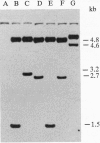
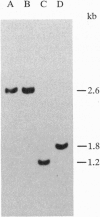
Abstract
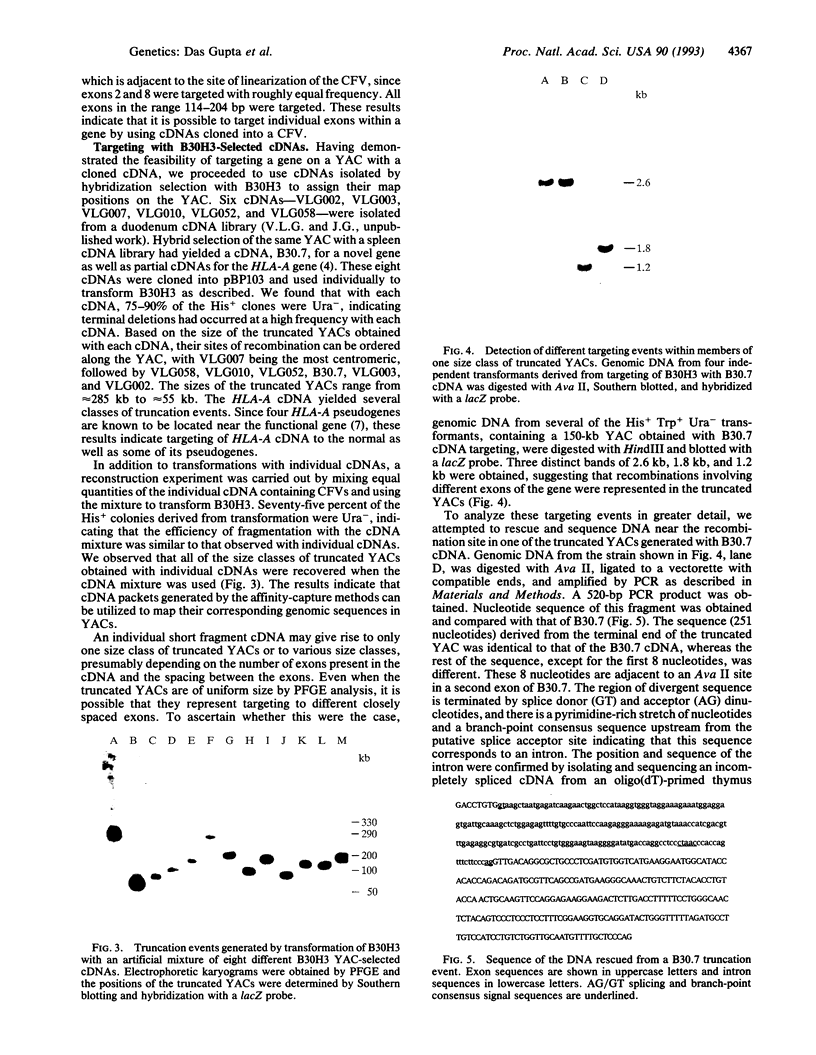
We have developed a method for generating expressed-sequence maps of human chromosomes. The method involves several steps that begin with libraries of highly representative short cDNAs prepared by using random oligomers as primers. The cDNA inserts are amplified by PCR with flanking vector primers. Chromosomal region-specific cDNA packets are prepared by hybridization of the cDNA inserts to DNA derived from yeast artificial chromosomes (YACs) assigned to defined regions of human chromosomes. The cDNA packets are cloned into yeast chromosome fragmentation vectors and used for transformation of yeast bearing the YAC used for affinity purification. Sequences in the cDNAs undergo homologous recombination with the corresponding exons in the genomic DNA yielding a set of truncated YACs. Each unique truncation specifies the location of an exon in the YAC. Since all of the truncation events end with the same vector sequence, it is possible to rescue and sequence these ends to generate expressed sequence tags. The method couples rapid purification of region-specific cDNAs with precise mapping of their genes on YACs. Appropriately truncated YACs also provide easy access to gene regulatory sequences. We describe the feasibility of individual steps of the method using the factor IX (F9) gene as a model system and we present the mapping of several expressed sequences corresponding to a 330-kb YAC containing DNA from human chromosome 6p21. In addition, we obtained the sequence, including an intron-exon junction, flanking a particular truncation event.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abidi F. E., Wada M., Little R. D., Schlessinger D. Yeast artificial chromosomes containing human Xq24-Xq28 DNA: library construction and representation of probe sequences. Genomics. 1990 Jul;7(3):363–376. doi: 10.1016/0888-7543(90)90170-y. [DOI] [PubMed] [Google Scholar]
- Adams M. D., Kelley J. M., Gocayne J. D., Dubnick M., Polymeropoulos M. H., Xiao H., Merril C. R., Wu A., Olde B., Moreno R. F. Complementary DNA sequencing: expressed sequence tags and human genome project. Science. 1991 Jun 21;252(5013):1651–1656. doi: 10.1126/science.2047873. [DOI] [PubMed] [Google Scholar]
- Bodmer W. F. Human genetics: the molecular challenge. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):1–13. doi: 10.1101/sqb.1986.051.01.003. [DOI] [PubMed] [Google Scholar]
- Buckler A. J., Chang D. D., Graw S. L., Brook J. D., Haber D. A., Sharp P. A., Housman D. E. Exon amplification: a strategy to isolate mammalian genes based on RNA splicing. Proc Natl Acad Sci U S A. 1991 May 1;88(9):4005–4009. doi: 10.1073/pnas.88.9.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell C., Gulati R., Nandi A. K., Floy K., Hieter P., Kucherlapati R. S. Generation of a nested series of interstitial deletions in yeast artificial chromosomes carrying human DNA. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5744–5748. doi: 10.1073/pnas.88.13.5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. W., Thomas M., Cameron J., St John T. P., Scherer S., Padgett R. A. Rapid DNA isolations for enzymatic and hybridization analysis. Methods Enzymol. 1980;65(1):404–411. doi: 10.1016/s0076-6879(80)65051-4. [DOI] [PubMed] [Google Scholar]
