Abstract
Aim:
To investigate the effects of S-allylcysteine (SAC), a water-soluble garlic derivative, on human ovarian cancer cells in vitro.
Methods:
Human epithelial ovarian cancer cell line A2780 was tested. Cell proliferation was examined with CCK-8 and colony formation assays. Cell cycle was analyzed with flow cytometry. Cell apoptosis was studied using Hoechst 33258 staining and Annexin V/PI staining with flow cytometry. The migration and invasion of A2780 cells were examined with transwell and wound healing assays. The expression of relevant proteins was detected with Western blot assays.
Results:
SAC (1−100 mmol/L) inhibited the proliferation of A2780 cells in dose- and time-dependent manners (the IC50 value was approximately 25 mmol/L at 48 h, and less than 6.25 mmol/L at 96 h). Furthermore, SAC dose-dependently inhibited the colony formation of A2780 cells. Treatment of A2780 cells with SAC resulted in G1/S phase arrest and induced apoptosis, accompanied by decreased expression of pro-caspase-3, Parp-1 and Bcl-2, and increased expression of active caspase-3 and Bax. SAC treatment significantly reduced the migration of A2780 cells, and markedly decreased the protein expression of Wnt5a, p-AKT and c-Jun, which were the key proteins involved in proliferation and metastasis.
Conclusion:
SAC suppresses proliferation and induces apoptosis in A2780 ovarian cancer cells in vitro.
Keywords: ovarian cancer, anticancer drug, garlic, S-allylcysteine, proliferation, apoptosis, cancer metastasis
Introduction
Ovarian cancer is one of the most lethal gynecologic malignancies in women. Despite advances in the diagnosis and treatment of ovarian cancer, the survival rate of ovarian cancer patients remains low. This poor survival rate is partly due to the lack of sensitive and specific methods for early detection and, more importantly, the metastatic and invasive capabilities of ovarian cancer cells1. Although platinum-based chemotherapy drugs are initially effective2,3, recurrent ovarian tumors are more aggressive, metastasize to secondary target tissues, and acquire resistance to conventional chemotherapies. Therefore, continued development of novel chemotherapy drugs for ovarian cancer is urgently required.
Natural compounds derived from food have been investigated as new sources of chemotherapy drugs4. These agents act in a manner similar to the conventional chemotherapy drugs by disrupting the cell cycle or inducing apoptosis. For example, the medicinal properties of compounds found in garlic, a member of the Allium species, have been well-documented throughout history5. Specifically, large-scale epidemiological studies within the past few decades have suggested a correlation between garlic consumption and a reduced incidence of cancer6,7. Further investigation showed that organosulfur compounds naturally found in garlic are likely to be responsible for the decreased cancer risk8. These previous reports encouraged efforts to investigate whether the compounds from garlic have specific anti-cancer activities.
Sulfur-containing compounds in garlic can be broadly categorized as oil-soluble, such as diallylsulphide, diallyldisulphide (DADS) and diallyltrisulfide (DATS), or water-soluble, such as S-allylcysteine (SAC). During the process of garlic aging, resistant lipid-soluble compounds are naturally converted into more stable and bioavailable water-soluble compounds9. Several lines of evidence have demonstrated that SAC can inhibit the proliferation, as well as induce the apoptosis, of various cancer cells, including human prostate cancer10, colon cancer11, gastric cancer12, breast cancer13, neuroblastoma14 and ovarian cancer15 cells. Moreover, SAC appeared to have no adverse effects when administered to nude mice at the dosage required to suppress the growth of prostate tumor xenografts10. However, the anti-proliferative mechanism of SAC in the treatment of ovarian carcinoma remains unclear. In this paper, we investigated the antitumor effects of SAC in vitro using the human ovarian epithelial cancer cell line A2780 with the aim of unraveling the molecular mechanisms that drive the SAC-induced antitumor activity in A2780 cells.
Materials and methods
Materials
RPMI-1640 medium and fetal bovine serum (FBS) were purchased from Thermo Scientific (South Logan, UT, USA). CCK-8 was purchased from Sigma-Aldrich (St Louis, MO, USA). Giemsa solution was purchased from Solarbio (Beijing, China). The Cycletest Plus DNA Reagent Kit was purchased from BD Biosciences (Franklin Lakes, NY, USA). Hoechst 33258 was purchased from Sigma-Aldrich (St Louis, MO, USA). The Annexin-V-Fluor Staining Kit was purchased from BD Biosciences (Franklin Lakes, NY, USA). BD BioCoat™ BD Matrigel™ Invasion Chambers were purchased from BD Biosciences (Franklin Lakes, NY, USA). Gentian violet was purchased from Huyu Biotech Co, Ltd (Shanghai, China). Cell Lysis Buffer was purchased from Cell signaling (Danvers, MA, USA). PVDF membrane was purchased from Millipore (Billerica, MA, USA). ECL Plus substrate was purchased from Thermo Scientific Pierce (Rockford, IL,USA). The internal reference antibody against β-actin and the primary antibodies against pro-caspase-3, active caspase-3, caspase-9, Parp-1 Bcl-2, Bax, Akt, p-Akt-ser473, PI3K, c-Jun, and Wnt5a were purchased from Abcam Inc (Cambridge, MA, USA).
