Abstract
Objective
Predicting outcome in patients with primary intracerebral haemorrhage (ICH) in the acute stage can provide information to determine the best therapeutic and rehabilitation strategies. We prospectively investigated the predictive value of the functional diffusion map (fDM) in the acute stage of ICH.
Methods
47 patients with ICH were enrolled for clinical evaluation and MRI within 24 h of symptom onset and 5 days after ICH. Functional diffusion mapping prospectively monitored the apparent diffusion coefficient (ADC) maps of perihaematomal oedema. Consequently, the change in perihaematomal oedema was classified into three categories: increased, decreased, or no significant change. Clinical outcomes were evaluated 6 months after ICH according to the modified Rankin Scale. Correlation between clinical outcome and the fDMs was performed.
Results
Among the clinical variables, thalamic haematoma, serum glucose level and National Institutes of Health Stroke Scale scores were significantly different between the good- and poor-outcome groups. The percentage of oedematous tissue undergoing significant change between baseline and Day 5 was also significantly different between the groups.
Conclusion
fDMs allow for spatial voxel-by-voxel tracking of changes in ADC values. It may be feasible to use fDMs to predict the functional outcome of patients with ICH during the acute stage.
Advances in knowledge
The use of fDMs for stroke study is demonstrated. fDMs may be more suitable to reflect the pathophysiological heterogeneity within oedemas and may facilitate another thinking process for imaging study of stroke and other neurological diseases.
Primary intracerebral haemorrhage (ICH) is associated with greater mortality and more severe neurological deficits than any other subtype of stroke [1]. Given the emphasis placed on the early introduction of rehabilitation programmes for improving function, prediction of functional outcome in the acute stage of ICH is important. Perihaematomal oedema develops immediately after ICH and peaks several days to weeks later [2,3]. Whether or not perihaematomal oedema contributes to ICH-induced neurological deficits and patient outcome is still controversial and warrants further investigation [4,5]. The pathophysiology of perihaematomal oedema is complicated and may provide valuable clues [4,6]. Diffusion MRI, a technique that can probe tissue microstructure by measuring the diffusion properties of water within tissues, has been used to study perihaematomal injury in patients with ICH, but the results have been inconsistent [7-12].
By monitoring changes in the apparent diffusion coefficient (ADC) over time, functional diffusion maps (fDMs) have been developed to monitor regional variations (both increases and decreases) in ADC values in order to provide early stratification of the clinical brain tumour response. Based on the relative change in the ADC value, fDMs can further classify the regions of interest (ROIs) into three categories, which correlate highly with pathological change [13].
Given the inconsistent results of previous studies of perihaematomal injury by diffusion-weighted imaging, the diffusion changes within oedematous tissue should be rapid and heterogeneous. In this study, we hypothesised that the early diffusion changes in perihaematomal oedema may correlate with functional outcome in patients with ICH, and that the fDM approach may be a predictive imaging biomarker in the acute stage of ICH.
Methods and materials
Patients
We prospectively examined 47 patients hospitalised with acute ICH within 24 h of symptom onset. Patients were enrolled in our study after their ICH was confirmed by CT. Exclusion criteria were large haematomas requiring emergent surgery, history or imaging findings of past ICH or other neurological trauma, and evidence of intraventricular haemorrhage on CT. The following clinical data were recorded within 24 h after ICH: age, sex, blood pressure, blood sugar level, haemoglobin level, white cell count, platelet count, creatinine level, haematoma volume, volume of perihaematomal oedema and relative oedema volume (volume of oedema divided by volume of haematoma). National Institutes of Health Stroke Scale (NIHSS) scores and Glasgow Coma Scale (GCS) scores were estimated by two neurosurgeons within 24 h after ICH. The duration of intensive care unit stay and the overall duration of hospital admission, as well as whether a patient had received rehabilitation, were also recorded. The modified Rankin Scale (mRS) and Barthel Index (BI) scores were estimated by the same two neurosurgeons 6 months after ICH.
Patients were divided into either good- or poor-outcome groups according to their functional outcome. For the good-outcome group, the mRS score estimated 6 months after ICH ranged between 0 and 2. For the poor-outcome group, the mRS score at 6 months after ICH ranged between 3 and 6. The study was part of an integrated stroke project at Chang Gung Memorial Hospital, Chiayi, Taiwan, and was approved by the Institutional Review Board of Chang Gung Memorial Hospital. All patients, or their families, gave their written informed consent prior to participation in the study.
