haemoptysis until definitive intervention is performed to prevent relapse. The long-term management for active PTB infection is effective medical therapy to reduce the local inflammatory environment and thus prevent recurrence. For associated aetiology such as the presence of aspergilloma, surgical management to remove the site of the pathological lung is needed to prevent recurrence, as this condition is resistant to other therapies and is a cause of relapse in haemoptysis. Chronic PTB is definitively managed medically, with BAE serving as a palliative procedure. In patients with sequelae of previous treated PTB who present with haemoptysis, BAE is effective in controlling haemoptysis but recurrence following BAE is an indicator for considering surgical management, as even extensive re-embolisation has been shown to be non-effective [12].
The role of BAE in massive or life-threatening haemoptysis specifically in the context of PTB coinfection with HIV appears not to have been investigated in the literature reviewed, but is investigated in this study. For the purposes of this study, massive haemoptysis is defined as the expectoration of 300–600 ml of blood per 24 h, or less if the result is life threatening and/or requires blood transfusion for a significant reduction in haemoglobin level, and is not controllable by conservative measures. Because BAE is an expensive, time-consuming procedure requiring operator skill, this study also investigates the rate of technical success of the procedure, compares it with the rate of clinical success and correlates these with HIV status. By investigating technical events during the procedure, the study aims to determine reasons for failed BAE.
The procedure is not without risk to both patient and operator. These include blood and blood-product exposure, and exposure to airborne PTB/multidrug-resistant (MDR) PTB, as the procedure is performed in a closed environment over prolonged periods in the presence of actively expectorating patients.
Methods and materials
A retrospective observational study on 107 of the 198 BAE procedures performed between January 2006 and December 2007 at Inkosi Albert Luthuli Central Hospital (IALCH), Berea, South Africa, was performed. Non-surgical candidates whose HIV status was known and who had active (confirmed on microbiology or radiology) or a history of PTB were included if they presented with life-threatening or massive haemoptysis on their first two attempts at embolisation. Demographic data, clinical presentation and outcome, laboratory parameters and procedural outcomes were reviewed. The radiological images were reviewed blind by an independent radiologist.
Radiologically, active PTB is suggested by the presence of a secondary focus or tuberculoma or nodules in the lung, pleural effusion or lymphadenopathy (LAP). Sequelae of PTB are suggested by fibroarchitectural distortion (fibrotic bands, calcified nodules, atelectasis, emphysema, bronchiectasis and volume loss) or the presence of cavitation and mycetoma in the setting of known previous PTB. LAP of any size was deemed significant because the possibility of LAP in HIV-negative patients with post-primary tuberculosis was considered to be small. Clinical outcome was considered unsuccessful if further definitive management was needed to staunch the haemoptysis. This included sedation and medical therapy, repeat bronchial artery embolisation procedures, surgery or re-admission at a later date or if a serious complication such as non-target organ embolisation or death occurred.
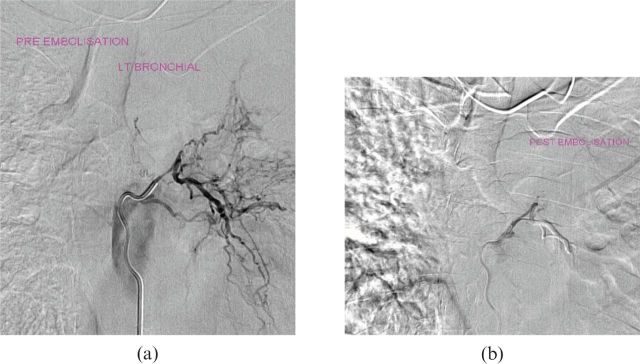
The bronchial arteries were selected with or without subselection (depending on operator confidence and equipment) and pathological vessels were embolised using large embospheres. Technical reasons for failure of the procedure included the inability to select the primary artery (bronchial artery), inability to subselect divisions of the artery for safe embolisation, instability of the catheter tip, reflux of the contrast medium into the parent vessel, visualisation of a contraindication such as a spinal artery, bronchial–pulmonary vein fistula, bronchial–pulmonary artery fistula, other bronchial–artery fistulae or if a pathological vessel was not identified. The procedure was concluded if there was successful embolisation of the vessel (Figure 1) or increased resistance to flow during injection, or if a decision was made to discontinue owing to contraindications (such as reflux or a clearer visualisation of the fistulae) or complications (Figures 2, 3 and 4).
