Abstract
Background:
Adipokines and inflammation may provide a mechanistic link between obesity and postmenopausal breast cancer, yet epidemiologic data on their associations with breast cancer risk are limited.
Methods:
In a case-cohort analysis nested within the Women’s Health Initiative Observational Study, a prospective cohort of postmenopausal women, baseline plasma samples from 875 incident breast cancer case patients and 839 subcohort participants were tested for levels of seven adipokines, namely leptin, adiponectin, resistin, interleukin-6, tumor necrosis factor-α, hepatocyte growth factor, and plasminogen activator inhibitor-1, and for C-reactive protein (CRP), an inflammatory marker. Data were analyzed by multivariable Cox modeling that included established breast cancer risk factors and previously measured estradiol and insulin levels. All statistical tests were two-sided.
Results:
The association between plasma CRP levels and breast cancer risk was dependent on hormone therapy (HT) use at baseline (P interaction = .003). In a model that controlled for multiple breast cancer risk factors including body mass index (BMI), estradiol, and insulin, CRP level was positively associated with breast cancer risk among HT nonusers (hazard ratio for high vs low CRP levels = 1.67, 95% confidence interval = 1.04 to 2.68, P trend = .029). None of the other adipokines were statistically significantly associated with breast cancer risk. Following inclusion of CRP, insulin, and estradiol in a multivariable model, the association of BMI with breast cancer was attenuated by 115%.
Conclusion:
These data indicate that CRP is a risk factor for postmenopausal breast cancer among HT nonusers. Inflammatory mediators, together with insulin and estrogen, may play a role in the obesity–breast cancer relation.
Obesity is an established risk factor for breast cancer in postmenopausal women (1,2); however, the biologic mechanisms underlying this relationship are not fully understood. In postmenopausal women, adiposity is associated with increased levels of estrogen and insulin, and both experimental and observational evidence support a role for these factors in breast tumorigenesis (3,4). Nonetheless, additional factors that are associated with obesity may also play a role in breast cancer development (5). Adipose tissue is a highly active endocrine and metabolic organ that secretes a variety of cytokines and hormones, termed adipokines. In the obese, adipokine levels may be abnormal, leading to the promotion of pathways implicated in breast tumorigenesis. For example, inflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor (TNF)-α are upregulated in obesity and have been demonstrated to promote breast tumor initiation and progression (6,7). Adipokines and associated inflammatory mediators may, therefore, provide an important mechanistic link between obesity and breast cancer.
Epidemiologic data on the association of adipokines and inflammatory factors with breast cancer are limited. Adiponectin, a hormone that improves insulin resistance and has been shown to have anti-mitogenic activity in vitro, was inversely associated with breast cancer incidence in some prior prospective investigations (8,9) but not in other studies (10,11), though three recent meta-analyses that included both prospective cohort and case-control studies reported an inverse relationship between adiponectin levels and breast cancer risk (12–14). Data on the association of other adipokines with breast cancer risk, such as leptin, plasminogen activator inhibitor (PAI)–1, and resistin are also mixed (9–11). C-reactive protein (CRP), a sensitive yet nonspecific marker of the inflammatory response, has also generally not been found to be statistically significantly associated with breast cancer risk (15–21). However, most studies evaluated a relatively small number of breast cancer case patients and most included both pre- and postmenopausal breast cancers, tumors that are known to differ in their association with body habitus.
To advance knowledge on the role of adipokines and inflammation in breast cancer development, we conducted a case-cohort study within the Women’s Health Initiative Observational Study (WHI-OS) to comprehensively evaluate the associations of postmenopausal breast cancer with circulating levels of CRP and seven adipokines, namely IL-6, TNF-α, adiponectin, leptin, resistin, hepatocyte growth factor (HGF), and PAI-1. This study builds upon previous findings on the role of insulin and estrogen in breast cancer development obtained from WHI-OS participants (3,22) by comprehensively assessing key adipokines and inflammatory mediators in a well-characterized study population of postmenopausal women. With existing measurements of other obesity-related factors, this study had the unique opportunity to assess whether adipokines, inflammatory factors, estradiol, and insulin could be part of the mechanisms linking obesity with breast cancer risk in postmenopausal women. Evaluating these obesity-related pathways simultaneously in one study has, to our knowledge, not been done previously.
Methods
Study Population
Women’s Health Initiative (WHI-OS)
This study was conducted among women enrolled in the WHI-OS, a prospective cohort of 93 676 postmenopausal women age 50 to 79 years who were recruited through 40 clinical centers across the United States between 1993 and 1998 (23). At enrollment, WHI-OS participants provided written informed consent and completed questionnaires regarding demographic and behavioral factors, medical history, and use of medications (including hormone therapy [HT]). Each woman underwent a physical examination that included waist, hip, height, and weight measurements and provided a blood sample following an overnight fast of at least eight hours; the blood samples were processed within two hours of collection and stored at -80°C (24). Cancer outcomes (including breast cancer) were initially ascertained through annual self-administered questionnaires; breast cancer status and the detailed diagnosis were subsequently formally determined through centralized review of medical records. Breast cancer case patients were coded according to the National Cancer Institute’s Surveillance, Epidemiology, and End Results program guidelines (25,26). As of June 2004, when the participants included in this case-cohort study were selected, 1.6% of the WHI-OS women had been lost to follow-up, and 4.7% were deceased.
Study Participants
WHI-OS participants were eligible for this case-cohort study if they had more than one year of follow-up and had no history of breast cancer before the end of year one. From these eligible participants, case patients were women who had a subsequent incident diagnosis of breast cancer after the first year of follow-up. We randomly selected 903 of the approximately 1800 eligible case participants available at the time this study was initiated. The comparison group was a subcohort of 892 participants who were randomly chosen from the eligible WHI-OS participants regardless of their subsequent breast cancer status. As such, 18 subcohort participants were also included in the case group. We also excluded participants who were using diabetes treatments at baseline (n = 28 case patients and 53 subcohort participants) because these treatments can have an impact on levels of the factors measured in our study. Therefore, the final analytic data set included 875 incident breast cancer case patients and 839 subcohort participants.
Laboratory Methods
EDTA plasma levels of adiponectin, PAI-1, and resistin were measured using Milliplex Human Adipokine Panel-A (EMD Millipore, Billerica, MA), and their interassay coefficients of variation (CV) were 13%, 12%, and 12%, respectively. Leptin, HGF, and TNF-α levels were assayed with Milliplex Human Adipokine Panel-B (interassay CVs = 9%, 12% and 13%, respectively). IL-6 levels were measured using an ultrasensitive solid-phase sandwich ELISA (R&D Systems, Minneapolis, MN; interassay CV = 10%), while CRP was assessed with latex-enhanced immunonephelometry on the Behring nephelometer II analyzer (Behring Diagnostics, San Jose, CA; interassay CV = 4%). Assay methods for insulin and estradiol have been described previously (3). The proportion of participants with measures below the limit of detection (LOD) was very low and ranged from 0% for leptin to 2.3% for TNF-α. For samples with an undetectable level below the assay LOD, the values were imputed using 0.5*LOD. The three-year intraclass correlation coefficients (ICC) for the seven adipokines and for insulin and estradiol have been reported previously and ranged from 0.39 for TNF-α to 0.95 for resistin (27–29). The five-year ICC of CRP was reported to be 0.83 (30).
