Abstract
Hematopoietic cell transplantation (HCT) using CCR5-Δ32/Δ32 stem cells from an adult donor has resulted in the only known cure of HIV infection. However, it is not feasible to repeat this procedure except rarely because of the low incidence of the CCR5-delta 32 allele, the availability of only a small number of potential donors for most patients and the need for a very close HLA match between donor and recipient. In contrast, cord blood (CB) transplants require significantly less stringent HLA matching. Therefore, our hypothesis is that cure of HIV infections by HCT can be accomplished much more readily using umbilical cord blood stem cells obtained from a modestly sized inventory of cryopreserved CCR5-Δ32/Δ32 CB units. To test this hypothesis we have developed a screening program for cord blood units and are developing an inventory of CCR5-Δ32/Δ32 cryopreserved units available for HCT. 300 such units are projected to provide for Caucasian pediatric patients a 73.6% probability of finding an adequately HLA matched unit with a cell dose of ≥ 2.5 x 107TNC/kg and a 27.9% probability for Caucasian adults. With a cell dose of ≥ 1 x 107TNC/kg the corresponding projected probabilities are 85.6% and 82.1%. The projected probabilities are lower for ethnic minorities. Impetus for using CB HCT was provided by a transplant of an adult with acute myelogenous leukemia who was not HIV infected. The HCT was performed with a CCR5-Δ32/Δ32 CB unit, and post-transplant in vitro studies indicated that the patient’s peripheral blood mononuclear cells (PBMCs) were resistant to HIV infection.
INTRODUCTION
Infection with the human immunodeficiency virus type 1 (HIV-1) requires both a CD4 receptor and a chemokine receptor, principally chemokine receptor 5 (CCR5) 1. In humans, the CCR5 protein is encoded by the CCR5 gene located on the short arm (p) of chromosome 3 at position 21. Certain populations have inherited the Δ-32 mutation which results in the genetic deletion of a portion of the CCR5 gene. Homozygous carriers of this mutation (CCR5-Δ32/Δ32) are resistant to HIV-1 infection 2–4. In 2009, Hütter et al 5 reported long-term control of HIV infection by hematopoietic cell transplantation (HCT) using peripheral blood stem cells from a CCR5-Δ32/Δ32 donor. More than 5½ years after this treatment the patient is still off antiretroviral medication and in the analysis of peripheral blood cells, and different tissue samples including gut, liver, and brain, no viral load or proviral DNA could be detected 6. Indeed, Allers et al 7 have suggested that these results strongly suggest that cure of HIV is achieved in this patient. Deeks and McCune 8 have commented that “the HIV research community is hesitant to use the word ‘cure’, but this single case could very well be the first example to fit the bill.”
Although it is compelling, this concept of performing HCT using stem cells from CCR5-Δ32/Δ32 adult donors of bone marrow or peripheral blood for treatment of HIV-infected patients cannot be readily generalized. This is true because the prevalence of the homozygous variant allele is low, i.e., only about 0.8–1% of individuals of northern European descent have the CCR5-Δ32/Δ32 genotype 9, 10, and it is much less prevalent in other ethnic groups 11. Furthermore, most patients in need of an HCT have only a small number of potential donors from among registries of adult donors because a very close HLA match for 8 of 8 or 7 of 8 high-resolution alleles at 4 loci (A, B, C, DRB1) is required between adult donors and recipients 12. Indeed, Hütter and Thiel have reported no further cases of HCT for HIV-infected patients because of the inability to match such patients with adult CCR5-Δ32/Δ32 donors in spite of a diligent search for “patient number 2” 13, and to date no other such patients have been reported.
In marked contrast, HCT using umbilical cord blood does not require such stringent HLA matching between donor and recipient 14. Acceptable HLA-matched units include those that are matched at 4 of 6, 5 of 6, or 6 of 6 alleles at 3 loci using low resolution testing at the A and B loci and high resolution testing at the DRB1 locus, disallowing 2 mismatches at the same locus for 4 of 6 matching.
Our hypothesis, therefore, is that cure of HIV by HCT can be readily accomplished only by using CCR5-Δ32/Δ32 umbilical cord blood stem cells. To test this hypothesis, we have developed a cord blood screening program and are developing an inventory of CCR5-Δ32/Δ32 units.
While testing was being performed, one of the units subsequently found to be CCR5-Δ32/Δ32 was discovered to have been released for HCT for an adult patient with acute myelogenous leukemia. This provided us the opportunity to document that a CCR5-Δ32/Δ32 cord blood unit resulted in donor cell engraftment and that the patient’s peripheral blood mononuclear cells following the transplant were resistant in vitro to infection with HIV-1.
