Abstract
The immune system constitutes one of the host factors modifying outcomes in ovarian cancer. Regulatory T cells (Tregs) are believed to be a major factor in preventing the immune response from destroying ovarian cancers. Understanding mechanisms that regulate Tregs in the tumor microenvironment could lead to the identification of novel targets aimed at reducing their influence. In this study, we used immunofluorescence-based microscopy to enumerate Tregs, total CD4 T cells, and CD8+ cytotoxic T cells in fresh frozen tumors from over 400 patients with ovarian cancer (>80 % high-grade serous). We sought to determine whether Tregs were associated with survival and genetic variation in 79 genes known to influence Treg induction, trafficking, or function. We used Cox regression, accounting for known prognostic factors, to estimate hazard ratios (HRs) associated with T cell counts and ratios. We found that the ratios of CD8 T cells and total CD4 T cells to Tregs were associated with improved overall survival (CD8/Treg HR 0.84, p = 0.0089; CD4/Treg HR 0.88, p = 0.046) and with genetic variation in IL-10 (p = 0.0073 and 0.01, respectively). In multivariate analyses, the associations between the ratios and overall survival remained similar (IL-10 and clinical covariate-adjusted CD8/Treg HR 0.85, p = 0.031; CD4/Treg HR 0.87, p = 0.093), suggesting that this association was not driven by variation in IL-10. Thus, integration of novel tumor phenotyping measures with extensive clinical and genetic information suggests that the ratio of T cells to Tregs may be prognostic of outcome in ovarian cancer, regardless of inherited genotype in genes related to Tregs.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-015-1753-x) contains supplementary material, which is available to authorized users.
Keywords: Immune suppression, Tumor microenvironment, Treg, CD8
Introduction
While there have been significant improvements in surgical resection and chemotherapy for ovarian cancer, the disease remains challenging cancer and survival has improved only marginally over the last two decades [1–3]. It is now understood that there are host characteristics, in addition to common clinical and pathologic characteristics (e.g., grade, tumor histology), which contribute to survival [4–6]. Ovarian cancer is naturally immune reactive, and the nature and extent of the immune responses have a pronounced effect on clinical outcomes [7–9]. Although the immune response against ovarian cancer is regulated at multiple levels, several studies have demonstrated that the malignancy is capable of establishing a dominant, complex, and hostile immune suppressive microenvironment, which likely limits the effectiveness of both the natural anti-tumor immune response as well of immunotherapeutic interventions [10, 11]. One potential strategy for targeted therapy in ovarian cancer is to disrupt this immune suppressive environment, thereby enabling the anti-tumor immune response to eradicate the malignancy.
Various immune cells are known to mediate immune suppression in ovarian cancer and include regulatory T cells (Tregs), dendritic cells (DCs), myeloid derived suppressor cells (MDSCs), and macrophages [10, 12–30]. The connection between pathogenesis of Tregs and prognosis in ovarian cancer was first suggested by Curiel who showed that infiltration of Tregs (as assessed by CD4 and CD25 staining) was associated with poor patient survival [15]. Other subsequently published studies have also suggested that Tregs have an important role in ovarian cancer pathogenesis and outcome [9, 26]. With the development of better marker sets for Tregs, our group recently demonstrated, in a small pilot study (n = 51 patients) leading up to the present study, that high-grade serous ovarian cancer is infiltrated with CD4+CD25+FOXp3+ Tregs and that a low ratio of infiltrating CD8+ T cells to these triple-positive Tregs is associated with poor survival [24]. Thus, Tregs are one potential cell to target and to disrupt local immune suppression in ovarian cancer. There are agents that are capable of depleting Tregs, including cyclophosphamide, IL-2, denileukin diftitox, and anti-CD25 antibodies; however, most fail to selectively deplete Tregs and have unacceptable adverse event profiles [31–35]. There need to be continued efforts at identifying targets that can be engaged for selective depletion of Tregs and reversal of the immune suppressive microenvironment in ovarian cancer.
We have recently undertaken large SNP-based genetic association studies aimed at identifying proteins that are critical to the trafficking, function, and/or generation of Tregs and reported several associations of genotypes only with ovarian cancer outcomes [36, 37]. Utilizing a subset of that genotyped data set, our main objective in the current study was to examine whether there is an association of Treg genotypes with the levels of tumor-infiltrating CD4+CD25+FOXp3+ Tregs and the combined associations correlate with clinical outcomes in epithelial ovarian cancer patients.
Materials and methods
Patients and clinical characteristics
Eligible patients were women with pathologically confirmed invasive epithelial ovarian, fallopian tube, or primary peritoneal cancer seen at the Mayo Clinic in Rochester, MN, between 1999 and 2010. Patients (n = 405) were enrolled within 1 year of diagnosis, provided a blood sample as a source of germline DNA, and gave written informed consent for use of fresh frozen tumor specimens as well as active and passive follow-up for vital status changes. The study was approved by the Mayo Institutional Review Board. Enrollment and biospecimen processing procedures have been published previously [24, 38].