Statistical Analyses
Differences in the distributions of baseline characteristics between case patients and the subcohort members without breast cancer were evaluated using the Wilcoxon rank sum test (for continuous data) or the chi-square test (for categorical data). To examine the associations between the serologic factors and risk of breast cancer, we estimated hazard ratios (HRs) and 95% confidence intervals (CIs) using Cox proportional hazard regression models that employed the Self-Prentice method for computing robust standard error estimates to account for the case-cohort design (31). The proportionality of the data was verified by graphical inspection and by Schoenfeld residuals. All serologic variables were expressed as quartiles with cutpoints based on the distributions of data in the subcohort. Trend tests were performed using Wald tests associated with fitting the quartile categories as continuous variables in the regression model.
In the primary analysis, we computed two models for each analyte: a base model that included age (50–54 [referent], 55–64, 65–74, or 75+ years) and race/ethnicity (white [referent], black, others) and an extended multivariable model that additionally included other breast cancer risk factors that were significantly associated with breast cancer risk in multivariable modeling in the study population, namely physical activity (metabolic equivalent tasks [METs], defined as the caloric need per kilogram of body weight per hour of activity divided by the caloric need per kilogram of body weight per hour at rest) and categorized as quartiles (<3.75, 3.75–9.99, 10–19.99, ≥20); body mass index (BMI; <25.0 [referent], 25.0–29.9, ≥30.0kg/m2); alcohol consumption, assessed as the number of servings per week (none [referent], <1.57, ≥1.57); family history of breast cancer in first-degree relatives (yes/no); parity (0 [referent], ≥1 live births); age at first child’s birth (<25 [referent], ≥25 years); years of menstrual cycling (≤33, 34–38, ≥39); history of benign breast disease (yes/no), estrogen status (serum estradiol among women who were not using HT [<8, ≥8 pg/mL] or women using HT at baseline). In another multivariable model, insulin was included as an additional covariate (quartiles of insulin, ≤3.3 [referent], 3.4–5.3, 5.4–8.5, or ≥8.6 μIU/mL), thus, controlling for both insulin and estradiol, in addition to other covariates. Variables that were not significantly associated with breast cancer risk in multivariable analysis in our study population, including smoking status, use of nonsteroidal anti-inflammatory drugs (NSAIDs), oral contraceptive use and educational history, were not included as covariates in the final multivariable model.
We explored whether the associations of breast cancer risk with the adipokines and inflammatory markers differed by baseline HT use (current user [n = 836] or nonuser [n = 859]) and BMI (<30 [n = 1279] or ≥30 [n = 417] kg/m2) because both HT use and BMI are known to have substantial effects on adipokine and inflammatory factor levels and are statistically significant risk factors for postmenopausal breast cancer. These stratified analyses were conducted by introducing interaction terms into multivariable models that also included the main effect variables. In addition, we conducted separate analyses for estrogen receptor (ER)–positive and ER-negative breast cancers.
All tests of statistical significance were two-sided, and P values under .05 were considered statistically significant.
Results
The baseline characteristics of the incident breast cancer case patients and subcohort are presented in Table 1. In univariable analyses, compared with women in the subcohort, case patients were older, had a later onset of menopause, gave birth to their first child at a later age, had greater alcohol consumption, had a higher frequency of NSAID use, and more often reported being a former smoker. Case patients were also more likely than women in the subcohort to have a first-degree relative with breast cancer, to be using HT, and to have a history of benign breast disease (Table 1).
Table 1.
Distribution of selected baseline characteristics among the breast cancer case patients and subcohort members without breast cancer*
| Variable | Case patients (n = 875) |
Subcohort members (n = 821)† |
P‡ |
|---|---|---|---|
| Median age, y (IQR) | 64.0 (59.0–69.0) | 63.0 (57.0–69.0) | .001 |
| Race/ethnicity, No. (%) | |||
| White | 766 (87.5) | 701 (85.4) | .402 |
| Black | 58 (6.6) | 55 (6.7) | |
| Hispanic | 25 (2.9) | 32 (3.9) | |
| Asian/other | 26 (3.0) | 33 (4.0) | |
| Highest education level, No. (%) | |||
| High school or less | 152 (17.4) | 167 (20.3) | .082 |
| Some college | 304 (34.7) | 303 (36.9) | |
| College and above | 409 (46.7) | 342 (41.7) | |
| Missing | 10 (1.1) | 9 (1.1) | |
| Age at menarche in y, No. (%) | |||
| ≤11 | 216 (24.7) | 185 (22.5) | .479 |
| 12–13 | 449 (51.3) | 444 (54.1) | |
| ≥14 | 205 (23.4) | 189 (23.0) | |
| Missing | 5 (0.6) | 3 (0.4) | |
| Age at menopause in y, No. (%) | |||
| ≤42 | 127 (14.5) | 180 (21.9) | < .001 |
| 43–48 | 189 (21.6) | 195 (23.8) | |
| 49–51 | 219 (25.0) | 182 (22.2) | |
| ≥52 | 260 (29.7) | 199 (24.2) | |
| Missing | 80 (9.1) | 65 (7.9) | |
| Years of menstrual cycling, No. (%) | |||
| ≤33 | 237 (27.1) | 287 (35.0) | .001 |
| 34–38 | 267 (30.5) | 252 (30.7) | |
| ≥39 | 289 (33.0) | 216 (26.3) | |
| Missing | 82 (9.37) | 66 (8.0) | |
| Parity, No. (%) | |||
| No live births | 120 (13.7) | 120 (14.6) | .586 |
| 1 | 63 (7.2) | 72 (8.8) | |
| 2–3 | 446 (51.0) | 402 (49.0) | |
| ≥4 | 241 (27.5) | 222 (27.0) | |
| Missing | 5 (0.6) | 5 (0.6) | |
| Age at first child’s birth in y, No. (%) | |||
| ≤19 | 83 (11.0) | 78 (11.1) | .002 |
| 20–24 | 288 (38.2) | 320 (45.7) | |
| 25–29 | 234 (31.0) | 170 (24.3) | |
| ≥30 | 79 (10.5) | 58 (8.3) | |
| Missing | 71 (9.4) | 75 (10.7) | |
| Ever use of oral contraceptives, No. (%) | 356 (40.7) | 337 (41.1) | .880 |
| Hormone therapy at baseline, No. (%) | |||
| Not using hormone therapy | 412 (47.1) | 447 (54.4) | .007 |
| Combined estrogen + progestin therapy | 239 (27.3) | 182 (22.2) | |
| Unopposed estrogen therapy | 224 (25.6) | 191 (23.3) | |
| Missing | 0 | 1 (0.1) | |
| Smoking status, No. (%) | |||
| Never | 419 (47.9) | 439 (53.5) | .005 |
| Former | 404 (46.2) | 320 (39.0) | |
| Current | 39 (4.5) | 52 (6.3) | |
| Missing | 13 (1.5) | 10 (1.2) | |
| Median alcohol consumption, servings per week (IQR) | 0.6 (0.0–3.8) | 0.4 (0.0–2.7) | .006 |
| NSAID use ≥ 2 wks, No. (%) | 371 (42.4) | 298 (36.3) | .010 |
| Median body mass index, kg/m2 (IQR) | 25.9 (23.2–30.3) | 26.2 (23.3–29.7) | .894 |
| Median physical activity, METs/ wk (IQR) | 10.5 (3.4–19.8) | 10.0 (3.8–20.0) | .784 |
| First-degree relative with breast cancer, No. (%) | 217 (24.8) | 150 (18.3) | .005 |
| History of benign breast disease, No. (%) | 329 (37.6) | 259 (31.6) | .009 |
| Estrogen receptor status, No. (%) | -- | ||
| Positive | 554 (63.3) | -- | |
| Negative | 253 (28.9) | -- | |
| Missing | 68 (7.8) | -- | |
| Median levels of analytes (IQR) | |||
| Leptin, pg/mL | 13950 (7433–25025) | 14406 (6974–24698) | .497 |
| Adiponectin, ng/mL | 28597 (20109–40085) | 29317 (20498–39929) | .571 |
| Resistin, ng/mL | 12.1 (9.7–15.1) | 12.3 (9.8–15.6) | .454 |
| CRP, μg/mL | 2.3 (1.0–4.7) | 1.9 (0.9–4.4) | .057 |
| IL-6, pg/mL | 1.4 (0.9–2.3) | 1.4 (0.9–2.2) | .794 |
| TNF-α, pg/mL | 2.5 (1.8–3.4) | 2.6 (1.8–3.6) | .756 |
| PAI-1, pg/mL | 14237 (9300–21076) | 14076 (8898–20726) | .409 |
| HGF, pg/mL | 611 (401–873) | 604 (404–855) | .588 |
| Insulin, µIU/mL | 5.7 (3.5–8.8) | 5.3 (3.3–8.5) | .196 |
| Estradiol, pg/mL§ | 11.9 (8.0–16.0) | 11.0 (7.0–16.0) | .025 |
* All statistical tests were two-sided. IQR = interquartile range; METs = metabolic equivalent tasks (defined as the caloric need per kilogram of body weight per hour of activity divided by the caloric need per kilogram of body weight per hour at rest); NSAID = nonsteroidal anti-inflammatory drug.