Figure 1.
(a) Pre-embolisation and (b) post-embolisation digital subtraction angiography images demonstrating the pathology and desired outcome of the procedure. LT, left.
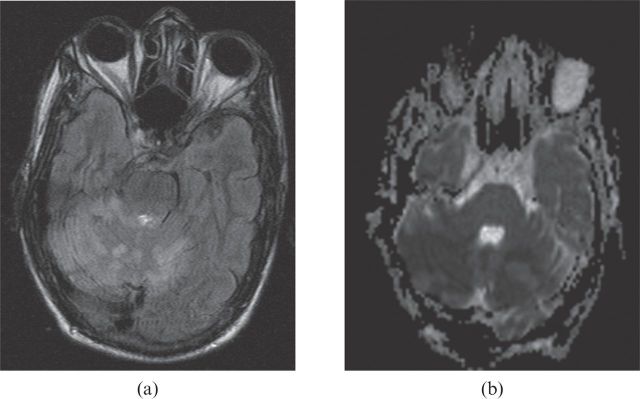
Figure 2.
(a) Fluid-attenuated inversion–recovery image and (b) diffusion-weighted axial MR image confirming bilateral cerebellar infarction.
Figure 3.
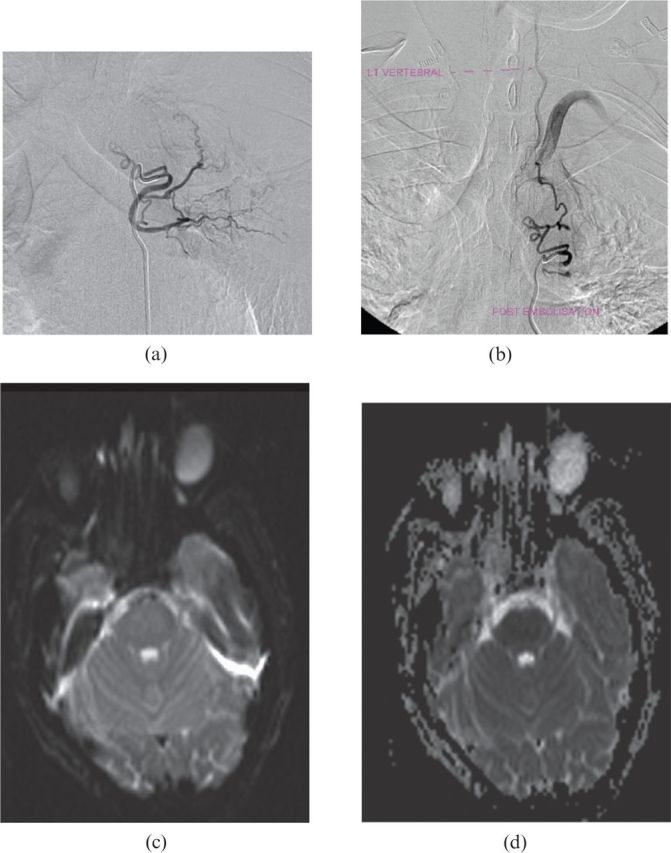
(a) Pre-embolisation and (b) post-embolisation angiogram demonstrating a fistula between the left bronchial artery and the left subclavian artery. (c) T2 weighted and (d) apparent diffusion coefficient map axial MRI confirming pontine infarction resulting in clinical locked-in syndrome.
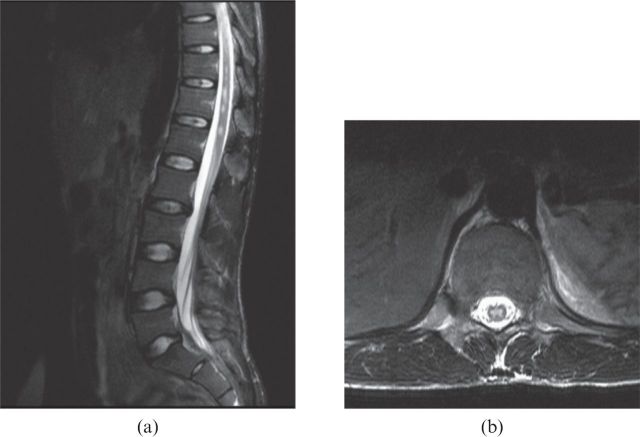
Figure 4.
