Abstract
In the developing neocortex, pyramidal neurons use molecular cues to form axonal arbors selectively in the correct layers. Despite the utility of mice for molecular and genetic studies, little work has been done on the development of layer-specific axonal arborizations of pyramidal neurons in mice. We intracellularly labeled and reconstructed the axons of layer 2/3 and layer 5 pyramidal neurons in slices of primary somatosensory cortex from C57Bl6 mice aged postnatal day 7–21. For all neurons studied, the development of the axonal arborizations in mice follows a pattern similar to that seen in other species; laminar specificity of the earliest axonal branches is similar to that of mature animals. At P7, pyramidal neurons are very simple, having only a main descending axon and few primary branches. Between P7 and P10 there is a large increase in the total number of axonal branches and axons continue to increase in complexity and total length from P10 to P21. Unlike observations in ferrets, cats, and monkeys, two types of layer 2/3 pyramidal neurons are present in both mature and developing mice; cells in superficial layer 2/3 lack axonal arbors in layer 4 while cells close to the layer 4 border have substantial axonal arbors within layer 4. We also describe axonal and dendritic arborization patterns of three pyramidal cell types in layer 5. The axons of tall-tufted layer 5 pyramidal neurons arborize almost exclusively within deep layers while tall-simple and short layer 5 pyramidal neurons also project axons to superficial layers.
Indexing Terms: local circuits, intracellular label, biocytin, pyramidal neuron, axonal branching, barrel cortex
Introduction
Information processing in the mammalian neocortex requires precise connectivity of the underlying neural circuits. Laminar specificity of axonal arbors is believed to be an important factor contributing to the specificity of cortical connections (Gilbert, 1983; Martin, 1984; Lund, 1988; Burkhalter, 1989; Callaway, 1998a). During development, laminar specificity of the local axons of pyramidal neurons emerges precisely from the onset, with axonal arbors forming in the correct layers while avoiding the incorrect layers (Lund et al., 1977; Katz and Callaway, 1992; Burkhalter et al., 1993; Callaway and Lieber, 1996; Katz and Shatz, 1996; Callaway, 1998a; Borrell and Callaway, 2002). For example, pyramidal neurons in layer 2/3 and layer 5 preferentially branch in layer 2/3 and layer 5, while avoiding layer 4; and pyramidal neurons in layer 6 preferentially branch in layer 6 and superficial layers, while avoiding layer 5. These local layer-specific branching patterns can also develop in a slice culture in vitro to mimic the correct in vivo pattern (Bolz et al., 1993; Bolz et al., 1996; Dantzker and Callaway, 1998; Butler et al., 2001; Borrell and Callaway, 2002). In addition to the local axonal arbors, both thalamocortical axonal projections and corticocortical axonal projections can also arborize in their correct target layers in slice culture in vitro (Yamamoto et al., 1989; Bolz et al., 1990; Bolz et al., 1992; Gotz et al., 1992; Yamamoto et al., 1992; Annis et al., 1993; Novak and Bolz, 1993; Hubener et al., 1995; Yamamoto et al., 1997; Skaliora et al., 2000). These observations suggest that molecular mechanisms are involved in the initial establishment of the layer-specific connections and that patterned neuronal activity is not instructive for the initial development of these projections.
With the molecular genetics available in transgenic mice it will be possible to direct gene expression to specific cell types and to identify, characterize and manipulate molecules that play a role in the establishment of the laminar-specific axonal arborizations. However, to date, very little work has been done on the normal development or the adult patterns of the layer-specific axonal arborization of pyramidal neurons in mice. The few studies that have been performed infer the normal development of the laminar-specific arbors based on bulk injections of biocytin into cortex (Bernardo et al., 1990; McCasland et al., 1992; Rhoades et al., 1996; Miller et al., 2001; Dagnew et al., 2003). In order to use transgenic mice to identify the role of specific molecules we need to first determine the mature specificity and the developmental timeline of the layer-specific connections from individual neurons in this species.
The development of the mouse cortex occurs over a shorter time period than in ferrets, cats, and monkeys, suggesting that some developmental processes might be unique to mouse (Angevine and Sidman, 1961; Caviness, 1982; Micheva and Beaulieu, 1996; De Felipe et al., 1997; Polleux et al., 1997; Takahashi et al., 1999; Levers et al., 2001). For example, there could be greater temporal overlap in the generation of cell types with different laminar fates or some cells might start to grow axons before layer specific markers are expressed; this is suggested by the informal observation that some mouse layer 2/3 pyramidal neurons make more axonal branches in layer 4 than is observed in other species (Yabuta et al., 2000).
We have individually labeled and reconstructed pyramidal neurons in layer 2/3 and layer 5 from primary somatosensory cortex in C57BL6 mice aged postnatal day 7 (P7) to postnatal day 21 (P21). The laminar specificities of axonal and dendritic arbors from these cells have been analyzed. We find that for each of the pyramidal cell types studied, axonal arbors develop correctly from the onset. However, we also find a subset of mature pyramidal neurons with arborization patterns distinct from previous reports in other species. Most notably we describe a pyramidal cell type in layer 2/3 located close to the border with layer 4 with substantial axonal arbors in layer 4 unlike typical layer 2/3 pyramids. We also find that layer 5 pyramidal neurons can be divided into at least three types; tall-tufted, tall-simple, and short, rather than just two types that are typically described; tall and short. Each of these three layer 5 pyramidal cell types has a unique pattern of axonal and dendritic arborization. As described previously, dendritic arborizations distinguish tall from short pyramids. We further separate tall layer 5 pyramidal neurons into tall-tufted cells that project axons only in deep layers and tall-simple pyramidal neurons that also project axons to superficial layers.
Material and Methods
Cortical Slices
C57BL6 mice were obtained from Harlan and kept on a 12 hr light/dark cycle. All animals were treated in accordance with institutional and NIH guidelines for the Care and Use of Laboratory Animals. Mice were deeply anesthetized with Nembutal (100 mg/kg i.p.) and decapitated. Brains were removed and submerged in ice-cold artificial cerebral spinal fluid (ACSF; in mM: 125 NaCl, 5 KCl, 2.5 CaCl2, 1.3 MgCl2, 1.25 NaH2PO4, 26 NaHCO3, 10 glucose, 1 Kynurenic Acid, saturated with 95% O2 and 5% CO2). The brain was cut along the midline to separate the two hemispheres and then the diencephalon was removed to leave a shell of the overlaying cortex. The anterior and posterior poles of the cortex were cut off, leaving somatosensory cortex intact within the block of tissue. The block of cortex was then placed on a bed of agar and 400 μm thick slices were made with a tissue slicer (Katz, 1987) at approximately 30 degrees relative to the sagittal plane and perpendicular to the pia surface. The slices were maintained over ACSF in a humidified, oxygenated interface chamber heated to 34ºC.
Intracellular Biocytin Fills
Slices were submerged in a recording chamber in oxygenated ACSF maintained at room temperature. Whole-cell recordings were made in current clamp mode using patch electrodes filled with intracellular solution (in mM: 130 potassium gluconate, 10 EGTA, 2 MgCl2, 0.5 CaCl2, 10 HEPES, 2.54 sodium ATP) and neurons were labeled by iontophoresis with biocytin (2%). We targeted areas in live slices where we could visualize barrels using DIC optics. Positions of cells in barrel cortex were later verified (see below). After neuronal labeling, the slices were placed back in the interface chamber and allowed to recover for 1–2 hours to reduce the background from any spilled biocytin. The slices were then fixed with 2.5% paraformaldehyde, 4% sucrose in 0.1 M phosphate buffer, pH 7.4 overnight at 4ºC.
Immunohistochemistry
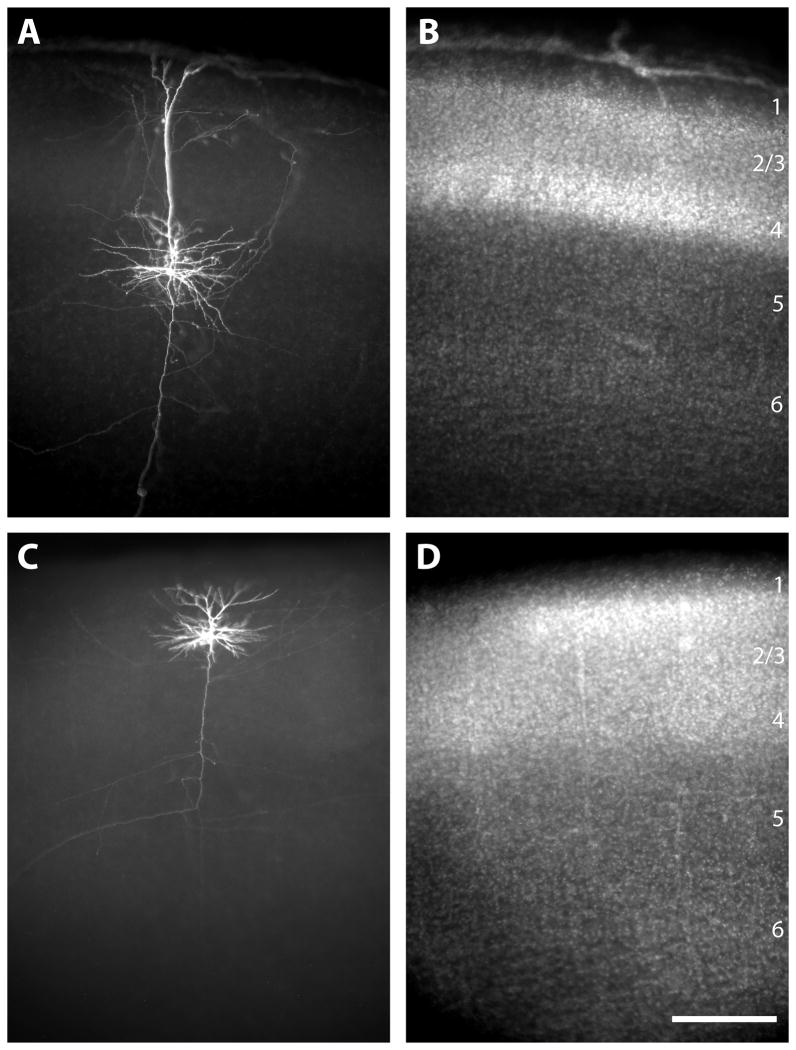
After fixation slices were cryoprotected in 30% sucrose and then freeze-thawed. The slices were incubated in blocking solution (10% NGS, 2% BSA, 0.25% Triton-X 100, 0.1M PB) for 2 hr. Slices were then incubated in Streptavidin-Cy3 (1:1000; Jackson ImmunoResearch Laboratories, Inc) in blocking solution for 4 hr. After staining, the slices were counterstained with DAPI (10 uM, Sigma) to allow for the determination of laminar and areal borders. Slices were then mounted onto subbed slides, dehydrated, and coverslipped with Krystalon mounting medium (Fisher). The typical labeling quality of neurons is illustrated in figure 1.