Preparation of SAC
SAC was purchased from Shanghai Fundamental Industrial Co, Ltd (Shanghai, China). A 500 mmol/L stock solution of SAC was freshly prepared in phosphate-buffered saline (PBS) according to the manufacturer's instructions and was diluted accordingly as needed.
Cell culture
The human epithelial ovarian cancer cell line A2780 was kindly provided by the Zhejiang Cancer Hospital. The cells were cultured in RPMI-1640 medium supplemented with 10% FBS and 1% penicillin/streptomycin in a 37 °C incubator supplied with 5% CO2.
Cell Count Kit-8 (CCK-8) assay
Cells were seeded at a density of 5000 cells per well in 96-well plates in 100 μL of medium and were incubated for 48 h before treatment. The cells were treated with different concentrations of SAC for 1, 2, 3, or 4 d. The medium was then removed, and 200 μL of fresh medium containing 5% CCK-8 was added for an further 1.5 h. The color intensity was measured using a Multiskan Spectrum spectrophotometer (Thermo Scientific, Rockford, IL,USA) at 450 nm. Each experiment consisted of eight replicates, and at least three individual experiments were performed.
Colony formation assay
A2780 cells in single-cell suspension (200 cells per well) were seeded in 6-well plates and incubated for 48 h. The cells were treated with different concentrations of SAC for 24 h. The medium was then replaced with 5 mL of fresh medium, and the cells were cultured for another 7 d. The cells were fixed with methyl alcohol and glacial acetic acid (3:1) for 10 min and stained with 10%–15% Giemsa solution for 10 min. The colonies consisting of more than 50 cells were counted directly on the plate.
Cell cycle analysis
A2780 cells (3×105) were cultured in 6-well plates for 48 h prior to the experiments. The cells were treated with different concentrations of SAC, ranging from 0 to 29.59 mmol/L, for 24 h. The cells were trypsinized and fixed with 75% ice-cold ethanol for several hours and then stained with the Cycletest Plus DNA Reagent Kit according to the manufacturer's instructions. The DNA content of 10 000 cells was analyzed by flow cytometry for each experiment (FACSCalibur, Becton Dickinson, Franklin Lakes, NJ, USA). Each experiment was analyzed in duplicate, and at least three independent experiments were performed.
Apoptosis
A2780 cells (3×105) were cultured in a 6-well plate for 48 h prior to treatment. The cells were treated with different concentrations of SAC, ranging from 0 to 29.59 mmol/L, for 24 h. The cells were then fixed and stained with Hoechst 33258 for 30 min in the dark and photographed using an inverted fluorescence microscope (Nikon Ti-s, Tokyo, Japan). The cells that contained brightly stained, condensed spots of chromatin were counted as apoptotic cells. Each experiment was analyzed in triplicate, and at least three independent experiments were performed.
Cell apoptosis was analyzed by flow cytometry. After the cells were seeded in a 6-well plate for 24 h, the cells were treated with 29.59 mmol/L SAC for 6, 12, 24, or 48 h. The cells were harvested and stained with the Annexin-V-Fluor Staining Kit according to the manufacturer's instructions and were analyzed by flow cytometry. At least three independent experiments were performed.
Transwell invasion assay
The invasion assay was performed using BD BioCoat™ BD Matrigel™ Invasion Chambers. Briefly, 5×104 A2780 cells were suspended in 300 μL of serum-free DMEM and seeded into the migration chamber. The migration chamber was placed into a 24-well plate and treated with 500 μL of SAC at the indicated concentrations. After 24 h of incubation, the treatment was removed, and the cells on the upper surface of the chamber were carefully scraped off using a cotton swab. The cells that had migrated through the chamber were stained with gentian violet and were subsequently counted under the microscope. At least three independent experiments were performed.
Wound healing assay
A2780 cells (3×105) were seeded in 24-well plates and grown to 100% confluence. A wound was created by scraping the tip of a glass pipette across the monolayer, and the resulting cell debris was washed away with PBS. Fresh medium was then added to the wells along with 0, 2.17, 6.42, or 12.4 mmol/L SAC. After 24 h, the cells were fixed and observed under a microscope. The number of cells that had crossed the wound gap was quantified. At least three independent experiments were performed.
Western blot
After treatment with different concentrations of SAC, A2780 cells were harvested with 0.02% EDTA and 0.025% trypsin, rinsed 3 times in PBS, and then treated with cell lysis buffer. Protein extracts were separated by 10% SDS-PAGE and transferred to a PVDF membrane. The blots were blocked with 5% non-fat milk for 2 h. Primary antibodies at the appropriate dilutions were hybridized to the membrane overnight at 4 °C. The membranes were washed 3 times with TBS-T and were then incubated with a horseradish peroxidase-linked secondary antibody for 1 h at room temperature. The protein bands were visualized using ECL Plus substrate. The BioRad Laboratories Quantity One software (BioRad, Hercules, CA, USA) was used to quantify the blots.
