Abstract
Objective
To reduce dosing errors when administering orally-ingested over-the-counter (OTC) liquid medications, the US Food and Drug Administration (FDA) and the Consumer Healthcare Products Association (CHPA) released voluntary recommendations for dosing directions and dosing devices. This study assessed recommendation adherence for national brand-name orally-ingested OTC liquid pediatric analgesics/antipyretics and cough, cold, and allergy medications available after the FDA Guidance was finalized in 2011 in order to identify and prioritize specific improvements to dosing directions and dosing devices.
Methods
Recommendations were categorized as top tier or low tier based on potential to directly address ≥3-fold dosing errors. Labeled dosing directions and accompanying dosing devices were assessed by 2 independent reviewers for adherence to specific recommendations.
Results
Of 68 products, 91% of dosing directions and 62% of dosing devices adhered to all top tier recommendations; 57% of products adhered to every top tier recommendation and 93% adhered to all or all but one. A dosing was included with all products. No dosing directions used atypical volumetric units (e.g., drams), and no devices used volumetric units that did not appear in dosing directions. Six products used trailing zeros or failed to use leading zeros with decimal doses and 8 did not use small font for fractions. Product adherence to low tier recommendations ranged from 26% to 91%.
Conclusion
Products adhered to most recommendations in the final FDA Guidance and CHPA Guideline suggesting that these voluntary initiatives promote adherence to recommendations. Improving adherence to recommendations should be prioritized based on potential to reduce harm.
Keywords: dosing error, unintentional overdose, medication label, dosing device, over-the-counter medicines
In response to reports of unintentional overdoses of orally-ingested over-the-counter (OTC) liquid medications due to dosing devices with markings that were inconsistent or incompatible with labeled dosing directions, the US Food and Drug Administration (FDA) released a draft guidance for industry.1 This voluntary guidance, “Dosage Delivery Devices for Orally Ingested OTC Liquid Drug Products” (hereafter “FDA Guidance”), finalized in May 2011, outlines specific recommendations for aligning dosing devices with the accompanying dosing directions for orally-ingested OTC liquid medications.2 Since many OTC liquid medications are intended for pediatric use, minimizing potential errors during dose measurement and administration by caregivers is a key focus of the guidance.
In 2009, concurrent to the initial draft FDA Guidance, the Consumer Healthcare Products Association (CHPA), a trade organization representing OTC medication manufacturers, released a voluntary guideline, “Volumetric Measures for Dosing of Over-the-Counter Oral Liquid Drug Products for Children ≤12 years of Age” (hereafter “CHPA Guideline”), to standardize volumetric measures used in dosing directions as well as devices.3 The following year, using a sample of “baseline” products, Yin et al reported the concerning finding that 98.6% of evaluated OTC liquid medications had “inconsistencies” between dosing directions and device markings.4
We assessed adherence to recommendations in the final FDA Guidance and CHPA Guideline in a sample of national brand-name orally-ingested OTC liquid medications with pediatric dosing available on the market after the final FDA Guidance was released. To prioritize areas for improvement in labeled dosing directions and accompanying devices, recommendations were categorized based on their potential to directly address ≥3-fold dosing errors.
Methods
Sample Selection
In December 2011, CHPA member manufacturers were asked to submit sample products for all currently available orally-ingested OTC liquid medications with specified dosing for children <12 years of age. National brand-name analgesics/antipyretics and cough, cold, and allergy products (e.g., PediaCare®, Robitussin®) were included in the study; generic products, including those branded for specific retailers (e.g., Walgreens®, Wal-Mart®) were not included. Market share of individual brands within each drug class was determined using SymphonyIRI InfoScan Tracking data on units sold to consumers from food, drug, and mass (FDM) merchandisers (excluding Wal-Mart) for the 1-year period ending January 22, 2012.
Definitions
Drug classification (analgesics/antipyretics or cough, cold, and allergy products) was based on labeled indications. Medications were categorized as infants’, children’s, or family products based on the age group indicated on the front panel of the outer packaging (i.e., the outer box or medication bottle), since such visual cues are used by consumers when deciding which medication to purchase.5 Within each brand, unique products were identified based on the product trade name and targeted age group. If products were available in multiple flavors, bottle sizes, or dye-free versions, one version (e.g., a single flavor) was randomly selected, so that each unique product would be given equal weight.
Standard abbreviations for volumetric units were identified by recommended or customary use. The FDA Guidance, CHPA Guideline, US Pharmacopeial Convention, the Institute for Safe Medication Practices, and others specify that milliliters should be abbreviated as “mL”.2,3,6,7 The FDA Guidance and CHPA Guideline specify that teaspoon should be abbreviated as “tsp”, but as there is no uniformly recommended abbreviation for tablespoon units, “TBSP” was considered the standard abbreviation based on common use.8 Pluralization of abbreviations is not addressed by the FDA Guidance or CHPA Guideline and was considered acceptable.
