Abstract
Burkholderia glumae PG1 is a soil-associated motile plant-pathogenic bacterium possessing a cell density-dependent regulation system called quorum sensing (QS). Its genome contains three genes, here designated bgaI1 to bgaI3, encoding distinct autoinducer-1 (AI-1) synthases, which are capable of synthesizing QS signaling molecules. Here, we report on the construction of B. glumae PG1 ΔbgaI1, ΔbgaI2, and ΔbgaI3 mutants, their phenotypic characterization, and genome-wide transcriptome analysis using RNA sequencing (RNA-seq) technology. Knockout of each of these bgaI genes resulted in strongly decreased motility, reduced extracellular lipase activity, a reduced ability to cause plant tissue maceration, and decreased pathogenicity. RNA-seq analysis of all three B. glumae PG1 AI-1 synthase mutants performed in the transition from exponential to stationary growth phase revealed differential expression of a significant number of predicted genes. In comparison with the levels of gene expression by wild-type strain B. glumae PG1, 481 genes were differentially expressed in the ΔbgaI1 mutant, 213 were differentially expressed in the ΔbgaI2 mutant, and 367 were differentially expressed in the ΔbgaI3 mutant. Interestingly, only a minor set of 78 genes was coregulated in all three mutants. The majority of the QS-regulated genes were linked to metabolic activities, and the most pronounced regulation was observed for genes involved in rhamnolipid and Flp pilus biosynthesis and the type VI secretion system and genes linked to a clustered regularly interspaced short palindromic repeat (CRISPR)-cas gene cluster.
INTRODUCTION
Quorum sensing (QS) is a cell density-dependent gene regulation system in bacteria (1) in which the population density is sensed through the accumulation of bacterially produced signaling molecules called autoinducers (AIs). This cell-to-cell signaling process allows the microbial population to synchronize group behavior and alter its gene expression accordingly. QS is involved in a wide array of regulatory circuits, among which are pathogenicity, secretion of extracellular proteins, secondary metabolite production, and others (2). Key QS signaling molecules in many Gram-negative bacteria are N-acyl-homoserine lactones (AHLs) (3–5), synthesized mainly through LuxI homologs (EC 2.3.1.184) using S-adenosylmethionine (SAM), and an acyl-acyl carrier protein (acyl-ACP) from the fatty acid biosynthesis pathway (6). LuxR-type receptor/regulator proteins are involved in AHL signal perception. Together with LuxR, other proteins may be part of this regulatory circuit.
The motile, rod-shaped Gram-negative soil bacterium Burkholderia glumae is considered to be a seed-borne pathogen that causes panicle blight of rice (7). B. glumae has also been reported to infect other plant species, like tomato, sunflower, and pepper (8, 9). Although it is not classified as a human pathogen, a single case of the isolation of B. glumae from a clinical sample was reported (10), indicating that at least some strains of this pathogen may be associated with opportunistic infections in humans. The phytopathogenicity of B. glumae is caused by multiple factors, including the biosynthesis of toxoflavin (11, 12); motility (13); the secretion of virulence factors by a type III secretion system (T3SS) (14); and the production of lipase (15), catalase (16), and pectate lyase (17). Since the expression of many of the respective genes is controlled by AHLs, QS plays a major role during plant infection processes as well (11, 18).
The first evidence of the presence of QS in the Burkholderia genus was obtained in 1995 for a strain within the Burkholderia cepacia complex (collectively called Bcc) from cross-feeding experiments with Pseudomonas aeruginosa (19). Since then, all described Burkholderia species have been found to employ at least one AHL-mediated QS system. Several species, like Burkholderia thailandensis, Burkholderia mallei, and Burkholderia pseudomallei (the so-called Bptm group), hold multiple QS systems driven by diverse AHL signaling molecules (20–22). Strain B. glumae BGR1 was reported to possess a single QS system homologous to LuxI/LuxR, designated TofI/TofR, involved in the biosynthesis and transport of toxoflavin (11). TofI, which is an AHL synthase belonging to the LuxI protein family (15, 23), catalyzes the synthesis of an N-hexanoyl-homoserine lactone (C6-AHL) and an N-octanoyl-homoserine lactone (C8-AHL) (8, 11, 24, 25). The tofR gene encodes its cognate LuxR-type receptor protein. In BGR1, C8-AHL forms a complex with TofR which regulates subordinated cellular processes like motility and toxoflavin biosynthesis, which are pivotal for the pathogenicity of B. glumae.
Recently, the complete genome sequence of B. glumae strain PG1 (here called BGPG1) was determined (26). Interestingly and in contrast to the findings for BGR1, the 7.9-Mbp genome of BGPG1 codes for three distinct AHL synthase genes designated bgaI1 to bgaI3. BgaI1 is highly similar to the AHL synthase TofI found in BGR1 (95% identity at the amino acid level). BgaI2 is similar to the AI synthase from B. thailandensis (GenBank accession no. WP_006029278) with 53% identity at the amino acid level, and BgaI3 has 46% identity at the amino acid level to an AI synthase from B. mallei (GenBank accession no. WP_004195479) (see Fig. S1 in the supplemental material). Although the presence of multiple AHL synthase genes is a common feature within the Burkholderia genus (20), the presence of three AHL synthase genes within the species B. glumae is currently a unique feature of BGPG1 only.
Until now, only a few high-resolution RNA sequencing (RNA-seq) studies that give a detailed first insight into the expression profiles of QS-regulated genes in selected Burkholderia species have been published. These studies analyzed the QS-dependent expression profiles in Burkholderia cenocepacia (27), B. thailandensis (28), Burkholderia gladioli (29), and B. mallei and B. pseudomallei (30). While B. thailandensis is a nonpathogenic tropical soil microorganism, B. cenocepacia, B. mallei, and B. pseudomallei are considered human and animal pathogens (31–33). Interestingly, the B. thailandensis and B. pseudomallei strains that have been analyzed were found to harbor three AHL-based systems, while the genome of the B. mallei isolate analyzed codes only for two AHL synthases. Very recently, the results of an RNA-seq analysis for B. gladioli, which causes diseases in both plants and humans and possesses two systems homologous to LuxI and LuxR, were published (29). Also, a low-coverage RNA-seq analysis of the plant-associated strain B. glumae BGR1 was reported (34, 35).
This report describes the expression profiling by high-resolution RNA-seq of the BGPG1 wild-type strain and compares the profile obtained to the profiles of three newly constructed deletion mutants, namely, BGPG1 ΔbgaI1 (BGPG2), BGPG1 ΔbgaI2 (BGPG3), and BGPG1 ΔbgaI3 (BGPG4). Additionally, the phenotypes of the wild-type and mutant strains were comparatively studied, and a common set of orthologous QS-regulated genes was identified in B. glumae PG1 and the recently studied model organisms from the Bptm group (28, 30).
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains and plasmids used in the present study are listed in Table 1. B. glumae PG1 strains and Chromobacterium violaceum CV026 were grown in lysogeny broth (LB) medium (36) at 30°C; Escherichia coli strains were cultivated in LB medium at 37°C. Agrobacterium tumefaciens NTL4 (37) was grown in LB medium or AT medium (38) at 30°C. Antibiotics were added to the cultures, when required, at the following final concentrations: for B. glumae, chloramphenicol was added at 25 μg/ml and gentamicin (Gm) was added at 20 μg/ml; for C. violaceum, chloramphenicol was added at 25 μg/ml; for E. coli, ampicillin was added at 100 μg/ml, gentamicin was added at 10 μg/ml, and kanamycin was added at 25 μg/ml; and for A. tumefaciens, tetracycline was added at 5 μg/ml and spectinomycin was added at 50 μg/ml. For the growth of E. coli WM3064, meso-diaminopimelic acid (DAP) was added to a final concentration of 300 μM. Motility assays were done as previously described (39), but the incubation time was 3 days and a glucose concentration of 45 mM was used. Agar concentrations were 0.45% (wt/vol) for swarming tests and 0.25% (wt/vol) for swimming tests. Sedimentation assays were performed in LB medium according to the previously described method (40), and the sedimentation time was extended to 42 h. For the tributyrin (TBT) plate assay, LB medium was homogenized with 1% TBT as an indicator substrate for lipolytic activity prior to autoclaving. Tetrazolium chloride (TZC) medium was prepared as described previously (41).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant trait(s)a | Source or reference |
|---|---|---|
| Strains | ||
| B. glumae | ||
| PG1 | Wild-type strain CBS 322.89 (CBS, Utrecht, The Netherlands) | 84 |
| PG2 | ΔbgaI1 mutant of B. glumae PG1; Gmr | This study |
| PG3 | ΔbgaI2 mutant of B. glumae PG1; Gmr | This study |
| PG4 | ΔbgaI3 mutant of B. glumae PG1; Gmr | This study |
| PG2_c | BGPG2 carrying the bgaI1 gene in pBBR1MCS-2 | This study |
| PG3_c | BGPG3 carrying the bgaI2 gene in pBBR1MCS-2 | This study |
| PG4_c | BGPG4 carrying the bgaI3 gene in pBBR1MCS-2 | This study |
| C. violaceum CV026 | Reporter strain for AI-1; mini-Tn5 mutant of C. violaceum ATCC 31532 | 44 |
| E. coli | ||
| WM3064 | thrB1004 pro thi rpsL hsdS lacZΔM15 RP4-1360 Δ(araBAD)567 ΔdapA1341::[erm pir(wt)] | W. Metcalf, University of Illinois, Urbana-Champaign, USA |
| DH5α | F− ϕ80dlacZΔM15 Δ(argF-lacZYA)U169 endA1 hsdR17(rK− mK−) supE44 thi-1 recA1 gyrA96 relA1 | 85 |
| A. tumefaciens NTL4(pCF218)(pCF372) | Reporter strain for AHL detection; traI::lacZ Tetr Spr | 86 |
| Plasmids | ||
| pDrive | Vector for PCR cloning, Qiagen PCR cloning kit; Ampr Kmr | Qiagen, Hilden, Germany |
| pGEM-T | Vector for PCR cloning; Promega Easy Vector systems; Ampr | Promega, Mannheim, Germany |
| pNPTS138-R6KT | Suicide plasmid for in-frame deletions; mobRP4+ ori-R6K sacB Kmr | 43 |
| pNPTS138-R6KT::ΔbgaI1-Gm | Deletion cassette ΔbgaI1-Gm in pNPTS138-R6KT | This study |
| pNPTS138-R6KT::ΔbgaI2-Gm | Deletion cassette ΔbgaI2-Gm in pNPTS138-R6KT | This study |
| pNPTS138-R6KT::ΔbgaI3-Gm | Deletion cassette ΔbgaI3-Gm in pNPTS138-R6KT | This study |
| pBBR1MCS-2 | Broad-host-range vector, low copy no.; Kmr | 42 |
| pBBR1MCS-5 | Broad-host-range vector, low copy no.; Gmr | 42 |
| pBBR1MCS-2::bgaI1 | pBBR1MCS-2 carrying the bgaI1 gene in the MCS | This study |
| pBBR1MCS-2::bgaI2 | pBBR1MCS-2 carrying the bgaI2 gene in the MCS | This study |
| pBBR1MCS-2::bgaI3 | pBBR1MCS-2 carrying the bgaI3 gene in the MCS | This study |
| pBBR1MCS-2::Pabc1::mCherry | pBBR1MCS-2 carrying the promoter of the Abc1 gene and mCherry in the MCS | This study |
| pBBR1MCS-2::PcysB::mCherry | pBBR1MCS-2 carrying the promoter of the cysB gene and mCherry in the MCS | This study |
| pBBR1MCS-2::PrhlA::mCherry | pBBR1MCS-2 carrying the promoter of the rhlA gene and mCherry in the MCS | This study |
| pDrive::bgaI1 | pDrive vector carrying the bgaI1 gene in the MCS | This study |
| pDrive::bgaI2 | pDrive vector carrying the bgaI2 gene in the MCS | This study |
| pDrive::bgaI3 | pDrive vector carrying the bgaI3 gene in the MCS | This study |
wt, wild type; MCS, multiple-cloning site.