Figure 1.
Photomicrographs illustrating examples of typical intracellular labeling results at P10 (A, B) and P14 (C, D). For each age the labeled neurons are visualized with strepavidin-Cy3, which binds the biocytin (A, C) and laminar borders are determined by a DAPI counterstain (B, D). The reconstruction of the neuron at P10 (A, B) is in figure 5 (first column for P10). The scale bar is 250 microns and applies to all panels.
Data Analysis and Figure Preparation
Neurons were reconstructed by using a Nikon fluorescence microscope in concert with a Neurolucida computerized reconstruction system (MicroBrightfield, Colchester VT). First, low power (10× objective, 0.5 NA) maps were drawn of each selected slice. Maps included locations of the cell bodies of all labeled cells, laminar borders, and borders of barrel cortex, identified by the presence of cell dense barrel septa visualized by DAPI counterstain. The axonal and dendritic arbors of selected cells located in barrel cortex were then reconstructed at higher power (60× oil immersion objective, 1.4 NA) without the knowledge of cortical layers. Cells located close to the surface of the slice in which a large number of either the axons or dendrites were missing, evidenced by cut ends, especially when cut off close to the main descending axon, were not included in the analysis. After completion, the neuronal reconstructions were placed into the map file for analysis. Each neuron was analyzed for the number of axonal and dendritic branch points per layer and the total distance of axonal and dendritic arbor length per layer using custom designed MatLab-based programs. All distance measurements were made in the Neurolucida software package or obtained in the analysis portion of the NeuroExplorer software package. No correction for shrinkage of the tissue was taken into account for distance measurements. For the analysis, layer 1 is included with layer 2/3 since the layer 1/2 border varies with depth. The determinations of the laminar identification for the apical dendritic tuft of layer 5 neurons included layer 1.
To assess possible differences in functional connectivity for type II layer 2/3 pyramidal neurons (see results) synaptic boutons were counted along 500–600 microns of axon located in layer 2/3 and layer 4 from 3 cells. Boutons were easily recognized using a 60× objective (oil immersion objective, 1.4 NA). The axonal length measurements and counts of boutons were made in the NeuroExplorer software package to give density of boutons per unit of axon length.
A total of 70 layer 2/3 pyramidal neurons and 64 layer 5 pyramidal neurons were reconstructed. The break down of ages and cell types (see results for complete descriptions of classifications) are as follows (see also tables 1 and 2). For layer 2/3 type I pyramidal neurons: P7, n=10; P10, n=8; P14/15, n=8, P21/22, n=9. For layer 2/3 type II pyramidal neurons: P7, n=9; P10, n=9; P14/15, n=7; P21/22, n=10. For layer 5 tall-tufted pyramidal neurons: P7, n=7; P10, n=6; P14/15, n=5; P21/22, n=6. For layer 5 tall-simple pyramidal neurons: P7, n=1; P10, n=5; P14/15, n=5; P21/22, n=3. For layer 5 short pyramidal neurons: P7, n=7; P10, n=5; P14/15, n=8; P21/22, n=6.
Table 1.
Number of dendritic branch points (top number) and lengths (mm, bottom number), per cell in each cortical layer (mean ± SEM) for layer 2/3 pyramidal neurons.
| Cell Type | Apical Dendrites | Basal Dendrites | |||
|---|---|---|---|---|---|
| Layer 2/3 | Layer 4 | Layer 2/3 | Layer 4 | ||
| P7 | All Cells n=19 |
18.79 ± 2.05b | 0.00 ± 0.00 | 20.95 ± 2.05f | 0.11 ± 0.07 |
| 0.82 ± 0.09c | 0.001 ± 0.001 | 1.01 ± 0.10g | 0.02 ± 0.01i | ||
| Type I n=10 |
17.90 ± 3.43 | 0.00 ± 0.00 | 22.00 ± 3.52 | 0.00 ± 0.00 | |
| 0.77 ± 0.15 | 0.00 ± 0.00 | 1.06 ± 0.16 | 0.00 ± 0.00 | ||
| Type II n=9 |
19.78 ± 2.23 | 0.00 ± 0.00 | 19.78 ± 2.01 | 0.22 ± 0.15 | |
| 0.87 ± 0.10 | 0.001 ± 0.001 | 0.97 ± 0.12 | 0.04 ± 0.02 | ||
| P10 | All Cells n=17 |
24.18 ± 2.58 | 0.00 ± 0.00 | 33.53 ± 2.59f | 1.00 ± 0.48 |
| 1.35 ± 0.15c,d,e | 0.01 ± 0.01 | 1.87 ± 0.15g,h | 0.16 ± 0.06i | ||
| Type I n=8 |
25.25 ± 4.12 | 0.00 ± 0.00 | 39.13 ± 3.19a | 0.00 ± 0.00a | |
| 1.24 ± 0.24 | 0.00 ± 0.00 | 2.08 ± 0.18 | 0.004 ± 0.004a | ||
| Type II n=9 |
23.22 ± 3.41 | 0.00 ± 0.00 | 28.56 ± 3.30a | 1.89 ± 0.81a | |
| 1.44 ± 0.19 | 0.01 ± 0.01 | 1.69 ± 0.22 | 0.29 ± 0.08a | ||
| P14 | All Cells n=15 |
26.31 ± 3.56 | 0.00 ± 0.00 | 33.94 ± 3.94f | 1.06 ± 0.91 |
| 2.13 ± 0.27c,d | 0.003 ± 0.002 | 2.53 ± 0.28g,h | 0.20 ± 0.10i | ||
| Type I n=8 |
24.25 ± 3.67 | 0.00 ± 0.00 | 40.75 ± 5.82 | 0.00 ± 0.00 | |
| 1.93 ± 0.30 | 0.00 ± 0.00a | 3.06 ± 0.40 | 0.00 ± 0.00a | ||
| Type II n=7 |
23.29 ± 3.71 | 0.00 ± 0.00 | 27.14 ± 4.51 | 2.43 ± 1.97 | |
| 1.95 ± 0.24 | 0.01 ± 0.003a | 2.02 ± 0.31 | 0.45 ± 0.18a | ||
| P21 | All Cells n=19 |
25.21 ± 1.62b | 0.05 ± 0.05 | 29.95 ± 3.09f | 0.63 ± 0.34 |
| 2.04 ± 0.11c,e | 0.02 ± 0.01 | 2.47 ± 0.24g,h | 0.11 ± 0.05 | ||
| Type I n=9 |
25.44 ± 1.85 | 0.00 ± 0.00 | 38.22 ± 4.11a | 0.00 ± 0.00a | |
| 2.08 ± 0.17 | 0.00 ± 0.00 | 3.02 ± 0.34 | 0.00 ± 0.00 | ||
| Type II n=10 |
25.00 ± 2.68 | 0.10 ± 0.10 | 22.50 ± 3.12a | 1.20 ± 0.59 | |
| 1.99 ± 0.15 | 0.03 ± 0.02 | 1.97 ± 0.25 | 0.21 ± 0.08a | ||
p<0.05 between Type I and Type II neurons at indicated age
p<0.05 between All Cells at P7 and P21
p<0.05 between All Cells at P7 and P10; p<0.001 between All Cells at P7 and P14, and P7 and P21
p<0.05 between All Cells at P10 and P14
p<0.001 between All Cells at P10 and P21
p<0.001 between All Cells at P7 and P10; p<0.05 between All Cells at P7 and P14, and P7 and P21
p<0.001 between All Cells at P7 and P10, P7 and P14, and P7 and P21
p<0.05 between All Cells at P10 and P14, and P10 and P21
p<0.05 between All Cells at P7 and P10, and P7 and P14
Table 2.
Number of dendritic branch points (top number) and lengths (mm, bottom number), per cell in each cortical layer (mean ± SEM) for layer 5 pyramidal neurons.
| Cell Type | Apical Dendrites | Basal Dendrites | ||||
|---|---|---|---|---|---|---|
| Layer 2/3 | Layer 4 | Layer 5 | Layer 5 | Layer 6 | ||
| P7 | Tall-Tufted n=7 |
13.14 ± 4.97a | 3.14 ± 1.19 | 41.14 ± 15.55 a | 44.14 ± 5.92a | 0.14 ± 0.14 |
| 1.03 ± 0.14a | 0.24 ± 0.08 | 2.95 ± 0.22a | 3.44 ± 0.40a | 0.08 ± 0.03 | ||
| Tall-Simple n=1 |
4.00 | 1.00 | 37.00 | 29.00 | 0.00 | |
| 0.41 | 0.14 | 2.14 | 1.50 | 0.04 | ||
| Short n=7 |
3.57 ± 1.07a | 3.14 ± 1.30 | 22.71 ± 2.13a | 28.71 ± 3.80a | 0.14 ± 0.14 | |
| 0.34 ± 0.46a | 0.24 ± 0.05 | 1.37 ± 0.14 a | 1.92 ± 0.22a | 0.10 ± 0.06 | ||
| P10 | Tall-Tufted n=6 |
18.50 ± 2.11a,b | 3.33 ± 1.38 | 32.17 ± 4.81b | 35.50 ± 8.42 | 0.00 ± 0.00 |
| 1.31 ± 0.10a,b | 0.30 ± 0.73 | 2.94 ± 0.33a,b | 3.06 ± 0.49 | 0.00 ± 0.00 | ||
| Tall-Simple n=5 |
8.80 ± 1.20b | 1.20 ± 0.80 | 16.60 ± 3.08b | 29.20 ± 4.45 | 0.00 ± 0.00 | |
| 0.71 ± 0.11b,c | 0.17 ± 0.02 | 1.41 ± 0.29b | 2.79 ± 0.17 | 0.03 ± 0.02 | ||
| Short n=5 |
3.20 ± 1.20a | 6.20 ± 0.80 | 19.20 ± 3.08 | 28.00 ± 4.45 | 0.20 ± 0.00 | |
| 0.21 ± 0.11a,c | 0.45 ± 0.02 | 1.25 ± 0.29a | 2.20 ± 0.17 | 0.05 ± 0.02 | ||
| P14 | Tall-Tufted n=5 |
17.20 ± 2.06a | 2.80 ± 1.59 | 22.80 ± 1.46a,b | 35.60 ± 2.94a | 0.00 ± 0.00 |
| 1.59 ± 0.26a,b | 0.41 ± 0.14 | 2.40 ± 0.19a,b | 4.54 ± 0.50a | 0.02 ± 0.01 | ||
| Tall-Simple n=5 |
12.40 ± 1.29c | 0.20 ± 0.20 | 15.00 ± 1.92b | 30.20 ± 3.37c | 0.00 ± 0.00 | |
| 0.95 ± 0.12a,c | 0.17 ± 0.02c | 1.74 ± 0.19b | 3.96 ± 0.56c | 0.01 ± 0.01 | ||
| Short n=8 |
0.50 ± 0.27a,c | 1.63 ± 0.65 | 15.63 ± 2.00a | 19.38 ± 2.43a,c | 0.13 ± 0.13 | |
| 0.18 ± 0.05a,c | 0.27 ± 0.04c | 1.51 ± 0.14a | 2.30 ± 0.23a,c | 0.16 ± 0.07 | ||
| P21 | Tall-Tufted n=6 |
14.83 ± 3.30a | 2.83 ± 0.60b | 28.50 ± 0.76a,b | 19.67 ± 1.33 | 0.00 ± 0.00 |
| 1.45 ± 0.37a | 0.38 ± 0.10b | 2.96 ± 0.14b | 2.70 ± 0.12a | 0.08 ± 0.03 | ||
| Tall-Simple n=3 |
6.00 ± 2.89 | 0.67 ± 0.33b | 14.33 ± 0.88b | 26.67 ± 8.57 | 0.00 ± 0.00 | |
| 0.73 ± 0.22 | 0.22 ± 0.02b | 1.44 ± 0.14b | 2.98 ± 0.77 | 0.00 ± 0.00 | ||
| Short n=6 |
2.17 ± 0.70a | 1.33 ± 0.71 | 12.33 ± 1.36a | 17.00 ± 2.46 | 0.17 ± 0.17 | |
| 0.38 ± 0.11a | 0.36 ± 0.12 | 1.33 ± 0.12a | 2.08 ± 0.22a | 0.27 ± 0.16 | ||
p<0.05 between Tall-tufted and Short groups of neurons at indicated age
p<0.05 between Tall-tufted and Tall-simple groups of neurons at indicated age
p<0.05 between Tall-simple and Short groups of neurons at indicated age
Figure 1 was prepared by importing images from software provided with Optronics MicroFire camera (Optronics, Goleta, CA), into Adobe Photoshop and subsequently into Adobe Illustrator. Minimal alterations were made in the brightness and contrast of these images.