Statistical analysis
Statistical analysis was carried out using SPSS 16.0 for Windows. A two-tailed Student's t-test was used for analysis of continuous variables. P<0.05 was considered statistically significant.
Results
SAC suppressed the proliferation of A2780 cells
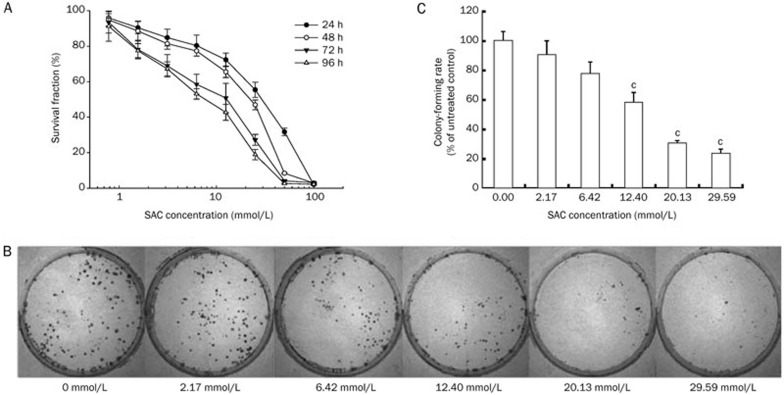
The dose-dependent anti-proliferative effect of SAC on A2780 cells was investigated using cell viability and colony forming assays. As the concentration of SAC increased, a corresponding decrease in cell viability was observed (Figure 1A). Treatment with 100 mmol/L SAC for 24 h resulted in negligible cell viability. Similarly, treatment with 50 mmol/L SAC resulted in less than 10% viable cells after 48 h treatment. Although the half maximal inhibitory concentration (IC50) was approximately 25 mmol/L for the 48 h treatment, the calculated 96 h IC50 value dropped to less than 6.25 mmol/L. This result indicated that prolonged exposure to SAC would eventually be detrimental to A2780 cells, even at low concentrations.
Figure 1.
The effect of SAC on the proliferation and colony formation ability of A2780 cells. (A) The anti-proliferative effect of SAC in A2780 cells after 24, 48, 72, and 96 h treatment. (B) Representative images of A2780 cell colonies after treatment with SAC for 24 h. (C) Colony formation rate of A2780 cells after treatment with SAC for 24 h. cP<0.01 vs control.
The A2780 cell colony formation rate followed a negative trend with the increasing dose of SAC (Figure 1B). Although there was only a slight decrease in the colony formation of A2780 cells after treatment with 2.17 mmol/L SAC, a significant inhibitory effect was observed when the cells were treated with 12.4 mmol/L SAC, and a marked inhibition of colony formation was observed (< 10%) at 29.59 mmol/L SAC (Figure 1C).
SAC resulted in G1/S phase cell cycle arrest of A2780 cells
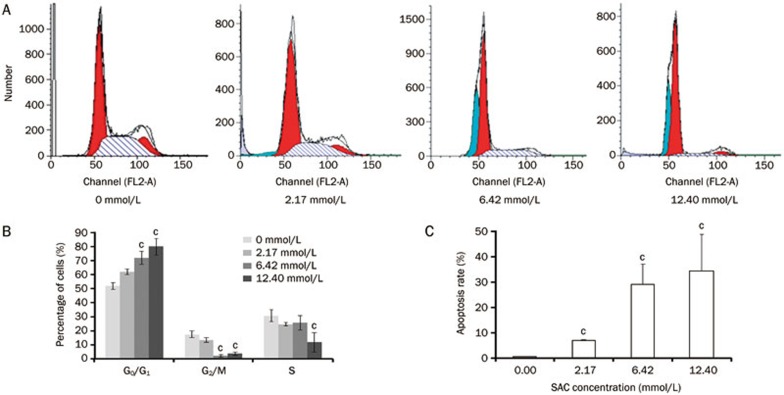
The cell cycle progression of A2780 cells treated with SAC was analyzed by quantitating the cells' DNA content using flow cytometry (Figure 2A). Treatment of A2780 cells with 2.17 mmol/L of SAC resulted in a small decrease in the percentage of G2/M phase cells (Figure 2B). As the SAC concentration increased to 6.4 and 12.4 mmol/L, almost none of these cells stayed in the G2/M phase. At a dose of 12.4 mmol/L, the fraction of cells in S phase was significantly lower than that in the untreated cells. Interestingly, the decreased percentage of cells in both G2/M phase and S phase resulted from a cell cycle arrest in G0/G1 phase, which may have caused the marked reduction in A2780 proliferation described in the previous section.
Figure 2.