MATERIALS AND METHODS
Developing an inventory of CCR5-Δ32/Δ32 cord blood units
Samples of cord blood units for testing were primarily from Caucasian donors in the inventories of StemCyte International Cord Blood Center (“StemCyte”), and from numerous collaborating cord blood banks: St. Louis Cord Blood Bank, Carolinas Cord Blood Bank at Duke University, University of Colorado Cord Blood Bank, MD Anderson Cancer Center Cord Blood Bank, the Barcelona, Spain Cord Blood Bank and the Sydney, Australia Cord Blood Bank. Samples from collaborating cord blood banks were sent to StemCyte, usually as ~500μl of blood from the pre- or post-processing sample. DNA is extracted from these samples, and the DNA specimens were then tested for CCR5-Δ32 at the City of Hope Medical Center to determine if the units were homozygous, heterozygous or wild type. Some CB samples were sent from the collaborating cord blood banks as DNA.
DNA isolation was carried out using QlAamp® DNA Mini and QlAmp DNA Blood Mini Kits. These are designed for purification of an average of 6 μg of total DNA from 200 μl of whole human blood, and up to 50 μg of DNA from 200 μl of buffy coat.
CCR5 Genotype analysis was performed at the City of Hope Medical Center on DNA preparations (1 μl, 100 ng DNA) extracted from cord blood using a PCR-based assay for homozygosity of CCR5-Δ32 bp deletion.
DNA oligonucleotides were purchased from Integrated DNA Technologies.
Primer 1 TTCATTACACCTGCAGCTCTC
Primer2 CCTGTTAGAGCTACTGCAATTAT
Primer 3 TGCAGCTCTCATTTTCCATACATTA
To identify the CCR5 genes with an internal 32–base pair deletion (CCR5-Δ32 allele) the DNAs were amplified using a real-time polymerase chain reaction on a BioRad C1000 Thermocycler. 1.5μl of genomic DNA was used as template and each PCR reaction was carried out in a 25-μl volume in 1x SYBR Green supermix (Bio-Rad 170-8882) with 15 pmol each of primer 2 and primer 3 for 3 min at 94°C (one cycle); 20 seconds at 94°C, 1 min at 68°C, (30 cycles); 7 min at 72°C (one cycle).
A second round of PCR was performed on samples that amplified with the primer 2 and 3 pair which is specific for the deletion. The second round used primers 1 and 2 which are positioned outside of the deleted region to allow us to discriminate whether the deletions are homozygous or heterozygous. Reaction mixtures consisted of 15 pmol of each primer, 1.5 μl of the first round amplicons, 0.2μM dNTP mix, 3mM MgCl2 and 1U Taq gold DNA polymerase (Applied Biosystems-Life Technologies) in a total reaction volume of 25μl at 95°C for 3 minutes (one cycle), 95°C for 45 seconds, 60°C for 45 seconds, 72°C for 45 seconds for 40 cycles, and 72°C for 7 minutes (one cycle). After the PCR amplification, the products were separated by electrophoresis on a 2% agarose gel and visualized by ethidium bromide staining. The products from deletion-containing samples generate either a single band of 157 bp from the homozygous deletions (Δ32/Δ2), or two bands of 189 and 157 bp from the heterozygous samples.
Projections of the probability of finding an appropriately HLA matched cord blood unit with an adequate cell dose for a patient in need of a HCT
HLA match rates for an inventory of 300 CCR5-Δ32/Δ32 cord blood units were estimated using population HLA haplotype frequencies using a population genetic model developed by Kollman and previously applied to project match rates for planning the National Cord Blood Inventory (NCBI) 15, 16. Simulation of HLA genotypes of patients and cord blood units not yet collected requires the use of HLA haplotype frequencies, which were calculated at the allele-family level for HLA-A and -B, and allele-level for DRB1 on a cohort of 679,519 European-Caucasian donors from the Be The Match Registry 17. We simulated cord blood units using distribution of CBU TNC of units banked in the Be The Match Registry and simulated patients using separate distributions of weights of pediatric (under 16) and adult (age 16 and over) patients who have searched the Be The Match Registry. We incorporated the dose requirement into the model by calculating the percentage of cords with the commonly used minimum adequate total nucleated cell (TNC) dose of 2.5 x 107/kg for each simulated patient based on their weight, and considered matched units with inadequate dose as unavailable. The simulated HLA genotypes consisted of a pool of 10,000 simulated European-American patients searching 10 replicate simulated European-American 300-unit CBU inventories, with reported match rates averaged over the results from the 10 inventories. This model assumes that HLA haplotypes in patients and CCR5-Δ32/Δ32 variant cords are drawn from the same overall European-American HLA frequency distribution. However, because the geographic distribution of the CCR5 variant is centered over Northern Europe 18, the HLA match rates for European-American patients will be slightly higher than the projection if the patient has Northern European ancestry, and lower if the patient has Southern European ancestry.
