Abstract
Understanding viral dynamics in arthropods is of great importance when designing models to describe how viral spread can influence arthropod populations. The endosymbiotic bacterium Wolbachia spp., which is present in up to 40% of all insect species, has the ability to alter viral dynamics in both Drosophila spp. and mosquitoes, a feature that in mosquitoes may be utilized to limit spread of important arboviruses. To understand the potential effect of Wolbachia on viral dynamics in nature, it is important to consider the impact of natural routes of virus infection on Wolbachia antiviral effects. Using adult Drosophila strains, we show here that Drosophila-Wolbachia associations that have previously been shown to confer antiviral protection following systemic viral infection also confer protection against virus-induced mortality following oral exposure to Drosophila C virus in adults. Interestingly, a different pattern was observed when the same fly lines were challenged with the virus when still larvae. Analysis of the four Drosophila-Wolbachia associations that were protective in adults indicated that only the w1118-wMelPop association conferred protection in larvae following oral delivery of the virus. Analysis of Wolbachia density using quantitative PCR (qPCR) showed that a high Wolbachia density was congruent with antiviral protection in both adults and larvae. This study indicates that Wolbachia-mediated protection may vary between larval and adult stages of a given Wolbachia-host combination and that the variations in susceptibility by life stage correspond with Wolbachia density. The differences in the outcome of virus infection are likely to influence viral dynamics in Wolbachia-infected insect populations in nature and could also have important implications for the transmission of arboviruses in mosquito populations.
INTRODUCTION
Arthropods harbor a wide range of viruses that can be transmitted between individuals or populations of the same species or can bridge the interspecies gap to infect plants or other animals. The outcome of viral infections can be modulated by tripartite interactions between arthropods, viruses, and bacteria (1). One such interaction is the tripartite interaction between insects, viruses, and the endosymbyotic bacterium Wolbachia pipientis.
Wolbachia spp. have gained much attention due to the antiviral effects they confer to their host. The impact of Wolbachia spp. on virus infection was first described in the Drosophila melanogaster host, where it was shown to protect against mortality induced by diverse viruses, including Drosophila C virus (DCV), cricket paralysis virus, and Flock House virus (2, 3). Since that discovery, Wolbachia-mediated antiviral effects have been demonstrated in a number of insect hosts and are being investigated as a way of limiting spread of arboviruses (reviewed in references 1, 4, 5, and 6). Notably, Wolbachia-mediated antiviral effects have been demonstrated in adult mosquitoes artificially infected with Wolbachia; in mosquitoes, Wolbachia can interfere with accumulation and transmission of important human pathogens, including dengue and Chikungunya viruses (7–17). While in many cases Wolbachia confers antiviral effects to its host organism, in some cases the presence of Wolbachia can enhance viral susceptibility (18–23). The impact of the presence of Wolbachia on virus infection can include two main effects: (i) interference with viral replication/accumulation, and/or (ii) protection against virus-induced mortality. In mosquitoes, Wolbachia interferes with viral replication/accumulation, while in Drosophila, Wolbachia can interfere with viral replication/accumulation and/or protect flies from virus-induced mortality. In this paper, we focus on the effect of Wolbachia on the survival of the host, and we define protection as a reduction/delay in virus-induced mortality.
The mechanisms involved in Wolbachia-mediated antiviral effects have not yet been fully elucidated. There is some evidence that microRNAs (24, 25), competition for host-derived resources (26), and elevated reactive oxygen species (27, 28) may influence antiviral effects. Drosophila-Wolbachia associations can be subdivided into two groups: protective and nonprotective. The Drosophila-Wolbachia pairings CO-wAu, DSR-wRi, w1118-wMel, and the overreplicating and life-shortening w1118-wMelPop association all show a delay in DCV-induced mortality when DCV is injected into adult flies, while the N7NO-wNo and DSH-wHa combinations did not (2, 3, 11, 29). A feature that all protective Wolbachia strains share is high density within their respective host organism, indicating that high Wolbachia density may serve as a prerequisite for antiviral protection (7, 12, 29–34).
Wolbachia is estimated to infect 40% of all insects (35); therefore, the effect it exerts on natural viral dynamics could be pronounced. The understanding of natural tripartite Drosophila-virus-Wolbachia interactions is very limited at present, partially due to a lack of a method for orally delivering the virus. Recently, three methods for oral infection of larvae and adults were described, and these will allow us to study the effects of the oral route of infection on antiviral protection mechanisms in Drosophila (36–38).
To investigate the effects of Wolbachia on virus-induced mortality following oral infection, we used DCV, a natural Drosophila pathogen and the most widely studied Drosophila virus (39). DCV is a positive-sense RNA virus that belongs to the Dicistroviridae family (40). When injected into flies, DCV is pathogenic, causing mortality within 4 to 6 days postinjection (41). Injection of DCV is a useful method to study Wolbachia-DCV interactions; however, injection bypasses the fly's natural immune barriers present within the midgut and can cause a differential immune response compared to exposure via virus feeding alone (37). DCV infection by ingestion is less pathogenic than by injection (36, 37) and represents a more natural Drosophila-DCV interaction. While Wolbachia-mediated protection has been extensively studied in adult flies following a systemic infection, it is not yet clear whether the Drosophila-Wolbachia associations that are protective for virus-induced host mortality following viral injection also exhibit a similar protective characteristic following the oral route of infection. Ingestion of infected cadavers is thought to be one of the mechanisms through which DCV transmission occurs naturally within an insect population (42); therefore, Wolbachia-mediated antiviral protection following the oral route of infection could have a direct impact on viral transmission and maintenance of the virus within a population.
Understanding the potential of Wolbachia to affect viral dynamics in natural populations will be facilitated by insights into the impact of antiviral protection on susceptibility throughout the life cycle of the host, following exposure via a natural route of infection. Both Drosophila and mosquitoes are holometabolous insects, as they undergo metamorphosis between the larval and adult stages. A wide range of genes coordinate the disintegration of larval structures, where some larval organs are histolyzed and major new growth takes place, altering the morphology and in some cases pathogen susceptibility (43–45). Pathogen susceptibility is often age or life stage dependent and can have a large effect on population dynamics, viral spread, and maintenance of the virus within the population (45–48). Studies focusing on the antiviral effects of Wolbachia have to date been conducted solely on adult flies and mosquitoes, without consideration of other developmental stages.