Molecular methods.
DNA cloning and PCR procedures were performed according to standard protocols (36); the primers are listed in Table 2. Genomic DNA of BGPG1 and the corresponding mutants was isolated using an Aqua Pure genomic DNA kit (Bio-Rad Laboratories, Hercules, Canada). Isolation of plasmid DNA was performed with a high-speed plasmid minikit (Geneaid Biotech Ltd., Taiwan, China). Plasmid transformation in E. coli was done following standard heat shock protocols (36). Conjugations in B. glumae were performed by biparental mating using E. coli WM3064 as the donor and incubation at 30°C overnight (36).
TABLE 2.
Primers used in this study
| Oligonucleotide | Sequencea |
|---|---|
| M13-20 for | GTAAAACGACGGCCAGT |
| M13 rev | CAGGAAACAGCTATGACC |
| T7 promoter | TAATACGACTCACTATAGGG |
| SP6 promoter | CATTTAGGTGACACTATAG |
| BgaI1_f | ACGACATCGAGTTCGGCGTGTTC |
| BgaI1_r | AGCAGACCGTGTCTTCGGCATTG |
| BgaI2_f | GAGGCGGCGCGATACTATCAAC |
| BgaI2_r | CGCGAGATCGACGTGCTCAAGTG |
| BgaI3_f | AAAGATTGGGCACGCGATCGAATCC |
| BgaI3_r | ATCTTCAGCTTCCGCAGCTACCG |
| BgaI1_uf | CGGATCCGCGGACTATCCGGTTGCGATCCAC |
| BgaI1_ur | CAAGCTTGATCGACATCGACGCGCAGAC |
| BgaI1_df | CAAGCTTGCGGGAACACTTCCTGCAACAGGTAG |
| BgaI1_dr | GACGCGTCGTCGGCTGGGACTGGTATCTCGAAC |
| BgaI2_uf | GGGATCCGAGCTGCTCGAGGAATAC |
| BgaI2_ur | AGCAAGCTTCCAGTTTCTCGACGAACAC |
| BgaI2_df | ACTAAGCTTGCTTCAGCGCAGCAAAC |
| BgaI2_dr | GGAATTCGGGATCGTCGAGGGATG |
| BgaI3_uf | TGGATCCGTCATCGCTTGATGCTTGG |
| BgaI3_ur | CGAAAGCTTCAGGTGCTTGACGAAC |
| BgaI3_df | ACAAAGCTTACCGGAAGAAGGGATTCAG |
| BgaI3_dr | AGAATTCAGACCGCCGAGAACATCGTG |
| BgaI1_in_1f | GAACAGCCGCTCGATGCTGCAGAAC |
| BgaI1_in_1r | GTTCTGCAGCATCGAGCGGCTGTTC |
| BgaI1_out_2f | CGTGACGAACATGAGCGAACCCATC |
| BgaI1_out_2r | ACAGCTCCCACGCTGTCATTCTTGC |
| BgaI2_in_1f | CCTATCTGCTCTCCGACGTGTTC |
| BgaI2_in_1r | TGAGCTCGATCCAGCAGGCGAAG |
| BgaI2_out_2f | AGGCGGACTTCTTCGGCTACCAG |
| BgaI2_out_2r | CAGACCGTGATGATCTCGAACTACC |
| BgaI3_in_1f | AAGTGGGACCTGCCGATGGTCTC |
| BgaI3_in_1r | CTTCCGGTAGAGCCGCATCATGG |
| BgaI3_out_2f | GCTTGTTCGCAGTGTAGTCCGAAGC |
| BgaI3_out_2r | GTCGCGCTGATCTCGACGATCAACG |
| BGPG1_abc1_f | CGGGATCCCGTTGGGCTTGAAGTCGTTGAG |
| BGPG1_abc1_r | GGAATTCCATCGCCACCAGCACGAACAC |
| BGPG1_cysB_f | CGGGATCCCGACGTGCAGAACAAGAAGGTC |
| BGPG1_cysB_r | GGAATTCCAATTCGCACTTGCCGTGCAG |
| BGPG1_rhlA_f | CGGGATCCCGACATAGCACGGAATGCATGG |
| BGPG1_rhlA_r | GGAATTCCTCTCGAACGACGGATCGTAG |
Underlined nucleotides indicate restriction sites.
Construction of B. glumae PG1 AHL synthase mutants.
Three insertion cassettes, namely, ΔbgaI1-Gm, ΔbgaI2-Gm, and ΔbgaI3-Gm, were assembled using the flanking regions of the bgaI1, bgaI2, and bgaI3 genes (400- to 770-bp up- and downstream fragments of the respective genes) and cloned into a gentamicin resistance gene derived from the broad-host-range cloning vector pBBR1MCS-5 (42). The three cassettes were then separately inserted into the suicide vector pNPTS138-R6KT (43), and the three constructs obtained were introduced into BGPG1 by conjugation. Mutants resulting from the first crossover event were selected on LB plates containing gentamicin and kanamycin and subsequently plated onto LB plates containing 10% (wt/vol) sucrose but lacking kanamycin. Kanamycin-sensitive colonies were selected and verified by direct colony PCR using different primer pairs flanking the respective bgaI1, bgaI2, and bgaI3 genes. The mutations obtained were confirmed by DNA sequencing, and the mutants were designated BGPG2 for B. glumae PG1 ΔbgaI1, BGPG3 for B. glumae PG1 ΔbgaI2, and BGPG4 for B. glumae PG1 ΔbgaI3 (Table 1). The corresponding deletion positions or the locations of the gentamicin resistance gene insertions in all three mutants are shown in Fig. 1A. Complementation of the mutants was achieved by reintroducing the three AHL synthase genes (bgaI1 to bgaI3) back into the respective mutant strains, BGPG2 to BGPG4, using the broad-host-range vector pBBR1MCS-2 (42). The complemented mutants were designated BGPG2_c for complemented B. glumae PG2, BGPG3_c for complemented B. glumae PG3, and BGPG4_c for complemented B. glumae PG4 (Table 1), and their correctness was verified by PCR. Recombinant E. coli strains carrying the bgaI1 to bgaI3 genes were constructed using a Qiagen PCR cloning kit (Qiagen, Hilden, Germany) and employed for determination of the acyl side chain length as outlined below.
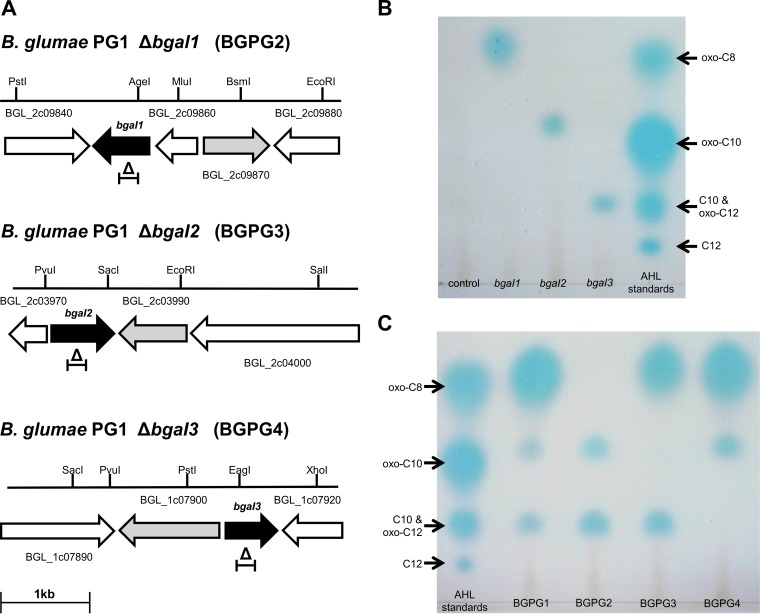
FIG 1.
Partial physical maps of the three B. glumae PG1 AHL synthase mutants and AHL detection on TLC plates using reporter strain A. tumefaciens NTL4. (A) Physical maps showing the AHL synthase genes bgaI1, bgaI2, and bgaI3, the positions of their deletions, and their flanking regions. Black arrows, the bgaI1, bgaI2, and bgaI3 genes; gray arrows, their putative LuxR-type regulatory genes; white arrows, flanking open reading frames. Open reading frames are designated using the numbers from the sequence with NCBI GenBank database accession no. CP002580 and CP002581. Triangles, the positions of the deletions, which were located from 1,237,902 bp to 1,238,222 bp (chromosome 2) for the ΔbgaI1 mutant, 486,784 bp to 487,479 bp (chromosome 2) for the ΔbgaI2 mutant, and 899,130 bp to 99,528 bp (chromosome 1) for the ΔbgaI3 mutant. The bars at the top show the identified restriction sites and their positions. (B) TLC chromatogram of AHL profiles of B. glumae PG1. The chromatogram illustrates the AHLs obtained from recombinant E. coli clones carrying bgaI1 to bgaI3 separated by TLC and detected by using the A. tumefaciens NTL4 biosensor as an overlay. (C) TLC analysis of the AHLs produced by parent strain BGPG1 and mutants BGPG2 to BGPG4 using the A. tumefaciens NTL4 biosensor as an overlay. AHL standards consist of oxo-C8 (3-oxo-C8-AHL), oxo-C10 (3-oxo-C10-AHL), C10 (C10-AHL), oxo-C12 (3-oxo-C12-AHL), and C12 (C12-AHL). All AHL standards used were obtained from Sigma-Aldrich and Biomol GmbH, Hamburg, Germany.
Detection of AHLs by C. violaceum CV026 and A. tumefaciens NTL4.
BGPG1 and the three mutant strains were grown in 100 ml LB for 28 h at 30°C, and E. coli harboring the bgaI1 to bgaI3 genes was grown in 100 ml LB for 18 h at 37°C. AHLs were extracted from stationary-phase cultures with a 3-fold volume of ethyl acetate, concentrated in vacuo, and resuspended in 500 μl ethyl acetate. The reporter strain, C. violaceum CV026 (44), was used for the detection of AHL molecules in cell extracts by quantification of violacein as previously described (45) with minor modifications. For the initial separation and subsequent detection of AHLs, thin-layer chromatography (TLC) was carried out, followed by AHL detection using the A. tumefaciens reporter strain NTL4 as previously described (40).
Onion maceration and rice pathogenicity assays.
The plant maceration capabilities of BGPG1 and the mutant strains were tested by an onion maceration assay (46) after incubation for 72 h at 30°C. Rice (Oryza sativa cv. Baldo) seeds were sterilized with 70% (vol/vol) ethanol for 5 min and washed once with sterile water, followed by 20 min of incubation in 3% H2O2 and rinsing three times with sterile water. Pretreated seeds were infected with 107 cells/ml of BGPG1 or the mutants and pregerminated for 2 days at 37°C, transferred into sterile petri dishes containing three layers of filter paper (diameter, 90 mm; Whatman no. 1) that had been wetted with 60 ml of sterile water, and subjected to growth cycles of 16 h in the light and 8 h in the dark at 28°C. Germination rates were calculated after 7 days, and seedling lengths were measured after 2 weeks.
Preparation of B. glumae transcriptome samples.
The culture samples of BGPG1 used for the transcriptome analysis are summarized in Table 3. Prior to cultivation of 100-ml cultures, precultures were established from cryocultures in 5 ml LB medium and cultivated at 30°C and 200 rpm. For the transcriptome analyses of early-stationary-growth-phase cultures, 100 ml LB medium was inoculated with freshly grown precultures of the BGPG1 wild type as well as the BGPG1 mutant strains, and the bacteria were cultivated as batch cultures for approximately 28 h at 30°C and 200 rpm. After 28 h the cultures were separated into fractions of 45 ml, which were then transferred into Falcon tubes, chilled on ice, and centrifuged at 13,000 rpm for 5 min. The supernatants were discarded, and samples were directly frozen in liquid nitrogen and stored at −70°C until further use.