The CCR5-Δ32/Δ32 units in the special inventory are primarily from Caucasian donors, and it is true that patients in need of transplant more commonly find a matched donor from among those in their own ethnic group. However, this is not invariably true and HLA matched donors are not uncommonly found among those in other ethnic groups. To assess match rates for minority populations, we simulated 10,000 African-American, Mexican-Hispanic, and Chinese-American patients searching European-origin cord inventory replicates.
Recent data indicate that a minimum CB cell dose of ≥ 1 x 107 TNC/kg body weight is adequate when a combined haploidentical and cord blood transplant is performed 19. Accordingly, similar projections were also carried out using this minimum cell dose.
Testing the cells in vitro of a patient with AML who was transplanted with CCR5-Δ32/Δ32 cord blood stem cells for resistance to HIV infection with laboratory strains BAL and NL4-3
Peripheral blood samples were collected from an HIV-negative AML patient on day +123 post-HCT at a time when chimerism studies indicated complete engraftment by CCR5-Δ32/Δ32 cells. The cells were cryopreserved and, when thawed, were shown to have >90% viability by trypan blue exclusion and 7-ADD flow cytometry. The Ficoll-Hypaque-separated PBMCs from the transplant recipient, a normal adult control and CCR5-Δ32 heterozygous and homozygous cord blood units were tested for susceptibility to infection with the laboratory strains HIV1 BAL, and the transplant recipient’s PBMCs were also infected with NL4-3 at MOI of .01 in vitro following standard PHA stimulation. Supernatants were collected and measured for p24Ag (Perkin–Elmer) at 3 and 7 days post-infection. No additional PBMC’s were added to cultures during the 7 day incubation. Patient cells were tested for CCR5-Δ32/Δ32 chimerism and again for viability by 7-ADD by flow cytometry at the termination of the experiment.
RESULTS
Identifying CCR5-Δ32/Δ32 units in inventories of cryopreserved cord blood units
Thus far, we have identified 121 cord blood units that are CCR5-Δ32/Δ32s after having tested approximately 18,000 units, primarily from Caucasians, for an incidence of approximately 0.7%. In addition, we searched 8,000 Asian units in the inventory of StemCyte Taiwan, but found no homozygous units. Testing of an additional ~25,000 cord blood units from Caucasians is expected to increase the special inventory to about 300 units.
Probabilities of finding adequately matched cord blood units with an adequate cell dose in an inventory of 300 CCR5-Δ32/Δ32 cord blood units
Table 1 indicates the projected probabilities of finding an adequately HLA matched unit with a total nucleated cell count (TNC) of ≥ 2.5 x 107/kg or with a TNC of ≥ 1 x 107 cells/kg in an inventory of 300 CCR5-Δ32/Δ32 units for pediatric and adult Caucasian patients, and for patients of other ethnic groups.
Table 1.
PROJECTED HLA MATCH RATES WITH A 300 UNIT INVENTORY OF CCR5-Δ32/Δ32 CB UNITS
| CAUCASIANS: | |
|---|---|
| INCLUDES NEED FOR TNC OF 2.5 X 10(7) CELLS/KG: | |
| ADULT PATIENTS | PEDIATRIC PATIENTS |
| 6 OF 6 MATCHES: 0.01% | 6 OF 6 MATCHES: 0.01% |
| 5 OF 6 MATCHES: 4.5% | 5 OF 6 MATCHES: 10.6% |
| 4 OF 6 MATCHES: 27.9% | 4 OF 6 MATCHES: 73.6% |
| INCLUDES NEED FOR TNC OF 1 X 10(7) CELLS/KG: | |
| ADULT PATIENTS | PEDIATRIC PATIENTS |
| 6 OF 6 MATCHES: 0.09% | 6 OF 6 MATCHES: 1.01% |
| 5 OF 6 MATCHES: 10.7% | 5 OF 6 MATCHES: 10.8% |
| 4 OF 6 MATCHES: 82.1% | 4 OF 6 MATCHES: 85.6% |
| MINORITY ETHNIC GROUPS: | |
| INCLUDES NEED FOR TNC OF 2.5 X 10(7) CELLS/KG: | |
| ADULT PATIENTS | PEDIATRIC PATIENTS |
| AFRICAN-AMERICAN | |
| 4 OF 6 MATCHES: 9.9% | 4 OF 6 MATCHES: 28.6% |
| MEXICAN-AMERICAN | |
| 4 OF 6 MATCHES: 14% | 4 OF 6 MATCHES: 44.1% |
| CHINESE-AMERICAN | |
| 4 OF 6 MATCHES: 2.7% | 4 OF 6 MATCHES: 12.3% |
| INCLUDES NEED FOR TNC OF 1 X 10(7) CELLS/KG: | |
| AFRICAN-AMERICAN | |
| 4 OF 6 MATCHES: 31.6% | 4 OF 6 MACHES: 34.1% |
| MEXICAN-AMERICAN | |
| 4 OF 6 MATCHES: 48.9% | 4 OF 6 MATCHES: 52.5% |
| CHINESE-AMERICAN | |
| 4 OF 6 MATCHES: 13.9% | 4 OF 6 MATCHES: 15.7% |
Projected match rates for Caucasian patients using a minimum necessary TNC of ≥ 2.5 x 107/kg were 73.6% for pediatric patients and 27.9% for adults. Using a minimum necessary TNC of ≥ 1.0 x 107/kg, the projected match rates were 82.1% for adults and 85.6% for pediatric patients. Probable match rates were significantly lower for patients of minority ethnic groups (Table 1).