MRI
MRI scans were obtained using a 1.5 T MRI scanner (Gyroscan Intera; Philips Medical Systems, Best, Netherlands). Patients were scanned at baseline (within 24 h after symptom onset) and again 5 days after symptom onset. Standard sequences for depiction of anatomy and haematoma and oedema extent included axial T2* weighted gradient echo images for location and haematoma volume [repetition time (TR)/echo time (TE) 355/13.81 ms; excitations 1; flip angle 18°; section thickness 6.5 mm with a gap of 1.5 mm; and matrix size 512×256] and axial fluid-attenuated inversion recovery (FLAIR) images for extension of perihaematomal oedema (TR/TE 6000/120 ms; excitations 2; flip angle 90°, using the same section thickness and matrix size). Diffusion-weighted images were acquired using a single-shot spin-echo diffusion-sensitised echo-planar imaging pulse sequence (TR/TE 2952/69 ms; excitations 1; section thickness 6.5 mm with a gap of 1.5 mm; and matrix size 128×128). Three-direction (x, y and z gradient directions) standard diffusion-weighted imaging with multiple b-values (b-value=0 and 1000 s mm−2) was acquired. The ADC map was derived directly from these diffusion-weighted images.
Functional diffusion map analysis
The ADC maps on Day 5 were coregistered to the baseline ADC maps (acquired within 24 h) using Statistical Parametric Mapping 2 (SPM2; www.fil.ion.ucl.ac.uk/spm). Following coregistration, areas of perihaematomal oedema were manually contoured on the baseline ADC maps by a neuroradiologist. The ROIs were marked using all available imaging data, including CT, T2* weighted gradient echo and FLAIR imaging. Prospective evaluation of the ADC maps from different days was performed using in-house software. Voxels in the perihaematomal oedema regions were stratified into three categories based on the ADC change from baseline for each time point. Red voxels indicated a significant increase in the ADC value, blue voxels indicated a significant decrease in the ADC value and green voxels indicated no significant change in the ADC value.
The thresholds for determining whether there was a significant change in the ADC value within a voxel were determined using the 95% confidence intervals (CIs) calculated from the normal contralateral brain of each patient on the baseline ADC map, including white matter and grey matter [13]. The percentage of perihaematomal oedema within each of the three categories was then calculated as VR (percentage of red voxels), VB (percentage of blue voxels), and VT (VR+VB).
Statistical methods
The baseline characteristics were presented as mean and standard deviation. Univariate and multiple stepwise linear regression model analysis was used to analyse the relationship between candidate predictor variables and categorical clinical outcome. Effect values were summarised as odds ratios per 1.0 unit of each respective independent variable with 95% CIs. A p-value of <0.05 was considered statistically significant. All statistical analyses were performed using Stata® v. 11.0 (StataCorp, College Station, TX).
Results
Clinical and radiological features of patients
Baseline demographic, clinical and radiological characteristics of the 47 enrolled patients are presented in Table 1. The upper portion of Table 2 shows the correlation of baseline clinical and laboratory variables with functional outcome 6 months after ICH. From the univariate linear analysis, age, thalamic location of haematoma, serum glucose and NIHSS score correlated positively with poor functional outcome (p=0.039, p=0.022, p=0.016 and p=0.005, respectively).
Table 1. Baseline characteristics of patients with good and poor outcomes.
| Outcome group |
|||
| Characteristics | All patients (n=47) | Good (n=18) | Poor (n=29) |