The cell cycle arrest of A2780 cells in response to SAC treatment. (A) Cell cycle analysis of A2780 cells treated with SAC for 24 h. (B) Quantitative cell cycle analysis of A2780 cells. (C) Quantitative analysis of the apoptotic A2780 cells. cP<0.01.
SAC induced the apoptosis of A2780 cells
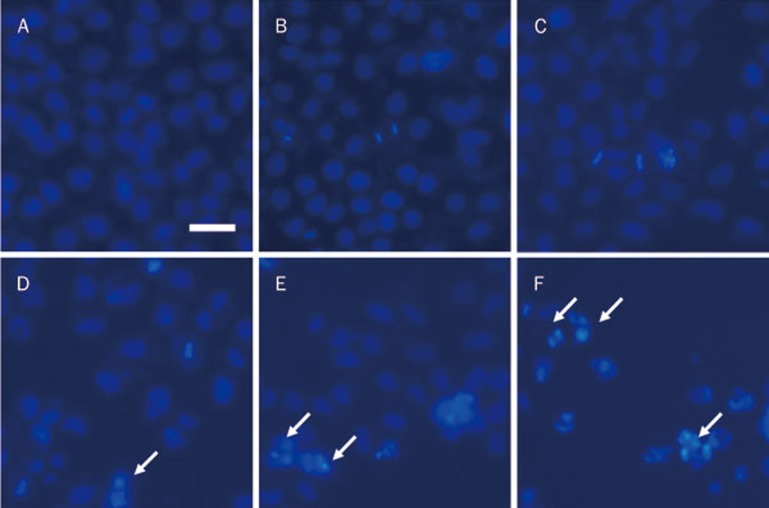
Hoechst nuclear staining can be used to visualize apoptotic A2780 cells because apoptotic cells have condensed chromatin and fragmented nuclei, which appear as bright dots that can be observed under a fluorescence microscope. As shown in Figure 3, the overall cell density decreased in response to increasing concentrations of SAC; additionally, more cells with bright spots indicative of dense chromatin could be seen in the 12.4 mmol/L SAC or higher doses of SAC treated cells, and these results were consistent with the flow cytometric analysis (Figure 2C).
Figure 3.
Hoechst 33258 staining of A2780 cells. The cells were treated with 0 mmol/L (A), 2.17 mmol/L (B), 6.42 mmol/L (C), 12.4 mmol/L (D), 20.13 mmol/L (E), or 29.59 mmol/L (F) SAC for 24 h. The arrows represent the apoptotic cells. Scale bar=10 μm.
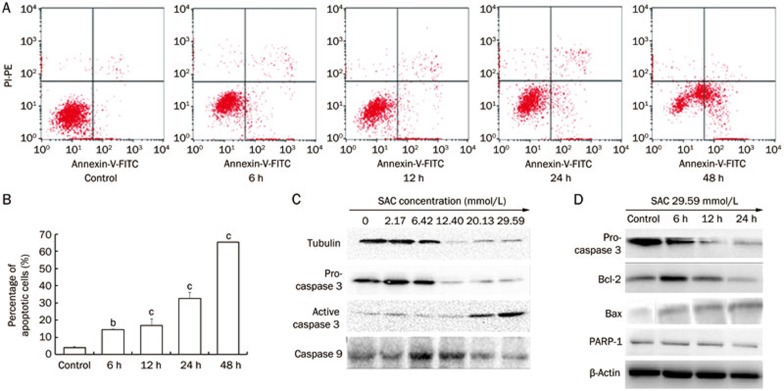
Annexin V/PI staining is generally used to investigate early and late apoptotic cells. Our results showed that the percentages of early and late apoptotic A2780 cells were significantly increased after treatment with 29.59 mmol/L SAC in a time-dependent manner (Figure 4A and 4B). There were 3.6-, 4.2-, 8.0-, and 16.1-fold increases in the number of total apoptotic A2780 cells after 6, 12, 24, and 48 h treatment with SAC, respectively, compared to the untreated cells (Figure 4B). Western blot analysis showed that pro-caspase-3 expression was downregulated in a time- and dose-dependent manner after A2780 cells were treated with SAC (Figure 4C, 4D). The expression of active caspase-3 was elevated after SAC treatment; furthermore active caspase cleaved PARP-1 and caused the downregulation of PARP-1. Moreover, the effects of SAC on Bcl-2 and Bax expression were shown to be time-dependent (Figure 4C). The expression of the anti-apoptotic protein Bcl-2 was suppressed by SAC treatment of A2780 cells, whereas the expression of the apoptosis promoting protein Bax increased.
Figure 4.
The apoptotic effect of SAC on A2780 cells. (A) Annexin V/PI staining of A2780 cells treated with 29.59 mmol/L SAC. (B) Quantitative analysis of apoptotic A2780 cells. bP<0.05, cP<0.01. (C) Protein expression of pro-caspase-3, active caspase-3, caspase-9, PARP-1, Bcl-2, and Bax in A2780 cells treated with SAC.