Testing the cells of the patient who was transplanted with a CCR5-Δ32/Δ32 unit for resistance to HIV infection
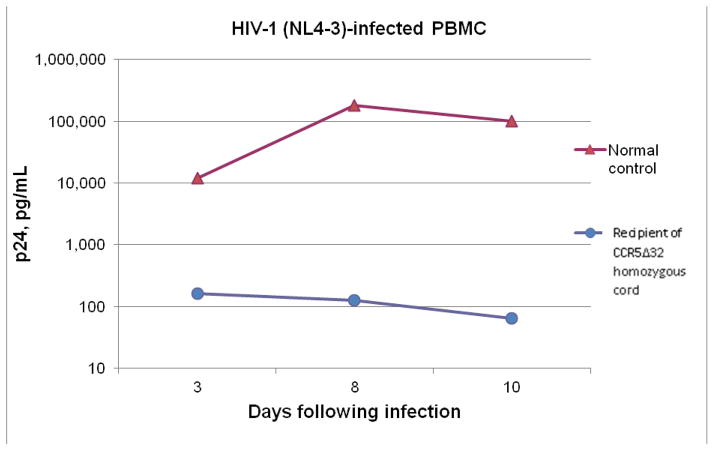
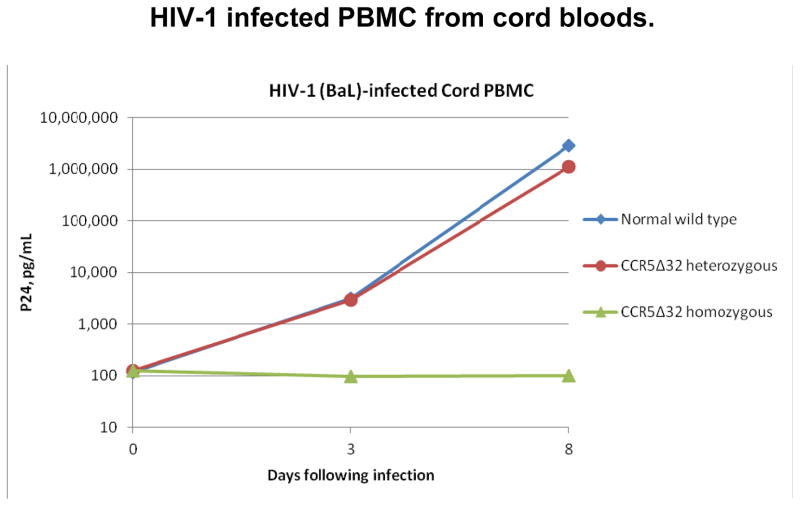
As seen in figure 1A, the recipient’s PBMC’s on day +123 showed no significant HIV replication with in vitro infection with either of the laboratory strains of HIV1, BAL (CCR5 tropic), or NL4-3 (CXCR4) compared to a normal control. Figure 1B shows a comparison of HIV replication in vitro with the laboratory CCR5 strain HIV-1BAL for cord blood PBMCs from CCR5 wild type, heterozygous and homozygous units. There was no detectable HIV replication in vitro in the CCR5-Δ32/Δ32 cord blood cells compared to significant replication in the heterozygous and wild type cord blood cells.
Figure 1.
Figure 1A In vitro study of infectivity by HIV-1 of peripheral blood mononuclear cells (PBMCs) in the posttransplant period from an HIV uninfected patient transplanted with a CCR5-Δ32/Δ32 cord blood. The recipient’s PBMCs showed no significant infection with either lab strains of HIV-1, BAL (CCR tropic) and NL4-3 (CXCR4) compared to a normal adult control.
Figure 1B A comparison of HIV replication in vitro with the laboratory CCR5 strain HIV-1 BAL for cord blood PBMCs from normal CCR5 wild type, heterozygous and homozygous units. There was no detectable replication in vitro in the CCR5-Δ32/Δ32 cord cells compared to significant replication in the heterozygote and wild type cord cells.