| Age (years) | 65.5±12.7 | 60.5±11.7 | 68.6±12.4 |
| Male | 26 (55.3) | 11 (61.1) | 15 (51.7) |
| Location of haematoma | |||
| Thalamus | 18 (38.3) | 3 (16.7) | 15 (51.7) |
| Basal ganglia | 12 (25.5) | 6 (33.3) | 6 (20.7) |
| Putamen | 10 (21.3) | 5 (27.8) | 5 (17.2) |
| Lobar | 6 (12.8) | 3 (16.7) | 3 (10.3) |
| Cerebellum | 1 (2.1) | 1 (5.9) | 0 (0.0) |
| Blood pressure (mmHg) | |||
| Systolic | 189.7±22.8 | 193.4±19.8 | 187.5±24.6 |
| Diastolic | 106.1±16.6 | 107.6±13.3 | 105.2±18.5 |
| Mean arterial | 134.0±16.4 | 136.2±13.0 | 132.6±18.3 |
| Serum glucose (mmol l−1) | 144.7±59.7 | 118.2±24.3 | 161.7±69.3 |
| Haemoglobin (g dl−1) | 14.1±1.6 | 14.6±1.2 | 13.8±1.7 |
| Platelet count (1000 per μl) | 216.7±93.9 | 209.0±65.7 | 221.5±108.6 |
| Creatinine (mg dl−1) | 1.0±0.4 | 0.8±0.3 | 1.0±0.5 |
| White cell count (1000 per μl) | 8.2±2.6 | 7.7±2.2 | 8.5±2.8 |
| NIHSS score within 24 h | 10.7±5.4 | 7.7±5.2 | 12.5±4.7 |
| GCS score within 24 h | 13.2±2.7 | 13.9±2.2 | 12.7±2.9 |
| Days in ICU | 6.3±3.0 | 5.4±2.1 | 6.8±3.3 |
| Days of admission | 18.9±7.8 | 16.4±6.6 | 20.5±8.2 |
| Rehabilitation | 18 (38.3) | 6 (33.3) | 12 (41.4) |
| mRS score at 6 months | 3.0±1.6 | 1.3±0.8 | 4.1±1.0 |
| BI score at 6 months | 57.0±39.1 | 96.9±3.5 | 32.2±29.1 |
| Haematoma volume (ml) | |||
| Baseline | 19.6±13.8 | 18.6±15.4 | 20.2±13.0 |
| Day 5 | 19.4±13.7 | 18.4±14.8 | 20.0±13.1 |
| Change between Day 5 and baseline | −0.2±3.4 | −0.2±3.4 | −0.1±3.5 |
| Oedema volume (ml) | |||
| Baseline | 20.4±16.2 | 17.6±13.1 | 22.1±17.9 |
| Day 5 | 27.8±22.5 | 27.1±21.6 | 28.3±23.4 |
| Change between Day 5 and baseline | 7.4±11.3 | 9.5±10.6 | 6.1±11.6 |
| Relative oedema volume | |||
| Baseline | 1.2±0.7 | 1.2±0.6 | 1.2±0.8 |
| Day 5 | 1.7±1.2 | 2.0±1.3 | 1.6±1.1 |
| Change between Day 5 and baseline | 0.6±0.9 | 0.8±1.0 | 0.4±0.9 |
BI, Barthel Index; GCS, Glasgow Coma Scale; ICU, intensive care unit; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale.
Data are given as mean ± standard deviation or number (percentage).
Table 2. Predictors of poor functional outcome at 6 months.
| Univariate analysis |
Multivariate analysis |
||||
| Predictors | Odds ratio (95% CI) | p-value | Odds ratio (95% CI) | p-value | |
| Age | 1.056 (1.003–1.111) | 0.039a | |||
| Thalamic location | 5.357 (1.272–22.560) | 0.022a | 15.637 (1.401–174.570) | 0.026a | |
| Systolic blood pressure | 0.988 (0.962–1.015) | 0.386 | |||
| Diastolic blood pressure | 0.991 (0.956–1.027) | 0.626 | |||
| Mean arterial blood pressure | 0.986 (0.951–1.023) | 0.465 | |||
| Serum glucose | 1.032 (1.006–1.060) | 0.016a | 1.042 (1.007–1.078) | 0.019a | |
| Haemoglobin | 0.704 (0.465–1.066) | 0.098 | |||
| Platelet count | 1.001 (0.995–1.008) | 0.657 | |||
| Creatinine | 4.030 (0.653–24.854) | 0.133 | |||
| White cell count | 1.129 (0.885–1.442) | 0.328 | |||
| NIHSS score within 24 h | 1.213 (1.059–1.390) | 0.005a | 1.321 (1.078–1.618) | 0.007a | |
| GCS score within 24 h | 0.806 (0.613–1.061) | 0.124 | |||
| Baseline haematoma volume | 1.000 (1.000–1.000) | 0.711 | |||
| Change in haematoma volume | 1.000 (1.000–1.000) | 0.971 | |||
| Baseline oedema volume | 1.000 (1.000–1.000) | 0.354 | |||
| Change in oedema volume | 1.000 (1.000–1.000) | 0.325 | |||
| Relative oedema volume | 0.977 (0.431–2.214) | 0.956 | |||
| Change in relative oedema ratio | 0.632 (0.326–1.225) | 0.174 | |||
| VR | 0.000 (0.000–1.194) | 0.054 | |||
| VB | 0.011 (0.000–1.805) | 0.083 | |||
| VT | 0.008 (0.000–0.485) | 0.021a | 0.004 (0.000–1.321) | 0.062a | |
CI, confidence interval; GCS, Glasgow Coma Scale; NIHSS, National Institutes of Health Stroke Scale; VB, percentage of blue voxels; VR, percentage of red voxels; VT, VR+VB.
aSignificant differences were defined as those with p-values of <0.05.