Outcomes
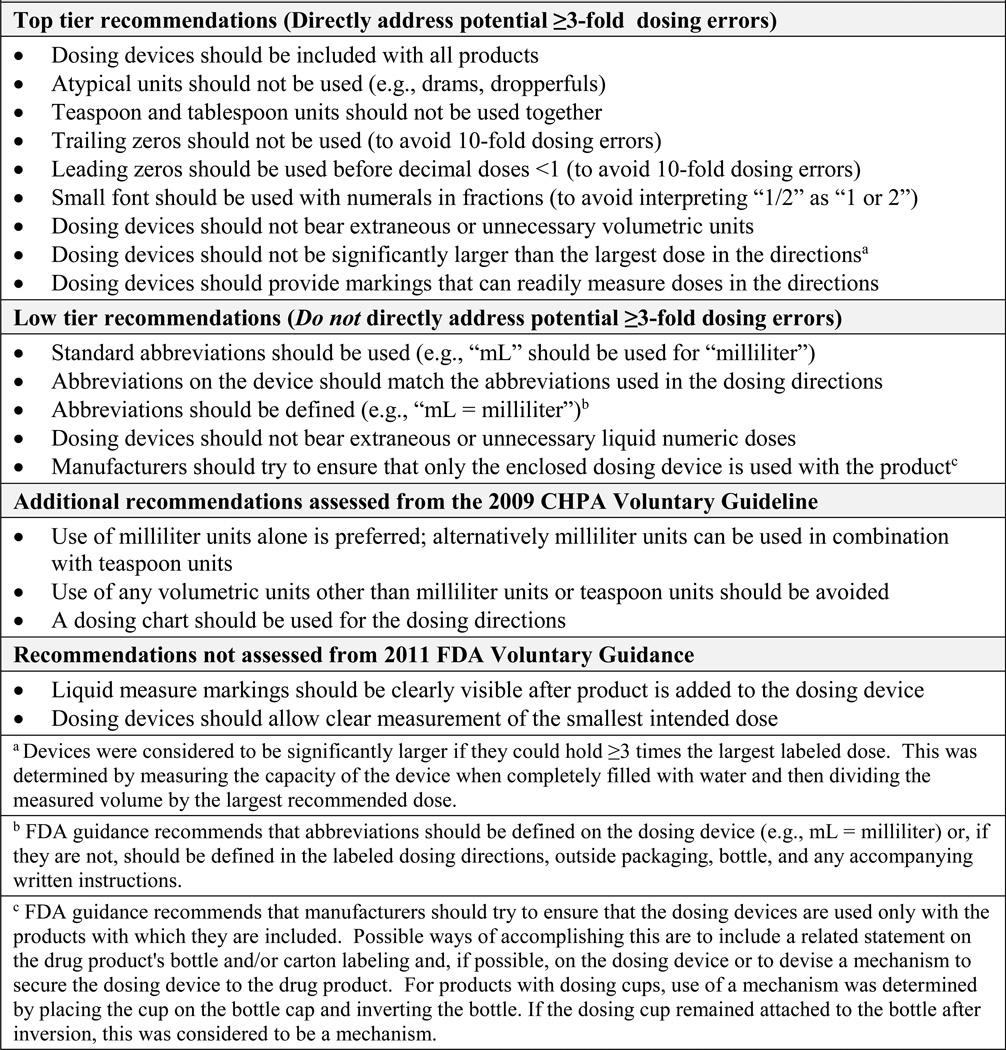
Adherence to specific recommendations in the final FDA Guidance and CHPA Guideline was assessed (Figure 1). Recommendations were categorized as “top tier” or “low tier” by the authors based on potential for reducing clinically meaningful dosing errors (Supplemental Appendix). Top tier recommendations directly address potential dosing errors of 3-fold or more. For example, use of trailing zeros in the dosing directions can lead to 10-fold overdoses if the decimal point is overlooked (i.e., a labeled dose of 1.0 mL is mistaken for 10 mL).6,7,9–12 Low tier recommendations improve consistency and maintain conventional standards of abbreviation and capitalization, but do not directly address ≥3-fold dosing errors. For example, milliliters should always be abbreviated “mL” (i.e., not “ml” or “ML”).2,3,6,7
FIGURE 1.
Recommendations from the 2011 FDA Voluntary Guidance and 2009 CHPA Voluntary Guideline
The recommendation that dosing devices should not be significantly larger than the largest dose in the dosing directions does not quantify “significantly larger.” For this study a dosing device with total volume ≥3-times the largest labeled dose was considered significantly larger. Two other recommendations without objective parameters (device markings should be “clearly visible” after product is added and devices should allow “clear measurement” of the smallest intended dose) were not assessed.