DISCUSSION
Long term control of HIV infection has been accomplished by Hütter et al 5 with hematopoietic cell transplantation (HCT) using peripheral blood stem cells from a CCR5-Δ32/Δ32 matched unrelated adult donor. The patient has remained without any evidence for HIV infection for more than 5½ years after discontinuation of antiretroviral drug therapy 13. However, using CCR5-Δ32/Δ32 stem cells from adult donors as in bone marrow or peripheral blood stem cell transplants cannot be readily generalized as a treatment option because of the rarity of the variant allele and the need for a very close HLA match between recipient and donor. As noted, the unsuccessful attempt by Hütter et al. to perform a HCT for a second patient confirms the futility of this approach 13. In contrast, umbilical cord blood transplants require significantly less stringent requirements for HLA matching.
Accordingly, our hypothesis is that cure of HIV infections by HCT can be much more readily accomplished using umbilical cord blood stem cells obtained from a modestly sized inventory of cryopreserved CCR5-Δ32/Δ32 CB units.
Furthermore, the numerous reports of essentially equivalent post-transplant outcomes using cord blood as compared to the results of bone marrow and peripheral blood cell transplants lend impetus to this approach 20–26. In spite of these reports, there are plentiful data indicating that cord blood transplantation is significantly underutilized 27. Starting in 2003, StemCyte cord blood inventories have been screened for CCR5-Δ32/Δ32 donors.
In addition, the transplantation of a CCR5-Δ32/Δ32 cord blood unit to an adult patient with AML as part of a double cord blood transplant provided the opportunity to collect data which indicated engraftment of the CCR5-Δ32/Δ32 unit as the dominant unit. At a time when chimerism studies indicated 100% engraftment by the CCR5-Δ32/Δ32 unit, in vitro studies indicated that the patient’s peripheral blood mononuclear cells were resistant to HIV1 BAL and NL4-3 strains (Figures 1A and 1B).
Projections regarding the probability of finding an appropriately HLA matched unit with an adequate cell dose for a Caucasian patient using a population of simulated patients are based on the HLA frequencies within the U.S. Caucasian population and the cell doses of units presently in the special inventory. We arbitrarily chose an inventory of 300 CCR5-Δ32/Δ32units for our initial calculations, and are developing such an inventory. Collaboration among numerous cord blood banks makes the development of a special inventory of this size eminently feasible and, if needed, additional units can readily be added. Indeed, Gonzales et al 11 have estimated that there are approximately 400,000 cord blood units cryopreserved around the globe, and among them are 2,000–4,000 CCR5-Δ32/Δ32 units.
The projections took into consideration the need for an adequate cell dose which is generally accepted to be in the range of ≥ 2.5 x 107 TNC/kg. The projections indicate that an inventory of 300 units would provide a 73.6% probability of finding an adequately HLA matched unit for Caucasian pediatric patients and a probability of 27.9% for Caucasian adult patients (Table 1). Also, since Liu et al19 have reported that a cord blood cell dose as low as 1 x 107TNC/kg body weight is adequate for cord blood transplants done in association with a haploidentical transplant, we have made projections using this as the minimum necessary cell dose. These projections indicate the probability of finding an adequately HLA matched unit for 85.6% of Caucasian pediatric patients and 82.1% for Caucasian adult patients (Table 1). The use of combined haploidentical and cord blood transplants19 provides important, and probably essential, advantages when considering use of cord blood transplantation to cure HIV in adults. Finding two adequately matched units from an inventory of 300 CB units would be very problematic, whereas the use of a single cord blood unit combined with a haploidentical transplant is much more feasible. Further, in such transplants, engraftment is rapid and chimerism studies have shown that cells from the cord blood unit almost always are the only ones present several months post-transplant19. However, it must be noted that combined haploidentical/cord blood transplants are not commonly performed at this time in the United States, and the adequacy of a cell dose of ≥ 1 x 107TNC/kg has not yet been confirmed.
To assess match rates for minority populations, we simulated 10,000 African-American, Mexican-Hispanic, and Chinese-American patients searching European-origin cord inventory replicates. As expected, the projected probabilities are significantly lower (Table 1).
The most obvious patient population for the transplantation of CCR5-Δ32/Δ32 cord blood units is that group of patients who are in need of a HCT for a hematologic malignancy or other indication, and are also infected with HIV. The incidence of patients meeting these criteria has not been determined and would appear to be relatively small. However, Hütter and Zaia 28 point out that “the lifetime expectancy of HIV-infected patients has improved substantially, but nevertheless the incidence rate of malignancies in these patients has increased considerably. Therefore, it can be assumed that there will be a rising necessity for HIV-1 infected patients with malignancies for allogeneic HCT.” Krishnan and Forman also indicate that the incidence of Hodgkin’s lymphoma and non-Hodgkin’s lymphoma is increased in HIV-infected patients compared to the general population 29. Indeed, AIDS-related malignancies remain a leading cause of mortality in HIV-infected patients 30.
