Abstract
Aim:
Sinomenine (SIN) is an alkaloid found in the roots and stems of Sinomenium acutum, which has been used to treat rheumatic arthritis in China and Japan. In this study we investigated the effects of SIN on osteoclast survival in vitro and the mechanisms of the actions.
Methods:
Mature osteoclasts were differentiated from murine monocyte/macrophage cell line RAW264.7 through incubation in the presence of receptor activator of NF-κB ligand (RANKL, 100 ng/mL) for 4 d. The cell viability was detected using the CCK-8 method. The survival and actin ring construction of the osteoclasts were scored using TRACP staining and phalloidin-FITC staining, respectively. The apoptosis of the osteoclasts was detected by DNA fragmentation and Hoechst 33258 staining, and the cell necrosis was indicated by LDH activity. The activation of caspase-3 in osteoclasts was measured using Western blotting and the caspase-3 activity colorimetric method.
Results:
SIN (0.25–2 mmol/L) inhibited the viability of mature osteoclasts in dose-dependent and time-dependent manners, but did not affect that of RAW264.7 cells. Consistently, SIN dose-dependently suppressed the survival of mature osteoclasts. The formation of actin ring, a marker associated with actively resorbing osteoclasts, was also impaired by the alkaloid. SIN (0.5 mmol/L) induced the apoptosis of mature osteoclasts, which was significantly attenuated in the presence of the caspase-3 inhibitor Ac-DEVD-CHO. SIN increased the cleavage of caspase-3 in mature osteoclasts in dose-dependent and time-dependent manners. Furthermore, SIN dose-dependently enhanced caspase-3 activity, which was blocked in the presence of Ac-DEVD-CHO.
Conclusion:
Sinomenine inhibits osteoclast survival in vitro through caspase-3-mediated apoptosis, thus it is a potential agent for treating excessive bone resorption diseases.
Keywords: sinomenine, bone, osteoclast, RAW264.7 cell, NF-κB, RANKL, apoptosis, sinomenine, osteoclast, apoptosis, caspase-3
Introduction
Bone disorders are commonly accompanied with an increase in the number and activation of osteoclasts, which results in bone loss. Because osteoclasts are primary cells involved in bone resorption1, an imbalance in the regulation of the number or function of osteoclasts could lead to the development of severe skeletal disorders2. Excessive bone resorption is clinically the most intractable issue in bone diseases such as osteoporosis, periodontitis and bone metastatic tumors3. However, osteoclasts rapidly die via apoptosis in the absence of trophic factors, such as receptor activator of NF-κB ligand (RANKL)4. The regulation of osteoclast apoptosis influences bone resorptive activity and bone homeostasis, which can be a potential therapeutic target5. Currently, anti-osteoclast drugs, such as bisphosphonates and calcitonin, typically represent the front line treatment in patients suffering from bone loss or skeletal complications of a disease6.
Sinomenine (SIN), or 7,8-didehydro-4-hydroxy-3,7-dimethoxy-17-methyl- morphinane-6-one (Figure 1A), is an alkaloid found in the roots and stems of Sinomenium acutum, which has been used to treat rheumatic arthritis for more than 1000 years in China and Japan7. In addition, a highly pure drug made of hydrochloric sinomenine has shown therapeutic efficacy and few side effects in patients with rheumatoid arthritis in China since the 1990s8. We found that SIN can significantly inhibit osteoclastogenesis and resorptive activity in vitro and in vivo (data not shown). Notably, SIN is known to induce apoptosis in various types of cells9,10,11,12,13,14. Therefore, we hypothesized that SIN could also inhibit bone resorption and the survival of mature osteoclasts by inducing osteoclast apoptosis.
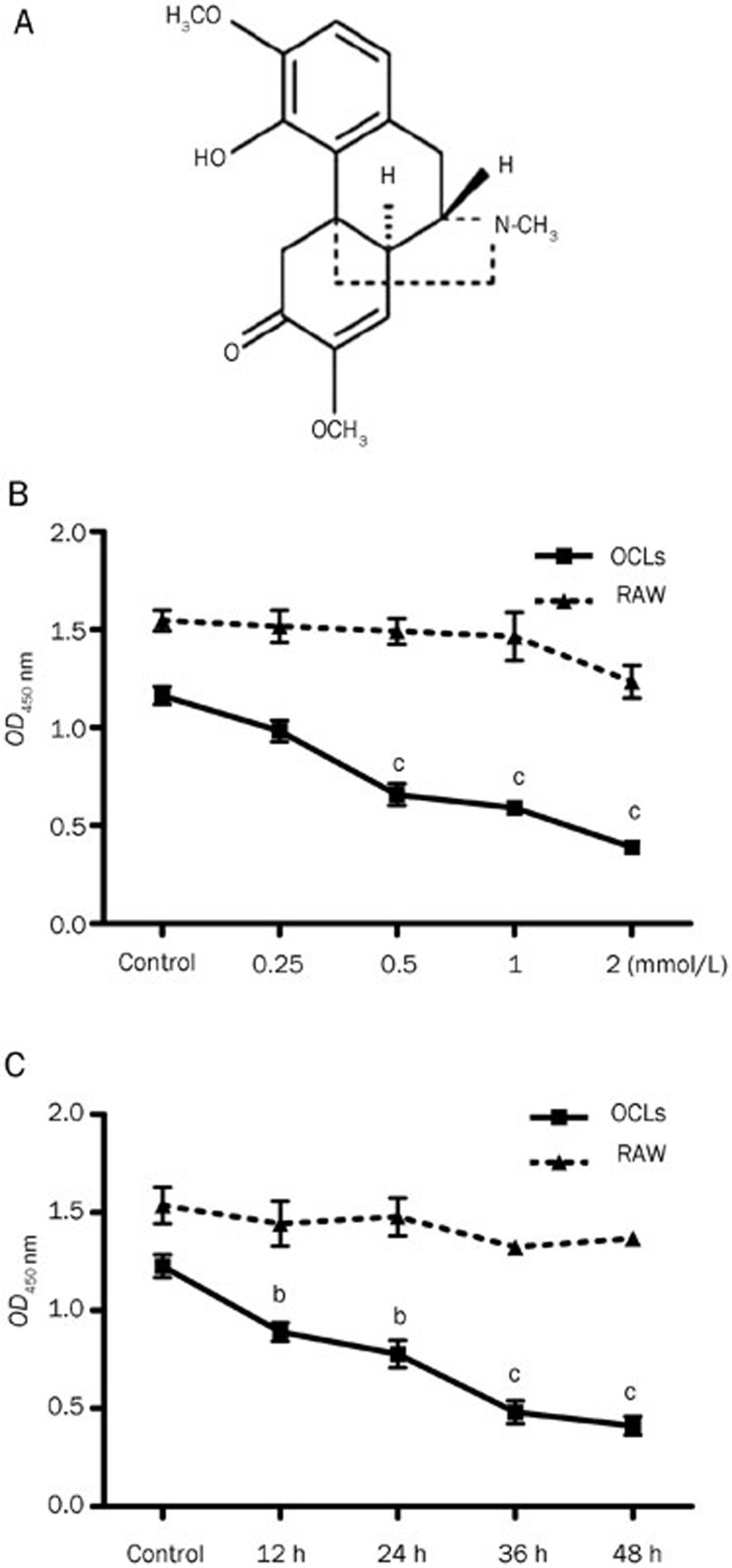
Figure 1.
