Abstract
Lager beer is the most consumed alcoholic beverage in the world. Its production process is marked by a fermentation conducted at low (8 to 15°C) temperatures and by the use of Saccharomyces pastorianus, an interspecific hybrid between Saccharomyces cerevisiae and the cold-tolerant Saccharomyces eubayanus. Recent whole-genome-sequencing efforts revealed that the currently available lager yeasts belong to one of only two archetypes, “Saaz” and “Frohberg.” This limited genetic variation likely reflects that all lager yeasts descend from only two separate interspecific hybridization events, which may also explain the relatively limited aromatic diversity between the available lager beer yeasts compared to, for example, wine and ale beer yeasts. In this study, 31 novel interspecific yeast hybrids were developed, resulting from large-scale robot-assisted selection and breeding between carefully selected strains of S. cerevisiae (six strains) and S. eubayanus (two strains). Interestingly, many of the resulting hybrids showed a broader temperature tolerance than their parental strains and reference S. pastorianus yeasts. Moreover, they combined a high fermentation capacity with a desirable aroma profile in laboratory-scale lager beer fermentations, thereby successfully enriching the currently available lager yeast biodiversity. Pilot-scale trials further confirmed the industrial potential of these hybrids and identified one strain, hybrid H29, which combines a fast fermentation, high attenuation, and the production of a complex, desirable fruity aroma.
INTRODUCTION
With an annual production exceeding 1.97 billion hectoliters a year, beer is the most-produced fermented beverage in the world (1). The vast majority of currently produced beer is classified as either ale or lager beer, each type being produced by a unique fermentation process (2). Specifically, ale beer production uses the common brewer's yeast Saccharomyces cerevisiae and relatively high fermentation temperatures (typically 18 to 25°C) (2–5).
In contrast, lager beer (with Pilsner beer as the most popular and commonly known type of lager beer) is fermented at lower temperatures (5 to 15°C), followed by a period of cold storage (lagering), which is a traditional practice vital for the beer's characteristically clean flavor and aroma. Lagers are not fermented by S. cerevisiae but by the closely related species Saccharomyces pastorianus (formerly known as Saccharomyces carlsbergensis), which combines the desirable fermentation characteristics of S. cerevisiae with the cold tolerance of its other parent, S. eubayanus (6). Lager beer currently accounts for more than 90% of the global beer market but has a much more recent origin than ales. The lager beer production process was developed in the 16th century in Bavaria (Germany), where brewing was only allowed during wintertime to minimize the microbial spoilage of beer. Later, in the 19th century, the advent of refrigeration enabled lager brewing throughout the whole year (2, 3, 7).
Several recent studies have focused on analyzing the S. pastorianus genome. The results revealed that S. pastorianus is not a clean yeast lineage but, rather, harbors the genomes of two different species (2, 8–10). Whereas it was generally assumed that lager yeasts were hybrids between S. cerevisiae and S. bayanus, Libkind et al. (11) showed that the second parent is actually S. eubayanus, a species originally discovered in Patagonia but later also isolated in other regions, such as North America and China (11–13). Although conclusive molecular evidence is not available (14), it is currently believed that the S. cerevisiae/S. eubayanus hybridization event took place 500 to 600 years ago, when German laws forced brewers to use lower fermentation temperatures, promoting the selection of rare natural hybrids between the common brewer's yeast S. cerevisiae and the more cold-tolerant S. eubayanus (11, 15).
Genetic analysis of a representative set of 17 lager strains revealed that all currently commercially used lager strains likely originated from two separate hybridization events that yielded two distinct genotypes, dubbed “Saaz” and “Frohberg” (16–18). Saaz-type S. pastorianus yeasts are allotriploids (∼3n), while Frohberg-type are allotetraploids (∼4n), and each type has a slightly different phenotype and fermentation characteristics. Apart from showing a better tolerance toward low temperatures, Saaz-type yeasts are unable to ferment maltotriose, resulting in lower growth and fermentation rates (18–20). Additionally, Saaz-type strains produce lower concentrations of aroma compounds like ethyl acetate, isoamyl alcohol, and isoamyl acetate (IA) than the more aroma-rich Frohberg yeasts (16, 19, 20). This phenotypic difference may partly explain why Frohberg-type lager yeasts are generally preferred over Saaz-type lager yeasts in today's beer industry.
The limited genetic diversity of lager yeasts is reflected in the relative limited influence of the yeast on the aroma profile of lager beer (7), especially compared to the immense genetic and aromatic diversity of S. cerevisiae ale strains (21–23). While the characteristically clean, fresh flavor and aroma of lager beers is one of their most distinctive and praised traits, diversification and differentiation have become increasingly important in today's market. The development of new lager hybrids may help to generate a set of distinct beers that in some ways bridge the gap between diverse, aromatic ales and fresh and drinkable lagers (7, 19, 24). The development of interspecific hybrids has proven to be a powerful approach to generate novel yeast variants with enhanced characteristics for wine making. Typically, an industrial strain of S. cerevisiae yeast is crossed with a wild, non-cerevisiae member of the Saccharomyces sensu stricto group, such as S. bayanus (15, 25–27), S. kudriavzevii (28, 29), S. uvarum (30, 31), or S. mikatae (32).
Here, we report the development and extensive testing of 31 new lager yeast variants. Our results show that some of these novel interspecific hybrids between S. cerevisiae yeasts and S. eubayanus can outperform both of the parental strains under lager beer conditions, yielding beers with very diverse aromatic profiles and thereby enriching the potential aroma spectrum of lager beer.
MATERIALS AND METHODS
Yeast strains used in this study.
Parental strains for the generation of interspecific hybrid yeasts were selected from a collection of 301 industrial and wild Saccharomyces strains, described by Steensels and coworkers (22). Six industrial S. cerevisiae strains were selected based on their production of desirable aromatic compounds, sporulation capacity, and spore viability (Fig. 1). Additionally, two wild S. eubayanus strains were included as parental strains. Two different S. pastorianus strains, corresponding to the two types of lager yeasts (Saaz and Frohberg type), were included in the different experiments as reference strains (Table 1) (16). An extra set of 15 different industrially used S. pastorianus strains were tested for their aroma and ethanol production in laboratory-scale lager beer fermentation tests. The yeast strains BY4742 (n) and BY4743 (2n) and a confirmed tetraploid strain, Y243, were included as references for fluorescence-activated cell sorting (FACS) analysis.
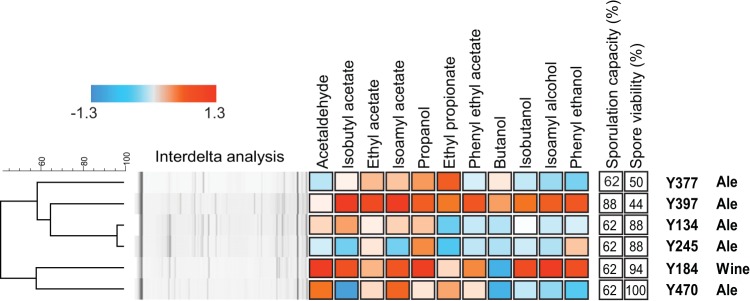
FIG 1.
Overview of selected S. cerevisiae parental strains. Genetic relatedness was determined by interdelta DNA fingerprinting, and subsequent clustering was performed using BioNumerics software. Sporulation capacity and spore viability are represented as percentages, whereas aroma production is visualized relative to a representative set of 104 ale beer strains (represented as Z-scores) previously screened by Steensels et al. (22), with color coding (as shown in the key) indicating the following range of results: white, same as average; blue, lower than average; red, higher than average.
TABLE 1.
Overview of yeast strains used in this study
| Straina | Culture collection aliasb | Species | Industry | Origin |
|---|---|---|---|---|
| Y134 | NAc | S. cerevisiae | Ale beer | NA |
| Y184 | NA | S. cerevisiae | Wine | NA |
| Y245 | NA | S. cerevisiae | Ale beer | Belgium |
| Y397 | NA | S. cerevisiae | Ale beer | Belgium |
| Y470 | NA | S. cerevisiae | Ale beer | NA |
| Y243 | NA | S. cerevisiae | Bread (confirmed tetraploid; 4n) | NA |
| BY4742 | NA | S. cerevisiae | Laboratory strain (n) | NA |
| BY4743 | NA | S. cerevisiae | Laboratory strain (2n) | NA |
| Y565 | NA | S. eubayanus | Wild isolate | Argentina |
| Y567 | NA | S. eubayanus | Wild isolate | Argentina |
| GSY129 | CBS1513 | S. pastorianus | Saaz-type lager beer | Denmark |
| GSY131 | CBS1538 | S. pastorianus | Saaz-type lager beer | Denmark |
| GSY133 | CBS1486 | S. pastorianus | Saaz-type lager beer | NA |
| GSY134 | CBS1503 | S. pastorianus | Saaz-type lager beer | Denmark |
| GSY137 | DBVPG6284 | S. pastorianus | Saaz-type lager beer | Denmark |
| GSY501 | CBS1174 | S. pastorianus | Saaz-type lager beer (reference) | NA |
| GSY509 | CBS2440 | S. pastorianus | Saaz-type lager beer | NA |
| GSY132 | CBS1260 | S. pastorianus | Frohberg-type lager beer (reference) | The Netherlands |
| GSY135 | DBVPG6282 | S. pastorianus | Frohberg-type lager beer | Canada |
| GSY515 | CBS5832 | S. pastorianus | Frohberg-type lager beer | The Netherlands |
| GSY516 | CBS6903 | S. pastorianus | Frohberg-type lager beer | The Netherlands |
| Y5 | NA | S. pastorianus | Frohberg-type lager beer | Belgium |
| Y447 | NA | S. pastorianus | Frohberg-type lager beer | Germany |
| Y449 | NA | S. pastorianus | Frohberg-type lager beer | Germany |
| Y453 | NA | S. pastorianus | Frohberg-type lager beer | Germany |
| Y454 | NA | S. pastorianus | Frohberg-type lager beer | Germany |
| Y473 | NA | S. pastorianus | Frohberg-type lager beer | Czech Republic |
Strains denoted with a “Y” code are derived from the historical yeast collection stored at KU Leuven University. “GSY” strain codes are the codes used in the previous research of Dunn and Sherlock (16).