Results
Overview of General Developmental Sequence
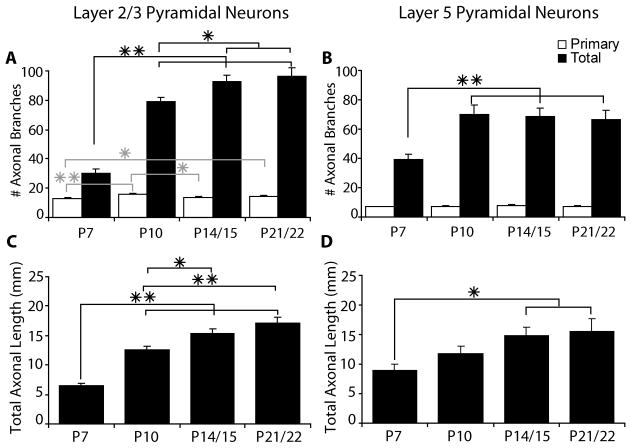
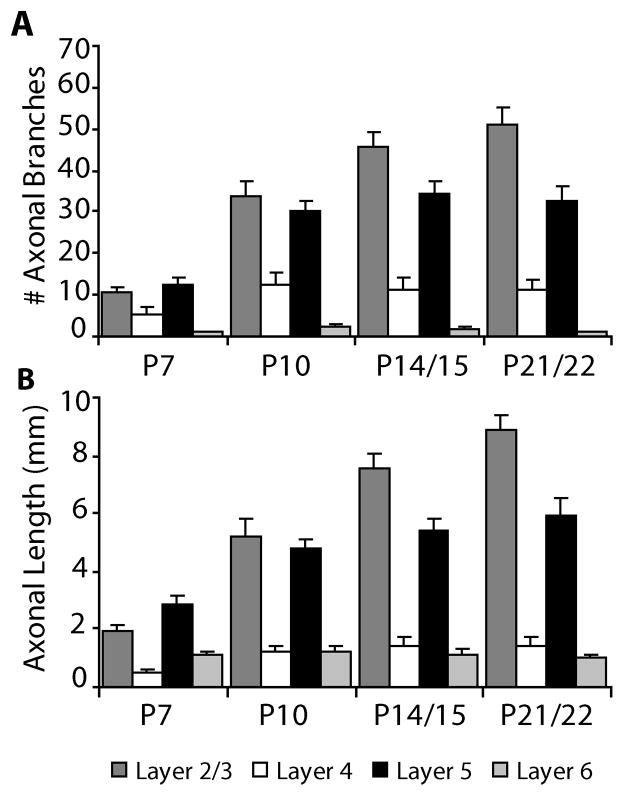
The development of axonal arbors of pyramidal neurons in mice follows the same overall pattern that has been observed in other species examined. We also observe a similar developmental timeline for both layer 2/3 and layer 5 pyramidal neurons. At the earliest age examined (P7) the axons of pyramidal neurons are very simple, including only the main descending axon and a small number of primary branches, which can be seen in both the quantification of the number of axonal branches (Fig. 2) and in the reconstructions of individual neurons (Figs. 3–6). The numbers of primary axonal branches are already close to adult levels at P7 and change very little after P7 (Fig. 2). Between P7 and P10, however, there is a rapid increase in the complexity of the pyramidal neurons. For layer 2/3 pyramidal neurons the total number of axonal branches per cell more than doubles between P7 and P10 (from 30.0 ± 3.2 to 78.9 ± 3.3 branches per cell; mean ± SEM, p<0.001, t-test) and it nearly doubles for layer 5 pyramidal neurons (from 39.1 ± 3.9 to 70.3 ± 6.3 branches per cell; mean ± SEM, p<0.001, t-test) (Fig. 2). These changes in numbers of axonal branches are paralleled by comparable changes in the total axonal length per cell (Fig. 2). Total axon length per cell increases from 6.46 mm ± 0.49 at P7 to 12.54 mm ± 0.64 at P10 for layer 2/3 pyramids (p<0.001, t-test) and from 9.02 mm ± 0.94 at P7 to 11.81 mm ± 1.15 at P10 for layer 5 pyramids (p=0.07, t-test) (mean ± SEM). From P10 through P22, layer 2/3 pyramidal neurons continue to have more modest increases in both their total axon length and total numbers of axonal branches (Fig. 2). During this same time period, total axonal branches do not change for layer 5 pyramidal neurons, but total axonal length does continue to increase (Fig. 2).
Figure 2.
Bar graphs indicate the number of primary (white bars) and total (black bars) axonal branch points per cell (A, B) and the total axonal length per cell (C, D) for layer 2/3 pyramidal neurons (A, C) and layer 5 pyramidal neurons (B, D). Primary axonal branches are defined as branches from the main descending axon. All values are mean ± SEM. Asterisks denote statistical significance between ages, with the grey asterisks for primary axonal branches and black asterisks for total axonal branches and axonal length (* = p<0.05 ** = p<0.001).
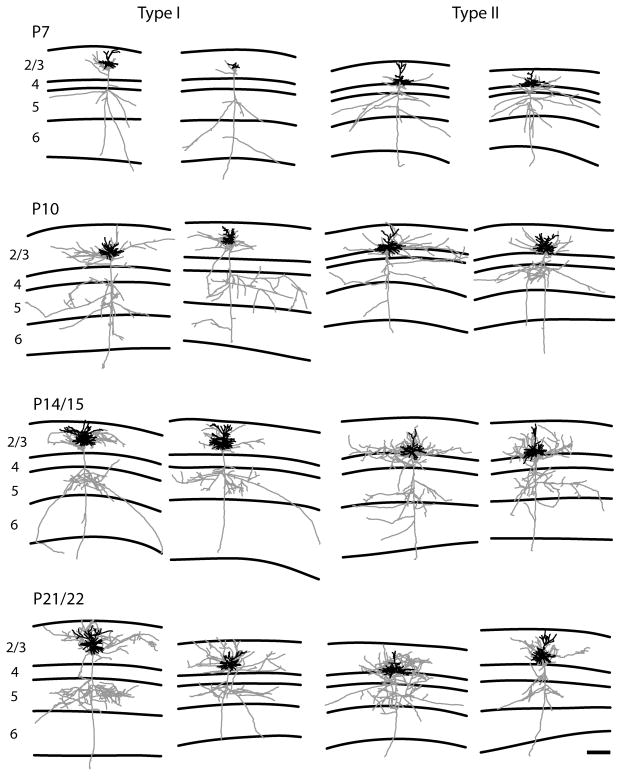
Figure 3.
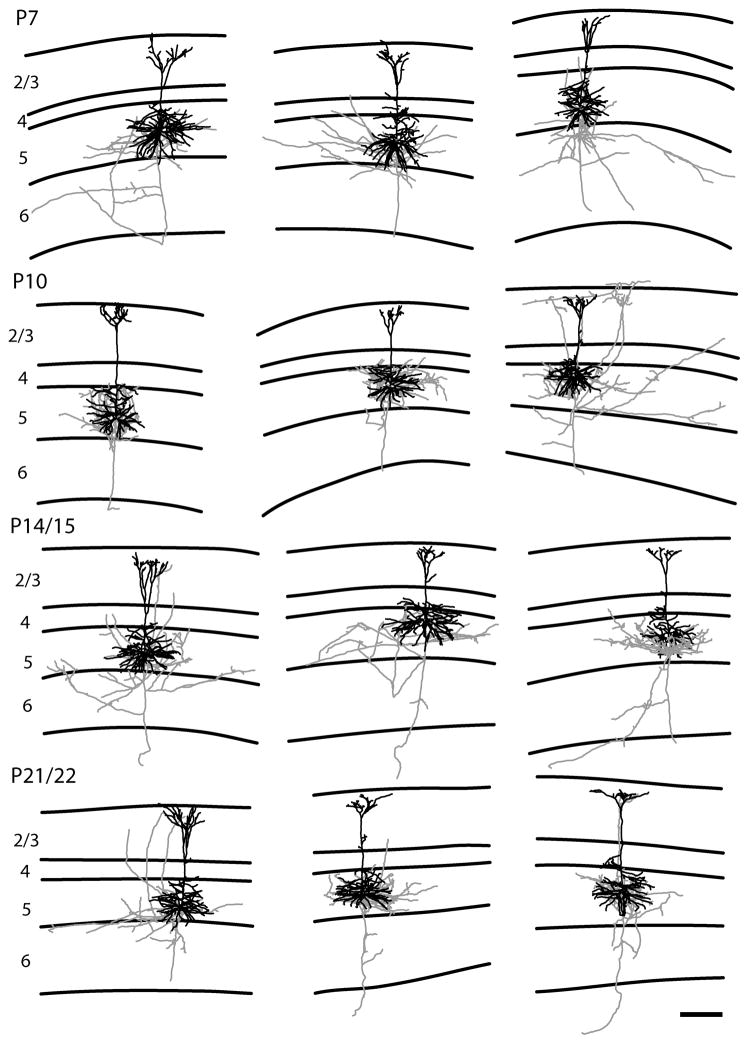
Representative examples of reconstructions of both type I (two columns on the left) and type II (two columns on the right) layer 2/3 pyramidal neurons. Neurons are illustrated with axons depicted in gray and dendrites in black. Ages are indicated to the left. Lines define the laminar boundaries for each cortical layer. The upper line indicates the top of the slice, with layer 1 included with layer 2/3. The type I neurons show the classic pattern with the descending axon crossing layer 4 with a few or no branches. In contrast the type II neurons show a large number of axonal branches within layer 4. The scale bar is 250 microns and applies to all drawings.