Here, we investigated the effect of Wolbachia on virus-induced mortality of Drosophila larvae and adults following oral challenge with DCV. By using four Drosophila-Wolbachia associations that have previously been shown to be protective for adult insects following viral injection, we show that the Drosophila-Wolbachia associations that are protective against virus-induced mortality following injection are also protective following oral infection of adults. In contrast, Wolbachia protection at the adult stages is not indicative of protection at larval stages, as only one out of four Drosophila-Wolbachia associations that were protective at the adult stage showed protection at the larval stage.
MATERIALS AND METHODS
Drosophila and Wolbachia.
Two Drosophila melanogaster and three Drosophila simulans fly lines were reared on a standard cornmeal medium at a constant temperature of 25°C with a 12-hour light/dark cycle. Paired populations of flies were used that either contained Wolbachia (w1118-wMel, w1118-wMelPop, N7NO-wNo, DSR-wRi, and Co-wAu) or had been cured of Wolbachia by tetracycline treatment (w1118-T, N7NO-T, DSR-T, and CO-T); flies were maintained on a standard cornmeal medium for at least five generations before use. Gut flora was reconstituted and normalized across fly lines by using standardized methods (31). Briefly, Drosophila embryos were transferred to vials containing 150 μl of a bacterial inoculum, which was prepared by adding 2 g of 10-day-old food containing w1118-wMelPop flies to 5 ml of sterile water and strained through a fine sterile mesh to remove larvae and embryos. The newly treated flies were checked for the presence of Wolbachia by using PCR, to make sure that no cross-contamination had occurred.
Virus.
Plaque-purified DCV isolate EB (49, 50) was propagated and purified from Schneider's Drosophila line 2 cells (51), and virus titers were determined based on the 50% tissue culture infective dose (TCID50), as described previously (29, 49).
DNA extraction.
Thirty 0- to 4-h-old larvae or 10 newly emerged male adult flies were pooled to perform DNA extraction. The flies were homogenized using a pestle in 180 μl of extraction buffer and 20 μl of proteinase K. The DNeasy blood and tissue kit (Qiagen) was used to extract the DNA as per the manufacturer's protocol. Three replicates on independent cohorts were performed for each treatment.
Quantitative PCR.
The abundance of Wolbachia was assessed by quantitative PCR (qPCR) to determine the abundance of the Wolbachia surface protein gene (wsp) relative to that of either the D. melanogaster RrpL32 or D. simulans Act5C genes. Platinum SYBR green qPCR SuperMix-UDG (Invitrogen) was used per the manufacturer's instruction using the wsp-specific primer pair 5′-GCATTTGGTTAYAAAATGGACGA-3′ and 5′-GGAGTGATAGGCATATCTTCAAT-3′ (producing a 185-bp PCR product) (29), RpL32-specific primers 5′-GACGCTTCAAGGGACAGTATCTG-3′ and 5′-AAACGCGGTTCTGCATGAG-3′ (producing a 141-bp PCR product) (49), and Act5C-specific primers 5′-GACGAAGAAGTTGCTGCTCTGGTTG-3′ and 5′-TGAGGATACCACGCTTGCTCTGC-3′ (producing a 192-bp PCR product) (30). The Rotor-Gene 6000 thermal cycler (Corbett Life Sciences, Qiagen) was used with the following profile: 95°C for 2 min, followed by 40 cycles of 95°C for 10 s, 52°C for 10 s, and 72°C for 20 s. This was followed by a standard melt analysis to assess the specificity of the amplified product. Two technical replicates (separate qPCRs on the same DNA) were performed for each sample (with a third performed where necessary), and DNA extracted from flies without Wolbachia was used as a negative control. Mean normalized wsp:RpL32 DNA ratios were calculated using qGENE software (52), and statistical analysis included a two-tailed Student's t test to compare differences of the means.
Survival bioassay.
Virus for larval and adult feeding assays was prepared by injecting flies with either 5,000 infectious units (IU) of DCV or an equivalent volume of phosphate-buffered saline (PBS), which acted as a control. Live flies were collected at 4 days postinjection and stored at −20°C until further use. Thirty PBS- or DCV-injected flies were pooled and homogenized in 300 μl of PBS, and the supernatant was filter sterilized using a Millex GV 22-μm filter (Merck Millipore). Homogenates prepared in this way were used for both adult and larval bioassays. The titers for DCV-injected fly homogenates were measured on four occasions and ranged between 4.4 × 1010 and 2 × 1011 IU/ml.
For adult infections, a modified version of a previously described method was used (37). A mix (250-μl volume) containing 75% fly homogenate (DCV or PBS treated, as described above) and 25% dry yeast was applied to a 1.5- by 1.5-cm filter paper and placed in a vial containing 10 4- to 7-day-old male flies. Flies were incubated with the medium for 24 h at 25°C with high humidity to prevent the food from drying out. Following this period, the flies were transferred to standard cornmeal medium, and mortality was scored daily for 15 days. Three replicates of independent cohorts were performed for each treatment.
Larval infections were performed by spreading DCV- or mock-infected fly homogenates onto petri dishes containing 10 ml of standard cornmeal medium (36). One hundred eggs were collected for each treatment on a wet piece of sterile filter paper and transferred onto petri dishes containing homogenates from either PBS- or DCV-injected flies. Larvae were maintained on the treatment medium until adult emergence, and they were counted 3 days postemergence. Egg-to-adult survival was determined as the proportion of adults postemergence relative to the initial number of eggs at the start of the treatment, and each survival bioassay was replicated 3 times with independent cohorts of insects.
Statistical analysis of the survival bioassay results.
We used generalized linear mixed-effects regression (GLMER) models based on a binomial distribution to examine the effect of feeding treatment and coinfection on the mortality of five D. melanogaster and D. simulans larvae, and we employed the lme4 R package in R 2.15.3 (53) (R Foundation for Statistical Computing, Vienna, Austria). The mortality response, as the binomial count of flies that survived or died for each line, was determined by fitting the feeding treatment (PBS or DCV) and coinfection treatment (absence of Wolbachia [-wol] or with a Wolbachia strain), as well as the interaction between the two factors. The interaction term compared across the mortality values of each of the feeding treatments across the absence (−wol) or presence (+ indicated Wolbachia strain) of Wolbachia. Each model included an experimental replicate as a random factor for analysis of the replicate variance component in each model. For adult survival bioassays, the survival curves were compared using Kaplan-Meier analysis and log rank statistics within the GraphPad Prism program.
RESULTS
Wolbachia protection in adult flies following oral challenge with DCV.