Data collection and analysis
Products were evaluated independently by two investigators (MCL and KOR). A third reviewer (DSB) resolved discordant assessments. Adherence to recommendations was assessed by reviewing dosing directions on bottle labels and attributes of the accompanying dosing devices. Dosing directions on the outer boxes and other written materials were not reviewed since some products are packaged only in the immediate container (i.e., medication bottle) and outer packaging and other written materials may be discarded after purchase. Adherence to recommendations was tabulated and analyzed using SAS version 9.2 (SAS Institute, Cary, NC). Product-specific findings were shared with respective manufacturers.
Results
A total of 89 national brand-name analgesic/antipyretic and cough, cold, and allergy products were collected from January – April 2012. Of these, 68 products representing 21 brands from 12 manufacturers were included in the final analysis. Four products did not meet study inclusion criteria and 17 were identical to an included product except for flavor, bottle size, or use of dye. The final sample included 100% of analgesic/antipyretic national brands and 98.6% of cough, cold, and allergy product national brands available during the study period based on units sold from FDM stores. Of the 68 products, 81% were cough, cold, and allergy medications and 88% were marketed as infants’ or children’s products (Table 1). Of the 55 cough, cold, and allergy medications, 9 (16%) were homeopathic products. A dosing device was provided with all products, most often a dosing cup (85%); all infants’ products were analgesics/antipyretics packaged with oral syringes. Across the 68 products, agreement between the two reviewers on adherence to 22 specific top tier and low tier recommendations was high; only 8 of 1,496 independent assessments required resolution by a third reviewer.
TABLE 1.
Characteristics of Orally-ingested OTC Liquid Products Assessed for Adherence to Recommendations from the 2011 FDA Voluntary Guidance and 2009 CHPA Voluntary Guideline
| Characteristic | n | % |
|---|---|---|
| Drug Class | ||
| Analgesic/antipyretic | 13 | 19 |
| Cough, cold, and allergy | 55 | 81 |
| Age Category | ||
| Infants | 5 | 7 |
| Children | 55 | 81 |
| Family | 8 | 12 |
| Device Type | ||
| Printed cup | 38 | 56 |
| Etched cup | 20 | 29 |
| Oral syringe | 6 | 9 |
| Dosing spoon | 2 | 3 |
| Dropper | 2 | 3 |
| Total | 68 | 100 |
Ninety-one percent (62/68) of dosing directions and 62% (42/68) of devices adhered to all top tier recommendations. Over half of products (57%; 39/68) adhered to all top tier recommendations for both dosing directions and devices, and 93% (63/68) adhered to all or all but one top tier recommendation. Milliliters, teaspoons, and tablespoons were the only volumetric units used; atypical units, such as drams or dropperfuls, were never used (Table 2). All products avoided using teaspoon and tablespoon units together on devices; however, 2 products used both units in the dosing directions.
TABLE 2.
Product Adherence to Top Tier Recommendations from the 2011 FDA Voluntary Guidance and 2009 CHPA Voluntary Guidelinea
| Dosing Directions | Dosing Device | ||||
|---|---|---|---|---|---|
| Recommendation | Relevant sample | No./Total No. | % of Relevant Sample |
No./Total No. | % of Relevant Sample |
| Dosing device included | All products | -- | -- | 68/68 | 100 |
| Atypical units not used (e.g., drams) | All products | 68/68 | 100 | 68/68 | 100 |
| Teaspoon and tablespoon units not used together | Products using teaspoon or tablespoon units | 47/49 | 96 | 43/43 | 100 |
| Trailing zeros not used | Products using decimals | 15/17 | 88 | 35/40 | 88 |
| Leading zeros used | Products using decimal doses <1 | 1/1 | 100 | 8/9 | 89 |
| Small font used for numerals in fractions (e.g., “½” instead of "1/2") | Products using fractions | 8/10 | 80 | 20/27 | 74 |
| No extraneous units appear on the dosing device that do not correspond to units in the directions | Products with dosing devices | -- | -- | 68/68 | 100 |
| Dosing device not significantly larger than largest recommended dose (≥3-fold) | Products with dosing devices | -- | -- | 56/68 | 82 |
| All doses from directions marked on dosing device | Products with dosing devices | -- | -- | 64/68 | 94 |
Not applicable denoted by (--)
Top Tier recommendations are those that directly address potential ≥3-fold dosing errors. Products were assessed for adherence to recommendations in "Guidance for Industry: Dosage Delivery Devices for Orally Ingested OTC Liquid Drug Products", US Food and Drug Administration (FDA), May 2011 and "Volumetric Measures for Dosing of Over-the-Counter Oral Liquid Drug Products for Children ≤ 12 years of Age", Consumer Healthcare Products Association (CHPA), November 2009.
