Abstract
Purpose
Resistance to glucocorticoid (GC) is a significant problem in the clinical management of lymphoid malignancies. Addressing this issue via a mechanistic understanding of relevant signaling pathways is more likely to yield positive outcomes.
Experimental Design
We used gene set enrichment analysis (GSEA), multiple genetic models of gain and loss of function in B-cell lymphoma cell lines, in vitro and in vivo, and primary patient samples to characterize a novel relationship between the cyclic AMP/phosphodiesterase 4B (cAMP/PDE4B), AKT/ mTOR activities, and GC responses.
Results
Starting from the GSEA, we found that overexpression of the PDE4B in diffuse large B-cell lymphoma (DLBCL) impinge on the same genes/pathways that are abnormally active in GC-resistant tumors. We used genetically modified cell lines to show that PDE4B modulates cAMP inhibitory activities toward the AKT/mTOR pathway and defines GC resistance in DLBCL. In agreement with these data, pharmacologic inhibition of PDE4 in a xenograft model of human lymphoma unleashed cAMP effects, inhibited AKT, and restored GC sensitivity. Finally, we used primary DLBCL samples to confirm the clinical relevance and biomarker potential of AKT/mTOR regulation by PDE4B.
Conclusions
Together, these data mechanistically elucidated how cAMP modulates GC responses in lymphocytes, defined AKT as the principal transducer of the growth inhibitory effects of cAMP in B cells, and allowed the formulation of genomics-guided clinical trials that test the ability of PDE4 inhibitors to restore GC sensitivity and improve the outcome of patients with B-cell malignancies.
Introduction
In recent years, it has become apparent that the successful development of novel therapeutic approaches for the treatment of cancer needs to be guided by an improved understanding of disease pathogenesis. Although these rational strategies have recently yielded important results (1), they are only rarely conceived with emphasis on overcoming acquired or innate resistance to pharmacologic agents that are already part of the therapeutic armamentarium. In this context, a pressing issue is the identification and validation of strategies aimed at improving or reestablishing glucocorticoid (GC) sensitivity in lymphoid malignancies. This is an important goal because GCs remain an important agent in the treatment of these tumors and, despite all advances in the molecular classification of these entities (2–4), the in vitro and in vivo responses to GCs continue to be one of the determinants of clinical outcome, in particular in acute lymphoid leukemia and multiple myeloma (5–7).
Cyclic AMP (cAMP) is a ubiquitous second messenger with marked growth inhibitory and proapoptotic properties in lymphocytes (8–11). At the termination point, the intracellular levels of cAMP are controlled by phosphodiesterases (PDE). In immune cells, members of the PDE4 family, particularly PDE4B in B lymphocytes, account for most of the cAMP hydrolysis and inactivation (12). This feature, combined with pharmacologic and structural principles that make PDEs excellent drug targets, prompted the testing of PDE4 inhibitors for inflammatory conditions such as asthma and chronic obstructive pulmonary disease (13).
We have previously shown that PDE4B expression was significantly elevated in patients with fatal diffuse large B-cell lymphoma (DLBCL; refs. 4, 9). Subsequently, we used in vitro genetic and pharmacologic modulation of PDE4B activity to confirm that this enzyme abrogates the growth inhibitory effects of cAMP in DLBCL, explaining why elevated PDE4B expression contributes to the poor outcome of subsets of B-cell tumors (9, 11). These data pointed to the potential of PDE4 inhibitors as antilymphoma agents and highlighted the addiction of subsets of DLBCL to low cAMP levels. Importantly, in these initial investigations, we uncovered an interplay between cAMP/PDE4B and the PI3K/AKT pathway in DLBCL, whereby cAMP-induced apoptosis was found to be dependent on the downmodulation of this growth-promoting pathway.
Recently, an unbiased chemical genomic approach unveiled a critical role for the AKT/mTOR pathway in modulating GC resistance in acute lymphoid leukemia (ALL; ref. 14). These observations led us to put forward the novel hypothesis that the cAMP-mediated downregulation of AKT could significantly influence GC response and that PDE4B inhibition may restore GC sensitivity in malignant lymphocytes. Herein, we comprehensively examined this hypothesis and show that (i) high expression of PDE4B in primary DLBCL impinges on the same genes and pathways associated with GC resistance in ALL, (ii) PDE4B expression directly correlates with GC resistance in B-cell lymphomas, (iii) genetic models of gain and loss of function for PDE4B confirmed the role of this enzyme in controlling GC effects in B-cell lymphoma, (iv) genetic modulation of AKT activity indicated that this kinase is the principal transducer of cAMP inhibitory effects in malignant B cells, and (v) pharmacologic inhibition of PDE4 restored GC sensitivity in B-cell lymphomas, in vitro and in vivo. Importantly, we showed that the correlation between PDE4B expression and AKT/mTOR activity was also present in primary DLBCL.
Together, these data link PDE4B to AKT/mTOR-mediated GC resistance, mechanistically explain how elevation of intracellular cAMP levels improves GC sensitivity, and provide further preclinical support for expanding the testing of PDE4 inhibitors in lymphoid cancers.
Materials and Methods
Cell lines, primary tumors, and normal B cells
Human DLBCL cell lines (DHL6, DHL7, DHL10, Ly1; refs. 15, 16) and Ramos (human Burkitt lymphoma cell line) were cultured in RPMI 1640 (Invitrogen) supplemented with 10% heat-inactivated FBS (Hyclone), 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mmol/L L-glutamine, and 10 mmol/L HEPES buffer in a 5% humidified CO2 incubator at 37°C, as previously reported (11). The HEK293 cell line was cultured in Dulbecco’s modified Eagle’s medium supplemented as described previously. The DLBCL cell line Ly3 was grown in Iscove’s modified Dulbecco’s medium supplemented with 20% human serum, as we described elsewhere (9). The primary DLBCLs were obtained from our tumor bank and reported before (17); the detailed features of the samples used in this study are described in Supplementary Table S3. These studies were approved by the Institutional Review Board of the University of Texas Health Science Center at San Antonio (UTHSCSA). Murine mature B cells were isolated and purified (>90% purity) from wild-type (WT) C57BL/6 mice spleens, as we recently described (11) and according to approval from the Institutional Animal Care and Use Committee (IACUC) of the UTHSCSA.
Gene set enrichment analysis
A gene collection containing 101 probe sets expressed at significantly higher levels in GC-resistant than in GC-sensitive ALL samples (14) was analyzed within the context of a cohort of primary DLBCL (n = 56) previously investigated by gene expression profiling on microarrays (18). PDE4B expression below and above the median, as we determined previously by quantitative real-time reverse transcriptase PCR (qRT-PCR; ref. 9), dichotomized the tumors in PDE4B low and high, respectively.