† Excludes women who developed breast cancer during follow-up.
‡ Missing are excluded for P value calculation.
§ Among women not using hormone therapy at baseline.
Correlations of the adipokines with each other and with insulin, estradiol, and BMI among the subcohort members have been reported previously (32,33), and these correlations among participants not using HT at baseline are presented in Supplementary Table 1 (available online). In brief, among HT nonusers in the representative subcohort, BMI had a moderate correlation with leptin (r = 0.70), insulin (r = 0.59), CRP (r = 0.50), and IL-6 (r = 0.41). Among the serologic factors, a moderate, inverse correlation was found between insulin and adiponectin (r = -0.46), while moderate, positive correlations were observed between insulin and leptin (r = 0.58), CRP and IL-6 (r = 0.59), CRP and insulin (r = 0.37), and CRP and leptin (r = 0.43).
Associations of Adipokines With Breast Cancer
Overall, we did not observe any statistically significant associations between leptin, resistin, IL-6, TNF-α, and HGF with breast cancer risk in age- and ethnicity-adjusted or multivariable models that included established breast cancer risk factors (Table 2). There was a suggestive inverse association between adiponectin and breast cancer with the hazard ratios for the third and fourth adiponectin quartiles, compared with the first quartile, of borderline statistical significance (P trend = .078). When combined as a single joint parameter, the upper two quartiles of adiponectin were associated with a modest reduction in breast cancer risk (HRq4+q3-q1 = 0.76, 95% CI = 0.57 to 1.02, P trend = .06). However, the association between adiponectin and breast cancer risk was weakened following inclusion of insulin in the multivariable model (HRq4+q3-q1 = 0.81, 95% CI = 0.59 to 1.12, P trend = .222, data not shown). Levels of CRP and PAI-1 were positively associated with breast cancer risk, but the associations differed by HT use (see below). We detected no significant heterogeneity in any of the results when stratified by BMI at baseline. The associations of the adipokines and inflammatory markers with breast cancer risk did not differ by breast tumor ER status.
Table 2.
Adjusted hazard ratios (and 95% CIs) for associations of incident breast cancer with baseline levels of adipokines and inflammatory markers in the WHI-OS participants
| Factor, model | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P trend |
|---|---|---|---|---|---|
| Leptin | |||||
| Quartile cutpoints, pg/mL | ≤7294 | 7295-15 007 | 15 008–25 517 | ≥25 518 | |
| No. case patients/No. subcohort members | 211/216 | 260/208 | 190/204 | 213/193 | |
| Age and ethnicity adjusted HR (95% CI) | 1.00 (referent) | 1.26 (0.96 to 1.65) | 0.97 (0.73 to 1.28) | 1.20 (0.91 to 1.59) | .554 |
| Multivariable-adjusted HR* (95% CI) | 1.00 (referent) | 1.56 (1.13 to 2.15) | 1.14 (0.79 to 1.65) | 1.39 (0.93 to 2.09) | .279 |
| Adiponectin | |||||
| Quartile cutpoints, ng/mL | ≤19 678 | 19 679–28 326 | 28 327–39 526 | ≥39 527 | |
| No. case patients/No. subcohort members | 210/183 | 223/206 | 220/213 | 222/218 | |
| Age and ethnicity adjusted HR (95% CI) | 1.00 (referent) | 0.92 (0.69 to 1.22) | 0.88 (0.66 to 1.17) | 0.84 (0.63 to 1.11) | .212 |
| Multivariable-adjusted HR* (95% CI) | 1.00 (referent) | 0.88 (0.65 to 1.20) | 0.76 (0.55 to 1.06) | 0.76 (0.55 to 1.06) | .078 |
| Resistin | |||||
| Quartile cutpoints, ng/mL | ≤9.86 | 9.87–12.46 | 12.47–15.70 | ≥15.8 | |
| No. case patients/No. subcohort members | 229/212 | 234/209 | 221/200 | 191/200 | |
| Age and ethnicity adjusted HR (95% CI) | 1.00 (referent) | 1.03 (0.79 to 1.35) | 0.96 (0.73 to 1.26) | 0.85 (0.64 to 1.13) | .227 |
| Multivariable-adjusted HR* (95% CI) | 1.00 (referent) | 1.01 (0.75 to 1.37) | 0.99 (0.73 to 1.35) | 0.93 (0.68 to 1.27) | .664 |
| CRP | |||||
| Quartile cutpoints, µg/mL | ≤0.91 | 0.92–2.01 | 2.02–4.57 | ≥4.58 | |
| No. case patients/No. subcohort members | 199/208 | 195/207 | 238/195 | 223/188 | |
| Age and ethnicity adjusted HR (95% CI) | 1.00 (referent) | 0.99 (0.75 to 1.31) | 1.34 (1.02 to 1.77) | 1.32 (1.00 to 1.75) | .012 |
| Multivariable-adjusted HR* (95% CI) | 1.00 (referent) | 1.01 (0.73 to 1.39) | 1.31 (0.93 to 1.85) | 1.24 (0.86 to 1.80) | .120 |
| IL-6 | |||||
| Quartile cutpoints, pg/mL | ≤0.92 | 0.93–1.47 | 1.48–2.31 | ≥2.32 | |
| No. case patients/No. subcohort members | 226/206 | 230/209 | 187/202 | 213/178 | |
| Age and ethnicity adjusted HR (95% CI) | 1.00 (referent) | 0.92 (0.70 to 1.21) | 0.82 (0.62 to 1.09) | 1.08 (0.82 to 1.43) | .801 |
| Multivariable-adjusted HR* (95% CI) | 1.00 (referent) | 1.03 (0.75 to 1.42) | 0.87 (0.63 to 1.22) | 1.20 (0.85 to 1.69) | .528 |
| TNF-α | |||||
| Quartile cutpoints, pg/mL | ≤1.81 | 1.82–2.62 | 2.63–3.62 | ≥3.63 | |
| No. case patients/No. subcohort members | 220/204 | 235/204 | 222/203 | 179/188 | |
| Age and ethnicity adjusted HR (95% CI) | 1.00 (referent) | 1.03 (0.78 to 1.35) | 0.97 (0.73 to 1.27) | 0.80 (0.60 to 1.08) | .144 |
| Multivariable-adjusted HR* (95% CI) | 1.00 (referent) | 1.01 (0.75 to 1.37) | 1.00 (0.74 to 1.36) | 0.82 (0.59 to 1.14) | .292 |
| PAI-1 | |||||
| Quartile cutpoints, pg/mL | ≤9187 | 9188-14 395 | 14 396–21 348 | ≥21 349 | |
| No. case patients/No. subcohort members | 207/210 | 231/204 | 212/198 | 208/187 | |
| Age and ethnicity adjusted HR (95% CI) | 1.00 (referent) | 1.20 (0.92 to 1.58) | 1.09 (0.83 to 1.44) | 1.14 (0.86 to 1.50) | .513 |
| Multivariable-adjusted HR* (95% CI) | 1.00 (referent) | 1.29 (0.95 to 1.74) | 1.18 (0.86 to 1.63) | 1.33 (0.96 to 1.86) | .145 |
| HGF | |||||
| Quartile cutpoints, pg/mL | ≤407 | 408–613 | 614–869 | ≥870 | |
| No. case patients/No. subcohort members | 223/209 | 217/212 | 214/210 | 220/189 | |
| Age and ethnicity adjusted HR (95% CI) | 1.00 (referent) | 0.93 (0.70 to 1.22) | 0.93 (0.71 to 1.22) | 1.03 (0.78 to 1.36) | .867 |