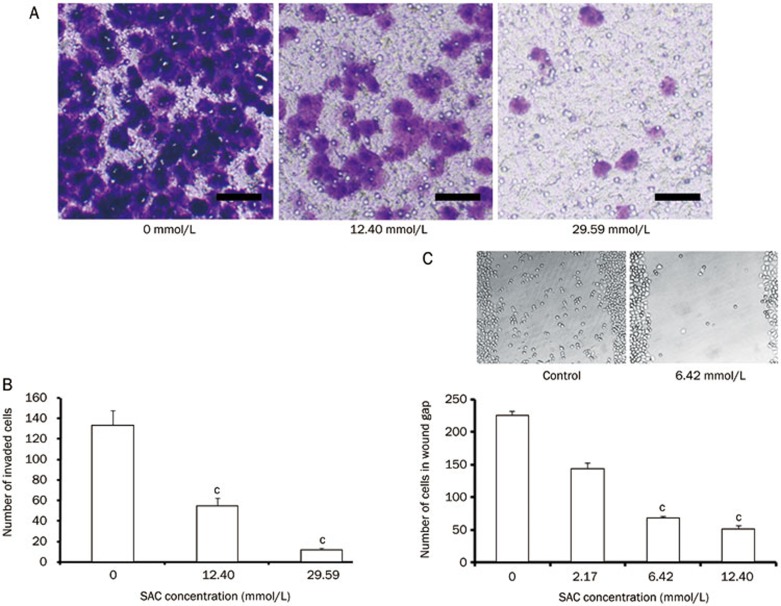
SAC suppressed the migration and invasion of A2780 cells
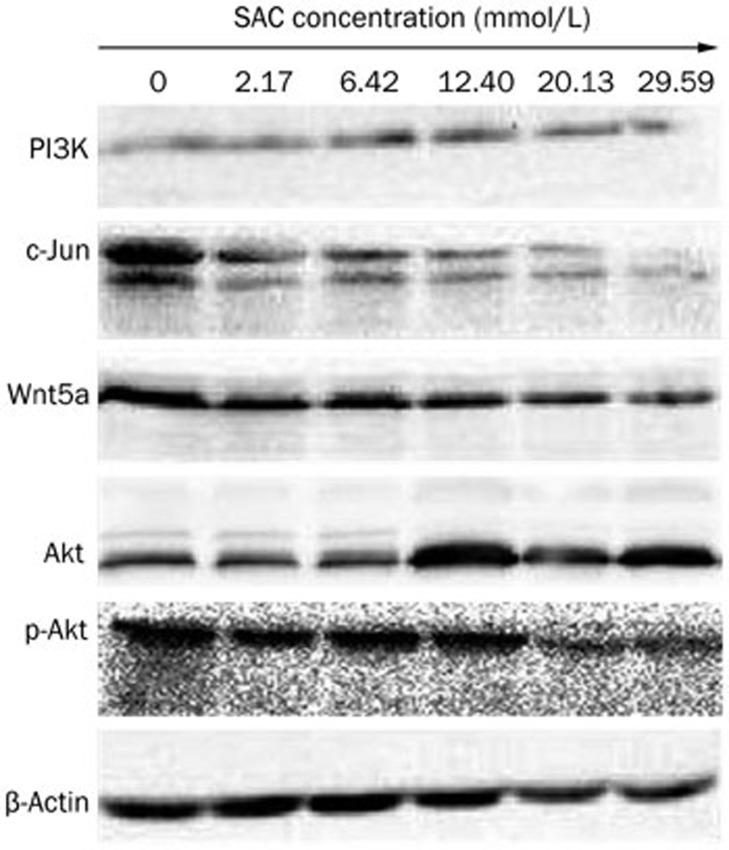
One important feature of ovarian cancer cells is their ability to metastasize to the secondary locations in the body. SAC is capable of inhibiting cell motility and migration. In the transwell invasion assay, treatment with 12.40 mmol/L SAC decreased the fraction of invasive cells by 60%, while 29.59 mmol/L SAC further reduced this fraction to less than 10% (Figure 5A and 5B). Similar anti-migration effects were observed in the wound healing assay (Figure 5C). Treatment with 6.42 mmol/L SAC reduced the migration of over 70% of the cells, and 12.40 mmol/L SAC reduced the migration of over 80% of the A2780 cells. The expression level of Wnt5a, a pivotal protein in the metastasis pathway, was also downregulated in the SAC treated cells (Figure 6).
Figure 5.
The inhibitory effect of SAC on the migration and invasion of A2780 cells. (A) The effect of SAC on the invasion and migration of A2780 cells as determined by Gentian violet staining. Scale bar=20 μm. (B) Quantitative analysis of the invading cells. (C) Quantitative analysis and images of the cells migrating into the wound following SAC treatment for 24 h. cP<0.01.
Figure 6.
Protein expression of PI3K, c-Jun, Wnt5a, Akt, and p-Akt in A2780 cells after treatment with various concentrations of SAC for 24 h.