Cell proliferation and apoptosis
The inhibitory activities of forskolin (2–40 μmol/L), 8-Br-cAMP (2 mmol/L), dexamethasone (1–1,000 nmol/L), etoposide (2–40 μmol/L), staurosporine (0.05–20 nmol/ L), and rolipram (2–20 μmol/L), all from Sigma-Aldrich or A.G. Scientific, on cell proliferation and viability were determined with the CellTiter 96 Aqueous Non-Radioactive Cell Proliferation Assay (MTS; Promega). The apoptosis rate was measured with Annexin V–phycoerythrin staining followed by fluorescence activated cell-sorting (FACS) analysis according to the manufacturer’s guidelines (Apoptosis Detection Kit I; BD Biosciences) and propidium iodide (Sigma-Aldrich) staining also followed by FACS, as we described elsewhere (9). In these assays, the relevant cell lines were cultured either in FBS or in human serum–containing media, and data were collected at 48, 72, or 96 hours. Specific details on culture conditions, time points, and range of drugs concentration are indicated in the figures and figure legends.
cAMP quantification
Intracellular cAMP levels were measured using the Parameter cAMP Assay Kit (R&D Systems), according to the manufacturer’s instructions. To modulate cAMP concentrations, the relevant cell lines were treated with forskolin, an adenylyl cyclase activator, and/or rolipram, a pan-PDE4 inhibitor (both purchased from Sigma). In brief, the PDE4B-high cell lines (Ly1, Ly3, and Ramos) were treated with forskolin (20–40 μmol/L) in the presence or absence of rolipram (20 μmol/L) for up to 72 hours. The genetically modified PDE4B-low DHL6 cell line was treated with forskolin (40 μmol/L) for 15 minutes.
Genetic modulation of PDE4B and AKT in DLBCL
The generation of DHL6 cell lines stably expressing a retrovirus construct (MSCV-eGFP) encoding the PDE4B-WT, PDE4B-PDE inactive (PDE4B-PI), or constitutively active (CA)-AKT has been reported previously (9). To create Ramos cell lines stably expressing PDE4B-specific short hairpin RNA (shRNA) constructs, we cloned 2 previously validated targeting sequences (PDE4B#2, 5′-GCCUAAA-CAAUACAAGCAU-3′, PDE4B#5, 5′-GCAUCUCACGCUU-UGGAGU-3′) into the pSilencer 4.1-CMV vector (Ambion). These constructs, and a shRNA-control–containing vector, were electroporated into Ramos cells with a Gene Pulser II system (Bio-Rad Laboratories) at 250 V and 950 μF. Subsequently, puromycin-resistant populations were obtained and clones were generated by limiting dilution. Confirmation of the PDE4B knockdown was done with PDE4B-specific qRT-PCR, as we described (9, 11). Expression of the glucocorticoid receptor (GR) in these cell models was determined by qRT-PCR, at baseline and following exposure to forskolin, using primers that amplify all GR iso-forms (GR forward, 5′-GGATCATGACTACGCTCAAC-3′; reverse, 5′-TGCAGTAGGGTCATTTGGTC-3′).
AKT and mTOR activity
The impact of PDE4B and cAMP levels on AKT/mTOR was determined by immunoblotting with anti-phospho-AKT (S473), phospho-S6K (T389), phospho-4E-BP1 (T37/ 46), all from Cell Signaling Technology, and anti-MCL1 antibody (Santa Cruz Biotechnology). Loading control antibodies included β-actin and α-tubulin (Sigma-Aldrich).
Xenograft model of human B-cell lymphoma and in vivo imaging
Ramos cells were transduced with a retrovirus encoding the luciferase gene, and stable populations were established with neomycin selection. Subsequently, 2 independent cohorts (n = 20 and n = 9) of 5-week-old nude mice (Harlan) were sublethally irradiated (400 cGy) and inoculated with 2 × 106 cells in the right flank. Three days after cell implantation, mice were subjected to noninvasive bioluminescent imaging (IVIS Spectrum; Caliper Life Sciences, as we described (19), and randomized into the following treatment groups: vehicle [1% dimethyl sulfoxide (DMSO) in distilled water], dexamethasone (15 mg/kg in distilled water), rolipram (10 mg/kg, 1% DMSO in distilled water), or combination of dexamethasone and rolipram, all administered daily in the peritoneal cavity. Treatment efficacy was monitored with weekly imaging and photon flux quantifications, and experiments were terminated when tumors were larger than 1 cm3 or mice became moribund. These studies were approved by the IACUC of the UTHSCSA.
Statistics
The statistical significance of all in vitro assays was determined with a 2-tailed Student’s t test or with one-way ANOVA with the Student–Newman–Keuls multiple comparisons test. The statistical values of the differences between the multiple treatment groups studied in vivo were determined with the Kruskal–Wallis test. In all instances, P < 0.05 was considered significant. Data analyses were done with Prism software (version 5.0; GraphPad) and Excel software (Microsoft). Dose–effect curves were calculated with the CalcuSyn software (Biosoft) and used to generate the combination index (CI), reflecting the synergistic activity of the drugs tested.
Results
A gene expression signature shared by DLBCL expressing high levels of PDE4B and ALL resistant to GC
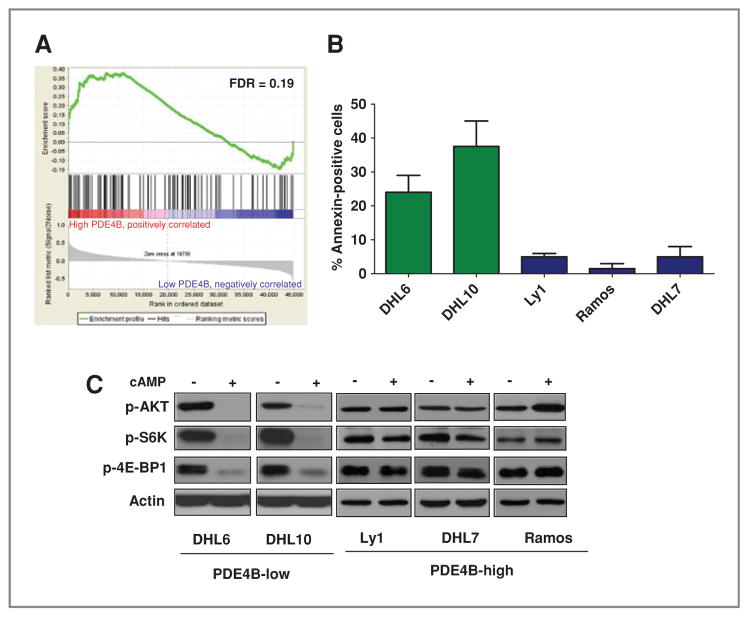
We showed that PDE4B activity controls PI3K/AKT signals in DLBCL (9), whereas Wei and colleagues independently determined that the AKT/mTOR pathway regulates GC sensitivity in ALL (14) Together, these data led us to hypothesize that PDE4B, by regulating the inhibitory effects of cAMP toward AKT, may play an important role in the resistance to GCs found in subsets of lymphoid cancers. We reasoned that if this hypothesis was correct, PDE4B expression should impinge on the same genes/ pathways that control GC response in malignant lymphocytes and that the gene signatures of GC resistance and PDE4B overexpression should be related, even if determined in distinct tumor types. To test this concept, we used gene set enrichment analysis (GSEA) (20) and found a significant enrichment of "GC resistance" genes in the expression signature of PDE4B-high DLBCLs [false discovery rate (FDR) = 0.19; Fig. 1A and Supplementary Table S1).