(a) T2 weighted sagittal and (b) axial MR images showing hyperintensity in the cord, suggestive of cord infarction. LT, left.
The findings were analysed using the χ2 test, Fisher's exact test or the Mann–Whitney U-test, as appropriate, using SPSS® v.15 for Windows (IBM, Chicago, IL). A p-value of ≤0.05 was considered significant.
Results
The study included 107 patients. Demographic data of the included patients are shown in Table 1. The average age was 36.6 years. The median age of the patients who were HIV positive was 32 years, and the average age of those who were HIV negative was 40.85 years. Unknown HIV status was the most common reason for exclusion and accounted for 53 (58.2%) of the 91 excluded cases. Data regarding methods of diagnosis of PTB are shown in Table 2.
Table 1. Demographic data for gender and HIV status.
| Gender | HIV-positive patients [n=74 (59.8%)] | HIV-negative patients [n=33 (30.8%)] | Total patients (n=107) |
| Male | 44 | 30 | 63 |
| Female | 29 | 14 | 44 |
HIV, human immunodeficiency virus.
Data are given as numbers.
Table 2. Method of diagnosis of PTB correlated with HIV status.
| Method of diagnosis | HIV-negative patients | HIV-positive patients |
| History of previous PTB [n=71 (66.4%); p=0.120] | 18 | 53 |
| Sputum AFB positive [n=28 (26.2%); p=1.000] | 9 | 19 |
| Sputum multidrug-resistant PTB [n=9 (8.4%); p=0.718] | 2 | 7 |
| Radiographic evidence of old or new PTB [n=101 (94.4%); p=0.664] | 32 | 69 |
| Clinical diagnosis [n=19 (17.8%); p=1.000] | 6 | 13 |
| Referral diagnosis [n=61 (57%); p=0.292] | 16 | 45 |
AFB, acid-fast bascilli; HIV, human immunodeficiency virus; PTB, pulmonary tuberculosis.
Data are given as numbers.
There were 93 high-resolution CT (HRCT) chest studies and 13 chest radiographs available for review and all were abnormal. The correlation of imaging technique availability, zones involved or features of active or old PTB and HIV status was not significant. The majority of cases showed radiographic evidence of active (101/106) or old (102/106) PTB. The most common imaging feature (regardless of HIV status) was architectural distortion. LAP was present in 18.9% of patients who were HIV positive and in 42.2% of those who were HIV negative, and was the only feature of significance. There was no significant difference in the incidence of imaging findings when correlating HIV status and active disease (p=0.664) or disease sequelae (p=1.000) (Table 3).
Table 3. Pathology identified on imaging correlated with HIV status.
| HIV status | Active disease (n=101, p=0.664) |
Sequelae of disease (n=102, p=1.000) |
|||
| Acute infection (n=101; p=0.664) | Pleural effusion (n=17; p=0.576) | LAP (n=28; p=0.017) | Architectural distortion (n=102; p=1.000) | Mycetoma (n=46; p=0.676) | |
| HIV-positive patients (n=73) | 69 | 13 | 14 | 70 | 33 |
| HIV-negative patients (n=33) | 32 | 4 | 14 | 32 | 13 |
HIV, human immunodeficiency virus; LAP, lymphadenopathy.
Data are given as numbers.
The difference in the clinical and procedural success rates for the entire caseload was significant (p=0.008). 15 cases were considered procedurally successful but did not have the desired clinical outcome. Of these patients, 3 were HIV negative and 12 were HIV positive, but the HIV status was not considered significant (p=0.183). 51 (47.7%) cases were clinically successful. 3 (5.9%) could not be accounted for by procedural success; this may have been the result of conservative and/or medical management. All three of those patients were HIV positive. 56 (52.3%) cases were clinically unsuccessful and required alternative management. There was no difference in the likelihood of any of the clinical follow-up noted for either HIV-positive or HIV-negative patients.
There was no significance in the correlation of a successful clinical outcome and HIV status (p=0.469). A total of 63 (58.9%) cases were classified as procedurally successful and there was no statistical significance in correlating procedural outcome with HIV status (p=1.069) (Table 4).
Table 4. Comparing procedural and clinical success based on HIV status.
| HIV status | Clinical outcome | Procedural success (n=63) | Procedural failure (n=44) |
| HIV-negative patients (n=33) | Clinical success | 14 | 0 |
| Clinical failure | 3 | 16 | |
| HIV-positive patients (n=74) | Clinical success | 34 | 3 |
| Clinical failure | 12 | 25 |
HIV, human immunodeficiency virus.