Staining and immunofluorescence analysis of tumor tissue specimens
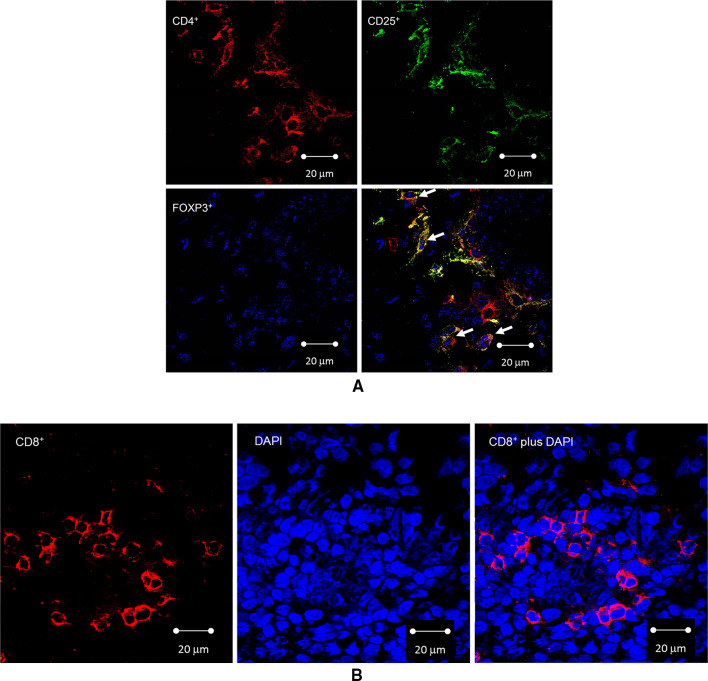
Frozen tumor specimen blocks were cryosectioned (5 µm), fixed in acetone for 10 min, air-dried for 1 h, stored at −80 °C until use, and stained following procedures described previously in our pilot study [24]. Antibodies for FOXp3 (Abcam, Cambridge, MA), CD4 (Abcam), and CD25 (Abd Serotec, Raleigh, NC, USA) were used for triple staining of Tregs, and an antibody for CD8 (BD Pharmingen, San Diego, CA, USA) was used as a single stain. Single-stained cells were also counterstained with 4′,6-diamidino-2-phenylindole (DAPI) for 30 min. Representative images are shown in Fig. 1.
Fig. 1.
Confocal imaging of CD4+CD25+FOXp3+ Tregs and intraepithelial infiltrating CD8+ cytotoxic T cells in ovarian tumors. a Picture (×40) of CD4+CD25+FOXp3+ Tregs and other infiltrating cells. Top left panel shows CD4 expression (red signal), top right panel shows CD25 expression (green signal), bottom left panel shows FOXp3 expression (blue signal, dotted intranuclear pattern), and bottom right panel shows the combined signals with arrows pointing to triple-stained CD4+CD25+FOXp3+ Tregs. CD4+CD25+FOXp3−, CD4+CD25−FOXp3+, and CD4−CD25−FOXp3+ cells are also seen. b Picture (×40) of intraepithelial infiltrating CD8+ cytotoxic T cells. Left panel shows CD8 expression (red signal), center panel shows intranuclear DAPI expression (blue signal), and right panel shows the combined signals
Confocal microscopy and cell quantification
Stained slides were evaluated at 400× by confocal laser scanning microscopy as previously described [24]. The quantification of Tregs and CD8 T cells was performed manually by observers blinded to survival, demographic, and subtype data. A subset of randomly chosen specimens was read by two observers to ensure agreement/reproducibility. Total counts of cells from each field were recorded in either the triple-stained (CD4, CD25, and FOXp3) or double-stained (CD8 and DAPI) samples. Intraepithelial tumor-infiltrating Tregs were defined as CD4+CD25+FOXp3+ cells that displayed nuclear but not cytoplasmic FOXp3 [24]. The primary assessment of Treg or CD8 T cell quantification was the average Treg or CD8 T cell counts, respectively, across ten fields from each tumor sample.
Genetic data
Procedures for selection of genes and SNPs and for germline genotyping and quality control have been previously published [36, 37]. In brief, 79 genes some with known roles in induction, trafficking, or function of regulatory T cells were identified, and tagging variants were chosen using HapMap and 1000 Genomes Project data (Supplemental Table S1). Genotyping was done in two experiments using a custom Illumina GoldenGate array for 54 genes [36] and a custom Illumina Infinium iSelect BeadArray for 25 genes [37]; IDO1 was included in both experiments and combined here. We excluded variants with call rate <95 % (minor allele frequency ≥0.05) or <99 % (minor allele frequency <0.05) and samples with call rate <95 % or predicted European ancestry ≤90 % [36, 37].
Statistical analysis
Staining count data quality was assessed via box-and-whisker and scatter plots to evaluate possible trends over time and distributional characteristics. We determined that the FOXp3 stain failed during 4 weeks of the study. FOXp3 staining during this period was determined to be abnormally low during this period as compared to all other time periods. Thus, analyses for the FOXp3 endpoint are based on a subset of n = 348 patients. Three patients failed CD8 staining and three failed CD4 staining. Associations between staining counts and clinical characteristics were assessed using Wilcoxon rank-sum tests and Spearman correlation coefficients, as appropriate. Overall survival was defined as the time from initial diagnosis until death due to any cause; patients still alive were censored at the date of last follow-up. Associations between staining counts and overall survival were assessed using Cox proportional hazards models and Kaplan–Meier curves. Adjusted Cox models included the following variables multivariately associated with overall survival at p < 0.05: age at diagnosis (continuous), stage (I or II, III or IV), and residual disease following debulking surgery (<1 cm, ≥1 cm, unknown); staining counts were transformed to the log2(count + 1) scale for modeling.
Associations between inherited variation and staining counts were assessed on the gene level using linear models and principal components (PCs). Variants within each gene were summarized using PCs agnostic to staining results. The minimum PCs needed to account for 90 % of the total variation within each gene were then placed in a linear regression model with staining count as the dependent variable, again on the log2(count + 1) scale. Finally, for genes with PCs significantly associated with staining counts, the gene PCs and staining counts were jointly modeled in prediction of overall survival to assess multivariable associations between staining counts, host genetics, and outcome.