Many physicians still automatically consider HIV status a barrier to transplant, and transplant centers often exclude these patients from their protocols 30. However, experience during the last 25 years indicates successful HCT in HIV-infected patients with hematological disease, including not only leukemia and relapsed lymphoma but also successful treatment of non-malignant disorders such as aplastic anemia 28. Indeed, the outcome of allografted HIV-positive patients is probably only negligibly poorer in comparison to HIV-negative patients 28.
An alternative approach to cure of HIV infection is offered by CCR5-targeted gene therapy of either T cells or stem cells, using siRNA based therapeutics as recently reported 8, 29, 31–33. Another highly innovative approach relies on engineered zinc-finger nucleases (ZFNs) specific for the CCR5 gene 34. ZFNs are a powerful tool that can be used to edit the human genome ad libitum 35. The technology has experienced remarkable development in the last few years with regard to both the target site specificity and the engineering platforms used to generate zinc-finger proteins 35. To date, clinical benefit has not been demonstrated in clinical trials 32, but recent progress in the field provides optimism that some of the promises of gene may finally be realized 36, 37.
In addition to HCT for HIV-infected patients with a hematologic malignancy or other indication for a transplant, selected patients with AIDS who have no other illness should also be considered for a clinical trial of CCR5-Δ32/Δ32 cord blood transplantation. The optimism of antiretroviral treatment is dampened by the current impossibility of viral eradication, the sustained medication with complex regimens, the potential toxic effects, and the prevalence of drug-resistant isolates 38. Patients with AIDS who are responding poorly to antiretroviral regimens and are informed of the significant risks of HCT and the potential benefits should be allowed to participate in a clinical trial of HCT if an appropriately HLA matched CCR5-Δ32/Δ32 unit of adequate cell dose is available.
The seemingly logical approach to a cure using adults who volunteer to be bone marrow/peripheral blood donors and who are found to have the variant CCR5 allele is an improbable solution. The testing for CCR5-Δ32/Δ32 at the time donors are registered with the NMDP is expensive and not likely to be effective because of the statistical improbability of finding both the high grade HLA match, as is required when HCT is done using stem cells from adults, and a CCR5-Δ32/Δ32 genotype in the donor. Thus, it is more reasonable to suggest large-scale testing of newly donated and currently inventoried cord blood for CCR5-Δ32/Δ32 units.
Footnotes
Conflict of Interest Disclosures:
L.P., D.S., R.T., S,S., A.B., and R.C. were employees of StemCyte International Cord Blood Center (“StemCyte”) while this work was being done. L.P., R.T. and S.S are still employed by StemCyte. L.P. and R.C. have stock options in StemCyte. J.K. is a consultant to StemCyte. The following authors have no conflicts of interest to report: I.R., Y.B., D.R., E.S., J.G., S.Q., P.C., S.S., M.B., L.G., J.R., S.L., H.L., J.R., J.Z. and S.F.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Lawrence Petz, StemCyte International Cord Blood Center
Istvan Redei, Midwestern Regional Medical Center, Zion, Illinois
Yvonne Bryson, David Geffen UCLA School of Medicine
Donna Regan, St. Louis Cord Blood Bank.
Joanne Kurtzberg, Duke University Medical Center
Elizabeth Shpall, The University of Texas MD Anderson Cancer Center
Jonathan Gutman, University of Colorado
Sergio Querol, Barcelona, Spain Cord Blood Bank
Pamela Clark, Sydney, Australia Cord Blood Bank
Richard Tonai, StemCyte International Cord Blood Center.
Sarah Santos, StemCyte International Cord Blood Center.
Aide Bravo, StemCyte international Cord Blood Center.
Stephen Spellman, National Marrow Donor Program
Michael Boo, National Marrow Donor Program.
Loren Gragert, National Marrow Donor Program.
John Rossi, City of Hope Medical Center
Shirley Li, City of Hope Medical Center
Haitang Li, City of Hope Medical Center.
David Senitzer, City of Hope Medical Center.