Most products adhered to recommendations specifying how numeric doses should be expressed. Where applicable, leading zeros were used and trailing zeros were omitted on 88% (15/17) of dosing directions and 85% (34/40) of devices. The 6 devices that used trailing zeros or failed to use leading zeros were oral syringes or droppers. Smaller font was used for fractional doses (e.g., “½”) on 80% (8/10) of dosing directions and 74% (20/27) of devices. All devices that did not use smaller font for fractions were dosing cups.
No dosing devices used extraneous units; all 68 dosing devices only used volumetric units that were specified in the dosing directions. Twelve children’s products included dosing cups with total volumes that were ≥3-times larger than the largest dose in the directions; the 12 cups averaged 3.4-times larger than the largest labeled dose (range 3.3 – 3.8-times larger). All doses from the dosing directions were explicitly marked on devices for all but 4 products (94%; 64/68); these 4 products included devices (2 droppers and 2 syringes) that needed to be filled >1 time to measure labeled doses.
Adherence to low tier recommendations varied (Table 3). Most products used standard abbreviations in the dosing directions (91%; 59/65) and on devices (72%; 49/68); all nonstandard abbreviations only differed in capitalization. Of 19 products that used non-standard abbreviations, 14 used “ml” and 2 used “ML” for milliliters (instead of “mL”) and 3 used “TSP” for teaspoons (instead of “tsp”). Of 65 products that used abbreviations for volumetric units both in dosing directions and on devices, 80% used exactly the same abbreviation in both locations. Again, all differences were related to capitalization (e.g., use of “mL” in dosing directions and “ml” on the device).
TABLE 3.
Product Adherence to Low Tier Recommendations from the 2011 FDA Voluntary Guidance and 2009 CHPA Voluntary Guidelinea
| Dosing Directions | Dosing Device | ||||
|---|---|---|---|---|---|
| Recommendation | Relevant sample | No./Total No. |
% of Relevant Sample |
No./Total No. |
% of Relevant Sample |
| Standard abbreviations used for volumetric units (e.g., "mL" used instead of "ml") | Products using abbreviations | 59/65 | 91 | 49/68 | 72 |
| Abbreviations for volumetric units on dosing device match the directions (e.g., if “mL” used in directions, “mL” used on device) | Products using abbreviations in directions and on dosing device | -- | -- | 52/65 | 80 |
| Abbreviations for volumetric units defined (e.g., “mL=milliliter”)b | Products using abbreviations | 18/68 | 26 | 0/68 | 0 |
| No extraneous numeric doses appear on the dosing device that do not correspond to amounts in the directions | Products with dosing devices | -- | -- | 19/68 | 28 |
| Statement included that only enclosed dosing device should be used or mechanism used to secure dosing device to the bottlec | Products with dosing devices | 33/68 | 49 | 32/68 | 47 |
Not applicable denoted by (--)
Low Tier recommendations maintain conventional standards of abbreviation and capitalization. They improve clarity and consistency but are not directly linked to potential ≥3-fold dosing errors. Products were assessed for adherence to recommendations in "Guidance for Industry: Dosage Delivery Devices for Orally Ingested OTC Liquid Drug Products", US Food and Drug Administration (FDA), May 2011 and "Volumetric Measures for Dosing of Over-the-Counter Oral Liquid Drug Products for Children ≤ 12 years of Age", Consumer Healthcare Products Association (CHPA), November 2009.
FDA guidance recommends that abbreviations should be defined on the dosing device (e.g., mL = milliliter) or, if they are not, should be defined in the labeled dosing directions, outside packaging, bottle, and any accompanying written instructions.
FDA guidance recommends that manufacturers should try to ensure that the dosing devices are used only with the products with which they are included. Possible ways of accomplishing this are to include a related statement on the drug product's bottle and/or carton labeling and, if possible, on the dosing device or to devise a mechanism to secure the dosing device to the drug product.
Few devices (28%; 19/68) only had the numeric markings for doses specified in the directions (e.g., directions specify doses of 5 mL or 10 mL; accompanying device only has 5 mL and 10 mL markings); Most devices had multipurpose numeric dosing scales (e.g., 2.5 mL increments starting with 5 mL and ending with 20 mL). Seventy-two percent of products (49/68) included a statement to only use the enclosed device with the product, used a physical mechanism (e.g., dosing cup that attaches to bottle cap) to link devices with accompanying products, or had both. The 6 cough, cold, and allergy products with dosing directions that used tablespoon units, in addition to other units, included a statement that doses could be measured using the device provided or a spoon.