Results
Patient clinical characteristics
For the present study, a total of 405 patients with epithelial ovarian cancer were germline genotyped and their associated tumor specimens were analyzed for tumor-infiltrating Tregs (CD4+CD25+FOXp3+), CD4, and CD8 T cells. The patient characteristics are given in Table 1. The median age at diagnosis was 63 years (range 28–86 years). The majority of patients (80 %) had high-grade serous carcinoma (HGSC), 86 % had advanced stage (III and IV) disease, and 84 % were optimally debulked. At a median follow-up of 42.2 months (range 0.3–165.6), 287 patients (71 %) were deceased.
Table 1.
Characteristics of epithelial ovarian cancer patients
| n | (%) | |
|---|---|---|
| Morphology | ||
| High-grade serous | 325 | 80.2 |
| Endometrioid | 54 | 13.4 |
| Clear cell | 15 | 3.7 |
| Mucinous | 6 | 1.5 |
| Low-grade serous | 3 | 0.7 |
| Serous/unknown grade | 2 | 0.5 |
| Grade | ||
| Low | 10 | 2.5 |
| High | 393 | 97 |
| Unknown | 2 | 0.5 |
| Stage | ||
| I | 41 | 10.1 |
| II | 15 | 3.7 |
| III | 265 | 65.4 |
| IV | 84 | 20.7 |
| Debulking | ||
| Optimal (≤1 cm) | 338 | 83.5 |
| Sub-optimal (>1 cm) | 60 | 14.8 |
| Unknown | 7 | 1.7 |
Higher intratumoral CD8 T cell/Treg and CD4 T cell/Treg ratios correlate with improved survival in ovarian cancer
Of the 348 evaluable specimens stained, the median average counts for CD4+CD25+FOXp3+ Tregs (hereinafter referred to as Tregs) were 2.4 Treg cells (Interquartile range, IQR 1.1–4.2) per field and 97 % of the specimens stained positive for Tregs on at least one field. The median counts for total CD4+ T cells (which include Tregs) were 12.8 (Interquartile range, IQR 7.3–19.0) cells per field and 100 % stained positive for CD4 T cells on at least one field. Tregs represented approximately 24.8 % ± 17.7 % (mean ± SD) of the total CD4 T cells. The median levels of CD8+ T cells were 3.4 (IQR 1.9–6.3) cells per field and 99.8 % stained positive for CD8 on at least one field.
We then examined whether there were any correlations between levels of intratumoral Tregs, CD4 T cells, CD8 T cells, and standard clinical variables. We found that there was no association between Treg counts and stage (p = 0.469), subtype (p = 0.831), or presence of ascites (p = 0.672). Similarly, there was no association between CD8 counts and stage (p = 0.608), subtype (p = 0.088), or presence of ascites (p = 0.268). However, patients with optimal surgical debulking (<1 cm remaining) had lower Treg counts (median 2.3, IQR 1.0–3.8 vs. 3.3 per field; IQR 1.6–5.0, p = 0.007) and lower CD8 counts (3.1 per field; IQR 1.9–6.1 vs. 4.4; IQR 2.5–6.6, p = 0.051) compared to patients with suboptimal debulking. Lower CD4 counts (12.3; IQR 7.2–19.0 vs. 13.4 per field; IQR 8.4–18.8, p = 0.29) were, however, not different comparing suboptimal and optimal debulking. Similar to our smaller prior pilot study [24], we also examined for associations between the various ratios and clinical variables. The median CD8/Treg ratio was 1.6 (IQR 0.8–3.4), 4.7 (IQR 2.8–9.0) for the CD4/Treg ratio and 0.3 (IQR 0.2–0.5) for CD8/CD4. We found that there was no association between any of the ratios and stage (p = 0.312, p = 0.47, p = 0.664, respectively), subtype (p = 0.787, p = 0.198, p = 0.589), presence of ascites (p = 0.923, p = 0.41, p = 0.188), or debulking status (p = 0.325, p = 0.043, p = 0.307).
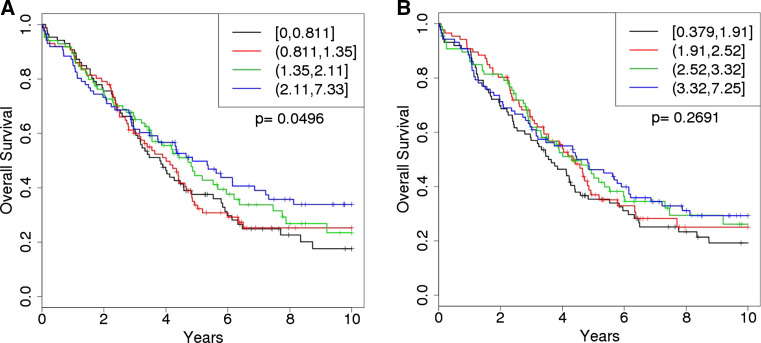
We next examined cell counts and ratios for associations with patient outcome as measured by overall survival (Table 2). Consistent with what we previously described in the smaller pilot study [24], Treg counts (log2) alone were not associated with overall survival either univariately (HR 1.06, 95 % CI 0.94–1.20, p = 0.35) or after adjustment for clinical factors (HR 1.11, 95 % CI 0.96–1.27, p = 0.15). However, also consistent with our prior report, we did observe that higher CD8/Treg ratios (log2) were associated with improved survival (HR 0.89, 95 % CI 0.79–1.00, p = 0.050), and this relationship strengthened considerably after adjusting for age, stage, and debulking (HR 0.84, 95 % CI 0.73–0.96, p = 0.0089) (Table 2). Kaplan–Meier analysis between CD8/Treg ratio quartiles and overall survival is shown in Fig. 2, showing that higher CD8/Treg ratios appear to influence overall survival in patients that live longer than 3 years. Patients in the higher quartile demonstrated a median survival of ~58 months, while those in the lowest quartile had a median survival of ~46 months. Log2 CD8 counts (HR 0.89, 95 % CI 0.80–1.01, p = 0.065) and log2 CD8/CD4 ratio (HR 0.71, 95 % CI 0.49–1.04, p = 0.080) were also marginally albeit nonsignificantly associated with overall survival after adjusting for age, stage, and debulking. Lastly we also observed that higher CD4/Treg ratios (log2) were associated with improved survival (HR 0.88, 95 % CI 0.77–0.998, p = 0.050) but only after adjusting for age, stage, and debulking (Table 2). Although not significant, Kaplan–Meier analysis between CD4/Treg ratio quartiles and overall survival is shown in Fig. 2b, showing a similar stepwise trend in survival.