Signaling pathways involved in SAC-mediated suppression of proliferation in A2780 cells
An overactive PI3K/Akt/mTOR signaling pathway is a characteristic associated with the hyperproliferative potential of many cancers. Thus, we analyzed the expression of key factors in this signaling pathway (Figure 6). As the SAC dose increased, Akt expression in the A2780 cells increased, together with a corresponding suppression of Ser473-phosphorylated Akt. By contrast, expression of PI3K, which is required upstream of Akt phosphorylation, was not affected by SAC treatment. Additionally, the expression of the apoptosis-related protein c-Jun was dramatically suppressed by SAC treatment in a dose-dependent manner.
Discussion
Chemotherapy with platinum-based drugs, such as carboplatin alone or carboplatin in combination with paclitaxel, is currently the preferred treatment for ovarian cancer. Unfortunately, the likelihood of relapse following chemotherapy is extremely high, particularly for patients in advanced stages of disease, necessitating a secondary round of “salvage chemotherapy”. However, a number of challenges still need to be overcome to improve the efficacy of salvage chemotherapy. First, cancer cells develop gradual resistance to platinum drugs; second, the cumulative toxicity of platinum often leads to negative outcomes in patients. Therefore, it is of great interest to develop novel chemotherapy agents for the treatment of ovarian cancer.
The anti-cancer properties of SAC, a water-soluble garlic derivative, that exhibits low toxicity and high anti-cancer activity, have been investigated in a variety of cancer cell lines. In this study, we sought to study the antitumor activity and the underlying mechanisms of SAC activity in A2780 cells. We demonstrated that SAC inhibited the proliferation rate of A2780 cells in a dose- and time-dependent manner. We found that treatment with 12.4 mmol/L SAC effectively suppressed the proliferation and colony formation of A2780 cells, which is consistent with studies of human hepatocellular carcinoma16 and breast cancer cell lines17.
The interruption of proper apoptosis signaling pathways is a hallmark of many cancers. Naturally derived organosulfur compounds have been shown to induce apoptosis in a variety of cancer cells18, such as human leukemia cells19, human colon cancer cells20, and breast cancer cells21. Here, we report that SAC induces apoptosis in A2780 cells in a time-dependent manner. Moreover, SAC treatment led to the downregulation of pro-caspase-3 and the activation of cleaved caspase-3 and PARP-1 in A2780 cells. SAC treatment also resulted in decreased expression of Bcl-2 and increased expression of Bax. These results are similar to the findings of Velmurugan et al in experimental gastric cancer12. The proteins of the Bcl-2 family play primary roles in regulating apoptosis by inducing mitochondrial outer membrane permeabilization22. This process is antagonized by members of the anti-apoptotic Bcl-2 family, such as Bcl-2 and Bcl-xL, which inhibit the permeabilization functions of Bax and Bak23. The ratio of Bcl-2/Bax is recognized as a good indicator of whether a cell will undergo apoptosis. Therefore, our findings indicated that the SAC-induced apoptosis was possibly mediated through the mitochondrion pathway.
Rapid and early metastasis of ovarian carcinoma into the peritoneal cavity is a key characteristic of ovarian cancer and is associated with poor outcomes at the point of diagnosis. Wnt5a is a target gene of the Wnt/β-catenin signaling pathway. High expression of Wnt5a usually correlates with poor prognosis due to its ability to stimulate cell migration and invasion24,25,26,27,28,29,30. Studies have shown that SAC treatment could affect the expression of β-catenin, which plays an important role in the Wnt signaling pathway15. Here, we observed a high degree of invasiveness by A2780 cells, which was abrogated by SAC treatment. Wnt5a was downregulated in the SAC-treated cells, which could potentially contribute to the inhibited invasiveness and metastatic ability of A2780 cells15,16.
The PI3K/Akt/mTOR pathway is a prototypical survival pathway that is over-activated in many cancers31. Activation of PI3K in response to extracellular stimuli allows for the subsequent downstream phosphorylation of Akt, which can then activate NF-κB, resulting in the transcription of pro-survival genes. Downregulation of p-Akt result in a G1/S phase cell cycle arrest in breast cancer MCF-7 cells32. Western blot analysis of Akt and p-Akt showed that SAC treatment greatly decreased the amount of Ser473-p-Akt in the A2780 cells compared to the untreated cells, and we observed a similar G1/S arrest in A2780 cells that corresponded to the decreased levels of p-Akt in response to SAC treatment. Additionally, the expression of c-Jun was dramatically downregulated by SAC treatment in a dose-dependent manner in this study, suggesting that the activation of JNK1/2 could be regulated by p-Akt through the PI3K pathway33.
Conclusion
In summary, we demonstrated the potential therapeutic effects of a naturally derived organosulfur compound, SAC, on the human ovarian carcinoma cell line A2780 in vitro. SAC suppresses cell proliferation while simultaneously inducing apoptosis. In the near future, our results will contribute to the validation of SAC as a potential, potent anti-cancer drug candidate.
