Abstract
Peripheral vascular malformations encompass a wide spectrum of lesions that can present as an incidental finding or produce potentially life- or limb-threatening complications. They can have intra-articular and intraosseous extensions that will result in more diverse symptomology and present greater therapeutic challenges. Developments in classification, imaging and interventional techniques have helped to improve outcome. The onus is now placed on appropriate detailed preliminary imaging, diagnosis and classification to direct management and exclude other more common mimics. Radiologists are thus playing an increasingly important role in the multidisciplinary teams charged with the care of these patients. By fully understanding the imaging characteristics and image-guided procedures available, radiologists will be armed with the tools to meet these responsibilities. This review highlights the recent advances made in imaging and the options available in interventional therapy.
Peripheral vascular malformations (PVMs) encompass a wide spectrum of lesions that can present as an incidental finding or produce potentially life- or limb-threatening complications. PVMs are relatively common within the extremities and usually confined to the subcutaneous tissues and muscles. They can have, however, intra-articular and intraosseous extensions that will result in more diverse symptomology and present greater therapeutic challenges. Any misdiagnosis can lead to inappropriate management and treatment.1
Clinical history and physical examination remain an essential facet in the diagnosis of these lesions but can underestimate their nature and size. With improvements in imaging and interventional techniques, radiologists are now playing an increasingly important role in their exact characterization and treatment. The management of complex PVMs is best undertaken in dedicated vascular centres with close communication and co-operation between diagnostic musculoskeletal and interventional radiologists and vascular surgeons.
CLASSIFICATION
Until recently, the vague terminology applied for these lesions produced a confusing collection of semantic diagnoses and varied and poor outcomes.1,2
Several classification systems have been proposed. Mulliken and Glowacki1,2 initially described a biologically focused system, based on cellular turnover, histological features, natural history and physical findings. This simply classified anomalies into haemangiomas or vascular malformations.1–5
Jackson et al6 later developed a radiological classification, which took account of earlier work, but further subclassified these lesions by their flow dynamics. This included low-flow malformations such as venous, lymphatic and capillary lesions, and high-flow malformations, including arteriovenous malformations (AVMs) and arteriovenous fistulas (AVFs)7 (Table 1).
Table 1.
Classification of vascular malformations according to flow dynamics
| Vascular malformations | |
|---|---|
| Low flow | High flow |
| Venous malformations | Arteriovenous malformations |
| Lymphatic malformations | Arteriovenous fistulas |
| Mixed malformations | |
These systems were eventually expanded upon by the International Study of Vascular Anomalies (ISSVA) and have been widely adopted. This incorporated cellular features, flow characteristics and clinical behaviour to allow a systematic approach to treatment options8,9 (Tables 2 and 3).
Table 2.
International Study of Vascular Anomalies classification of vascular anomalies into tumours and simple malformations5
| Vascular tumour | Simple vascular malformations |
|---|---|
| Haemangioma | Capillary malformation |
| Venous malformation | |
| Other | Lymphatic malformation |
Table 3.
International Study of Vascular Anomalies classification of vascular anomalies into complex malformations5
| Complex vascular malformations | |
|---|---|
| Vascular | • Arteriovenous fistula • Arteriovenous malformation |
| • Capillary–venous malformation • Capillary–lymphatic–venous malformation | |
| Lymphatic | • Lymphatic–venous malformation • Capillary–lymphatic–arteriovenous malformation |
More recently, Houdart10 and later Cho et al11 highlighted that some of the most complex high-flow lesions, such as AVMs, were more common (>60%). Because of the difficulties faced in treating these high-flow peripheral lesions, a modified angiographic classification was proposed, in an attempt to aid treatment outcome.12,13
CLINICAL FINDINGS
Haemangiomas
Haemangiomas are benign vascular tumours of infancy and childhood that consist of cellular proliferation and hyperplasia and are characterized by a rapid early proliferative stage and a later involution.1,7,14 In comparison, vascular malformations arise from dysplastic vascular channels and exhibit normal endothelial turnover, growing commensurately with the child without regression.1,7,14
Low-flow venous and lymphatic malformations
Low-flow lesions such as venous malformations (VM) are some of the most common types of vascular malformations, with an overall prevalence of up to 1% in the general population.7,15 The extremities encompass approximately 40% of the sites involved, with low-flow venous PVMs being the most common types encountered. Other locations include the head and neck (40% of cases) and the trunk (20%).7,15
They have a variable morphology and consist of abnormal venous channels, adipose tissue and degenerative muscle matrix.7,9,16 They are composed of dysplastic post-capillary thin-walled venous channels of varying sizes and wall thickness.7 The proportion of vascular spaces and matrix differ from lesion to lesion, with limited mural smooth muscle content and variable amounts of thrombi or phleboliths.9,16
They usually present at childhood or early adulthood with functional or cosmetic symptoms related to their size and location, including pain, reduced joint mobility and skeletal deformity.7,9,16 On examination, they are soft and compressible masses with blue discolourations of the skin. They characteristically decompress with elevation and local compression and enlarge on valsalva manoeuvre. They can cross tissue planes and invade adjacent tissues, including fat, muscle, tendon and even bone. Joint swelling can be caused by venous engorgement, localized thrombosis/thrombophlebitis, mass effect or local haemorrhage. Any connection to the deeper limb venous system can also precipitate the risk of deep vein thrombosis.7,9,16
High-flow vascular arteriovenous malformations and fistulas
High-flow malformations make up approximately 10% of malformations in the extremities.7,17 AVMs and AVFs are typically congenital and acquired malformations, respectively. AVFs are formed by a single vascular channel between an artery and a vein. AVMs consist of feeding arteries, draining veins and a nidus composed of multiple dysplastic vascular channels that connect the arteries and veins, with the absence of a normal capillary bed. Although there are a cluster of channels, there is no significant solid identifiable mass.7
AVMs are present in a dormant stage during birth but can show rapid growth over a relatively short period of time during childhood or adulthood, as they increase in size proportionally with the growth of the patient.18 This growth rate can be exacerbated by hormonal changes during puberty or pregnancy or as a result of thrombosis, infection and trauma.16,17 AVMs may be single, multiple or part of a genetic disorder such as hereditary haemorrhagic telangiectasia syndrome (Osler–Weber–Rendu) (Table 4).
Table 4.
| Regional syndromes with associated vascular malformations |
| 1. Sturge–Weber: characterized by facial capillary malformations with “port wine stains” and intracranial malformation such as pial angiomas 2. Klippel–Trenaunay: observe limb and trunk overgrowth from combination involving soft-tissue or bone hypertrophy, varicose veins and venous or lymphatic malformations |
| Diffuse syndromes associated slow-flow malformations |
| 1. Proteus syndrome: multisystemic congenital hamartomatous overgrowth condition characterized by asymmetrical vascular malformations (capillary or venous), cerebriform connective tissue and epidermal naevi, hemihypertrophy, macrodactyly and lipomas 2. Blue rubber bleb nevus (Bean) syndrome: rare multifocal syndrome with venous malformations most prominent in the skin and soft tissues, but also gastrointestinal tract 3. Epidermal nevus syndrome (Solomon syndrome): vascular malformations (intracranial arteriovenous malformation), epidermal nevi, various developmental abnormalities of the skin, eyes, nervous, skeletal, cardiovascular and urogenital systems |
| Diffuse syndromes associated fast-flow malformations |
| 1. Hereditary haemorrhagic telangiectasia (Osler–Weber–Rendu): multiple telangiectasias, including skin, mucous membranes and gastrointestinal tract mucosa. The presence of multiorgan arteriovenous malformation, including lungs, liver, brain and spine |
The high blood flow in the lesions produces a pulsatile, red, warm mass with a thrill on examinations. If related to joints, they may lead to bone overgrowth, arterial steal phenomenon and cutaneous ischaemia. Ulceration and haemorrhage may be seen in extreme cases. Less commonly, high-output cardiac failure can occur with large arteriovenous shunts.18
Intra-articular malformations
Intra-articular vascular anomalies are relatively rare and can represent a subset of both high- or low-flow malformations. Although congenital, they are often not discovered until later in life. Within the literature, these lesions are often misrepresented as synovial haemangiomas.21–23
Crucially, any intra-articular extension of malformations will produce features of a pre-mature destructive arthropathy secondary to repeated haemarthrosis. Any haemarthroses can produce effusion and potential flexion contracture, muscular atrophy, equinus deformity of the foot and progressive ankylosis and early osteoarthritis. These intra-articular features mimic those typically encountered in haemophilic arthropathy or other causes of articular degeneration, such as inflammatory arthropathy or pigmented villonodular synovitis, which require different management.21,23
Intraosseous malformations
Skeletal changes are commonly associated with vascular malformations. Boyd et al24 described up to 30% of vascular malformations in the limbs appeared to have osseous transformations.25 If asymptomatic, intra-osseous lesions can be often discovered by chance when imaging for other reasons. The most common presenting reason, however, includes pain and leg-length discrepancy with associated muscular involvement. In a small series, Breugem et al25 described 55% of patients with a leg-length discrepancy of 2 cm or more.
Associated syndromes
As alluded to earlier, vascular malformations can be associated with a number of syndromes of which the Klippel–Trenaunay syndrome and Parkes–Weber syndrome are well recognized. A number can be specific to the type of malformation present and should always be considered on first presentations8,26,27 (Table 4).19,20
Imaging modalities
A variety of imaging modalities, most notably Doppler ultrasonography and MRI are used to evaluate the nature, extent and complexity of the malformation and to help plan appropriate treatment.