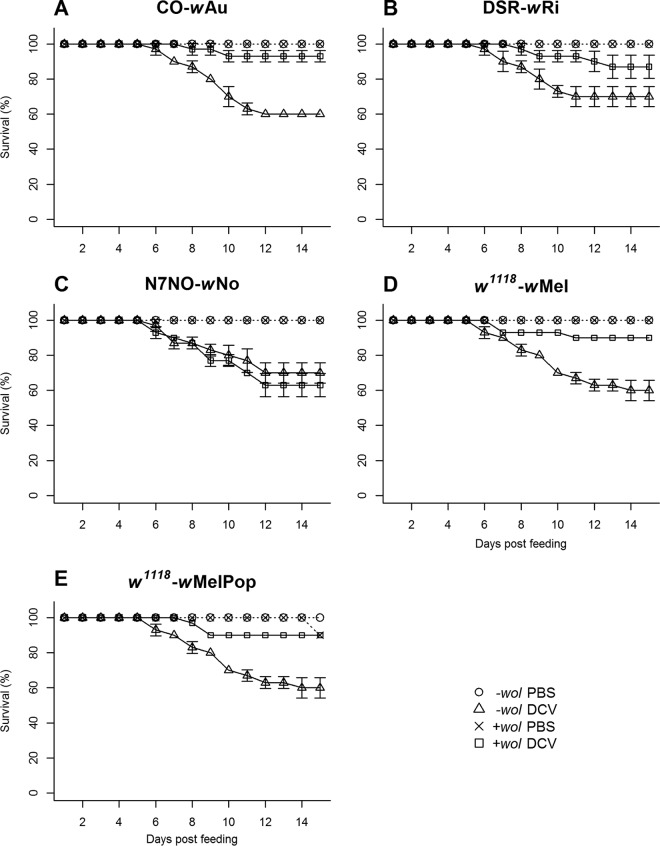
Initially, we tested the protective effects of the Wolbachia strain wAu in a CO fly background (CO-wAu) due to a strong antiviral protection observed previously following systemic DCV infection (29). Wolbachia-free CO flies challenged with DCV by oral infection showed 40% mortality within 15 days postfeeding. In contrast, CO-wAu flies showed a significant reduction in mortality during the same time period, to 7% (Fig. 1A; Kaplan-Meyer analysis, P < 0.05). We investigated an additional three Drosophila-Wolbachia associations, DSR-wRi, w1118-wMel, and w1118-wMelPop, all of which have previously been shown to confer protection against DCV-induced mortality in adult flies following a systemic infection (3, 29, 49), and the results indicated that all three Drosophila-Wolbachia associations conferred protection against DCV-induced mortality following the oral route of infection (Fig. 1B, D, and E; Kaplan-Meyer analysis, P < 0.05). Because not all Drosophila-Wolbachia associations protect against systemic viral infections, we tested a nonprotective association, N7NO-wNo, to see whether protection would occur following oral virus challenge (29). Feeding the nonprotective N7NO-wNo flies with DCV led to a nonsignificant difference in virus-induced mortality compared to Wolbachia-free flies (Fig. 1C; Kaplan-Meyer analysis, P > 0.05). Taken together, these results indicate that Wolbachia-mediated protection against virus-induced mortality in adults infected through the oral route was consistent with what was previously reported following injection of virus.
FIG 1.
Survival of adult flies following oral challenge with DCV. Each fly strain contained a Wolbachia strain (+wol) or was tetracycline treated to remove Wolbachia (−wol). Adult flies were exposed to either homogenates from DCV-infected or mock-infected (PBS) flies for 24 h before being transferred to vials containing standard cornmeal medium. Survival of flies is shown from 3 biological replicates of 10 flies (or from 1 replicate of 10 flies for PBS controls). Statistically significant differences in survival were determined by using the log rank test on Kaplan-Meier curves.
Wolbachia protection in larvae following oral challenge with DCV.
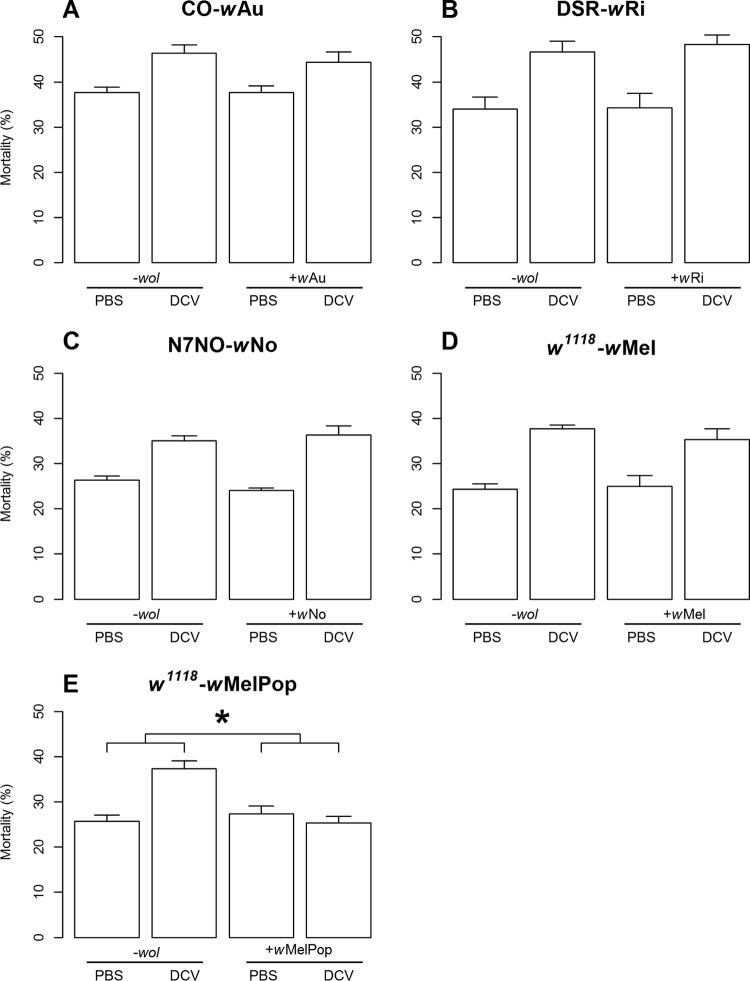
To determine whether exposure to Wolbachia sp. protects larvae from virus-induced mortality, we orally challenged CO-wAu larvae with DCV. We found that in Wolbachia-free flies, larvum-to-adult mortality increased from about 37% in mock-infected flies to about 46% in DCV-infected flies and that the presence of Wolbachia had no significant effect on DCV-induced mortality (Fig. 2A and Table 1). This suggests that the Wolbachia strain wAu may not protect its host against DCV-induced mortality following this route of infection at the larval developmental stages.