CBS, Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands; DBVPG, Dipartimento Biologia Vegetale Perugia, Yeast Industrial Collection, Perugia, Italy.
NA, the culture collection alias is nonexistent or the exact origin is unknown.
Sporulation and tetrad dissection of possible parental strains.
Sporulation of selected parental strains was induced on acetate medium (1% [wt vol−1] potassium acetate, 0.05% [wt vol−1] amino acid mix, and 2% [wt vol−1] agar) after 5 to 10 days at 25°C. Subsequently, sporulation capacity was assessed using a light microscope (magnification of ×40). The ascus wall was digested with 4 mg · ml−1 Zymolyase (Seikagaku, Japan) in suspension (dissolved in 2 M sorbitol), incubated for 3 min at room temperature. Tetrads were dissected using a micromanipulator (MSM 400; Singer Instruments, Watchet, United Kingdom) on YPD agar (2% [wt vol−1] Bacto peptone, 1% [wt vol−1] yeast extract, 2% [wt vol−1] glucose, and 2% [wt vol−1] agar).
Hybrid generation through spore-to-spore mating and confirmation of hybrid nature.
Sporulation was induced as described previously (22, 33). Spores were isolated en masse as described by Snoek and coworkers (33) and stored at −80°C in glycerol-yeast extract-peptone-dextrose (GYPD) medium (2% [wt vol−1] Bacto peptone, 1% [wt vol−1] yeast extract, 2% [wt vol−1] glucose, and 25% [wt vol−1] glycerol).
Hybridization was induced by placing single spores from both parental strains together with a micromanipulator (MSM; Singer Instruments) on YPD agar (2% [wt vol−1] Bacto peptone, 1% [wt vol−1] yeast extract, 2% [wt vol−1] glucose, and 1.5% [wt vol−1] agar), followed by visual inspection of zygote formation after 6 to 8 h of incubation at room temperature. Candidate interspecific hybrids were purified by streaking on synthetic 12 degrees Plato (°P) malt agar medium (12% [wt vol−1] synthetic malt extract [8EBC; Brouwland, Belgium] and 1.5% [wt vol−1] agar). Hybrids were confirmed through a species-specific multiplex PCR (see below). PCR-confirmed interspecific hybrids were streaked another three consecutive times on 12°P wort medium prior to long-term storage at −80°C to ensure strain purity.
Species-specific multiplex PCR.
Two primer pairs were used for the species-specific multiplex PCR, each targeting a specific part of one of the parental species' genome (34, 35). Primers Scer F2 (5′-GCG CTT TAC ATT CAG ATC CCG AG-3′) and Scer R2 (5′-TAA GTT GGT TGT CAG CAA GAT TG-3′) amplify a 150-bp amplicon of the S. cerevisiae genome. Primers Seub F3 (5′-GTC CCT GTA CCA ATT TAA TAT TGC GC-3′) and Seub R2 (5′-TTT CAC ATC TCT TAG TCT TTT CCA GAC G-3′) generate a 228-bp S. eubayanus-specific amplicon (see Fig. S1 in the supplemental material). The PCR conditions were as follows: 3 min at 95°C, 30 cycles of 30 s at 95°C, 30 s at 58°C, and 30 s of 72°C, followed by a final cycle of 5 min at 72°C and subsequent cooling to room temperature (RT). Candidate hybrids showing two bands were considered to be interspecific hybrids (see Fig. S1).
Amplified interdelta-sequence DNA polymorphism analysis.
Primers delta12 (5′-TCA ACA ATG GAA TCC CAA C-3′) and delta21 (5′-CAT CTT AAC ACC GTA TAT GA-3′) were used for interdelta-sequence DNA polymorphism analysis, as previously described by Legras and Karst (see Fig. S1) (36).
RAPD analysis.
Random amplified polymorphic DNA (RAPD) analysis was carried out by using the R3 primer (5′-ATG CAG CCA C-3′), as described previously (29, 37), using the following PCR protocol: 4 min at 94°C, 35 cycles of 25 s at 94°C, 30 s at 42°C, and 1.30 min at 72°C, followed by a final cycle of 5 min at 72°C and subsequent cooling to RT (see Fig. S1).
Flow cytometry.
Cells were grown overnight to stationary phase in 1 ml YPD medium at RT on a shaker set at 300 rpm. Next, cells were washed in 1 ml deionized water, resuspended in 1 ml 70% vol vol−1 ethanol, and incubated at 4°C for at least 16 h while rotating. The cultures were washed once with 500 μl SC buffer (50 mM trisodium citrate dihydrate; pH 7.4), resuspended in 1 ml SC buffer containing 0.25 mg · ml−1 RNase A (Thermo Fisher Scientific), and incubated for 1 h at 50°C, after which 50 μl proteinase K solution (20 mg · ml−1; Fisher Bioreagents) was added, followed by an additional incubation of 1 h at 50°C. Cell suspensions were washed once with 500 μl SC buffer and resuspended in 1 ml SC buffer. Propidium iodide (Sigma-Aldrich, Belgium) was added to a final concentration of 16 μg · ml−1, and cells were incubated overnight at 4°C while rotating to stain the DNA content of the cells. Finally, the cells were washed once with SC buffer and the DNA contents of single yeast cells were analyzed using a FACS system (BD Biosciences, Belgium). Analysis of the FACS data obtained, based on mixed Gaussian models, was performed in R (38).
Genetic stabilization of generated hybrids.
The interspecific hybrids obtained were subjected to a high-throughput genetic stabilization protocol. Each hybrid was individually inoculated into 750 μl industrial-grade high-glucose-content 12°P wort medium (provided by a Belgian brewery) in a 96-well deep-well plate and incubated statically at 16°C for 6 days. At this stage, cultures were diluted 10 times in 742.5 μl of fresh wort medium. This incubation/dilution procedure was repeated 12 times, and then the genetic stability of the hybrids was assessed by genetic fingerprinting PCRs of four biological replicates (interdelta-sequence DNA polymorphism analysis and R3-RAPD for four single-colony isolates, described above; see also Fig. S1 in the supplemental material) (29).
Laboratory-scale lager fermentations.
The fermentation protocol was designed to mimic industrial lager fermentations. First, yeast was propagated by inoculation into 5 ml 4% yeast extract-peptone-maltose (YPM; 2% [wt vol−1] Bacto peptone, 1% [wt vol−1] yeast extract, and 4% [wt vol−1] maltose) medium at RT and 300 rpm. After 16 h of incubation, 1 ml of the culture was transferred to 50 ml YPM (4%) medium in a 250-ml Erlenmeyer flask and incubated at 20°C and 200 rpm for 16 h. After this second propagation, the optical density at 600 nm (OD600) was measured and the pregrowth culture was used to inoculate 150 ml of industrial-grade wort medium (high-glucose-content 12°P wort, sparged with pressurized air for 1 h to saturation, provided by a Belgian brewery) to a starting OD600 of 0.3 (approximately 2.1 × 107 cells · ml−1). The 250-ml bottles were equipped with Ankom system gas monitors for online measurement of gas production (Ankom, USA). The headspace was flushed with nitrogen gas prior to incubation at 16°C. During fermentation, a constant overpressure of 5 × 104 Pa (to atmospheric pressure) was applied. CO2 production was monitored in real time with the Ankom systems for all fermentations, and fermentations were stopped when all strains stopped fermenting (after 13 days). Next, the fermentations were cooled on ice to prevent evaporation of the volatile compounds, and samples for chromatographic analysis and ethanol measurements were taken. The leftover fermented medium was used for sensory analysis.
Temperature tolerance assay.
Yeasts were propagated in 200 μl YPD for 16 h at 30°C (shaking at 900 rpm). Next, the yeast cultures were diluted to OD600 values of 1, 0.1, 0.01, and 0.001 in isotonic phosphate saline buffer. Subsequently, 5 μl amounts of the dilution series were spotted in biological duplicates on YPD agar plates using the Rotor HDA (Singer Instruments, United Kingdom) and incubated at eight different temperatures until sufficient growth could be observed, as follows: 4°C (15 days), 8°C (5 days), 10°C (5days), 16°C (2 days), 25°C (2 days), 30°C (2 days), 37°C (2 days), and 41°C (2 days). Plates were scanned and colony sizes were quantified using the ScreenMill software in ImageJ, as described in reference 39. Colony sizes at different temperatures were represented as Z-scores (calculated per strain and per temperature).
Determination of 4-VG production capacity.
Yeasts were grown for 48 h at 30°C in 5 ml 2% YPD medium to which 0.1 mg · ml−1 ferulic acid (Sigma-Aldrich, Belgium) was added. Production of 4-vinyl guaiacol (4-VG) was measured using head space gas chromatography with flame ionization detector.
Pilot-scale fermentation tests.