Radiographs
Radiographs play a limited part in classification of vascular lesions but are often the first test requested in a patient with pain or a lump. Although often non-diagnostic, the presence of phleboliths ought to alert the radiologist to the likely diagnosis of haemangiomas or VM5 (Figures 1 and 2). Vascular malformations with associated osseous involvement often encompass the metadiaphysis and diaphysis of long bones but rarely the epiphysis, especially if there is an intra-articular component.21–25
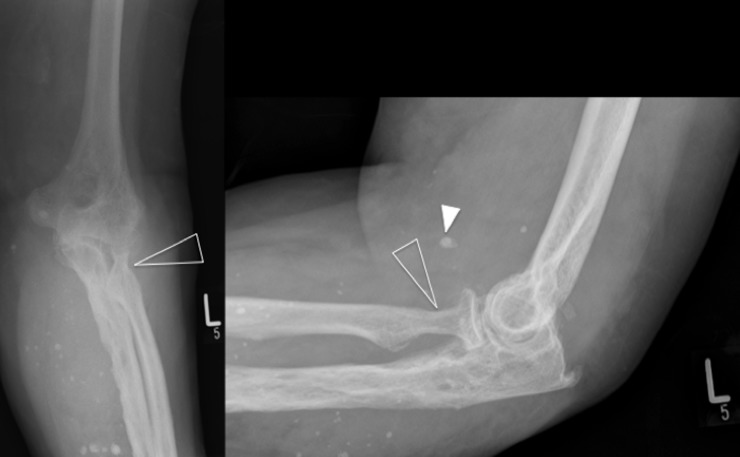
Figure 1.
Plain radiographs of the left elbow demonstrate multiple calcific densities consistent with vascular phleboliths (white arrowhead) suggestive of venous malformations. There is also degenerative osteoarthritic change, with joint space loss and subtle scalloping of the radial neck (open arrowheads). These arthropathic findings are highly suggestive of a destructive arthropathy secondary to recurrent haemarthrosis.
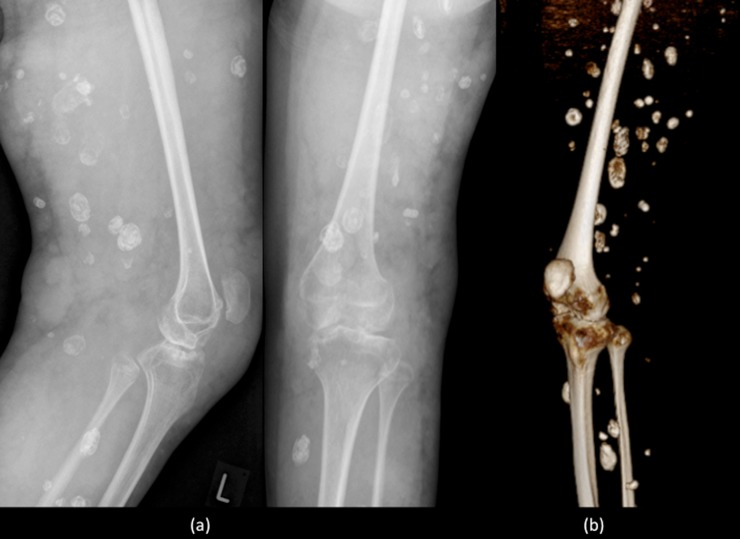
Figure 2.
Plain radiographs (a) and equivalent reconstructed three-dimensional CT image (b) of the left knee demonstrating multiple rounded calcific densities within the soft tissues, consistent with phleboliths, later confirmed to be within extensive slow-flow venous malformations.
Intraosseous and lytic changes can be characteristic of high-flow lesions. On plain radiographs, a distinctive thickened irregular trabecular pattern with well-defined lesions, containing lattice-like trabecular pattern is seen.23–25 The presence of any erosion, sclerotic or periosteal reaction, scalloping of the cortex or pathological fracture all help direct further investigation towards an intra-articular and osseous involvement5 (Figure 3).
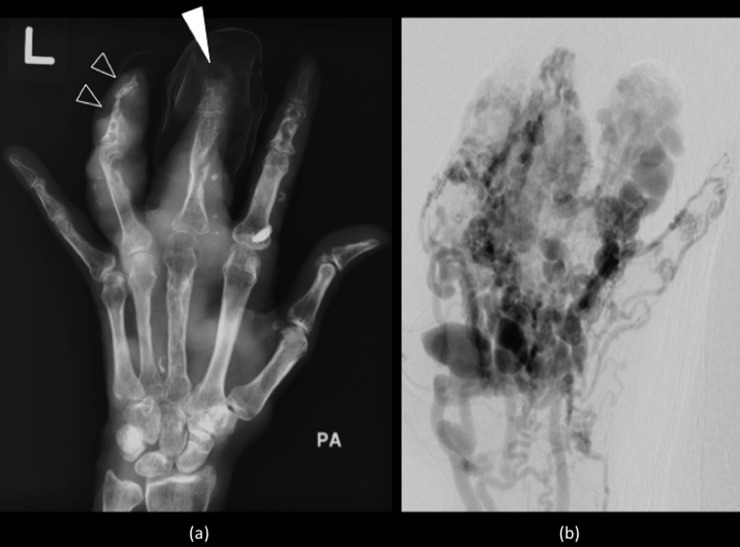
Figure 3.
Plain radiograph (a) of the left hand demonstrating lytic lesions, cortical scalloping (open arrowheads), remodelling and acro-osteolysis of the distal phalanges (white arrowhead) caused by multiple malformations, confirmed to be high-flow arteriovenous malformations on angiography (b).
Ultrasonography
Ultrasound has always been a key non-invasive tool used to examine superficial vascular lesions, especially in the initial outpatient setting. The diagnosis of a PVM is often first made with ultrasound, particularly when the patient presents with a lump. Anomalies can be assessed by colour Doppler ultrasound for their flow and velocity characteristics. They are also an essential part of real-time evaluation during ultrasound-guided intervention procedures and post-therapy monitoring.11,28
For deep intramuscular or periosteal malformations, a 5- to 7-MHz probe can be more useful, especially in obese patients. For very small or superficial lesions, for example, in the hand, fingers or feet, a higher frequency range (up to 17 MHz) provides better resolution (Figure 4).11,28,29
Figure 4.
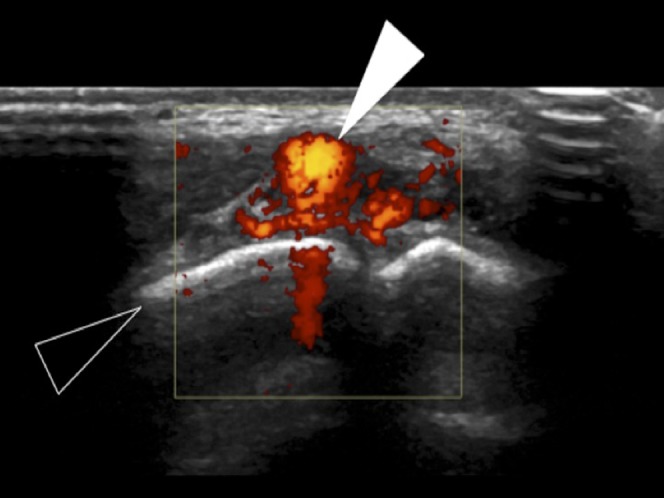
Ultrasound of the hand, demonstrating avid colour Doppler flow features of a high-velocity arteriovenous malformation (white arrowhead), seen to be dorsal to the proximal interphalangeal joint of the little finger (open arrowhead).
The modality is, however, limited by operator experience and assessment of deep lesions, especially adjacent to the bone, can be challenging with underestimation of the complexity and size of lesions.11,28
CT
Contrast-enhanced CT allows speedy evaluation of malformations and their complications such as acute haemorrhage and confirms the existence of calcification, thrombus or concomitant lesions.5 The high-resolution allows for extremely accurate measurement and mapping of feeding and draining compartments (Figure 5). However, because of the heavy burden of ionizing radiation and the minimal flow velocity information provided, its role is limited to the emergency presentations, equivocal MRI findings or adjunct to interventional or surgical planning.11,30
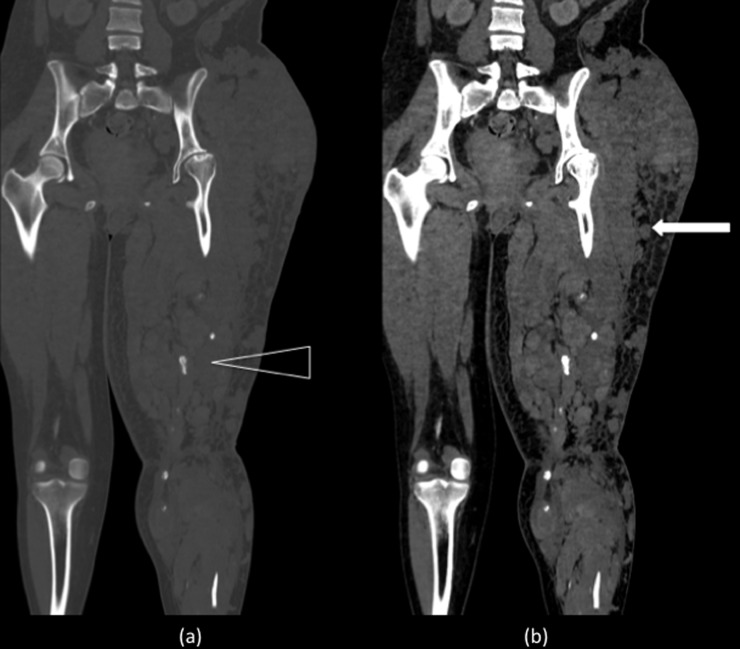
Figure 5.
Coronal reformat CT of the pelvis and lower limbs in bone (a) and soft tissue (b) windows showing multiple slow-flow malformations (white arrow) producing a large left thigh. The partially seen left femur is osteopenic and gracile. There are a number of phlebliths suggesting multiple venous channels (open arrowhead).