FIG 2.
The impact of Wolbachia on virus-induced mortality in DCV-infected Drosophila larvae. Each fly line was exposed to a different Wolbachia strain or was tetracycline treated to remove Wolbachia (−wol), as indicated on the x axis. Larvae were exposed to homogenates from either DCV-infected or mock-infected (PBS) flies. Graphs display means and standard errors from three replicates with 100 individuals per fly strain. *, significant interaction (P < 0.05) between the feeding treatment and presence or absence of Wolbachia for mortality.
TABLE 1.
Analysis of mortality of Drosophila larvae in response to DCV in feed with or without Wolbachiaa
| Drosophila line and basis of analysis | Parameter estimate | SE | Z score | P value |
|---|---|---|---|---|
| CO | ||||
| Intercept | −0.147 | 0.116 | −1.269 | 0.204 |
| Feeding treatments | −0.357 | 0.166 | −2.147 | <0.05 |
| Wolbachia treatments | −0.081 | 0.164 | −0.492 | 0.623 |
| Treatment interaction | −0.081 | 0.235 | 0.343 | 0.731 |
| Replicate variance component | 0 | 0 | ||
| DSR | ||||
| Intercept | −0.134 | 0.116 | −1.154 | 0.249 |
| Feeding treatments | −0.53 | 0.168 | −3.152 | <0.01 |
| Wolbachia treatments | 0.067 | 0.164 | 0.409 | 0.683 |
| Treatment interaction | −0.052 | 0.237 | −0.219 | 0.827 |
| Replicate variance component | 2.4 × 10−11 | 4.8 × 10−6 | ||
| N7NO | ||||
| Intercept | −0.619 | 0.121 | −5.114 | <0.0001 |
| Feeding treatments | −0.41 | 0.178 | −2.296 | <0.05 |
| Wolbachia treatments | 0.058 | 0.17 | 0.341 | 0.733 |
| Treatment interaction | −0.182 | 0.254 | −0.717 | 0.474 |
| Replicate variance component | 9.7 × 10−15 | 9.8 × 10−8 | ||
| w1118 | ||||
| Intercept | −0.518 | 0.119 | −4.339 | <0.0001 |
| Feeding treatments | −0.545 | 0.178 | −3.062 | <0.01 |
| Wolbachia treatments | −0.563 | 0.179 | −3.154 | <0.01 |
| Treatment interaction | 0.649 | 0.257 | 2.522 | <0.05 |
| Replicate variance component | 5.9 × 10−12 | 2.4 × 10−6 | ||
| w1118 | ||||
| Intercept | −0.504 | 0.119 | −4.228 | <0.0001 |
| Feeding treatments | −0.631 | 0.18 | −3.51 | <0.001 |
| Wolbachia treatments | −0.101 | 0.17 | −0.594 | 0.553 |
| Treatment interaction | 0.137 | 0.254 | 0.537 | 0.591 |
| Replicate variance component | 0 | 0 |
Generalized linear mixed-effects regression (GLMER) analysis of the percent mortality in five Drosophila lines in response to feeding treatment with PBS or DCV. Each Drosophila line was either coinfected with a Wolbachia strain (wAu, wRi, wNo, wMelPop, or wMel) or was not exposed to Wolbachia (−wol). Treatment interaction is a comparison of percent mortality across all combinations of feeding treatment (DCV or PBS) and the presence/absence of Wolbachia.
As no protection was observed in CO-wAu larvae, we then investigated whether the lack of protection was specific to this Drosophila-Wolbachia association. We investigated other protective Drosophila-Wolbachia associations, DSR-wRi and w1118-wMel, and one nonprotective association, N7NO-wNo. None of these associations showed a significant difference in DCV-induced mortality between larvae with and without Wolbachia (Fig. 2B to D and Table 1), suggesting that the lack of Wolbachia-mediated protection at the larval stages is not confined to CO-wAu flies.
The Wolbachia strain wMelPop has a strong protective effect in both adult flies and mosquitoes, so we investigated whether w1118-wMelPop larvae exhibited a protective phenotype. In this Drosophila-Wolbachia association, there was a statistically significant difference in DCV-induced mortality between flies with and without Wolbachia (25% and 37% mortality, respectively) (Fig. 2E and Table 1). Unlike the other Drosophila-Wolbachia associations, wMelPop provided complete protection against DCV-induced mortality (Fig. 2E). Because the ability to confer antiviral effects is strongly associated with Wolbachia density in adult flies and mosquitoes and because wMelPop is known to be an overreplicative strain, we investigated whether the observed differences in Wolbachia protection were associated with differences in Wolbachia densities.
Wolbachia densities.
Wolbachia densities have previously been determined in adults but not in larvae for different Drosophila-Wolbachia associations. Using qPCR, we determined Wolbachia densities at both the larval and adult stages for all five Drosophila-Wolbachia associations used in this study (Fig. 3). In adults, the densities of the protective Wolbachia strains wAu, wRi, wMel, and wMelPop were significantly higher than that of the nonprotective wNo strain, providing an association between Wolbachia density and protection. In contrast, Wolbachia strains wAu, wRi, and wMel showed lower abundance at the larval stage than at the adult stage (two-tailed Student's t test, P < 0.05) (Fig. 3A and B), while the Wolbachia strain wMelPop showed high densities at both the larval and adult stages (Fig. 3A). Wolbachia density in the nonprotective N7NO-wNo larvae remained lower than in either the wRi- or wAu-treated insects at both developmental stages (Fig. 3B), consistent with a lack of protection.
FIG 3.

The densities of six different Wolbachia strains during larval and adult stages of development. (A) Relative abundance of the Wolbachia surface protein gene (wsp) in D. melanogaster, using RpL32 as the reference gene. (B) Relative abundance of the wsp gene in D. simulans, using Act5C as the reference gene.
DISCUSSION
The importance of the route of pathogen entry on the outcome of infection has been well-documented following bacterial infections in Drosophila. Injecting bacteria into the hemocoel induces a systemic immune response (54–57), while oral infections often lead to localized immune induction in the gut, often making the bacteria less pathogenic (58–60). A recent paper that showed the involvement of the Toll immune pathway in mediating resistance to oral viral infection with DCV, Flock House virus, cricket paralysis virus, or Nora virus, however, showed no influence of the viral exposure pathway following a systemic bacterial infection (37), indicating that the route of viral entry can have an effect on the host's response to viral infection.