Figure 6.
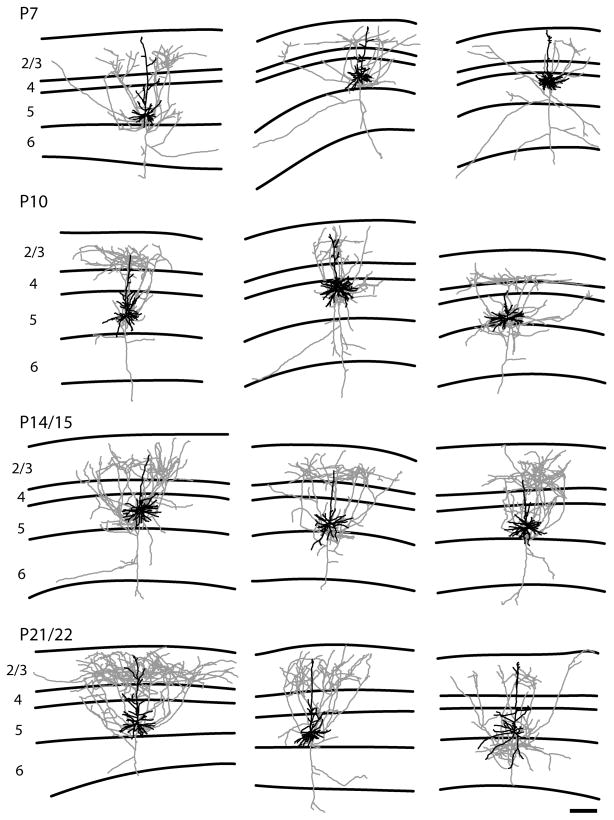
Representative examples of reconstructions of short layer 5 pyramidal neurons. The short layer 5 pyramidal neurons have a significant axonal projection to the superficial layers in contrast to the pattern observed in the tall-tufted layer 5 pyramidal neurons. Conventions are the same as figure 3. The scale bar is 250 microns and applies to all drawings.
Dendritic development also follows a similar timeline as axonal development. For layer 2/3 pyramidal neurons (Table 1) there is a modest increase in the number of apical dendritic branches occurring between P7 and P10 (from 18.79 ± 2.05 to 24.18 ± 2.58; mean ± SEM, p=0.1, t-test) and a larger increase for basal dendrites (from 21.05 ± 2.05 to 34.53 ± 2.29; mean ± SEM, p<0.001, t-test). For apical dendrites the only significant increase for the number of branches occurs between ages P7 and P21 (p<0.05, t-test). Despite the modest changes in branch points, we do observe large changes in the total apical dendritic length. Between P7 and P10 there is a significant increase in total apical dendritic length from 0.82 ± 0.09 mm to 1.36 ± 0.15 mm (mean ± SEM; p<0.001, t-test). There is also a significant increase between P10 and P14, with the apical dendritic length increasing to 2.14 ± 0.27 mm (mean ± SEM; p<0.05, t-test). For basal dendritic branches of layer 2/3 pyramidal neurons there are no significant increases in numbers of branch points after P10, but basal dendritic length continues to increase with significant increases occurring between P7 and P10 (Table 1; p<0.001, t-test) and between P10 and P14 (Table 1; p<0.05, t-test) before leveling off.
For layer 5 pyramidal neurons, there was not a significant increase in the number of apical dendritic branches between P7 and P10. The only significant differences found for the number of apical dendritic branches was between P7 and both P14 and P21 (Table 2; p<0.05, t-test). There was also no difference in the total apical dendritic length for all cells across all ages examined. For basal dendritic branches we see significant increases between P7 and P14 (Table 2; p<0.05, t-test), P7 and P21 (Table 2; p<0.001, t-test), P10 and P21 (Table 2; p<0.05, t-test), and P14 and P21 (Table 2; p<0.05, t-test). We only observe a significant increase for total basal dendritic length between the ages of P14 and P21 (Table 2; p<0.05, t-test).
Development of axonal laminar specificity
Layer 2/3 Pyramidal Neurons
The development of layer 2/3 pyramidal neurons has previously been described in cats, ferrets, and monkeys (Katz, 1991; Callaway, 1998b; Borrell and Callaway, 2002). Layer 2/3 pyramidal neurons in these studies show a high degree of specificity at all time points, with axons arborizing within layer 2/3 and layer 5 and avoiding layer 4 and layer 6. In contrast, we identified two cell types in mice based on soma position (defined further below). For many layer 2/3 pyramids in mouse barrel cortex we found the same general pattern of axonal arborization as in other species at all ages examined (Fig. 3, Type I cells), based on both the number of axonal branches (Fig. 7A) and axonal length measurements (Fig. 7B). However, we also noticed a major difference that was not observed in the previous studies of other species. For some layer 2/3 pyramids in mice, there were many axonal branches and substantial axonal length found in layer 4 (Fig. 3, Type II cells).
Figure 7.
Bar graphs indicate, for all layer 2/3 pyramidal neurons, the number of axonal branch points (A) and the axonal length (B) per cell present in each cortical layer (mean ± SEM).
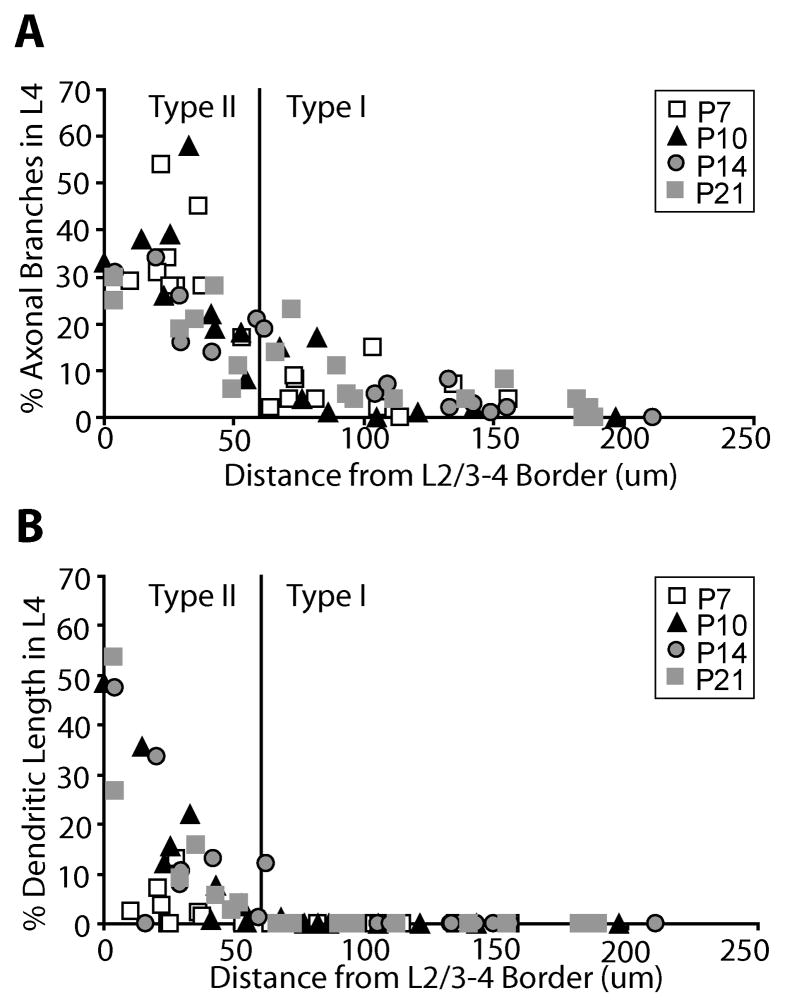
Informal observations suggested that the layer 2/3 pyramids with axonal arbors in layer 4 were located close to the border between layer 2/3 and layer 4. We therefore examined this relationship quantitatively, plotting the percentage of axonal branches in layer 4 against the distance between the soma and the top of layer 4 (Fig. 8A). We found that layer 2/3 pyramidal neurons located less than 60 microns from the layer 4 border have increased axonal arbors in layer 4. In addition we also see a similar trend when we analyze the relationship between the percentage of dendritic length in layer 4 and the distance of the soma from the top of layer 4 (Fig. 8B), but this trend becomes more pronounced at older ages when cells have more extensive dendritic arbors. Comparing figure 8A to figure 8B it can be seen that cell body position correlates with axonal branching in layer 4 at all ages examined while the correlation with dendritic length emerges only at later ages when more extensive dendrites have developed. Therefore, since cell position relative to the laminar border is less likely to change with age than dendritic complexity (Table 1), we consider cell body position a better predictor of whether an immature layer 2/3 neuron is likely to have axonal and dendritic arbors in layer 4 later in development. We also examined if there was a correlation with the two types of neurons and the underlying barrels and found none. Neurons from both groups were found above both barrels and septa.
Figure 8.
Each layer 2/3 pyramidal neuron reconstructed in this study is plotted as a function of its distance from the top of layer 4 to the bottom of the soma against the percentage of axonal branches located in layer 4 (A) or the percentage of basal dendritic length located in layer 4 (B). Based on these plots we separated the neurons into two groups according to the distance from the top of layer 4, with neurons found at a distance more than 60 microns being termed type I neurons and neurons found at a distance less than 60 microns being termed type II neurons (see text). The cut-off point for the cell types are denoted by the black line in both graphs A and B.
Based on these scatter plots (Fig. 8), we have divided our data set into two types of layer 2/3 pyramidal neurons, that we term type I and type II. Type I neurons have their cell body located at a distance greater than 60 microns from the top of layer 4 and typically lack dendritic arbors in layer 4, regardless of age (Fig. 8B). Type II neurons are those with the cell body located at a distance less than 60 microns from the top of layer 4.