MRI
MRI has become by far the most valuable modality in the confirmation, characterization and differentiation of vascular malformation.12 The majority of protocols include the addition of T1 spin echo (SE) sequences and T2 fat-saturated and/or short tau inversion recovery (STIR) sequence. The SE or fast SE (FSE) T1 weighted (T1W) imaging is used for basic anatomic evaluation, and the high accuracy of heavily T2 weighted (T2W) imaging helps to demonstrate the extent of vascular malformations. The STIR images improve the definition of the lesion from the surrounding high-signal-intensity fat and neurovascular bundles. This is crucial for interventional planning7,25,26 (Table 5, Figure 6).
Table 5.
Typical MRI protocols applied in the characterization of vascular malformation, with diagnostic and imaging features7
| Sequence | Diagnostic role | Imaging |
|---|---|---|
| SE or fast T1W | Anatomical analysis | Relation of surrounding structures, including muscle, tendon and neurovascular bundles |
| Fat-suppressed SE T2W or STIR | Evaluation of lesion and flow dynamics | High-flow lesion produces signal voids Low-flow lesion maintains high signal |
| T2W GRE | Assess foci of calcification and haemosiderin deposition | Calcification produces low-signal foci Haemosiderin produces “blooming” artefact |
| T1W gadolinium-based contrast-enhanced GRE | Differentiation of time-lapsed enhancement patterns of arterial vs venous vessels | Early enhancement and “arterialization” of vessels in arteriovenous malformations vs delayed venous malformations |
GRE, gradient-recalled echo; SE, spin echo; STIR, short tau inversion recovery; T1W, T1 weighted; T2W, T2 weighted.
Figure 6.
Sagittal short tau inversion recovery (a) and T1 weighted (b) images of the left knee showing multiple low-flow venous malformations (white arrowheads) within the supra patellar fat pad and patella femoral joint.
Options for unenhanced MRI angiographic techniques such as time-of-flight (TOF) and phase contrast (PC) have also been available for sometime for vascular assessment. In clinical practice, two-dimensional TOF angiography is well established in characterization of peripheral lower limb vessels including tibial and pedal arteries.31–33 PC sequences can also be applied to quantify velocities within a vessel and calculate flow, helping to assess occlusive vascular disease and potential flow to any AVMs. Selective saturation pulses can also be used to isolate arterial supply to AVMs.31,33 These sequences can be used to assess the effects of embolization procedures.
However, these two techniques have their limitations and can suffer from long acquisition times for the comparable spatial resolution obtained. Relative slow flow and in-plane saturation can also be a challenge for TOF imaging and the selection of appropriate velocity encoding is a real challenge for PC MR angiography.31,32
Although gadolinium-enhanced techniques have overcome some of these limitations, in resent years, the application of non-enhanced techniques are becoming more desirable because of the potential risk of nephrogenic systemic fibrosis in patients with impaired renal function.31,32
Improvements in both hardware and software technology have allowed the development of alternative techniques, including the application of electrocardiographically gated three-dimensional (3D) partial Fourier FSE. This method relies on subtraction of systolic from diastolic acquisitions for angiography.32–35 Single-shot partial Fourier FSE sequences (i.e. single-shot FSE, half-Fourier rapid acquisition with relaxation enhancement or fast asymmetric FSE) have allowed shortened acquisition times to provide clinically feasible imaging of the peripheral arterial vascular system, especially the lower limb. Issues of blurring and relative insensitivity to slow arterial flow vessels present with the distal upper limbs, such as the hand, have been an issue. The application of variable flip angle FSE-based techniques, with sampling perfection with application of optimized contrasts using different flip angle evolutions (SPACE) has been described in an attempt to reduce the degree of this blurring. Spoiler gradients have also been used to enhance differences between arterial and venous signal.31,35
An alternative is the use of arterial spin labelling (ASL), with partial-Fourier FSE imaging. This technique of subtracting a tag-on and a tag-off scan allows background signal to be removed and a significant improvement on the visualization of small vessels, especially the fingers. ASL provides information on function and blood flow and the partial-Fourier FSE produces information on morphology.31
Gadolinium-based contrast-enhanced angiography, usually with 3D T1W fast gradient echo (GRE), however, allows for perfusion assessment with dynamic time-resolved MR angiography giving high resolution and enables differentiation between arterial inflow and venous drainage or shunting, while reducing motion artefacts.5,7
Images are obtained before contrast material administration for post-subtraction of contrast-enhanced images and 3D reformation, followed by post-contrast single arterial and several venous phase sequences to evaluate perfusion of the lesion.5,7,36,37
Angiography
Angiography is a critical part of the anatomical assessment of vascular malformations, especially in complex high-flow AVMs. The use of direct puncture of the nidus can also be used to evaluate the volume and flow pattern of a lesion.5 The invasive nature of the investigation, pain and radiation exposure must be balanced with the key information needed for management. Its use is usually reserved as a part of pre-operative assessment and as an immediate precursor during an interventional procedure5 (Figure 7).
Figure 7.
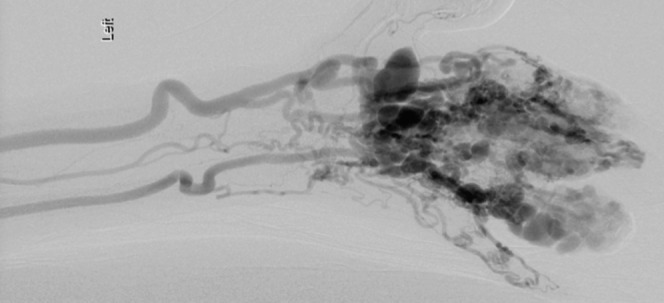
Digital subtracted angiogram of the left hand showing contrast-filled multiple dilated tortious arteriovenous malformations within in the digits, supplied by dilated radial and ulnar arteries.
IMAGING FINDINGS
Low-flow malformations—ultrasound
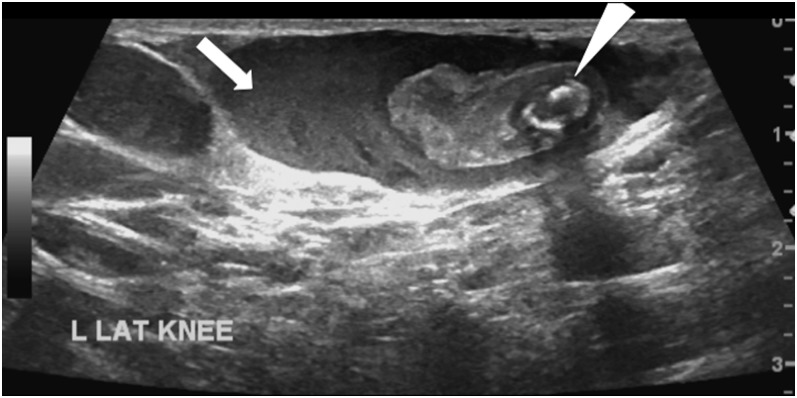
Ultrasound is valuable to differentiate vascular and lymphatic malformations that are both classified as low-flow lesions. VM demonstrate heterogeneous echo texture that are more commonly hypoechoic (80%) rather than hyper- or isoechoic.12,29 Occasionally, more anechoic tubular structures with echogenic phleboliths and acoustical shadowing allow a pathognomonic diagnosis12,15 (Figure 8).
Figure 8.
Ultrasound image showing isoechoic and echo poor tubular structures, the largest (arrow) containing an echogenic focus representing likely thrombus with a central smaller phlebolith, with characteristic post-acoustic shadowing, pathognomonic of a venous peripheral vascular malformation (arrowhead). L Lat Knee, left lateral knee.
Lymphatic malformations are typically cystic lesions containing fibrous septae, and ultrasound is highly sensitive at demonstrating this feature9 (Table 6). On colour Doppler imaging, no flow is detected in lymphatic lesions, whereas low flow can be detected in 85% of VM. The majority of venous lesions will demonstrate monophasic or biphasic flow on Doppler, and only a significant minority demonstrate no detectable flow.12,29
Table 6.
Greyscale and Doppler ultrasound imaging features of common vascular malformations8
| Vascular anomalies | Greyscale | Colour Doppler |
|---|---|---|
| Haemangioma | • Well-defined • Solid • Homogenous • Variable echogenicity |
• Hypervascular • Arterial and venous waveforms • High-vessel density (>5 vessels cm−2) • High Doppler shift (>2 kHz) |
| Low-flow vascular malformations | ||
| Venous | • Solid echogenic mass • Phleboliths • Often multispacial and compressible |
• Monophasic (venous) or no flow pattern |
| Lymphatic | • Variable multicystic • Multispacial masses, with or without fluid and/or debris levels |
• Multispacial, macrocystic mass with no flow except in septa |
| High-flow vascular malformations | ||
| Arteriovenous malformations and fistulas | • Cluster of vessels with no intervening well-defined mass | • Arterial and venous signals from vessels in the lesions with arterialization of venous structures |
Lesions with greater proportion of vascular or lymphatic spaces are more likely to benefit from interventional sclerotherapy than do those with more cellular content, where the outcome is less predictable. Ultrasound can help define this composition and aid in management.9
Low-flow malformations—MRI
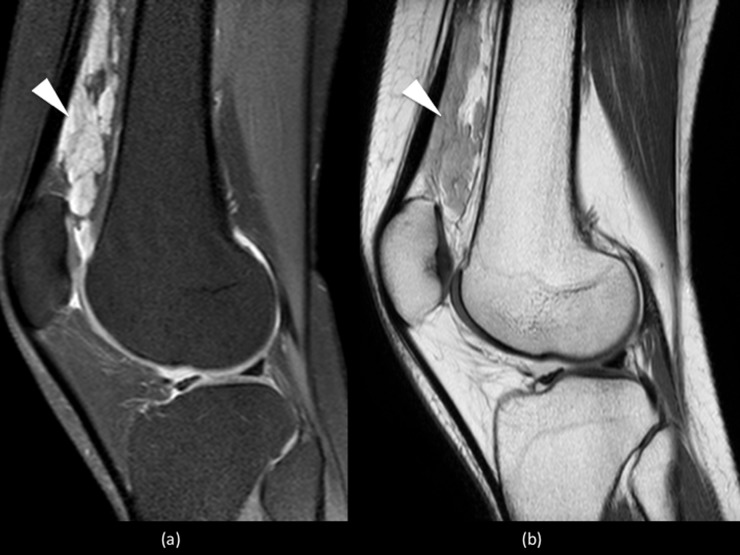
VM are usually serpiginous septated lesions with intermediate-to-decreased signal intensity on T1W images and increased signal intensity on T2 and STIR images, owing to their high water content7,16 (Figure 9).