A commercial Belgian Pilsner malt was used to produce wort in the 5-hl pilot brewery of KU Leuven, Technology Campus Ghent. Eighty-seven kilograms of fine milled Pilsner malt (wet disc milling; Meura) was mixed with 1.91 hl deaerated, reverse-osmosis-filtered brewing water containing 80 mg · liter−1 CaCl2 and 30% vol vol−1 lactic acid (the precise volume to be added is malt dependent). Mashing-in occurred at 64°C and pH 5.3. The following brewing scheme was used: 64°C for 30 min, 72°C for 20 min, and 78°C for 1 min, with the rises in temperature at 1°C · min−1, after which the wort was filtered through a membrane-assisted thin-bed filter (Meura 2001) and sparged with 2.5 liter · kg−1 water (extract of last runnings 1.5°P and 1°P after final compression). The extract of the combined sweet wort was 14.5°P. The sweet wort was mixed with brewing water at the onset of boiling to obtain an extract content of 11.5°P. Wort boiling was conducted for 60 min at the atmospheric boiling point in a boiling kettle with internal boiler (∼5% evaporation). At the end of boiling, 0.2 mg · liter−1 Zn2+ ions was added, as well as 3.85 g · hl−1 iso-α-acid extract, aiming at 25 mg · liter−1 iso-α-acids in the finished beer (utilization, ∼65%). The wort was clarified in an open whirlpool as follows: filling for 6 min, 20 min of rest, and emptying in 20 min at 95°C. After cooling and aeration, the wort (12°P) was divided among ten 50-liter fermenters and 107 cells · ml−1 of the appropriate yeast strain was pitched on each. Starters were propagated at 25°C in 125 g · liter−1 wort extract (Brouwland, Belgium) until the cell titer was high enough for pitching. The duration of the primary fermentation at 12°C in cylindroconical tanks was strain dependent: fermentations were stopped if a minimal apparent degree of fermentation (ADF) of 72% was achieved or if the respective yeast stopped fermenting within 3 weeks after pitching. Fermentations that took longer than 3 weeks were omitted from further analysis. Green beer was matured for 10 days at −0.5°C in 50-liter beer kegs. The final beer was filtered using a cellulose sheet filter system (1-μm pore size) prior to CO2 saturation up to 5.6 g · liter−1 and packaging with a 6-head rotating counter-pressure filler (monobloc; Cimec, Italy), using double preevacuation with intermediate CO2 rinsing and overfoaming with hot water injection before capping (final oxygen levels below 50 ppb). Finished beers were sampled for chromatographic analysis and ethanol measurements. Also, a professional tasting panel assessed the different finished beers for their aroma, flavor, taste/mouthfeel, and overall impression.
Data analysis and data visualization.
To correct for noise, the headspace gas chromatography-flame ionization detector and temperature tolerance data obtained were converted to Z-scores as follows: Z-score = (X − μ)/σ, where X is the concentration measurement or colony size, μ is the mean value of all strains per measured component or temperature (column Z-scores) or mean colony growth per strain at each different temperature (row Z-scores), and σ is the standard deviation of values per tested aroma compound or per tested temperature of all strains or standard deviation per strain at the different temperatures (row Z-scores).
BioNumerics (Applied Maths, Belgium) was used to analyze and cluster the strains based on their phenotypes. A similarity matrix was built based on Euclidean distances, and an unweighted pair group method with arithmetic mean (UPGMA) algorithm was used for clustering.
The temperature tolerance data and aroma production in the laboratory-scale fermentation tests were statistically assessed using a Kruskal-Wallis one-way analysis of variance test (40) in combination with post hoc Dunn tests with false discovery rate (FDR) corrected P values, based on the Benjamini-Hochberg correction (41, 42). Statistical tests were performed in R (38).
RESULTS
To expand the genetic and phenotypic diversity of lager yeasts, we generated 31 new interspecific yeast hybrids between S. cerevisiae and S. eubayanus. These hybrids were assessed for their temperature tolerance and their fermentation capacity and aroma production in laboratory-scale fermentations. Subsequently, the fermentation performance of four selected hybrids was tested in a pilot-scale brewery.
Selection of parental strains for the interspecific hybrids.
Careful selection of optimal parental strains is vital for successful breeding experiments. Previously, our research group screened 301 industrial and wild strains from a large Saccharomyces yeast collection for different industrially relevant traits (22, 33, 43). Based on these data, six S. cerevisiae strains were selected using four selection criteria: production of a diverse aroma profile, high sporulation capacity, high spore viability, and efficient maltose fermentation (Fig. 1). Strains Y565 and Y567 were chosen as the S. eubayanus parental strains, since they were able to generate viable spores and also showed high tolerance toward cold temperatures (Fig. 2).
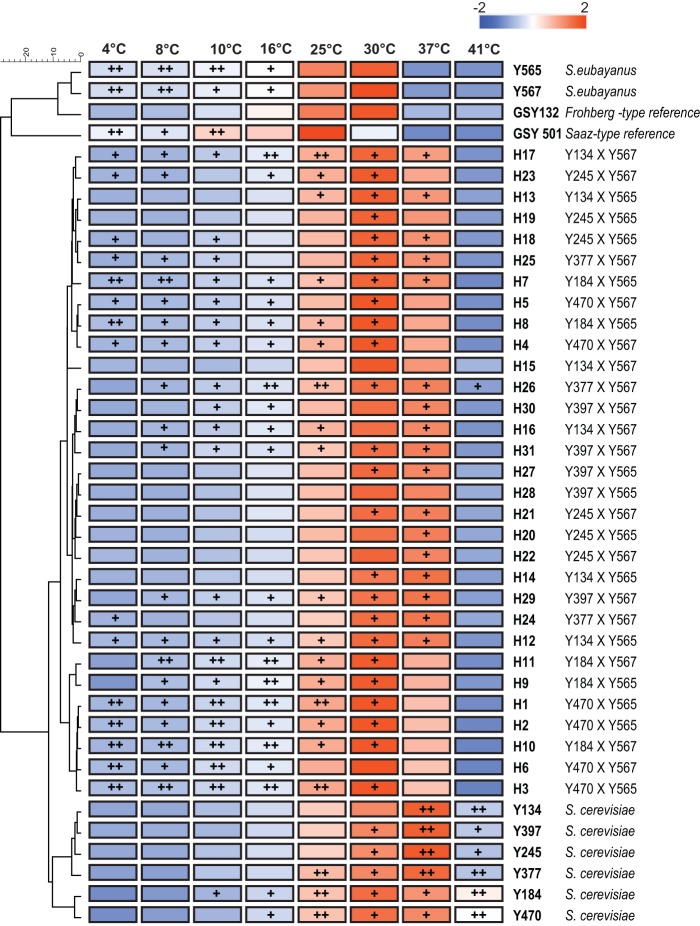
FIG 2.
Relative growth rates of the parental strains, the newly generated hybrids, and two commercial lager yeasts at different temperatures (4°C, 8°C, 10°C, 16°C, 30°C, 37°C, and 41°C). Growth rates were calculated as Z-scores. Pairwise similarities were calculated by Euclidean distance, and a UPGMA clustering algorithm was applied to cluster the data. Colors represent the calculated Z-score per strain (as shown in the key), with blue indicating a lower-than-average growth rate, white indicating an average growth rate, and red indicating a higher-than-average growth rate (calculated over the rows). Additionally, plus signs indicate whether a particular strain has a growth capacity that is better than the average growth capacity of all tested strains at a particular temperature (Z-score calculated over the columns; +, between zero and one; ++, above one). Note that some hybrids share the relatively high growth rate at lower temperatures of S. eubayanus with the capacity of S. cerevisiae to grow at temperatures of 37°C and above.
Development of interspecific yeast hybrids using spore-to-spore mating.
Previous studies indicate that the success rate of interspecific mass mating experiments is very low, likely because of the prezygotic barrier between species from the Saccharomyces genus (24, 29). To overcome this limitation, spore-to-spore mating was used to cross yeasts of different species (21). However, even this approach proved to be relatively inefficient, with 2,061 mating attempts yielding a total of 31 different interspecific hybrids (overall hybridization yield of 1.5%; hybrids were confirmed through the species-specific multiplex PCR developed here) (Table 2 and Materials and Methods; see also Fig. S1 in the supplemental material). Moreover, the mating efficiency seemed to depend on the parental strains: while mating Y134 with Y567 resulted in a hybridization yield of 3.85%, none of the 135 attempts to cross Y377 with Y565 yielded viable hybrids.
TABLE 2.
Overview of interspecific hybrid yeasts developed
| Ploidy | Strain | Result for hybridization with strain: |
|||
|---|---|---|---|---|---|
| Y565 |
Y567 |
||||
| No. of PCR-confirmed hybrids (designations) | Hybridization success rate (%) | No. of PCR-confirmed hybrids (designations) | Hybridization success rate (%) | ||
| Allodiploid | Y470 | 3 (H1 to H3) | 2.34 | 3 (H4 to H6) | 1.17 |
| Y184 | 3 (H7 to H9) | 2.56 | 2 (H10 to H11) | 3.33 | |
| Allotriploid | Y134 | 3 (H12 to H14) | 1.75 | 3 (H15 to H17) | 3.85 |
| Y245 | 3 (H18 to H20) | 2.22 | 3 (H21 to H23) | 1.93 | |
| Y377 | 0 | 0 | 3 (H24 to H26) | 1.39 | |
| Y397 | 2 (H27 to H28) | 1.23 | 3 (H29 to H31) | 2.47 | |
It is known that newly generated interspecific hybrids may show temporary genome instability, with several chromosomal rearrangements taking place in the first cell divisions after the hybridization event. Prior to phenotypic characterization, the genomes of the newly developed hybrids were therefore stabilized by growing interspecific hybrids for approximately 70 generations in industrial lager beer medium (see Materials and Methods for details). This number is shown to be sufficient to stabilize the genomes of newly formed interspecific yeast hybrids within the Saccharomyces genus (29–32). Genetic stability was confirmed by genetic fingerprinting, and hybrids were considered stable when, after stabilization, both the interdelta and R3-RAPD band patterns were identical for the four biological replicates tested (see Fig. S1 in the supplemental material).