Figure 1.
PDE4B expression correlates with GC response in B-cell lymphomas. A, enrichment plot from GSEA conducted with 101 probe sets expressed at high levels in GC-resistant ALL samples (14) in a collection of primary DLBCLs dichotomized high versus low PDE4B expression (9, 18). Statistically significant enrichment (FDR = 0.19) of "GC resistance" genes was found in DLBCLs expressing high PDE4B levels when compared with tumors with low PDE4B expression. B, Annexin V staining shows that in the presence of cAMP (forskolin 5 μmol/L), DLBCL lymphoma cell lines expressing low levels of PDE4B were markedly more sensitive to dexamethasone (100 nmol/L) than B-cell lymphomas expressing high PDE4B levels (P < 0.05, one-way ANOVA test). Data shown were collected 48 hours after drug exposure, represent the mean of 2 independent experiments, and normalized to apoptosis rate of cells exposed exclusively to dexamethasone; normalization by forskolin yields mostly the same result. C, Western blot analyses of PDE4B-low and -high cell lines following rapid elevation of intracellular cAMP levels (forskolin 40 μmol/L for 60 minutes) show significant inhibition of AKT (S473), S6K, and 4E-BP1 phosphorylation in a PDE4B-dependent manner. Actin immunoblotting confirms equal protein loading.
PDE4B expression and cAMP levels associate with AKT/mTOR activity and GC response in B-cell lymphomas
The positive results obtained in the GSEA gave us the impetus to test the impact of PDE4B expression/activity on the response to GC found in lymphomas. Toward this end, we used a panel of B lymphoma cell lines expressing low/null or high levels of PDE4B as defined by mRNA expression (Supplementary Fig. S1A), which is highly correlated with previously defined protein expression (9). As we have reported earlier (9, 11), these cell lines present with low basal activity of adenylyl cyclase and, without proper stimulation, lack intracellular cAMP. Thus, to fully capture the relevance of PDE4B activity in this context, we experimentally increased intracellular cAMP with the adenylyl cyclase activator forskolin or with the cell-permeable synthetic cAMP 8-Br-cAMP.
On examining 5 independent B lymphoma cell lines, we found that in the presence of cAMP, tumors expressing low levels of PDE4B were markedly more sensitive to dexamethasone-induced apoptosis than lymphomas expressing high PDE4B levels (P < 0.05, one-way ANOVA test) (Fig. 1B and Supplementary Fig. S1B, measurements done 48 hours after drug exposure with Annexin V and propidium iodide, respectively). Noticeably, at the concentrations used, neither forskolin (5 μmol/L) nor synthetic cAMP (2 mmol/L) significantly affected cell proliferation or viability, indicating that in this context, cAMP acted primarily as a sensitizer of GC activities in a PDE4B-dependent manner (Supplementary Fig. S1C and D). Furthermore, in agreement with the important role of cAMP levels and PDE4B activity in regulating GC efficacy in lymphomas, we detected a progressive dexamethasone-induced growth inhibition when concomitantly augmenting the intracellular levels of cAMP (Supplementary Fig. S1E).
To further define the relationship between cAMP/ PDE4B, AKT/mTOR, and GC sensitivity in B-cell lymphomas, we also determined the phosphorylation levels of AKT and 2 mTOR-regulated proteins in our model. As shown in Figure 1C (and Supplementary Fig. S1F), cAMP markedly inhibited the phosphorylation of AKT, S6K, and 4E-BP1 (the latter two are surrogate markers for mTOR inhibition) in a PDE4B-dependent manner. Furthermore, we showed that the cAMP-mediated downregulation of this pathway is readily detectable in normal mature B cells (Supplementary Fig. S1G), thus defining the physiologic relevance of these signals and highlighting the role of the abnormally high PDE4B expression in blocking this growth inhibitory axis in B-cell lymphomas.
In addition, because recent data have suggested a correlation between the pretreatment levels of the antiapoptotic protein MCL1 and GC response in MLL-translocated ALLs (21), we also measured the expression of this antiapoptotic protein in our lymphoma models. In the group of cell lines analyzed, MCL1 baseline expression did not per se predicted GC response (Supplementary Fig. S1H), although, as we will show later, MCL1 can be downregulated by cAMP in a PDE4B- and AKT-dependent fashion. Finally, c-MYC expression has been suggested to influence GC sensitivity in ALL (22). However, in the DLBCL cell lines studied here, c-MYC status [activated by a translocation t(8;14) in Ramos and DHL10, or wild type in Ly1, Ly3, and DHL6; refs. 23, 24], appear to not segregate with the degree of GC sensitivity.