We used a natural route of DCV infection, oral feeding, to investigate the effect of Wolbachia on protection against virus-induced mortality, in order to determine whether Wolbachia-mediated protection is confined to systemic viral infections in Drosophila. By examining Wolbachia-mediated protection in adult flies across four Drosophila-Wolbachia associations that had previously been shown to be protective following systemic bacterial infection, we found that oral DCV infections led to a reduction in virus-induced mortality in adult flies infected with Wolbachia compared to Wolbachia-free flies (Fig. 1). These findings are consistent with a recently published report (37) and support the idea that Wolbachia-mediated protection extends beyond systemic viral infections and could be used in future experiments to better understand the effects of Wolbachia on viral dynamics in natural insect populations.
While in adults the Wolbachia-mediated reduction in virus-induced mortality is comparable between systemically and orally infected flies, the same is not always true in larvae. Of the four Drosophila-Wolbachia associations that showed protection following DCV infection in adults, only the w1118-wMelPop flies showed protection against DCV-induced mortality during the larval stages (Fig. 2E). These results suggest that Wolbachia-mediated protection may vary between different life stages of the same Drosophila-Wolbachia association, although it is possible that the amounts of virus ingested by larvae and adults are different. Since Wolbachia density has previously been shown to be important for mediating antiviral effects, we measured Wolbachia density in adults and found that there was congruence between Wolbachia density and protection against DCV-induced mortality following the oral route of infection. Similarly to adults, Wolbachia-protection in larvae was associated with Wolbachia density, however interestingly high Wolbachia density was only observed in w1118-wMelPop larvae, which was also the only association to show protection against DCV-induced mortality at the larval stages. The wMelPop strain causes a life-shortening phenotype and is present in relatively high densities in both mosquitoes and Drosophila (7, 61–63). The relatively high density and the life-shortening effects of the wMelPop strain have been reported to be due to the high copy number of 8 Wolbachia genes referred as the Octomom region (31, 62). It remains to be seen whether other strains will be protective in larvae and what controls the differences in density between larvae and adults. The finding that Wolbachia-protection correlates with Wolbachia density is consistent with previous findings in adult flies following a systemic infection (29–32). Gradually reducing Wolbachia density in both Drosophila adults and mosquito cell culture using tetracycline leads to a dose-dependent loss of antiviral protection (12, 30).
Wolbachia-mediated antiviral protection is not limited to Drosophila, and since Wolbachia infects up to 40% of all arthropod species (35) it may be important to consider the impact of life-stage susceptibility on arthropod population dynamics and viral transmission. Similarly to Drosophila, mosquitoes also undergo metamorphosis, a change that can result in life-stage-dependent differences in viral susceptibility. Mosquitoes are known to form natural associations with Wolbachia; however, it is artificial Wolbachia transinfections that have shown promise as a tool for limiting spread of human pathogenic viruses (5, 6). Commonly, there is a focus on transmission of arboviruses that occurs between mosquitoes and human hosts. While this horizontal transmission is responsible for the major health concerns in humans, vertical transmission of arboviruses within mosquito populations can affect the maintenance of the virus within the population (64, 65). Viruses such as dengue virus and Chikungunya virus can be vertically transmitted from an infected adult female to its offspring. Dengue virus can spread vertically under both natural (66–69) and laboratory conditions (70–72). Furthermore, transovarially infected female mosquitoes can transmit dengue virus orally (73). Chikungunya is also capable of vertical transmission under laboratory conditions, which would suggest that similar transmission is possible in nature (64).
Various models have been applied to try to understand the impact of Wolbachia on the transmission of dengue virus in its mosquito host (74–76). These models do not consider the effects of vertical transmission on the maintenance of dengue virus within a population, which has been suggested to be an important factor affecting the ability of the virus to persist within the population in rural areas with low human population densities (65). Furthermore, vertical transmission could allow the survival of arboviruses during adverse climatic conditions and has been suggested to be an important mechanism of maintenance of the virus during interepidemic periods (64). Given the importance of vertical transmission on virus dynamics and the possible life-stage-dependent variations in Wolbachia-mediated protection, it is important to consider the impact of Wolbachia antiviral protection, or the lack of thereof, on the maintenance of a virus within a population. Understanding the impact of Wolbachia antiviral protection at different insect life stages is likely to be an important consideration when designing programs to minimize the spread of insect-borne viruses.
ACKNOWLEDGMENTS
We thank David Merritt and members of the Johnson lab for useful comments, and we thank Craig White for help with the statistical analysis.
REFERENCES
- 1.Johnson KN. 2015. Bacteria and antiviral immunity in insects. Curr Opin Insect Sci 8:97–103. doi: 10.1016/j.cois.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 2.Hedges LM, Brownlie JC, O'Neill SL, Johnson KN. 2008. Wolbachia and virus protection in insects. Science 322:702. doi: 10.1126/science.1162418. [DOI] [PubMed] [Google Scholar]