Table 2.
Association between T cell counts and ratios and overall survival in epithelial ovarian cancer
| Count or ratio | n patients | Unadjusted | Adjusted for age, stage, debulking | ||||
|---|---|---|---|---|---|---|---|
| HR | 95 % CI | p value | HR | 95 % CI | p value | ||
| CD8+ | 402 | 0.92 | 0.82–1.04 | 0.18 | 0.89 | 0.80–1.01 | 0.065 |
| CD4+ | 402 | 1.00 | 0.89–1.13 | 0.96 | 0.98 | 0.87–1.11 | 0.79 |
| Tregs | 346 | 1.06 | 0.94–1.20 | 0.35 | 1.11 | 0.96–1.27 | 0.15 |
| CD4 +/Tregs | 346 | 0.94 | 0.84–1.05 | 0.27 | 0.88 | 0.77–0.998 | 0.046 |
| CD8+/CD4+ | 399 | 0.75 | 0.54–1.06 | 0.10 | 0.71 | 0.49–1.04 | 0.080 |
| CD8+/Tregs | 343 | 0.89 | 0.79–1.00 | 0.050 | 0.84 | 0.73–0.96 | 0.0089 |
log2 of each stain or ratio was used; three patients failed staining for CD8, three patients failed CD4 staining, and 57 patients failed FOXp3
Fig. 2.
Overall survival by CD8/Treg and CD4/Treg ratio quartiles. Among 405 invasive epithelial ovarian cancer patients, Kaplan–Meier survival curves by quartiles of the log2 ratio of a CD8+ cytotoxic T cells to CD4+CD25−FOXp3+ Tregs (CD8+/CD4+CD25−FOXp3+) or b CD4+ T cells to CD4+CD25−FOXp3+ Tregs (CD4+/CD4+CD25−FOXp3+). The legends indicate quartile cut-points, where ‘[’ is inclusive of the interval endpoint and ‘(’ is exclusive of the interval endpoints. Patients with higher ratios show improved overall survival (p = 0.0496)
Inherited variation shows modest association with Treg infiltration or the T cell/Treg ratios in ovarian cancer
One of the primary unique goals of this study was to potentially identify novel gene products that influence the role of Tregs in ovarian cancer outcomes, with our hypothesis that we could do this through examining for an association between gene variation and either the levels of Tregs or effector T cell to Treg ratios. To assess whether levels of Treg infiltration or respective ratios are associated with inherited genetic variability, we first summarized the gene variations across all of the variants within a gene using PCs analysis, agnostic to the observed Treg infiltration counts. The gene variation PCs were then modeled with measures of Treg infiltration (counts and ratios) in linear models to assess association between inherited gene variation and observed Treg infiltration. Table 3 lists the top genes associated with each infiltrating cell or ratios. Only two genes were found to be associated, albeit weakly, with the levels of Tregs, IL-15RA (p = 0.021), and IL6ST (p = 0.023). The genes most strongly associated (p < 0.05) with the CD8/Treg ratio were IL-10, CCL3, CD46, CCL19, and INHBA. The genes most strongly associated with the CD4/Treg ratio were IL-10, IL-8, CCL4, MDFIC, and CCL3. For other infiltration measures, genes with highly suggestive associations included CCL11 (CD8, p = 0.0086) and CCL22 (CD8/CD4, ratio p = 0.0058) (Table 3). However, after accounting for multiple testing (79 genes and six different cell counts and ratios), there were no significant (p < 0.05) associations between gene-level variation and infiltration ratios. The complete list of genes of interest that were examined are detailed in Supplemental Table S1, including the putative functions of the genes in Treg biology, the SNPs examined as well as identifiers. Supplemental Table S2 provides a complete list of PC analysis of all the genes.
Table 3.