TABLE 3.
Overall transcriptome statistics for the 12 samples from strains BGPG1 to BGPG4 analyzeda
| Sample no. | BGPG1 genotype | OD600 | No. of reads generated (106) | No. of uniquely mapped reads (106) |
|---|---|---|---|---|
| 1 | wt | 3.13 | 39.3 | 32.7 |
| 2 | wt | 3.15 | 45.3 | 37.9 |
| 3 | wt | 3.09 | 39.8 | 34.4 |
| 4 | ΔbgaI1 | 2.84 | 43.4 | 33.0 |
| 5 | ΔbgaI1 | 2.84 | 32.5 | 25.0 |
| 6 | ΔbgaI1 | 2.81 | 32.8 | 28.6 |
| 7 | ΔbgaI2 | 3.08 | 44.8 | 32.1 |
| 8 | ΔbgaI2 | 3.05 | 33.5 | 26.1 |
| 9 | ΔbgaI2 | 3.07 | 34.7 | 26.4 |
| 10 | ΔbgaI3 | 2.98 | 42.1 | 33.4 |
| 11 | ΔbgaI3 | 2.92 | 34.6 | 26.8 |
| 12 | ΔbgaI3 | 2.91 | 33.5 | 24.6 |
Cultures were harvested after 28 h of growth in the transition from exponential to stationary growth phase. wt, wild type.
RNA extraction, library construction, sequencing, and analysis of transcriptome samples.
For the BGPG1 wild-type and mutant strains, RNA-seq libraries were constructed from independent biological triplicates of RNA samples. Total RNA was extracted using an RNeasy minikit (Qiagen, Hilden, Germany) and the protocol for purification of total RNA from the bacteria, with the following exceptions: to include the small RNA fraction (<200 nucleotides), the proportion of ethanol to supernatant was raised to 1.5:1 (vol/vol) after cell disruption (step 6), and buffer RWT was used instead of buffer RW1 to wash the column (step 8). The residual genomic DNA was removed from the isolated total RNA by DNase I (Fermentas, St. Leon-Rot, Germany) treatment. To reduce the amount of rRNA-derived sequences, the samples were subjected to rRNA depletion using a Ribo-Zero magnetic kit (Epicentre Biotechnologies, Madison, WI, USA). The strand-specific cDNA libraries for sequencing were constructed with a NEBNext Ultra directional RNA library preparation kit for Illumina (New England BioLabs, Frankfurt am Main, Germany). The cDNA libraries obtained were sequenced by using a GAIIx instrument (Illumina Inc., San Diego, CA, USA) in the single-read mode and running 75 cycles. For the 12 samples analyzed, we retrieved between 32.5 million and 45.3 million raw reads (Table 3). To ensure high sequence quality, the remaining sequencing adaptors were removed and the reads with a cutoff phred-33 score of 15 were trimmed by the program Trimmomatic (47). The remaining sequences were mapped with the Bowtie (version 2) program (48) using the implemented end-to-end mode, which requires that the entire read align from one end to the other. Differential expression analyses were performed with the baySeq program (49). Genes with a fold change in expression of ≥2.0, a likelihood value of ≥0.9, and an adjusted P value of ≤0.05 (the P value was corrected by the false discovery rate [FDR] on the basis of the Benjamini-Hochberg procedure) were considered differentially expressed.
Quantitative RT-PCR (qRT-PCR).
RNA from a 2-ml culture was routinely extracted using the RNeasy minikit (Qiagen, Hilden, Germany). DNase I digestion was performed both on column with an RNase-free DNase set (Qiagen, Hilden, Germany) and after RNA elution with DNase I (RNase-free) from Ambion (Life Technologies, Darmstadt, Germany) according to the manufacturers' instructions. The transcription of isolated mRNA into cDNA was carried out with a Maxima first-strand cDNA synthesis kit for reverse transcription (RT)-quantitative PCR (qPCR) (Thermo Scientific, Vilnius, Lithuania) according to the manufacturer's protocol. In a separate reaction, each sample was treated without reverse transcription to exclude the possibility of DNA contamination. The analysis of the transcriptional levels of selected genes was performed by real-time qPCR (35 cycles) using the ΔΔCT threshold cycle (CT) method (50). Here, 50 ng of the reverse-transcribed cDNA was used as the template in a real-time 7900HT Fast real-time PCR system (Applied Biosystems, Foster City, CA, USA) with Maxima SYBR green–carboxy-X-rhodamine qPCR master mix (2×; Thermo Scientific, Vilnius, Lithuania), primers specific for the genes of interest (Table 4), and the constitutively expressed gene rpoD as a reference (see Fig. S2 in the supplemental material). Primers were designed using the Primer3 tool (51). The amount of PCR product was calculated as the CT value by a sequence detection system (version 2.3; Applied Biosystems, Foster City, CA, USA). PCR efficiencies were determined with the tool LinRegPCR (52). Calculations of the changes in transcript levels were performed and statistically analyzed with REST software (53). A change in the transcript level was assumed to be significantly different from that in the control sample if the P value was ≤0.05.
TABLE 4.
Oligonucleotides used for qRT-PCR
| Gene locus | Gene name | Primer sequence (5′-3′) |
|
|---|---|---|---|
| Upstream | Downstream | ||
| BGL_2c18660 | lipA | CTATCCGGTGATCCTCGTC | GAGAGATTCGCGACGTACAC |
| BGL_2c18650 | lipB | GTGGCAGACGCGCTATCAAG | CGTGAAAGTCTGCTGCCTGAG |
| BGL_2c21380 | rpoD | GATGACGACGCAACCCAGAG | GAACGCTTCCTTCAGCAGCA |
| BGL_2c07470 | rhlA | TGAAGCCGGAGGCCTATCTC | TTGCCGATCGTCTCGAACTC |
| BGL_2c07480 | rhlB | TACGTGTCGGTGCAGGTGTC | GTGATGAGCCCCGTCTTCAG |
| BGL_1c18830 | csy1 | TCGCCGTGCAGAAACTTGGC | GCAGATGGTTGAGGCGGCTG |
| BGL_1c18840 | csy2 | TATCGAGGCGCTGCTGGTCC | TTGCAGCGCCCACATCAACC |
| BGL_1c01710 | flhA1 | TCAAGCGGATCAAGAGCATCC | GAGGTTGTCGCGGATATGGA |
| BGL_1c35020 | flgB2 | CGTTCGCTCGTACCGGCAG | CGACGTCGCGGGCCTGGTAG |
Analysis of gene expression using mCherry-based promoter fusions.
Promoter fusions were constructed employing the red fluorescent protein mCherry (designated mCherry in the fusions). All constructs were inserted in the broad-host-range cloning vector pBBR1MCS-2 (42) and mobilized via conjugation into strains BGPG1 to BGPG4. Strains BGPG1 to BGPG4 harboring the constructs were grown in LB medium at 30°C for 48 h, and then culture aliquots of 200 μl were transferred into microtiter plates and analyzed. The fluorescence of mCherry was measured with an excitation filter (590/20-nm filter set) and an emission filter (645/40-nm filter set) and by determination of the absorbance at 600 nm with a Synergy HT multimode microplate reader, and the results were analyzed with Gen5 software (BioTek Instruments Inc., Winooski, VT, USA). The number of relative fluorescence units (RFUs) were corrected by the optical density at 600 nm (OD600) of the analyzed cultures. Data were recorded for a minimum of three independently grown cultures, and each measurement was repeated three times.
SRA accession number.
The trimmed reads have been deposited in the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA) under accession no. SRP047507.
RESULTS AND DISCUSSION
AHL profiles of B. glumae PG1 and bgaI1 to bgaI3 deletion mutants.
The genome of wild-type strain B. glumae PG1 encodes three AHL synthases, and these are flanked by their cognate LuxR-type regulators (Fig. 1A). This is an unusual feature within the species B. glumae, since the genomes of all other currently sequenced strains contain only a single AHL synthase gene. To investigate the QS-mediated gene regulation of these three QS regulons in BGPG1, we initially constructed mutants with a deletion of each of the three identified AHL synthase genes. The obtained mutant strains were designated BGPG2 for B. glumae PG1 ΔbgaI1, BGPG3 for B. glumae PG1 ΔbgaI2, and BGPG4 for B. glumae PG1 ΔbgaI3 (Fig. 1A). Each mutant strain was verified by DNA sequencing and phenotypic analyses.
Production of AI by the B. glumae PG1 wild-type strain and the ΔbgaI mutant strains was tested by TLC analysis with subsequent AHL detection using reporter strain NTL4. Recombinant E. coli strains carrying the bgaI1 to bgaI3 genes produced single spots on the TLC plates (Fig. 1B). For E. coli carrying the bgaI1 gene, a signal that most likely corresponded to the oxo-C8 standard was detected, the clone carrying bgaI2 produced a spot that most likely corresponded to the oxo-C10 standard, and the clone carrying bgaI3 produced a spot that corresponded to either the C10 or the oxo-C12 standard. The parent strain BGPG1 reproducibly produced three spots on such TLC plates which corresponded to the above-described spots of the individual recombinant E. coli clones. Further, each of the ΔbgaI mutants lacked one of these spots, and the individual AHL profiles of strains BGPG2, BGPG3, and BGPG4 were different (Fig. 1C).
Finally, production of AI by the B. glumae PG1 wild-type strain and the ΔbgaI mutants was tested with the reporter strain CV026 (Table 1), which is unable to synthesize AHLs and is therefore impaired in QS-regulated violacein production. While a culture extract of BGPG1 could reproducibly restore violacein production in the reporter strain, extracts of BGPG2 could not complement CV026 at all. However, culture extracts of BGPG3 and BGPG4 complemented CV026 at significantly reduced levels (data not shown). Since CV026 can detect only AHLs with an acyl side chain length ranging from C6 to C8, the data obtained fit nicely with the chain lengths of AHLs estimated using TLC analyses.
Phenotypic analyses of B. glumae PG1 and its AHL synthase mutants. (i) Bacterial motility.
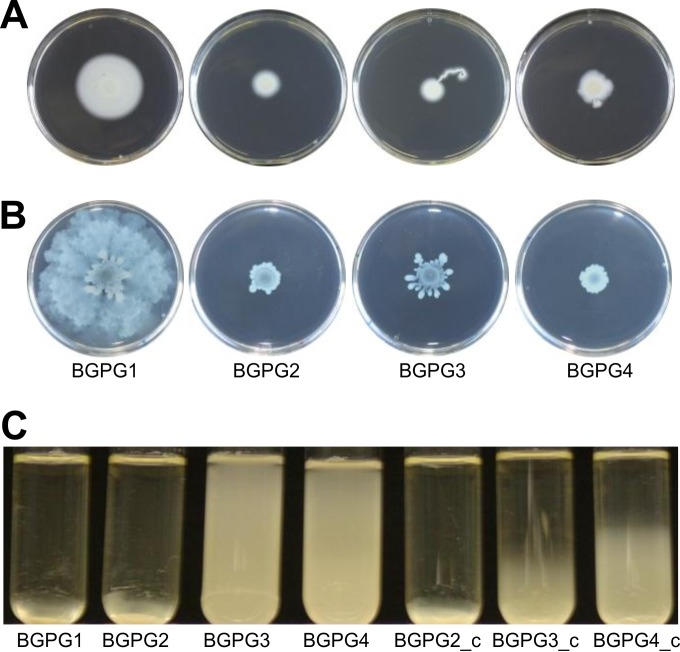
Swimming and swarming assays were performed with BGPG1 and the mutant strains BGPG2 to BGPG4, and it was found that both types of motility were clearly affected in the AHL synthase mutant strains. Figure 2A and B indicate typical results of the motility assays for strains BGPG1 to BGPG4. Complementation analyses revealed that swarming motility could be restored (see Fig. S3 in the supplemental material).