PDE4B and AKT are central regulators of GC sensitivity in DLBCL
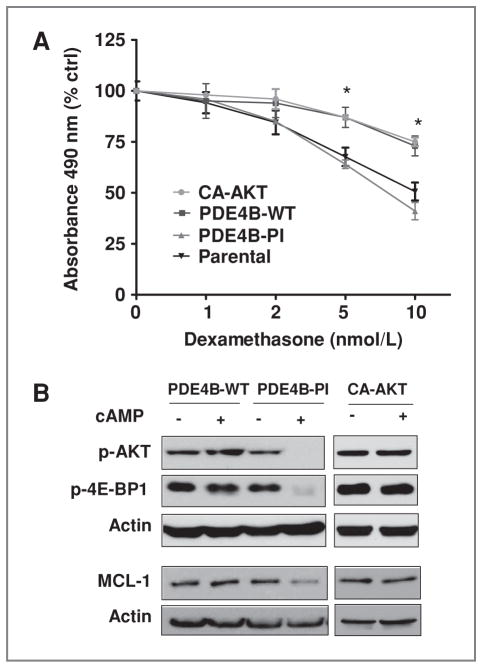
The aforementioned data defined an association between PDE4B expression and GC sensitivity, which related to cAMP-mediated inhibition of AKT/mTOR. To move beyond these correlative analyses, and firmly establish the roles of PDE4B and AKT in regulating GC response in B-cell lymphomas, we generated DLBCL models stably expressing PDE4B (WT and PI) or a constitutively active (myristoylated) AKT protein (CA-AKT). We found that reconstitution of PDE4B-WT expression in the PDE4B-null and GC-sensitive DHL6 cell line rendered these cells significantly more resistant to dexamethasone (P < 0.05, 2-tailed Student’s t test) than their isogenic counterpart expressing a PI mutant, or the parental cell line (Fig. 2A), thus indicating that the regulation of GC response in this context is related to the enzymatic activity of PDE4B, as confirmed by measurements of intracellular cAMP levels (Supplementary Fig. S2A). In addition, ascertaining the central role of AKT in transducing cAMP effects and controlling GC responses in B-cell lymphomas, we showed that the GC-sensitive DHL6 cell line stably expressing CA-AKT became resistant to dexamethasone to the same extent as their isogenic counterparts ectopically expressing PDE4B (Fig. 2A). These results indicate that most, if not all, of the cAMP-mediated PDE4B-controlled GC responses observed in B-cell lymphomas are transduced by AKT. In agreement with this concept, in the PDE4B-WT and CA-AKT expressing cells, cAMP did not inhibited AKT/mTOR phosphor-ylation, whereas these effects were preserved in PI-mutant expressing cells (Fig. 2B). Furthermore, we show that cAMP, in a PDE4B- and AKT-dependent manner, also inhibits the expression of MCL1 (Fig. 2B). These data agree with a previous observation that placed MCL1 downstream to AKT in regulating GC resistance in ALL (14), but they also highlight the novel role of cAMP/PDE4B in controlling these events. Together, our data suggest that high PDE4B expression in B-cell lymphomas contributes to GC resistance by blocking cAMP inhibitory effect on the AKT/ mTOR pathway and its downstream components.
Figure 2.
Interplay between PDE4B and AKT controls GC response in DLBCL. A, cell proliferation assays show that reconstitution of PDE4B-WT expression or a CA-AKT mutant equally induces dexamethasone resistance in the GC-sensitive DHL6 cell line (*, P < 0.05, 2-tailed Student's t test for WT vs. PDE4B-PI, and CA-AKT vs. PI). PDE4B-PI cell line response is similar to that of the DHL6 parental cell line. Growth inhibition curves include escalating doses of dexamethasone in the presence of forskolin (2 μmol/L); data were normalized by cells exposed solely to forskolin (2 μmol/L), hence controlling for the effects of this small increment in cAMP levels on cell growth. Results shown are mean and SD of 4 independent experiments done in triplicate and collected at 72 hours. B, Western blot–based determination of the phosphorylation levels of AKT (S473) and 4E-BP1 (T37/46), and total MCL1, shows that isogenic cells expressing PDE4B-WT or CA-AKT, but not a PDE4B-PI mutant, become resistant to the marked inhibitory effects of cAMP (forskolin 40 μmol/L for 60 minutes) toward this pathway. β-Actin immunoblots confirm equal protein loading.
Because AKT has a wide range of activities, we found necessary to establish that in the present context, its constitutive activation was primarily leading to a blockade in the transduction of cAMP signals and not interfering with other relevant physiologic processes. To address this possibility, we first compared the intracellular levels of cAMP in PDE4B-WT DHL6, PDE4B-PI, and CA-AKT cells. Upon exposure to forskolin, cAMP levels were similarly raised in the PDE4B-PI and CA-AKT cells, despite the fact that the latter were resistant to cAMP-controlled GC sensitization, and in both cases at significantly higher levels than in the PDE4B-WT expressing cells (P < 0.05, one-way ANOVA test, Supplementary Fig. S2A); these data show that AKT does not interfere with the generation of cAMP. Next, we examined whether the previously reported link between induction of GR expression by cAMP and restoration of GC sensitivity (25, 26) accounted for our results. Here, we tested whether CA-AKT expression was interfering with cAMP-mediated GR regulation. Our data showed that despite being resistant to cAMP-controlled GC sensitization, in the CA-AKT lymphoma cells, GR expression was promptly induced by cAMP (Supplementary Fig. S2B). Finally, we showed that the GC resistance acquired by the CA-AKT expressing cells was not a general growth advantage phenomenon; in these assays, we found that in the absence of cAMP, CA-AKT lymphomas proliferated at the same rate and responded to dexamethasone in the same manner as their isogenic counterparts expressing PDE4B-WT and PDE4B-PI cells (Supplementary Fig. S2C).
Taken together, these results establish PDE4B as an important regulator of GC efficacy in lymphomas, indicate that a functional AKT pathway is critical for the cAMP/ PDE4B-controlled restoration of GC activity, and show that induction of GR expression does not fully account for the improved GC response that follows PDE4B inhibition/ cAMP elevation in lymphocytes.