Genes most associated with T cells, Tregs, and ratios
| p value | |
|---|---|
| Tregs | |
| IL-15RA | 0.021 |
| IL6ST | 0.023 |
| AGTR1 | 0.067 |
| CCL2 | 0.109 |
| CXCR5 | 0.121 |
| CD8+/Tregs | |
| IL-10 | 0.0073 |
| CCL3 | 0.011 |
| CD46 | 0.034 |
| CCL19 | 0.048 |
| INHBA | 0.057 |
| CD8+ T cells | |
| CCL11 | 0.0086 |
| LRRC32 | 0.016 |
| CCL19 | 0.028 |
| SLC22A2 | 0.036 |
| CCL2 | 0.038 |
| CD4+ T cells | |
| IDO1 | 0.025 |
| CD28 | 0.029 |
| CCL11 | 0.033 |
| CCL2 | 0.042 |
| MDFIC | 0.051 |
| CD4+/Tregs | |
| IL-10 | 0.010 |
| IL-8 | 0.017 |
| CCL4 | 0.033 |
| MDFIC | 0.039 |
| CCL3 | 0.039 |
| CD8+/CD4+ | |
| CCL22 | 0.0058 |
| IL23R | 0.011 |
| CXCL10 | 0.061 |
| TNFRSF18 | 0.068 |
| CCL17 | 0.093 |
Treg ratios, inherited variation, and correlation with clinical outcome in ovarian cancer
Given the observed association between the CD8/Treg ratio and overall survival in the study, we further explored this relationship in the context of inherited variation in IL-10, the gene most associated (p = 0.0073) with the CD8/Treg ratio in order to examine whether host genetics may be influencing patient outcome through the intermediate of cellular infiltration. In a multivariable Cox model for overall survival adjusting for all three IL-10 SNPs, as well as the clinical covariates of age, stage, and surgical debulking status, the CD8/Treg ratio still remained associated with overall survival with a similar effect size (HR 0.85, 95 % CI 0.74–0.99, p = 0.031). Furthermore, in this model, the set of IL-10 SNPs was not associated with overall survival (p = 0.84) which was also observed when the CD8/Treg ratio was excluded from the model (p = 0.70). We also assessed this relationship with IL-10 SNPs and the CD4/Treg ratio. Adjusting for all three IL-10 SNPs and the clinical covariates of age, stage, and surgical debulking status, the CD4/Treg ratio still remained marginally associated with overall survival with a similar effect size (HR 0.87, 95 % CI 0.74–1.02, p = 0.093) as the unadjusted models, while the set of IL-10 SNPs was not associated with overall survival (p = 0.79). Thus, although we did see a statistically significant interaction between IL-10 gene variation and both the CD4/Treg and CD8/Treg ratios, this variation in the IL-10 gene did not appear to influence the association between the Treg infiltration ratios and patient outcomes.
Discussion
Our comprehensive analysis of tumors from over 400 invasive epithelial ovarian cancer patients has shown an association between improved outcome and a higher ratio of both infiltrating cytotoxic CD8+ T cells and total CD4 T cells to Tregs and represents the largest study assessing triple-stained CD4+CD25+FOXp3+ Treg cells. Importantly, this relationship was apparent after adjusting for several known prognostic factors. However, we were unable to find strong evidence that the levels of Treg infiltration are modified by inherited genetic variation in genes that have previously been associated with Tregs.
Although we previously reported that triple-positive Tregs are associated with outcome when examined as a ratio with CD8 T cells in a pilot study, the present study has many significant differences. The pilot study only examined 52 high-grade serous patients (including 30 who were genotyped and included here), whereas the present studies examined 405 samples derived from all subtypes of ovarian cancers [24]. Furthermore, the design of the pilot was limited in that we selected two groups of optimally debulked advanced-stage patients, those with good outcomes (>60 months survival), and those with poor outcomes (<12 months), avoiding those with survival between 12 and 60 months. In the current study, we examined survival as a continuous variable, providing additional statistical power. The present study also more broadly represents invasive epithelial ovarian cancer as it included sub-optimally debulked patients and those at earlier stages and with low-grade malignancies.
It is well established that optimal debulking of advanced-stage (III and IV) ovarian cancer is associated with greatly improved survival [39–41]. A unique finding in the present study was the association between reduced Treg and CD8 infiltration with optimal debulking. This finding points to a biological reason that related the ability to optimally debulk ovarian cancer patients. Factors that are known to impact the ability to optimally debulk patients with ovarian cancer, however, are largely technical and include assigning of a gynecologic oncology surgeon during the initial diagnostic surgery and the surgeon’s ability and overall preference for conducting radical cytoreductive surgery, the latter of which results in higher rates of optimal debulking [39, 42]. However, it is highly unlikely that the extent of T cell or Treg infiltration would be associated with the technical variables described above. The findings, however, may point to the interpretation that tumors that are not optimally resectable may interact less with a patient’s immune system for various reasons that could include lack of chemokine/chemokine production, reduced expression of tumor associated antigens, or anatomic location.
Another unique finding from this study is that the total CD4 T cell to Treg ratio was statistically associated with improved survival. Research over the past three decades has shown that CD4 T cells are a markedly heterogeneous group of T cells representing multiple subsets such as Th1, Th2, Th17, as well as Tregs [43]. This heterogeneity is the likely reason that the CD4 marker, unlike CD8, has not been linked to improved survival in ovarian cancer. Importantly, staining with CD4 alone captures Tregs which in the present study we demonstrate constitute nearly one quarter of the infiltrating CD4 T cells. To better differentiate effector CD4 T cells from Tregs, additional markers need to be validated which would further provide important information as to which helper CD4 T cells (Th1, Th2, or Th17) have a prognostic role. The need to further evaluate the role of helper CD4 T cells is exemplified by a recent study, demonstrating that the levels of IL-17, a dominant cytokine product of Th17 T cells, have been associated with improved survival in ovarian cancer [44].