| Multivariable-adjusted HR* (95% CI) | 1.00 (referent) | 1.03 (0.76 to 1.39) | 0.97 (0.71 to 1.31) | 1.20 (0.87 to 1.65) | .369 |
*Multivariable model adjusted for age, ethnicity, alcohol consumption, family history of breast cancer, parity, years of menstrual cycling, age at first child’s birth, use of hormone therapy, endogenous estradiol levels (in non–hormone therapy users only), history of benign breast disease, body mass index, and physical activity. All statistical tests were two-sided. CI = confidence interval; CRP = C-reactive protein; HGF = hepatocyte growth factor; HR = hazard ratio; IL-6 = interleukin-6; PAI-1 = plasminogen activator inhibitor–1; TNF-α = tumor necrosis factor-α; WHI-OS = Women’s Health Initiative Observational Study.
CRP and PAI-1 Results Stratified by HT Use at Baseline
The association of CRP level with breast cancer risk differed according to HT use at baseline (P interaction = .003), and mean CRP levels varied statistically significantly between HT nonusers (1.48 ug/mL) and HT users (2.65 ug/mL for estrogen-alone and 2.23 ug/mL for estrogen plus progestin; P ≤ .001). Among women using HT at baseline, CRP levels were unrelated to breast cancer risk (HRq4-q1 = 0.90, 95% CI = 0.53 to 1.53, P trend = .509), while in non-HT users, CRP levels were positively associated with breast cancer incidence after controlling for established breast cancer risk factors (Table 3). Specifically, compared with women in the lowest quartile of CRP, those in the third quartile had a statistically significantly increased incidence of breast cancer (HRq3-q1 = 2.28, 95% CI = 1.36 to 3.81), while the hazard ratio for the highest quartile was of borderline statistical significance (HRq4-q1 = 1.63, 95% CI = 0.95 to 2.80, P trend = .010) (Table 3). However, when combined, the upper two quartiles of CRP were positively and significantly associated with breast cancer risk (HRq4+q3-q1 = 1.98, 95% CI = 1.25 to 3.13, P trend = .003). Given that CRP can be a marker of acute inflammation, we further excluded participants with CRPs 10 µg/mL or higher (considered evidence for acute infection) and observed no change in the risk estimates (HRq4+q3-q1 = 1.85, 95% CI = 1.13 to 3.05, P trend= .014). High CRP levels remained statistically significantly associated with breast cancer risk in non-HT users even when insulin was added into the multivariable model (HRq4+q3-q1 = 1.67, 95% CI = 1.04 to 2.68, P trend = .029) (Table 4). In addition, we restricted our analysis to women who reported that they had never used HT (71.4% of HT nonusers at baseline) and found the association of CRP with breast cancer risk to be essentially unaltered (HRq4+q3-q1 = 1.77, 95% CI = 0.95 to 3.29), albeit the association was of borderline significance because of the reduced sample size. We also investigated whether the association of CRP with breast cancer risk differed by tumor ER status in HT nonusers separately. Among HT nonusers, and in a multivariable model that included insulin, estradiol, BMI, and other breast cancer risk factors, the association between CRP and breast cancer incidence was not substantially different for ER+ (HRq4+q3-q1 = 1.68, 95% CI = 0.97 to 2.89) and ER- tumors (HRq4+q3-q1 = 2.10, 95% CI = 1.04 to 4.23).
Table 3.
Adjusted hazard ratios (and 95% CIs) for associations of incident breast cancer with baseline levels of CRP and PAI-1 with stratification by HT use
| Factor, model | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P trend |
|---|---|---|---|---|---|
| Non-HT users at baseline | |||||
| CRP | |||||
| Quartile cutpoints, µg/mL | ≤0.91 | 0.92–2.01 | 2.02–4.57 | ≥4.58 | |
| No. case patients/No. subcohort members | 113/149 | 97/122 | 110/84 | 81/79 | |
| Age and ethnicity adjusted HR (95% CI) | 1.00 (referent) | 1.02 (0.71 to 1.48) | 1.82 (1.23 to 2.68) | 1.40 (0.93 to 2.10) | .010 |
| Multivariable-adjusted HR* (95% CI) | 1.00 (referent) | 1.00 (0.65 to 1.56) | 2.28 (1.36 to 3.81) | 1.63 (0.95 to 2.80) | .010 |
| PAI-1 | |||||
| Quartile cutpoints, pg/mL | ≤9187 | 9188-14 395 | 14 396–21 348 | ≥21 349 | |
| No. case subjects/No. subcohort members | 55/79 | 96/102 | 114/125 | 138/129 | |
| Age and ethnicity adjusted HR (95% CI) | 1.00 (referent) | 1.38 (0.88 to 2.15) | 1.35 (0.88 to 2.09) | 1.62 (1.06 to 2.48) | .045 |
| Multivariable-adjusted HR* (95% CI) | 1.00 (referent) | 1.47 (0.88 to 2.46) | 1.49 (0.89 to 2.52) | 1.71 (1.02 to 2.89) | .077 |
| HT users at baseline | |||||
| CRP | |||||
| Quartile cutpoints, µg/mL | ≤0.91 | 0.92–2.01 | 2.02–4.57 | ≥4.58 | |
| No. case patients/No. subcohort members | 86/59 | 98/85 | 128/111 | 142/108 | |
| Age- and ethnicity-adjusted HR (95% CI) | 1.00 (referent) | 0.82 (0.52 to 1.28) | 0.80 (0.52 to 1.22) | 0.94 (0.62 to 1.45) | .918 |
| Multivariable-adjusted HR* (95% CI) | 1.00 (referent) | 0.96 (0.56 to 1.66) | 0.75 (0.45 to 1.24) | 0.90 (0.53 to 1.53) | .509 |
| PAI-1 | |||||
| Quartile cutpoints, pg/mL | ≤9187 | 9188-14 395 | 14 396–21 348 | ≥21 349 | |
| No. case patients/No. subcohort members | 152/131 | 135/101 | 98/73 | 70/58 | |
| Age- and ethnicity-adjusted HR (95% CI) | 1.00 (referent) | 1.26 (0.87 to 1.81) | 1.18 (0.80 to 1.75) | 1.04 (0.68 to 1.59) | .706 |
| Multivariable-adjusted HR* (95% CI) | 1.00 (referent) | 1.31 (0.86 to 1.97) | 1.06 (0.65 to 1.72) | 1.17 (0.71 to 1.93) | .663 |
* Multivariable model adjusted for age, ethnicity, alcohol consumption, family history of breast cancer, parity, years of menstrual cycling, age at first child’s birth, type of hormone therapy (HT; in HT users only), endogenous estradiol levels (in non-HT users only), history of benign breast disease, body mass index, and physical activity. All statistical tests were two-sided. CI = confidence interval; CRP = C-reactive protein; HR = hazard ratio; HT = hormone therapy; PAI-1 = plasminogen activator inhibitor–1.