The effects of SIN on the cell viability of OCLs. (A) The chemical structure of SIN. (B) The effects of SIN on the cell viability of RAW264.7 cells and OCLs for 24 h, measured using the CCK-8 method. (C) The effects of SIN (0.5 mmol/L) on the cell viability of RAW264.7 cells and OCLs during different time periods, measured using the CCK-8 method. The data represent the mean±SEM from 3 independent experiments. bP<0.05, cP<0.01 compared with control group.
Recently, SIN has been shown to modulate caspase activation to induce apoptosis9,12,13. However, there is currently no report about the effect of SIN on the apoptosis of osteoclasts. Such an understanding could be critical for developing SIN as an agent for the prevention and treatment of bone diseases. Therefore, this study focused on the effect of SIN on the apoptosis of osteoclasts.
Materials and methods
Cell culture
Sinomenine (SIN, > 98% purity by HPLC) was purchased from Ronghe Inc (Shanghai, China). Soluble recombinant RANKL and the TACSTM apoptotic DNA laddering kit were obtained from R&D (Lorton, VA, USA). The tartrate-resistant acid phosphatase staining kit (TRACP) and phalloidin-FITC were obtained from Sigma-Aldrich (St Louis, MO, USA). Dulbecco's modified Eagle's medium (DMEM), alpha modification of Eagle's medium (α-MEM), fetal bovine serum (FBS), penicillin and streptomycin were purchased from Gibco (Rockville, MD, USA). The Cell Counting Kit-8 (CCK-8) and Hoechst 33258 were obtained from Dojindo Molecular Technologies (Shanghai, China). The caspase-3 colorimetric activity assay kit was purchased from Chemicon International Inc (Temecula, CA, USA). All of the antibodies were obtained from Cell Signaling Technology (Danvers, MA, USA).
The murine monocyte/macrophage cell line RAW264.7 (obtained from the American Type Culture Collection, USA) was maintained in DMEM supplemented with 10% FBS and antibiotics (100 units/mL of penicillin G sodium and 100 μg/mL of streptomycin sulfate) at 37 °C in an atmosphere of 5% CO2. RAW264.7 cells were seeded in 96-well plates at a density of 2×103 cells/well (for the viability assay, the LDH activity assay, TRACP staining, Hoechst 33258 staining and actin rings staining) or in 6-well plates at a density of 2×105 cells/well (for the DNA-fragmentation assay, the caspase activity assay and Western blot analysis) in α-MEM containing 10% FBS and antibiotics. The differentiation of osteoclasts from RAW264.7 cells was induced after 4 d incubation with 100 ng/mL of RANKL. The differentiated multi-nucleic RAW264.7 cells were regarded as osteoclast-like cells (OCLs).
Cell viability assay
To evaluate the effect of SIN on the viability of OCLs, the multinucleated OCLs were incubated with different concentrations of SIN (0.25, 0.5, 1, and 2 mmol/L) for 24 h or with 0.5 mmol/L SIN for different time periods (12, 24, 36, and 48 h). RAW264.7 cells were treated under the same conditions as the control. The viability of the cells was determined using a CCK-8 assay and the absorbance was measured at 450 nm.
LDH activity assay
Lactate dehydrogenase (LDH) is a stable cytosolic enzyme released upon membrane damage in necrotic cells. To detect the effect of SIN on necrosis of OCLs, the multinucleated OCLs were incubated with 0.5 mmol/L SIN for 24 h. The culture supernatants were collected, and LDH was detected using the LDH activity assay kit (Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer's instructions. The absorbance was measured at 450 nm.
TRACP staining
TRACP-positive multinucleated cells were scored as OCLs. To study the effect of SIN on OCLs, SIN at a concentration of 0.25, 0.5 or 1 mmol/L was added to the multinucleated OCLs on d 4, and the cells were incubated for 24 h. After treatment, the cells were fixed and stained for TRACP according to the manufacturer's instructions.
Actin rings staining
On differentiation day 4, OCLs derived from RAW264.7 cells were incubated with SIN (1 mmol/L) and RANKL (100 ng/mL) for 6 h and then washed twice with PBS. The cells were fixed with 10% formalin for 5 min, permeabilized with 0.1% Triton X-100 for 5 min and washed again with PBS. The actin rings were then stained with 50 mg/mL phalloidin-FITC for 1 h under light protection15. After two washes with PBS, the nuclei were stained with DAPI. Images of the stained cells were captured with a fluorescent microscope (Nikon, Japan). The number of osteoclasts with actin rings was counted in each well.