FIG 2.
Assays of B. glumae PG1 and mutant strain motility. (A) Altered swimming motility of BGPG2, BGPG3, and BGPG4 compared to that of wild-type strain BGPG1 on agar plates containing 0.25% Eiken agar (Eiken, Tokyo, Japan). The plates were inoculated with 1 × 107 cells and incubated for 3 days at 28°C. (B) Phenotypes of reduced swarming of BGPG2, BGPG3, and BGPG4 compared to that of wild-type BGPG1 on agar plates containing 0.45% Eiken agar. The plates were inoculated with 1 × 107 cells and incubated for 3 days at 28°C. (C) Sedimentation phenotypes of BGPG1, BGPG2, BGPG3, and BGPG4 in liquid tryptone-yeast extract medium. BGPG1 and BGPG2 show clear sedimentation phenotypes, whereby cultures of BGPG3 and BGPG4 did not settle after 42 h of incubation at room temperature. The sedimentation phenotypes of the BGPG3_c and BGPG4_c strains were partially restored by reintroducing the wild-type bgaI2 and bgaI3 genes into BGPG3 and BGPG4, respectively.
Interestingly, BGPG1 and BGPG2 settled to the bottom of the test tubes after incubation for 42 h. BGPG3 and BGPG4 did not sediment within 42 h. These phenotypes were in part restored by complementation (Fig. 2C), and they indicated a different regulation of the flagellar genes in these QS mutants. Similar sedimentation phenotypes were observed for Sinorhizobium fredii NGR234 (40) and Legionella pneumophila (54, 55) AI synthase mutants.
(ii) Lipolytic activity.
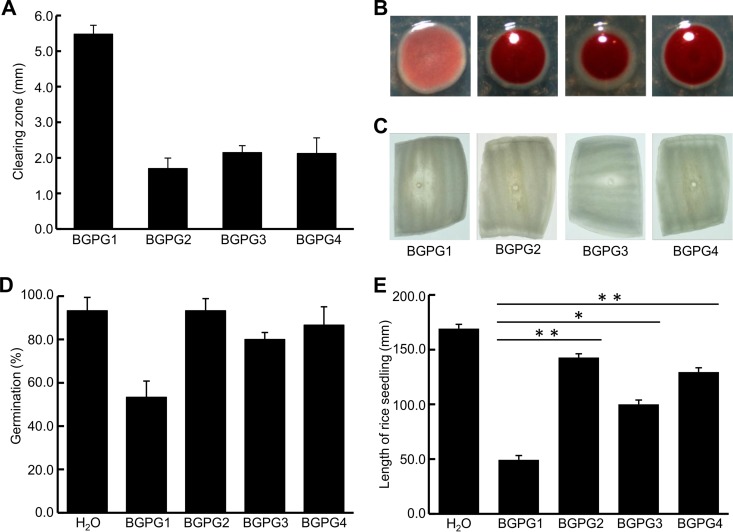
All bgaI deletion mutants were significantly affected in the production and/or secretion of lipolytic enzymes. Clearing zones on indicator plates were, in general, reduced 2.5-fold in the mutant strains compared to the sizes of the clearing zones for the parental strain (Fig. 3A). This observation was confirmed by activity assays using p-nitrophenyl octanoate as the substrate (see Fig. S4 in the supplemental material). Thus, our experimental findings are in line with data obtained for the B. glumae strains AU6208 and ATCC 33617 (15).
FIG 3.
(A) Decreased lipase production. Clearing zones were assayed on agar plates containing TBT. Data are mean values for five colonies analyzed. (B) Colony color and morphology analyzed after growing BGPG1 to BGPG4 on TZC-containing medium for 3 days at 30°C (41). (C) Maceration of onion tissue caused by BGPG1 to BGPG4. Slices of onion bulbs were infected with the wild-type and mutant strains and incubated for 72 h at 30°C. (D and E) AHL synthase mutations reduce inhibition of seed germination (D) and favor fruit development (E). The data in panels D and E are mean values for 10 individual seedlings analyzed per treatment. *, P < 0.05; **, P < 0.01.
(iii) Colony variation and plant-pathogenic phenotypes.
The colony variation of the B. glumae parent strain and the bgaI deletion mutants was assayed by cultivation of the strains on TZC agar plates. In this assay, the colony morphology and color were influenced by the formation of extracellular or capsular polysaccharides, which were, furthermore, directly correlated with virulence (41). Colonies of the AHL synthase mutants could be readily distinguished by a deep red color (Fig. 3B). Since this test is well-known to differentiate between avirulent and virulent strains (41), we speculated that all the mutations would affect the ability to macerate plant tissue. Therefore, we set up onion maceration assays, where the wild-type strain as well as the mutant strains was tested for pathogenicity on detached onion bulb scales. As expected, each of the constructed mutant strains appeared to be attenuated in its ability to macerate onion tissue (Fig. 3C). Here, clearly macerated tissue around the wound was shown for BGPG1, whereas no maceration could be observed for BGPG2 and BGPG4 and clearly reduced maceration could be observed for BGPG3. These tests were done at least three to five times.
Furthermore, to study the virulence of B. glumae PG1 mutants for rice, we monitored the germination rate and recorded the length of developing rice seedlings. All mutant strains showed a reduced pathogenicity on rice seedlings compared to that of BGPG1 (Fig. 3D and E).
Altogether, these findings confirm earlier reports on QS-dependent gene regulation in B. glumae with respect to the role of TofI-dependent genes (11, 13, 15, 18, 56). Since mutations in bgaI2 and bgaI3 also had strong effects on motility, lipase production, and pathogenicity, our findings suggest that the QS-dependent regulatory network is more complex in BGPG1 than in BGR1 and other currently studied B. glumae isolates (15, 56, 57).
Global pattern of QS-dependent gene expression in BGPG1.
The global patterns of gene expression in wild-type strain BGPG1 and the three AHL synthase mutants (BGPG2 to BGPG4) were analyzed at the transition from exponential to stationary growth phase (see Fig. S5 in the supplemental material). We choose this time point, since we speculated that many QS-dependent processes are turned on during the onset of the stationary growth phase. For these experiments, cells were grown for 28 h at 30°C to an OD600 ranging from 2.81 to 3.15 prior to total RNA extraction. Twelve individual samples representing three independent biological samples for each of the four strains were analyzed by RNA-seq (Table 3). Alignments were established, and for each sample a minimum of 24.6 million cDNA reads could be uniquely mapped to the B. glumae genome, resulting in 24.6 million to 37.9 million uniquely mapped reads per treatment.
In the comparative analysis of the RNA-seq data, we considered genes with a fold change of ≥2.0, a likelihood value of ≥0.9, and an FDR value of ≤0.05 to be statistically significantly differentially expressed between the BGPG1 parent strain and the mutant strains. Only values that complied with these three requirements were used for subsequent analyses (see Table S1 in the supplemental material). Expression analysis by use of the qRT-PCR technology was used in part to confirm the RNA-seq data. For this, we analyzed the expression profiles of the genes lipA, lipB, rhlA, rhlB, csy1, csy2, flhA1, flgB2, and rpoD as an internal control in the early-stationary-growth phase. The lipA gene (BGL_2c18660) codes for a lipase, and the lipB gene (BGL_2c18650) codes for a corresponding foldase. The rhlA (BGL_2c07470) and rhlB (BGL_2c07480) genes are involved in rhamnolipd biosynthesis and motility, the csy1 (BGL_1c18830) and csy2 (BGL_1c18840) genes are part of a clustered regularly interspaced short palindromic repeat (CRISPR)-cas system, and the flhA1 (BGL_1c01710) and flgB2 (BGL_1c35020) genes are involved in the buildup of the bacterial flagella. The expression data obtained for these eight genes and the data obtained by qRT-PCR largely confirmed the data obtained by RNA-seq (see Fig. S2 and Table S1 in the supplemental material).
Furthermore, we constructed fusions of the promoters of selected and differentially regulated genes (e.g., the Abc1 gene, cysB, and rhlA) and mCherry and monitored their fluorescence in the BGPG1 wild-type strain and the three QS mutants. The Abc1 gene (BGL_2c03920) codes for a predicted ABC transporter family protein, the cysB gene (BGL_2c03850) codes for a predicted cysteine synthase, and the rhlA gene (BGL_2c07470) codes for the predicted rhamnosyltransferase I subunit A (see Table S1 in the supplemental material). All these genes were chosen because of their assumed QS-dependent regulation and importance for B. glumae metabolism. Comparison of the number of RFUs of strain BGPG1 to the number of RFUs of strains BGPG2 to BGPG4 confirmed in part the observed RNA-seq data and the QS-dependent regulation of these genes (see Fig. S6 in the supplemental material). Thereby, the downregulation of the Abc1 gene could be confirmed for BGPG2 and BGPG3 but not for BGPG4. The use of the PcysB::mCherry promoter fusion confirmed the downregulation of the cysB gene only in BGPG2 and not in BGPG3 and BGPG4. Lastly, the PrhlA::mCherry promoter fusion demonstrated the downregulation of the rhlA gene in the QS mutant BGPG2. This finding is in line with the RNA-seq data obtained for the rhlA gene, where no downregulation was also observed in the background of BGPG3 and BGPG4.
The partly different results in the fluorescence measurements obtained for BGPG3 and BGPG4 may be explained in part by the multicopy effects caused by the use of the promoter fusions in a self-replicable plasmid with multiple copies, which thereby outcompeted the corresponding single-copy regulator. Furthermore, the deviation of the results might also be linked in part to the phenotypic heterogeneous expression of these genes.
bgaI1-, bgaI2-, and bgaI3-specific gene regulation.
The genome of BGPG1 consists of a total of 6,502 predicted genes located on two large chromosomes. Chromosome 1, with a size of 4.164 Mbp, codes for 3,562 genes, and chromosome 2 has a size of 3.733 Mbp and codes for 2,940 genes.
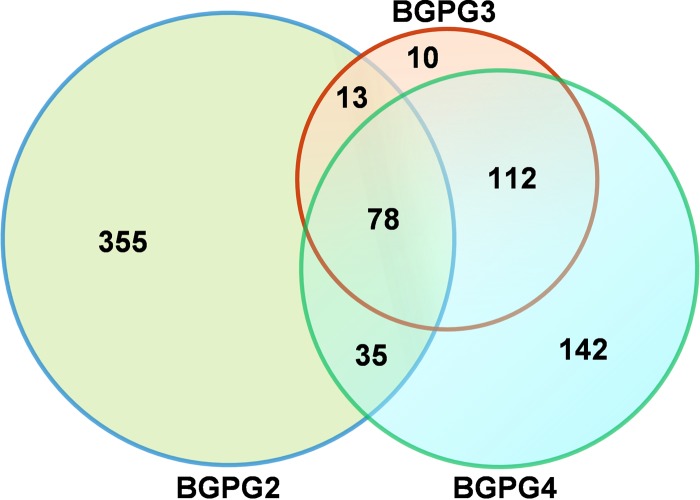
A total of 481 genes (6.5% of all predicted genes) were found to be differentially regulated in the BGPG2 deletion mutant compared to their regulation in the wild-type strain, and the expression of a total of 213 genes (2.9% of all predicted genes) was significantly altered in the BGPG3 mutant. Moreover, the expression of a set of 367 genes (4.9% of all predicted genes) was specifically altered in the BGPG4 mutant strain (Fig. 4). Altogether, a common subset of 78 genes was differentially regulated in the three mutant strains in comparison to their expression in the wild type. In total, 355 genes appeared to be specifically regulated in the BGPG2 strain, 10 genes appeared to be specifically regulated in the BGPG3 strain, and 142 genes appeared to be uniquely regulated in the BGPG4 deletion mutant. Thus, a total of 745 genes (11.5% of all predicted genes) were regulated in a QS-dependent way (Fig. 4). However, with 340 QS-regulated genes being on chromosome 1 and 405 being on chromosome 2, the majority of all QS-regulated genes were located on the second replicon.