Figure 9.
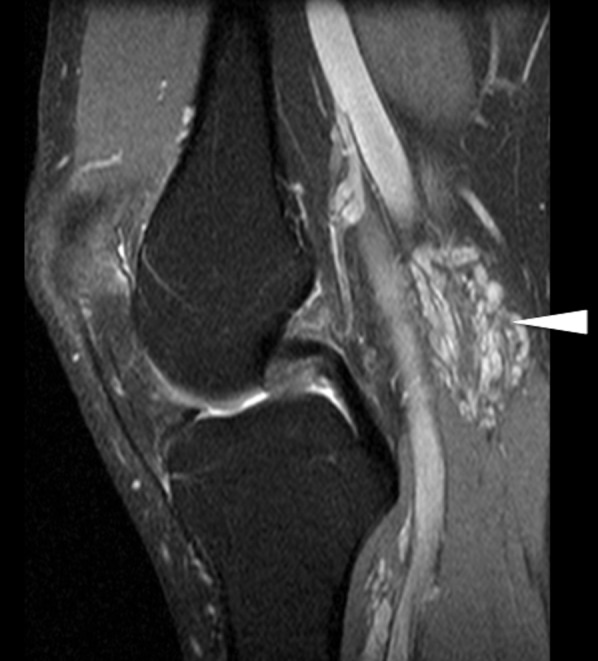
Sagittal proton density spectral pre-saturation with inversion recovery image of the knee showing a small cluster of high-signal slow-flow malformations, with the absence of flow voids, posterior to the popliteal vessels (white arrowhead). There is no intra-articular extension.
They tend to have more uniform signal intensity than do lymphatic malformations, which can demonstrate layering within more cystic components. They can also be associated with surrounding oedema or fibro fatty stroma. Rarely a confirmatory biopsy may be needed because of unusual clinical or imaging features.7,16
The T1W imaging allows exact definition of anatomy and also information on any areas of acute haemorrhage or high protein content, which can cause internal fluid–fluid levels. Any areas of thrombosis or haemorrhage can also produce heterogeneous signal intensity on T1W images.7 Fat-suppressed T2W and STIR images help to further define the full extent of the malformations. Refer to Figure 10.
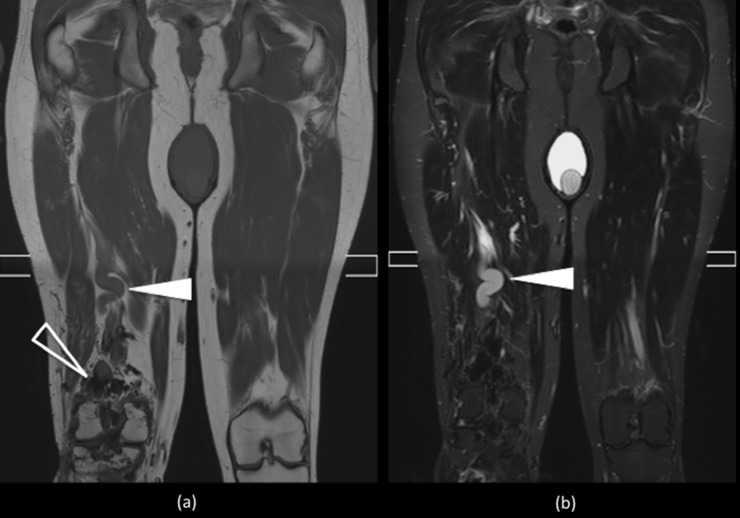
Figure 10.
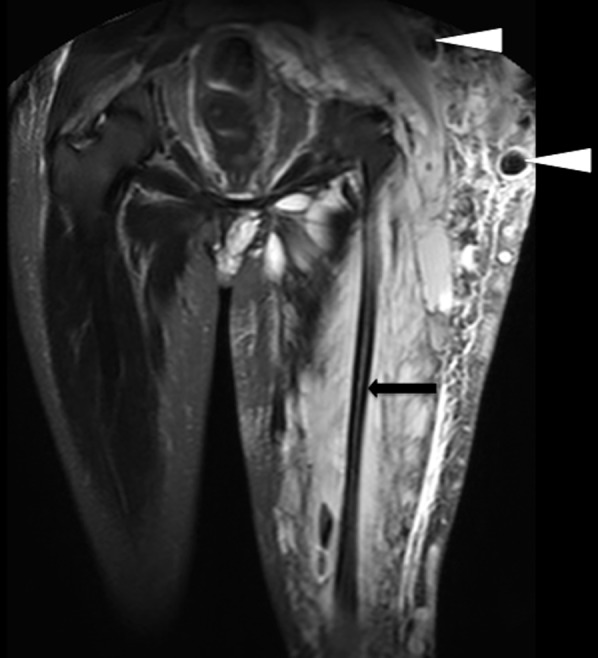
Coronal short tau inversion recovery image of the pelvis and upper thighs shows high-intensity heterogeneous masses involving the subcutaneous tissue and muscle surrounding a gracile left hemi pelvis and femur (black arrow). The lack of signal void indicates slow-flow venous malformations. There are numerous phleboliths lying within the vascular channels seen as signal voids in the lesions (arrowheads).
The presence of phleboliths, seen as small low-signal-intensity foci in all pulse sequences, can provide further diagnosis clues. Low-flow malformation will show the absence of flow voids on SE images, but occasionally, low-signal-intensity striations, septa, thrombosed vessels or phleboliths can produce flow voids7 (Figure 10).
Contrast-enhanced and GRE images are used to problem solve in certain cases. High-flow lesions will show enhancement and high signal intensity on GRE images, leaving phleboliths and calcifications to appear as signal voids with all pulse sequences. VM will show gradual filling with contrast material and may demonstrate characteristic nodular enhancement of tortuous vessels with delayed phase images and diffuse enhancement of the slow flowing venous channels.37 The contrast filling time into these VMs is much longer than into high-flow AVMs, reaching up to 90 s. This combined with the fact that VMs have no significant arterial or early venous enhancement, enlarged feeding vessels or arteriovenous shunting, helps to define their nature.38
Delayed contrast-enhanced sequences are also helpful in demonstrating any connection between the malformations to deeper venous vessels. This is an important detail to confirm prior to interventional treatment, as these lesions have been linked to a greater risk of deep venous thrombosis. A total imaging time of 45 min for a standard examination protocol has been described.7,38
High-flow malformation—ultrasound
High-flow lesions such as AVMs can present as mixed echogenicity lesions with feeding vessels large enough to be seen on greyscale. Colour Doppler and spectral analysis can be used to confirm high-systolic flow. Arterialization of veins will also produce pulsatile venous flow, confirming arteriovenous shunting12,29,39 (Table 6).
High-flow malformation—MRI
MRI/angiography is a key aspect of defining the morphology of these lesions. Typically, high-flow serpentine feeding arteries and draining veins will appear as large flow voids on SE images or high-signal-intensity foci on GRE images. There is usually no mass effect but fatty hypertrophy and muscular atrophy can be seen7,9,39–41 (Figures 11 and 12).
Figure 11.
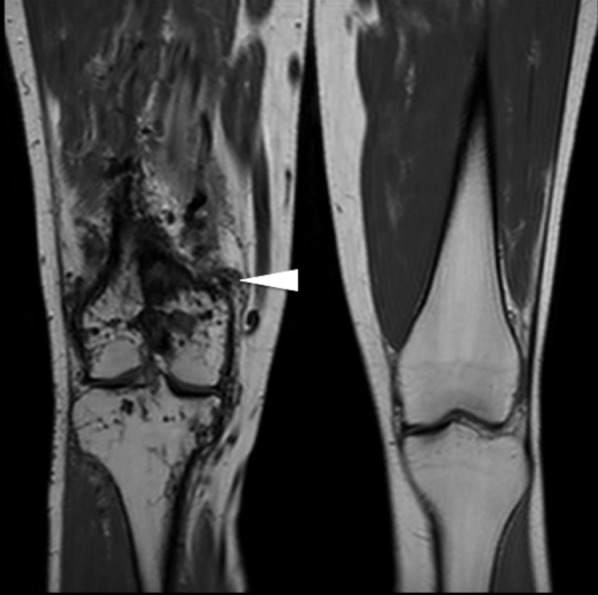
Coronal T1 weighted images of the knees showing multiple flow voids surrounding the right knee (white arrowhead), consistent with complex high-flow arterial malformations.
Figure 12.
Coronal T1 weighted (a) and short tau inversion recovery (b) images show a large cluster of small high-flow vessels seen as signal voids (open arrowhead) within the left popliteal fossa (a), communicating superiorly with a large calibre slow-flow superficial femoral vein (white arrowheads).
On T1W images, any areas of haemorrhage or thrombosis will produce high-signal intensity. By contrast, intraosseous extension will produce decreased marrow signal intensity and should be used as a trigger to evaluate any intra-articular component further.7
T1 post-gadolinium enhancement is used to evaluate the feeding arteries and draining veins with time-resolved dynamic 3D MR angiography. Early venous filling is typically seen in AVMs and a contrast rise within 5–10 s should be expected7 (Table 7).
Table 7.