DNA-fragmentation assay
The OCLs were incubated with RANKL followed by different concentrations of SIN (0.25, 0.5, 1 and 2 mmol/L) for 24 h or 0.5 mmol/L SIN for different time periods (12, 24, 36 and 48 h). After treatment, the TACSTM apoptotic DNA laddering kit was used to test for apoptosis by detecting inter-nucleosomal DNA fragmentation and displaying the DNA laddering. After washing with cold PBS, the DNA was isolated by lysing the cells, and the DNA was purified by organic extraction and isopropanol precipitation. The precipitated DNA was pelleted by centrifugation (12 000×g, 10 min at 4 °C), washed with 70% ethanol, dried and dissolved in DNase-free water. The purified DNA was quantified using a spectrophotometer and was resolved by electrophoresis through the 1.5% Trevi-Gel included in the kit. The gel was stained with ethidium bromide (EB) and examined under ultraviolet illumination.
Hoechst 33258 staining
OCLs were incubated with RANKL and treated with 0.5 mmol/L SIN with or without the specific caspase-3 inhibitor Ac-DEVD-CHO (10 μmol/L) for 24 h. At the end of the treatment, the cells were washed with PBS and were stained for 15 min with 10 μmol/L Hoechst 33258 dye. Images of the stained cells were captured with a fluorescent microscope (Nikon, Tokyo, Japan). The differences were evaluated by counting the number of cells with apoptotic nuclear condensation in each well.
Western blot analysis
OCLs were treated with SIN as in the DNA-fragmentation assay. After incubation, the whole cell extract was prepared using radioimmunoprecipitation assay buffer (50 mmol/L Tris-HCl, pH 7.5, 150 μmol/L sodium chloride, 0.5% cholic acid, 0.1% SDS, 2 mmol/L EDTA, 1% Triton, and 10% glycerol) containing protease and phosphatase inhibitors (1 mmol/L phenylmethylsulfonyl fluoride, 1 mmol/L Na3VO4, and 1 mmol/L NaF). The protein concentration was determined by the BCA method. The samples (20–50 μg total protein) were separated using 12% SDS–PAGE and were transferred to polyvinylidene difluoride membranes. After blocking with 5% no-fat milk in Tris-buffered saline and 0.05% Tween-20 (TBST) for 2 h at room temperature, the membranes were incubated with primary antibody overnight and then incubated with the conjugated secondary antibody for 2 h. The membrane was incubated with ECL and then exposed to X-ray flim. The films were scanned and quantified using Quantity One software (Bio-Rad, USA).
Caspase-3 activity assay
OCLs were incubated with RANKL followed by different concentrations of SIN (0.5 and 1 mmol/L) or camptothecin (4 μmol/L) with or without Ac-DEVD-CHO (10 μmol/L) for 24 h. Camptothecin was used as a positive control to induce apoptosis. Caspase-3 activity in the cells was measured using the caspase-3 colorimetric activity assay kit according to the manufacturer's protocol. Briefly, the cells were washed with cold PBS and lysed with the cell lysis buffer included in the kit. The cell lysates were centrifuged (10 000×g for 5 min at 4 °C), and the supernatants were collected. Equal amounts of protein (100 μg), 10 μL of colorimetric caspase-3 substrate (Ac-DEVD-pNA, 2 mmol/L) and assay buffer were added to each reaction mixture, and the reaction mixtures were incubated for 2 h at 37 °C. Caspase-3 activity was determined by measuring the absorbance at 405 nm.
Statistics analysis
All data were analyzed using GraphPad Prism 4.0 software. The results are shown as the mean±SEM. The paired t test was used for group comparison. A one-way ANOVA and Turkey's multiple comparison test were used to test statistical significance among groups. In all cases, P<0.05 was considered to be statistically significant.
Results
SIN inhibits the viability of RAW264.7 cell-derived OCLs, but not the viability of control RAW264.7 cells
The CCK-8 assays showed that SIN at concentrations of greater than 0.5 mmol/L could significantly decrease the viability of RAW264.7 cell-derived OCLs after 24 h of treatment (Figure 1B). Additionally, 0.5 mmol/L SIN significantly inhibited the viability of OCLs in a time-dependent manner from 12 h to 48 h (Figure 1C). Interestingly, there were no significant effects on the viability of the undifferentiated RAW264.7 cells, even when the cells were treated with 2 mmol/L SIN for 24 h (Figure 1B). These findings suggest that SIN might specifically suppress cell survival in mature osteoclasts but not in osteoclast precursors. These data also indicate that the appropriate concentration of SIN for treating RAW264.7 cell cultures should be less than 2 mmol/L in the subsequent studies.
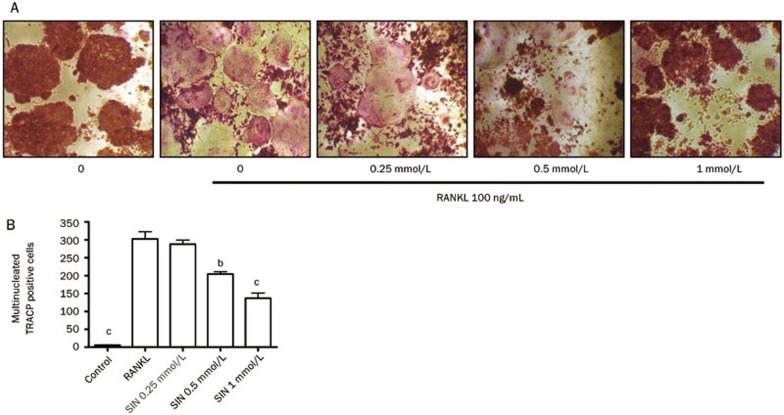
SIN decreases the survival of RAW 264.7 cell-derived OCLs
Mature osteoclasts have ring-like cytoskeletal structures that are crucial for bone resorption, and the presence of this structure is considered to be a marker for mature osteoclasts survival16. To examine the effect of SIN on osteoclast survival, OCLs were treated with various concentrations of SIN for 24 h. At concentrations of 0.5 and 1 mmol/L, SIN significantly decreased the number of mature osteoclasts with ring-like structures (Figure 2A), and fewer nuclei were found when compared with positive controls. After counting the number of TRACP-positive multinucleated OCLs, it was observed that the number of OCLs decreased in a dose-dependent manner when treated with SIN (Figure 2B). These results suggest that SIN inhibits the survival of RAW 264.7 cell-derived OCLs.