- 3.Teixeira L, Ferreira A, Ashburner M. 2008. The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol 6:e1000002. doi: 10.1371/journal.pbio.1000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brownlie JC, Johnson KN. 2009. Symbiont-mediated protection in insect hosts. Trends Microbiol 17:348–354. doi: 10.1016/j.tim.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Rainey SM, Shah P, Kohl A, Dietrich I. 2014. Understanding the Wolbachia-mediated inhibition of arboviruses in mosquitoes: progress and challenges. J Gen Virol 95:517–530. doi: 10.1099/vir.0.057422-0. [DOI] [PubMed] [Google Scholar]
- 6.Bourtzis K, Dobson SL, Xi ZY, Rasgon JL, Calvitti M, Moreira LA, Bossin HC, Moretti R, Baton LA, Hughes GL, Mavingui P, Gilles JRL. 2014. Harnessing mosquito-Wolbachia symbiosis for vector and disease control. Acta Trop 132:S150–S163. doi: 10.1016/j.actatropica.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Moreira LA, Iturbe-Ormaetxe I, Jeffery JA, Lu GJ, Pyke AT, Hedges LM, Rocha BC, Hall-Mendelin S, Day A, Riegler M, Hugo LE, Johnson KN, Kay BH, McGraw EA, van den Hurk AF, Ryan PA, O'Neill SL. 2009. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and plasmodium. Cell 139:1268–1278. doi: 10.1016/j.cell.2009.11.042. [DOI] [PubMed] [Google Scholar]
- 8.Blagrove MSC, Arias-Goeta C, Failloux AB, Sinkins SP. 2012. Wolbachia strain wMel induces cytoplasmic incompatibility and blocks dengue transmission in Aedes albopictus. Proc Natl Acad Sci U S A 109:255–260. doi: 10.1073/pnas.1112021108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bian GW, Xu Y, Lu P, Xie Y, Xi ZY. 2010. The endosymbiotic bacterium Wolbachia induces resistance to dengue virus in Aedes aegypti. PLoS Pathog 6:e1000833. doi: 10.1371/journal.ppat.1000833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frentiu FD, Zakir T, Walker T, Popovici J, Pyke AT, van den Hurk A, McGraw EA, O'Neill SL. 2014. Limited dengue virus replication in field-collected Aedes aegypti mosquitoes infected with Wolbachia. PLoS Negl Trop Dis 8:e2688. doi: 10.1371/journal.pntd.0002688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rances E, Ye YXH, Woolfit M, McGraw EA, O'Neill SL. 2012. The relative importance of innate immune priming in Wolbachia-mediated dengue interference. PLoS Pathog 8:e1002548. doi: 10.1371/journal.ppat.1002548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu P, Bian GW, Pan XL, Xi ZY. 2012. Wolbachia induces density-dependent inhibition to dengue virus in mosquito cells. PLoS Negl Trop Dis 6:e1754. doi: 10.1371/journal.pntd.0001754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walker T, Johnson PH, Moreira LA, Iturbe-Ormaetxe I, Frentiu FD, McMeniman CJ, Leong YS, Dong Y, Axford J, Kriesner P, Lloyd AL, Ritchie SA, O'Neill SL, Hoffmann AA. 2011. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature 476:450–453. doi: 10.1038/nature10355. [DOI] [PubMed] [Google Scholar]
- 14.Hoffmann AA, Montgomery BL, Popovici J, Iturbe-Ormaetxe I, Johnson PH, Muzzi F, Greenfield M, Durkan M, Leong YS, Dong Y, Cook H, Axford J, Callahan AG, Kenny N, Omodei C, McGraw EA, Ryan PA, Ritchie SA, Turelli M, O'Neill SL. 2011. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 476:454–457. doi: 10.1038/nature10356. [DOI] [PubMed] [Google Scholar]
- 15.Blagrove MSC, Arias-Goeta C, Di Genua C, Failloux AB, Sinkins SP. 2013. A Wolbachia wMel transinfection in Aedes albopictus is not detrimental to host fitness and inhibits Chikungunya virus. PLoS Negl Trop Dis 7:e2152. doi: 10.1371/journal.pntd.0002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mousson L, Martin E, Zouache K, Madec Y, Mavingui P, Failloux AB. 2010. Wolbachia modulates Chikungunya replication in Aedes albopictus. Mol Ecol 19:1953–1964. doi: 10.1111/j.1365-294X.2010.04606.x. [DOI] [PubMed] [Google Scholar]
- 17.van den Hurk AF, Hall-Mendelin S, Pyke AT, Frentiu FD, McElroy K, Day A, Higgs S, O'Neill SL. 2012. Impact of Wolbachia on infection with Chikungunya and yellow fever viruses in the mosquito vector Aedes aegypti. PLoS Negl Trop Dis 6:e1892. doi: 10.1371/journal.pntd.0001892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zele F, Nicot A, Berthomieu A, Weill M, Duron O, Rivero A. 2014. Wolbachia increases susceptibility to Plasmodium infection in a natural system. Proc R Soc B 281:20132837. doi: 10.1098/rspb.2013.2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graham RI, Grzywacz D, Mushobozi WL, Wilson K. 2012. Wolbachia in a major African crop pest increases susceptibility to viral disease rather than protects. Ecol Lett 15:993–1000. doi: 10.1111/j.1461-0248.2012.01820.x. [DOI] [PubMed] [Google Scholar]
- 20.Dodson BL, Hughes GL, Paul O, Matacchiero AC, Kramer LD, Rasgon JL. 2014. Wolbachia enhances West Nile Virus (WNV) infection in the mosquito Culex tarsalis. PLoS Negl Trop Dis 8:e2965. doi: 10.1371/journal.pntd.0002965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hughes GL, Vega-Rodriguez J, Xue P, Rasgon JL. 2012. Wolbachia strain wAlbB enhances infection by the rodent malaria parasite Plasmodium berghei in Anopheles gambiae mosquitoes. Appl Environ Microbiol 78:1491–1495. doi: 10.1128/AEM.06751-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baton LA, Pacidonio EC, Goncalves DD, Moreira LA. 2013. wFlu: characterization and evaluation of a native Wolbachia from the mosquito Aedes fluviatilis as a potential vector control agent. PLoS One 8:e59619. doi: 10.1371/journal.pone.0059619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murdock CC, Blanford S, Hughes GL, Rasgon JL, Thomas MB. 2014. Temperature alters Plasmodium blocking by Wolbachia. Sci Rep 4:3932. doi: 10.1038/srep03932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hussain M, Frentiu FD, Moreira LA, O'Neill SL, Asgari S. 2011. Wolbachia uses host microRNAs to manipulate host gene expression and facilitate colonization of the dengue vector Aedes aegypti. Proc Natl Acad Sci U S A 108:9250–9255. doi: 10.1073/pnas.1105469108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang GM, Hussain M, O'Neill SL, Asgari S. 2013. Wolbachia uses a host microRNA to regulate transcripts of a methyltransferase, contributing to dengue virus inhibition in Aedes aegypti. Proc Natl Acad Sci U S A 110:10276–10281. doi: 10.1073/pnas.1303603110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caragata EP, Rances