Propidium iodide staining reveals ploidy differences within the interspecific hybrids generated.
Propidium iodide staining and flow cytometry revealed ploidy differences between the selected S. cerevisiae parental strains, which in turn led to ploidy differences within the interspecific hybrids that were developed (Table 2; see also Table S1 in the supplemental material). S. cerevisiae strains Y470 and Y184 showed DNA contents of, respectively, 1.55 and 1.77, indicating that these strains are likely aneuploid but are probably derived from a diploid strain. Therefore, mating the haploid segregants of these strains with haploid segregants from S. eubayanus resulted in allodiploid hybrids. However, the remaining four parental S. cerevisiae strains harbored larger genomes (>2n). Strain Y134 was (allo)triploid, whereas the genome sizes of Y245, Y377, and Y397 were similar to the genome size of the tetraploid reference strain (see Table S1). As expected, hybridization of (diploid) segregants of these strains with haploid S. eubayanus segregants yielded hybrids with an allotriploid genome. Interestingly, differences in genome size were observed between hybrids originating from the same parental strains. For example, the genome of hybrid H27 was 1.44 times larger than the genome of hybrid H28, even though both hybrids were the result of crossing the same two parental yeast strains, Y397 and Y565 (Table 2; see also Table S1). This phenomenon indicates that segregants from the same alloploid S. cerevisiae parent can differ in ploidy or that the genomes of interspecific hybrids originating from the same parents can stabilize differently, resulting in different genomic configurations, as reported in previous studies targeting interspecific hybrids (30, 32, 44, 45).
Newly developed interspecific hybrids show a broad temperature tolerance range.
The growth capacity of the developed hybrids was assessed at a wide range of different temperatures (4°C, 8°C, 10°C, 16°C, 25°C, 30°C, 37°C, and 41°C) and compared to the growth of their parental strains and two reference S. pastorianus strains (one Frohberg type [GSY132] and one Saaz type [GSY501]). These data revealed that temperature tolerance was species specific, with S. cerevisiae showing optimal growth at high temperatures (37°C and 41°C), while S. eubayanus (and the S. pastorianus reference strains) showed good performance at low temperatures but proved unable to grow at 37°C (Fig. 2).
At 4°C, almost all of the interspecific hybrids (except for H9, H15, and H28, which were not able to grow at this low temperature) showed a higher growth capacity than their corresponding S. cerevisiae parental strain (only the S. cerevisiae Y184 wine strain showed some growth at this temperature). The S. eubayanus parental strains and the Saaz-type reference S. pastorianus strain showed relatively good growth at this low temperature, confirming their cold-tolerant nature.
The growth capacity of the newly generated interspecific hybrids at 37°C resembled the growth capacity of their corresponding S. cerevisiae parental strains, while the reference S. pastorianus strains and the S. eubayanus parental strains were not able to grow under this condition. At 41°C, only the selected parental S. cerevisiae strains and hybrids H26 and H27 were able to grow.
Overall, the generated hybrids showed a significantly higher capacity for growth at low temperatures (4°C, 8°C, 10°C, and 16°C) than the selected S. cerevisiae parents and a higher capacity for growth at high temperatures (30°C and 37°C) than both S. eubayanus parents (Dunn test with Benjamini Hochberg-corrected P values of <0.05; see Table S2 in the supplemental material). These results confirm that interspecific mating between S. cerevisiae and S. eubayanus yeasts can generate hybrids that show a broad temperature tolerance, equipping them with a competitive advantage over the corresponding S. cerevisiae parental strain at low temperatures.
Interspecific hybrid yeasts outperform their parental strains in terms of fermentation capacity in lager fermentations.
Next, we assessed the potential of the new interspecific hybrids to produce aromatic lager beer. All 31 interspecific hybrids and their respective parental strains were tested in parallel laboratory-scale lager beer fermentations (see Materials and Methods for details). Seven commercial Saaz-type and 10 Frohberg-type S. pastorianus strains were included as reference strains.
To determine whether the strains had completed the beer fermentation, ethanol concentrations were measured at the end of fermentation. Ten of 11 allodiploid (90.9%) and 15 of 20 allotriploid interspecific hybrids (75.0%) were able to produce more ethanol than both their parental strains in a 13-day static fermentation process (Fig. 3; see also Table S1 in the supplemental material). On average, the hybrids showed 28.8% higher ethanol production than their corresponding best-performing parental strain, suggesting that the interspecific hybrids outperform their parental strains in terms of fermentation capacity under lager beer fermentation conditions (32).
FIG 3.
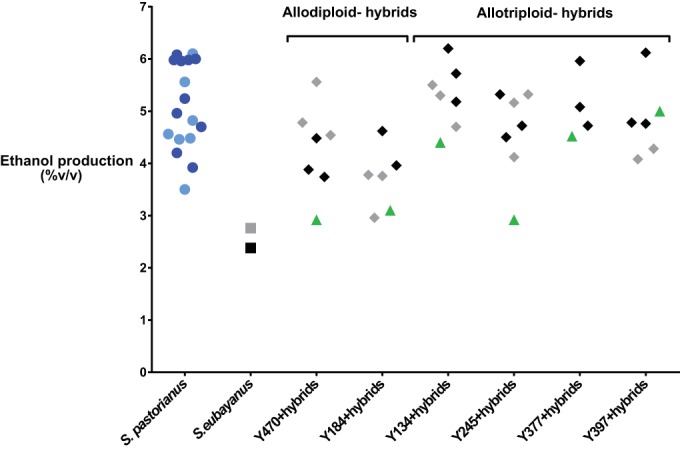
Ethanol production in laboratory-scale lager fermentations. The figure depicts the percentages (vol/vol) of ethanol produced by the different hybrids (black or gray diamonds; color corresponds to the corresponding S. eubayanus parental strain, described below), together with the results for their corresponding S. cerevisiae parental strain (green triangles), both S. eubayanus parental strains (Y565, gray square, and Y567, black square), and 7 Saaz-type (light blue circles) and 10 Frohberg-type (dark blue circles) reference S. pastorianus yeasts.
Moreover, the ethanol production by many of the new interspecific hybrids was similar to the concentrations obtained with the commercial reference S. pastorianus strains. Interestingly, three hybrids, H15, H29, and H27 (all hybrids from different S. cerevisiae parents crossed with Y567) showed an ethanol production capacity similar to that of the best reference S. pastorianus strains. Of these, H15 (Y134 × Y567) produced the highest final ethanol concentration (6.20% vol vol−1; see Table S1).
Interspecific hybrid yeasts show diversified aroma production in lager beer fermentations.
Both S. eubayanus parental strains produced a similar aroma profile, characterized by a relatively modest production of acetate and ethyl esters and higher concentrations of fusel alcohols (Fig. 4). The latter are often described as having alcoholic and solventlike aromas and are generally considered unpleasant in beer when present in high concentrations (46, 47). Additionally, sensorial analysis revealed the presence of strong sulfurlike off flavors in both S. eubayanus fermentations, which might be due to the fact that the beer was not maturated (lagered) (see Table S1 in the supplemental material). Interestingly, the pronounced aromatic diversity of the selected S. cerevisiae parental strains in ale fermentations is largely reduced when these strains are applied in lager beer fermentations, highlighting the strong influence of environmental parameters (medium composition, temperature, and agitation) on yeast aroma production. For instance, strain Y184 produced concentrations of aroma compounds similar to those of the S. eubayanus parental strains, whereas this yeast strain was one of the most aromatic strains in ale beer fermentations (Fig. 1). Strains Y134 and Y470 were characterized by an overall low aroma production. Strain Y245 (which, like Y184, showed a relative low fermentation capacity) produced larger amounts of acetate and ethyl esters and smaller amounts of undesirable higher alcohols, resulting in an overall fruity and pleasant beer. The aroma profile of Y377 highly resembled that of strain Y245, except for the production of isoamyl alcohol. Y397 was the only parental S. cerevisiae strain that was able to combine a good fermentation capacity with an overall high production of aroma compounds in these small-scale lager beer fermentations.
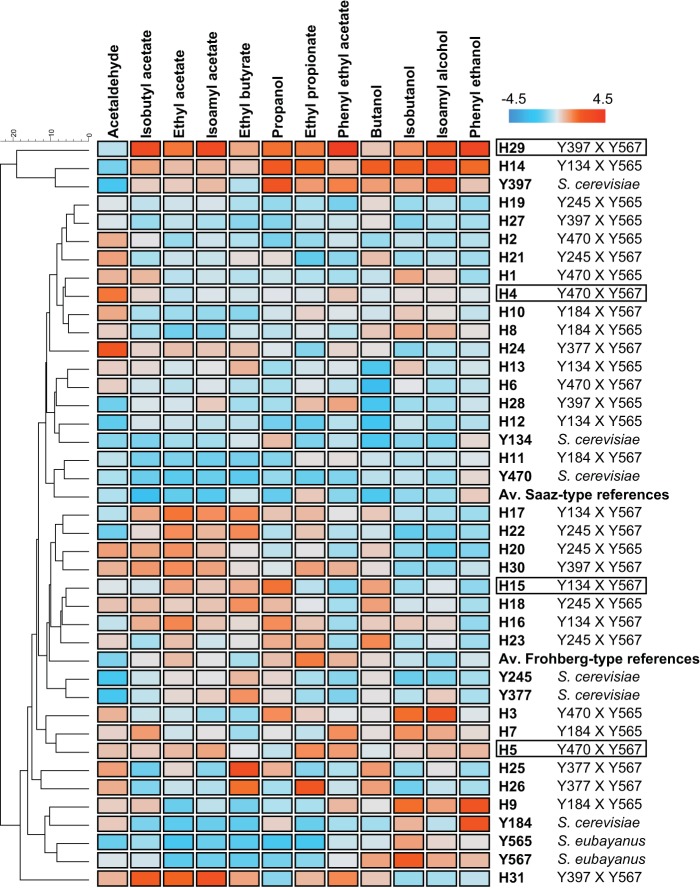
FIG 4.