Figure 2.
The effects of SIN on RANKL-induced OCL survival. (A) The effect of SIN on RANKL-induced multinucleated OCLs. RAW264.7 cells were plated in 96-well plates and induced by 100 ng/mL RANKL. After 4 d of induction, SIN was added, and the cells were incubated for 24 h. Multinucleated OCLs were evaluated by TRACP staining. Photographs were taken at a magnification of ×40. (B) The total number of OCLs, counted per well of each group. The data represent the mean±SEM from 3 independent experiments. bP<0.05, cP<0.01 compared with the RANKL-induced alone group.
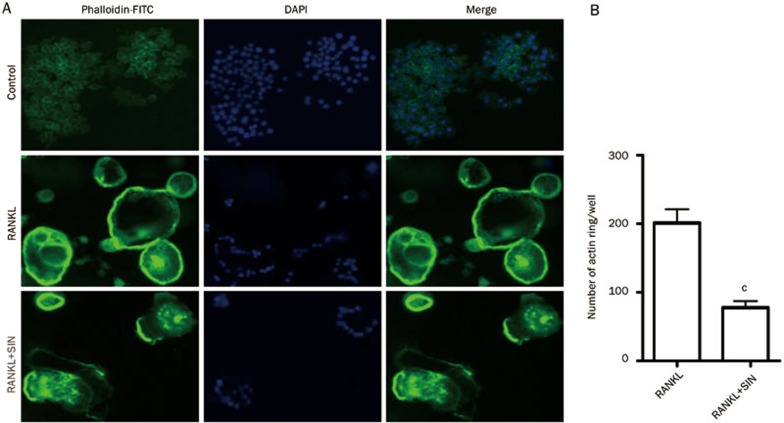
SIN disrupts the actin ring structure of RAW 264.7 cell-derived OCLs
To determine whether the inhibitory effect of SIN was due to the disruption of ring-like structure, we further evaluated the effect of SIN on the appearance of actin rings using fluorescent microscopy. The actin ring cytoskeleton was observed in RAW264.7 cells that were incubated with RANKL (100 ng/mL) for 4 d, but the actin rings were disrupted in OCLs treated with 1 mmol/L SIN for 6 h (Figure 3A). The number of actin rings was reduced by approximately 60% (Figure 3B) in the OCLs treated with SIN. These results suggest that SIN suppresses OCL survival via the reduction and disruption of actin ring development.
Figure 3.
The effects of SIN on the actin ring structure of OCLs. (A) OCLs derived from RAW264.7 cells were incubated with 1 mmol/L SIN for 6 h, and the actin rings and nuclei of osteoclasts were stained with phalloidin-FITC and DAPI, respectively. Photographs were taken at a magnification of ×100. (B) The number of osteoclasts with actin rings per well. The data represent the mean±SEM from 3 independent experiments. cP<0.01, compared with the RANKL-induced alone group.
SIN induces apoptosis in RAW 264.7 cell-derived OCLs
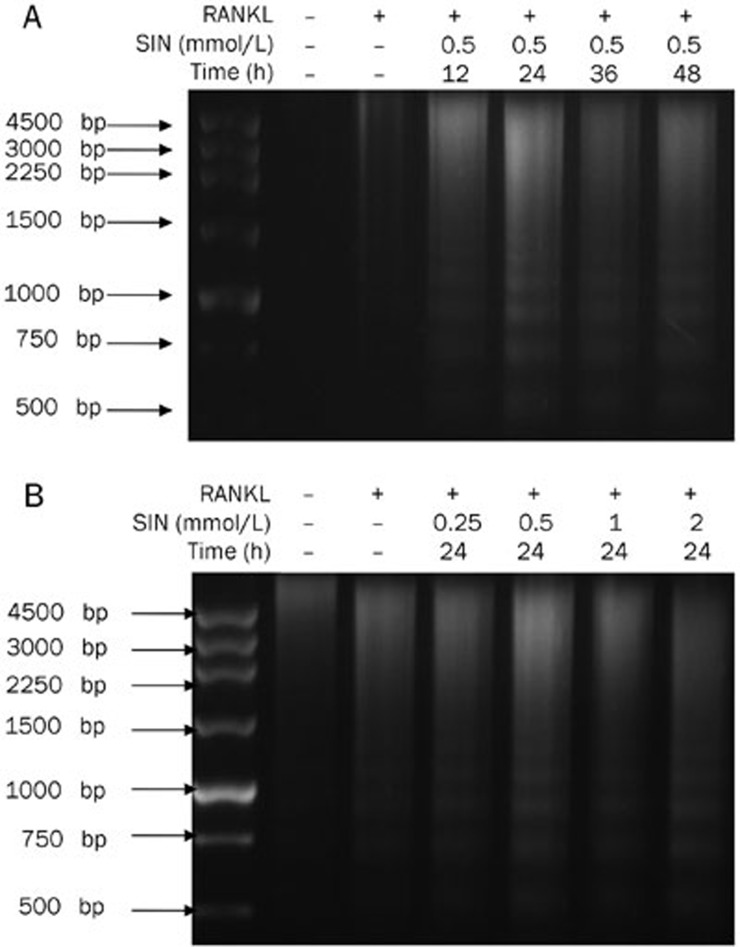
We further studied the mechanisms of action by which SIN inhibits OCL survival. To investigate whether SIN induces apoptosis in RAW 264.7 cell-derived OCLs, the OCLs were treated with SIN at different concentrations and for different time periods. In each case, nucleosomal DNA fragmentation, which is typical of apoptosis, was observed, and the fragmentation pattern was most prominent in the cells that were treated with 0.5 mmol/L SIN for 24 h (Figure 4). These results showed that SIN significantly induced apoptosis in osteoclasts.