E, Hedges LM, Gofton AW, Johnson KN, O'Neill SL, McGraw EA. 2013. Dietary cholesterol modulates pathogen blocking by Wolbachia. PLoS Pathog 9:e1003459. doi: 10.1371/journal.ppat.1003459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan XL, Zhou GL, Wu JH, Bian GW, Lu P, Raikhel AS, Xi ZY. 2012. Wolbachia induces reactive oxygen species (ROS)-dependent activation of the Toll pathway to control dengue virus in the mosquito Aedes aegypti. Proc Natl Acad Sci U S A 109:E23–E31. doi: 10.1073/pnas.1116932108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong ZS, Brownlie JC, Johnson KN. 2015. Oxidative stress correlates with Wolbachia-mediated antiviral protection in Wolbachia-Drosophila associations. Appl Environ Microbiol 81:3001–3005. doi: 10.1128/AEM.03847-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osborne SE, Leong YS, O'Neill SL, Johnson KN. 2009. Variation in antiviral protection mediated by different Wolbachia strains in Drosophila simulans. PLoS Pathog 5:e1000656. doi: 10.1371/journal.ppat.1000656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Osborne SE, Iturbe-Ormaetxe I, Brownlie JC, O'Neill SL, Johnson KN. 2012. Antiviral protection and the importance of Wolbachia density and tissue tropism in Drosophila simulans. Appl Environ Microbiol 78:6922–6929. doi: 10.1128/AEM.01727-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chrostek E, Marialva MSP, Esteves SS, Weinert LA, Martinez J, Jiggins FM, Teixeira L. 2013. Wolbachia variants induce differential protection to viruses in Drosophila melanogaster: a phenotypic and phylogenomic analysis. PLoS Genet 9:e1003896. doi: 10.1371/journal.pgen.1003896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martinez J, Longdon B, Bauer S, Chan YS, Miller WJ, Bourtzis K, Teixeira L, Jiggins FM. 2014. Symbionts commonly provide broad spectrum resistance to viruses in insects: a comparative analysis of Wolbachia strains. PLoS Pathog 10:e1004369. doi: 10.1371/journal.ppat.1004369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bian GW, Zhou GL, Lu P, Xi ZY. 2013. Replacing a native Wolbachia with a novel strain results in an increase in endosymbiont load and resistance to dengue virus in a mosquito vector. PLoS Negl Trop Dis 7:e2250. doi: 10.1371/journal.pntd.0002250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frentiu FD, Robinson J, Young PR, McGraw EA, O'Neill SL. 2010. Wolbachia-mediated resistance to dengue virus infection and death at the cellular level. PLoS One 5:e13398. doi: 10.1371/journal.pone.0013398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zug R, Hammerstein P. 2012. Still a host of hosts for Wolbachia: analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PLoS One 7:e38544. doi: 10.1371/journal.pone.0038544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stevanovic A, Johnson KN. 2015. Infectivity of Drosophila C virus following oral delivery in Drosophila larvae. J Gen Virol 96:1490–1496. doi: 10.1099/vir.0.000068. [DOI] [PubMed] [Google Scholar]
- 37.Ferreira AG, Naylor H, Esteves SS, Pais IS, Martins NE, Teixeira L. 2014. The Toll-dorsal pathway is required for resistance to viral oral infection in Drosophila. PLoS Pathog 10:e1004507. doi: 10.1371/journal.ppat.1004507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu J, Hopkins K, Sabin L, Yasunaga A, Subramanian H, Lamborn I, Gordesky-Gold B, Cherry S. 2013. ERK signaling couples nutrient status to antiviral defense in the insect gut. Proc Natl Acad Sci U S A 110:15025–15030. doi: 10.1073/pnas.1303193110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huszart T, Imler JL. 2008. Drosophila viruses and the study of antiviral host-defense. Adv Virus Res 72:227–265. doi: 10.1016/S0065-3527(08)00406-5. [DOI] [PubMed] [Google Scholar]
- 40.Johnson KN, Christian PD. 1998. The novel genome organization of the insect picorna-like virus Drosophila C virus suggests this virus belongs to a previously undescribed virus family. J Gen Virol 79:191–203. doi: 10.1099/0022-1317-79-1-191. [DOI] [PubMed] [Google Scholar]
- 41.Jousset FX, Plus N, Croizier G, Thomas M. 1972. Existence in Drosophila of 2 groups of picornavirus of different serological and biological properties. C R Acad Sci Hebd Seances Acad Sci D 275:3043–3046. (In French.) [PubMed] [Google Scholar]
- 42.Gomariz-Zilber E, Jeune B, Thomas-Orillard M. 1998. Limiting conditions of the horizontal transmission of the Drosophila C virus in its host (D. melanogaster). Acta Oecol 19:125–137. doi: 10.1016/S1146-609X(98)80016-7. [DOI] [Google Scholar]
- 43.Bainbridge SP, Bownes M. 1981. Staging the metamorphosis of Drosophila melanogaster. J Embryol Exp Morphol 66:57–80. [PubMed] [Google Scholar]
- 44.White KP, Rifkin SA, Hurban P, Hogness DS. 1999. Microarray analysis of Drosophila development during metamorphosis. Science 286:2179–2184. doi: 10.1126/science.286.5447.2179. [DOI] [PubMed] [Google Scholar]
- 45.Briggs CJ, Godfray HCJ. 1995. The dynamics of insect-pathogen interactions in stage-structured populations. Am Nat 145:855–887. doi: 10.1086/285774. [DOI] [Google Scholar]
- 46.Bernal A, Simon O, Williams T, Caballero P. 2014. Stage-specific insecticidal characteristics of a nucleopolyhedrovirus isolate from Chrysodeixis chalcites enhanced by optical brighteners. Pest Manag Sci 70:798–804. doi: 10.1002/ps.3617. [DOI] [PubMed] [Google Scholar]
- 47.McNeil J, Cox-Foster D, Gardner M, Slavicek J, Thiem S, Hoover K. 2010. Pathogenesis of Lymantria dispar multiple nucleopolyhedrovirus in L. dispar and mechanisms of developmental resistance. J Gen Virol 91:1590–1600. doi: 10.1099/vir.0.018952-0. [DOI] [PubMed] [Google Scholar]
- 48.Milks ML. 1997. Comparative biology and susceptibility of cabbage looper (Lepidoptera: Noctuidae) lines to a nuclear polyhedrosis virus. Environ Entomol 26:839–848. doi: 10.1093/ee/26.4.839. [DOI] [Google Scholar]
- 49.Hedges LM, Johnson KN. 2008. Induction of host defence responses by Drosophila C virus. J Gen Virol 89:1497–1501. doi: 10.1099/vir.0.83684-0. [DOI] [PubMed] [Google Scholar]
- 50.Johnson KN, Christian PD. 1999. Molecular characterization of Drosophila C virus isolates. J Invertebr Pathol 73:248–254. doi: 10.1006/jipa.1998.4830. [DOI] [PubMed] [Google Scholar]