Visual representation of the aroma production of the hybrids generated, together with the results for their corresponding parental strains and the average results for 7 Saaz-type and 10 Frohberg-type S. pastorianus strains. Colors represent the range of calculated Z-scores (calculated over the columns), with blue indicating lower-than-average production, white indicating average production, and red indicating higher-than-average production of the aroma compound in question. Pairwise similarities were calculated by Euclidean distance, and a UPGMA clustering algorithm was applied to cluster the data. The hybrids selected for further pilot-scale fermentation experiments are highlighted by boxes.
Interestingly, the newly developed interspecific hybrids produced widely diverse aroma profiles. For example strain H9 (Y184 × Y565) produced an aroma profile similar to those of the S. eubayanus parental strains, whereas other hybrids, like H31 (Y397 × Y567), produced higher concentrations of esters and lower concentrations of higher alcohols, reminiscent of some S. cerevisiae strains used in ale fermentations. Strain H29 (also Y397 × Y567) was not only one of the hybrids with the best fermentation capacity but was also characterized by a high production of the aromatic compounds that were quantified. Interestingly, hybrids from the same parents produced different aromas. For example, strain H14 turned out to be one of the most aromatic hybrids, whereas hybrid H12, which shares its parental strains with strain H14 (Y134 × Y565), showed an overall low aroma production.
In general, the hybrids produced significantly more isoamyl acetate (IA), a fruity aroma compound, than their respective parental strains. The IA levels were increased in 21 of the 31 hybrids (10/11 allodiploid and 11/21 allotriploid), resulting in lager beers with distinctive fruity banana and pineapple notes (see Table S1 in the supplemental material). Moreover, the isoamyl acetate concentrations obtained with some of the newly generated hybrids were also markedly higher than those obtained with the 17 reference S. pastorianus strains (Mann-Whitney U test, P < 0.05) (see Fig. S2). Strain H29 showed the highest IA production (2.66 mg · liter−1), which was 5.11 and 2.11 times more IA than the average of the Saaz-type and Frohberg-type reference S. pastorianus strains, respectively. Besides its high IA production, the production of ethyl acetate, which confers a solventlike flavor, was only slightly increased compared to that of the corresponding parents and still below its reported flavor threshold in lager beer (30 mg · liter−1) (46). Since this strain also showed a good fermentation capacity, it was earmarked as an interesting candidate for the production of highly aromatic lager beer.
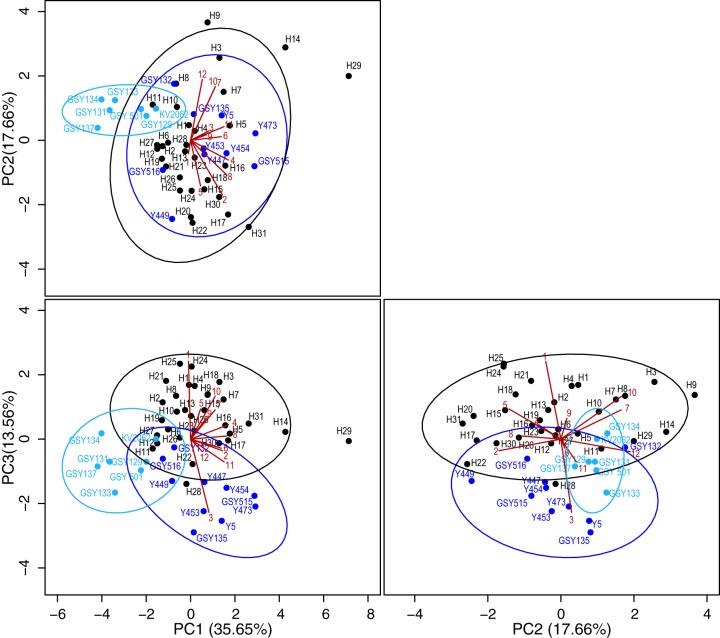
Principal component analysis (PCA) of all measured aroma compounds further revealed how the novel interspecific hybrids increased the aromatic diversity of lager beer altogether (Fig. 5). Plotting principal component 1 (PC1) versus PC2 revealed a clear separation in the aroma profiles of both types of reference S. pastorianus strains, with the Saaz-type reference strains showing a narrower aromatic diversity. Interestingly, the aromatic diversity of the new interspecific hybrids covers and expands the aromatic diversity of the more aromatic Frohberg-type reference S. pastorianus strains. Six hybrids (H3, H9, H14, H17, H29, and H31) were characterized by an aroma profile that was clearly differentiated from those of both types of reference S. pastorianus strains. More interestingly, plotting PC1 versus PC3 and PC2 versus PC3 revealed a clear difference in aroma production between the interspecific hybrids and the reference Frohberg-type S. pastorianus strains.
FIG 5.
Three two-dimensional (2-D) principal component analyses (PCA), visualizing the aromatic diversity introduced by the newly developed interspecific hybrids (black dots) and 7 reference Saaz-type (light blue dots) and 10 reference Frohberg-type (dark blue dots) S. pastorianus strains. Ninety-percent confidence ellipses are drawn in the corresponding colors. The component scores for the 12 aroma compounds are depicted as red lines numbered as follows: 1, acetaldehyde; 2, ethyl acetate; 3, ethyl propionate; 4, isobutyl acetate; 5, ethyl butyrate; 6, propanol; 7, isobutanol; 8, isoamyl acetate; 9, butanol; 10, isoamyl alcohol; 11, phenylethyl acetate; 12, phenyl ethanol. The three 2-D PCA plots represent 66.87% of the total variance of the data set.
The differences in aroma production were also confirmed by statistical analyses. Except for isobutanol and phenyl ethanol, the Kruskal-Wallis one-way analysis of variance test indicated stochastic dominance for the production of the 10 remaining aroma compounds for at least one of the three groups (P < 0.05; see Table S3 in the supplemental material), indicating significant differences between the lowest and highest median production levels of the three groups for these compounds.
Furthermore, nonparametric post hoc analysis revealed that Frohberg-type reference S. pastorianus strains produced significantly higher concentrations of acetate esters, such as ethyl acetate, isobutyl acetate, isoamyl acetate, and phenyl-ethyl acetate, than the Saaz-type S. pastorianus reference strains, which is in line with the previous findings of Gibson and coworkers (19) and Walther et al. (20).
The interspecific hybrids in their turn produced significantly more acetaldehyde, ethyl butyrate, and isoamyl alcohol and significant less ethyl propionate than the Frohberg-type reference S. pastorianus strains (Dunn test with FDR-corrected P value, <0.01; see Table S3 in the supplemental material). Interspecific hybrids were also significantly more aromatic than the Saaz-type reference S. pastorianus strains, showing a higher production of esters like isoamyl acetate, isobutyl acetate, ethyl acetate, and phenyl ethyl acetate. Additionally, they also produced significantly more fusel alcohols, such as isoamyl alcohol, propanol, and butanol.
Finally, the ability of the strains to produce 4-VG, a compound associated with a phenolic or smoky flavor, was investigated (see Materials and Methods for details). It was shown that all of the hybrids, as well as the S. eubayanus strains and 3/6 of the S. cerevisiae parents were able to produce this compound (see Table S1 in the supplemental material). While production of this compound is generally undesired in lager beers, it was not detected sensorially in the small-scale lager fermentations.
Pilot-scale fermentation confirms the potential of new hybrids for commercial production of lager beer with a distinct aromatic profile.
To assess the industrial applicability of the interspecific hybrids generated, the performance of four hybrids (H4, H5, H15, and H29) was tested in 50-liter pilot-scale fermentations and compared to the performance of their corresponding parental strains and two reference commercial S. pastorianus strains (GSY132 and GSY501). These four hybrids were selected based on their ploidy (two allodiploid hybrids and two allotriploid hybrids were included), their fermentation efficiency, and desirable aroma production in the laboratory-scale fermentation experiments (Fig. 4 and 5).
Unfortunately, 4 of the 10 yeast strains tested (S. eubayanus Y567 and hybrids H4 and H5, as well as the selected Frohberg-type reference S. pastorianus strain GSY132) yielded stuck fermentations and were unable to finish the fermentation within 3 weeks after pitching. These strains were therefore omitted from further analysis.
After fermentation and 10 days of cold storage at −0.5°C (lagering), the remaining six beers that were produced showed ethanol contents ranging from 4.93 to 5.19% vol vol−1, with hybrid H29 producing the highest ethanol concentration (Table 3). The difference in ethanol production between strains was largely due to the inability of some strains to efficiently ferment the maltotriose present in the wort. Strains producing less than 5% alcohol by volume (ABV) ethanol only fermented 50% (Y134, Y470, and GSY501) or 60% (H15) of the maltotriose, whereas strains producing more than 5% ABV (Y397 and H29) fermented up to 70% of the maltotriose (Table 3).
TABLE 3.