John Zaia, City of Hope Medical Center
Stephen Forman, City of Hope National Med Center
Robert Chow, HMD Consulting, Irvine, California
Reference List
- 1.Berger EA, Doms RW, Fenyo EM, et al. A new classification for HIV-1. Nature. 1998;391:240. doi: 10.1038/34571. [DOI] [PubMed] [Google Scholar]
- 2.Samson M, Libert F, Doranz BJ, et al. Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996;382:722–725. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- 3.Liu R, Paxton WA, Choe S, et al. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–377. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 4.Zimmerman PA, Buckler-White A, Alkhatib G, et al. Inherited resistance to HIV-1 conferred by an inactivating mutation in CC chemokine receptor 5: studies in populations with contrasting clinical phenotypes, defined racial background, and quantified risk. Mol Med. 1997;3:23–36. [PMC free article] [PubMed] [Google Scholar]
- 5.Hutter G, Nowak E, Mossner M. Long-term control of HIV by CCR5 Delta32/Delta32 stem cell transplantation. New Eng J Med. 2009;360:692–698. doi: 10.1056/NEJMoa0802905. [DOI] [PubMed] [Google Scholar]
- 6.Hutter G, Ganepola S. Eradication of HIV by transplantation of CCR5-deficient hematopoietic stem cells. ScientificWorldJournal. 2011;11:1068–1076. doi: 10.1100/tsw.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allers K, Hutter G, Hofmann J, et al. Evidence for the cure of HIV infection by CCR5{Delta}32/{Delta}32 stem cell transplantation. Blood. 2011;117:2791–2799. doi: 10.1182/blood-2010-09-309591. [DOI] [PubMed] [Google Scholar]
- 8.Deeks SG, McCune JM. Can HIV be cured with stem cell therapy? Nat Biotechnol. 2010;28:807–810. doi: 10.1038/nbt0810-807. [DOI] [PubMed] [Google Scholar]
- 9.Martinson JJ, Chapman NH, Rees DC, Liu YT, Clegg JB. Global distribution of the CCR5 gene 32-basepair deletion. Nat Genet. 1997;16:100–103. doi: 10.1038/ng0597-100. [DOI] [PubMed] [Google Scholar]
- 10.Ruiz-Ferrer M, Barroso N, Antinolo G, guilar-Reina J. Analysis of CCR5-Delta 32 and CCR2-V64I polymorphisms in a cohort of Spanish HCV patients using real-time polymerase chain reaction and fluorescence resonance energy transfer technologies. J Viral Hepat. 2004;11:319–323. doi: 10.1111/j.1365-2893.2004.00510.x. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez G, Park S, Chen D, Armitage S, Shpall E, Behringer R. Identification and frequency of CCR5Delta32/Delta32 HIV-resistant cord blood units from Houston area hospitals. HIV Med. 2011 doi: 10.1111/j.1468-1293.2010.00911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee SJ, Klein J, Haagenson M, et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110:4576–4583. doi: 10.1182/blood-2007-06-097386. [DOI] [PubMed] [Google Scholar]
- 13.Hutter G, Thiel E. Allogeneic transplantation of CCR5-deficient progenitor cells in a patient with HIV infection: an update after 3 years and the search for patient no. 2. AIDS. 2011;25:273–274. doi: 10.1097/QAD.0b013e328340fe28. [DOI] [PubMed] [Google Scholar]
- 14.Smith AR, Wagner JE. Alternative haematopoietic stem cell sources for transplantation: place of umbilical cord blood. Br J Haematol. 2009;147:246–261. doi: 10.1111/j.1365-2141.2009.07828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kollman C, Abella E, Baitty RL, et al. Assessment of optimal size and composition of the U.S. National Registry of hematopoietic stem cell donors. Transplantation. 2004;78:89–95. doi: 10.1097/01.tp.0000132327.40702.97. [DOI] [PubMed] [Google Scholar]
- 16.Howard DH, Meltzer D, Kollman C, et al. Use of cost-effectiveness analysis to determine inventory size for a national cord blood bank. Med Decis Making. 2008;28:243–253. doi: 10.1177/0272989X07308750. [DOI] [PubMed] [Google Scholar]
- 17.Kollman C, Maiers M, Gragert L, et al. Estimation of HLA-A, -B, -DRB1 haplotype frequencies using mixed resolution data from a National Registry with selective retyping of volunteers. Hum Immunol. 2007;68:950–958. doi: 10.1016/j.humimm.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Novembre J, Galvani AP, Slatkin M. The geographic spread of the CCR5 Delta32 HIV-resistance allele. PLoS Biol. 2005;3:e339. doi: 10.1371/journal.pbio.0030339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu H, Rich ES, Godley L, et al. Reduced-intensity conditioning with combined haploidentical and cord blood transplantation results in rapid engraftment, low GVHD, and durable remissions. Blood. 2011;118:6438–6445. doi: 10.1182/blood-2011-08-372508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith AR, Baker KS, DeFor TE, Verneris MR, Wagner JE, MacMillan ML. Hematopoietic cell transplantation for children with acute lymphoblastic leukemia in second complete remission: similar outcomes in recipients of unrelated marrow and umbilical cord blood versus marrow from HLA matched sibling donors. Biol Blood Marrow Transplant. 