Genetic and pharmacologic inhibition of PDE4B in B-cell lymphoma restores GC sensitivity
In vitro
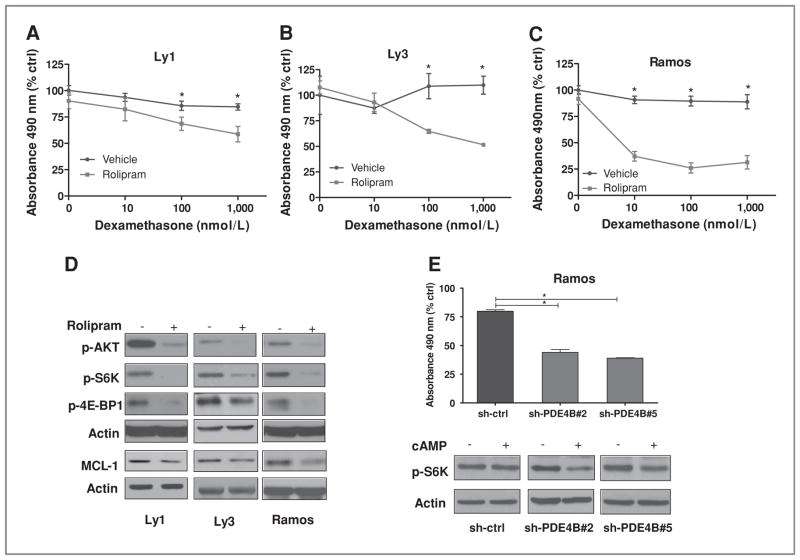
Up to this point, we have used genetic models of PDE4B gain of function to establish the role of this enzyme in controlling GC responses in B-cell lymphomas. However, our ultimate objective is to test in the clinic the potential of pharmacologic PDE4B inhibition as a rational strategy to reverse GC resistance in lymphoid malignancies. To address this translational aspect of our research, we examined the effects of rolipram, a prototypical PDE4 inhibitor (13), in 3 independent GC-resistant lymphoma cell lines that express high levels of PDE4B. In each instance, PDE4 inhibition with rolipram, in the presence of cAMP, significantly increased the antilymphoma effects of dexamethasone (Fig. 3A–C, P < 0.05, 2-tailed Student’s t test, and Supplementary Fig. S3A) in a highly synergistic manner (CI <0.1 in all 3 cell lines tested, as determined by isobologram analysis using the CalcuSyn Software, Supplementary Table S2 and Supplementary Fig S3B). Showing the specificity of these effects, exposure to rolipram was accompanied by elevation of intracellular levels of cAMP (Supplementary Fig. S3C) but did not impact on the effectiveness of 2 broad-spectrum chemotherapeutic agents (Supplementary Fig. S3D). Finally, confirming the central role of PDE4B in regulating cAMP effects toward AKT/ mTOR, and on the relevance of this pathway in controlling GC sensitivity in B-cell lymphomas, rolipram treatment was accompanied by a decrease in the phospho levels of AKT, S6K, and 4EPB1 (Fig. 3D).
Figure 3.
Pharmacologic and genetic inhibition of PDE4B improves GC sensitivity in B-cell lymphoma in vitro. A–C, in 3 independent B-cell lymphoma cell lines (Ly1, Ly3, and Ramos) that express high levels of PDE4B, treatment with the PDE4-specific inhibitor rolipram significantly improved the antilymphoma activity of dexamethasone in a dose-dependent manner (10–1,000 nmol/L; *, P < 0.05, 2-tailed Student's t test). In agreement with the high expression of PDE4B and low basal activity of adenylyl cyclases in these cells, forskolin (20–40 μmol/L) or rolipram alone (10–20 μmol/L) had limited effect on cell proliferation (see Supplementary Fig. S3A). Data shown are mean and SD of all data points from experiments done in duplicate and collected at 72 hours (Ly1 and Ramos) or in triplicate and collected at 96 hours (Ly3), normalized by vehicle-treated cells (no dexamethasone). D, Western blot analyses of the phosphorylation of AKT (S473), S6K (T389), 4E-BP1 (T37/46), and total MCL1 levels in 3 aggressive lymphoma cell lines show that PDE4 inhibition with rolipram (10–20 μmol/L) significantly inhibits this pathway. In lanes labeled (−), cells were exposed to forskolin (20–40 μmol/L) alone, and in (+), forskolin and rolipram. E, top, in the PDE4B-high, GC-resistant B-cell lymphoma cell line Ramos, stable expression of 2 independent PDE4B-specific shRNA constructs significantly increased GC sensitivity (dexamethasone 100 nmol/L; P < 0.05, one-way ANOVA test) in comparison with cells expressing a shRNA-control (sh-ctrl) construct; all cells were also exposed to forskolin (10 μmol/L). Results shown are the mean and SD of data generated in triplicate, collected at 96 hours, and representative of 3 independent experiments. Bottom, Western blot detection of S6K phosphorylation (T389) in an aggressive B-cell lymphoma stably expressing control- or PDE4B-shRNA confirm the role of this enzyme in regulating cAMP effects (forskolin 40 μmol/L for 60 minutes) on the mTOR pathway and GC resistance.
Our earlier data pointed to PDE4B as the critical PDE in the controlling cAMP signals in malignant B cells (4, 9, 11). Thus, to confirm the specificity of the pharmacologic observations derived from the use of rolipram, we created B lymphoma cell lines stably expressing 2 independent shRNA constructs targeting the PDE4B gene. These cells showed downregulation of PDE4B, elevated intracellular cAMP levels (Supplementary Fig. S3E and F), and more importantly became sensitive to dexamethasone activity (Fig. 3E). Of note, we were consistently unable to isolate stable polyclonal or monoclonal B-cell populations displaying a substantial knockdown of PDE4B expression (e.g., >80%), using multiple RNA interference strategies, and only those cells with more modest downregulation of this gene (~40%) were rescued for downstream experiments. We attribute these findings to the addiction of B lymphoma cells to low levels of cAMP and loss of viability with continuous suppression of this gene. Nonetheless, although in the cell populations that we analyzed the shRNA effects on PDE4B expression were only moderate (Supplementary Fig. S3E), they readily led to increase in intracellular cAMP levels (Supplementary Fig. S3F) and improvement in dexamethasone activity (Fig. 3E).
In vivo
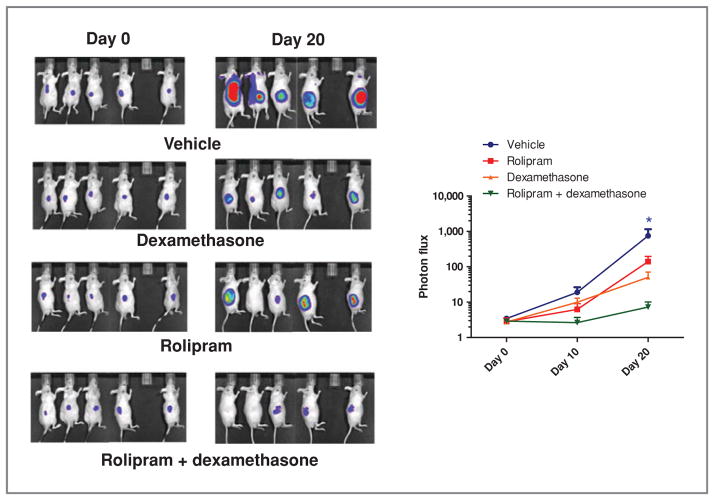
To determine whether PDE4 inhibition could also reverse GC resistance in a more relevant setting, we tested the efficacy of dexamethasone, rolipram, or their combination for the treatment of a xenograft model of human lymphoma with noninvasive luminescent imaging capability. In these assays, the mice were inoculated subcutaneously with the aggressive B lymphoma cell line Ramos (PDE4B-high, GC-resistant) constitutively expressing the luciferase gene. Seventy-two hours after tumor implantation, the mice were imaged, assigned to distinct treatment group, and followed weekly with luminescence imaging. Consistent with our in vitro results, within 2 to 3 weeks of treatment, mice from two2 independent cohorts that received a combination of rolipram plus dexamethasone had a significantly better clinical response with a pronounced inhibition in tumor growth than those receiving vehicle or single agents, in association with down-modulation of phospho-AKT signals (Fig. 4A and Supplementary Fig. S3G; P < 0.05, Kruskal–Wallis test).