Author contribution
Neng-ming LIN and Dan SU designed the research; Jian-guo FENG, Dan ZHANG, Bo ZHANG, Min LUO, and Ya-si XU performed the research; Ya-si XU, Jian-guo FENG, Dan ZHANG, Bo ZHANG, Min LUO, Dan SU, and Neng-ming LIN analyzed the data and wrote the paper.
Acknowledgments
This work was supported by the Zhejiang Provincial Program for the Cultivation of High-level Innovative Health talents (2010-190-4) and the Zhejiang Key Laboratory of Diagnosis and Treatment Technology on Thoracic Oncology (Lung and Esophagus) (2012-02).
References
- 1Yancik R. Ovarian cancer. Age contrasts in incidence, histology, disease stage at diagnosis, and mortality. Cancer 1993; 71: 517–23. [DOI] [PubMed] [Google Scholar]
- 2Pujade-Lauraine E, Wagner U, Aavall-Lundqvist E, Gebski V, Heywood M, Vasey PA, et al. Pegylated liposomal doxorubicin and carboplatin compared with paclitaxel and carboplatin for patients with platinum–sensitive ovarian cancer in late relapse. J Clin Oncol 2010; 28: 3323–3329. [DOI] [PubMed] [Google Scholar]
- 3Hamilton CA, Miller A, Miller C, Krivak TC, Farley JH, Chernofsky MR, et al. The impact of disease distribution on survival in patients with stage III epithelial ovarian cancer cytoreduced to microscopic residual: a Gynecologic Oncology Group study. Gynecol Oncol 2011; 122: 521–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4Nobili S, Lippi D, Witort E, Donnini M, Bausi L, Mini E, et al. Natural compounds for cancer treatment and prevention. Pharmacol Res 2009; 59: 365–78. [DOI] [PubMed] [Google Scholar]
- 5Iciek M, Kwiecien I, Wlodek L. Biological properties of garlic and garlic-derived organosulfur compounds. Environ Mol Mutagen 2009; 50: 247–65. [DOI] [PubMed] [Google Scholar]
- 6Butt MS, Sultan MT, Iqbal J. Garlic: nature's protection against physiological threats. Crit Rev Food Sci Nutr 2009; 49: 538–51. [DOI] [PubMed] [Google Scholar]
- 7Zhou Y, Zhuang W, Hu W, Liu GJ, Wu TX, Wu XT. Consumption of large amounts of Allium vegetables reduces risk for gastric cancer in a meta-analysis. Gastroenterology 2011; 141: 80–9. [DOI] [PubMed] [Google Scholar]
- 8Iciek M, Kwiecien I, Chwatko G, Sokolowska-Jezewicz M, Kowalczyk–Pachel D, Rokita H. The effects of garlic-derived sulfur compounds on cell proliferation, caspase 3 activity, thiol levels and anaerobic sulfur metabolism in human hepatoblastoma HepG2 cells. Cell Biochem Funct 2012; 30: 198–204. [DOI] [PubMed] [Google Scholar]
- 9Lai KC, Kuo CL, Ho HC, Yang JS, Ma CY, Lu HF, et al. Diallyl sulfide, diallyl disulfide and diallyltrisulfide affect drug resistant gene expression in colo 205 human colon cancer cells in vitro and in vivo. Phytomedicine 2012; 19: 625–30. [DOI] [PubMed] [Google Scholar]
- 10Chu Q, Lee DT, Tsao SW, Wang X, Wong YC. S-allylcysteine, a water-soluble garlic derivative, suppresses the growth of a human androgen-independent prostate cancer xenograft, CWR22R, under in vivo conditions. BJU Int 2007; 99: 925–32. [DOI] [PubMed] [Google Scholar]
- 11Tanaka S, Haruma K, Yoshihara M, Kajiyama G, Kira K, Amagase H, et al. Aged garlic extract has potential suppressive effect on colorectal adenomas in humans. J Nutr 2006; 136: 821S–826S. [DOI] [PubMed] [Google Scholar]
- 12Velmurugan B, Mani A, Nagini S. Combination of S-allylcysteine and lycopene induces apoptosis by modulating Bcl-2, Bax, Bim and caspases during experimental gastric carcinogenesis. Eur J Cancer Prev 2005; 14: 387–93. [DOI] [PubMed] [Google Scholar]
- 13Na HK, Kim EH, Choi MA, Park JM, Kim DH, Surh YJ. Diallyltrisulfide induces apoptosis in human breast cancer cells through ROS-mediated activation of JNK and AP-1. Biochem Pharmacol 2012; 84: 1241–50. [DOI] [PubMed] [Google Scholar]
- 14Pagliei B, Aquilano K, Baldelli S, Ciriolo MR. Garlic-derived diallyl disulfide modulates peroxisome proliferator activated receptor gamma co-activator 1 alpha in neuroblastoma cells. Biochem Pharmacol 2013; 85: 335–44. [DOI] [PubMed] [Google Scholar]