Functional diffusion map analysis
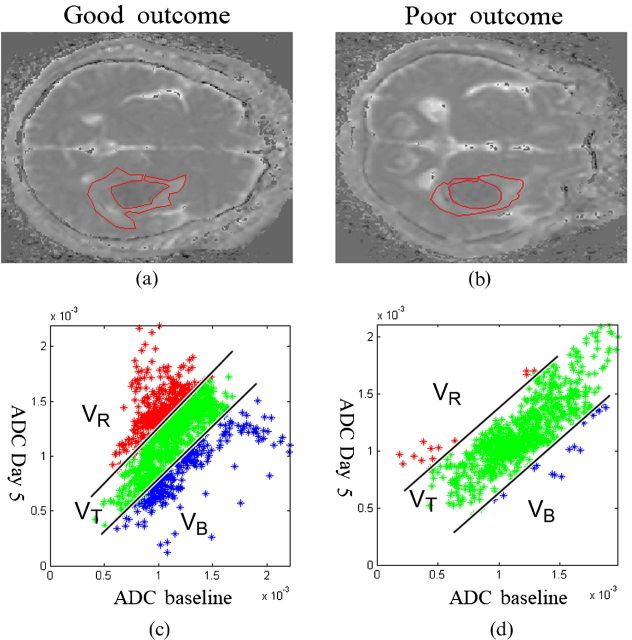
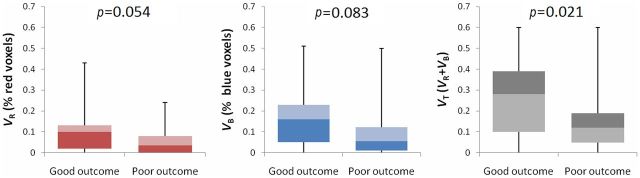
Examples of fDM analyses from each group are shown in Figure 1. Figure 1a,b illustrates selected ROIs from the baseline ADC map. The red, green and blue data points in the associated scatter plots (Figures 1c,d) represent three different categories of ADC change determined on the fifth day after ICH, as compared with the baseline. The ratios of diffusional change obtained from the fDM for each group are summarised in the lower portion of Table 2 and in Figure 2. In the good-outcome group, on the fifth day after ICH, VT=26.1±18.4% (mean ± standard error of the mean), which was significantly higher than in the poor-outcome group (VT=14.0±13.5%; p=0.021). From the multivariate logistic analyses (Columns 4 and 5 of Table 2), the observed association between VT and functional outcome remained significant (p=0.062). Thalamic location of haematoma, serum glucose level and NIHSS score were also associated with functional outcome based on the multivariate analysis (p=0.026, p=0.019 and p=0.007, respectively).
Figure 1.
Representative apparent diffusion coefficient (ADC) maps and functional diffusion map (fDM) scatter plots for patients in each group. (a,b) Following coregisteration of the ADC maps on Day 5 to the baseline, perihaematomal oedema was manually contoured on baseline ADC maps. (c,d) The scatter plots quantitatively show the distribution of ADC value changes for the entire contoured perihaematomal oedema. (a,c) A patient with good outcome who had fDM parameters of VR=28.7, VB=23.9 and VT=52.6. (b,d) A patient with poor outcome who had fDM parameters of VR=1.4, VB=3.0 and VT=4.4. VB, percentage of blue voxels; VR, percentage of red voxels; VT, VR+VB.
Figure 2.
Box plots summarising functional diffusion map (fDM) change volumes as percentages of perihaematomal volume for each patient group. The percentage of total voxels undergoing change of diffusion values (VT) within perihaematomal oedema is significantly different between the good- and poor-outcome groups. Error bars reflect 95% confidence intervals.
Discussion
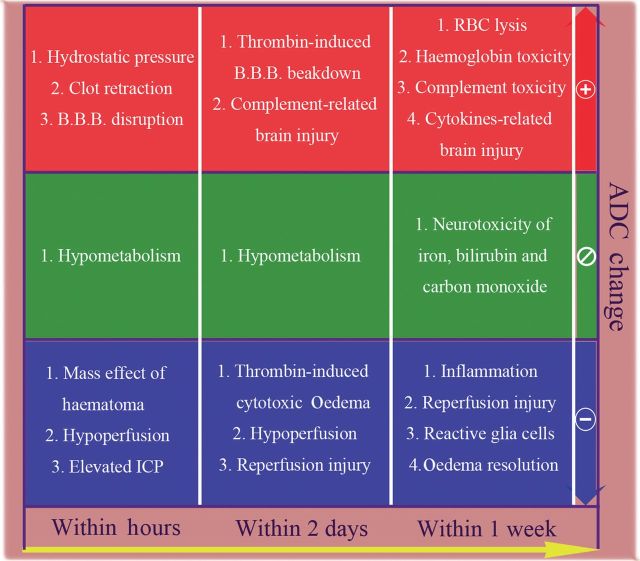
The capability of the fDM as an early imaging predictor of functional outcome in patients with acute ICH was demonstrated in this study. The development of perihaematomal oedema can be divided into three temporal phases. The early phase occurs during the first several hours after ICH and involves hydrostatic pressure changes during haematoma formation, clot retraction, mass effect of the haematoma and hypometabolism as the cause of reduced cerebral blood flow (diaschisis). A second phase occurs during the first 24–48 h and results from thrombin production, coagulation cascade activation and early reperfusion injury. Finally, the delayed phase occurs 3–5 days after ICH and involves haemolysis of red blood cells with haemoglobin-induced toxicity; brain injury related to complement and cytokine release; neurotoxicity from iron, bilirubin and carbon monoxide; inflammatory processes; and reperfusion, as well as oedema resolution [4,6,9,14,15]. These mechanisms lead to various types of oedema after ICH, even at the same time point. Vasogenic oedema, which follows increased permeability or disruption of the blood–brain barrier, is the primary type of oedema, but cytotoxic oedema due to reperfusion or inflammation as a result of sodium–potassium pump failure has also been proposed. In addition, interstitial oedema caused by clot retraction and osmotic oedema caused by liquefaction of haematoma are observed soon after ICH, as well as several days later [14,16].