FIG 4.
Venn diagram showing the relationship of differentially expressed genes (fold change, ≥2.0; likelihood, ≥0.9; adjusted FDR, ≤0.05) among the BGPG1 AHL synthase mutants. The circles display the number of genes uniquely regulated in each BGPG1 mutant compared with their regulation in the wild-type strain. The circles also show the number of commonly regulated genes within particular relationships. Overall, AHL synthase mutants BGPG2 to BGPG4 share a core set of 78 differentially regulated genes.
Interestingly, two of the QS systems of BGPG1 are found on chromosome 2 (i.e., bgaI1 and bgaI2), while the third one (bgaI3) is encoded on the larger chromosome 1, which harbors the housekeeping genes. This differs from the situation observed in B. thailandensis (28). Further distribution analyses of QS-regulated genes implied that no obvious correlation exists between the deleted AI synthase gene and the corresponding number of differentially regulated genes on the respective chromosome. Overall, the distribution over both replicons is balanced in the background of all three mutant strains.
To date, only a few studies have been performed using genome-wide transcriptome analyses to identify QS-regulated gene expression patterns. Each study focused on a different organism, and the growth conditions also differed; hence, the number of genes that were differentially regulated in response to QS processes varied. In recent studies, the QS regulons represented up to 6.2% of the coding sequences in the P. aeruginosa genome (for lasI and lasR and for rhlI and rhlR) (58), up to 8.1% of the coding sequences in the Yersinia pestis genome (59), up to 8.0% coding sequences in the B. thailandensis genome (28), 0.8% of the coding sequences in the B. mallei genome, 3.6% coding sequences in the B. pseudomallei genome (30), and between 4.9 and 7.3% of the coding sequences in the in S. fredii NGR234 genome (40). We observed that 11.5% of all genes were differentially regulated by the three BGPG1 QS systems, and it was recently reported that up to 19.6% of the BGR1 genes were regulated in a QS-dependent manner in a tofI mutant (34). This value, however, differs from our data, since a different cutoff was used and the overall read coverage of the BGR1 genome was 8- to 35-fold lower. Also, cells were harvested after 8 and 10 h of growth at 37°C in the study by Kim and colleagues (34), while RNA was extracted from our strains after 28 h of growth at 30°C. Finally, it is noteworthy that the data represent those from a comparison of the B. glumae PG1 wild-type strain to its AHL synthase deletion mutants and not those from a comparison of the B. glumae PG1 wild-type strain to its LuxR regulator deletion mutants. Thus, we cannot exclude the possibility of cross talk between the different regulons due to the promiscuity of the LuxR regulator proteins with respect to the recognition of the AHL signaling molecules.
Interplay between the three bgaI QS systems in BGPG1.
Results from our transcriptome analyses implied that deletion of the bgaI1 gene caused a 10.5-fold downregulation of the expression profile of the bgaI2 gene and a 2.9-fold downregulation of the bgaI3 gene (see Table S1 in the supplemental material). The deletion of bgaI2 had no obvious effect on expression of both bgaI1 and bgaI3. The deletion of bgaI3 caused a 5.7-fold downregulation of the expression of bgaI2 but had no obvious effect on the expression of bgaI1 (see Table S1 in the supplemental material). These data suggest that in BGPG1 the three QS systems form a network, with bgaI1 most likely being at the top of the hierarchy, followed by bgaI3. Similarly, other Gram-negative bacteria employ multiple AHL-dependent QS systems to control group behaviors during their life cycle, such as P. aeruginosa and members of the Bptm group but not rhizobial or other isolates (30, 40, 60, 61).
QS-regulated orthologous genes in other Burkholderia species.
Since B. glumae is a plant pathogen, we asked which of the QS-regulated genes are involved in plant infection and which ones are involved in life in the soil. Fortunately, Majerczyk and colleagues (28, 30) have recently described the QS regulons in the tropical soil bacterium B. thailandensis and the human- and animal-pathogenic species B. mallei and B. pseudomallei. The B. thailandensis genome codes for three AHL synthase genes, and B. thailandensis has a nonpathogenic saprophytic lifestyle. A comparison of the QS-dependent genes reported for B. thailandensis and the genes identified in this study uncovered a common set of 61 orthologous genes coregulated in both microbes. Of these, 41 genes were coregulated by bgaI1, 17 genes were coregulated by bgaI2, and 26 genes were coregulated by bgaI3. Further, a direct comparison of the data published by Majerczyk et al. (30) and our data allowed us to identify eight shared and QS-regulated genes in B. pseudomallei but no QS-regulated genes shared between B. mallei and BGPG1. Our data, together with the data set from Majerczyk et al. (28, 30), now allow us to draw the conclusion that no common core set of genes is shared and QS regulated in the four strains analyzed (Table 5). However, a small number of QS-regulated and shared genes can be identified in B. thailandensis, B. pseudomallei, and BGPG1. The absence of shared genes between BGPG1 and B. mallei may indicate a wider phylogenetic distance between these bacteria. It should be noted that a direct functional comparison may add shared functional homologs. Applying this strategy and comparing the functional homologs of QS-regulated genes in the Burkholderia isolates from our study and the two studies by Majerczyk et al. (28, 30), we identified two shared functional homologs in all strains, namely, nonribosomal peptide synthases genes and ABC transporters (Table 5).
TABLE 5.
Shared functional and QS-regulated homologous genes in BGPG1 versus selected members of the Bptm group with multiple AHL QS systems
| Predicted function | Presence of gene for the indicated protein ina: |
|||
|---|---|---|---|---|
| BGPG1 | B. thailandensis | B. pseudomallei | B. mallei | |
| Flagellum biosynthesis | + (21) | + (6) | − | − |
| AHL synthases | + (3) | + (3) | + (1) | − |
| LuxR proteins | + (5) | + (3) | − | + (1) |
| Polyketide biosynthesis (PKS) | + (5) | + (5) | + (4) | − |
| Nonribosomal peptide synthases (NRPS) | + (5) | + (1) | + (2) | + (2) |
| Rhamnosyltransferase I | + (3) | + (2) | − | − |
| T1SS | + (2) | + (1) | − | − |
| T2SS | − | + (1) | − | − |
| T3SS | + (4) | + (1) | + (3) | − |
| T4SS | − | + (2) | − | − |
| T6SS | + (13) | − | − | − |
| Flp pilus assembly | + (9) | − | − | − |
| Lipase A | + (1) | − | − | − |
| Histidine utilization system | + (3) | + (3) | + (1) | − |
| Phosphate metabolism | + (6) | − | − | − |
| Major facilitator family transporter | + (10) | + (5) | + (4) | − |
| Malleilactone biosynthesis | − | + (2) | + (2) | + (2) |
| ABC transporter | + (17) | + (14) | + (7) | + (2) |
| Polysaccharide biosynthesis | + (6) | + (6) | + (1) | − |
| Lipoproteins | + (11) | + (8) | + (1) | − |
| Ribosomal proteins | + (7) | + (1) | + (26) | − |
Function-based evaluation of the BGPG1 transcriptome data.
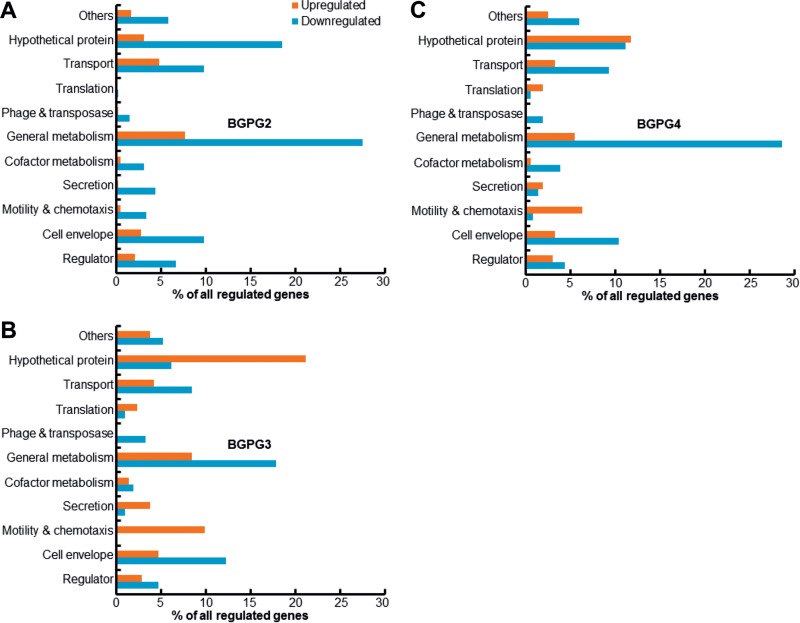
The genes differentially expressed in the three AHL synthase mutants and the parent strain were classified into 11 functional categories on the basis of the KEGG database (http://www.genome.jp/kegg/pathway.html). As indicated in Fig. 5A to C, the genome-wide transcriptome data analysis revealed that the regulated genes were mainly linked to general metabolism (Fig. 6A, cluster 11, and B, clusters 2, 5, and 10) and hypothetical proteins (Fig. 6A, cluster 9, and B, clusters 7 and 9; see also Table S1 in the supplemental material) in all three mutants. Striking changes in the expression of genes in selected important functional categories are discussed below. A complete list of all QS-regulated genes is given in Table S1 in the supplemental material. Some of the identified genes were mentioned in an earlier low-resolution RNA-seq study of B. glumae strain BGR1 and a corresponding AI synthase and receptor mutant (34). Since the BGR1 genome codes for only a single AI synthase gene, overall large differences in the regulatory networks associated with the QS-dependent gene regulation occur in BGR1 and our strain BGPG1.
FIG 5.
Genes differentially expressed (in percent) in BGPG1 mutant strains in the background of BGPG2 versus the wild-type strain (A), BGPG3 versus the wild-type strain (B), and BGPG4 versus the wild-type strain (C). The classification was based on the KEGG database (http://www.genome.jp/kegg/pathway.html).
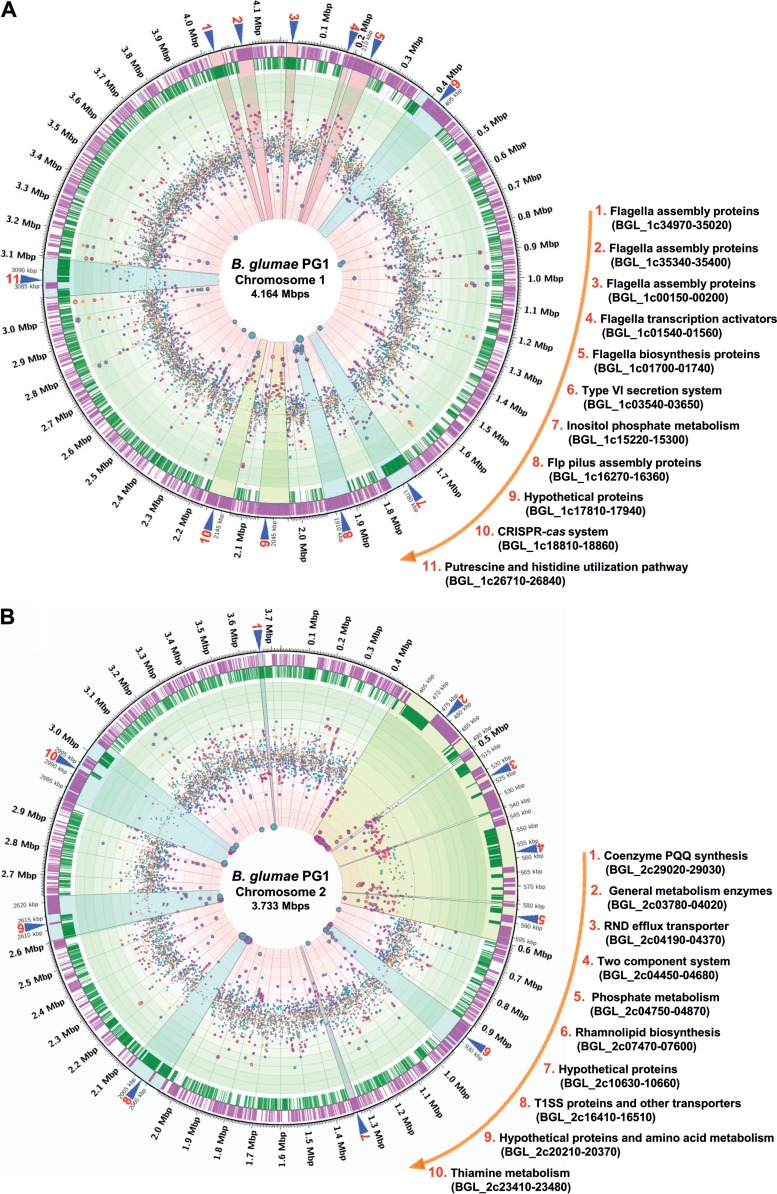
FIG 6.