Overview of fermentation capacity, aroma production, and sugar consumption during pilot-scale fermentation tests
| Parameter | Value for strainb: |
|||||
|---|---|---|---|---|---|---|
| GSY501 | Y134 | Y397 | Y470 | H15 | H29 | |
| Fermentation capacity | ||||||
| Fermentation time (days) | 16 | 7 | 8 | 16 | 8 | 7 |
| Ethanol production (% ABV) | 4.93 | 4.94 | 5.13 | 4.92 | 4.98 | 5.19 |
| Sugar consumption (%) | ||||||
| Glucose | 98.3 | 100.0 | 100.0 | 100.0 | 98.7 | 100.0 |
| Maltose | 98.6 | 98.9 | 98.9 | 98.8 | 98.5 | 98.8 |
| Maltotriose | 50.8 | 53.5 | 69.1 | 53.9 | 59.0 | 70.6 |
| Maltotetraose | 25.1 | 25.9 | 28.6 | 28.0 | 22.4 | 25.6 |
| Maltopentaose | 20.6 | 24.2 | 27.3 | 25.6 | 30.0 | 22.2 |
| Maltohexaose | 18.4 | 31.0 | 27.6 | 27.9 | 32.5 | 27.1 |
| Maltoheptaose | 39.8 | 42.1 | 42.9 | 38.5 | 41.8 | 41.4 |
| Aroma production (mg · liter−1a) | ||||||
| Acetaldehyde (10) | 11.01 | 2.58 | 4.29 | 4.29 | 3.64 | 1.42 |
| Ethyl acetate (30) | 16.23 | 34.22 | 36.34 | 30.83 | 38.59 | 30.21 |
| Ethyl propionate (10) | 0.32 | 0.28 | 0.37 | 0.39 | 0.28 | 0.42 |
| Isobutyl acetate (1.6) | 0.08 | 0.22 | 0.33 | 0.20 | 0.18 | 0.24 |
| Ethyl butyrate (0.5) | 0.24 | 0.21 | 0.19 | 0.13 | 0.19 | 0.17 |
| Propanol (800) | 4.97 | 13.13 | 12.81 | 12.97 | 12.75 | 11.69 |
| Isobutanol (65) | 8.51 | 11.56 | 16.23 | 10.66 | 12.21 | 16.06 |
| Isoamyl acetate (1.2) | 1.56 | 4.08 | 5.45 | 3.81 | 2.78 | 3.90 |
| Butanol (50) | ND | 0.20 | 0.21 | ND | 0.19 | 0.21 |
| Isoamyl alcohol (70) | 33.29 | 54.66 | 72.14 | 49.44 | 51.84 | 76.27 |
| Phenyl ethyl acetate (3) | 2.44 | 3.17 | 5.56 | 2.69 | 3.20 | 4.06 |
| Phenyl ethanol (100) | 25.72 | 12.51 | 36.32 | 14.00 | 23.93 | 20.13 |
Fermentations conducted with strains GSY132, Y567, H4, and H5 were not finished within 3 weeks and were therefore omitted from further analysis. Values are the averages of two independent measurements. Values in boldface are those higher than the reported flavor threshold in lager beer. ND, not determined.
Sensory analysis by an independent tasting panel of the beer that was produced showed that the use of non-S. pastorianus strains increased the aromatic diversity and complexity of lager beers (see Table S4 in the supplemental material for an overview of the sensory results). The reference Saaz-type S. pastorianus strain GSY501 produced a lager beer with clear fruity and pineapplelike aromatic notes and was well appreciated by the panel (see Table S4). This fruity character of GSY501 is likely due to the relatively high concentration of isoamyl acetate.
S. cerevisiae parental strains produced overall complex and aromatic lager beers, characterized by a high production of isoamyl acetate and ethyl acetate (Table 3), with fruity notes in the aroma and/or taste. In addition to these aromas, strain Y470 introduced a slightly grainy and grassy aroma and taste into the beer, which was well appreciated by the panel. Strains Y134 and Y397 produced slightly sulfury notes and an onion and sulfury off flavor, respectively, in the beers.
Strain H15 showed a fermentation capacity and production of aroma compounds similar to those of its S. cerevisiae parent Y134 but with a slightly lower isoamyl acetate production (2.78 versus 4.08 mg · liter−1). The panel described this beer as having a slightly grainy aroma and grainy taste, characterized by slightly sulfury and metallic notes, resulting in an overall undesirable aroma.
Hybrid H29 displayed a faster fermentation and higher attenuation (i.e., higher final ethanol concentration) than its corresponding S. cerevisiae parental strain (Y397) and the reference S. pastorianus yeast GSY501. Sensory analysis of the beer produced with this strain revealed a complex, fruity aroma profile. Indeed, chemical analysis revealed that strain H29 produced high concentrations of isoamyl acetate, ethyl acetate, isoamyl alcohol, and phenyl-ethyl acetate, well above the respective flavor thresholds of 1.2, 30, 70, and 3 mg · liter−1. Therefore, despite the very slightly sulfury notes detected, the beer produced with this strain was highly rated by the test panel. Another interesting thing to note is the low acetaldehyde production of this strain compared to the acetaldehyde production of its corresponding parental strain Y397 and reference strain GSY501, which might increase the stability of the beer (48).
DISCUSSION
Over the past decades, the beer industry has been increasingly dominated by fewer firms (e.g., in 2012, 50% of the beer sales and 70% of the revenues were accounted for by only four breweries) (49). However, recent years have brought a remarkable increase in the demand for specialty beer, turning the global beer market into a niche market where product diversification has become pivotal. Despite the fact that the clean flavor and aroma of lager beer still remains an important characteristic, new lager yeasts that can introduce aromatic diversity into Pilsner-type beers could be of considerable industrial importance and provide opportunities for breweries to expand their market share and diversify their product portfolio to fulfill the customer's demands. Moreover, the interspecific hybrids generated here can also be used to create a new niche beer market of aromatic, low-alcohol but still highly drinkable beers.
In this study, we generated 31 new interspecific hybrids through spore-to-spore mating of six carefully selected S. cerevisiae strain and two S. eubayanus strains. The overall yield of 1.5% obtained with the spore-to-spore mating technique is significantly higher than the yield obtained with mass mating approaches (e.g., the hybridization frequency of 2.6 × 10−6 reported by Krogerus and coworkers [24]). Another advantage of the spore-to-spore approach is that no auxotrophic mutants of the parental strains needed to be obtained prior to mating (24, 29). Moreover, because the experimental procedure only relies on natural mating and not on genetic modification, the interspecific hybrids generated are not considered to be genetically modified (GM) organisms and can be used by the beverage industry without restrictions.
Interestingly, none of the 135 mating attempts between Y377 and Y565 yielded interspecific hybrids, indicating that some strains within the Saccharomyces genus are less prone to mate (50) and/or show more efficient prezygotic or postzygotic barriers due to strain-specific (and not species-specific) differences in spore germination timing (51, 52). Further research is needed to elucidate the underlying genetic base of this phenomenon.
Previous research showed that newly formed interspecific hybrid genomes are characterized by high plasticity and that genome rearrangements, such as whole or partial chromosome losses and introgressions, are common and can be directed by the environmental stress conditions applied (30, 31, 44, 45). For example Dunn and coworkers (30) showed that newly formed interspecific hybrids between S. cerevisiae and S. uvarum exhibited a characteristic genome rearrangement pattern when hybrids were grown under continuous ammonium limitations (30). The work of Piotrowski et al. (31) supports the hypothesis that the genomic fate of new interspecific hybrids depends on the stress they encounter in their immediate environment (31). Especially in lager production, where it is common practice to recycle yeast for several consecutive fermentation cycles (53, 54), it is paramount that these strains remain genetically stable during the whole process. Therefore, genetic stabilization, based on vegetative growth under specific conditions mimicking typical lager beer fermentations, was a crucial step in the development of our strains (29).
The temperature tolerance profiles of the interspecific hybrids generated here are in line with the results of Bellon and coworkers, who discovered a similar trend in interspecific hybrids between S. cerevisiae and the cold-tolerant S. mikatae (28). Our results also line up with the recent findings of Hebly and coworkers, where one interspecific hybrid between S. cerevisiae and S. eubayanus showed a temperature range similar to those of most of our interspecific hybrids (55). However, despite the industrial importance of cold tolerance, relatively little is known about the underlying molecular mechanisms. Therefore, the interspecific hybrids obtained in this study could be of use to further investigate the underlying genetic mechanisms of the observed cold tolerance.
In addition, our interspecific hybrids also showed interesting fermentation characteristics in laboratory-scale lager beer fermentation experiments. On average, the interspecific hybrids produced more ethanol than their corresponding best parental strain. These results are in line with the work of Pérez-Través and coworkers and, more recently, Krogerus et al. in which interspecific hybridization between S. cerevisiae and cold-tolerant yeast species like S. kudriavzevii and S. eubayanus yielded variants able to outperform their parental strains in wine and beer fermentations at low temperatures (24, 29).
However, not all of the hybrids developed were able to outperform their respective parental strains in lager beer fermentations. This can be explained by a potential downside of the applied breeding strategy (spore-to-spore mating), where random haploid segregants of the parents are applied in the breeding experiments. Since industrial strains are often heterozygous, allelic segregation will cause great diversity within the haploid segregants originating from the same parent, some of which might be inferior in the desired characteristics (21). Other breeding techniques, such as cell-to-cell mating, allow phenotyping of the haploid segregants prior to breeding, but due to the homothallic nature of the S. eubayanus parents, the cell-to-cell mating technique was not applicable in this work.
Our results support the current hypothesis about the origin of lager yeasts, which states that the combination of the cold tolerance of S. eubayanus and the good fermentation capacity of S. cerevisiae provided the interspecific hybrids with a competitive advantage in ancient Bavarian lager beer production processes, which were typically conducted at very low temperatures (7, 13, 16, 55).