The volumetric units used on the dosing devices were exactly the same as the units used in the directions for 90% of products (61/68) (Table 4). Dosing directions on 7 other products included additional volumetric units not found on accompanying devices. Of the 68 products, 19 dosing directions (28%) and 25 devices (37%) followed the CHPA Guideline’s primary preference to use only milliliter units. Alternatively, the CHPA Guideline recommends using milliliters in combination with teaspoon units; 74% of products (50/68) used milliliters alone or in combination with teaspoons. A dosing chart was used to specify doses in the dosing directions on 76% of products.
TABLE 4.
Volumetric Units Used in Product Dosing Directions and on Accompanying Dosing Devices
| Units on Dosing Device, n | ||||||
|---|---|---|---|---|---|---|
| Units in Dosing Directions, n | Milliliters only | Teaspoons only |
Milliliters and teaspoons |
Milliliters and tablespoons |
Milliliters, teaspoons, and tablespoons |
Total |
| Milliliters only | 19 | 0 | 0 | 0 | 0 | 19 |
| Teaspoons only | 0 | 11 | 0 | 0 | 0 | 11 |
| Milliliters and teaspoons | 0 | 1 | 31 | 0 | 0 | 32 |
| Milliliters and tablespoons | 4 | 0 | 0 | 0 | 0 | 4 |
| Milliliters, teaspoons, and tablespoons | 2 | 0 | 0 | 0 | 0 | 2 |
| Total | 25 | 12 | 31 | 0 | 0 | 68 |
Discussion
This study is the first to assess dosing directions and dosing devices in a sample of products available after the voluntary FDA Guidance was finalized in 2011. Among 68 national brand-name orally-ingested OTC liquid medications, 91% of dosing directions and 62% of included devices adhered to all recommendations that directly address ≥3-fold dosing errors (top tier recommendations). Adherence to individual recommendations intended to improve the clarity and consistency of labeled doses and accompanying devices (low tier recommendations) ranged from 26% to 91%. Specific findings help identify areas for product improvement and recommendation refinement.
In this sample of 68 products, there was 100% adherence to several key recommendations which address issues that have been directly implicated in clinically significant errors. All 68 products included dosing devices to discourage use of household spoons or other non-calibrated devices.13,14 No dosing directions or devices used atypical volumetric units (e.g., drams, milligrams, or dropperfuls) and no devices had extraneous units that did not appear in the dosing directions.15,16 Two products mixed teaspoon and tablespoon units in the dosing directions (a cause of 3-fold errors),17,18 but both have since been discontinued.
There is opportunity to improve the expression of decimals and fractions. Most non-adherence to these recommendations occurred with dosing devices, but for overdose prevention, non-adherence in dosing directions is most critical. Two products used trailing zeros in the directions, which could lead to 10-fold overdosing errors (e.g., interpreting “1.0” as “10”).6,7,9–12 One product did not use leading zeros and five products used trailing zeros on dosing devices; however, overlooking decimal points on devices would likely lead to underdosing. Expressing fractional doses with small font has been suggested as a means to prevent errors from misinterpreting “1/2” as “1 or 2” (i.e., a potential 4-fold error) or overlooking the fraction bar altogether.9 Small font was not used for fractional doses in 2 dosing directions (potential for overdose) and on 7 devices (potential for underdose).
Two recommendations related to device size required interpretation to assess adherence. First, to limit the magnitude of overdoses from patients or caregivers assuming that a full device holds “one dose” or “one unit”,18 dosing devices should not be “significantly larger” than doses specified in the directions. Twelve devices were slightly larger than the 3-fold cutoff (3.3 - 3.8-times larger) used to define “significantly larger” in this study. Second, to prevent situations in which doses specified in the directions cannot be measured using the device provided,15,16 devices should include markings that can measure all labeled doses. We considered 4 products that needed to be filled >1 time to measure the largest dose to be non-adherent. Updated guidelines could define when larger devices are “significantly larger” than needed and clarify whether or not smaller devices that may need to be filled more than once are recommended.
Eliminating extraneous markings on devices is recommended to reduce potential for confusion, but some exceptions may be well-intentioned and this recommendation surpasses current practice for prescription products. Only 28% of devices assessed in this study included just the numeric doses specified in dosing directions, typically because the device had a general numeric scale. However, additional numeric dose markings on devices may be useful for accommodating professional dosing recommendations to use smaller doses than the labeled directions.2 It is notable that when devices are provided with prescription medications (and sometimes patients must explicitly request them) the large majority are not tailored to the prescription but are “off-the-shelf” devices that have general numeric scales and may have multiple volumetric units to accommodate numerous doses and units.19
One recommendation with <50% adherence is to link medications and accompanying devices. One rationale is that devices are calibrated to account for product viscosity and other factors; however, such fine measurement accuracy is unlikely to cause clinically significant overdoses of OTC products. Another rationale is to discourage use of household spoons which can vary considerably in fill capacity.14,20,21 The dosing directions for 6 products stated that the included dosing cup or a teaspoon and/or tablespoon could be used. While 2 of these products have been discontinued, the remaining products’ directions should not suggest use of household spoons.