Understanding the pathologic role of Tregs, which have the ability to suppress activated T cells in the ovarian cancer microenvironment, is important for developing new immune-based therapies and understanding a patient’s prognosis. There are several drugs that are known to be useful as Treg-depleting agents such as cyclophosphamide, anti-CD25 antibody, or denileukin diftitox and are available for depletion of Tregs alone or combination therapy with novel vaccine or adoptive T cell therapy approaches [28, 31]. However, the lack of specificity and the adverse event profile associated with these agents prevents widespread use. Thus, it is particularly important to continue to identify pathways and proteins that can be specifically targeted to selectively deplete Tregs, while retaining infiltration of other anti-tumor T cells, including CD8 cytotoxic T cells and CD4 helper T cells. The objective in the current study was to determine whether we could use genetic variation as a means of identifying new targets for Tregs. Specifically, we hypothesized that genetic variation may be responsible for the variation observed in Treg infiltration, which could then be linked with overall survival. We previously reported that overall survival was associated with inherited variation in Treg-associated genes IL2RA and CD80 (among patients with endometrioid subtype), CTLA4 and RGS1 (among patients with clear cell subtype), and LRRC32 and TNFRSF18/TNFRSF4 (among patients with mucinous subtype) [36, 37]. Despite these interesting findings, we did not find any compelling associations for the more predominant serous subtype of ovarian cancer in those studies, and because these associations occurred among patients with rare histologic subtypes, we could not examine them in relation to the Treg infiltration studied here. This trade-off of sample size and detailed tumor phenotyping exemplifies one of the challenges faced in integrative molecular epidemiology.
In the present study, when we examined the relationship between Treg infiltration and genetic variation using the entire group of specimens, we were unable to see any associations that were significant enough to withstand correction for multiple testing. This reinforces the observations that while inherited factors, including common variants [45–49], clearly play a role in the etiology of ovarian cancer, they have been more difficult to characterize in relation to survival outcomes, particularly as linked through histologic findings, such as Treg infiltration. The most likely reason for the lack of detectable association of genetic variation with Treg infiltration or the CD8/Treg ratio is statistical power. We believe that the penetrance of the genetic variation may be too subtle to pick up with a relatively small sample of only 400 patients and a complex immune measurement. Therefore, we cannot rule more modest effects of genetics on the Treg infiltration or CD8/Treg ratio. The most statistically significant association was between IL-10 gene variation and the CD8/Treg ratio. Interestingly, we also observed that genetic variation in the IL-10 gene was associated albeit more weakly with the CD4/Treg ratio. While power and multiple testing currently limit the interpretation of these results, the IL-10, which encodes the IL-10 protein cytokine, has well-established connections to CD8, CD4 T cell, and Treg biology [50–52]. Specifically, this would be consistent with a model in which Treg-derived IL-10 prevents proliferation of effector T cells in the tumor microenvironment, thereby modifying the respective effector T cell to Treg ratios.
In summary, through detailed analysis of genotyped ovarian cancer patients with immunofluorescence-stained fresh frozen tumors, we found that higher CD8 and CD4 T cell to CD4+CD25+FOXp3+ Treg ratios are predictive of longer overall survival time, regardless of inherited genetic variation.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported by US National Institute of Health Grants (P50-CA136393 [Mayo Clinic SPORE in ovarian cancer], R01-CA122443 and, P30-CA15083) and the Fred C. and Katherine B. Andersen Foundation. The authors would like to acknowledge the help and support of Duane Deal, the Mayo Clinic Cancer Center Flow Cytometry/Optical Morphology Shared Resource, and the Ovarian Cancer Association Consortium.
Abbreviations
- CI
Confidence interval
- DAPI
4′,6-Diamidino-2-phenylindole
- HGSC
High-grade serous carcinoma
- HR
Hazard ratio
- IQR
Interquartile range
- PC
Principal Component
- Treg
CD4 regulatory T cell
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Keith L. Knutson, Phone: 904-953-6657, Email: knutson.keith@mayo.edu
Ellen L. Goode, Phone: 507-266-7997, Email: egoode@mayo.edu
References
- 1.Aletti GD, Gallenberg MM, Cliby WA, Jatoi A, Hartmann LC. Current management strategies for ovarian cancer. Mayo Clin Proc. 2007;82:751–770. doi: 10.1016/S0025-6196(11)61196-8. [DOI] [PubMed] [Google Scholar]
- 2.Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519–2529. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- 3.Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2003;21:3194–3200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- 4.Hoskins P, Vergote I, Cervantes A, Tu D, Stuart G, Zola P, et al. Advanced ovarian cancer: phase III randomized study of sequential cisplatin-topotecan and carboplatin-paclitaxel vs carboplatin-paclitaxel. J Natl Cancer Inst. 2010;102:1547–1556. doi: 10.1093/jnci/djq362. [DOI] [PubMed] [Google Scholar]