Figure 4.
The effects of SIN on the apoptosis of OCLs measured with the DNA fragmentation assay. (A) The effect of SIN (0.5 mmol/L) on nucleosomal DNA fragmentation in OCLs for different time periods (12, 24, 36, and 48 h). (B) The effect of different concentrations (0.25, 0.5, 1, 2 mmol/L) of SIN on nucleosomal DNA fragmentation of OCLs for 24 h. Experiments were repeated 3 times.
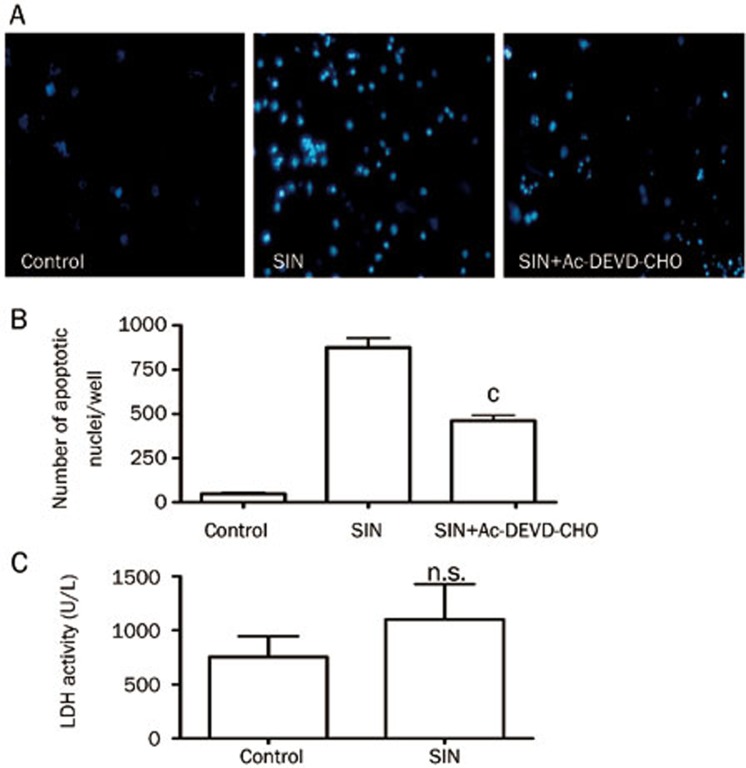
To further confirm that apoptosis in OCLs was induced by SIN, OCLs were treated with SIN (0.5 mmol/L) for 24 h in the presence or absence of the specific caspase-3 inhibitor Ac-DEVD-CHO. Apoptosis was analyzed using Hoechst 33258 staining, and necrosis was detected by measuring LDH activity. The nuclei of OCLs treated with SIN exhibited typical nuclei fragmentation, aggregation and condensation (Figure 5A). The number of cells with changed nuclei per well significantly increased approximately 20 fold after treatment with 0.5 mmol/L SIN compared with the control group during Hoechst 33258 staining (Figure 5B), but cell necrosis was not significantly increased by 0.5 mmol/L SIN in the LDH assay (Figure 5C). Remarkably, 10 μmol/L Ac-DEVD-CHO partially blocked the effect of SIN-induced apoptosis and reduced the number of apoptotic nuclei (Figure 5A-B). Therefore, SIN presumably destroys the mature OCLs partly by inducing apoptosis, and the mechanism of action might involve caspase-3 activation.
Figure 5.
The effects of SIN on the apoptosis of OCLs measured using the Hoechst assay. (A) The apoptotic nuclei of OCLs stained by Hoechst 33258. OCLs derived from RAW264.7 cells were treated with SIN (0.5 mmol/L) for 24 h in the presence or absence of the caspase-3 inhibitor Ac-DEVD-CHO (10 μmol/L), and the cells were stained with Hoechst 33258 (magnification ×100). (B) The number of cells with apoptotic nuclear condensation per well. (C) The effects of SIN on the necrosis of OCLs that were treated for 24 h, which was measured using the LDH activity assay. The data represent the mean±SEM (n=3) from 3 independent experiments. “NS” was no significant difference compared with control group. bP<0.05, cP<0.01 compared with the control group (RANKL alone).