MRI imaging features of the common vascular malformations7
| Haemangioma |
| • Proliferating phase: well-defined lobulated mass, low SI on T1WI, high SI on T2WI, flow voids on SE images. No perilesion oedema and can see early homogeneous enhancement |
| • Involuting phase: fat replacement (high SI on T1WI) and decreased enhancement |
| Low-flow vascular malformations |
| Venous |
| • Septated lobulated mass without mass effect |
| • Phleboliths (low SI), fluid–fluid levels, low SI on T1WI, high SI on T2WI |
| • No flow voids on SE images |
| • Infiltrates tissue planes and possible surrounding oedema |
| • No arterial or early venous enhancement |
| • Slow gradual enhancement and diffuse enhancement on delayed images with some late enhancement (≥5 s) |
| • Normal afferent arteries |
| • Contrast pooling in dilated stagnant venous spaces in later venous phase imaging |
| Lymphatic |
| • Septated lobulated mass with some fluid–fluid levels |
| • Can see infiltration of tissue planes |
| • Low SI on T1WI, high SI on T2WI |
| • No flow voids on SE images |
| • Rim and septal enhancement |
| High-flow vascular malformations |
| Arteriovenous malformations and fistulas |
| • No well-defined mass |
| • Infiltration of tissue planes |
| • Flow voids on SE images |
| • Enlarged feeding arteries and draining veins |
| • Hypertrophied tortuous afferent arteries and efferent veins |
| • Direct arteriovenous communications via vascular nidus |
| • Early enhancement of arteriovenous lesion (≤5 s) |
| • Early enhancement of enlarged feeding arteries and nidus with shunting to draining veins |
SE, spin echo; SI, signal intensity; T1WI, T1 weighted imaging; T2WI, T2 weighted imaging.
Intra-articular vessel malformations
As previously described, SE or FSE T1W sequences can be used for basic anatomical assessment supplemented with FSE T2W with fat saturation or STIR sequences to accurately identify extension of the lesions.16 These T2 sequences can also identify any intra-articular extension but can also be used to recognize areas of low-signal intensity (“signal drop-out”) consistent with haemosiderin deposition from repeated haemarthroses. STIR sequences also help to demonstrate degenerative changes, joint space narrowing, cartilage thinning, subchondral cyst formation and extensive bone marrow oedema1,42,43 (Figure 13).
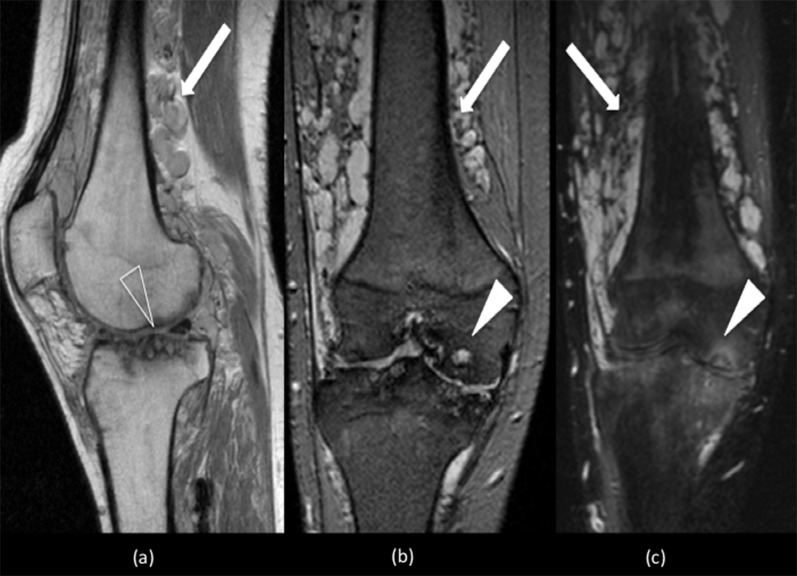
Figure 13.
Sagittal proton density (PD)-weighted (a) and coronal T2 weighted (b) and short tau inversion recovery (STIR) (c) sequences show multiple high-signal serpiginous structures (white arrows) within the lower thigh and intra-articular extension consistent with slow-flow peripheral venous malformations. The PD and STIR sequence demonstrate marked degenerative change within both tibiofemoral compartments. There is significant joint space narrowing with cartilage thinning, subchondral cysts (open arrowhead) (a) and bone marrow oedema (white arrowheads) (b and c).
Some also advocate the use of GRE T2* weighted images to establish the presence of calcification or haemosiderin, as well as high-flow vessels. On GRE images, the absence of signal in the blood vessel suggests a low-flow malformation, whereas high-flow vessels have high-signal intensity.40,42
Contrast-enhanced angiography with 3D T1W fast gradient-echo (GRE) allows perfusion assessment with dynamic time-resolved MR angiography enabling differentiation between arterial inflow and venous drainage or shunting. 3D T1 fast field echo with water excitation for cartilage scan and proton density spectral pre-saturation with inversion recovery sequences can be used to confirm intra-articular and bone extension of low-flow malformations16,19,44 (Figure 14).
Figure 14.
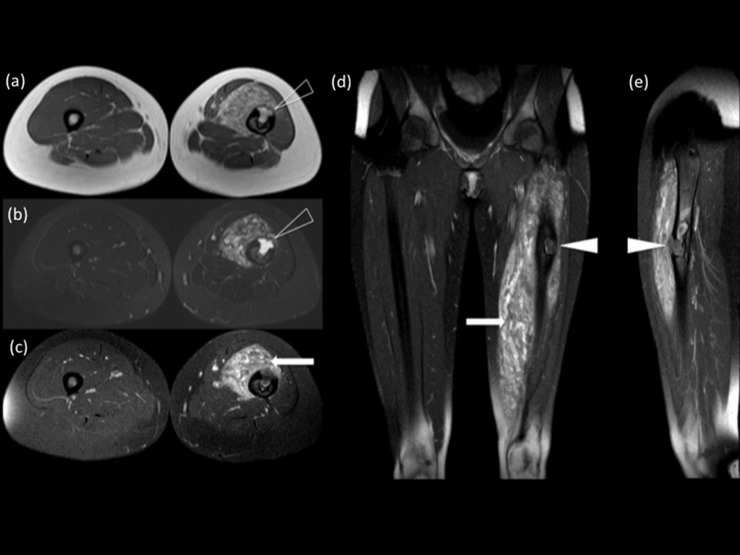
Axial T1 weighted (T1W) (a), short tau inversion recovery (b) and T1W gadolinium-enhanced (c) images of the thighs showing a large well-demarcated intra-osseous extending arteriovenous malformation (AVM) (open arrowheads) (a, b) with associated surrounding enhancing anterior intramuscular lesions (white arrow), within the right thigh (c). Coronal (d) and sagittal (e) T1W gadolinium-enhanced images of the same patient, confirmed the full extent of the AVM (white arrowheads) within the diaphysis of the femur and the enhancing intramuscular lesions (white arrow) (d).
IMAGING FINDINGS—KEY DIFFERENTIALS OF INTRA-ARTICULAR VESSEL MALFORMATIONS
Haemophilic arthropathy
Haemophilic arthropathy is more common than are vascular malformations. Pathognomonic features include overgrowth of the epi/metaphyses owing to hyperaemia and a gracile diaphyses. Typically, cartilage destruction, erosions and subchondral cysts will also be present secondary to an inflammatory synovitis. The most important differentiating features include characteristic low-signal synovial proliferation on T1W and T2W sequences, synovium enhancement with contrast and a “blooming” artefact on gradient-echo sequences.45,46
Pigmented villonodular synovitis
Pigmented villonodular synovitis is a monoarticular proliferation of haemorrhagic synovium. It may cause diffuse villonodular proliferation of the entire synovium or can produce a more focal nodular mass. It most often occurs in young to middle-aged adults. The knee is the most frequently involved joint; followed by the hip, ankle and shoulder.44,46 Local recurrence following surgical or arthroscopic synovectomy occurs in almost 50% of cases. Polyarticular involvement is extremely rare.22,45
The involved joints usually have preserved spaces and bone density, until very late in the disease. On MRI an extensive diffuse or single nodular mass with lobulated margin will be noted. These lesions have a tendency to bleed, causing haemosiderin deposition and a characteristic low-signal intensity with all pulse sequences. Classically, synovial thickening with nodular frond-like masses arising within the joint space will be seen.22,45 Areas of inflamed synovium or joint effusions will produce high-signal intensity on T2W images.22,46 The presence of repeat haemosiderin deposition again produces characteristic low-signal intensity with all pulse sequences, which can have serpiginous and nodular characteristics mimicking vascular malformations.
TREATMENT
Image-guided percutaneous and interventional treatments of vascular malformations are now widely accepted as first-line therapy when combined with a multidisciplinary approach to treatment. The results of treatment vary depending on the type of vascular malformation being treated, but, with the exception of capillary-based lesions, all types of vascular malformations can be considered.5,9,12,47
Treatment is very much based on the impact of the lesions on the patients' quality of life, weighed against the risk of complications. In most cases, conservative treatment is recommended, but when a patient suffers clinical complications, treatment needs to be considered. Lee13 outlined absolute and relative indications for therapy.12
Schobinger et al48 also adapted a clinical classification system to provide helpful indicators to determine the timing of intervention.12,13
Kawanabe et al49 developed a practical system, centred on the ISSVA classification, in which the treatment procedure can be selected according to the characteristic flow within the lesion, placing the emphasis not only on the correct diagnosis of the flow dynamics5 (Table 8).
Table 8.
Classification of vascular malformations according to flow dynamics and treatment5
| Vascular anomalies | Treatment |
|---|---|
| Capillary | None |
| Haemangioma | None (propranolol) |
| Slow-flow venous and lymphatic malformations | Percutaneous sclerotherapy |
| Fast flow AVM and AVF | Transarterial embolization |
AVF, arteriovenous fistula; AVM, arteriovenous malformations.