Moreover, some of the interspecific hybrids developed here (e.g., H15, H29, and H27) showed a higher fermentation efficiency at 16°C than the best reference S. pastorianus strains that are currently used for commercial production, and others (e.g., H29, which also showed the fastest fermentation, and H15) showed higher fermentation efficiency in pilot-scale lager beer fermentation tests conducted at 12°C. The use of these interspecific hybrids in industrial lager beer production could potentially lead to a shortened fermentation time and, consequently, lead to higher profits.
Gibson and Liti (7) and, more recently, Krogerus and coworkers (24) hypothesized that the development of interspecific hybrids between S. cerevisiae and S. eubayanus could lead to aromatic diversity in lager beer production. This study provides the first proof for this hypothesis, showing that newly developed interspecific hybrids produced aromatic profiles that were significantly different from the aroma production of currently available lager yeasts, making them interesting new yeast strains for commercial lager beer production.
For example, strain H29, a hybrid of the ale-type S. cerevisiae Y397 and S. eubayanus Y567, outperformed its parents with respect to its fermentation capacity and production of fruity flavors. This strain also performed extremely well at pilot scale in a 50-liter lager beer fermentation at 12°C, where it exhibited the fastest fermentation profile and reached the highest final ethanol titer. The resulting beer showed a complex fruity aroma and was highly appreciated by a trained, independent expert panel, further highlighting its industrial potential for the production of aromatic lager beer.
An interesting observation was the presence of smoky flavors in some but not all hybrids and parental strains in pilot-scale fermentation experiments. This sensorial attribute, typically associated with the presence of 4-VG, is an important factor in beer brewing and is often negatively perceived in the beer industry (and therefore also called “phenolic off flavor” [POF]). Interestingly, our experiments indicated that while all of the hybrids we developed and most of the parental strains have the intrinsic potential to produce 4-VG (see Table S1 in the supplemental material), it was only perceived in the products of two pilot-scale fermentations (H4 and S. eubayanus strain Y567). The strains for which no smoky flavor was detected but that do possess the potential to produce 4-VG (hybrids H5, H15, and H29, as well as S. cerevisiae parent strain Y397) most probably produce this compound below the threshold level in Pilsner beer (300 ppb) (56), or it is masked by other flavor attributes, such as fruitiness.
In conclusion, we describe the development of 31 novel interspecific yeast hybrids, resulting from large-scale breeding experiments between six carefully selected S. cerevisiae strains and two feral S. eubayanus strains. The best newly generated hybrids showed growth at a broader range of temperatures, high fermentation capacity in laboratory-scale lager beer fermentations, and desirable aromatic profiles that were significantly different from the profiles produced by the currently applied lager yeasts. Importantly, these industrially interesting characteristics were also confirmed in pilot-scale trials, with hybrid H29 showing perhaps the most interesting profile, due to its combination of a fast fermentation, high attenuation (i.e., higher final ethanol concentration), and the production of a complex, desirable fruity aroma palate.
Supplementary Material
ACKNOWLEDGMENTS
We thank all Verstrepen laboratory members for their help and suggestions.
Research in the laboratory of K.J.V. is supported by Vlaams Instituut voor Biotechnologie (VIB), European Molecular Biology Organization Young Investigator Programme (EMBO YIP), InBev-Baillet Latour Fonds, European Research Council (ERC) starting grant 241426, Human Frontier Science Program (HFSP) grant RGP0050/2013, Fonds Wetenschappelijke Onderzoeks (FWO) grants G.12491.14N, G.0C59.14N, and G.0910.08, and Agentschap voor Innovatie door Wetenschap en Technologie (IWT) grants 100697 and 131210.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.02464-15.
REFERENCES
- 1.Statistica. 2014. Beer production worldwide from 1998 to 2014 (in billions of hectoliters). Statistica, New York, NY. [Google Scholar]
- 2.Kodama Y, Kielland-Brandt MC, Hansen J. 2006. Lager brewing yeast. Top Curr Genet 15:145–164. doi: 10.1007/b106370. [DOI] [Google Scholar]
- 3.Hornsey IS. 2003. A history of beer and brewing, 1st ed The Royal Society of Chemistry, Cambridge, United Kingdom. [Google Scholar]
- 4.Sicard D, Legras JL. 2011. Bread, beer and wine: yeast domestication in the Saccharomyces sensu stricto complex. C R Biol 334:229–236. doi: 10.1016/j.crvi.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 5.Preedy VR. 2009. Beer in health and disease prevention, 1st ed Elsevier, Burlington, MA. [Google Scholar]
- 6.Dequin S, Casaregola S. 2011. The genomes of fermentative Saccharomyces. C R Biol 334:687–693. doi: 10.1016/j.crvi.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 7.Gibson B, Liti G. 2015. Saccharomyces pastorianus: genomic insights inspiring innovation for industry. Yeast 28:17–27. doi: 10.1002/yea.3033. [DOI] [PubMed] [Google Scholar]
- 8.Vaughan Martini A, Kurtzman CP. 1985. Deoxyribonucleic acid relatedness among species of the genus Saccharomyces sensu stricto. Int J Syst Bacteriol 35:508–511. doi: 10.1099/00207713-35-4-508. [DOI] [Google Scholar]
- 9.Tamai Y, Momma T, Yoshimoto H, Kaneko Y. 1998. Co-existence of two types of chromosome in the bottom fermenting yeast, Saccharomyces pastorianus. Yeast 14:923–933. doi:. [DOI] [PubMed] [Google Scholar]
- 10.Nilsson-Tillgren T, Gjermansen C, Kielland-Brandt MC, Petersen JGL, Holmberg S. 1981. Genetic differences between Saccharomyces carlsbergensis and S. cerevisiae. Analysis of chromosome III by single chromosome transfer. Carlsberg Res Commun 46:65–76. doi: 10.1007/BF02906199. [DOI] [Google Scholar]
- 11.Libkind D, Hittinger CT, Valério E, Gonçalves C, Dover J, Johnston M, Gonçalves P, Sampaio JP. 2011. Microbe domestication and the identification of the wild genetic stock of lager-brewing yeast. Proc Natl Acad Sci U S A 108:14539–14544. doi: 10.1073/pnas.1105430108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peris D, Sylvester K, Libkind D, Gonçalves P, Sampaio JP, Alexander WG, Hittinger CT. 2014. Population structure and reticulate evolution of Saccharomyces eubayanus and its lager-brewing hybrids. Mol Ecol 23:2031–2045. doi: 10.1111/mec.12702. [DOI] [PubMed] [Google Scholar]
- 13.Bing J, Han P-J, Liu W-Q, Wang Q-M, Bai F-Y. 2014. Evidence for a Far East Asian origin of lager beer yeast. Curr Biol 24:R380–R381. doi: 10.1016/j.cub.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 14.Baker E, Wang B, Bellora N, Peris D, Hulfachor AB, Koshalek JA, Adams M, Libkind D, Hittinger CT. 11 August 2015. The genome sequence of Saccharomyces eubayanus and the domestication of lager-brewing yeasts. Mol Biol Evol. doi: 10.1093/molbev/msv168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sato M, Kishimoto M, Watari J, Takashio M. 2002. Breeding of brewer's yeast by hybridization between a top-fermenting yeast Saccharomyces cerevisiae and a cryophilic yeast Saccharomyces bayanus. J Biosci Bioeng 93:509–511. doi: 10.1016/S1389-1723(02)80101-3. [DOI] [PubMed] [Google Scholar]
- 16.Dunn B, Sherlock G. 2008. Reconstruction of the genome origins and evolution of the hybrid lager yeast Saccharomyces pastorianus. Genome Res 18:1610–1623. doi: 10.1101/gr.076075.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liti G, Peruffo A, James SA, Roberts IN, Louis EJ. 2005. Inferences of evolutionary relationships from a population survey of LTR-retrotransposons and telomeric-associated sequences in the Saccharomyces sensu stricto complex. Yeast 22:177–192. doi: 10.1002/yea.1200. [DOI] [PubMed] [Google Scholar]
- 18.Monerawela C, James TC, Wolfe KH, Bond U. 2015. Loss of lager specific genes and subtelomeric regions define two different Saccharomyces cerevisiae lineages for Saccharomyces pastorianus group I and II strains. FEMS Yeast Res 15:fou008. doi: 10.1093/femsyr/fou008. [DOI] [PubMed] [Google Scholar]
- 19.Gibson BR, Storgårds E, Krogerus K, Vidgren V. 2013. Comparative physiology and fermentation performance of Saaz and Frohberg lager yeast strains and the parental species Saccharomyces eubayanus. Yeast 30:255–266. doi: 10.1002/yea.2960. [DOI] [PubMed] [Google Scholar]