Diffusion MRI has contributed greatly to the diagnosis and understanding of the natural history of ischaemic stroke by allowing for early definition of deeply ischaemic or infarcted brain tissue. It has also been reported to predict outcome in ischaemic stroke [17]. This technique has additionally been used to study perihaematomal injury in patients with ICH, but the results have been inconsistent [9,12,18,19]. In general, the mean ADC values in the perihaematomal regions relative to contralateral, homologous brain regions may increase quickly during the acute stage, with peak increases noted 2–3 days after ICH [12]. However, decreased relative mean ADC values can be observed in some patients during the hyperacute stage (within 6 h) after haemorrhage, and have been reported to be associated with poor clinical outcome [10]. Significant correlation between ICH volume and degree of ADC elevation in perihaematomal oedema and ADC values in contralateral, corresponding healthy brain tissue has also been noted [9]. A number of factors have been reported to affect ADC values. Although ADC values are increased in vasogenic oedema, extracellular methaemoglobin and gliosis, they are reduced in cytotoxic oedema, ischaemia, high cellularity and high viscosity [20,21]. Therefore, the diffusion signal of oedema at different areas with variable ADC changes (Figure 3) would be obscured using the overall mean ADC, thus reducing the sensitivity and specificity of ADC in predicting outcome [22,23].
Figure 3.
Biological processes proposed to induce changes in oedema apparent diffusion coefficient (ADC) values during acute stage of intracerebral haemorrhage. The pathophysiological process within perihaematomal oedema is complicated and relates to both systemic and local responses. This will induce various changes in the ADC values. Even a single biological response, such as thrombin formation, will lead to different kinds of ADC changes. Therefore, the diffusion signal of oedema at different areas with increasing or decreasing changes would be obscured in the overall mean ADC but easily discriminated by a functional diffusion map. B.B.B., blood–brain barrier; ICP, intracranial pressure; RBC, red blood cell.
Functional diffusion maps quantify regional variations in structural diffusivity by classifying voxels into three colour regions based on the magnitude and trend of ADC change. They allow spatial voxel-by-voxel tracking of changes in water diffusion values over time. The fDM has been reported to provide an imaging biomarker for the early prediction of treatment response and overall survival for patients with bone metastases from prostate cancer [23], diverse primary brain tumours [13] and high-grade gliomas [24]. In these studies, early changes in ADC values indicated cell death within tumours and were associated with good tumour response to therapy. The results of our study indicated that a large percentage change in ADC value during the acute stage of perihaematomal oedema formation may lead to better functional outcome. Changes in the ADC value indicate changes in tissue microstructure and may represent an intrinsic response, such as inflammation, hypometabolism, or autoimmune response, aimed at reducing cellular damage and neurotoxicity. In addition, changes in the ADC value may reflect a tissue response to medical treatment such as osmotic agents, steroids, hypovolaemia, controlled hyperventilation and arterial blood pressure control. Brain tissues without significant changes in the ADC value may suffer from irreversible cellular damage such as cytotoxic oedema, necrosis and even apoptosis. Further studies closely investigating different physiological and therapeutic factors are necessary to better understand how these intrinsic and extrinsic factors affect ADC values and how they are related to the patient's functional outcome. Based on the results of this study and previous studies using fDM, we hypothesise that changes in ADC value may reflect cellular response or plasticity toward changes in the biological environment. Therefore, fDM may also be used to study both the natural course and the therapeutic response of disease entities such as inflammation, demyelination and traumatic brain injury.