A variety of percutaneous sclerotic and transarterial embolizing agents have been advocated in numerous combinations, depending on location, severity and the extent of the malformations. Absolute ethanol, bleomycin, 3% sodium tetradecyl sulfate (STS), polidocanol, ethanolamine oleate, n-butyl cyanoacrylate, polyvinyl alcohol foam and various types of coils and polymer microspheres have all been used.5,9,12
Of the agents described, ethanol sclerotherapy has been the most successfully applied, in suitable low-flow lesions, as single therapy or as part of pre-operative surgery. It has been shown to be an effective means of treating these malformations with a 64–96% response rate, defined as improvement in symptoms or reduction in the size of the lesion.5,50
The aim of treatment of high-flow lesions is the complete occlusion of the AVM nidus or fistulous connection. Sclerotherapy has not been shown to be as effective in treating these lesions, in part owing to the rapid outflow of the infused agents. There is no unified agreement on the ideal treatment of these more complex malformations and a case-by-case basis multidisciplinary approach has been advocated.5,9,12,51
Ethanol
Ethanol is one of the main agents used in the treatment of surgically inaccessible lesions. The anatomic or more importantly the haemodynamic status of the individual vascular malformation largely determines the method of injection, which can be via direct puncture, transarterial or venous routes. It causes vascular occlusion by endothelial damage, denaturation of blood proteins and thrombus formation5,11,51–53 (Figure 15).
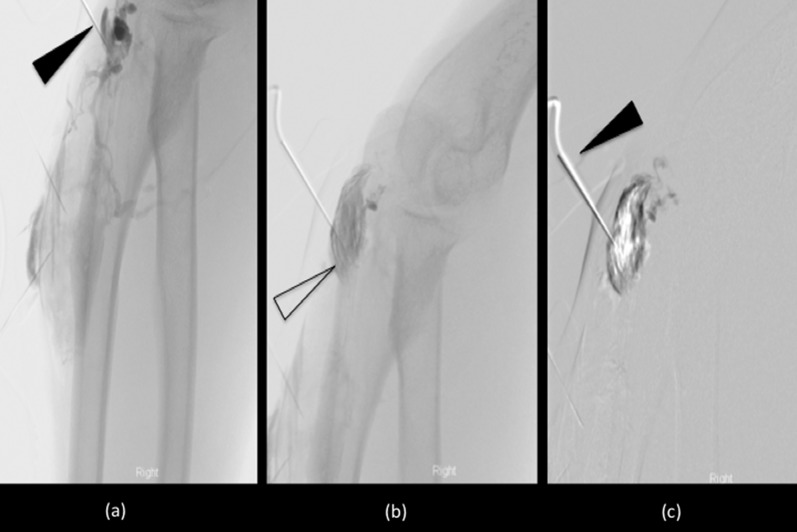
Figure 15.
Digital subtraction angiogram images of a complex arteriovenous malformation surrounding the right elbow. The initial contrast run (a) confirmed the correct position of the needle (arrowhead) within the malformation, overlying the olecranon. A delayed run (b) also established a larger component of the malformation (open arrowhead). Percutaneous injection of sclerosant (c) (arrowhead), delivered as foam and air mixture, shows gradual vessel “ghosting” of the malformation.
A variety of complications have been reported (7.5–27.9%) ranging from minor skin blisters and transient pain to permanent nerve damage, necrosis, muscle contracture, deep vein thrombosis, pulmonary embolus and, in rare but severe cases, cardiopulmonary collapse.5,54
Bleomycin
Bleomycin is an antitumour cytotoxic antibiotic that has sclerosing properties, initially used in the early treatment of macrocystic low-flow lymphatic malformations.55 Because of concerns regarding associated complications of pulmonary fibrosis its use was limited. There is now increasing evidence that reduced dosing separated by 2 weeks between sessions, with no more than 5 mg kg−1 lifetime dose, can limit this complication.12
Sodium tetradecyl sulfate
STS is an ionic surfactant that has a soapy consistency, which causes vascular closure by intimal necrosis. The toxic impact is not permanent and because of its better safety profile, it has been advocated as an alternative to ethanol. It is strategically injected under image guidance into the lesions in association with limb tourniquet to reduce inadvertent involvement of adjacent tissue. Often more than one treatment session is required to obtain the desired level of control.11,56
Polidocanol
Polidocanol consists of 95% hydroxypolyethoxydodecane and has been used for sclerotherapy of lower limb varices. It has detergent properties, which dehydrates endothelial cells, leading to vascular injury and occlusion. Because of its inherent anaesthetic effect, it can also make general anaesthesia less necessary.5,57
Ethanolamine oleate
Ethanolamine oleate is used as a sclerosing largely because it has excellent thrombosing properties. It has an added advantage of less effect on the deep vascular layers with no penetrative effect and is less likely to be associated with neurological side effects despite the proximity of the nervous system to the vascular system5,58 (Table 9).
Table 9.
Advantages and disadvantages of common sclerosants5
| Sclerosant | Advantages | Disadvantages |
|---|---|---|
| Absolute ethanol | • Strong endothelial damage • High response rate • Less expensive • Easy to obtain |
• Painful during procedure • High complication rate • Penetrative effect on deep vascular layer |
| Ethanolamine oleate | • Excellent thrombosing effect • Chemical damage to vessel wall • Less toxic effect than absolute ethanol |
• May induce acute renal failure owing to haemolytic effect • Less endothelial damage than absolute ethanol |
| Polidocanol | • Over hydration of endothelial cells • Nearly painless procedure |
• May induce reversible cardiac arrest |
Ethylene vinyl alcohol copolymer
Ethylene vinyl alcohol copolymer (Onyx; Micro Therapeutics, Irvine, CA) is a non-adhesive biocompatible polymer, which causes occlusion of the vessel by precipitation. It has been primarily utilized in embolization of intracranial high-flow malformations owing to the ease of control during administration and lower rates of distant complications, particularly in the case of AVMs.9,11 It has, however, been used for the treatment of peripheral high low-flow malformations, via an intravascular approach59 (Figure 16–18).
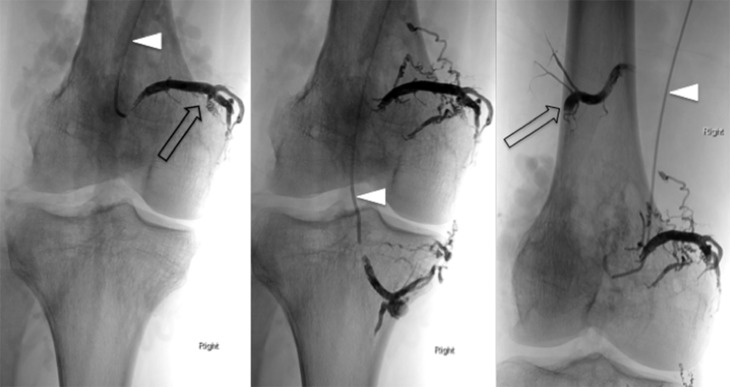
Figure 16.
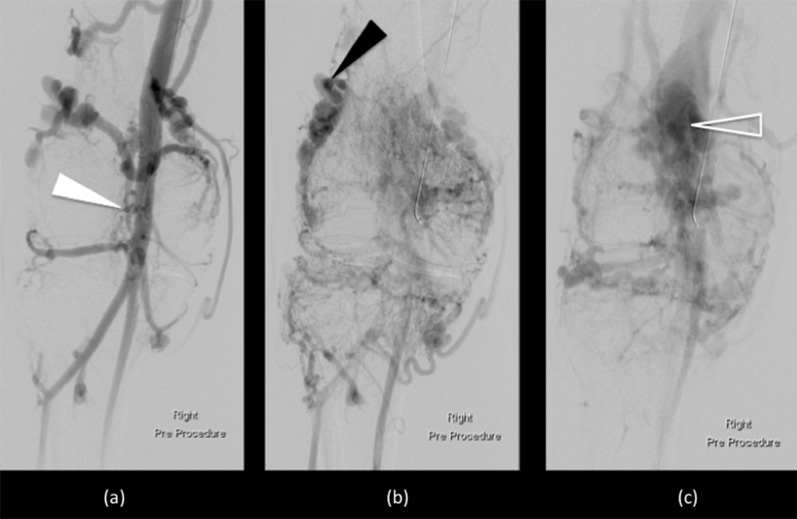
Digital subtraction angiograms of high-flow arteriovenous malformations surrounding the right knee. The early contrast run (a) demonstrates filling of a hyperdynamic and enlarged superficial femoral artery (white arrowhead). Intraosseous and intra-articular components supplied by high-flow perigeniculate branches were noted (black arrowhead) (b). On the delayed phase images (c), rapid shunting was seen into the efferent dilated and partially aneurysmal veins (open arrowhead).
Figure 18.
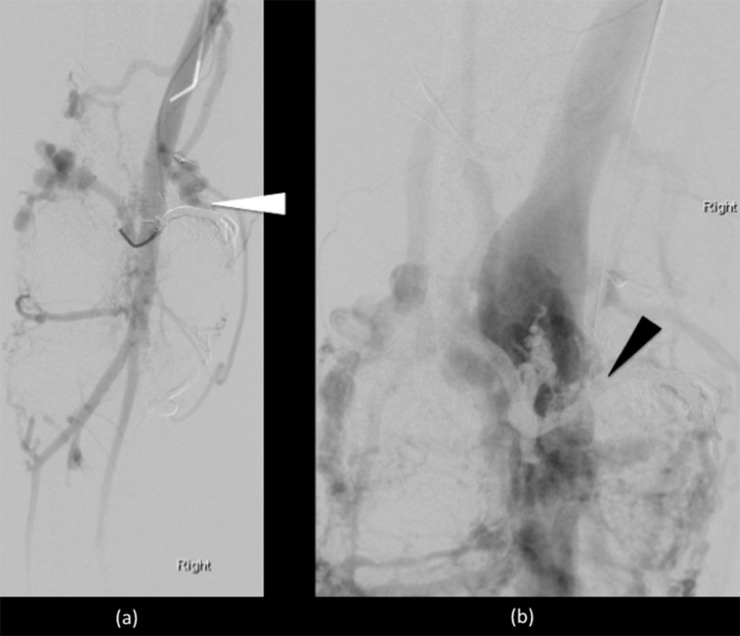
Post-embolization confirmatory angiogram showed markedly reduced filling and shunting with expected “ghosting” artefact within the obliterated vessels in the arterial phase (white arrowhead) (a) and delayed venous phase images (black arrowhead) (b).