Circular transcriptome map representing the genome-wide RNA-seq data for mutants BGPG2 to BGPG4 versus parent strain BGPG1 obtained with Circos software (version 0.64) (83). Cyan dots, orange dots, and deep purple dots, genes from strains BGPG2, BGPG3, and BGPG4, respectively; dots with red-violet circles, differentially expressed genes. The cutoff was set to a fold change of 2.0 with an FDR of ≤0.05 (the sizes of the dots correspond to the values obtained). The circles from the outside to the innermost circle are as follows: the first circle indicates the genome coordinates of BGPG1 in mega-base pairs; the second and third circles indicate open reading frames on the leading (purple) and the lagging (deep green) strands. The light green circles represent genes with log2 fold changes of 4, 3, 2, and 1 (from the outside to inside); the light red circles represent genes with log2 fold changes of −1, −2, −3, and −4 (from outside to inside). Highlighted areas (labeled with numbers) are magnified 5-fold and show gene clusters which are differentially expressed in the mutant strains. Clusters in light cyan and light purple, genes differentially expressed only in BGPG2 and BGPG4, respectively; clusters in light pink, genes differentially expressed in both BGPG3 and BGPG4; clusters in pale green, genes differentially expressed in BGPG2, BGPG3, and BGPG4. (A) Circular map representing RNA-seq data from chromosome 1 of BGPG1; (B) circular map representing RNA-seq data from chromosome 2 of BGPG1.
QS-dependent regulation of flagellar genes and their regulators.
In many bacteria, motility on surfaces and in liquids is controlled by QS (13, 62–64). While in BGR1 a single AHL synthase is involved in the QS-dependent regulation of flagellar movement, our experimental data suggested that in BGPG1 all three different AHL synthase genes are involved in the regulation of swimming and swarming motility (Fig. 2A and B). The QS-dependent phenotype observed in this work is in line with the QS-dependent BGR1 motility (13, 18). In B. glumae BGR1, flagellum-driven motility is essential for the infection of rice plants and subject to QS- and temperature-dependent regulation. Nonmotile mutants are attenuated in their virulence (13). In BGR1, flagellar motility is subject to a complex system of regulation, where FlhDC together with the QsmR regulatory protein is a key regulator of flagellum-dependent motility (13, 18). Thereby, TofR, the AHL receptor/regulator, activates QsmR transcription, and QsmR activates the flagellar master activator FlhDC, which subsequently activates flagellar biosynthesis (13, 18). In BGPG1, BGL_1c10570 encodes the QsmR homologue, and our data indicate that qsmR in BGPG1 is not affected by QS.
Interestingly, our RNA-seq data indicate that the majority of the structural genes were upregulated 2.0- to 5.3-fold in the bgaI2 and bgaI3 mutant strains compared to their level of regulation in the parent strain (Fig. 6A, clusters 1 to 5; see also Table S1 in the supplemental material). The higher level of transcription of the flagellar genes is in line with reports from S. fredii NGR234 in the background of two AHL mutants (40) and is consistent with the increased transcription of flagellar genes in BGR1 (34) and B. gladioli tofI mutants (29). The overall stronger transcription of the flagellar genes is most likely responsible for the delayed sedimentation of the mutants BGPG3 and BGPG4 compared with the time to sedimentation of the parent strain that was observed (Fig. 2C).
Within this framework, it is noteworthy that in other Gram-negative bacteria, the qseBC genes are involved in the regulation of the flagellum regulator FlhDC (65–68). While the direct involvement of the qseBC genes in motility has not been shown for B. glumae, it is perhaps reasonable to suggest that they are involved in the regulation of motility in BGPG1 as well. Within this framework, we observed that qseBC genes were downregulated in the three mutants analyzed in this work (Fig. 6B, cluster 4; see also Table S1 in the supplemental material). This finding implies that the expression levels of the different regulators rather than their structural features were responsible for the flagellum-dependent motility phenotypes observed on agar plates (Fig. 2A and B).
The BGPG1 genome encodes a QS-regulated type IVb/Flp pilus on chromosome 1.
We further asked to what extent genes involved in the synthesis or formation of type IVb/Flp pili are regulated in a QS-dependent manner. Genes for these pili have been identified at up to four copies per genome in a wide variety of different bacteria and archaea (69–71). Flp pili are involved in surface attachment and in pathogenic interactions with eukaryotic hosts. The tad (tight adherence) macromolecular transport system represents an ancient subtype of the type II secretion system (T2SS) and is essential for pilus biogenesis (72, 73). The BGPG1 genome encodes a single cluster of Flp pilus genes on chromosome 1. In the three mutants, the flp gene cluster was significantly downregulated (Fig. 6A, cluster 8). The strongest effects were observed in BGPG2, with this gene cluster being downregulated more than 10-fold compared to the levels of expression in the wild-type strain (see Table S1 in the supplemental material). We can only speculate that the B. glumae Flp pilus plays a crucial role during the infection process, as recently shown for the plant pathogen Ralstonia solanacearum (73). It is likely that the downregulation of Flp pilus genes is also responsible in part for the motility phenotypes observed in Fig. 2A and B.
T1SS and T6SS are subject to QS-dependent regulation.
Although the BGPG1 genome encodes many genes linked to the formation of secretion systems, only genes for the type I secretion system (T1SS) and T6SS were regulated in a QS-dependent manner in BGPG1. We identified four QS-regulated T1SS-associated genes, one on chromosome 1 and three on chromosome 2 (Fig. 6B, cluster 8; see also Table S1 in the supplemental material). T1SS is widely distributed in Gram-negative bacteria and is able to transport a variety of substrates, including proteins, antibiotics, toxins, and metal ions, by a one-step mechanism (74). Further, we observed T6SS-affiliated and QS-regulated genes on both chromosomes, but they were spread out in several clusters. One larger cluster (T6SS-1) was identified on chromosome 1 (Fig. 6A, cluster 6; see also Table S1 in the supplemental material). This cluster is similar to a cluster identified in B. thailandensis strain E264 (75). This secretion pathway has been shown to be involved in bacterial virulence and interaction with other organisms using a contact-dependent protein translocation mechanism (76).
Genes linked to a CRISPR-cas gene cluster are QS dependent.
Clustered regularly interspaced short palindromic repeats (CRISPRs) are observed in the genomes of many prokaryotes, and they protect them from invasion by bacteriophages or foreign plasmid DNA (77, 78). Each cluster is linked to a subset of specific CRISPR-associated cas genes (79, 80). CRISPR systems have been associated with virulence and biofilm formation in different human-pathogenic microorganisms, and their expression can be modulated through stress, temperature shifts, and other environmental stimuli (81). Three CRISPR arrays were identified in BGPG1 (26). Two of the clusters were accompanied by cas genes. Interestingly, our data searches indicated that only two other B. glumae/B. gladioli isolates (i.e., B. glumae 3242-8 and B. glumae A.1) have a CRISPR-cas system. This feature appears to be unique to a small group of B. glumae/B. gladioli isolates and may be of importance for the plant interaction.
In the course of the transcriptome data analysis, we identified a CRISPR cluster together with the associated cas genes spanning from BGL_1c18810 to BGL_1c18860. The cluster was differentially transcribed in all three AHL mutants compared to their transcription in the wild-type strain (Fig. 6A, cluster 10; see also Table S1 in the supplemental material). Thereby, we observed 2- to 10-fold decreased transcription in BGPG2, 1.2- to 3.8-fold decreased transcription in BGPG3, and 1.1- to 5.6-fold decreased transcription in BGPG4. Data from qRT-PCR analyses verified these findings (see Fig. S2 in the supplemental material).
Altogether this finding suggests the QS-dependent regulation of cell immunity in bacteria. In fact, this is the first report on the QS-dependent regulation of CRISPR-cas genes.
Genes linked to secondary metabolite synthesis and general metabolism.
Altogether, 284 QS-regulated genes were linked to the main metabolic activities of BGPG1. Similar to the findings for B. thailandensis (28), several polyketide biosynthesis clusters were subject to QS regulation. Further, rhamnolipid biosynthesis genes were differentially regulated (Fig. 6B, cluster 6; see also Table S1 in the supplemental material). The rhamnolipid synthesis genes rhlABC are known to be controlled by QS in, for example, P. aeruginosa (82). This is in line with our findings for BGPG2 as well (see Fig. S2 in the supplemental material). Further, selected genes from phosphate metabolism (Fig. 6B, cluster 5; see also Table S1 in the supplemental material) as well as a large cluster involved in inositol phosphate biosynthesis (Fig. 6A, cluster 7; see also Table S1 in the supplemental material) were subject to QS-dependent regulation. The observation that genes linked to central phosphate metabolism are QS regulated shows the importance of AHL-dependent regulatory circuits, and especially, the observation that genes involved in inositol phosphate biosynthesis are QS regulated raises the question of whether inositol phosphate is involved in plant-microbe signaling.
In addition, several clusters involved in amino acid and cofactor biosynthesis were QS regulated (BGL_1c26710 to BGL_1c26840 and BGL_2c20210 to BGL_2c20370; Fig. 6A, cluster 11, and B, cluster 9; see also Table S1 in the supplemental material). With respect to the QS-dependent biosynthesis of cofactors, coenzyme pyrroloquinoline quinone (PQQ) and thiamine biosynthesis appeared to be regulated in a QS-dependent manner as well (Fig. 6B, clusters 1 and 10; see also Table S1 in the supplemental material).
Because of the relatively high number of QS-regulated genes which are linked to metabolite biosynthesis and general metabolism, one can speculate that QS is of strong importance for growth and survival in soil or saprophytic growth on the plant surface.
Conclusions.
A set of 745 QS-regulated genes was identified in BGPG1 and its AHL synthase mutants using RNA-seq. Among them were the genes linked to flagellum, Flp pilus, T1SS, T6SS, and metabolite biosynthesis as well as a CRISPR-cas gene cluster. With respect to the QS-dependent expression of motility, pathogenicity, and lipase-related genes, phenotypic analyses confirmed these findings. Finally, it is noteworthy that BGPG1 shared most QS-dependent functional orthologs with B. thailandensis, suggesting a common evolutionary origin of QS-dependent regulatory circuits in these microorganisms.
Supplementary Material
ACKNOWLEDGMENTS
This work was funded by the German Federal Ministry of Education and Research (BMBF) within the framework of the collaborative project ExpresSys (FKZ 0315586). R.G. was funded by the China Scholarship Council (http://en.csc.edu.cn/). Part of the work in the laboratory of K.-E.J. was funded by the Deutsche Forschungsgemeinschaft through EXC 1028.