- 15Chu Q, Ling MT, Feng H, Cheung HW, Tsao SW, Wang X, et al. A novel anticancer effect of garlic derivatives: inhibition of cancer cell invasion through restoration of E-cadherin expression. Carcinogenesis 2006; 27: 2180–9. [DOI] [PubMed] [Google Scholar]
- 16Ng KT, Guo DY, Cheng Q, Geng W, Ling CC, Li CX, et al. A garlic derivative, S-allylcysteine (SAC), suppresses proliferation and metastasis of hepatocellular carcinoma. PLoS One 2012; 7: e 31655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17Gapter LA, Yuin OZ, Ng KY. S-Allylcysteine reduces breast tumor cell adhesion and invasion. Biochem Biophys Res Commun 2008; 367: 446–51. [DOI] [PubMed] [Google Scholar]
- 18Powolny AA, Singh SV. Multitargeted prevention and therapy of cancer by diallyltrisulfide and related Allium vegetable-derived organosulfur compounds. Cancer Lett 2008; 269: 305–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19Wong WW, Boutros PC, Wasylishen AR, Guckert KD, O'Brien EM, Griffiths R, et al. Characterization of the apoptotic response of human leukemia cells to organosulfur compounds. BMC Cancer 2010; 10: 351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20Bat-chen W, Golan T, Peri I, Ludmer Z, Schwartz B. Allicin purified from fresh garlic cloves induces apoptosis in colon cancer cells via Nrf2. Nutr Cancer 2010; 62: 947–57. [DOI] [PubMed] [Google Scholar]
- 21Malki A, El-Saadani M, Sultan AS. Garlic constituent diallyltrisulfide induced apoptosis in MCF7 human breast cancer cells. Cancer Biol Ther 2009; 8: 2175–85. [DOI] [PubMed] [Google Scholar]
- 22Llambi F, Green DR. Apoptosis and oncogenesis: give and take in the BCL-2 family. Curr Opin Genet Dev 2011; 21: 12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23Reed JC. Proapoptotic multidomain Bcl-2/Bax-family proteins: mechanisms, physiological roles, and therapeutic opportunities. Cell Death Differ 2006; 13: 1378–86. [DOI] [PubMed] [Google Scholar]
- 24Peng C, Zhang X, Yu H, Wu D, Zheng J. Wnt5a as a predictor in poor clinical outcome of patients and a mediator in chemoresistance of ovarian cancer. Int J Gynecol Cancer 2011; 21: 280–8. [DOI] [PubMed] [Google Scholar]
- 25Kurayoshi M, Oue N, Yamamoto H, Kishida M, Inoue A, Asahara T, et al. Expression of Wnt-5a is correlated with aggressiveness of gastric cancer by stimulating cell migration and invasion. Cancer Res 2006; 66: 10439–48. [DOI] [PubMed] [Google Scholar]
- 26Huang CL, Liu D, Nakano J, Ishikawa S, Kontani K, Yokomise H, et al. Wnt5a expression is associated with the tumor proliferation and the stromal vascular endothelial growth factor — an expression in non-small-cell lung cancer. J Clin Oncol 2005; 23: 8765–73. [DOI] [PubMed] [Google Scholar]
- 27Zeng ZY, Zhou YH, Zhang WL, Xiong W, Fan SQ, Li XL, et al. Gene expression profiling of nasopharyngeal carcinoma reveals the abnormally regulated Wnt signaling pathway. Hum Pathol 2007; 38: 120–33. [DOI] [PubMed] [Google Scholar]
- 28Ripka S, Konig A, Buchholz M, Wagner M, Sipos B, Kloppel G, et al. WNT5A — target of CUTL1 and potent modulator of tumor cell migration and invasion in pancreatic cancer. Carcinogenesis 2007; 28: 1178–87. [DOI] [PubMed] [Google Scholar]
- 29Kikuchi A, Yamamoto H, Sato A, Matsumoto S. Wnt5a: its signalling, functions and implication in diseases. ActaPhysiol (Oxf) 2012; 204: 17–33. [DOI] [PubMed] [Google Scholar]
- 30Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer 2013; 13: 11–26. [DOI] [PubMed] [Google Scholar]
- 31LoPiccolo J, Blumenthal GM, Bernstein WB, Dennis PA. Targeting the PI3K/Akt/mTOR pathway: effective combinations and clinical considerations. Drug Resist Updat 2008; 11: 32–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32Noori S, Hassan ZM. Tehranolide inhibits proliferation of MCF-7 human breast cancer cells by inducing G0/G1 arrest and apoptosis. Free Radic Biol Med 2012; 52: 1987–99. [DOI] [PubMed] [Google Scholar]
- 33Hui L, Pei DS, Zhang QG, Guan QH, Zhang GY. The neuroprotection of insulin on ischemic brain injury in rat hippocampus through negative regulation of JNK signaling pathway by PI3K/Akt activation. Brain Res 2005; 1052: 1–9. [DOI] [PubMed] [Google Scholar]