Table 4.
Multivariable hazard ratios (and 95% CIs) for the associations of obesity-related factors with breast cancer risk among non-HT users at baseline
| Covariates* | HR (95% CI) | P trend |
|---|---|---|
| Estradiol, pg/mL | ||
| <8 | 1.00 (referent) | |
| ≥8 | 1.52 (1.02 to 2.26) | .040 |
| Insulin, μIU/mL | ||
| ≤3.3 | 1.00 (referent) | .003 |
| 3.4–5.3 | 1.20 (0.69 to 2.07) | |
| 5.4–8.5 | 1.60 (0.93 to 2.73) | |
| ≥8.6 | 2.37 (1.30 to 4.30) | |
| CRP, μg/mL | ||
| ≤0.91 | 1.00 (referent) | .029 |
| 0.92–2.01 | 0.98 (0.63 to 1.54) | |
| ≥2.02 | 1.67 (1.04 to 2.68) | |
*Multivariable model included estradiol, insulin, C-reactive protein, and the following covariates: age, race/ethnicity, body mass index, alcohol consumption, family history of breast cancer, parity, years of menstrual cycling, age at first child’s birth, history of benign breast disease, and physical activity. All statistical tests were two-sided. CI = confidence interval; CRP = C-reactive protein; HR = hazard ratio; HT = hormone therapy.
We also observed heterogeneity in the association of PAI-1 with breast cancer by HT use such that among non-HT users, PAI-1 levels were positively associated with breast cancer risk (HRq4-q1 = 1.71, 95% CI = 1.02 to 2.89, P trend = .077), while among HT-users there was no association with breast cancer (HRq4-q1 = 1.17, 95% CI = 0.17 to 1.93, P trend = .663); however, the formal test of interaction was not significant (P = .238). Further, including insulin in the multivariable model attenuated the positive association between PAI-1 and breast cancer among non-HT users (HRq4-q1 = 1.49, 95% CI = 0.85 to 2.61, P trend = .270).
Mediation of the Obesity–Breast Cancer Association by Estradiol, Insulin, and CRP
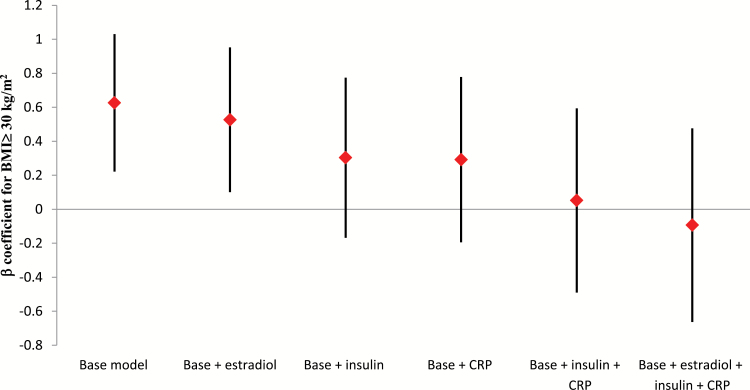
As shown in Table 4, among non-HT users, insulin, estradiol, and CRP were each positively and statistically significantly associated with breast cancer, even after mutual adjustment for one another. We next evaluated whether the obesity-related factors that were statistically significantly associated with breast cancer risk could provide a mechanistic link between obesity and breast cancer. We examined, among non-HT users, whether the strength of association between obesity and breast cancer risk would change after accounting for the potential mediating effects of estradiol, insulin, and/or CRP. Table 5 shows that a BMI of 30kg/m2 or more, compared with a BMI under 25kg/m2, was statistically significantly associated with an increased breast cancer risk (HR = 1.87, 95% CI = 1.25 to 2.80, P trend = .003) while controlling for other breast cancer risk factors, except estradiol, insulin, and CRP. When estradiol was added into the model, the obesity association with breast cancer was weakened, but remained statistically significant (HR = 1.69, 95% CI = 1.11 to 2.59, P trend = .018). However, following inclusion of insulin or CRP in the model, the hazard ratio for BMI of 30 or more vs under 25kg/m2 was attenuated and no longer significant. The hazard ratio for BMI of 30kg/m2 or more vs under 25kg/m2 was reduced to 0.91 (95% CI = 0.52 to 1.61, P trend = .780) when all three obesity-related factors were entered into the model simultaneously. Figure 1 shows that the β-coefficient for BMI of 30kg/m2 or more decreased 16%, 52%, 53%, 92%, and 115% following adjustment for estradiol, insulin, CRP, both insulin and CRP, or all three factors simultaneously, respectively. On the other hand, the associations of CRP, insulin, and estradiol each remained statistically significantly associated with breast cancer after mutual adjustment for one another (Table 4). The association of BMI with breast cancer did not materially change when we excluded women with a BMI under 18.5kg/m2 or when BMI was modeled as a continuous variable (data not shown). Finally, the degree of attenuation of the BMI–breast cancer association by insulin, estradiol, and CRP did not differ depending on ER status of the breast tumor: for ER+, the hazard ratio comparing a BMI of more than 30kg/m2 with BMI between 18.5 and 25.0kg/m2 was 1.80 (95% CI = 1.12 to 2.88); for inclusion of insulin, estradiol, and CRP, the hazard ratio comparing BMI of more than 30kg/m2 with BMI between 18.5 and 25.0kg/m2 was 0.82 (95% CI = 0.43 to 1.57); for ER-, the hazard ratio comparing BMI of more than 30kg/m2 with BMI between 18.5 and 25.0kg/m2 was 1.80 (95% CI = 1.02 to 3.16); for inclusion of insulin, estradiol, and CRP, the hazard ratio comparing BMI of more than 30kg/m2 with BMI between 18.5 and 25.0kg/m2 was 0.95 (95% CI = 0.41 to 2.19).