Three low tier recommendations focus on capitalization conventions and definition of abbreviations. Capitalization differences (e.g., “ml” instead of “mL”) accounted for all instances of non-adherence to the recommendations to use standard abbreviations for volumetric units and to ensure device abbreviations match dosing direction abbreviations. While abbreviations should generally be defined, definitions for common abbreviations may not be necessary,22 particularly when the same abbreviation is used both in the dosing directions and on the device. Fifty-six products used milliliters both in the directions and on devices; all 56 used an abbreviation in both locations. In this situation, it is unclear if defining the abbreviation aids in error prevention.
Using milliliters (expressed as mL), as the primary volumetric unit could address many guidance/guideline goals. An “mL only” approach discourages use of household spoons, avoids confusion between teaspoons and tablespoons, and limits confusion from use of multiple units. Milliliters are the standard units for dosing orally-ingested liquid medications in inpatient settings,6,11,23 and there is increasing consensus that use of milliliters for dosing orally-ingested liquid medications is preferred for outpatient settings as well.24–27 Nearly three-fourths of products in this study (74%) followed CHPA’s recommendation to use milliliters alone or in combination with teaspoon units, and success in adopting milliliters on OTC products has facilitated efforts to encourage use of milliliters on prescription product labels.28–30 Nonetheless, ongoing monitoring would be appropriate to identify unintended consequences of milliliter-only dosing.
The manner in which results are reported can substantially impact interpretation of findings. A previous study by Yin et al evaluated a sample of products available prior to release of the draft FDA Guidance and concluded that 98.6% had at least one “inconsistency.”4 However, aggregating inconsistencies by combining serious issues (e.g., representation of decimal doses) with less serious issues (e.g., inconsistent capitalization for milliliter abbreviations) and giving equal weight to serious and less serious issues could lead to over-statement of problems. In addition, reporting measures of inconsistency that combine issues with the dosing directions and issues with dosing devices clouds rather than clarifies where dosing directions improvements are needed and where devices improvements are needed. While differences in study design and inclusion criteria do not allow direct comparisons, findings from this study suggest that, overall, products collected after the CHPA Guideline and final FDA Guidance adhered to most recommendations, particularly those addressing clinically meaningful errors. After analyses were completed for this study, product-specific findings were shared with respective manufacturers and several label and device updates have been made.
Study findings are subject to several limitations. This study assessed national brand-name analgesic/antipyretic and cough, cold, and allergy medications with pediatric dosing available on the market during the study period. Findings may not be generalizable to national brand-name products that were not available during the study period (e.g., due to product recalls) or to generic products. Adherence of generic products available after the final FDA Guidance should be assessed. Findings also may not be generalizable to other OTC drug classes, but analgesic/antipyretic and cough, cold, and allergy medications are the OTC medications involved in most emergency visits for therapeutic errors involving children ≤5 years of age.31 Products were collected through a request sent to CHPA member manufacturers, and possibly eligible products from non-member manufacturers were not included. However, the products evaluated represented over 98% of units of national brand-name products in the included drug classes sold during the study period. Lastly, we did not evaluate other product characteristics such as use of concentration (mg/mL) or pictures or graphics on product packaging.
Conclusion
Findings suggest that these voluntary initiatives promote adherence to label and device recommendations. Further improving adherence to top tier recommendations addressing potential for ≥3-fold errors should be prioritized, but detailed reporting by patients and care providers is needed to identify the specific ways packaging and dose devices contribute to errors. Additional opportunities for standardization include design and marking of dosing devices and promotion of milliliter as the standard unit for dosing orally-ingested liquid medications. Evaluation and continued improvement of labels and devices for OTC liquid medications should be ongoing and transparent as new products are introduced and recommendations are revised.
Supplementary Material
What’s Known on This Subject
Due to reports of unintentional overdoses, in 2011 the US Food and Drug Administration finalized voluntary recommendations for dosing devices included with orally-ingested over-the-counter (OTC) medications. The Consumer Healthcare Products Association previously endorsed similar recommendations for devices and dosing directions.
What This Study Adds
This study assessed dosing directions and devices for national brand-name OTC liquid medications, available after a voluntary FDA guidance, and found high levels of adherence to most recommendations. Further improvement efforts should prioritize recommendations that directly address potential dosing errors.