We are grateful to Peter Greenberg and Charlotte Majerczyk for giving us access to transcriptome data from B. mallei and B. pseudomallei prior to their publication.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.01043-15.
REFERENCES
- 1.Waters CM, Bassler BL. 2005. Quorum sensing: cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol 21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- 2.Frederix M, Downie AJ. 2011. Quorum sensing: regulating the regulators. Adv Microb Physiol 58:23–80. doi: 10.1016/B978-0-12-381043-4.00002-7. [DOI] [PubMed] [Google Scholar]
- 3.Ng WL, Bassler BL. 2009. Bacterial quorum-sensing network architectures. Annu Rev Genet 43:197–222. doi: 10.1146/annurev-genet-102108-134304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shank EA, Kolter R. 2009. New developments in microbial interspecies signaling. Curr Opin Microbiol 12:205–214. doi: 10.1016/j.mib.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller MB, Bassler BL. 2001. Quorum sensing in bacteria. Annu Rev Microbiol 55:165–199. doi: 10.1146/annurev.micro.55.1.165. [DOI] [PubMed] [Google Scholar]
- 6.Val DL, Cronan JE Jr. 1998. In vivo evidence that S-adenosylmethionine and fatty acid synthesis intermediates are the substrates for the LuxI family of autoinducer synthases. J Bacteriol 180:2644–2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ham JH, Melanson RA, Rush MC. 2011. Burkholderia glumae: next major pathogen of rice? Mol Plant Pathol 12:329–339. doi: 10.1111/j.1364-3703.2010.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho HS, Park SY, Ryu CM, Kim JF, Kim JG, Park SH. 2007. Interference of quorum sensing and virulence of the rice pathogen Burkholderia glumae by an engineered endophytic bacterium. FEMS Microbiol Ecol 60:14–23. doi: 10.1111/j.1574-6941.2007.00280.x. [DOI] [PubMed] [Google Scholar]
- 9.Jeong Y, Kim J, Kim S, Kang Y, Nagamatsu T, Hwang I. 2003. Toxoflavin produced by Burkholderia glumae causing rice grain rot is responsible for inducing bacterial wilt in many field crops. Plant Dis 87:890–895. doi: 10.1094/PDIS.2003.87.8.890. [DOI] [PubMed] [Google Scholar]
- 10.Weinberg JB, Alexander BD, Majure JM, Williams LW, Kim JY, Vandamme P, LiPuma JJ. 2007. Burkholderia glumae infection in an infant with chronic granulomatous disease. J Clin Microbiol 45:662–665. doi: 10.1128/JCM.02058-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim J, Kim JG, Kang Y, Jang JY, Jog GJ, Lim JY, Kim S, Suga H, Nagamatsu T, Hwang I. 2004. Quorum sensing and the LysR-type transcriptional activator ToxR regulate toxoflavin biosynthesis and transport in Burkholderia glumae. Mol Microbiol 54:921–934. doi: 10.1111/j.1365-2958.2004.04338.x. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki F, Sawada H, Azegami K, Tsuchiya K. 2004. Molecular characterization of the tox operon involved in toxoflavin biosynthesis of Burkholderia glumae. J Gen Plant Pathol 70:97–107. doi: 10.1007/s10327-003-0096-1. [DOI] [Google Scholar]
- 13.Kim J, Kang Y, Choi O, Jeong Y, Jeong JE, Lim JY, Kim M, Moon JS, Suga H, Hwang I. 2007. Regulation of polar flagellum genes is mediated by quorum sensing and FlhDC in Burkholderia glumae. Mol Microbiol 64:165–179. doi: 10.1111/j.1365-2958.2007.05646.x. [DOI] [PubMed] [Google Scholar]
- 14.Kang Y, Kim J, Kim S, Kim H, Lim JY, Kim M, Kwak J, Moon JS, Hwang I. 2008. Proteomic analysis of the proteins regulated by HrpB from the plant pathogenic bacterium Burkholderia glumae. Proteomics 8:106–121. doi: 10.1002/pmic.200700244. [DOI] [PubMed] [Google Scholar]
- 15.Devescovi G, Bigirimana J, Degrassi G, Cabrio L, LiPuma JJ, Kim J, Hwang I, Venturi V. 2007. Involvement of a quorum-sensing-regulated lipase secreted by a clinical isolate of Burkholderia glumae in severe disease symptoms in rice. Appl Environ Microbiol 73:4950–4958. doi: 10.1128/AEM.00105-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chun H, Choi O, Goo E, Kim N, Kim H, Kang Y, Kim J, Moon JS, Hwang I. 2009. The quorum sensing-dependent gene katG of Burkholderia glumae is important for protection from visible light. J Bacteriol 191:4152–4157. doi: 10.1128/JB.00227-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Degrassi G, Devescovi G, Kim J, Hwang I, Venturi V. 2008. Identification, characterization and regulation of two secreted polygalacturonases of the emerging rice pathogen Burkholderia glumae. FEMS Microbiol Ecol 65:251–262. doi: 10.1111/j.1574-6941.2008.00516.x. [DOI] [PubMed] [Google Scholar]
- 18.Jang MS, Goo E, An JH, Kim J, Hwang I. 2014. Quorum sensing controls flagellar morphogenesis in Burkholderia glumae. PLoS One 9:e84831. doi: 10.1371/journal.pone.0084831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKenney D, Brown KE, Allison DG. 1995. Influence of Pseudomonas aeruginosa exoproducts on virulence factor production in Burkholderia cepacia: evidence of interspecies communication. J Bacteriol 177:6989–6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eberl L. 2006. Quorum sensing in the genus Burkholderia. Int J Med Microbiol 296:103–110. doi: 10.1016/j.ijmm.2006.01.035. [DOI] [PubMed] [Google Scholar]
- 21.Choudhary KS, Hudaiberdiev S, Gelencser Z, Goncalves Coutinho B, Venturi V, Pongor S. 2013. The organization of the quorum sensing luxI/R family genes in Burkholderia. Int J Mol Sci 14:13727–13747. doi: 10.3390/ijms140713727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Majerczyk C, Greenberg E, Chandler J. 2013. Quorum sensing in Burkholderia, p 40–57. In Vasil ML, Darwin AJ (ed), Regulation of bacterial virulence. ASM Press, Washington, DC. [Google Scholar]
- 23.Lim J, Lee TH, Nahm BH, Choi YD, Kim M, Hwang I. 2009. Complete genome sequence of Burkholderia glumae BGR1. J Bacteriol 191:3758–3759. doi: 10.1128/JB.00349-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goo E, Kang Y, Kim H, Hwang I. 2010. Proteomic analysis of quorum sensing-dependent proteins in Burkholderia glumae. J Proteome Res 9:3184–3199. doi: 10.1021/pr100045n. [DOI] [PubMed] [Google Scholar]
- 25.Kim J, Oh J, Choi O, Kang Y, Kim H, Goo E, Ma J, Nagamatsu T, Moon JS, Hwang I. 2009. Biochemical evidence for ToxR and ToxJ binding to the tox operons of Burkholderia glumae and mutational analysis of ToxR. J Bacteriol 191:4870–4878. doi: 10.1128/JB.01561-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Voget S, Knapp A, Poehlein A, Vollstedt C, Streit WR, Daniel R, Jaeger KE. 2015. Complete genome sequence of the lipase producing strain Burkholderia glumae PG1. J Biotechnol 204:3–4. doi: 10.1016/j.jbiotec.2015.03.022. [DOI] [PubMed] [Google Scholar]
- 27.Schmid N, Pessi G, Deng Y, Aguilar C, Carlier AL, Grunau A, Omasits U, Zhang LH, Ahrens CH, Eberl L. 2012. The AHL- and BDSF-dependent quorum sensing systems control specific and overlapping sets of genes in Burkholderia cenocepacia H111. PLoS One 7:e49966. doi: 10.1371/journal.pone.0049966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Majerczyk C, Brittnacher M, Jacobs M, Armour CD, Radey M, Schneider E, Phattarasokul S, Bunt R, Greenberg EP. 2014. Global analysis of the Burkholderia thailandensis quorum sensing-controlled regulon. J Bacteriol 196:1412–1424. doi: 10.1128/JB.01405-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim S, Park J, Choi O, Kim J, Seo YS. 2014. Investigation of quorum sensing-dependent gene expression in Burkholderia gladioli BSR3 through RNA-seq analyses. J Microbiol Biotechnol 24:1609–1621. doi: 10.4014/jmb.1408.08064. [DOI] [PubMed] [Google Scholar]
- 30.Majerczyk CD, Brittnacher MJ, Jacobs MA, Armour CD, Radey MC, Bunt R, Hayden HS, Bydalek R, Greenberg EP. 2014. A cross-species comparison of the Burkholderia pseudomallei, Burkholderia thailandensis, and Burkholderia mallei quorum sensing regulons. J Bacteriol 196:3862–3871. doi: 10.1128/JB.01974-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lipuma JJ. 2005. Update on the Burkholderia cepacia complex. Curr Opin Pulm Med 11:528–533. doi: 10.1097/01.mcp.0000181475.85187.ed. [DOI] [PubMed] [Google Scholar]
- 32.Scholz HC, Pearson T, Hornstra H, Projahn M, Terzioglu R, Wernery R, Georgi E, Riehm JM, Wagner DM, Keim PS, Joseph M, Johnson B, Kinne J, Jose S, Hepp CM, Witte A, Wernery U. 2014. Genotyping of Burkholderia mallei from an outbreak of glanders in Bahrain suggests multiple introduction events. PLoS Negl Trop Dis 8:e3195. doi: 10.1371/journal.pntd.0003195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiersinga WJ, van der Poll T. 2009. Immunity to Burkholderia pseudomallei. Curr Opin Infect Dis 22:102–108. doi: 10.1097/QCO.0b013e328322e727. [DOI] [PubMed] [Google Scholar]
- 34.Kim S, Park J, Kim J, Lee J, Bang B, Hwang I, Seo YS. 2013. RNAseq-based transcriptome analysis of Burkholderia glumae quorum sensing. Plant Pathol J 29:249–259. doi: 10.5423/PPJ.OA.04.2013.0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim S, Park J, Lee J, Shin D, Park DS, Lim JS, Choi IY, Seo YS. 2014. Understanding pathogenic Burkholderia glumae metabolic and signaling pathways within rice tissues through in vivo transcriptome analyses. Gene 547:77–85. doi: 10.1016/j.gene.2014.06.029. [DOI] [PubMed] [Google Scholar]
- 36.Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual, 3rd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 37.Fuqua C, Winans SC. 1996. Conserved cis-acting promoter elements are required for density-dependent transcription of Agrobacterium tumefaciens conjugal transfer genes. J Bacteriol 178:435–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tempe J, Petit A, Holsters M, Montagu M, Schell J. 1977. Thermo-sensitive step associated with transfer of the Ti plasmid during conjugation: possible relation to transformation in crown gall. Proc Natl Acad Sci U S A 74:2848–2849. doi: 10.1073/pnas.74.7.2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bijtenhoorn P, Mayerhofer H, Müller-Dieckmann J, Utpatel C, Schipper C, Hornung C, Szesny M, Grond S, Thürmer A, Brzuszkiewicz E, Daniel R, Dierking K, Schulenburg H, Streit WR. 2011. A novel metagenomic short-chain dehydrogenase/reductase attenuates Pseudomonas aeruginosa biofilm formation and virulence on Caenorhabditis elegans. PLoS One 6:e26278. doi: 10.1371/journal.pone.0026278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krysciak D, Grote J, Rodriguez Orbegoso M, Utpatel C, Förstner KU, Li L, Schmeisser C, Krishnan HB, Streit WR. 2014. RNA-seq in the broad host range strain Sinorhizobium fredii NGR234 identifies a large set of genes linked to quorum sensing-dependent regulation in the background of a traI and ngrI deletion mutant. Appl Environ Microbiol 80:5655–5671. doi: 10.1128/AEM.01835-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kelman A. 1954. The relationship of pathogenicity in Pseudomonas solanacearum to colony appearance on a tetrazolium medium. Phytopathology 44:693–695. [Google Scholar]