Table 5.
Multivariable hazard ratios (and 95% CIs) for the association of incident breast cancer and BMI among non-HT users at baseline
| Factor, model | Quantile 1 | Quantile 2 | Quantile 3 | P trend |
|---|---|---|---|---|
| BMI | ||||
| Category cutpoints, kg/m2 | ≤24.9 | 25.0–29.9 | ≥30.0 | |
| No. case patients /No. subcohort members | 135/162 | 135/155 | 137/116 | |
| Base model* | 1.00 (referent) | 1.17 (0.81 to 1.69) | 1.87 (1.25 to 2.80) | .003 |
| Base + estradiol | 1.00 (referent) | 1.13 (0.77 to 1.64) | 1.69 (1.11 to 2.59) | .018 |
| Base + insulin | 1.00 (referent) | 0.89 (0.59 to 1.34) | 1.35 (0.85 to 2.17) | .201 |
| Base + CRP | 1.00 (referent) | 1.01 (0.67 to 1.52) | 1.34 (0.82 to 2.18) | .251 |
| Base + insulin + CRP | 1.00 (referent) | 0.79 (0.51 to 1.23) | 1.05 (0.61 to 1.81) | .823 |
| Base + estradiol + insulin + CRP | 1.00 (referent) | 0.74 (0.47 to 1.17) | 0.91 (0.52 to 1.61) | .780 |
*Base model adjusted for age, race/ethnicity, alcohol consumption, family history of breast cancer, parity, years of menstrual cycling, age at first child’s birth, history of benign breast disease, and physical activity. All statistical tests were two-sided. BMI = body mass index; CI = confidence interval; CRP = C-reactive protein; HT = hormone therapy.
Figure 1.
Beta coefficients for the association between body mass index ≥ 30kg/m2 and breast cancer after accounting for possible mediation by estradiol, insulin, and/or C-reactive protein (CRP) among non-HT users at baseline. Vertical lines represent the 95% confidence intervals. Base model adjusted for age, race/ethnicity, alcohol consumption, family history of breast cancer, parity, years of menstrual cycling, age at first child’s birth, history of benign breast disease, and physical activity.
Discussion
In this prospective investigation of postmenopausal women, higher circulating CRP levels were associated with increased incidence of breast cancer among women not using HT. Specifically, the breast cancer incidence rate in these women was two-fold greater among those in the highest two quartiles compared with those in the lowest quartile of CRP, even after controlling for estradiol, insulin, BMI, and established breast cancer risk factors.
CRP is an acute phase, liver-derived peptide that is routinely measured clinically as a nonspecific inflammatory marker. Circulating levels of CRP have been shown to be predictive of risk of cardiovascular disease (34) and type II diabetes (35), and CRP levels have also been associated with colorectal cancer risk (36). However, epidemiologic data relating circulating CRP concentrations to breast cancer are more limited and the majority of prospective studies have reported null findings (16,18). However, a nested case-control study conducted in the Multiethnic Cohort that included 706 postmenopausal breast cancer case patients and 706 matched control patients reported a positive, albeit nonlinear, association between circulating CRP and breast cancer risk, even after adjustment for BMI and other breast cancer risk factors (37). More recently, a prospective analysis in the E3N cohort in France did not report a statistically significant association between CRP levels and breast cancer risk overall, though there was evidence for a statistical interaction with BMI, such that higher CRP levels were positively associated with breast cancer incidence among overweight and obese women (21). In the current study, there was no evidence of heterogeneity in the association of CRP levels with breast cancer by BMI, though we found a positive CRP association among non-HT users but not in HT users. It is difficult to discern the discrepancies between the E3N and WHI results, particularly when data stratified by HT use were not available in the E3N study. If women who were overweight or obese tended not to use HT after menopause, as was the case in the WHI postmenopausal women, then the interaction between CRP and BMI observed in the E3N study could be explained by HT use. In our study, the relationship between CRP and breast cancer persisted even after simultaneous adjustment for BMI, estradiol, and insulin levels. This finding suggests that CRP may represent an inflammatory mechanism or a closely linked pathway that is associated with breast cancer development that is independent of hyperinsulinemia and estrogen.
A possible explanation for why CRP levels were not associated with breast cancer in HT users is that HT use is a major risk factor for breast cancer and further exposure to inflammation in the presence of HT may be relatively unimportant. In addition, the hepatic first-pass effect of HT can artificially raise the levels of circulating CRP such that a relatively high CRP level may not necessarily reflect a proinflammatory status in HT users (32). Indeed, in the current analysis, CRP levels were statistically significantly raised among HT users with the highest geometric mean levels observed in estrogen-alone users, intermediate in estrogen-plus-progestin users, and lowest in HT nonusers.
In this study, we found null to modest associations between breast cancer risk and circulating levels of adipokines, despite the fact that they can have significant biological impact on insulin sensitivity, inflammatory response, cell proliferation and apoptosis, and estrogen metabolism—all processes that have been implicated in breast cancer tumorigenesis (38–40). Adiponectin, an adipokine that is downregulated in obesity and has insulin-sensitizing properties and antiproliferative effects on breast epithelial cells, tended to be inversely associated with breast cancer risk in our analysis—a finding that is consistent with prior data (8,12–14). However, following inclusion of insulin in the multivariable model this association was attenuated and became null, suggesting that the effects of adiponectin on breast cancer risk are partly explained by insulin. Similarly, PAI-1, an adipokine that is upregulated in obesity, was positively associated with breast cancer risk in non-HT users; however, this association was attenuated and lost statistical significance following adjustment for insulin. We previously reported a positive and robust association between insulin levels and postmenopausal breast cancer risk in the same participants of the WHI-OS who were tested as part of the current investigation (3). In that analysis, women in the highest quartile of insulin were at a greater-than-two-fold higher risk of developing breast cancer than those in the lowest quartile, and this association was independent of BMI and endogenous estradiol levels. Given that both adiponectin and PAI-1 have effects on insulin sensitivity and that hyperinsulinemia is a statistically significant positive risk factor for breast cancer, it may be inferred that any effects of these adipokines on breast cancer risk may be mediated through insulin.
One of the advantages of our case-cohort study was that we had measurements on various obesity-related factors and were able to evaluate whether those statistically significantly associated with breast cancer risk, namely insulin, estradiol, and CRP, could be intervening variables linking obesity with development of breast cancer. Our data showed that the relation of obesity with breast cancer risk was entirely attenuated after adjustment for endogenous estradiol, insulin, and CRP, with the latter two being the major factors contributing to the attenuation of the obesity–breast cancer association. Our observations are consistent with the hypothesis that obesity-induced hyperinsulinemia and chronic inflammation are part of the biological mechanisms leading to breast cancer. Given that CRP is a nonspecific inflammatory marker, our findings raise questions regarding the precise mechanism by which inflammation could be related to breast cancer development. A potential limitation in the evaluation of adipokines in relation to breast cancer is that adipokines primarily function in a paracrine manner and the measurement of circulating levels may not accurately reflect processes at the tissue level. It is not known to what extent circulating levels of cytokines are associated with levels in breast or adipose tissue, and it is possible that plasma levels may be a poor surrogate for local levels, at least for some of the cytokines.