- 20.Walther A, Hesselbart A, Wendland J. 2014. Genome sequence of Saccharomyces carlsbergensis, the world's first pure culture lager yeast. G3 (Bethesda) 4:783–793. doi: 10.1534/g3.113.010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steensels J, Snoek T, Meersman E, Nicolino MP, Voordeckers K, Verstrepen KJ. 2014. Improving industrial yeast strains: exploiting natural and artificial diversity. FEMS Microbiol Rev 38:947–995. doi: 10.1111/1574-6976.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steensels J, Meersman E, Snoek T, Saels V, Verstrepen KJ. 2014. Large-scale selection and breeding to generate industrial yeasts with superior aroma production. Appl Environ Microbiol 80:6965–6975. doi: 10.1128/AEM.02235-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steensels J, Verstrepen KJ. 2014. Taming wild yeast: potential of conventional and nonconventional yeasts in industrial fermentations. Annu Rev Microbiol 68:61–80. doi: 10.1146/annurev-micro-091213-113025. [DOI] [PubMed] [Google Scholar]
- 24.Krogerus K, Magalhães F, Vidgren V, Gibson B. 2015. New lager yeast strains generated by interspecific hybridization. J Ind Microbiol Biotechnol 42:769–778. doi: 10.1007/s10295-015-1597-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sebastiani F, Barberio C, Casalone E, Cavalieri D, Polsinelli M. 2002. Crosses between Saccharomyces cerevisiae and Saccharomyces bayanus generate fertile hybrids. Res Microbiol 153:53–58. doi: 10.1016/S0923-2508(01)01286-4. [DOI] [PubMed] [Google Scholar]
- 26.Marinoni G, Manuel M, Petersen RF, Hvidtfeldt J, Sulo P, Piskur J. 1999. Horizontal transfer of genetic material among Saccharomyces yeasts. J Bacteriol 181:6488–6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Serra A, Strehaiano P, Taillandier P. 2005. Influence of temperature and pH on Saccharomyces bayanus var. uvarum growth; impact of a wine yeast interspecific hybridization on these parameters. Int J Food Microbiol 104:257–265. doi: 10.1016/j.ijfoodmicro.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 28.Bellon JR, Eglinton JM, Siebert TE, Pollnitz AP, Rose L, De Barros Lopes M, Chambers PJ. 2011. Newly generated interspecific wine yeast hybrids introduce flavour and aroma diversity to wines. Appl Microbiol Biotechnol 91:603–612. doi: 10.1007/s00253-011-3294-3. [DOI] [PubMed] [Google Scholar]
- 29.Pérez-Través L, Lopes CA, Barrio E, Querol A. 2012. Evaluation of different genetic procedures for the generation of artificial hybrids in Saccharomyces genus for winemaking. Int J Food Microbiol 156:102–111. doi: 10.1016/j.ijfoodmicro.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 30.Dunn B, Paulish T, Stanbery A, Piotrowski J, Koniges G, Kroll E, Louis EJ, Liti G, Sherlock G, Rosenzweig F. 2013. Recurrent rearrangement during adaptive evolution in an interspecific yeast hybrid suggests a model for rapid introgression. PLoS Genet 9:e1003366. doi: 10.1371/journal.pgen.1003366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piotrowski JS, Nagarajan S, Kroll E, Stanbery A, Chiotti KE, Kruckeberg AL, Dunn B, Sherlock G, Rosenzweig F. 2012. Different selective pressures lead to different genomic outcomes as newly-formed hybrid yeasts evolve. BMC Evol Biol 12:46. doi: 10.1186/1471-2148-12-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bellon JR, Schmid F, Capone DL, Dunn BL, Chambers PJ. 2013. Introducing a new breed of wine yeast: interspecific hybridisation between a commercial Saccharomyces cerevisiae wine yeast and Saccharomyces mikatae. PLoS One 8:e62053. doi: 10.1371/journal.pone.0062053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Snoek T, Picca Nicolino M, Van den Bremt S, Mertens S, Saels V, Verplaetse A, Steensels J, Verstrepen KJ. 2015. Large-scale robot-assisted genome shuffling yields industrial Saccharomyces cerevisiae yeasts with increased ethanol tolerance. Biotechnol Biofuels 8:32. doi: 10.1186/s13068-015-0216-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pengelly RJ, Wheals AE. 2013. Rapid identification of Saccharomyces eubayanus and its hybrids. FEMS Yeast Res 13:156–161. doi: 10.1111/1567-1364.12018. [DOI] [PubMed] [Google Scholar]
- 35.Muir A, Harrison E, Wheals A. 2011. A multiplex set of species-specific primers for rapid identification of members of the genus Saccharomyces. FEMS Yeast Res 11:552–563. doi: 10.1111/j.1567-1364.2011.00745.x. [DOI] [PubMed] [Google Scholar]
- 36.Legras JL, Karst F. 2003. Optimisation of interdelta analysis for Saccharomyces cerevisiae strain characterisation. FEMS Microbiol Lett 221:249–255. doi: 10.1016/S0378-1097(03)00205-2. [DOI] [PubMed] [Google Scholar]
- 37.Corte L, Lattanzi M, Buzzini P, Bolano A, Fatichenti F, Cardinali G. 2005. Use of RAPD and killer toxin sensitivity in Saccharomyces cerevisiae strain typing. J Appl Microbiol 99:609–617. doi: 10.1111/j.1365-2672.2005.02631.x. [DOI] [PubMed] [Google Scholar]
- 38.R Development Core Team. 2013. R: a language and environment for statistical computing. R Foundation for Statistical Computing. http://www.r-project.org/.
- 39.Dittmar JC, Reid RJ, Rothstein R. 2010. ScreenMill: a freely available software suite for growth measurement, analysis and visualization of high-throughput screen data. BMC Bioinformatics 11:353. doi: 10.1186/1471-2105-11-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kruskal WH, Wallis WA. 1952. Use of ranks in one-criterion variance analysis. J Am Stat Assoc 47:583–621. doi: 10.1080/01621459.1952.10483441. [DOI] [Google Scholar]
- 41.Dunn OJ. 1964. Multiple comparisons using rank sums. Technometrics 6:241–252. doi: 10.1080/00401706.1964.10490181. [DOI] [Google Scholar]
- 42.Benjamini YY, Daniel. 2001. The control of the false discovery rate in multiple testing under dependency. Ann Stat 29:1165–1188. doi: 10.1214/aos/1013699998. [DOI] [Google Scholar]
- 43.Mukherjee V, Steensels J, Lievens B, Van de Voorde I, Verplaetse A, Aerts G, Willems KA, Thevelein JM, Verstrepen KJ, Ruyters S. 2014. Phenotypic evaluation of natural and industrial Saccharomyces yeasts for different traits desirable in industrial bioethanol production. Appl Microbiol Biotechnol 98:9483–9498. doi: 10.1007/s00253-014-6090-z. [DOI] [PubMed] [Google Scholar]
- 44.Kunicka-Styczyńska A, Rajkowska K. 2011. Physiological and genetic stability of hybrids of industrial wine yeasts Saccharomyces sensu stricto complex. J Appl Microbiol 110:1538–1549. doi: 10.1111/j.1365-2672.2011.05009.x. [DOI] [PubMed] [Google Scholar]
- 45.Morales L, Dujon B. 2012. Evolutionary role of interspecies hybridization and genetic exchanges in yeasts. Microbiol Mol Biol Rev 76:721–739. doi: 10.1128/MMBR.00022-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meilgaard MC. 1982. Prediction of flavor differences between beers from their chemical composition. J Agric Food Chem 30:1009–1017. doi: 10.1021/jf00114a002. [DOI] [Google Scholar]
- 47.Harrison GAF. 1970. The flavour of beer—a review. J Inst Brew 76:486–495. doi: 10.1002/j.2050-0416.1970.tb03333.x. [DOI] [Google Scholar]
- 48.Saison D, De Schutter DP, Uyttenhove B, Delvaux F, Delvaux FR. 2009. Contribution of staling compounds to the aged flavour of lager beer by studying their flavour thresholds. Food Chem 114:1206–1215. doi: 10.1016/j.foodchem.2008.10.078. [DOI] [Google Scholar]
- 49.Howard PH. 2014. To big to ale? Globalization and consolidation in the beer industry, p 155–165. In Patterson MW, Hoalst-Pullen N (ed), The geography of beer: regions, environment, and societies. Springer, Dordrecht, the Netherlands. [Google Scholar]
- 50.Greig D. 2009. Reproductive isolation in Saccharomyces. Heredity (Edinb) 102:39–44. doi: 10.1038/hdy.2008.73. [DOI] [PubMed] [Google Scholar]
- 51.Maclean CJ, Greig D. 2008. Prezygotic reproductive isolation between Saccharomyces cerevisiae and Saccharomyces paradoxus. BMC Evol Biol 8:1. doi: 10.1186/1471-2148-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liti G, Barton DBH, Louis EJ. 2006. Sequence diversity, reproductive isolation and species concepts in Saccharomyces. Genetics 174:839–850. doi: 10.1534/genetics.106.062166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Powell CD, Quain DE, Smart KA. 2003. The impact of brewing yeast cell age on fermentation performance, attenuation and flocculation. FEMS Yeast Res 3:149–157. doi: 10.1016/S1567-1356(03)00002-3. [DOI] [PubMed] [Google Scholar]
- 54.Ekberg J, Rautio J, Mattinen L, Vidgren V, Londesborough J, Gibson BR. 2013. Adaptive evolution of the lager brewing yeast Saccharomyces pastorianus for improved growth under hyperosmotic conditions and its influence on fermentation performance. FEMS Yeast Res 13:335–349. doi: 10.1111/1567-1364.12038. [DOI] [PubMed] [Google Scholar]
- 55.Hebly M, Brickwedde A, Bolat I, Driessen MRM, de Hulster EA, van den Broek FM, Pronk JT, Geertman J-M, Daran J-M, Daran-Lapujade P. 5 March 2015. S. cerevisiae × S. eubayanus interspecific hybrid, the best of both worlds and beyond. FEMS Yeast Res. doi: 10.1093/femsyr/fov005. [DOI] [PubMed] [Google Scholar]
- 56.Vanbeneden N, Gils F, Delvaux F, Delvaux FR. 2008. Formation of 4-vinyl and 4-ethyl derivatives from hydroxycinnamic acids: occurrence of volatile phenolic flavour compounds in beer and distribution of Pad1-activity among brewing yeasts. Food Chem 107:221–230. doi: 10.1016/j.foodchem.2007.08.008. [DOI] [Google Scholar]
- 57.Vanderhaegen B, Delvaux F, Daenen L, Verachtert H, Delvaux FR. 2007. Aging characteristics of different beer types. Food Chem 103:404–412. doi: 10.1016/j.foodchem.2006.07.062. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.