- 5.Trimble EL, Birrer MJ, Hoskins WJ, Marth C, Petryshyn R, Quinn M, et al. Current academic clinical trials in ovarian cancer: Gynecologic Cancer Intergroup and US National Cancer Institute Clinical Trials Planning Meeting, May 2009. Int J Gynecol Cancer. 2010;20:1290–1298. doi: 10.1111/IGC.0b013e3181ee1c01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winter WE, III, Maxwell GL, Tian C, Carlson JW, Ozols RF, Rose PG, et al. Prognostic factors for stage III epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:3621–3627. doi: 10.1200/JCO.2006.10.2517. [DOI] [PubMed] [Google Scholar]
- 7.Nelson BH. New insights into tumor immunity revealed by the unique genetic and genomic aspects of ovarian cancer. Curr Opin Immunol. 2015;33C:93–100. doi: 10.1016/j.coi.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 9.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Preston CC, Goode EL, Hartmann LC, Kalli KR, Knutson KL. Immunity and immune suppression in human ovarian cancer. Immunotherapy. 2011;3:539–556. doi: 10.2217/imt.11.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charbonneau B, Goode EL, Kalli KR, Knutson KL, Derycke MS. The immune system in the pathogenesis of ovarian cancer. Crit Rev Immunol. 2013;33:137–164. doi: 10.1615/CritRevImmunol.2013006813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karyampudi L, Lamichhane P, Scheid AD, Kalli KR, Shreeder B, Krempski JW, Behrens MD, Knutson KL. Accumulation of memory precursor CD8 T cells in regressing tumors following combination therapy with vaccine and anti-PD-1 antibody. Cancer Res. 2014;74:2974–2985. doi: 10.1158/0008-5472.CAN-13-2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Audia S, Nicolas A, Cathelin D, Larmonier N, Ferrand C, Foucher P, et al. Increase of CD4+CD25+ regulatory T cells in the peripheral blood of patients with metastatic carcinoma: a phase I clinical trial using cyclophosphamide and immunotherapy to eliminate CD4+CD25+ T lymphocytes. Clin Exp Immunol. 2007;150:523–530. doi: 10.1111/j.1365-2249.2007.03521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen F, Hou M, Ye F, Lv W, Xie X. Ovarian cancer cells induce peripheral mature dendritic cells to differentiate into macrophagelike cells in vitro. Int J Gynecol Cancer. 2009;19:1487–1493. doi: 10.1111/IGC.0b013e3181bb70c6. [DOI] [PubMed] [Google Scholar]
- 15.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 16.Hagemann T, Wilson J, Burke F, Kulbe H, Li NF, Pluddemann A, et al. Ovarian cancer cells polarize macrophages toward a tumor-associated phenotype. J Immunol. 2006;176:5023–5032. doi: 10.4049/jimmunol.176.8.5023. [DOI] [PubMed] [Google Scholar]
- 17.Huarte E, Cubillos-Ruiz JR, Nesbeth YC, Scarlett UK, Martinez DG, Buckanovich RJ, et al. Depletion of dendritic cells delays ovarian cancer progression by boosting antitumor immunity. Cancer Res. 2008;68:7684–7691. doi: 10.1158/0008-5472.CAN-08-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ko SY, Ladanyi A, Lengyel E, Naora H. Expression of the homeobox gene HOXA9 in ovarian cancer induces peritoneal macrophages to acquire an M2 tumor-promoting phenotype. Am J Pathol. 2014;184:271–281. doi: 10.1016/j.ajpath.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krempski J, Karyampudi L, Behrens MD, Erskine CL, Hartmann L, Dong H, et al. Tumor-infiltrating programmed death receptor-1+ dendritic cells mediate immune suppression in ovarian cancer. J Immunol. 2011;186:6905–6913. doi: 10.4049/jimmunol.1100274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lan C, Huang X, Lin S, Huang H, Cai Q, Wan T, Lu J, Liu J. Expression of M2-polarized macrophages is associated with poor prognosis for advanced epithelial ovarian cancer. Technol Cancer Res Treat. 2013;12:259–267. doi: 10.7785/tcrt.2012.500312. [DOI] [PubMed] [Google Scholar]
- 21.Le Page C, Marineau A, Bonza PK, Rahimi K, Cyr L, Labouba I, et al. BTN3A2 expression in epithelial ovarian cancer is associated with higher tumor infiltrating T cells and a better prognosis. PLoS ONE. 2012;7:e38541. doi: 10.1371/journal.pone.0038541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meloni F, Morosini M, Solari N, Passadore I, Nascimbene C, Novo M, et al. FOXp3 expressing CD4+CD25+ and CD8+CD28− T regulatory cells in the peripheral blood of patients with lung cancer and pleural mesothelioma. Hum Immunol. 2006;67:1–12. doi: 10.1016/j.humimm.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 23.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Preston CC, Maurer MJ, Oberg AL, Visscher DW, Kalli KR, Hartmann LC, Goode EL, Knutson KL. The ratios of CD8+ T cells to CD4+CD25+FOXp3+ and FOXp3− T cells correlate with poor clinical outcome in human serous ovarian cancer. PLoS ONE. 2013;8:e80063. doi: 10.1371/journal.pone.0080063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wei S, Kryczek I, Zou L, Daniel B, Cheng P, Mottram P, et al. Plasmacytoid dendritic cells induce CD8+ regulatory T cells in human ovarian carcinoma. Cancer Res. 2005;65:5020–5026. doi: 10.1158/0008-5472.CAN-04-4043. [DOI] [PubMed] [Google Scholar]
- 26.Wolf D, Wolf AM, Rumpold H, Fiegl H, Zeimet AG, Muller-Holzner E, et al. The expression of the regulatory T cell-specific forkhead box transcription factor FOXp3 is associated with poor prognosis in ovarian cancer. Clin Cancer Res. 2005;11:8326–8331. doi: 10.1158/1078-0432.CCR-05-1244. [DOI] [PubMed] [Google Scholar]
- 27.Yang R, Cai Z, Zhang Y, Yutzy WHt, Roby KF, Roden RB (2006) CD80 in immune suppression by mouse ovarian carcinoma-associated Gr-1 + CD11b + myeloid cells. Cancer Res 66:6807–6815. doi:10.1158/0008-5472.CAN-05-3755 [DOI] [PubMed]
- 28.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 29.Zou W, Machelon V, Coulomb-L’Hermin A, Borvak J, Nome F, Isaeva T, et al. Stromal-derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells. Nat Med. 2001;7:1339–1346. doi: 10.1038/nm1201-1339. [DOI] [PubMed] [Google Scholar]