Data in bold indicate cases of procedural success without the desired clinical outcome.
Data are given as numbers.
Only 106 of the 212 selected vessels were embolised. Not all selected vessels needed to be embolised; therefore, the two categories were not correlated. There was no significance in the correlation of selection or embolisation of any of the vessels (right bronchial artery, left bronchial artery, intercostobronchial artery, intercostals or internal mammary arteries) and HIV status.
Four factors were significant when correlated with overall failure of the procedure regardless of HIV status. These were “unable to select” (p=0.007), “unable to subselect” (p=0.015), “spinal feeder” (p=0.006) and “no bleeder identified” (p=0.010), but there was no difference in the likelihood of occurrence of any of the factors investigated when correlated with HIV status (Table 5).
Table 5. Reasons for failure to embolise correlated with HIV status.
| Reasons | HIV-negative patients | HIV-positive patients |
| Unable to select (n=11; p=0.503) | 5 | 6 |
| Unable to subselect (n=10; p=0.144) | 6 | 4 |
| Unable to engage securely (n=3; p=1.000) | 1 | 2 |
| Reflux observed (n=0) | 0 | 0 |
| Bronchiopulmonary artery fistula (n=4; p=1.000) | 1 | 3 |
| Bronchiopulmonary vein fistula (n=8; p=1.000) | 3 | 5 |
| Bronchiovisceral fistula (n=5; p=1.000) | 2 | 3 |
| Spinal feeder identified (n=15; p=0.646) | 6 | 9 |
| No bleeder identified (n=5; p=0.651) | 1 | 4 |
| Other (n=1; p=1.000) | 0 | 1 |
HIV, human immunodeficiency virus.
Data are given as numbers.
Of the 74 patients who were HIV positive, the cluster of differentiation cell type 4 (CD4) level was available in 57 (77.0%) cases only. The median level of 176 was used for comparative purposes. Using a CD4 level of 200 as a cut-off value, 35 (47.3%) patients had a CD4 level consistent with World Health Organization Stage 4 clinical disease. Neither the total number of clinically successful cases (25/57) nor the total number of cases of procedural success (35/57) correlated significantly with the CD4 level.
There were 31 patients with a CD4 level at or below 176. Of these, 14 (45.2%) patients had a clinically successful outcome. 17 (54.8%) were procedurally successful. In 5 (16.1%) cases, the procedure was considered successful but the patient did not have a good clinical outcome (p=0.002). 26 patients had a CD4 level above 176. 15 (57.7%) of these cases had a clinically successful outcome and 11 (42.3%) were procedurally successful. In 7 (26.9%) cases, the procedure was considered successful but the patient did not have a good clinical outcome (p=0.007) (Table 6). The likelihood of a procedure being successful with poor clinical outcome was significant for both categories of CD4 levels.
Table 6. Correlating CD4 value and clinical and procedural success.
| CD4 value | Clinical success | Procedure successful |
Total | |
| No (n=22) | Yes (n=35) | |||
| CD4<176 (n=31) | Unsuccessful | 12 | 5 (p=0.002) | 17 |
| Successful | 2 | 12 | 14 | |
| CD4>176 (n=26) | Unsuccessful | 8 | 7 (p=0.007) | 15 |
| Successful | 0 | 11 | 11 | |
CD4, cluster of differentiation cell type 4.
Data are given as numbers unless otherwise indicated.
Discussion
The fear of stigmatisation and concerns of the patient regarding how they would be managed may have accounted for the large proportion of cases that were excluded because of unknown or untested HIV status. Only two cases were excluded for other causes of haemoptysis (asthma and metastatic cancer), confirming that PTB is the most common cause for massive or life-threatening haemoptysis in KZN. Most patients either had a history of PTB or were referred from another healthcare facility with diagnosis of active PTB and were being treated at the time of referral. A large proportion of cases showed radiological evidence of active disease and/or the sequelae of disease. Comparatively fewer cases were diagnosed primarily by sputum examination, but a large (32.1%) proportion of these confirmed MDR PTB infection.