SIN activated caspase-3 in RAW 264.7 cell-derived OCLs
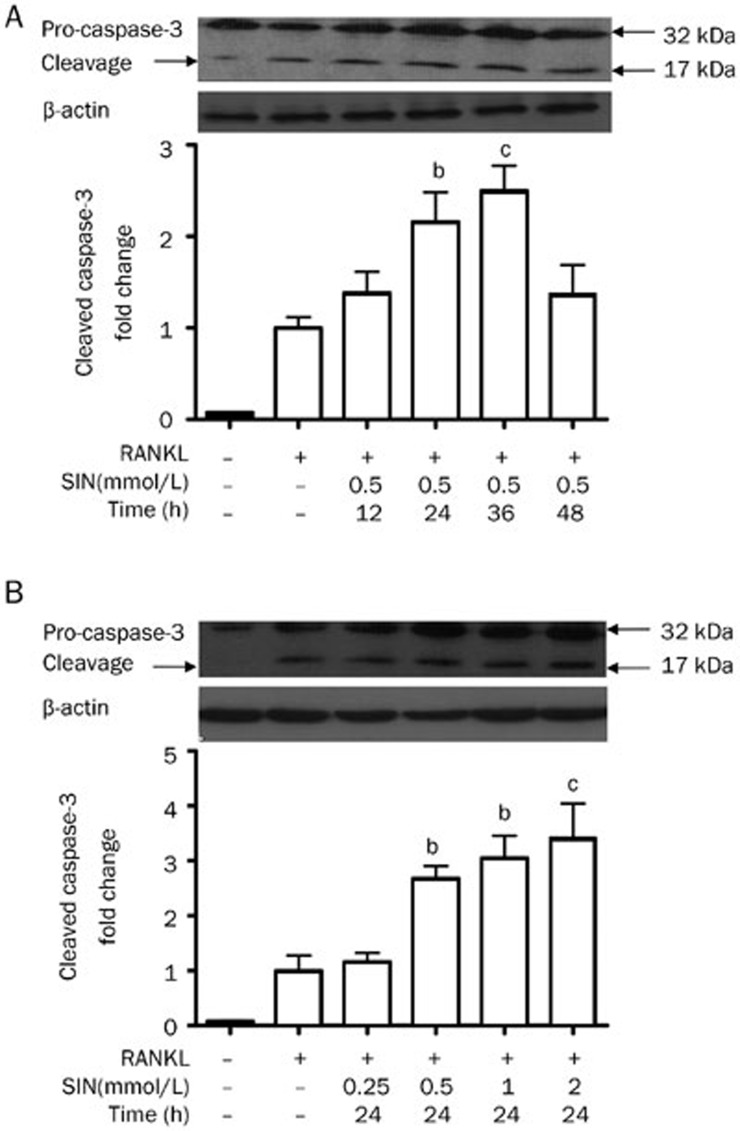
To further verify the hypothesis that SIN induces apoptosis in OCLs via the caspase-3 activation, Western blotting and the colorimetric caspase-3 activity assay were implemented. The antibody in the Western blot used recognizes both the intact 32-kDa pro-form and the cleaved 17-kDa active form of caspase-3. Compared with untreated cells and cells treated with only RANKL, treatment with 0.5 mmol/L SIN increased the amount of the cleaved caspase-3 in a time-dependent manner from 12-26 h, indicating the activation of caspase-3 (Figure 6A). Additionally, the amount of the cleaved caspase-3 was also increased in a dose-dependent manner (Figure 6B). These results verified our hypothesis.
Figure 6.
The effect of SIN on the protein expression of caspase-3 in OCLs. (A) SIN (0.5 mmol/L) was added to OCLs for different time periods. (B) Different concentrations of SIN were added into OCLs for 24 h. The protein levels of the intact pre-form (32 kDa) and cleaved active form (17 kDa) of caspase-3 were measured using Western blotting. The expression of cleaved caspase 3 was quantified using Quantity One software. β-actin was used to show equal protein loading. The data indicate the mean±SEM of 3 independent experiments. bP<0.05, cP<0.01 compared with the RANKL-induced alone group.
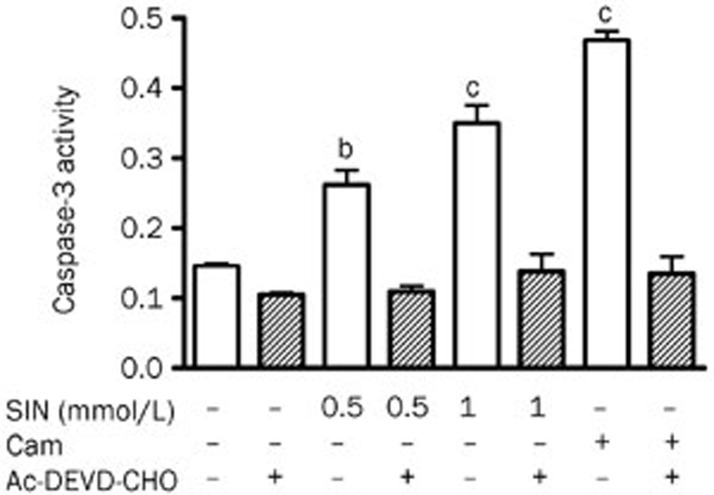
The activity of caspase-3 in OCLs was also measured. SIN at 0.5 and 1 mmol/L dose-dependently increased caspase-3 activity in RANKL-induced OCLs (Figure 7). These effects of SIN were blocked by the caspase-3 inhibitor Ac-DEVD-CHO. Camptothecin (4 μmol/L), a positive control, increased caspase-3 activity, which was also blocked by Ac-DEVD-CHO (Figure 7). These results further indicated that SIN induces caspase-3 activation, which is associated with the reduced survival and increased apoptosis of mature osteoclasts.
Figure 7.
The effects of SIN on the activity of caspase-3 in OCLs. Cells were cultured in the presence of SIN (0.5 and 1 mmol/L) or CAM (4 μmol/L) with or without the caspase-3 inhibitor Ac-DEVD-CHO (10 μmol/L) in triplicate for 24 h. The cell lysates were collected, and the caspase-3 activity was measured using Ac-DVED-pNA as a substrate. Caspase-3 activity was determined by measuring the absorbance at 405 nm. The data represent the mean±SEM (n=3) from 3 independent experiments. bP<0.05, cP<0.01, compared with control group.