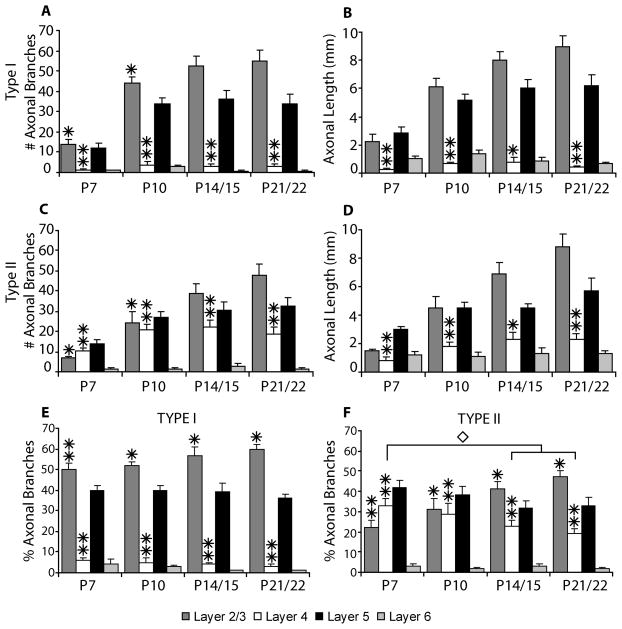
For the type I neurons we observe the classic arborization pattern for layer 2/3 pyramidal neurons as described in previous studies for cats, ferrets, and primates; axonal arbors are confined almost exclusively to layers 2/3 and 5 (Figs. 9A, 9B). This is illustrated in figures 9A and 9B which show that axonal branches occurring in layer 4 are rare and only small amounts of axonal length are present in layer 4, primarily due to the descending axon crossing layer 4. This pattern is present from the earliest ages examined with the percentage of axonal branches per layer staying consistent despite overall increases in branch number and length (Fig. 9E). In contrast, the type II neurons have extensive axonal branches and considerable axonal length in layer 4 (Figs. 9C, 9D). As seen with the type I neurons the laminar arborization pattern is observed at all ages (Figs. 9C, 9D). However, axonal branches and length in layer 4 reach a plateau at P10 while later branching and growth is predominantly in layers 2/3 and 5. As a result the percentage of axonal branches in layer 4 decreases between P10 and P22 (Fig. 9F). Differences in the morphology of the two types of cells are also readily apparent from the reconstructions illustrated in figure 3.
Figure 9.
Bar graphs indicate, for type I layer 2/3 pyramidal neurons (A, B) and type II layer 2/3 pyramidal neurons (C, D), the number of axonal branch points (A, C) and the axonal length (B, D) per cell present in each cortical layer (mean ± SEM). Panels E and F indicate the percentage of axonal branches per cell in each cortical layer (mean ± SEM) for type I and type II layer 2/3 pyramidal neurons respectively. Asterisks denote statistical significance between type I and type II neurons at indicated ages (* = p<0.05, ** = p<0.001). Diamond denotes statistical significance between ages for type II neurons (p<0.05).
To assess the possibility of functional connections for the type II neurons within layer 4 we counted the number of synaptic boutons across 500–600 microns of axon in both layer 4 and layer 2/3 for 3 type II neurons at P21. We found no difference in density of synaptic boutons per length of axon between the two layers (3.80 ± 0.36 um/bouton in layer 2/3; 4.15 ± 0.59 um/bouton in layer 4; mean ± SD). The distribution of values varied between neurons but the values across layers for the same neuron was similar, as previously described for other neurons (Yabuta and Callaway, 1998). Thus the relative axonal lengths in each layer also provide a good quantitative description of relative numbers of synaptic boutons.
Layer 5 Pyramidal Neurons
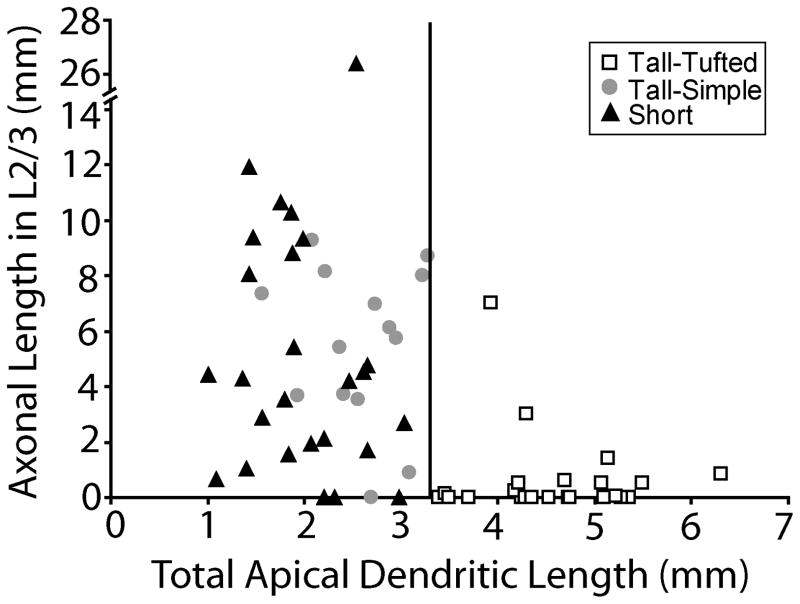
We examined the development of layer 5 pyramidal cells which we classified into three types. The first group is the tall-tufted layer 5 pyramidal neurons. These neurons are characterized by a large cell body and an apical dendrite that extends to layer 1 with a large dendritic tuft in layer 2/3 (Fig. 4, Table 2). The tall-tufted layer 5 neurons are known to be the subcortical projection neurons, projecting to targets such as the superior colliculus (Wise and Jones, 1977; Games and Winer, 1988; Hallman et al., 1988; Hubener and Bolz, 1988; Killackey et al., 1989; Hubener et al., 1990; Larkman and Mason, 1990; Tsiola et al., 2003), and are intrinsically bursting (Chagnac-Amitai et al., 1990; Mason and Larkman, 1990; Kim and Connors, 1993; Wang and McCormick, 1993). The second group is the tall-simple layer 5 pyramidal neurons. These neurons are characterized by having a smaller, more elongated cell body than the tall-tufted neurons and an apical dendrite that extends to layer 1 with a simple tuft at the top of layer 2/3 (Fig. 5, Table 2). Similar cells have been described previously (see discussion) (Chagnac-Amitai et al., 1990; Kim and Connors, 1993; Tsiola et al., 2003; Akemann et al., 2004). We distinguish between the tall-tufted and the tall-simple layer 5 pyramidal neurons both qualitatively, based on their morphology, and quantitatively, based on total apical dendritic length. The tall-tufted layer 5 neurons have greater apical dendritic length (range: 3.4–6.3 mm) than the tall-simple layer 5 neurons (range: 1.5–3.3 mm) at all ages examined with the values in the lower ranges occurring earlier in development (Fig. 10). There is a significant difference in total apical dendritic length between tall-tufted layer 5 neurons and both tall-simple (at all ages except P7; p < 0.05, t-test) and short layer 5 (p < 0.001, t-test) pyramidal neurons (described below).
Figure 4.
Representative examples of reconstructions of tall-tufted layer 5 pyramidal neurons. The tall-tufted layer 5 pyramidal neurons have little, if any, axonal projections to the superficial layers. Conventions are the same as figure 3. The scale bar is 250 microns and applies to all drawings.
Figure 5.
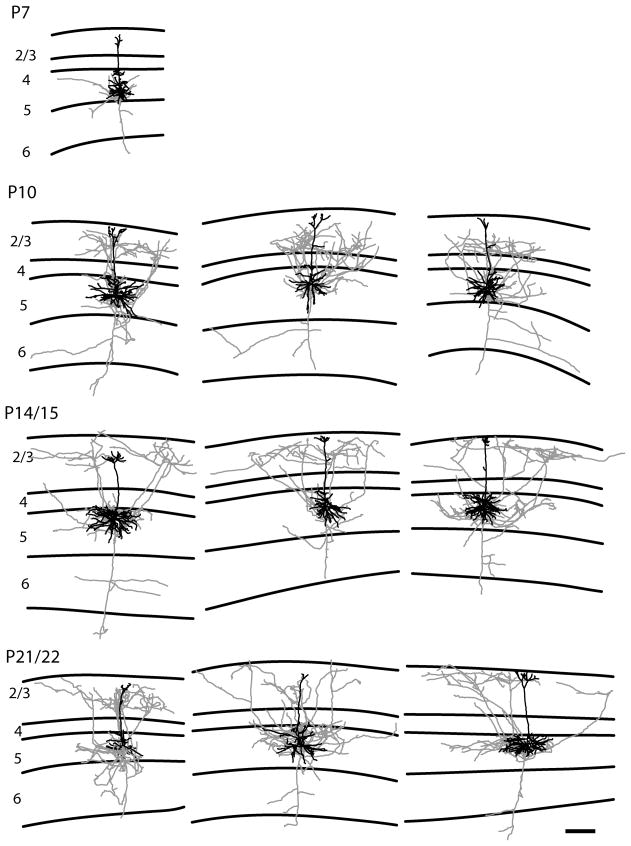
Representative examples of reconstructions of tall-simple layer 5 pyramidal neurons. The tall-simple layer 5 pyramidal neurons have a significant axonal projection to the superficial layers in contrast to the pattern observed in the tall-tufted layer 5 pyramidal neurons. Conventions are the same as figure 3. The scale bar is 250 microns and applies to all drawings.
Figure 10.
Each layer 5 pyramidal neuron reconstructed in this study is plotted as a function of its total apical dendritic length against the axonal length in layer 2/3. From this plot the separation of tall-simple and tall-tufted layer 5 pyramidal neurons can be seen with the tall-tufted neurons having greater apical dendritic length and less axonal length in layer 2/3. The vertical line denotes the cut-off between the tall-tufted and tall-simple layer 5 neurons. Note that the y-axis is split for one short layer 5 neuron with a very long axon in layer 2/3. There is a significant difference in total apical dendritic length between tall-tufted layer 5 neurons and both tall-simple (at all ages except P7; p < 0.05, t-test) and short (p < 0.001, t-test) layer 5 pyramidal neurons.
The final group we examined is the short layer 5 pyramidal neurons. These neurons are characterized by smaller cell bodies and an apical dendrite that only extends part way into layer 2/3 (Fig. 6, Table 2). The short layer 5 pyramidal neurons include corticocortical projection neurons (Games and Winer, 1988; Hallman et al., 1988; Hubener and Bolz, 1988; Hubener et al., 1990; Larkman and Mason, 1990).
Previous work allows us to use the dendritic morphology at all the ages we examined to classify each cell type in the study. In rats the dendrites of both tall-tufted and short layer 5 neurons start out as a common ‘tall’ pyramidal morphology early in development, but the apical dendrites are later refined to the class specific morphology observed in the adult (Koester and O’Leary, 1992). In this study the adult, class specific morphology is present by P10, while at P7 many neurons show a transitional morphology. One difficulty that we had was identifying tall-simple neurons at P7; our data set had only one tall-simple neuron at this age. This leaves open the possibility that we are unable to clearly distinguish between tall-tufted and tall-simple pyramidal neurons at P7, but it is also clear that short layer 5 pyramidal neurons already show a clear difference in axonal arborization pattern (see below). At all later ages we observe similar proportions of all three cell types (for complete descriptions of sample sizes see materials and methods and table 2). With this difficulty we can only describe the development of the tall-simple layer 5 pyramidal neurons starting at P10.