- 51.Schneider I. 1972. Cell lines derived from late embryonic stages of Drosophila melanogaster. J Embryol Exp Morphol 27:353–365. [PubMed] [Google Scholar]
- 52.Muller PY, Janovjak H, Miserez AR, Dobbie Z. 2002. Processing of gene expression data generated by quantitative real-time RT-PCR. Biotechniques 32:1372–1379. [PubMed] [Google Scholar]
- 53.Bates D, Mäechler M, Bolker B, Walker S, Christensen RHB, Singmann H, Dai B, Grothendieck G. 2014. lme4: linear mixed-effects models using Eigen and S4. R package version 1.1-7. R Foundation for Statistical Computing, Vienna, Austria: http://cran.r-project.org/package=lme4. [Google Scholar]
- 54.Lemaitre B, Meister M, Govind S, Georgel P, Steward R, Reichhart JM, Hoffmann JA. 1995. Functional-analysis and regulation of nuclear import of Dorsal during the immune-response in Drosophila. EMBO J 14:536–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. 1996. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 86:973–983. doi: 10.1016/S0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 56.Kylsten P, Samakovlis C, Hultmark D. 1990. The cecropin locus in Drosophila: a compact gene-cluster involved in the response to infection. EMBO J 9:217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wicker C, Reichhart JM, Hoffmann D, Hultmark D, Samakovlis C, Hoffmann JA. 1990. Characterization of a Drosophila cDNA-encoding a novel member of the diptericin family of immune peptides. J Biol Chem 265:22493–22498. [PubMed] [Google Scholar]
- 58.Bosco-Drayon V, Poidevin M, Boneca IG, Narbonne-Reveau K, Royet J, Charroux B. 2012. Peptidoglycan sensing by the receptor PGRP-LE in the Drosophila gut induces immune responses to infectious bacteria and tolerance to microbiota. Cell Host Microbe 12:153–165. doi: 10.1016/j.chom.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 59.Vodovar N, Vinals M, Liehl P, Basset A, Degrouard J, Spellman P, Boccard F, Lemaitre B. 2005. Drosophila host defense after oral infection by an entomopathogenic Pseudomonas species. Proc Natl Acad Sci U S A 102:11414–11419. doi: 10.1073/pnas.0502240102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Buchon N, Broderick NA, Poidevin M, Pradervand S, Lemaitre B. 2009. Drosophila intestinal response to bacterial infection: activation of host defense and stem cell proliferation. Cell Host Microbe 5:200–211. doi: 10.1016/j.chom.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 61.McGraw EA, Merritt DJ, Droller JN, O'Neill SL. 2002. Wolbachia density and virulence attenuation after transfer into a novel host. Proc Natl Acad Sci U S A 99:2918–2923. doi: 10.1073/pnas.052466499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chrostek E, Teixeira L. 2015. Mutualism breakdown by amplification of Wolbachia genes. PLoS Biol 13:e1002065. doi: 10.1371/journal.pbio.1002065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Min KT, Benzer S. 1997. Wolbachia, normally a symbiont of Drosophila, can be virulent, causing degeneration and early death. Proc Natl Acad Sci U S A 94:10792–10796. doi: 10.1073/pnas.94.20.10792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Agarwal A, Dash PK, Singh AK, Sharma S, Gopalan N, Rao PVL, Parida MM, Reiter P. 2014. Evidence of experimental vertical transmission of emerging novel ECSA genotype of Chikungunya virus in Aedes aegypti. PLoS Negl Trop Dis 8:e2990. doi: 10.1371/journal.pntd.0002990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Esteva L, Vargas C. 2000. Influence of vertical and mechanical transmission on the dynamics of dengue disease. Math Biosci 167:51–64. doi: 10.1016/S0025-5564(00)00024-9. [DOI] [PubMed] [Google Scholar]
- 66.Thongrungkiat S, Maneekan P, Wasinpiyamongkol L, Prummongkol S. 2011. Prospective field study of transovarial dengue-virus transmission by two different forms of Aedes aegypti in an urban area of Bangkok, Thailand. J Vector Ecol 36:147–152. doi: 10.1111/j.1948-7134.2011.00151.x. [DOI] [PubMed] [Google Scholar]
- 67.Khin MM, Than KA. 1983. Trans-ovarial transmission of dengue-2 virus by Aedes aegypti in nature. Am J Trop Med Hyg 32:590–594. [DOI] [PubMed] [Google Scholar]
- 68.Fouque F, Garinci R, Gaborit P. 2004. Epidemiological and entomological surveillance of the co-circulation of DEN-I, DEN-2 and DEN-4 viruses in French Guiana. Trop Med Int Health 9:41–46. doi: 10.1046/j.1365-3156.2003.01166.x. [DOI] [PubMed] [Google Scholar]
- 69.Gunther J, Martinez-Munoz JP, Perez-Ishiwara DG, Salas-Benito J. 2007. Evidence of vertical transmission of dengue virus in two endemic localities in the state of Oaxaca, Mexico. Intervirology 50:347–352. doi: 10.1159/000107272. [DOI] [PubMed] [Google Scholar]
- 70.Joshi V, Mourya DT, Sharma RC. 2002. Persistence of dengue-3 virus through transovarial transmission passage in successive generations of Aedes aegypti mosquitoes. Am J Trop Med Hyg 67:158–161. [DOI] [PubMed] [Google Scholar]
- 71.Rosen L. 1987. Sexual transmission of dengue viruses by Aedes albopictus. Am J Trop Med Hyg 37:398–402. [PubMed] [Google Scholar]
- 72.Buckner EA, Alto BW, Lounibos LP. 2013. Vertical transmission of Key West dengue-1 virus by Aedes aegypti and Aedes albopictus (Diptera:Culicidae) mosquitoes from Florida. J Med Entomol 50:1291–1297. doi: 10.1603/ME13047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mourya DT, Gokhale MD, Basu A, Barde PV, Sapkal GN, Padbidri VS, Gore MM. 2001. Horizontal and vertical transmission of dengue virus type 2 in highly and lowly susceptible strains of Aedes aegypti mosquitoes. Acta Virol 45:67–71. [PubMed] [Google Scholar]
- 74.Hughes H, Britton NF. 2013. Modelling the use of Wolbachia to control dengue fever transmission. Bull Math Biol 75:796–818. doi: 10.1007/s11538-013-9835-4. [DOI] [PubMed] [Google Scholar]
- 75.Ndii MZ, Hickson RI, Mercer GN. 2012. Modelling the introduction of Wolbachia into Aedes aegypti mosquitoes to reduce dengue transmission. ANZIAM J 53:213–227. doi: 10.1017/S1446181112000132. [DOI] [Google Scholar]
- 76.Ndii MZ, Hickson RI, Allingham D, Mercer GN. 2015. Modelling the transmission dynamics of dengue in the presence of Wolbachia. Math Biosci 262:157–166. doi: 10.1016/j.mbs.2014.12.011. [DOI] [PubMed] [Google Scholar]