Discussion
In our previous study, we found that SIN suppresses osteoclastogenesis and bone resorption by inhibiting NF-κB and MAPKs signaling pathways (data not shown). The activity of NF-κB and MAPKs is required for osteoclasts survival 17,18. Therefore, we presumed that SIN could suppress the survival of osteoclasts or osteoclast precursors. Interestingly, we found that SIN inhibits the viability of mature OCLs, but not the viability of OCL precursors, in a time- and concentration-dependent manner (Figure 1). Subsequently, our research has demonstrated that SIN decreases the number of mature OCLs (Figure 2) and disrupts the actin ring in OCLs (Figure 3), which suggests that SIN can suppress mature OCL survival. Osteoclast survival and bone resorption depend upon the actin cytoskeleton19. A previous report has shown that changes in the actin cytoskeleton were related to apoptotic osteoclast death20. Therefore, SIN might suppress the survival of mature osteoclasts by inducing apoptosis.
Osteoclasts are a type of multinuclear terminal cell, which have a limited life span4. Regulation of osteoclast apoptosis makes possible the prospect of osteoclast-targeted therapy as a novel treatment strategy for bone disorders21,22. Apoptosis is characterized by morphologic and biochemical features, such as chromatin condensation, internucleosomal cleavage of genomic DNA, and caspase activation23. Here, we demonstrated that the inhibition of OCL survival by SIN might be mediated by OCL apoptosis, as evidenced by genomic DNA fragmentation (Figure 4), which is the most significant biochemical hallmark of apoptosis, and chromatin condensation (Figure 5). Remarkably, nucleosomal DNA fragmentation was visible after treatment with SIN at higher concentrations (Figure 4); however, DNA ladder electrophoresis is not an assay that is used to quantify apoptosis. Additionally, Hoechst 33258 staining was used to observe apoptosis in OCLs, and the LDH activity was measured to exclude the necrotic cells (Figure 5). Necrosis was not significantly increased by 0.5 mmol/L SIN, which suggests that apoptosis was the primary cause of cell death at the concentration of 0.5 mmol/L, as indicated by DNA ladder assay and Hoechst staining. In addition, the apoptotic effect of SIN on osteoclasts was dramatically suppressed by the caspase-3 inhibitor Ac-DEVD-CHO, suggesting that caspase-3 is involved in the SIN-induced apoptosis of OCLs (Figure 5).
Caspases are a part of a growing family of cysteine proteases, and 14 mammalian caspases have been reported to date. Caspase-3 is a critical executioner of apoptosis in the caspase signalling pathway. Moreover, caspase-3 is the key caspase responsible for the majority of apoptotic effects24. A previous study reported that the activation of caspase-3 was required for osteoclast differentiation25. However, the excessive activation of caspases in mature osteoclasts induced the proteolytic cascade of apoptosis26,27. These results suggest that SIN stimulates the cleavage of the caspase-3 precursor and enhances caspase-3 activity (Figure 6 and 7). We also found that the SIN-induced activation of caspase-3 was completely abrogated by Ac-DEVD-CHO (Figure 7). Therefore, SIN induction of apoptosis in RAW264.7 cell-derived osteoclasts involves the activation of caspase-3. However, Ac-DEVD-CHO only partially suppressed SIN-induced OCL apoptosis (Figure 5), suggesting that other signaling pathways might be involved in the SIN-induced apoptosis of osteoclasts in addition to the caspase-3 pathway. Previous studies have shown that the inhibition of the NF-κB signal pathway in osteoclasts potently induces apoptosis, which is associated with an increase in caspase-3 activity and a decrease in IL-6 expression27,28. In our previous study, we found that SIN is capable of inhibiting the activation of the NF-κB signal pathway in RAW264.7 cells (data not shown). We hypothesized that SIN also inhibits the activation of NF-κB involved in osteoclast apoptosis. However, it remains to be determined which pathway is dominant in SIN-induced apoptosis in osteoclasts.
The results of this study showed that SIN inhibits osteoclast survival by inducing apoptosis in mature osteoclasts, at least in part through the activation of caspase-3. Because osteoclasts are essential for several pathologies associated with bone loss, the application of SIN could be an alternative tool for the treatment of bone loss-associated diseases, including rheumatoid arthritis, osteoporosis, bone metastasis of tumors and osteosarcoma.
Author contribution
Long-gang HE, Xiao-juan LI and Shu-wen LIU designed the research; Long-gang HE, Xiang-lian LI, Xiang-zhou ZENG, and Heng DUAN performed the research; Long-gang HE, Xiao-juan LI and Song WANG analyzed the data; and Long-gang HE, Lin-sheng LEI, Xiao-juan LI and Shu-wen LIU wrote the paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81073119, 30801413) to Xiao-juan LI and the Guangdong Pearl River Scholar Funded Scheme to Shuwen LIU.