Figure 17.
Endovascular embolization performed using liquid embolic agent (Onyx). Two medial and two lateral vessels feeding a high-flow intra-articular component were targeted with selective catherization (white arrowheads). At the end of the procedure, there was markedly reduced filling and reduced shunting with the embolic agent producing a black filling of the occluded vessels (open arrows).
Devices and embolic material
Transarterial deployment of coils or small particles, as method of embolization, is more likely to be applied for high-flowing vessels and for treatment of complex vascular malformations in controlling of acute bleeding. Coil embolization is especially effective at clot induction of fast-flowing vessels but can be dislodged from the deployed position.
Balloon occlusion devises and coils have also been used as means of altering flow rates in intermediate-flow lesions. By the application of these flow-control procedures, the time for reaction between the inner surface of the nidus and the sclerosant agent is extended and the dilution effect of high flow minimized. In this way, the well-tried injection methods used for slow-flow vascular can be applied to increase the success rate. Flow can also be reduced by means of n-butyl cyanoacrylate embolotherapy, but such treatment is usually palliative, because avascular malformation can include multiple AVFs.
Post-treatment imaging and follow-up
After the immediate post-procedure clinical monitoring, some authors have recommended imaging approximately 3 days after.5 Colour Doppler ultrasound, post-contrast CT can be used, but again, MRI is an excellent tool for assessing intermediate results and long-term management.5,60
MR provides extra advantages in evaluating any change in size and flow, with specific attention made to the nidus, which should decrease in volume and signal intensity on T2W sequences. Follow-up for 2 years has been advocated to monitor for recurrence and complications, ideally within the context of a multidisciplinary vascular/plastic team.5
Immediate post-treatment VM will show heterogeneous signal intensity on both T1W and T2W images and high-signal intensity in the treated and intermuscular septa areas on T2W and STIR images.7,15 MR angiography is important to show the absence of enhancement in the central portion of the lesion. Intense peripheral hyper enhancement produced by the surrounded reactive hyperaemia can be expected.7,15 This enhancement will reduce over the following months and be replaced by darker T1W and STIR images without gadolinium enhancement, owing to scar tissue formation. The lesion should then shrink in size.7,15
A delay of up to several months may be necessary to allow time for the transient inflammatory response to resolve and full a response to treatment to be appreciated.7,15 Gadolinium-enhanced imaging is useful in this context, to demonstrate any residual perfusion of the post-treatment malformation, to target any further management.7,15
With arterial malformations, a reduction or absence of shunting is used as an indication that thrombosis has occurred after embolization. This is seen as a reduced or absent early opacification of the venous system on MR angiography. Early post-treatment imaging is recommended to highlight residual lesions, but ferromagnetic coils can cause susceptibility artefacts, which can obscure areas of potential residual vascular malformations. This should be taken into account in further follow-up imaging.5
CONCLUSION
Peripheral vascular anomalies are rare but an important spectrum of abnormalities. The symptomatology and imaging appearances of these lesions can be complex, especially with rarely described intra-articular and osseous forms, which can complicate diagnosis further.
Developments in classification, imaging and interventional techniques have helped to improve management and outcome in these potentially life- or limb-threatening lesions. Image-guided percutaneous and interventional treatments are now widely accepted as first-line therapy.
REFERENCES
- 1.Mulliken JB, Glowacki J. Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plast Reconstr Surg 1982; 69: 412–22. [DOI] [PubMed] [Google Scholar]
- 2.Mulliken JB, Glowacki J. Classification of pediatric vascular lesions. Plast Reconstr Surg 1982; 70: 120–1. [PubMed] [Google Scholar]
- 3.Legiehn GM, Heran MK. Venous malformations: classification, development, diagnosis, and interventional radiologic management. Radiol Clin North Am 2008; 46: 545–97. doi: 10.1016/j.rcl.2008.02.008 [DOI] [PubMed] [Google Scholar]
- 4.Legiehn GM, Heran MK. Classification, diagnosis, and interventional radiologic management of vascular malformations. Orthop Clin North Am 2006; 37: 435–74. [DOI] [PubMed] [Google Scholar]
- 5.Hyodoh H, Hori M, Akiba H, Tamakawa M, Hyodoh K, Hareyama M. Peripheral vascular malformations imaging, treatment approaches, and therapeutic issues. Radiographics 2005; 25(Suppl 1): S159–71. [DOI] [PubMed] [Google Scholar]
- 6.Jackson IT, Carreño R, Potparic Z, Hussain K. Hemangiomas, vascular malformations, and lymphovenous malformations: classification and methods of treatment. Plast Reconstr Surg 1993; 91: 1216–30. [DOI] [PubMed] [Google Scholar]
- 7.Flors L, Leiva-Salinas C, Maged IM, Norton PT, Matsumoto AH, Angle JF, et al. MR imaging of soft-tissue vascular malformations: diagnosis, classification, and therapy follow-up. Radiographics 2011; 31: 1321–40. doi: 10.1148/rg.315105213 [DOI] [PubMed] [Google Scholar]
- 8.Lowe LH, Marchant TC, Rivard DC, Scherbel AJ. Vascular malformations: classification and terminology the radiologist needs to know. Semin Roentgenol 2012; 47: 106–17. doi: 10.1053/j.ro.2011.11.002 [DOI] [PubMed] [Google Scholar]
- 9.McCafferty IJ, Jones RG. Imaging and management of vascular malformations. Clin Radiol 2011; 66: 1208–18. doi: 10.1016/j.crad.2011.06.014 [DOI] [PubMed] [Google Scholar]
- 10.Houdart E, Gobin YP, Casasco A, Aymard A, Herbreteau D, Merland JJ. A proposed angiographic classification of intracranial arteriovenous fistulae and malformations. Neuroradiology 1993; 35: 381–5. [DOI] [PubMed] [Google Scholar]
- 11.Cho SK, Do YS, Shin SW, Kim DI, Kim YW, Park KB, et al. Arteriovenous malformations of the body and extremities: analysis of therapeutic outcomes and approaches according to a modified angiographic classification. J Endovasc Ther 2006; 13: 527–38. [DOI] [PubMed] [Google Scholar]
- 12.Legiehn GM, Heran MK. A step-by-step practical approach to imaging diagnosis and interventional radiologic therapy in vascular malformations. Semin Intervent Radiol 2010; 27: 209–31. doi: 10.1055/s-0030-1253521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee BB. New approaches to the treatment of congenital vascular malformations (CVMs)—a single centre experience. Eur J Vasc Endovasc Surg 2005; 30: 184–97. [DOI] [PubMed] [Google Scholar]
- 14.Moukaddam H, Pollak J, Haims AH. MRI characteristics and classifications of peripheral vascular malformations and tumours Skeletal Radiol 2009; 38: 535–47. doi: 10.1007/s00256-008-0609-2 [DOI] [PubMed] [Google Scholar]
- 15.Dubois J, Soulez G, Oliva VL, Berthiaume MJ, Lapierre C, Therasse E. Soft-tissue venous malformations in adult patients: imaging and therapeutic issues. Radiographics 2001; 21: 1519–31. [DOI] [PubMed] [Google Scholar]
- 16.Donnelly LF, Adams DM, Bisset GS, 3rd. Vascular malformations and hemangiomas: a practical approach in a multidisciplinary clinic. AJR Am J Roentgenol 2000; 174: 597–608. [DOI] [PubMed] [Google Scholar]
- 17.Dobson MJ, Hartley RW, Ashleigh R, Watson Y, Hawnaur JM. MR angiography and MR imaging of symptomatic vascular malformations. Clin Radiol 1997; 52: 595–602. [DOI] [PubMed] [Google Scholar]
- 18.Ernemann U, Kramer U, Miller S, Bisdas S, Rebmann H, Breuninger H, et al. Current concepts in the classification, diagnosis and treatment of vascular anomalies. Eur J Radiol 2010; 75: 2–11. doi: 10.1016/j.ejrad.2010.04.009 [DOI] [PubMed] [Google Scholar]
- 19.E-medicine home page. Schwartz RA. Epidermal nevus syndrome. [Cited 28 September 2014.] Available from: http://emedicine.medscape.com/article/1117506-overview
- 20.Carvalho S, Barbosa V, Santosa N, Machadoa E. Blue rubber-bleb nevus syndrome: report of a familial case with a dural arteriovenous fistula. AJNR Am J Neuroradiol 2003; 24: 1916–18. [PMC free article] [PubMed] [Google Scholar]
- 21.Chhaya N, Farrant J, Anwar I, Marmery H, Platts A, Holloway B. Intra-articular vascular malformations mimicking haemophilic arthropathy. European Society Musculoskeletal Radiology 2013 Annual Scientific Meeting:13–15 June 2013; Marbella, Spain: 2013. doi: 10.1594/essr2013/P-0115. [Google Scholar]