Figure 4.
Pharmacologic inhibition of PDE4B improves GC sensitivity in B-cell lymphoma in vivo. Bioluminescent imaging of a cohort of 20 mice inoculated with Ramos cells stably expressing the luciferase gene. Images shown are from pretreatment (day 0–72 hours postinoculation) or at the 20th day of treatment with vehicle [1% DMSO in water, intraperitonealy (i.p.) daily], dexamethasone (15 mg/kg, i.p. daily), rolipram (10 mg/kg, i.p. daily), or the combination of both agents. The panel on the right is a photon flux–based quantification of tumor size and spread and confirms the statistically significant improvement in GC activity following its rational combination with a PDE4 inhibitor (*, P < 0.05, Kruskal–Wallis test). The combined analysis of 2 independent cohorts (n = 29) is shown in Supplementary Figure S3G.
PDE4B expression and AKT activity in primary DLBCLs
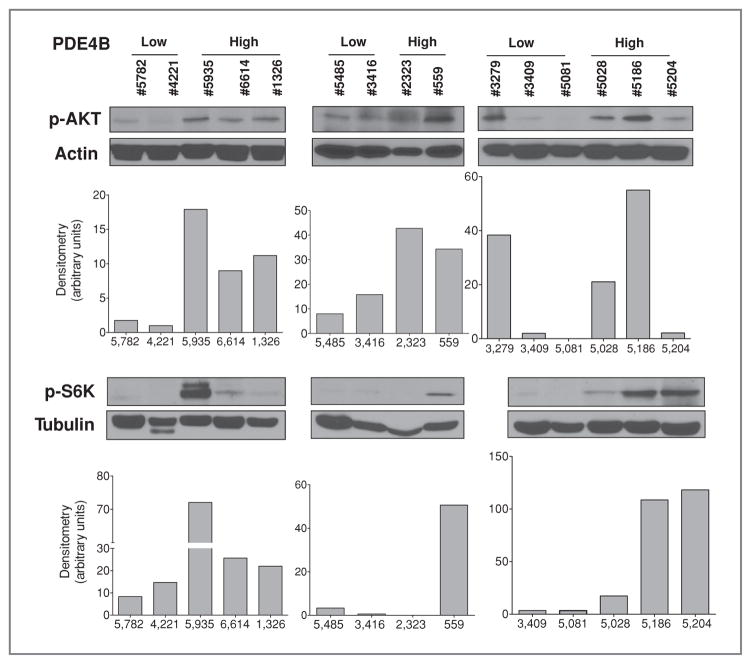
We mechanistically defined using in silico investigations, and in vitro and in vivo studies in lymphoma cell lines, a novel interplay between PDE4B-controlled cAMP effects and AKT/mTOR activities, with major relevance to GC sensitivity. As a next step in our investigation, we aimed to confirm that this cross-talk is also present in primary lymphoma samples. This is also relevant because it could establish the potential of PDE4B measurements in association with AKT/mTOR activity in the design of biomarker-guided trials aimed at restoring GC response in B-cell malignancies. To achieve these goals, we recently identified a collection of primary DLBCLs and obtained enough good quality matching RNAs and proteins from a subset of tumors (n = 15). PDE4B expression was determined by real-time qRT-PCR (Supplementary Table S3), a methodology that we have extensively shown to correlate with the activity of this enzyme (9, 11), and phospho-AKT and phospho-S6K levels were determined by Western blotting. In agreement with our cell line data, a significantly higher expression of these phospho-proteins was found in primary DLBCLs expressing high levels of PDE4B (P < 0.05, Mann–Whitney test for the densitometric values normalized by 2 independent control proteins, β-actin and α-tubulin), thus suggesting that the cAMP/PDE4B axis could play a role in controlling this oncogenic pathway in primary DLBCLs (Fig. 5).
Figure 5.
AKT/mTOR activity correlates with PDE4B expression in primary DLBCL. Western blot analyses of phospho-AKT (S473) and phospho-S6K (T389) were done in primary DLBCLs categorized by PDE4B expression (see Supplementary Table S3). Densitometric analysis, normalized by 2 independent proteins (β-actin and α-tubulin), is also shown and points to a correlation between PDE4B expression and activity of the AKT/mTOR pathway in the majority of primary DLBCLs analyzed and a significantly higher expression of these phospho-proteins in PDE4B-high DLCBLs (P < 0.05, Mann–Whitney test for the densitometric values). Note that protein from sample 3279 was available for only one of the Western blot analysis.
Discussion
The mechanistic basis for GC resistance in B-cell tumors remains ill defined. Early investigations had tentatively linked this event to loss-of-function mutation, defective expression, or specific promoter usage of the GR gene (27, 28). However, studies in large and well-characterized ALL cohorts did not confirm these hypotheses (29, 30).
Interestingly, cAMP has long been known to modulate GR expression, and initial studies suggested that the benefits of this second messenger on GC sensitivity were related to GR induction (25). Still, we found that although GR expression in DLBCL is indeed induced by cAMP, it did not influence GC sensitivity. Rather, we showed that the cAMP-mediated inhibition of AKT/mTOR is the central event needed to reinstate GC sensitivity in B-cell lymphomas. Additional studies will ascertain whether the cAMP-mediated induction of GR expression could also play role in the downmodulation of AKT/mTOR functions. In fact, our results agree with recent reports implicating this pathway in GC-resistant ALL (14) but refine the picture by showing that PDE4B is an important upstream regulator of AKT activity in this context. Downstream to AKT/mTOR, the antiapoptotic protein MCL1 has been suggested to be a predictor of GC resistance in MLL-rearranged infant acute lymphoblastic leukemia (21). Our preliminary data in DLBCL did not find a correlation between baseline MCL1 levels and GC response, albeit cAMP lowered MCL1 levels in an AKT-dependent manner. The reason for this discrepancy is presently unclear, but it could reflect the distinct biology of immature (ALL) and mature B-cell malignancies (DLBCL) or indicate a differential contribution of effector proteins downstream of AKT in these tumor types, with MCL1 more relevant in specific subsets of ALL than in DLBCL. Further studies should help clarify this issue.