Acknowledgements
We thank the participating Consumer Healthcare Products Association (CHPA) member manufacturers for submitting products for assessment. In particular, we thank Barbara Kochanowski, PhD, and Jay Sirois, PhD, of CHPA for coordinating the submission of products for evaluation and providing market data obtained from Hamacher Resource Group, Inc (HRG). HRG used best efforts to provide current, complete, and accurate information reflecting data available as of April 2012 and assumes no responsibility or liability for, and gives no warranties concerning the information. We also thank Lee Hampton, MD, MSc of CDC for thoughtful review of the manuscript. Finally, prevention of unintentional medication overdoses in children has been a priority focus of the CDC-led public-private PROTECT Initiative (www.cdc.gov/medicationsafety/protect/protect_initiative.html) and we thank PROTECT members for valuable discussions that were instrumental in laying the groundwork for this study.
Funding source: No external funding was secured for this study
Abbreviations and Acronyms
- CHPA
Consumer Healthcare Products Association
- FDA
US Food and Drug Administration
- FDM
food, drug, and mass merchandiser
- OTC
over-the-counter
Footnotes
Financial disclosures: None reported
Conflict of Interest Disclosures: None reported
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC). The Consumer Healthcare Products Association (CHPA) contacted member manufacturers and requested products to be sent directly to CDC for review and provided SymphonyIRI InfoScan Tracking data on consumer sales from food, drug, and mass (FDM) merchandisers. CHPA members did not contribute to data abstraction, data analysis, writing or revising the manuscript for publication.
Contributors’ Statement:
Daniel S. Budnitz: conceptualized the study, assisted with study design, drafted the initial manuscript, reviewed and revised the manuscript, and approved the final manuscript as submitted
Maribeth C. Lovegrove: assisted with study design, data collection, and drafting the manuscript, conducted the analyses, reviewed and revised the manuscript, and approved the final manuscript as submitted
Kathleen O. Rose: assisted with study design and data collection, reviewed and revised the manuscript, and approved the final manuscript as submitted
References
- 1.US Food and Drug Administration. Draft Guidance for Industry on Dosage Delivery Devices for Over-The-Counter Liquid Drug Products. Federal Register. 2009;74(213) [Google Scholar]
- 2.US Food and Drug Administration. Guidance for industry: dosage delivery devices for orally ingested OTC liquid drug products. [Accessed June 20, 2013]; Available at: www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM188992.pdf.
- 3.Consumer Healthcare Products Association. Volumetric measures for dosing of over-the-counter oral liquid drug products for children ≤ 12 years of age. [Accessed June 20, 2013]; Available at: www.chpa.org/VolCodesGuidelines.aspx. [Google Scholar]
- 4.Yin HS, Wolf MS, Dreyer BP, Sanders LM, Parker RM. Evaluation of consistency in dosing directions and measuring devices for pediatric nonprescription liquid medications. JAMA. 2010;304(23):2595–2602. doi: 10.1001/jama.2010.1797. [DOI] [PubMed] [Google Scholar]
- 5.Lokker N, Sanders L, Perrin EM, et al. Parental misinterpretations of over-the-counter pediatric cough and cold medication labels. Pediatrics. 2009;123(6):1464–1471. doi: 10.1542/peds.2008-0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.United States Pharmacopeia. General notices and requirements applying to standards, test, assays, and other specifications of the United States Pharmacopeia: USP 34. [Accessed June 20, 2013]; Available at: www.usp.org/sites/default/files/usp_pdf/EN/USPNF/USP34-NF29General%20Notices.pdf. [Google Scholar]
- 7.Institute for Safe Medication Practices. ISMP’s list of error-prone abbreviations, symbols, and dose designations. [Accessed June 20, 2013]; Available at: www.ismp.org/tools/errorproneabbreviations.pdf. [Google Scholar]
- 8.American Academy of Pediatrics. Using Liquid Medicines. [Accessed June 20, 2013]; Available at: www.healthychildren.org/English/safety-prevention/at-home/medication-safety/Pages/Using-Liquid-Medicines.aspx. [Google Scholar]
- 9.Institute for Safe Medication Practices. ISMP’s Guidelines for Standard Order Sets. [Accessed June 20, 2013]; Available at: www.ismp.org/tools/guidelines/standardordersets.pdf. [Google Scholar]