- 30.Tran Janco JM, Lamichhane P, Karyampudi L, Knutson KL. Tumor-infiltrating dendritic cells in cancer pathogenesis. J Immunol. 2015;194:2985–2991. doi: 10.4049/jimmunol.1403134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knutson KL, Disis ML, Salazar LG. CD4 regulatory T cells in human cancer pathogenesis. Cancer Immunol Immunother. 2007;56:271–285. doi: 10.1007/s00262-006-0194-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Savage PA, Malchow S, Leventhal DS. Basic principles of tumor-associated regulatory T cell biology. Trends Immunol. 2013;34:33–40. doi: 10.1016/j.it.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Le DT, Jaffee EM. Regulatory T-cell modulation using cyclophosphamide in vaccine approaches: a current perspective. Cancer Res. 2012;72:3439–3444. doi: 10.1158/0008-5472.CAN-11-3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dannull J, Su Z, Rizzieri D, Yang BK, Coleman D, Yancey D, et al. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest. 2005;115:3623–3633. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knutson KL, Dang Y, Lu H, Lukas J, Almand B, Gad E, Azeke E, Disis ML. IL-2 immunotoxin therapy modulates tumor-associated regulatory T cells and leads to lasting immune-mediated rejection of breast cancers in neu-transgenic mice. J Immunol. 2006;177:84–91. doi: 10.4049/jimmunol.177.1.84. [DOI] [PubMed] [Google Scholar]
- 36.Goode EL, DeRycke M, Kalli KR, Oberg AL, Cunningham JM, Maurer MJ, et al. Inherited variants in regulatory T cell genes and outcome of ovarian cancer. PLoS ONE. 2013;8:e53903. doi: 10.1371/journal.pone.0053903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Charbonneau B, Moysich KB, Kalli KR, Oberg AL, Vierkant RA, Fogarty ZC, et al. Large-scale evaluation of common variation in regulatory T cell-related genes and ovarian cancer outcome. Cancer Immunol Res. 2014;2:332–340. doi: 10.1158/2326-6066.CIR-13-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goode EL, Maurer MJ, Sellers TA, Phelan CM, Kalli KR, Fridley BL, et al. Inherited determinants of ovarian cancer survival. Clin Cancer Res. 2010;16:995–1007. doi: 10.1158/1078-0432.CCR-09-2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aletti GD, Dowdy SC, Gostout BS, Jones MB, Stanhope CR, Wilson TO, Podratz KC, Cliby WA. Aggressive surgical effort and improved survival in advanced-stage ovarian cancer. Obstet Gynecol. 2006;107:77–85. doi: 10.1097/01.AOG.0000192407.04428.bb. [DOI] [PubMed] [Google Scholar]
- 40.Bristow RE, Montz FJ, Lagasse LD, Leuchter RS, Karlan BY. Survival impact of surgical cytoreduction in stage IV epithelial ovarian cancer. Gynecol Oncol. 1999;72:278–287. doi: 10.1006/gyno.1998.5145. [DOI] [PubMed] [Google Scholar]
- 41.Chang SJ, Bristow RE. Evolution of surgical treatment paradigms for advanced-stage ovarian cancer: redefining ‘optimal’ residual disease. Gynecol Oncol. 2012;125:483–492. doi: 10.1016/j.ygyno.2012.02.024. [DOI] [PubMed] [Google Scholar]
- 42.Jelovac D, Armstrong DK. Recent progress in the diagnosis and treatment of ovarian cancer. CA Cancer J Clin. 2011;61:183–203. doi: 10.3322/caac.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakayamada S, Takahashi H, Kanno Y, O’Shea JJ. Helper T cell diversity and plasticity. Curr Opin Immunol. 2012;24:297–302. doi: 10.1016/j.coi.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S, et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114:1141–1149. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Song H, Ramus SJ, Tyrer J, Bolton KL, Gentry-Maharaj A, Wozniak E, et al. A genome-wide association study identifies a new ovarian cancer susceptibility locus on 9p22.2. Nat Genet. 2009;41:996–1000. doi: 10.1038/ng.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bolton KL, Tyrer J, Song H, Ramus SJ, Notaridou M, Jones C, et al. Common variants at 19p13 are associated with susceptibility to ovarian cancer. Nat Genet. 2010;42:880–884. doi: 10.1038/ng.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goode EL, Chenevix-Trench G, Song H, Ramus SJ, Notaridou M, Lawrenson K, et al. A genome-wide association study identifies susceptibility loci for ovarian cancer at 2q31 and 8q24. Nat Genet. 2010;42:874–879. doi: 10.1038/ng.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pharoah PD, Tsai YY, Ramus SJ, Phelan CM, Goode EL, Lawrenson K et al (2013) GWAS meta-analysis and replication identifies three new susceptibility loci for ovarian cancer. Nat Genet 45:362–370, 70e1–2. doi:10.1038/ng.2564 [DOI] [PMC free article] [PubMed]
- 49.Permuth-Wey J, Lawrenson K, Shen HC, Velkova A, Tyrer JP, Chen Z et al (2013) Identification and molecular characterization of a new ovarian cancer susceptibility locus at 17q21.31. Nat Commun 4:1627. doi:10.1038/ncomms2613 [DOI] [PMC free article] [PubMed]
- 50.Yang AS, Lattime EC. Tumor-induced interleukin 10 suppresses the ability of splenic dendritic cells to stimulate CD4 and CD8 T-cell responses. Cancer Res. 2003;63:2150–2157. [PubMed] [Google Scholar]
- 51.Groux H, Bigler M, de Vries JE, Roncarolo MG. Inhibitory and stimulatory effects of IL-10 on human CD8+ T cells. J Immunol. 1998;160:3188–3193. [PubMed] [Google Scholar]
- 52.Jarnicki AG, Lysaght J, Todryk S, Mills KH. Suppression of antitumor immunity by IL-10 and TGF-beta-producing T cells infiltrating the growing tumor: influence of tumor environment on the induction of CD4+ and CD8+ regulatory T cells. J Immunol. 2006;177:896–904. doi: 10.4049/jimmunol.177.2.896. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.