We evaluated a number of variables previously reported to be associated with perihaematomal oedema formation and clinical outcome. From our stepwise multivariate analysis, we found that thalamic location of haematoma, serum glucose level, NIHSS scores and VT were independently associated with functional outcome. There are various factors and scales used to predict prognosis after ICH [20,25]. The prognosis after ICH or other acute neurological disorders is a fundamental issue; therefore, it is important to weigh treatment risks against benefits and provide patients and their families with information regarding the severity of the illness. Besides determining the prognosis, predictive methods also provide a framework for clinical decision making and also provide reliable criteria for assessing the efficacy of new treatments. A predictor such as fDM could be used as part of a risk stratification for ICH studies, but not as a precise predictor of outcome.
This study had several limitations. First, we excluded patients who underwent surgical evacuation during the first 5 days after ICH, and patients with intraventricular haemorrhage. The range of haematoma volumes in this study was between 1.5 and 65.9 ml (mean 19.6 ml; standard deviation 13.8 ml), which was relatively small, and may be the reason why haematoma size did not correlate with functional outcome in our study. Also, our results may not be applicable to studies involving larger haematoma volumes. Second, the number of patients in our study was small and further subgroup analysis could not be achieved. In addition, we tested the reliability of different pulse sequences to measure the haematoma volume and the results showed that T2* weighted images have the highest intraobserver reliability and most significant correlation with CT for measuring ICH volume (Appendix A). However, using T2* weighted imaging to measure haematoma volume may overestimate the haematoma volume, owing to blooming artefacts. Third, because perihaematomal oedema develops immediately and peaks between 10 and 20 days after ICH in humans [2-4], a repeat MRI scan during the early acute phase (Day 1 or 2) may help to highlight early diffusion change and improve outcome prediction at an earlier stage. Also, an additional MRI scan at 10–20 days after ICH may provide more information regarding the diffusional and pathological changes within the oedema zone.
Conclusion
The pathophysiological process that occurs within perihaematomal oedema is complicated and relates to both systemic and local responses. fDMs allow for spatial voxel-by-voxel tracking of changes in ADC values over time that more precisely reflect the pathophysiological heterogeneity within oedemas. Based on this study, the use of fDMs to evaluate perihaematomal oedema appears promising. It may be feasible to use fDMs as part of a risk stratification to predict the functional outcome of patients with ICH during the acute stage. Further research should be performed in order to understand how the intrinsic and extrinsic factors after ICH affect the ADC and how they are related to functional outcome.
Appendix A
CT is used as a standard to measure the size of an intracerebral haematoma. The signal intensity of haematomas on different pulse sequences of MRI depends significantly on the age and internal content of the haematoma. The extension of the haematoma and the interface of the haematoma and oedema are not always easily identified on every pulse sequence or even using CT.
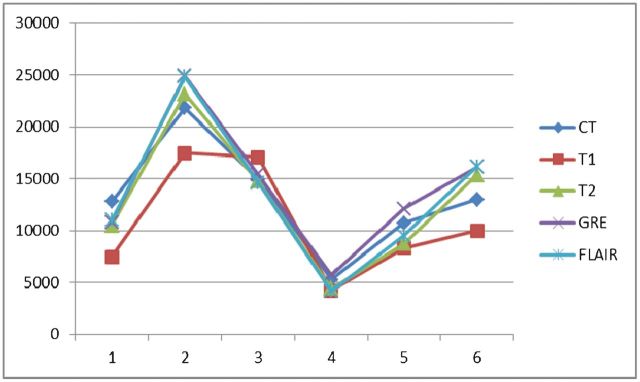
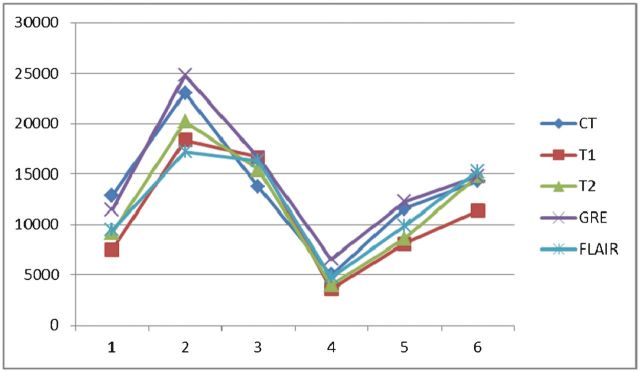
In order to test the intraobserver reliability and which MRI pulse sequence is most suitable to measure haematoma size, we measured haematoma volumes using T1 weighted imaging, T2 weighted imaging, T2* weighted imaging, fluid-attenuated inversion recovery (FLAIR) imaging and CT for the first 6 subjects before analysing data from all the subjects (the CT and MRI scans were not performed at the same time—the CT scan was carried out once the patient had arrived at the emergency department and the MRI scan was performed once the patient was enrolled in the study, all within 24 h after the CT scan). The results are shown in Tables A1, A2 and A3. The first interpretation is shown in Figure A1, and the second interpretation in Figure A2. Both interpretations were undertaken by the same neuroradiologist.