- Duyk G. M., Kim S. W., Myers R. M., Cox D. R. Exon trapping: a genetic screen to identify candidate transcribed sequences in cloned mammalian genomic DNA. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8995–8999. doi: 10.1073/pnas.87.22.8995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Geraghty D. E., Pei J., Lipsky B., Hansen J. A., Taillon-Miller P., Bronson S. K., Chaplin D. D. Cloning and physical mapping of the HLA class I region spanning the HLA-E-to-HLA-F interval by using yeast artificial chromosomes. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2669–2673. doi: 10.1073/pnas.89.7.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagadeeswaran P., Lavelle D. E., Kaul R., Mohandas T., Warren S. T. Isolation and characterization of human factor IX cDNA: identification of Taq I polymorphism and regional assignment. Somat Cell Mol Genet. 1984 Sep;10(5):465–473. doi: 10.1007/BF01534851. [DOI] [PubMed] [Google Scholar]
- Orr-Weaver T. L., Szostak J. W., Rothstein R. J. Yeast transformation: a model system for the study of recombination. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6354–6358. doi: 10.1073/pnas.78.10.6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parimoo S., Patanjali S. R., Shukla H., Chaplin D. D., Weissman S. M. cDNA selection: efficient PCR approach for the selection of cDNAs encoded in large chromosomal DNA fragments. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9623–9627. doi: 10.1073/pnas.88.21.9623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patanjali S. R., Parimoo S., Weissman S. M. Construction of a uniform-abundance (normalized) cDNA library. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1943–1947. doi: 10.1073/pnas.88.5.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavan W. J., Hieter P., Sears D., Burkhoff A., Reeves R. H. High-efficiency yeast artificial chromosome fragmentation vectors. Gene. 1991 Sep 30;106(1):125–127. doi: 10.1016/0378-1119(91)90576-w. [DOI] [PubMed] [Google Scholar]
- Riley J., Butler R., Ogilvie D., Finniear R., Jenner D., Powell S., Anand R., Smith J. C., Markham A. F. A novel, rapid method for the isolation of terminal sequences from yeast artificial chromosome (YAC) clones. Nucleic Acids Res. 1990 May 25;18(10):2887–2890. doi: 10.1093/nar/18.10.2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiestl R. H., Petes T. D. Integration of DNA fragments by illegitimate recombination in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7585–7589. doi: 10.1073/pnas.88.17.7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D. C., Cantor C. R. Separation of yeast chromosome-sized DNAs by pulsed field gradient gel electrophoresis. Cell. 1984 May;37(1):67–75. doi: 10.1016/0092-8674(84)90301-5. [DOI] [PubMed] [Google Scholar]
- Vollrath D., Davis R. W., Connelly C., Hieter P. Physical mapping of large DNA by chromosome fragmentation. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6027–6031. doi: 10.1073/pnas.85.16.6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshitake S., Schach B. G., Foster D. C., Davie E. W., Kurachi K. Nucleotide sequence of the gene for human factor IX (antihemophilic factor B). Biochemistry. 1985 Jul 2;24(14):3736–3750. doi: 10.1021/bi00335a049. [DOI] [PubMed] [Google Scholar]