In conclusion, our data indicate that relatively high levels of CRP are positively associated with risk of postmenopausal breast cancer in women not using HT, and this relationship is largely independent of other obesity-related pathways such as hyperinsulinemia and estrogen. Further, CRP, insulin, and estradiol together appear to explain all of the association of BMI with breast cancer development. Interventions aimed at lowering CRP levels, alongside insulin and estrogen, may be effective at reducing risk of breast cancer in postmenopausal women.
Funding
This work was supported by contract N01WH74310 (to GYFH) with the National Heart, Lung, and Blood Institute (NHLBI). The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, and US Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C.
Supplementary Material
Women’s Health Initiative Investigators
Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Jacques Rossouw, Shari Ludlam, Dale Burwen, Joan McGowan, Leslie Ford, Nancy Geller.
Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA)
Ross Prentice, Garnet Anderson, Andrea LaCroix, Charles L. Kooperberg, Barbara Cochrane, Julie Hunt, Marian Neuhouser, Lesley Tinker, Susan Heckbert, Alex Reiner.
Regional Centers: (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson, Kathryn M. Rexrode, Brian Walsh, J. Michael Gaziano, Maria Bueche; (MedStar Health Research Institute/Howard University, Washington, DC) Barbara V. Howard, Lucile Adams-Campbell, Lawrence Lessin, Cheryl Iglesia, Brian Walitt, Amy Park; (The Ohio State University, Columbus, OH) Rebecca Jackson, Randall Harris, Electra Paskett, W. Jerry Mysiw, Michael Blumenfeld; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick, Mark A. Hlatky, Manisha Desai, Jean Tang, Stacy T. Sims; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thomson, Tamsen Bassford, Cheryl Ritenbaugh, Zhao Chen, Marcia Ko; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende, Maurizio Trevisan, Ellen Smit, Amy Millen, Michael LaMonte; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher, Michael Perri, Andrew Kaunitz, R. Stan Williams, Yvonne Brinson; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace, James Torner, Susan Johnson, Linda Snetselaar, Jennifer Robinson; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller, Jane Cauley, N. Carole Milas; (University of Tennessee Health Science Center, Memphis, TN) Karen C. Johnson, Suzanne Satterfield, Rongling Li, Stephanie Connelly, Fran Tylavsky; (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker, Stephen Rapp, Claudine Legault, Mark Espeland, Laura Coker, Michelle Naughton.
Women’s Health Initiative Memory Study: (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker, Stephen Rapp, Claudine Legault, Mark Espeland, Laura Coker, Michelle Naughton.
Former Principal Investigators and Project Officers: (Albert Einstein College of Medicine, Bronx, NY) Sylvia Wassertheil-Smoller (Baylor College of Medicine, Houston, TX) Haleh Sangi-Haghpeykar, Aleksandar Rajkovic, Jennifer Hays, John Foreyt; (Brown University, Providence, RI) Charles B. Eaton, Annlouise R. Assaf; (Emory University, Atlanta, GA) Lawrence S. Phillips, Nelson Watts, Sally McNagny, Dallas Hall; (Fred Hutchinson Cancer Research Center, Seattle, WA) Shirley A. A. Beresford, Maureen Henderson; (George Washington University, Washington, DC) Lisa Martin, Judith Hsia, Valery Miller; (Harbor-UCLA Research and Education Institute, Torrance, CA) Rowan Chlebowski (Kaiser Permanente Center for Health Research, Portland, OR) Erin LeBlanc, Yvonne Michael, Evelyn Whitlock, Cheryl Ritenbaugh, Barbara Valanis; (Kaiser Permanente Division of Research, Oakland, CA) Bette Caan, Robert Hiatt; (National Cancer Institute, Bethesda, MD) Carolyn Clifford*; (Medical College of Wisconsin, Milwaukee, WI) Jane Morley Kotchen (National Heart, Lung, and Blood Institute, Bethesda, MD) Linda Pottern; (Northwestern University, Chicago/Evanston, IL) Linda Van Horn, Philip Greenland; (Rush University Medical Center, Chicago, IL) Lynda Powell, William Elliott, Henry Black; (State University of New York at Stony Brook, Stony Brook, NY) Dorothy Lane, Iris Granek; (University at Buffalo, Buffalo, NY) Maurizio Trevisan; (University of Alabama at Birmingham, Birmingham, AL) Cora E. Lewis, Albert Oberman; (University of Arizona, Tucson/Phoenix, AZ) Tamsen Bassford, Cheryl Ritenbaugh, Tom Moon; (University of California at Davis, Sacramento, CA) John Robbins; (University of California at Irvine, CA) F. Allan Hubbell, Frank Meyskens Jr; (University of California at Los Angeles, CA) Lauren Nathan, Howard Judd*; (University of California at San Diego, LaJolla/Chula Vista, CA) Robert D. Langer; (University of Cincinnati, Cincinnati, OH) Michael Thomas, Margery Gass, James Liu; (University of Hawaii, Honolulu, HI) J. David Curb*; (University of Massachusetts/Fallon Clinic, Worcester, MA) Judith Ockene; (University of Medicine and Dentistry of New Jersey, Newark, NJ) Norman Lasser; (University of Miami, Miami, FL) Mary Jo O’Sullivan, Marianna Baum; (University of Minnesota, Minneapolis, MN) Karen L. Margolis, Richard Grimm; (University of Nevada, Reno, NV) Robert Brunner, Sandra Daugherty*; (University of North Carolina, Chapel Hill, NC) Gerardo Heiss, Barbara Hulka, David Sheps; (University of Tennessee Health Science Center, Memphis, TN) Karen Johnson, William Applegate; (University of Texas Health Science Center, San Antonio, TX) Robert Brzyski, Robert Schenken; (University of Wisconsin, Madison, WI) Gloria E. Sarto, Catherine Allen*; (Wake Forest University School of Medicine, Winston-Salem, NC) Mara Vitolins, Denise Bonds, Electra Paskett, Greg Burke; (Wayne State University School of Medicine/Karmanos Cancer Institute, Detroit, MI) Michael S. Simon, Susan Hendrix.