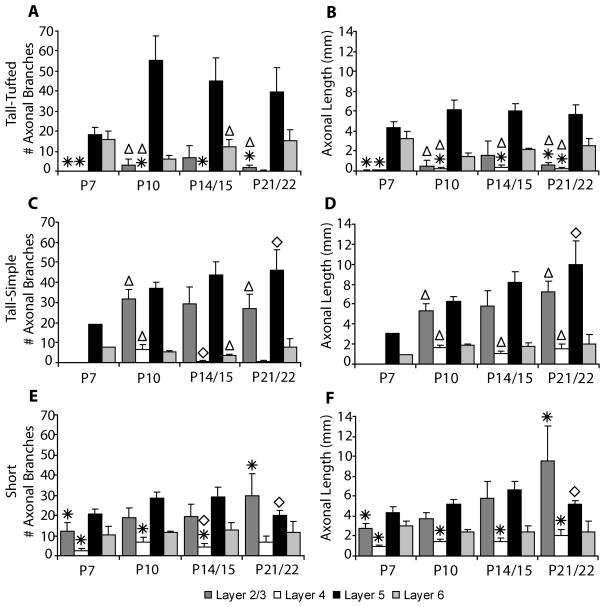
We observed two basic axonal arborization patterns for the three cell types examined. For the tall-tufted layer 5 pyramidal neurons the axonal arborization pattern is mostly in the deep layers with little or no projection into the superficial layers (Figs. 4, 11A). This basic pattern of arborizations is seen at all ages examined. In contrast, both the tall-simple (Figs. 5, 11B) and short (Figs. 6, 11C) layer 5 pyramidal neurons have a large superficial layer axonal projection in addition to the arborization found in the deep layers. As with the tall-tufted layer 5 pyramidal neurons these laminar patterns are observed at all ages examined. The only exception is the tall-simple layer 5 pyramidal neurons at P7 (see discussion). The separation of the groups and the differences in the extent of layer 2/3 axonal arborization can more clearly be seen in figure 10 when the total length of the apical dendrite is plotted verses the axonal length in layer 2/3. The total numbers of axonal branches per cell in layer 2/3 are lower for tall-tufted layer 5 cells than for either tall-simple (P7, p=0.736; P10, p=0.001; P14, p=0.065; P21, p=0.001; t-test) or short layer 5 pyramids (P7, p=0.024; P10, p=0.077; P14, p=0.172; P21, p=0.031; t-test).
Figure 11.
Bar graphs indicate the number of axonal branch points (A, C, E) and the axonal length (B, D, F) per cell present in each cortical layer (mean ± SEM) for tall-tufted layer 5 pyramidal neurons (A, B), tall-simple layer 5 pyramidal neurons (C, D), and short layer 5 pyramidal neurons (E, F). Statistical significance (p<0.05) between groups at the indicated ages is denoted by either an asterisk for tall-tufted vs. short, a triangle for tall-tufted vs. tall-simple, or a diamond for tall-simple vs. short layer 5 pyramidal neurons.
Discussion
In this study we have quantitatively described the normal development of layer-specific axonal arborizations for layer 2/3 and layer 5 pyramidal neurons in mice. We find that mice follow a similar developmental program to that previously described in other species, with the laminar specificity of each cell type developing correctly from the onset of development. However, we observe important differences between mice and previously described species. We characterize a population of layer 2/3 pyramidal neurons at the border of layer 4 that have extensive axons within layer 4. Additionally, we show that layer 5 tall pyramids can be further divided into three distinct populations that differ in both their dendritic morphologies and the laminar specificity of their axonal arbors.
Layer 2/3 pyramidal neurons are of particular interest because of the high degree of axonal laminar specificity observed in cats and primates (Katz, 1991; Callaway, 1998a). In cats layer 2/3 pyramidal cells in primary visual cortex avoid having axonal arbors in layer 4, even when their close proximity to the layer 4 border precludes the formation of primary branches in layer 2/3 (Katz, 1991). In ferrets, layer 2/3 pyramidal neurons in primary visual cortex also show a high degree of specificity at later development ages. An important difference observed in ferrets is that at earlier developmental ages, layer 2/3 pyramidal neurons at all depths in the layer have small numbers of transient branches within layer 4. These branches are pruned at later ages to give the expected adult arborization pattern (Borrell and Callaway, 2002). In mice we describe a striking difference from previously reported results; pyramidal neurons located just above the layer 4 border (type II neurons) have extensive axonal arborizations within layer 4, both in developing and mature animals. The remaining neurons located farther from the layer 4 border (type I neurons) avoid growing axons in layer 4.
The observation of two types of layer 2/3 pyramidal neurons raises questions about the mechanisms responsible. We envision two possible scenarios, the first related to activity and dendritic length in layer 4 and the second related to molecular cues and laminar position of the cell body. Both laminar position and layer 4 dendritic length of layer 2/3 pyramidal neurons correlated with the presence of layer 4 axons.
For the mature type II layer 2/3 pyramidal neurons we found that both soma position and the dendritic length within layer 4 correlate with the axonal length within layer 4 (Fig. 8). These relationships suggest a correlation-based, activity-dependent mechanism for maintenance of axons in layer 4. Because type II layer 2/3 pyramidal neurons with dendrites in layer 4 could receive direct thalamic input, their activity might be better correlated with that of layer 4 pyramidal and spiny stellate neurons than type I layer 2/3 pyramids, which lack axonal arborization in layer 4.
An alternative to activity-based mechanisms is that neurons located at the layer 3–4 border could have a unique molecular, genetic identity as shown for neurons in other layers (Arimatsu and Ishida, 2002; Hevner et al., 2003; Voelker et al., 2004). Previous studies strongly suggest that molecular cues are used by developing pyramidal neurons to establish their initial layer-specific axonal arbors (Bolz and Castellani, 1997; Castellani and Bolz, 1997; Castellani et al., 1998; Dantzker and Callaway, 1998; Butler et al., 2001; Borrell and Callaway, 2002). A distinct neuronal class would respond differently to cues found within layer 4 and arborize within this layer where growth of axons from other layer 2/3 pyramidal neurons is excluded.
Considering possible mechanisms for species differences in the development layer 2/3 pyramidal neurons, differences in the timing of cortical development might be important. In mice, neurogenesis (Angevine and Sidman, 1961; Polleux et al., 1997; Takahashi et al., 1999; Levers et al., 2001) occurs over a much shorter time period than in ferrets, cats, or monkeys (Rakic, 1974; Luskin and Shatz, 1985; Jackson et al., 1989) (approximately 1 week versus 4–8 weeks). With the compressed time frame in which neurogenesis occurs in mice, neurons that are born at the same time can occupy more than one cortical layer (Takahashi et al., 1999). Thus, neurons at the layer 3–4 border might be born at the same time as layer 4 neurons and therefore share some of their characteristics. It should be noted however, that the axonal arbors of type II layer 2/3 pyramidal neurons are distinctly different from those of both layer 4 neurons and type I layer 2/3 pyramidal neurons. Layer 4 neurons have more extensive axonal arbors in layer 4 than in layer 2/3 (Callaway and Katz, 1992; Bender et al., 2003), while our type II layer 2/3 pyramidal neurons average twice as many axons in layer 2/3 versus layer 4 (Fig. 9).
Interestingly we observed that type II layer 2/3 pyramids reach peak levels of axonal branching and growth in layer 4 by P10 (Fig. 9), while growth in other layers continues after P10. This raises the possibility that either the molecular environment in layer 4 changes at P10 or maturation of type II layer 2/3 neurons allows them to alter their response to these cues. Since type I neurons avoid layer 4 from the outset however, there are apparently sufficient cues present at P7 for these cells to avoid axonal growth in layer 4, suggesting more subtle interactions between multiple developmentally regulated mechanisms leading to the observed differences between the cell types.
The functional importance of connections in layer 4 that come from type II layer 2/3 pyramidal neurons is unclear. One possibility is that these synapses are formed preferentially on to apical dendrites of deep layer pyramidal neurons and thus do not contribute to any basic change in connectivity between the various cell types relative to other species. We think it is more likely, however, that some of the connections are made with layer 4 neurons. Whether this reflects a different mode of information processing in mouse depends in turn on the functional properties of the type II layer 2/3 neurons and their sources of input, whichremain unknown.
The development of layer 5 pyramidal neurons has also been described previously in several species. In ferrets and primates the layer-specific development of the local axonal arborizations have been described (Callaway, 1998b; Butler et al., 2001). In rodents however, there have been multiple studies on the development of dendrites (Miller, 1981; Miller and Peters, 1981; Miller, 1986; Koester and O’Leary, 1992; Kasper et al., 1994; Zhang, 2004) of layer 5 neurons and extrinsic projections (O’Leary and Terashima, 1988; O’Leary and Stanfield, 1989; Heffner et al., 1990; Koester and O’Leary, 1993), but not on local axonal laminar specificity.
Multiple cell types have been described in layer 5 based on physiology, morphology, and extrinsic axonal projections. We examined the development of three pyramidal cell types previously described. Previous studies have not, however, correlated dendritic morphology with axonal laminar specificity for each cell type. The first cell type is the tall-tufted layer 5 pyramidal neuron. These neurons are characterized by a large cell body, an apical dendrite that extends to layer 1, and a large dendritic tuft in layer 2/3. The tall-tufted layer 5 neurons are known to be the subcortical projection neurons, projecting to targets such as the superior colliculus (Games and Winer, 1988; Hallman et al., 1988; Hubener and Bolz, 1988; Hubener et al., 1990; Larkman and Mason, 1990; Tsiola et al., 2003). The tall-tufted layer 5 neurons are also known to be intrinsically bursting (Chagnac-Amitai et al., 1990; Mason and Larkman, 1990; Kim and Connors, 1993; Wang and McCormick, 1993). The second cell type is the tall, simple-tufted layer 5 pyramidal neuron. These neurons are characterized by having a smaller, elongated cell body than the tall-tufted neurons, an apical dendrite that extends to layer 1, and a simple tuft at the top of layer 2/3. This cell type was identified using principle component analysis and cluster analysis of dendritic morphology to find morphological classifications of layer 5 neurons (Tsiola et al., 2003). These neurons were in the same larger cluster of tall layer 5 neurons but were different from the tall-tufted in both the extent of the tuft found in at the top of layer 2/3 and the thickness and extent of apical dendritic branches. We show here that these neurons are also distinguished from tall-tufted layer 5 pyramidal neurons in their total apical dendritic length (Fig. 10). Several other studies mention cell types that have similar morphological features to our tall-simple class, but these cells were identified primarily based on their intrinsic firing properties rather than on morphology (Chagnac-Amitai et al., 1990; Kim and Connors, 1993); they are “regular spiking”. Similar cells were also described in a transgenic mouse line expressing YFP under control of the Kv3.1 promoter (Akemann et al., 2004). The YFP-positive cells appear similar to our tall-simple layer 5 pyramidal neurons and were shown to project to other cortical areas. The final cell type we examined was the short layer 5 pyramidal neuron. These neurons are characterized by smaller cell bodies and an apical dendrite that only extends part way into layer 2/3. The short layer 5 pyramidal neurons are the corticocortical projection neurons (Games and Winer, 1988; Hallman et al., 1988; Hubener and Bolz, 1988; Hubener et al., 1990; Larkman and Mason, 1990).