Our genetic models of gain or loss of function highlighted the principal role of the 4B member of the superfamily of PDEs in controlling cAMP levels in DLBCL. Nonetheless, other PDE families also have cAMP catalytic activity (e.g., PDE3, PDE7, PDE8) and a broader-spectrum PDE inhibitor may be of additional value, as recently suggested (31). Of note, considering exclusively the PDE4s, a dominance of PDE4B activities may come with an unexpected benefit, as PDE4D targeting has been linked to the most common side effect (emesis) found with the clinical use of broad-spectrum PDE4 inhibitors (32). Thus, development of compounds with preferential activity to PDE4B versus other PDE4s, in particular PDE4D, should be sought, as they may have improved tumor-suppressing properties with more limited adverse effects.
Our preclinical data and examination of primary tumors also instruct on the design of clinical trials aimed at testing PDE4 inhibitors in B-cell cancers; these should be genomic-driven and perhaps restricted to patients with high levels of PDE4B expression and cAMP-responsive PI3K/AKT/mTOR signals, with WT status of PI3K, AKT, and PTEN genes, amongst other regulators of this pathway. Furthermore, on the basis of our in vivo data, it is possible that greater clinical benefit will be achieved when PDE4 inhibitors and GCs are combined with classical chemotherapy and/or additional rationally developed agents.
Together, our data show that modulation of cAMP levels, primarily via inhibition of PDE, should be clinically tested for the treatment of lymphoid malignancies. In particular, our data highlight the role of PDE4 inhibitors in restoring GC sensitivity and show that insights into disease pathogenesis can be exploited not only to identify novel targets for treatment but also to rationally overcome resistance to classical pharmacologic agents. It is possible that further elucidation of the cAMP effects on B cells, in particular the events upstream to SYK and PI3K/AKT (9, 11), will improve our appreciation of the role of this second messenger in B-cell biology and create novel opportunities for the development of therapeutic strategies in malignant, inflammatory, and autoimmune conditions.
Supplementary Material
Translational Relevance.
Glucocorticoid (GC) resistance is a significant problem in the management of lymphoid malignancies. We have previously shown that modulation of intracellular cyclic AMP (cAMP) via inhibition of phosphodiesterase 4B (PDE4B) induces apoptosis in diffuse large B-cell lymphomas (DLBCL). Here, we show that over-expression of PDE4B in DLBCL impinges on the same genes/pathways that are abnormally active in GC-resistant tumors. In agreement with these data, we showed, in vitro and in vivo, that genetic and pharmacologic targeting of PDE4B restores the GC sensitivity in B-cell lymphomas, in association with cAMP-mediated inhibition of mTOR. A marked correlation between PDE4B levels and AKT/mTOR activity in primary DLBCLs confirmed the relevance of this interplay. Together, our data indicate that PDE4 inhibitors may be useful in the treatment of lymphoid malignancies, mechanistically explain how these agents could improve GC sensitivity, and show that insights into disease pathogenesis can be exploited to rationally overcome drug resistance.
Acknowledgments
We thank Dr. Kumaraguruparan Ramasamy for expert help with the animal studies.
Grant Support
This work was supported in part by a grant from the National Cancer Institute (NCI; R21CA112043), a CTSA grant (UL1RR025767), a grant from the Cancer Prevention and Research Institute of Texas (CPRIT, RP110200), all to R.C.T. Aguiar). Core resources used in this study (Flow Cytometry, Pathology and Optical Imaging) are supported by a NCI Cancer Center Grant, 2P30CA054174-17.
Footnotes
Authors' contributions
S-W. Kim designed assays, conducted experiments, and analyzed results; D. Rai conducted experiments; R.C.T. Aguiar, conceived the project, designed experiments, analyzed results, and wrote the manuscript.
Disclosure of Potential Conflicts of Interest
No conflicts of interest were disclosed.
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
References
- 1.Harris TJ, McCormick F. The molecular pathology of cancer. Nat Rev Clin Oncol. 2010;7:251–65. doi: 10.1038/nrclinonc.2010.41. [DOI] [PubMed] [Google Scholar]
- 2.Mullighan CG, Downing JR. Global genomic characterization of acute lymphoblastic leukemia. Semin Hematol. 2009;46:3–15. doi: 10.1053/j.seminhematol.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–11. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 4.Shipp MA, Ross KN, Tamayo P, Weng AP, Kutok JL, Aguiar RC, et al. Diffuse large B-cell lymphoma outcome prediction by gene-expression profiling and supervised machine learning. Nat Med. 2002;8:68–74. doi: 10.1038/nm0102-68. [DOI] [PubMed] [Google Scholar]
- 5.Greenstein S, Krett NL, Kurosawa Y, Ma C, Chauhan D, Hideshima T, et al. Characterization of the MM. 1 human multiple myeloma (MM) cell lines: a model system to elucidate the characteristics, behavior, and signaling of steroid-sensitive and -resistant. MM cells Exp Hematol. 2003;31:271–82. doi: 10.1016/s0301-472x(03)00023-7. [DOI] [PubMed] [Google Scholar]
- 6.Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354:166–78. doi: 10.1056/NEJMra052603. [DOI] [PubMed] [Google Scholar]
- 7.Catley L, Tai YT, Chauhan D, Anderson KC. Perspectives for combination therapy to overcome drug-resistant multiple myeloma. Drug Resist Updat. 2005;8:205–18. doi: 10.1016/j.drup.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Kammer GM. The adenylate cyclase-cAMP-protein kinase A pathway and regulation of the immune response. Immunol Today. 1988;9:222–9. doi: 10.1016/0167-5699(88)91220-0. [DOI] [PubMed] [Google Scholar]