Figure A1.
First interpretation. FLAIR, fluid-attenuated inversion recovery; GRE, T2* weighted gradient echo; T1, T1 weighted imaging; T2, T2 weighted imaging.
Figure A2.
Second interpretation (given by the same neuroradiologist as the first interpretation). FLAIR, fluid-attenuated inversion recovery; GRE, T2* weighted gradient echo; T1, T1 weighted imaging; T2, T2 weighted imaging.
Table A1. Measured haematoma volume.
| Volume (ml) |
|||||
| Interpretation | T1 weighted imaging | T2 weighted imaging | T2* weighted imaging | FLAIR | CT |
| First interpretation | 10.8±5.4 | 12.8±6.5 | 14.2±6.4 | 13.4±7.0 | 13.1±5.4 |
| Second interpretation | 10.9±5.7 | 12.0±5.8 | 14.4±6.1 | 12.2±4.9 | 13.4±5.8 |
FLAIR, fluid-attenuated inversion recovery.
Data are given as mean ± standard deviation.
Table A2. Results of one-way intraclass correlation coefficient (ICC) with absolute agreement.
| Results | T1 weighted imaging | T2 weighted imaging | T2* weighted imaging | FLAIR | CT |
| ICC | 0.991 | 0.975 | 0.990 | 0.854 | 0.985 |
FLAIR, fluid-attenuated inversion recovery.
Table A3. Correlations of the results of MRI with CT.
| Correlation with CT (p-value) |
||||
| Interpretation | T1 weighted imaging | T2 weighted imaging | T2* weighted imaging | FLAIR |
| First interpretation | 0.021 | 0.002 | 0.002 | 0.011 |
| Second interpretation | 0.027 | 0.007 | 0.001 | 0.031 |
FLAIR, fluid-attenuated inversion recovery.
If we use the volume measured by CT as the standard, the haematoma volume measured by T2* weighted imaging was overestimated, but the results measured by T2 weighted imaging and FLAIR were underestimated. As mentioned above, the MRI scans were performed within 24 h after the CT scans. The haematomas might have enlarged during this time period but would not have shrunk or become resolved. In addition, the T2* weighted image has the highest intraobserver reliability (intraclass correlation coefficient 0.990) and most significant correlation with CT (p=0.002 and 0.001 for the first and second interpretations, respectively) for measuring intracerebral haemorrhage volume. Based on the above findings, we used T2* weighted imaging to measure the haematoma. However, using T2* weighted imaging to measure haematoma volume is a limitation of this study, because there was a chance of it overestimating the haematoma volume, owing to blooming artefacts.
Footnotes
This research was partly supported by the Chang Gung Medical Research Fund, Chang Gung Memorial Hospital, Chiayi, Taiwan (CMRPG690462 and CMRPG690472).
The authors acknowledge technical support from the Laboratory of MRI Core Facility, National Yang-Ming University, Taipei, Taiwan.
References
- 1.Broderick J, Connolly S, Feldmann E, Hanley D, Kase C, Krieger D, et al. REPRINT: Guidelines for the management of spontaneous intracerebral haemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group: The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Circulation 2007;116:e391–413. [DOI] [PubMed] [Google Scholar]
- 2.Zazulia AR, Diringer MN, Derdeyn CP, Powers WJ. Progression of mass effect after intracerebral haemorrhage. Stroke 1999;30:1167–73. [DOI] [PubMed] [Google Scholar]
- 3.Venkatasubramanian C, Mlynash M, Finley-Caulfield A, Eyngorn I, Kalimuthu R, Snider RW, et al. Natural history of perihematomal edema after intracerebral haemorrhage measured by serial magnetic resonance imaging. Stroke 2011;42:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol 2006;5:53–63. [DOI] [PubMed] [Google Scholar]
- 5.Arima H, Wang JG, Huang Y, Heeley E, Skulina C, Parsons MW, et al. Significance of perihematomal edema in acute intracerebral haemorrhage: The INTERACT trial. Neurology 2009;73:1963–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehdiratta M, Kumar S, Hackney D, Schlaug G, Selim M. Association between serum ferritin level and perihematoma edema volume in patients with spontaneous intracerebral haemorrhage. Stroke 2008;39:1165–70. [DOI] [PubMed] [Google Scholar]
- 7.Olivot JM, Mlynash M, Kleinman JT, Straka M, Venkatasubramanian C, Bammer R, et al. MRI profile of the perihematomal region in acute intracerebral haemorrhage. Stroke 2010;41:2681–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schellinger PD. Stroke MRI in intracerebral haemorrhage: is there a perihemorrhagic penumbra? Stroke 2003;34:1674–9. [DOI] [PubMed] [Google Scholar]