2009;15:1086–1093. doi: 10.1016/j.bbmt.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomblyn MB, Arora M, Baker KS, et al. Myeloablative hematopoietic cell transplantation for acute lymphoblastic leukemia: analysis of graft sources and long-term outcome. J Clin Oncol. 2009;27:3634–3641. doi: 10.1200/JCO.2008.20.2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gutman JA, Leisenring W, Appelbaum FR, Woolfrey AE, Delaney C. Low relapse without excessive transplant-related mortality following myeloablative cord blood transplantation for acute leukemia in complete remission: a matched cohort analysis. Biol Blood Marrow Transplant. 2009;15:1122–1129. doi: 10.1016/j.bbmt.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takahashi S, Ooi J, Tomonari A, et al. Comparative single-institute analysis of cord blood transplantation from unrelated donors with bone marrow or peripheral blood stem-cell transplants from related donors in adult patients with hematologic malignancies after myeloablative conditioning regimen. Blood. 2007;109:1322–1330. doi: 10.1182/blood-2006-04-020172. [DOI] [PubMed] [Google Scholar]
- 24.Eapen M, Rocha V, Sanz G, et al. Effect of graft source on unrelated donor haemopoietic stem-cell transplantation in adults with acute leukaemia: a retrospective analysis. Lancet Oncol. 2010;11:653–660. doi: 10.1016/S1470-2045(10)70127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brunstein CG, Gutman JA, Weisdorf DJ, et al. Allogeneic hematopoietic cell transplantation for hematologic malignancy: relative risks and benefits of double umbilical cord blood. Blood. 2010;116:4693–4699. doi: 10.1182/blood-2010-05-285304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang MJ, Davies SM, Camitta BM, Logan B, Tiedemann K, Eapen M. Comparison of Outcomes after HLA-Matched Sibling and Unrelated Donor Transplantation for Children with High-Risk Acute Lymphoblastic Leukemia. Biol Blood Marrow Transplant. 2012;18:1204–1210. doi: 10.1016/j.bbmt.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petz LD, Spellman S, Gragert L. The underutilization of Cord Blood Transplantation - Extent of the problem, causes and methods of improvement. In: Broxmeyer H, editor. Cord Blood Biology, Transplantation, Banking, and Regulatory Considerations. AABB Press; 2011. pp. 557–584. [Google Scholar]
- 28.Hutter G, Zaia JA. Allogeneic haematopoietic stem cell transplantation in patients with human immunodeficiency virus: the experiences of more than 25 years. Clin Exp Immunol. 2011;163:284–295. doi: 10.1111/j.1365-2249.2010.04312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krishnan A, Forman SJ. Hematopoietic stem cell transplantation for AIDS-related malignancies. Curr Opin Oncol. 2010;22:456–460. doi: 10.1097/CCO.0b013e32833d2cf0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krishnan A, Palmer JM, Zaia JA, Tsai NC, Alvarnas J, Forman SJ. HIV status does not affect the outcome of autologous stem cell transplantation (ASCT) for non-Hodgkin lymphoma (NHL) Biol Blood Marrow Transplant. 2010;16:1302–1308. doi: 10.1016/j.bbmt.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DiGiusto DL, Krishnan A, Li L, et al. RNA-based gene therapy for HIV with lentiviral vector-modified CD34(+) cells in patients undergoing transplantation for AIDS-related lymphoma. Sci Transl Med. 2010;2:36ra43. doi: 10.1126/scitranslmed.3000931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krishnan A, Zaia J, Rossi J, et al. First in human engraftment of anti-HIV lentiviral vector gene modified peripheral blood progenitor cells in the treatment of AIDS related lymphoma. Blood. 2011;112:2348a. (Abstract) [Google Scholar]
- 33.Scherer LJ, Rossi JJ. Ex vivo gene therapy for HIV-1 treatment. Hum Mol Genet. 2011;20(R1):R100–R107. doi: 10.1093/hmg/ddr160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perez EE, Wang J, Miller JC, et al. Establishment of HIV-1 resistance in CD4+ T cells by genome editing using zinc-finger nucleases. Nat Biotechnol. 2008;26:808–816. doi: 10.1038/nbt1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rahman SH, Maeder ML, Joung JK, Cathomen T. Zinc-Finger Nucleases for Somatic Gene Therapy: The Next Frontier. Hum Gene Ther. 2011 doi: 10.1089/hum.2011.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kay MA. State-of-the-art gene-based therapies: the road ahead. Nat Rev Genet. 2011;12:316–328. doi: 10.1038/nrg2971. [DOI] [PubMed] [Google Scholar]
- 37.Riviere I, Dunbar CE, Sadelain M. Hematopoietic stem cell engineering at a crossroads. Blood. 2012;119:1107–1116. doi: 10.1182/blood-2011-09-349993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lai Y. Adopting autologous hematopoietic stem cells with non-functional CCR5 and CXCR4 against HIV. Bone Marrow Transplantation. 2010;45:770–771. doi: 10.1038/bmt.2009.201. (Abstract) [DOI] [PubMed] [Google Scholar]