- 22.Dalmonte P, Granata C, Fulcheri E, Vercellino N, Gregorio S, Magnano G. Intra-articular venous malformations of the knee. J Pediatr Orthop 2012; 32: 394–8. doi: 10.1097/BPO.0b013e31824b29ef [DOI] [PubMed] [Google Scholar]
- 23.Sheldon PJ, Forrester DM, Learch TJ. Imaging of intra-articular masses. Radiographics 2005; 25: 105–19. [DOI] [PubMed] [Google Scholar]
- 24.Boyd JB, Mulliken JB, Kaban LB, Upton J, 3rd, Murray JE. Skeletal changes associated with vascular malformations. Plast Reconstr Surg 1984; 74: 789–97. [DOI] [PubMed] [Google Scholar]
- 25.Breugem CC, Maas M, Breugem SJ, Schaap GR, van der Horst CM. Vascular malformations of the lower limb with osseous involvement. J Bone Joint Surg Br 2003; 85: 399–405. [DOI] [PubMed] [Google Scholar]
- 26.Nozaki T, Nosaka S, Miyazaki O, Makidono A, Yamamoto A, Niwa T, et al. Syndromes associated with vascular tumors and malformations: a pictorial review. Radiographics 2013; 33: 175–95. doi: 10.1148/rg.331125052 [DOI] [PubMed] [Google Scholar]
- 27.Elsayes KM, Menias CO, Dillman JR, Platt JF, Willatt JM, Heiken JP. Vascular malformation and hemangiomatosis syndromes: spectrum of imaging manifestations. AJR Am J Roentgenol 2008; 190: 1291–9. doi: 10.2214/AJR.07.2779 [DOI] [PubMed] [Google Scholar]
- 28.Gruber H, Peer S. Ultrasound diagnosis of soft tissue vascular malformations and tumours. Curr Med Imaging Rev 2009; 5: 55–61. [Google Scholar]
- 29.Trop I, Dubois J, Guibaud L, Grignon A, Patriquin H, McCauaig C, et al. Soft-tissue venous malformations in pediatric and young adult patients: diagnosis with Doppler US. Radiology 1999; 212: 841–5. [DOI] [PubMed] [Google Scholar]
- 30.Gulati MS, Paul SB, Batra A, Sarma D, Dadhwal V, Nath J. Uterine arteriovenous malformations: the role of intravenous ‘‘dual-phase’’ CT angiography. Clin Imaging 2000; 24: 10–14. [DOI] [PubMed] [Google Scholar]
- 31.Miyazaki M, Lee VS. Nonenhanced MR Angiography. Radiology 2008; 248: 20–43.doi: 10.1148/radiol.2481071497. [DOI] [PubMed] [Google Scholar]
- 32.University of California, San Diego, Department of Neuroradiology home page. UCSD neuroradiology teaching file database. Hesselink J R. MR Angiography: CNS applications. [Cited 28 September 2014.] Available from: http://spinwarp.ucsd.edu/neuroweb/Text/MR-ANGIO.htm [Google Scholar]
- 33.Massachusetts General Hospital, Radiology Rounds home page. Cochrane Miller J. Non-contrast MR angiography. [Cited 28 September 2014.] Available from: http://www.mghradrounds.org/index.php?src=gendocs&ref=2011_nov_dec#peripheral
- 34.Hodnett PA, Koktzoglou I, Davarpanah AH, Scanlon TG, Collins JD, Sheehan JJ, et al. Evaluation of peripheral arterial disease with nonenhanced quiescent-interval single-shot MR angiography. Radiology 2011; 260: 282–93. doi: 10.1148/radiol.11101336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klasen J, Blondin D, Schmitt P, Bi X, Sansone R, Wittsack HJ, et al. Nonenhanced ECG-gated quiescent-interval single-shot MRA (QISS MRA) of the lower extremities: comparison with contrast-enhanced MRA. Clin Radiol 2012; 67: 441–6. doi: 10.1016/j.crad.2011.10.014 [DOI] [PubMed] [Google Scholar]
- 36.Kramer U, Ernemann U, Mangold S, Seeger A, Bretschneider C, Miller S, et al. Diagnostic value of high spatial and temporal resolution time-resolved MR angiography in the workup of peripheral high-flow vascular malformations at 1.5 Tesla. Int J Cardiovasc Imaging 2012; 28: 823–34. doi: 10.1007/s10554-011-9887-1 [DOI] [PubMed] [Google Scholar]
- 37.Herborn CU, Goyen M, Lauenstein TC, Debatin JF, Ruehm SG, Kröger K. Comprehensive time-resolved MRI of peripheral vascular malformations. AJR Am J Roentgenol 2003; 181: 729–35. [DOI] [PubMed] [Google Scholar]
- 38.Ohgiya Y, Hashimoto T, Gokan T, Watanabe S, Kuroda M, Hirose M, et al. Dynamic MRI for distinguishing high-flow from low-flow peripheral vascular malformations. AJR Am J Roentgenol 2005; 185: 1131–7. [DOI] [PubMed] [Google Scholar]
- 39.Rózylo-Kalinowska I, Brodzisz A, Gałkowska E, Rózylo TK, Wieczorek AP. Application of Doppler ultrasonography in congenital vascular lesions of the head and neck. Dentomaxillofac Radiol 2002; 31: 2–6. [DOI] [PubMed] [Google Scholar]
- 40.Abernethy LJ. Classification and imaging of vascular malformations in children. Eur Radiol 2003; 13: 2483–97. [DOI] [PubMed] [Google Scholar]
- 41.Siegel MJ. Magnetic resonance imaging of musculoskeletal soft tissue masses. Radiol Clin North Am 2001; 39: 701–20. [DOI] [PubMed] [Google Scholar]
- 42.Konez O, Burrows PE. Magnetic resonance of vascular anomalies. Magn Reson Imaging Clin N Am 2002; 10: 363–e88.. [DOI] [PubMed] [Google Scholar]
- 43.Rak KM, Yakes WF, Ray RL, Dreisbach JN, Parker SH, Luethke JM, et al. MR imaging of symptomatic peripheral vascular malformations. AJR Am J Roentgenol 1992; 159: 107–12. [DOI] [PubMed] [Google Scholar]
- 44.Kramer U, Ernemann U, Fenchel M, Seeger A, Laub G, Claussen CD, et al. Pretreatment evaluation of peripheral vascular malformations using low-dose contrast-enhanced time-resolved 3D MR angiography: initial results in 22 patients. AJR Am J Roentgenol 2011; 196: 702–11. doi: 10.2214/AJR.10.5092 [DOI] [PubMed] [Google Scholar]
- 45.Goldman AB, DiCarlo EF. Pigmented villonodular synovitis: diagnosis and differential diagnosis. Radiol Clin North Am 1988; 26: 1327–47. [PubMed] [Google Scholar]
- 46.Llauger J, Palmer J, Rosón N, Bagué S, Camins A, Cremades R. Nonseptic monoarthritis: imaging features with clinical and histopathologic correlation. Radiographics 2000; 20: S263–78. [DOI] [PubMed] [Google Scholar]
- 47.Dabus G, Benenati JF. Interventional treatment options for vascular malformations. Approaches, techniques, and sclerosing and embolic agents that can be used in low- and high-flow vascular malformations. Endovasc Today 2013; 50–64. [Google Scholar]
- 48.Shobinger R. Proceedings of International Society for the Study of Vascular Anomalies Congress; 23–26 June 1996; Rome, Italy, 1996. [Google Scholar]
- 49.Kawanabe T, Wakita S, Harii K, Hayashi N, Inoue Y. Sclerotherapy of hemangiomas and vascular malformations in lips. Jpn J Plast Reconstr Surg 1996; 16: 852–62. [Google Scholar]
- 50.Goyal M, Causer PA, Armstrong D. Venous vascular malformations in pediatric patients: comparison of results of alcohol sclerotherapy with proposed MR imaging classification. Radiology 2002; 223: 639–44. [DOI] [PubMed] [Google Scholar]
- 51.Yakes WF, Haas DK, Parker SH, Hopper KD, Mulligan JS, Pevsner PH, et al. Symptomatic vascular malformations: ethanol embolotherapy. Radiology 1989; 170: 1059–66. [DOI] [PubMed] [Google Scholar]
- 52.Choi YH, Han MH, O-Ki K, Cha SH, Chang KH. Craniofacial cavernous venous malformations: percutaneous sclerotherapy with use of ethanolamine oleate. J Vasc Interv Radiol 2002; 13: 475–82. [DOI] [PubMed] [Google Scholar]
- 53.Fayad LM, Hazirolan T, Bluemke D, Mitchell S. Vascular malformations in the extremities: emphasis on MR imaging features that guide treatment options. Skeletal Radiol 2006; 35: 127–37. [DOI] [PubMed] [Google Scholar]
- 54.Yakes WF, Luethke JM, Parker SH, Stavros AT, Rak KM, Hopper KD, et al. Ethanol embolization of vascular malformations. Radiographics 1990; 10: 787–96. [DOI] [PubMed] [Google Scholar]
- 55.Tanigawa N, Shimomatsuya T, Takahashi K, Inomata Y, Tanaka K, Satomura K, et al. Treatment of cystic hygroma and lymphangioma with the use of bleomycin fat emulsion. Cancer 1987; 60: 741–9. [DOI] [PubMed] [Google Scholar]
- 56.Tan KT, Kirby J, Rajan DK, Hayeems E, Beecroft JR, Simons ME. Percutaneous sodium tetradecyl sulfate sclerotherapy for peripheral venous vascular malformations: a single-center experience. J Vasc Interv Radiol 2007; 18: 343–51. [DOI] [PubMed] [Google Scholar]
- 57.Jain R, Bandhu S, Sawhney S, Mittal R. Sonographically guided percutaneous sclerosis using 1% polidocanol in the treatment of vascular malformations. J Clin Ultrasound 2002; 30: 416–23. [DOI] [PubMed] [Google Scholar]
- 58.Hyodoh H, Fujita A, Hyodoh K, Furuse M, Kamisawa O, Hareyama M. High-flow arteriovenous malformation of the lower extremity: ethanolamine oleate sclerotherapy. Cardiovasc Intervent Radiol 2001; 24: 348–51. [DOI] [PubMed] [Google Scholar]
- 59.Igari K, Kudo T, Toyofuku T, Jibiki M, Inoue Y. Multidisciplinary approach to a peripheral arteriovenous malformation. Eur J Vasc Endovasc Surg 2012; 43: 365. [Google Scholar]
- 60.Yakes WF, Rossi P, Odink H. How I do it. Arteriovenous malformation management. Cardiovasc Intervent Radiol 1996; 19: 65–71. [DOI] [PubMed] [Google Scholar]