We find that each of the three pyramidal cell types described have a unique pattern of axonal and dendritic arborization. The tall-tufted layer 5 pyramidal neurons have axonal arbors mainly in the deep layers and the greatest apical dendritic length (Figs. 10, 11). The tall-simple and short layer 5 pyramidal neurons have large superficial layer axonal arbors in addition to the axons located in the deep layers (Figs. 10, 11) but differ in their apical dendritic morphology, as described above. The difference in axonal arborizations between the tall-tufted and short layer 5 neurons that we described has also been previously reported in a comparison of bursting versus regular spiking neurons (Chagnac-Amitai et al., 1990). In their study, the bursting neurons in layer 5, which are the tall-tufted neurons, only have axons within the deep layers, while the regular spiking neurons, which include both the short and tall-simple neurons, have axons in the superficial layers as well as the deep layers.
Thus, we distinguish three cell types, while previous anatomical studies usually group together tall-tufted with tall-simple and previous studies separating neurons based on intrinsic firing probably grouped together tall-simple with short layer 5 pyramidal neurons. These three neuron types clearly differ, however, in both the extent of the apical dendritic arborization and the laminar axonal arborization pattern. These observations could explain some confusion in previous descriptions of the axonal arborization pattern for the tall-tufted neurons in the adult.
CONCLUDING REMARKS
It is of general importance that the normal developmental sequence of specific processes be defined in mice. As more laboratories are turning to the use of transgenic mice in their research, understanding the similarities and differences between mice and other model systems is becoming more important. In the case of development of laminar specific axonal arborizations we have found similarities with other species as well as differences. Our observations also clarify the adult organization of axonal laminar specificity for layer 2/3 and layer 5 pyramidal neurons in mouse barrel cortex.
Acknowledgments
We would like to thank Dan Feldman, Todd McLaughlin, and David Lyon for critical reading and comments on the manuscript, and Victor Borrell for helpful discussions during the course of the project.
Supporting Grant Information: The work described here was supported by the National Institutes of Health EY010742 and MH063912 to EMC and AG00216 to DDL.
References
- Akemann W, Zhong YM, Ichinohe N, Rockland KS, Knopfel T. Transgenic mice expressing a fluorescent in vivo label in a distinct subpopulation of neocortical layer 5 pyramidal cells. J Comp Neurol. 2004;480:72–88. doi: 10.1002/cne.20338. [DOI] [PubMed] [Google Scholar]
- Angevine JB, Sidman RL. Autoradiographic Study of Cell Migration during Histogenesis of Cerebral Cortex in the Mouse. Nature. 1961;192:766–768. doi: 10.1038/192766b0. [DOI] [PubMed] [Google Scholar]
- Annis CM, Robertson RT, O’Dowd DK. Aspects of early postnatal development of cortical neurons that proceed independently of normally present extrinsic influences. J Neurobiol. 1993;24:1460–1480. doi: 10.1002/neu.480241103. [DOI] [PubMed] [Google Scholar]
- Arimatsu Y, Ishida M. Distinct neuronal populations specified to form corticocortical and corticothalamic projections from layer VI of developing cerebral cortex. Neuroscience. 2002;114:1033–1045. doi: 10.1016/s0306-4522(02)00201-4. [DOI] [PubMed] [Google Scholar]
- Bender KJ, Rangel J, Feldman DE. Development of columnar topography in the excitatory layer 4 to layer 2/3 projection in rat barrel cortex. J Neurosci. 2003;23:8759–8770. doi: 10.1523/JNEUROSCI.23-25-08759.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardo KL, McCasland JS, Woolsey TA, Strominger RN. Local intra- and interlaminar connections in mouse barrel cortex. J Comp Neurol. 1990;291:231–255. doi: 10.1002/cne.902910207. [DOI] [PubMed] [Google Scholar]
- Bolz J, Castellani V. How do wiring molecules specify cortical connections? Cell Tissue Res. 1997;290:307–314. doi: 10.1007/s004410050935. [DOI] [PubMed] [Google Scholar]
- Bolz J, Castellani V, Mann F, Henke-Fahle S. Specification of layer-specific connections in the developing cortex. Prog Brain Res. 1996;108:41–54. doi: 10.1016/s0079-6123(08)62531-5. [DOI] [PubMed] [Google Scholar]
- Bolz J, Gotz M, Hubener M, Novak N. Reconstructing cortical connections in a dish. Trends Neurosci. 1993;16:310–316. doi: 10.1016/0166-2236(93)90107-w. [DOI] [PubMed] [Google Scholar]
- Bolz J, Novak N, Gotz M, Bonhoeffer T. Formation of target-specific neuronal projections in organotypic slice cultures from rat visual cortex. Nature. 1990;346:359–362. doi: 10.1038/346359a0. [DOI] [PubMed] [Google Scholar]
- Bolz J, Novak N, Staiger V. Formation of specific afferent connections in organotypic slice cultures from rat visual cortex cocultured with lateral geniculate nucleus. J Neurosci. 1992;12:3054–3070. doi: 10.1523/JNEUROSCI.12-08-03054.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrell V, Callaway EM. Reorganization of exuberant axonal arbors contributes to the development of laminar specificity in ferret visual cortex. J Neurosci. 2002;22:6682–6695. doi: 10.1523/JNEUROSCI.22-15-06682.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhalter A. Intrinsic connections of rat primary visual cortex: laminar organization of axonal projections. J Comp Neurol. 1989;279:171–186. doi: 10.1002/cne.902790202. [DOI] [PubMed] [Google Scholar]
- Burkhalter A, Bernardo KL, Charles V. Development of local circuits in human visual cortex. J Neurosci. 1993;13:1916–1931. doi: 10.1523/JNEUROSCI.13-05-01916.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler AK, Dantzker JL, Shah RB, Callaway EM. Development of visual cortical axons: layer-specific effects of extrinsic influences and activity blockade. J Comp Neurol. 2001;430:321–331. doi: 10.1002/1096-9861(20010212)430:3<321::aid-cne1033>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Callaway EM. Local circuits in primary visual cortex of the macaque monkey. Annu Rev Neurosci. 1998a;21:47–74. doi: 10.1146/annurev.neuro.21.1.47. [DOI] [PubMed] [Google Scholar]
- Callaway EM. Prenatal development of layer-specific local circuits in primary visual cortex of the macaque monkey. J Neurosci. 1998b;18:1505–1527. doi: 10.1523/JNEUROSCI.18-04-01505.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway EM, Katz LC. Development of axonal arbors of layer 4 spiny neurons in cat striate cortex. J Neurosci. 1992;12:570–582. doi: 10.1523/JNEUROSCI.12-02-00570.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway EM, Lieber JL. Development of axonal arbors of layer 6 pyramidal neurons in ferret primary visual cortex. J Comp Neurol. 1996;376:295–305. doi: 10.1002/(SICI)1096-9861(19961209)376:2<295::AID-CNE10>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Castellani V, Bolz J. Membrane-associated molecules regulate the formation of layer-specific cortical circuits. Proc Natl Acad Sci U S A. 1997;94:7030–7035. doi: 10.1073/pnas.94.13.7030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani V, Yue Y, Gao PP, Zhou R, Bolz J. Dual action of a ligand for Eph receptor tyrosine kinases on specific populations of axons during the development of cortical circuits. J Neurosci. 1998;18:4663–4672. doi: 10.1523/JNEUROSCI.18-12-04663.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviness VS., Jr Neocortical histogenesis in normal and reeler mice: a developmental study based upon [3H]thymidine autoradiography. Brain Res. 1982;256:293–302. doi: 10.1016/0165-3806(82)90141-9. [DOI] [PubMed] [Google Scholar]
- Chagnac-Amitai Y, Luhmann HJ, Prince DA. Burst generating and regular spiking layer 5 pyramidal neurons of rat neocortex have different morphological features. J Comp Neurol. 1990;296:598–613. doi: 10.1002/cne.902960407. [DOI] [PubMed] [Google Scholar]
- Dagnew E, Latchamsetty K, Erinjeri JP, Miller B, Fox K, Woolsey TA. Glutamate receptor blockade alters the development of intracortical connections in rat barrel cortex. Somatosens Mot Res. 2003;20:77–84. doi: 10.1080/0899022031000083852. [DOI] [PubMed] [Google Scholar]
- Dantzker JL, Callaway EM. The development of local, layer-specific visual cortical axons in the absence of extrinsic influences and intrinsic activity. J Neurosci. 1998;18:4145–4154. doi: 10.1523/JNEUROSCI.18-11-04145.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felipe J, Marco P, Fairen A, Jones EG. Inhibitory synaptogenesis in mouse somatosensory cortex. Cereb Cortex. 1997;7:619–634. doi: 10.1093/cercor/7.7.619. [DOI] [PubMed] [Google Scholar]
- Games KD, Winer JA. Layer V in rat auditory cortex: projections to the inferior colliculus and contralateral cortex. Hear Res. 1988;34:1–25. doi: 10.1016/0378-5955(88)90047-0. [DOI] [PubMed] [Google Scholar]
- Gilbert CD. Microcircuitry of the visual cortex. Annu Rev Neurosci. 1983;6:217–247. doi: 10.1146/annurev.ne.06.030183.001245. [DOI] [PubMed] [Google Scholar]
- Gotz M, Novak N, Bastmeyer M, Bolz J. Membrane-bound molecules in rat cerebral cortex regulate thalamic innervation. Development. 1992;116:507–519. [Google Scholar]
- Hallman LE, Schofield BR, Lin CS. Dendritic morphology and axon collaterals of corticotectal, corticopontine, and callosal neurons in layer V of primary visual cortex of the hooded rat. J Comp Neurol. 1988;272:149–160. doi: 10.1002/cne.902720111. [DOI] [PubMed] [Google Scholar]
- Heffner CD, Lumsden AG, O’Leary DD. Target control of collateral extension and directional axon growth in the mammalian brain. Science. 1990;247:217–220. doi: 10.1126/science.2294603. [DOI] [PubMed] [Google Scholar]
- Hevner RF, Daza RA, Rubenstein JL, Stunnenberg H, Olavarria JF, Englund C. Beyond laminar fate: toward a molecular classification of cortical projection/pyramidal neurons. Dev Neurosci. 2003;25:139–151. doi: 10.1159/000072263. [DOI] [PubMed] [Google Scholar]
- Hubener M, Bolz J. Morphology of identified projection neurons in layer 5 of rat visual cortex. Neurosci Lett. 1988;94:76–81. doi: 10.1016/0304-3940(88)90273-x. [DOI] [PubMed] [Google Scholar]