References
- 1. van den Brandt PA, Spiegelman D, Yaun SS, et al. Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol. 2000;152:514–527. [DOI] [PubMed] [Google Scholar]
- 2. Ahn J, Schatzkin A, Lacey JV, Jr, et al. Adiposity, adult weight change, and postmenopausal breast cancer risk. Arch Intern Med. 2007;167:2091–2102. [DOI] [PubMed] [Google Scholar]
- 3. Gunter MJ, Hoover DR, Yu H, et al. Insulin, insulin-like growth factor-I, and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 2009;101:48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Key T, Appleby P, Barnes I, et al. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94:606–616. [DOI] [PubMed] [Google Scholar]
- 5. van Kruijsdijk RC, van der Wall E, Visseren FL. Obesity and cancer: the role of dysfunctional adipose tissue. Cancer Epidemiol Biomarkers Prev. 2009;18:2569–2578. [DOI] [PubMed] [Google Scholar]
- 6. Perks CM, Holly JM. Hormonal mechanisms underlying the relationship between obesity and breast cancer. Endocrinol Metab Clin North Am. 2011;40:485–507, vii. [DOI] [PubMed] [Google Scholar]
- 7. Roberts DL, Dive C, Renehan AG. Biological mechanisms linking obesity and cancer risk: new perspectives. Annu Rev Med. 2010;61:301–316. [DOI] [PubMed] [Google Scholar]
- 8. Tworoger SS, Eliassen AH, Kelesidis T, et al. Plasma adiponectin concentrations and risk of incident breast cancer. J Clin Endocrinol Metab. 2007;92:1510–1516. [DOI] [PubMed] [Google Scholar]
- 9. Gross AL, Newschaffer CJ, Hoffman-Bolton J, et al. Adipocytokines, inflammation, and breast cancer risk in postmenopausal women: a prospective study. Cancer Epidemiol Biomarkers Prev. 2013;22:1319–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gaudet MM, Patel AV, Teras LR, et al. Obesity-related markers and breast cancer in CPS-II Nutrition Cohort. Int J Mol Epidemiol Genet. 2013;4:156–166. [PMC free article] [PubMed] [Google Scholar]
- 11. Cust AE, Stocks T, Lukanova A, et al. The influence of overweight and insulin resistance on breast cancer risk and tumour stage at diagnosis: a prospective study. Breast Cancer Res Treat. 2009;113:567–576. [DOI] [PubMed] [Google Scholar]
- 12. Macis D, Guerrieri-Gonzaga A, Gandini S. Circulating adiponectin and breast cancer risk: a systematic review and meta-analysis. Int J Epidemiol. 2014;43:1226–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ye J, Jia J, Dong S, et al. Circulating adiponectin levels and the risk of breast cancer: a meta-analysis. Eur J Cancer Prev. 2014;23:158–165. [DOI] [PubMed] [Google Scholar]
- 14. Liu LY, Wang M, Ma ZB, et al. The role of adiponectin in breast cancer: a meta-analysis. PLoS One. 2013;8:e73183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Siemes C, Visser LE, Coebergh JW, et al. C-reactive protein levels, variation in the C-reactive protein gene, and cancer risk: the Rotterdam Study. J Clin Oncol. 2006;24:5216–5222. [DOI] [PubMed] [Google Scholar]
- 16. Heikkila K, Harris R, Lowe G, et al. Associations of circulating C-reactive protein and interleukin-6 with cancer risk: findings from two prospective cohorts and a meta-analysis. Cancer Causes Control. 2009;20:15–26. [DOI] [PubMed] [Google Scholar]
- 17. Trichopoulos D, Psaltopoulou T, Orfanos P, et al. Plasma C-reactive protein and risk of cancer: a prospective study from Greece. Cancer Epidemiol Biomarkers Prev. 2006;15:381–384. [DOI] [PubMed] [Google Scholar]
- 18. Zhang SM, Lin J, Cook NR, et al. C-reactive protein and risk of breast cancer. J Natl Cancer Inst. 2007;99:890–894. [DOI] [PubMed] [Google Scholar]
- 19. Allin KH, Bojesen SE, Nordestgaard BG. [C-reactive protein, risk of and survival after cancer--secondary publication]. Ugeskr Laeger. 2009;171:3510–3513. [PubMed] [Google Scholar]
- 20. Il’yasova D, Colbert LH, Harris TB, et al. Circulating levels of inflammatory markers and cancer risk in the health aging and body composition cohort. Cancer Epidemiol Biomarkers Prev. 2005;14:2413–2418. [DOI] [PubMed] [Google Scholar]
- 21. Dossus L, Jimenez-Corona A, Romieu I, et al. C-reactive protein and postmenopausal breast cancer risk: results from the E3N cohort study. Cancer Causes Control. 2014;25:533–539. [DOI] [PubMed] [Google Scholar]
- 22. Hvidtfeldt UA, Gunter MJ, Lange T, et al. Quantifying mediating effects of endogenous estrogen and insulin in the relation between obesity, alcohol consumption, and breast cancer. Cancer Epidemiol Biomarkers Prev. 2012;21:1203–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Design of the Women’s Health Initiative clinical trial and observational study. The Women’s Health Initiative Study Group. Control Clin Trials. 1998;19:61–109. [DOI] [PubMed] [Google Scholar]
- 24.http://www.whiscience.org/about/about_biospecimen.php.:
- 25. The SEER Program Code Manual. Cancer Statistics Branch, Surveillance Program, Division of Cancer Prevention and Control, National Cancer Institute, US Dept of Health and Human Services, Public Health Service, National Institutes of Health; June 1992. NIH Publication No. 92–1999. 1992.
- 26. SEER Program: Comparative Staging Guide for Cancer. Washington, DC: US Dept of Health and Human Services, Public Health Service, National Institutes of Health; version 1.1, June 1993. NIH Publication 93–3640. 1993. [Google Scholar]
- 27. Kaplan RC, Ho GY, Xue X, et al. Within-individual stability of obesity-related biomarkers among women. Cancer Epidemiol Biomarkers Prev. 2007;16:1291–1293. [DOI] [PubMed] [Google Scholar]
- 28. Hankinson SE, Manson JE, Spiegelman D, et al. Reproducibility of plasma hormone levels in postmenopausal women over a 2-3-year period. Cancer Epidemiol Biomarkers Prev. 1995;4:649–654. [PubMed] [Google Scholar]
- 29. Missmer SA, Spiegelman D, Bertone-Johnson ER, et al. Reproducibility of plasma steroid hormones, prolactin, and insulin-like growth factor levels among premenopausal women over a 2- to 3-year period. Cancer Epidemiol Biomarkers Prev. 2006;15:972–978. [DOI] [PubMed] [Google Scholar]
- 30. Gunter MJ, Cross AJ, Huang WY, et al. A prospective evaluation of C-reactive protein levels and colorectal adenoma development. Cancer Epidemiol Biomarkers Prev. 2011;20:537–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Prentice RL. A Case-Cohort design for epidemiologic studies and disease prevention trials. Biometrika. 1986;73:1–11. [Google Scholar]
- 32. Ho GY, Wang T, Gunter MJ, et al. Adipokines linking obesity with colorectal cancer risk in postmenopausal women. Cancer Res. 2012;72:3029–3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang T, Rohan TE, Gunter MJ, et al. A prospective study of inflammation markers and endometrial cancer risk in postmenopausal hormone nonusers. Cancer Epidemiol Biomarkers Prev. 2011;20:971–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ridker PM, Hennekens CH, Buring JE, et al. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. [DOI] [PubMed] [Google Scholar]
- 35. Pradhan AD, Manson JE, Rifai N, et al. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. [DOI] [PubMed] [Google Scholar]
- 36. Tsilidis KK, Branchini C, Guallar E, et al. C-reactive protein and colorectal cancer risk: a systematic review of prospective studies. Int J Cancer. 2008;123:1133–1140. [DOI] [PubMed] [Google Scholar]
- 37. Ollberding NJ, Kim Y, Shvetsov YB, et al. Prediagnostic leptin, adiponectin, C-reactive protein, and the risk of postmenopausal breast cancer. Cancer Prev Res (Phila). 2013;6:188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Grivennikov SI, Karin M. Inflammatory cytokines in cancer: tumour necrosis factor and interleukin 6 take the stage. Ann Rheum Dis. 2011;70(Suppl 1):i104–e108. [DOI] [PubMed] [Google Scholar]
- 39. Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. [DOI] [PubMed] [Google Scholar]
- 40. Hotamisligil GS, Arner P, Caro JF, et al. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95:2409–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.