There was lack of significance when correlating imaging tools used for diagnosis and the distribution of disease within the lung zones. The patients included in the study were those that were referred to the specialist unit for management and were likely to have more complicated disease and therefore more obvious imaging findings. In patients who were HIV positive there was a lower prevalence of parenchymal consolidation, cavitation and post-primary pattern, and a higher prevalence of extrapulmonary and miliary disease than in those who were HIV negative [18,19], but this imaging pattern was not demonstrated in this study. Notably, the incidence of mycetoma was equally likely amongst HIV-positive and HIV-negative cases.
The only feature of statistical significance was the presence of LAP, which was almost twice as likely in patients who were HIV negative as in those who were HIV positive. There is controversy in the literature regarding the presence of LAP and HIV infection. The literature suggests that LAP is a reflection of the host immune response in primary TB rather than in post-primary PTB [8]. However, the modality of imaging (namely chest radiography, HRCT scan or thicker-slice contrasted CT scan) must be considered to ensure that the results are not a reflection of the imaging limitations rather than actual incidence. LAP in HIV-negative patients who have post-primary TB may be under-reported because the LAP is small and more difficult to detect on chest radiography than on CT scan [20]. It is technically more difficult to detect small LAP on HRCT than on contrast-enhanced thicker-slice CT chest. In addition, the relatively lower prevalence of LAP may be a result of the predominance of post-primary TB in our adult population, or because of the degree of general poor immunity (not necessarily owing to HIV).
Although the literature suggests that it is more difficult to detect active disease when there are features of previous disease on chest radiographs and CT scans [8], many cases demonstrated features of both sequelae and active infection on HRCT regardless of HIV status. This is thought to reflect the severity of disease in the patients included in the study.
There is no difference in the likelihood of procedural or clinical success based on HIV status. This supports the findings of a local study by Corr [16], where it was first suggested that patients who were HIV positive tolerated BAE well. In that study, the HIV-positive rate was 32%, and 75% of those patients were successfully managed with BAE [16]. This study also confirmed that there was no statistical significance in the correlation of procedural or clinical success in the two categories of CD4 levels assessed. However, the likelihood of a procedure being technically successful with poor clinical outcome was significant for both categories of CD4 levels.
The poor correlation between the technical success of the procedure and the clinical outcome for the entire caseload encourages one to reassess the role of the procedure. In the present study, 44 (41.1%) cases were considered procedurally unsuccessful compared with reports of rates of technical failure from 0% to 56.5% by Chun and Belli [6]. At our centre, owing to limited human resources, registrars at different stages of training performed the procedures in the absence of a dedicated interventional unit, and the rate of procedural failure may have reflected suboptimal skill.
The four factors (“unable to select”, “unable to subselect”, “spinal feeder” and “no bleeder identified”) that were significant in overall procedural failure were not significant when correlated with HIV status; this could be the result of operator inexperience, anatomical remodelling following severe architectural distortion, lack of vessel dilatation and hypertrophy or the presence of vascular thrombosis, or non-availability of specific catheters, microcatheters or guidewires. Vascular fistulae were identified in 17 (38.6%) failed cases and served as the contraindication to embolisation, and would have been more relevant if smaller embospheres were available. Spinal feeders were identified approximately three times more than is quoted in the literature, but review of the images suggested that proper confirmation was not undertaken. Operator inexperience may have resulted in over-reporting and reluctance to embolise given the possible complications of non-target embolisation. This may be remedied with better training.
Conclusion
HIV infection (regardless of CD4 level) should not alter accessibility to BAE or affect the technical aspects of the procedure in a patient with massive haemoptysis as a result of PTB. The poor correlation between technical and clinical success of the procedure in this study and the reviewed literature calls for further research into the role of BAE in the management of haemoptysis once the procedure has been optimised. Current knowledge may be used to caution both clinician and patient expectations of the procedure.