- 9.Smith PG, Wang F, Wilkinson KN, Savage KJ, Klein U, Neuberg DS, et al. The phosphodiesterase PDE4B limits cAMP-associated PI3K/ AKT-dependent apoptosis in diffuse large B-cell lymphoma. Blood. 2005;105:308–16. doi: 10.1182/blood-2004-01-0240. [DOI] [PubMed] [Google Scholar]
- 10.Serezani CH, Ballinger MN, Aronoff DM, Peters-Golden M. Cyclic AMP: master regulator of innate immune cell function. Am J Respir Cell Mol Biol. 2008;39:127–32. doi: 10.1165/rcmb.2008-0091TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim SW, Rai D, McKeller MR, Aguiar RC. Rational combined targeting of phosphodiesterase 4B and SYK in DLBCL. Blood. 2009;113:6153–60. doi: 10.1182/blood-2009-02-206128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conti M, Richter W, Mehats C, Livera G, Park JY, Jin C. Cyclic AMP-specific PDE4 phosphodiesterases as critical components of cyclic AMP signaling. J Biol Chem. 2003;278:5493–6. doi: 10.1074/jbc.R200029200. [DOI] [PubMed] [Google Scholar]
- 13.Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev. 2006;58:488–520. doi: 10.1124/pr.58.3.5. [DOI] [PubMed] [Google Scholar]
- 14.Wei G, Twomey D, Lamb J, Schlis K, Agarwal J, Stam RW, et al. Gene expression-based chemical genomics identifies rapamycin as a modulator of MCL1 and glucocorticoid resistance. Cancer Cell. 2006;10:331–42. doi: 10.1016/j.ccr.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Hecht BK, Epstein AL, Berger CS, Kaplan HS, Hecht F. Histiocytic lymphoma cell lines: immunologic and cytogenetic studies. Cancer Genet Cytogenet. 1985;14:205–18. doi: 10.1016/0165-4608(85)90186-4. [DOI] [PubMed] [Google Scholar]
- 16.Tweeddale ME, Lim B, Jamal N, Robinson J, Zalcberg J, Lockwood G, et al. The presence of clonogenic cells in high-grade malignant lymphoma: a prognostic factor. Blood. 1987;69:1307–14. [PubMed] [Google Scholar]
- 17.Li C, Kim SW, Rai D, Bolla AR, Adhvaryu S, Kinney MC, et al. Copy number abnormalities, MYC activity, and the genetic fingerprint of normal B cells mechanistically define the microRNA profile of diffuse large B-cell lymphoma. Blood. 2009;113:6681–90. doi: 10.1182/blood-2009-01-202028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monti S, Savage KJ, Kutok JL, Feuerhake F, Kurtin P, Mihm M, et al. Molecular profiling of diffuse large B-cell lymphoma identifies robust subtypes including one characterized by host inflammatory response. Blood. 2005;105:1851–61. doi: 10.1182/blood-2004-07-2947. [DOI] [PubMed] [Google Scholar]
- 19.Rai D, Kim SW, McKeller MR, Dahia PL, Aguiar RC. Targeting of SMAD5 links microRNA-155 to the TGF-beta pathway and lymphomagenesis. Proc Natl Acad Sci U S A. 2010;107:3111–6. doi: 10.1073/pnas.0910667107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–50. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stam RW, Den Boer ML, Schneider P, de Boer J, Hagelstein J, Valsecchi MG, et al. Association of high-level MCL-1 expression with in vitro and in vivo prednisone resistance in MLL-rearranged infant acute lymphoblastic leukemia. Blood. 2010;115:1018–25. doi: 10.1182/blood-2009-02-205963. [DOI] [PubMed] [Google Scholar]
- 22.Tissing WJ, Meijerink JP, den Boer ML, Pieters R. Molecular determinants of glucocorticoid sensitivity and resistance in acute lymphoblastic leukemia. Leukemia. 2003;17:17–25. doi: 10.1038/sj.leu.2402733. [DOI] [PubMed] [Google Scholar]
- 23.Farrugia MM, Duan LJ, Reis MD, Ngan BY, Berinstein NL. Alterations of the p53 tumor suppressor gene in diffuse large cell lymphomas with translocations of the c-MYC and BCL-2 proto-oncogenes. Blood. 1994;83:191–8. [PubMed] [Google Scholar]
- 24.Chang H, Blondal JA, Benchimol S, Minden MD, Messner HA. p53 mutations, c-myc and bcl-2 rearrangements in human non-Hodgkin's lymphoma cell lines. Leuk Lymphoma. 1995;19:165–71. doi: 10.3109/10428199509059672. [DOI] [PubMed] [Google Scholar]
- 25.Dong Y, Aronsson M, Gustafsson JA, Okret S. The mechanism of cAMP-induced glucocorticoid receptor expression. Correlation to cellular glucocorticoid response. J Biol Chem. 1989;264:13679–83. [PubMed] [Google Scholar]
- 26.Gruol DJ, Rajah FM, Bourgeois S. Cyclic AMP-dependent protein kinase modulation of the glucocorticoid-induced cytolytic response in murine T-lymphoma cells. Mol Endocrinol. 1989;3:2119–27. doi: 10.1210/mend-3-12-2119. [DOI] [PubMed] [Google Scholar]
- 27.Hala M, Hartmann BL, Bock G, Geley S, Kofler R. Glucocorticoid-receptor-gene defects and resistance to glucocorticoid-induced apoptosis in human leukemic cell lines. Int J Cancer. 1996;68:663–8. doi: 10.1002/(SICI)1097-0215(19961127)68:5<663::AID-IJC17>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 28.Hillmann AG, Ramdas J, Multanen K, Norman MR, Harmon JM. Glucocorticoid receptor gene mutations in leukemic cells acquired in vitro and in vivo. Cancer Res. 2000;60:2056–62. [PubMed] [Google Scholar]
- 29.Tissing WJ, Meijerink JP, Brinkhof B, Broekhuis MJ, Menezes RX, den Boer ML, et al. Glucocorticoid-induced glucocorticoid-receptor expression and promoter usage is not linked to glucocorticoid resistance in childhood ALL. Blood. 2006;108:1045–9. doi: 10.1182/blood-2006-01-0261. [DOI] [PubMed] [Google Scholar]
- 30.Irving JA, Minto L, Bailey S, Hall AG. Loss of heterozygosity and somatic mutations of the glucocorticoid receptor gene are rarely found at relapse in pediatric acute lymphoblastic leukemia but may occur in a subpopulation early in the disease course. Cancer Res. 2005;65:9712–8. doi: 10.1158/0008-5472.CAN-05-1227. [DOI] [PubMed] [Google Scholar]
- 31.Spina D. PDE4 inhibitors: current status. Br J Pharmacol. 2008;155:308–15. doi: 10.1038/bjp.2008.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robichaud A, Stamatiou PB, Jin SL, Lachance N, MacDonald D, Laliberte F, et al. Deletion of phosphodiesterase 4D in mice shortens alpha(2)-adrenoceptor-mediated anesthesia, a behavioral correlate of emesis. J Clin Invest. 2002;110:1045–52. doi: 10.1172/JCI15506. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.