- Hubener M, Gotz M, Klostermann S, Bolz J. Guidance of thalamocortical axons by growth-promoting molecules in developing rat cerebral cortex. Eur J Neurosci. 1995;7:1963–1972. doi: 10.1111/j.1460-9568.1995.tb00719.x. [DOI] [PubMed] [Google Scholar]
- Hubener M, Schwarz C, Bolz J. Morphological types of projection neurons in layer 5 of cat visual cortex. J Comp Neurol. 1990;301:655–674. doi: 10.1002/cne.903010412. [DOI] [PubMed] [Google Scholar]
- Jackson CA, Peduzzi JD, Hickey TL. Visual cortex development in the ferret. I. Genesis and migration of visual cortical neurons. J Neurosci. 1989;9:1242–1253. doi: 10.1523/JNEUROSCI.09-04-01242.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper EM, Lubke J, Larkman AU, Blakemore C. Pyramidal neurons in layer 5 of the rat visual cortex. III. Differential maturation of axon targeting, dendritic morphology, and electrophysiological properties. J Comp Neurol. 1994;339:495–518. doi: 10.1002/cne.903390404. [DOI] [PubMed] [Google Scholar]
- Katz LC. Local circuitry of identified projection neurons in cat visual cortex brain slices. J Neurosci. 1987;7:1223–1249. doi: 10.1523/JNEUROSCI.07-04-01223.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LC. Specificity in the Development of Vertical Connections in Cat Striate Cortex. Eur J Neurosci. 1991;3:1–9. doi: 10.1111/j.1460-9568.1991.tb00805.x. [DOI] [PubMed] [Google Scholar]
- Katz LC, Callaway EM. Development of local circuits in mammalian visual cortex. Annu Rev Neurosci. 1992;15:31–56. doi: 10.1146/annurev.ne.15.030192.000335. [DOI] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Killackey HP, Koralek KA, Chiaia NL, Rhodes RW. Laminar and areal differences in the origin of the subcortical projection neurons of the rat somatosensory cortex. J Comp Neurol. 1989;282:428–445. doi: 10.1002/cne.902820309. [DOI] [PubMed] [Google Scholar]
- Kim HG, Connors BW. Apical dendrites of the neocortex: correlation between sodium- and calcium-dependent spiking and pyramidal cell morphology. J Neurosci. 1993;13:5301–5311. doi: 10.1523/JNEUROSCI.13-12-05301.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koester SE, O’Leary DD. Functional classes of cortical projection neurons develop dendritic distinctions by class-specific sculpting of an early common pattern. J Neurosci. 1992;12:1382–1393. doi: 10.1523/JNEUROSCI.12-04-01382.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koester SE, O’Leary DD. Connectional distinction between callosal and subcortically projecting cortical neurons is determined prior to axon extension. Dev Biol. 1993;160:1–14. doi: 10.1006/dbio.1993.1281. [DOI] [PubMed] [Google Scholar]
- Larkman A, Mason A. Correlations between morphology and electrophysiology of pyramidal neurons in slices of rat visual cortex. I. Establishment of cell classes. J Neurosci. 1990;10:1407–1414. doi: 10.1523/JNEUROSCI.10-05-01407.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levers TE, Edgar JM, Price DJ. The fates of cells generated at the end of neurogenesis in developing mouse cortex. J Neurobiol. 2001;48:265–277. doi: 10.1002/neu.1056. [DOI] [PubMed] [Google Scholar]
- Lund JS. Anatomical organization of macaque monkey striate visual cortex. Annu Rev Neurosci. 1988;11:253–288. doi: 10.1146/annurev.ne.11.030188.001345. [DOI] [PubMed] [Google Scholar]
- Lund JS, Boothe RG, Lund RD. Development of neurons in the visual cortex (area 17) of the monkey (Macaca nemestrina): a Golgi study from fetal day 127 to postnatal maturity. J Comp Neurol. 1977;176:149–188. doi: 10.1002/cne.901760203. [DOI] [PubMed] [Google Scholar]
- Luskin MB, Shatz CJ. Neurogenesis of the cat’s primary visual cortex. J Comp Neurol. 1985;242:611–631. doi: 10.1002/cne.902420409. [DOI] [PubMed] [Google Scholar]
- Martin KA. Neuronal Circuits in Cat Striate Cortex. In: Jones EG, Peters A, editors. Cerebral Cortex. New York: Plenum Press; 1984. pp. 241–284. [Google Scholar]
- Mason A, Larkman A. Correlations between morphology and electrophysiology of pyramidal neurons in slices of rat visual cortex. II. Electrophysiology. J Neurosci. 1990;10:1415–1428. doi: 10.1523/JNEUROSCI.10-05-01415.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCasland JS, Bernardo KL, Probst KL, Woolsey TA. Cortical local circuit axons do not mature after early deafferentation. Proc Natl Acad Sci U S A. 1992;89:1832–1836. doi: 10.1073/pnas.89.5.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheva KD, Beaulieu C. Quantitative aspects of synaptogenesis in the rat barrel field cortex with special reference to GABA circuitry. J Comp Neurol. 1996;373:340–354. doi: 10.1002/(SICI)1096-9861(19960923)373:3<340::AID-CNE3>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Miller B, Blake NM, Erinjeri JP, Reistad CE, Sexton T, Admire P, Woolsey TA. Postnatal growth of intrinsic connections in mouse barrel cortex. J Comp Neurol. 2001;436:17–31. [PubMed] [Google Scholar]
- Miller M. Maturation of rat visual cortex. I. A quantitative study of Golgi-impregnated pyramidal neurons. J Neurocytol. 1981;10:859–878. doi: 10.1007/BF01262658. [DOI] [PubMed] [Google Scholar]
- Miller M, Peters A. Maturation of rat visual cortex. II. A combined Golgi-electron microscope study of pyramidal neurons. J Comp Neurol. 1981;203:555–573. doi: 10.1002/cne.902030402. [DOI] [PubMed] [Google Scholar]
- Miller MW. Maturation of rat visual cortex. III. Postnatal morphogenesis and synaptogenesis of local circuit neurons. Brain Res. 1986;390:271–285. doi: 10.1016/s0006-8993(86)80236-0. [DOI] [PubMed] [Google Scholar]
- Novak N, Bolz J. Formation of specific efferent connections in organotypic slice cultures from rat visual cortex cocultured with lateral geniculate nucleus and superior colliculus. Eur J Neurosci. 1993;5:15–24. doi: 10.1111/j.1460-9568.1993.tb00200.x. [DOI] [PubMed] [Google Scholar]
- O’Leary DD, Stanfield BB. Selective elimination of axons extended by developing cortical neurons is dependent on regional locale: experiments utilizing fetal cortical transplants. J Neurosci. 1989;9:2230–2246. doi: 10.1523/JNEUROSCI.09-07-02230.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary DD, Terashima T. Cortical axons branch to multiple subcortical targets by interstitial axon budding: implications for target recognition and “waiting periods”. Neuron. 1988;1:901–910. doi: 10.1016/0896-6273(88)90147-x. [DOI] [PubMed] [Google Scholar]
- Polleux F, Dehay C, Kennedy H. The timetable of laminar neurogenesis contributes to the specification of cortical areas in mouse isocortex. J Comp Neurol. 1997;385:95–116. [PubMed] [Google Scholar]
- Rakic P. Neurons in rhesus monkey visual cortex: systematic relation between time of origin and eventual disposition. Science. 1974;183:425–427. doi: 10.1126/science.183.4123.425. [DOI] [PubMed] [Google Scholar]
- Rhoades RW, Crissman RS, Bennett-Clarke CA, Killackey HP, Chiaia NL. Development and plasticity of local intracortical projections within the vibrissae representation of the rat primary somatosensory cortex. J Comp Neurol. 1996;370:524–535. doi: 10.1002/(SICI)1096-9861(19960708)370:4<524::AID-CNE8>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Skaliora I, Adams R, Blakemore C. Morphology and growth patterns of developing thalamocortical axons. J Neurosci. 2000;20:3650–3662. doi: 10.1523/JNEUROSCI.20-10-03650.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Goto T, Miyama S, Nowakowski RS, Caviness VS., Jr Sequence of neuron origin and neocortical laminar fate: relation to cell cycle of origin in the developing murine cerebral wall. J Neurosci. 1999;19:10357–10371. doi: 10.1523/JNEUROSCI.19-23-10357.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiola A, Hamzei-Sichani F, Peterlin Z, Yuste R. Quantitative morphologic classification of layer 5 neurons from mouse primary visual cortex. J Comp Neurol. 2003;461:415–428. doi: 10.1002/cne.10628. [DOI] [PubMed] [Google Scholar]
- Voelker CC, Garin N, Taylor JS, Gahwiler BH, Hornung JP, Molnar Z. Selective Neurofilament (SMI-32, FNP-7 and N200) Expression in Subpopulations of Layer V Pyramidal Neurons In Vivo and In Vitro. Cereb Cortex. 2004;14:1276–1286. doi: 10.1093/cercor/bhh089. [DOI] [PubMed] [Google Scholar]
- Wang Z, McCormick DA. Control of firing mode of corticotectal and corticopontine layer V burst-generating neurons by norepinephrine, acetylcholine, and 1S,3R-ACPD. J Neurosci. 1993;13:2199–2216. doi: 10.1523/JNEUROSCI.13-05-02199.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise SP, Jones EG. Somatotopic and columnar organization in the corticotectal projection of the rat somatic sensory cortex. Brain Res. 1977;133:223–235. doi: 10.1016/0006-8993(77)90760-0. [DOI] [PubMed] [Google Scholar]
- Yabuta NH, Butler AK, Callaway EM. Laminar specificity of local circuits in barrel cortex of ephrin-A5 knockout mice. J Neurosci. 2000;20:RC88. doi: 10.1523/JNEUROSCI.20-15-j0005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabuta NH, Callaway EM. Cytochrome-oxidase blobs and intrinsic horizontal connections of layer 2/3 pyramidal neurons in primate V1. Vis Neurosci. 1998;15:1007–1027. doi: 10.1017/s0952523898156018. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Higashi S, Toyama K. Stop and branch behaviors of geniculocortical axons: a time-lapse study in organotypic cocultures. J Neurosci. 1997;17:3653–3663. doi: 10.1523/JNEUROSCI.17-10-03653.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N, Kurotani T, Toyama K. Neural connections between the lateral geniculate nucleus and visual cortex in vitro. Science. 1989;245:192–194. doi: 10.1126/science.2749258. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Yamada K, Kurotani T, Toyama K. Laminar specificity of extrinsic cortical connections studied in coculture preparations. Neuron. 1992;9:217–228. doi: 10.1016/0896-6273(92)90161-6. [DOI] [PubMed] [Google Scholar]
- Zhang ZW. Maturation of layer V pyramidal neurons in the rat prefrontal cortex: intrinsic properties and synaptic function. J Neurophysiol. 2004;91:1171–1182. doi: 10.1152/jn.00855.2003. [DOI] [PubMed] [Google Scholar]