References
- 1.Marshall TJ, Flower CDR, Jackson JE. The role of radiology in the investigation and management of patients with haemoptysis. Clin Radiol 1996;51:391–400. [DOI] [PubMed] [Google Scholar]
- 2.Day C, Gray A. Health and Related Indicators (16). South African Health Review [serial on the internet]. 2008 [cited August 2011] 239–396. Available from: http://www.hst.org.za/uploads/files/chap16_08.pdf. [Google Scholar]
- 3.Knott-Craig C, Oostuizen J, Rossouw G, Joubert J, Barnard P. Management and prognosis of massive hemoptysis. Recent experience with 120 patients. J Thorac Cardiovasc Surg 1993;105:394–7. [PubMed] [Google Scholar]
- 4.Alcantara-Peraza AR, Frias-Salcedo JA, Carrillo-Largaespada OL, Reza-Trujillo C, Castañeda F. Bronchial artery embolization for hemoptysis secondary to pulmonary tuberculosis. Semin Intervent Radiol 2000;17:185–92. [Google Scholar]
- 5.Chan VL, So LKY, Lam JYM, Lau KY, Chan CS, Lin AWN, et al. Major haemoptysis in Hong Kong: aetiologies, angiographic findings and outcomes of bronchial artery embolisation. Int J Tuberc Lung Dis 2009;13:1167–73. [PubMed] [Google Scholar]
- 6.Chun J-Y, Belli A-M. Immediate and long-term outcomes of bronchial and non-bronchial systemic artery embolisation for the management of haemoptysis. Eur Radiol 2010;20:558–65. [DOI] [PubMed] [Google Scholar]
- 7.Katoh O, Kishikawa T, Yamada H, Matsumoto S, Kudo S. Recurrent bleeding after arterial embolization in patients with hemoptysis. Chest 1990;97:541–6. [DOI] [PubMed] [Google Scholar]
- 8.Kim YG, Yoon H-K, Ko GY, Lim C-M, Kim WD, Koh Y. Long-term effect of bronchial artery embolization in Korean patients with haemoptysis. Respirology 2006;11:776–81. [DOI] [PubMed] [Google Scholar]
- 9.Lee S, Chan JWM, Chan SCH, Chan YH, Kwan TL, Chan MK, et al. Bronchial artery embolisation can be equally safe and effective in the management of chronic recurrent haemoptysis. Hong Kong Med J 2008;14:14–20. [PubMed] [Google Scholar]
- 10.Mal H, Rullon I, Mellot F, Brugière O, Sleiman C, Menu Y, et al. Immediate and long-term results of bronchial artery embolization for life-threatening hemoptysis. Chest 1999;115:996–1001. [DOI] [PubMed] [Google Scholar]
- 11.Poyanli A, Acunas B, Rozanes I, Guven K, Yilmaz S, Salmaslioglu A, et al. Endovascular therapy in the management of moderate and massive haemoptysis. Br J Radiol 2007;80:331–6. [DOI] [PubMed] [Google Scholar]
- 12.Ramakantan R, Bandekar VG, Gandhi MS, Aulakh BG, Deshmukh HL. Massive hemoptysis due to pulmonary tuberculosis: control with bronchial artery embolization. Radiology 1996;200:691–4. [DOI] [PubMed] [Google Scholar]
- 13.Rémy J, Arnaud A, Fardou H, Giraud R, Voisin C. Treatment of hemoptysis by embolization of bronchial arteries. Radiology 1977;122:33–7. [DOI] [PubMed] [Google Scholar]
- 14.Swanson KL, Johnson M, Prakash UBS, McKusick MA, Andrews JC, Stanson AW. Bronchial artery embolization. Chest 2002;121:789. [DOI] [PubMed] [Google Scholar]
- 15.Uflacker R, Kaemmerer A, Neves C, Picon PD. Management of massive hemoptysis by bronchial artery embolization. Radiology 1983;146:627–34. [DOI] [PubMed] [Google Scholar]
- 16.Corr PD. Bronchial artery embolization for life-threatening hemoptysis using acryl microspheres: short-term result. Cardiovasc Intervent Radiol 2005;28:439–41. [DOI] [PubMed] [Google Scholar]
- 17.Yoon W, Kim JK, Kim YH, Chung TW, Kang HK. Bronchial and nonbronchial systemic artery embolization for life-threatening hemoptysis: a comprehensive review. Radiographics 2002;22:1395–409. [DOI] [PubMed] [Google Scholar]
- 18.Beigelman C, Sellami D, Brauner M. CT of parenchymal and bronchial tuberculosis. Eur Radiol 2000;10:699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leung AN, Brauner MW, Gamsu G, Mlika-Cabanne N, Ben Romdhane H, Carette MF, et al. Pulmonary tuberculosis: comparison of CT findings in HIV-seropositive and HIV-seronegative patients. Radiology 1996;198:687–91. [DOI] [PubMed] [Google Scholar]
- 20.Burrill J, Williams CJ, Bain G, Conder G, Hine AL, Misra RR. Tuberculosis: a radiologic review. Radiographics 2007;27:1255–73. [DOI] [PubMed] [Google Scholar]