- 10.The Joint Commission. Facts about the Official “Do Not Use” List. [Accessed June 20, 2013]; Available at: http://www.jointcommission.org/assets/1/18/Do_Not_Use_List.pdf. [Google Scholar]
- 11.American Society of Health-System Pharmacists (ASHP) ASHP guidelines on preventing medication errors in hospitals. Am J Hosp Pharm. 1993;50(2):305–314. [PubMed] [Google Scholar]
- 12.Lesar TS. Tenfold medication dose prescribing errors. Ann Pharmacother. 2002;36(12):1833–1839. doi: 10.1345/aph.1C032. [DOI] [PubMed] [Google Scholar]
- 13.Dart RC, Paul IM, Bond GR, et al. Pediatric fatalities associated with over the counter (nonprescription) cough and cold medications. Ann Emerg Med. 2009;53(4):411–417. doi: 10.1016/j.annemergmed.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 14.Karch AM, Karch FE. A spoonful of medicine. Or is it more? Am J Nurs. 2000;100(11):24. [PubMed] [Google Scholar]
- 15.Parker RM, Wolf MS, Jacobson KL, Wood AJ. Risk of confusion in dosing Tamiflu oral suspension in children. N Engl J Med. 2009 Nov 5;361(19):1912–1913. doi: 10.1056/NEJMc0908840. [DOI] [PubMed] [Google Scholar]
- 16.Budnitz DS, Lewis LL, Shehab N, Birnkrant D. CDC and FDA response to risk of confusion in dosing Tamiflu oral suspension. N Engl J Med. 2009;361(19):1913–1914. doi: 10.1056/NEJMc0909190. [DOI] [PubMed] [Google Scholar]
- 17.Madlon-Kay DJ, Mosch FS. Liquid medication dosing errors. J Fam Pract. 2000;49(8):741–744. [PubMed] [Google Scholar]
- 18.Litovitz T. Implication of dispensing cups in dosing errors and pediatric poisonings: a report from the American Association of Poison Control Centers. Ann Pharmacother. 1992;26(7–8):917–918. doi: 10.1177/106002809202600710. [DOI] [PubMed] [Google Scholar]
- 19.Honey BL, Condren M, Phillips C, Votruba A. Evaluation of oral medication delivery devices provided by community pharmacies. Clin Pediatr (Phila) 2013;52(5):418–422. doi: 10.1177/0009922813479160. [DOI] [PubMed] [Google Scholar]
- 20.Dean BS, Krenzelok EP. Syrup of ipecac dosing... How much is a tablespoonful? Vet Hum Toxicol. 1986;28(2):155–156. [PubMed] [Google Scholar]
- 21.Falagas ME, Vouloumanou EK, Plessa E, Peppas G, Rafailidis PI. Inaccuracies in dosing drugs with teaspoons and tablespoons. Int J Clin Pract. 2010;64(9):1185–1189. doi: 10.1111/j.1742-1241.2010.02402.x. [DOI] [PubMed] [Google Scholar]
- 22.Iverson C, Christiansen S, Flanagin A, et al. AMA Manual of Style. 10th ed. New York, NY: Oxford University Press; 2007. [Google Scholar]
- 23.National Coordinating Council for Medication Error Reporting and Prevention. Recommendations to Enhance Accuracy of Prescription Writing. [Accessed June 20, 2013]; Available at: www.nccmerp.org/council/council1996-09-04.html. [Google Scholar]
- 24.ISMP Statement on Use of Metric Measurements to Prevent Errors with Oral Liquids. Horsham, PA: Institute for Safe Medication Practices; 2011. Oct, [Accessed June 20, 2013]. Available at: www.ismp.org/pressroom/PR20110808.pdf. [Google Scholar]
- 25.Johnson KB, Lehmann CU. Council on Clinical Information Technology of the American Academy of Pediatrics. Electronic prescribing in pediatrics: toward safer and more effective medication management. Pediatrics. 2013;131(4):e1350–e1356. doi: 10.1542/peds.2013-0193. Epub 2013 Mar 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paul IM, Yin HS. Out with teaspoons, in with metric units: Pediatricians urged to prescribe liquid medications in mLs only. AAP News. 2012;33(3):10. [Google Scholar]
- 27.American Academy of Family Physicians. Preferred Unit of Measurement for Liquid Medications. [Accessed June 20, 2013]; Available at: www.aafp.org/about/policies/all/preferred-unit.html. [Google Scholar]
- 28.US Food and Drug Administration. Draft guidance for industry: safety considerations for container labels and carton labeling design to minimize medication errors. [Accessed June 20, 2013]; Available at: www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM349009.pdf.
- 29.National Council for Prescription Drug Programs. NCPDP 2012 annual report: connecting healthcare to improve patient safety and health outcomes. [Accessed June 20, 2013]; Available at: www.ncpdp.org/annual-reports.aspx. [Google Scholar]
- 30.Budnitz DS, Salis S. Preventing medication overdoses in young children: an opportunity for harm elimination. Pediatrics. 2011;127(6):e1597–e1599. doi: 10.1542/peds.2011-0926. [DOI] [PubMed] [Google Scholar]
- 31.Bond GR, Woodward RW, Ho M. The growing impact of pediatric pharmaceutical poisoning. J Pediatr. 2012;160(2):265–270. doi: 10.1016/j.jpeds.2011.07.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.