References
- 1Baron R. Molecular mechanisms of bone resorption by the osteoclast. Anat Rec 1989; 224: 317–24. [DOI] [PubMed] [Google Scholar]
- 2Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature 2003; 423: 337–42. [DOI] [PubMed] [Google Scholar]
- 3Blair HC, Zaidi M, Huang CL, Sun L. The developmental basis of skeletal cell differentiation and the molecular basis of major skeletal defects. Biol Rev Camb Philos Soc 2008; 83: 401–15. [DOI] [PubMed] [Google Scholar]
- 4Tanaka S, Miyazaki T, Fukuda A, Akiyama T, Kadono Y, Wakeyama H, et al. Molecular mechanism of the life and death of the osteoclast. Ann N Y Acad Sci 2006; 1068: 180–6. [DOI] [PubMed] [Google Scholar]
- 5Stern PH. Antiresorptive agents and osteoclast apoptosis. J Cell Biochem 2007; 101: 1087–96. [DOI] [PubMed] [Google Scholar]
- 6Edwards JR, Weivoda MM. Osteoclasts: malefactors of disease and targets for treatment. Discov Med 2012; 13: 201–10. [PubMed] [Google Scholar]
- 7Xu M, Liu L, Qi C, Deng B, Cai X. Sinomenine versus NSAIDs for the treatment of rheumatoid arthritis: a systematic review and meta-analysis. Planta Med 2008; 74: 1423–9. [DOI] [PubMed] [Google Scholar]
- 8Zhao XX, Peng C, Zhang H, Qin LP. Sinomenium acutum: A review of chemistry, pharmacology, pharmacokinetics, and clinical use. Pharm Biol 2012; 50: 1053–61. [DOI] [PubMed] [Google Scholar]
- 9Lu XL, Zeng J, Chen YL, He PM, Wen MX, Ren MD, et al. Sinomenine hydrochloride inhibits human hepatocellular carcinoma cell growth in vitro and in vivo: involvement of cell cycle arrest and apoptosis induction. Int J Oncol 2013; 42: 229–38. [DOI] [PubMed] [Google Scholar]
- 10Lv Y, Li C, Li S, Hao Z. Sinomenine inhibits proliferation of SGC-7901 gastric adenocarcinoma cells via suppression of cyclooxygenase-2 expression. Oncol Lett 2011; 2: 741–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11Zhou L, Luan H, Liu Q, Jiang T, Liang H, Dong X, et al. Activation of PI3K/Akt and ERK signaling pathways antagonized sinomenine-induced lung cancer cell apoptosis. Mol Med Report 2012; 5: 1256–60. [DOI] [PubMed] [Google Scholar]
- 12Jiang T, Zhou L, Zhang W, Qu D, Xu X, Yang Y, et al. Effects of sinomenine on proliferation and apoptosis in human lung cancer cell line NCI-H460 in vitro. Mol Med Report 2010; 3: 51–6. [DOI] [PubMed] [Google Scholar]
- 13Shu L, Yin W, Zhang J, Tang B, Kang YX, Ding F, et al. Sinomenine inhibits primary CD4+ T-cell proliferation via apoptosis. Cell Biol Int 2007; 31: 784–9. [DOI] [PubMed] [Google Scholar]
- 14He X, Wang J, Guo Z, Liu Q, Chen T, Wang X, et al. Requirement for ERK activation in sinomenine-induced apoptosis of macrophages. Immunol Lett 2005; 98: 91–6. [DOI] [PubMed] [Google Scholar]
- 15Kim MH, Ryu SY, Choi JS, Min YK, Kim SH. Saurolactam inhibits osteoclast differentiation and stimulates apoptosis of mature osteoclasts. J Cell Physiol 2009; 221: 618–28. [DOI] [PubMed] [Google Scholar]
- 16Yonezawa T, Hasegawa S, Ahn JY, Cha BY, Teruya T, Hagiwara H, et al. Tributyltin and triphenyltin inhibit osteoclast differentiation through a retinoic acid receptor-dependent signaling pathway. Biochem Biophys Res Commun 2007; 355: 10–5. [DOI] [PubMed] [Google Scholar]
- 17Hwang YH, Lee JW, Hahm ER, Jung KC, Lee JH, Park CH, et al. Momordin I, an inhibitor of AP-1, suppressed osteoclastogenesis through inhibition of NF-kappaB and AP-1 and also reduced osteoclast activity and survival. Biochem Biophys Res Commun 2005; 337: 815–23. [DOI] [PubMed] [Google Scholar]
- 18Marie PJ. Signaling pathways affecting skeletal health. Curr Osteoporos Rep 2012; 10: 190–8. [DOI] [PubMed] [Google Scholar]
- 19Gu G, Mulari M, Peng Z, Hentunen TA, Vaananen HK. Death of osteocytes turns off the inhibition of osteoclasts and triggers local bone resorption. Biochem Biophys Res Commun 2005; 335: 1095–101. [DOI] [PubMed] [Google Scholar]
- 20Li X, Senda K, Ito A, Sogo Y, Yamazaki A. Effect of Zn and Mg in tricalcium phosphate and in culture medium on apoptosis and actin ring formation of mature osteoclasts. Biomed Mater 2008; 3: 045002. [DOI] [PubMed] [Google Scholar]
- 21Akiyama T, Dass CR, Choong PF. Novel therapeutic strategy for osteosarcoma targeting osteoclast differentiation, bone-resorbing activity, and apoptosis pathway. Mol Cancer Ther 2008; 7: 3461–9. [DOI] [PubMed] [Google Scholar]
- 22Xing L, Boyce BF. Regulation of apoptosis in osteoclasts and osteoblastic cells. Biochem Biophys Res Commun 2005; 328: 709–20. [DOI] [PubMed] [Google Scholar]
- 23Zimmermann KC, Bonzon C, Green DR. The machinery of programmed cell death. Pharmacol Ther 2001; 92: 57–70. [DOI] [PubMed] [Google Scholar]
- 24Nicholson DW. Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death Differ 1999; 6: 1028–42. [DOI] [PubMed] [Google Scholar]
- 25Szymczyk KH, Freeman TA, Adams CS, Srinivas V, Steinbeck MJ. Active caspase-3 is required for osteoclast differentiation. J Cell Physiol 2006; 209: 836–44. [DOI] [PubMed] [Google Scholar]
- 26Shandala T, Shen NY, Hopwood B, Yip YC, Foster BK, Xian CJ. The role of osteocyte apoptosis in cancer chemotherapy-induced bone loss. J Cell Physiol 2012; 227: 2889–97. [DOI] [PubMed] [Google Scholar]
- 27Piva R, Penolazzi L, Zennaro M, Bianchini E, Magri E, Borgatti M, et al. Induction of apoptosis of osteoclasts by targeting transcription factors with decoy molecules. Ann N Y Acad Sci 2006; 1091: 509–16. [DOI] [PubMed] [Google Scholar]
- 28Piva R, Penolazzi L, Borgatti M, Lampronti I, Lambertini E, Torreggiani E, et al. Apoptosis of human primary osteoclasts treated with molecules targeting nuclear factor-kappaB. Ann N Y Acad Sci 2009; 1171: 448–56. [DOI] [PubMed] [Google Scholar]