- 42.Kovach ME, Elzer PH, Hill DS, Robertson GT, Farris MA, Roop RM II, Peterson KM. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175–176. doi: 10.1016/0378-1119(95)00584-1. [DOI] [PubMed] [Google Scholar]
- 43.Lassak J, Henche AL, Binnenkade L, Thormann KM. 2010. ArcS, the cognate sensor kinase in an atypical Arc system of Shewanella oneidensis MR-1. Appl Environ Microbiol 76:3263–3274. doi: 10.1128/AEM.00512-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McClean KH, Winson MK, Fish L, Taylor A, Chhabra SR, Camara M, Daykin M, Lamb JH, Swift S, Bycroft BW, Stewart GS, Williams P. 1997. Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology 143(Pt 12):3703–3711. doi: 10.1099/00221287-143-12-3703. [DOI] [PubMed] [Google Scholar]
- 45.Blosser RS, Gray KM. 2000. Extraction of violacein from Chromobacterium violaceum provides a new quantitative bioassay for N-acyl homoserine lactone autoinducers. J Microbiol Methods 40:47–55. doi: 10.1016/S0167-7012(99)00136-0. [DOI] [PubMed] [Google Scholar]
- 46.Jacobs JL, Fasi AC, Ramette A, Smith JJ, Hammerschmidt R, Sundin GW. 2008. Identification and onion pathogenicity of Burkholderia cepacia complex isolates from the onion rhizosphere and onion field soil. Appl Environ Microbiol 74:3121–3129. doi: 10.1128/AEM.01941-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Langmead B, Salzberg SL. 2012. Fast gapped-read alignment with Bowtie 2. Nat Methods 9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hardcastle TJ, Kelly KA. 2010. baySeq: empirical Bayesian methods for identifying differential expression in sequence count data. BMC Bioinformatics 11:422. doi: 10.1186/1471-2105-11-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schmittgen TD, Livak KJ. 2008. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 51.Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, Rozen SG. 2012. Primer3—new capabilities and interfaces. Nucleic Acids Res 40:e115. doi: 10.1093/nar/gks596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ruijter JM, Ramakers C, Hoogaars WM, Karlen Y, Bakker O, van den Hoff MJ, Moorman AF. 2009. Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res 37:e45. doi: 10.1093/nar/gkp045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pfaffl MW, Horgan GW, Dempfle L. 2002. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res 30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tiaden A, Spirig T, Sahr T, Wälti MA, Boucke K, Buchrieser C, Hilbi H. 2010. The autoinducer synthase LqsA and putative sensor kinase LqsS regulate phagocyte interactions, extracellular filaments and a genomic island of Legionella pneumophila. Environ Microbiol 12:1243–1259. doi: 10.1111/j.1462-2920.2010.02167.x. [DOI] [PubMed] [Google Scholar]
- 55.Kessler A, Schell U, Sahr T, Tiaden A, Harrison C, Buchrieser C, Hilbi H. 2013. The Legionella pneumophila orphan sensor kinase LqsT regulates competence and pathogen-host interactions as a component of the LAI-1 circuit. Environ Microbiol 15:646–662. doi: 10.1111/j.1462-2920.2012.02889.x. [DOI] [PubMed] [Google Scholar]
- 56.Chen R, Barphagha IK, Karki HS, Ham JH. 2012. Dissection of quorum-sensing genes in Burkholderia glumae reveals non-canonical regulation and the new regulatory gene tofM for toxoflavin production. PLoS One 7:e52150. doi: 10.1371/journal.pone.0052150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kato T, Morohoshi T, Tsushima S, Ikeda T. 2014. Characterization of three types of quorum-sensing mutants in Burkholderia glumae strains isolated in Japan. J Agric Sci 6:16–26. [Google Scholar]
- 58.Chugani S, Kim BS, Phattarasukol S, Brittnacher MJ, Choi SH, Harwood CS, Greenberg EP. 2012. Strain-dependent diversity in the Pseudomonas aeruginosa quorum-sensing regulon. Proc Natl Acad Sci U S A 109:E2823–E2831. doi: 10.1073/pnas.1214128109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.LaRock CN, Yu J, Horswill AR, Parsek MR, Minion FC. 2013. Transcriptome analysis of acetyl-homoserine lactone-based quorum sensing regulation in Yersinia pestis. PLoS One 8:e62337. doi: 10.1371/journal.pone.0062337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee J, Zhang L. 2014. The hierarchy quorum sensing network in Pseudomonas aeruginosa. Protein Cell 6:26–41. doi: 10.1007/s13238-014-0100-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Venturi V. 2006. Regulation of quorum sensing in Pseudomonas. FEMS Microbiol Rev 30:274–291. doi: 10.1111/j.1574-6976.2005.00012.x. [DOI] [PubMed] [Google Scholar]
- 62.Huber B, Riedel K, Hentzer M, Heydorn A, Gotschlich A, Givskov M, Molin S, Eberl L. 2001. The cep quorum-sensing system of Burkholderia cepacia H111 controls biofilm formation and swarming motility. Microbiology 147:2517–2528. doi: 10.1099/00221287-147-9-2517. [DOI] [PubMed] [Google Scholar]
- 63.Atkinson S, Chang CY, Sockett RE, Camara M, Williams P. 2006. Quorum sensing in Yersinia enterocolitica controls swimming and swarming motility. J Bacteriol 188:1451–1461. doi: 10.1128/JB.188.4.1451-1461.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hoang HH, Gurich N, González JE. 2008. Regulation of motility by the ExpR/Sin quorum-sensing system in Sinorhizobium meliloti. J Bacteriol 190:861–871. doi: 10.1128/JB.01310-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sperandio V, Torres AG, Kaper JB. 2002. Quorum sensing Escherichia coli regulators B and C (QseBC): a novel two-component regulatory system involved in the regulation of flagella and motility by quorum sensing in E. coli. Mol Microbiol 43:809–821. doi: 10.1046/j.1365-2958.2002.02803.x. [DOI] [PubMed] [Google Scholar]
- 66.Hadjifrangiskou M, Kostakioti M, Chen SL, Henderson JP, Greene SE, Hultgren SJ. 2011. A central metabolic circuit controlled by QseC in pathogenic Escherichia coli. Mol Microbiol 80:1516–1529. doi: 10.1111/j.1365-2958.2011.07660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bearson BL, Bearson SM, Lee IS, Brunelle BW. 2010. The Salmonella enterica serovar Typhimurium QseB response regulator negatively regulates bacterial motility and swine colonization in the absence of the QseC sensor kinase. Microb Pathog 48:214–219. doi: 10.1016/j.micpath.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 68.Yang K, Meng J, Huang YC, Ye LH, Li GJ, Huang J, Chen HM. 2014. The role of the QseC quorum-sensing sensor kinase in epinephrine-enhanced motility and biofilm formation by Escherichia coli. Cell Biochem Biophys 70:391–398. doi: 10.1007/s12013-014-9924-5. [DOI] [PubMed] [Google Scholar]
- 69.Planet PJ, Kachlany SC, DeSalle R, Figurski DH. 2001. Phylogeny of genes for secretion NTPases: identification of the widespread tadA subfamily and development of a diagnostic key for gene classification. Proc Natl Acad Sci U S A 98:2503–2508. doi: 10.1073/pnas.051436598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kachlany SC, Planet PJ, Desalle R, Fine DH, Figurski DH, Kaplan JB. 2001. flp-1, the first representative of a new pilin gene subfamily, is required for non-specific adherence of Actinobacillus actinomycetemcomitans. Mol Microbiol 40:542–554. doi: 10.1046/j.1365-2958.2001.02422.x. [DOI] [PubMed] [Google Scholar]
- 71.Tomich M, Planet PJ, Figurski DH. 2007. The tad locus: postcards from the widespread colonization island. Nat Rev Microbiol 5:363–375. doi: 10.1038/nrmicro1636. [DOI] [PubMed] [Google Scholar]
- 72.Mattick JS. 2002. Type IV pili and twitching motility. Annu Rev Microbiol 56:289–314. doi: 10.1146/annurev.micro.56.012302.160938. [DOI] [PubMed] [Google Scholar]
- 73.Wairuri CK, van der Waals JE, van Schalkwyk A, Theron J. 2012. Ralstonia solanacearum needs Flp pili for virulence on potato. Mol Plant Microbe Interact 25:546–556. doi: 10.1094/MPMI-06-11-0166. [DOI] [PubMed] [Google Scholar]
- 74.Delepelaire P. 2004. Type I secretion in gram-negative bacteria. Biochim Biophys Acta 1694:149–161. doi: 10.1016/j.bbamcr.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 75.Schwarz S, West TE, Boyer F, Chiang WC, Carl MA, Hood RD, Rohmer L, Tolker-Nielsen T, Skerrett SJ, Mougous JD. 2010. Burkholderia type VI secretion systems have distinct roles in eukaryotic and bacterial cell interactions. PLoS Pathog 6:e1001068. doi: 10.1371/journal.ppat.1001068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bernard CS, Brunet YR, Gueguen E, Cascales E. 2010. Nooks and crannies in type VI secretion regulation. J Bacteriol 192:3850–3860. doi: 10.1128/JB.00370-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jansen R, Embden J, Gaastra W, Schouls L. 2002. Identification of genes that are associated with DNA repeats in prokaryotes. Mol Microbiol 43:1565–1575. doi: 10.1046/j.1365-2958.2002.02839.x. [DOI] [PubMed] [Google Scholar]
- 78.Mojica FJ, Díez-Villaseñor C, Soria E, Juez G. 2000. Biological significance of a family of regularly spaced repeats in the genomes of Archaea, Bacteria and mitochondria. Mol Microbiol 36:244–246. doi: 10.1046/j.1365-2958.2000.01838.x. [DOI] [PubMed] [Google Scholar]
- 79.Sorek R, Kunin V, Hugenholtz P. 2008. CRISPR—a widespread system that provides acquired resistance against phages in bacteria and archaea. Nat Rev Microbiol 6:181–186. doi: 10.1038/nrmicro1793. [DOI] [PubMed] [Google Scholar]
- 80.Marraffini LA, Sontheimer EJ. 2010. Self versus non-self discrimination during CRISPR RNA-directed immunity. Nature 463:568–571. doi: 10.1038/nature08703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Louwen R, Staals RHJ, Endtz HP, van Baarlen P, van der Oost J. 2014. The role of CRISPR-Cas systems in virulence of pathogenic bacteria. Microbiol Mol Biol Rev 78:74–88. doi: 10.1128/MMBR.00039-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Soberón-Chávez G, Lépine F, Déziel E. 2005. Production of rhamnolipids by Pseudomonas aeruginosa. Appl Microbiol Biotechnol 68:718–725. doi: 10.1007/s00253-005-0150-3. [DOI] [PubMed] [Google Scholar]
- 83.Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D, Jones SJ, Marra MA. 2009. Circos: an information aesthetic for comparative genomics. Genome Res 19:1639–1645. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Frenken LG, Egmond MR, Batenburg AM, Bos JW, Visser C, Verrips CT. 1992. Cloning of the Pseudomonas glumae lipase gene and determination of the active site residues. Appl Environ Microbiol 58:3787–3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hanahan D. 1983. Studies on transformation of Escherichia coli with plasmids. J Mol Biol 166:557–580. doi: 10.1016/S0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 86.Fuqua C, Burbea M, Winans SC. 1995. Activity of the Agrobacterium Ti plasmid conjugal transfer regulator TraR is inhibited by the product of the traM gene. J Bacteriol 177:1367–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.