- 9.Carhuapoma JR, Barker PB, Hanley DF, Wang P, Beauchamp NJ. Human brain haemorrhage: quantification of perihematoma edema by use of diffusion-weighted MR imaging. AJNR Am J Neuroradiol 2002;23:1322–6. [PMC free article] [PubMed] [Google Scholar]
- 10.Kidwell CS, Saver JL, Mattiello J, Warach S, Liebeskind DS, Starkman S, et al. Diffusion-perfusion MR evaluation of perihematomal injury in hyperacute intracerebral haemorrhage. Neurology 2001;57:1611–17. [DOI] [PubMed] [Google Scholar]
- 11.Carhuapoma JR, Wang PY, Beauchamp NJ, Keyl PM, Hanley DF, Barker PB. Diffusion-weighted MRI and proton MR spectroscopic imaging in the study of secondary neuronal injury after intracerebral haemorrhage. Stroke 2000;31:726–32. [DOI] [PubMed] [Google Scholar]
- 12.Kamal AK, Dyke JP, Katz JM, Liberato B, Filippi CG, Zimmerman RD, et al. Temporal evolution of diffusion after spontaneous supratentorial intracranial haemorrhage. AJNR Am J Neuroradiol 2003;24:895–901. [PMC free article] [PubMed] [Google Scholar]
- 13.Moffat BA, Chenevert TL, Lawrence TS, Meyer CR, Johnson TD, Dong Q, et al. Functional diffusion map: a noninvasive MRI biomarker for early stratification of clinical brain tumor response. Proc Natl Acad Sci U S A 2005;102:552–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu J. Iron and iron-handling proteins in the brain after intracerebral haemorrhage. Stroke 2003;34:2964–9. [DOI] [PubMed] [Google Scholar]
- 15.Zazulia AR, Videen TO, Powers WJ. Transient focal increase in perihematomal glucose metabolism after acute human intracerebral haemorrhage. Stroke 2009;40:1638–43. [DOI] [PubMed] [Google Scholar]
- 16.Betz AL, Iannotti F, Hoff JT. Brain edema: a classification based on blood-brain barrier integrity. Cerebrovasc Brain Metab Rev 1989;1:133–54. [PubMed] [Google Scholar]
- 17.Olivot JM, Mlynash M, Thijs VN, Kemp S, Lansberg MG, Wechsler L, et al. Relationships between infarct growth, clinical outcome, and early recanalization in diffusion and perfusion imaging for understanding stroke evolution (DEFUSE). Stroke 2008;39:2257–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fainardi E, Borrelli M, Saletti A, Schivalocchi R, Russo M, Azzini C, et al. Evaluation of acute perihematomal regional apparent diffusion coefficient abnormalities by diffusion-weighted imaging. Acta Neurochir Suppl 2006;96:81–4. [DOI] [PubMed] [Google Scholar]
- 19.Butcher KS, Baird T, MacGregor L, Desmond P, Tress B, Davis S. Perihematomal edema in primary intracerebral haemorrhage is plasma derived. Stroke 2004;35:1879–85. [DOI] [PubMed] [Google Scholar]
- 20.Yoshioka H, Horikoshi T, Aoki S, Hori M, Ishigame K, Uchida M, et al. Diffusion tensor tractography predicts motor functional outcome in patients with spontaneous intracerebral haemorrhage. Neurosurgery 2008;62:97–103; discussion 103. [DOI] [PubMed] [Google Scholar]
- 21.Schaefer PW, Grant PE, Gonzalez RG. Diffusion-weighted MR imaging of the brain. Radiology 2000;217:331–45. [DOI] [PubMed] [Google Scholar]
- 22.Galban CJ, Mukherji SK, Chenevert TL, Meyer CR, Hamstra DA, Bland PH, et al. A feasibility study of parametric response map analysis of diffusion-weighted magnetic resonance imaging scans of head and neck cancer patients for providing early detection of therapeutic efficacy. Transl Oncol 2009;2:184–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee KC, Bradley DA, Hussain M, Meyer CR, Chenevert TL, Jacobson JA, et al. A feasibility study evaluating the functional diffusion map as a predictive imaging biomarker for detection of treatment response in a patient with metastatic prostate cancer to the bone. Neoplasia 2007;9:1003–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamstra DA, Galban CJ, Meyer CR, Johnson TD, Sundgren PC, Tsien C, et al. Functional diffusion map as an early imaging biomarker for high-grade glioma: correlation with conventional radiologic response and overall survival. J Clin Oncol 2008;26:3387–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hemphill JC, 3rd, Farrant M, Neill TA., Jr Prospective validation of the ICH Score for 12-month functional outcome. Neurology 2009;73:1088–94. [DOI] [PMC free article] [PubMed] [Google Scholar]