Abstract
Aim:
To investigate whether human multiple myeloma (MM) cells secrete microvesicles (MVs) and whether the MVs secreted from MM cells (MM-MVs) promote angiogenesis.
Methods:
RPMI8226 human MM cells and EA.hy926 human umbilical vein cells were used. MVs isolated from RPMI8226 cells were characterized under laser confocal microscopy, electron microscopy and with flow cytometry. The fusion of MM-MVs and EA.hy926 cells was studied under confocal microscopy, and the transfer of CD138 to EA.hy926 cells was demonstrated with flow cytometry. The proliferation, invasion and tube formation of EA.hy926 cells in vitro were evaluated using MTT, transwell migration and tube formation assays, respectively. The vasculization of EA.hy926 cells in vivo was studied using Matrigel plug assay. The expression of IL-6 and VEGF was analyzed with PCR and ELISA.
Results:
MM-MVs from the RPMI 8226 cells had the characteristic cup-shape with diameter of 100–1000 nm. Most of the MM-MVs expressed phosphatidylserine and the myeloma cell marker CD138, confirming that they were derived from myeloma cells. After added to EA.hy926 cells, the MM-MVs transferred CD138 to the endothelial cells and significantly stimulated the endothelial cells to proliferate, invade, secrete IL-6 and VEGF, two key angiogenic factors of myeloma, and form tubes in vitro and in vivo.
Conclusion:
Our results confirm the presence of MVs in MM cells and support the idea that MM-MVs are newfound mediators for myeloma angiogenesis and may serve as a therapeutic target to treat MM.
Keywords: microvesicle, multiple myeloma, CD138, IL-6, VEGF, angiogenesis, endothelial cell
Introduction
The development of multiple myeloma (MM) is a complex, multistep process. Accumulating evidence has shown that the interaction and crosstalk of myeloma plasma cells with the surrounding host cells play key roles in disease progression1,2,3. Many findings suggested that MM cells possess angiogenic capabilities through the release of angiogenic cytokines, which leads to substantial bone marrow neovascularization4,5. However, the proangiogenic properties of myeloma cells and the biological mechanisms of MM-induced angiogenesis have not yet been elucidated5.
Microvesicles (MVs), also known as shedding vesicles or microparticles, are endosome-derived organelles that are spontaneously secreted from normal and neoplastic cells. These vesicles were originally considered to be inert cellular debris6,7,8,9,10; however, it is now known that both their cytoplasmic fraction and their membrane contain a variable spectrum of molecules, and this pattern specifically reflects the donor cell that secreted them9,11,12. MVs can reach the local target cells but can also travel over long distances12,13. A large number of studies have reported that tumor cell-derived MVs (TMVs) reflect the special potential of tumor cells for the expansion of the tumor, independently from cell to cell contact14,15. Recently, a growing body of evidence suggests that TMVs contain a selective enrichment of a discrete set of cellular molecules that can be transferred into the target cells16,17,18,19. Interestingly, Thompson et al20 recently confirmed that exosomes, which are a different kind of vesicle with different origins, sizes and content, are secreted by myeloma cells, and emerging evidence has shown that TMVs act as novel angiogenic mediators and can stimulate angiogenesis in tumors14,19,21,22,23; however, it is not clear whether MVs can be secreted by myeloma cells (MM-MVs).
In the present study, we investigated the presence and characteristics of MM-MVs from RPMI8226 myeloma cells, observed the fusion of MM-MVs and endothelial cells, and investigated the effects of MM-MVs on the neovasculization and angiogenesis of endothelial cells in vitro and in vivo. Our findings confirmed the presence of MM-MVs and demonstrated that these vesicles can act as novel mediators in myeloma angiogenesis. These results shed new light on the interaction of myeloma cells and the tumor environment.
Materials and methods
Cell culture
The RPMI 8226 human myeloma cell line and the EA.hy926 human umbilical vein cell line were purchased from the American Type Culture Collection (ATCC, Rockville, MD, USA) and cultured in a humidity-controlled incubator (37 °C, 5% CO2).
Isolation of MVs
MVs were isolated by differential ultracentrifugation13. Briefly, the RPMI 8226 cells (1×107) were washed twice with PBS and were then incubated in serum-free DMEM (20 mL) for 24 h. Then, the media were collected by centrifugation at 1000×g for 5 min. The supernatant was centrifuged at 4000×g for 1 h to remove the cellular debris, and the resulting supernatant was then distributed into EP tubes for an additional centrifugation at 16 000×g for 1 h. The supernatant was discarded to remove the exosomes, and the MV pellet was washed and resuspended in PBS, followed by another centrifugation at 16 000×g for 1 h to remove the remaining exosomes. The protein content of the MV preparation was quantified using the Bradford method (Bio-Rad, Hercules, CA, USA) as previously reported24. All of the centrifugations were performed at 4 °C, and the isolated MVs were stored in PBS at 4 °C until use.
Transmission electron microscopy (TEM) and fluorescence microscopy (FM)
TEM was performed as previously reported25. For FM, the purified MM-MVs were stained using the PKH26 Red Fluorescent Cell Linker Kit (Sigma-Aldrich, St Louis, MO, USA) according to the kit's instructions, and the MM-MVs were observed by FM (Olympus, Tokyo, Japan).
Membrane interaction assay
As previously reported13, the MM-MVs were stained with PKH26 (1 μmol/L), and the EA.hy926 cells were incubated with the PKH26-stained MM-MVs for 2, 4, 6, 12, 18, or 24 h at 37 °C. The cells were then washed twice with PBS, counterstained with DAPI (Beyotime, Shanghai, China) and visualized using a confocal microscope (NikonA1Si, Nikon, Tokyo, Japan). Furthermore, to analyze the MM-MV markers, standard microbeads with a diameter of 1 μm (Sigma-Aldrich, St Louis, MO, USA) were used to set the upper size limit of the MVs, and this population was used to gate the MVs. MM-MVs were stained with both annexin V and an anti-CD138 antibody and were analyzed by flow cytometry using a FACScan flow cytometer (Becton Dickinson, San Diego, CA, USA). The EA.hy926 cells were incubated with 5 μg/mL MM-MVs for 24 h, washed with PBS and then stained for CD138 to check the incorporation of the MM-MVs into the EA.hy926 cells by flow cytometry.
MTT analysis
Briefly, EA.hy926 cells (5×104 cells/mL) were seeded into each well of a 96-well microplate in a final volume of 200 μL and were cultured overnight at 37 °C in a humidified, 5% CO2 atmosphere (v/v). Then, 10 μL of MM-MVs were added at a final concentration of 5 μg/mL and cultured for an additional 12, 24, 36, or 48 h. The MTT assay was performed in triplicate.
Transwell migration assay
The transwell (Corning Inc, NY, USA) migration assay was performed as previously reported26. The bottom chambers were filled with 600 μL of DMEM supplemented with 10% FBS, and the top chambers were seeded with 5×104 EA.hy926 cells/well in 100 μL of medium; 5 μg/mL MM-MVs or PBS (control) was added to the cells in the top chamber. After 12, 24, 36, or 48 h of migration, the invasive cells found in the bottom chamber were fixed and counted.
Tube formation assay
Matrigel (growth factor reduced) was thawed at 4 °C, and each well of prechilled 96-well plates was coated with 50 μL Matrigel, then incubated at 37 °C for 1 h. EA.hy926 cells (2×103), in a volume of 100 μL of DMEM supplemented with 0.5% FBS, were added to each well, along with 10 μL of MM-MVs (at a final concentration of 5 μg/mL) or PBS. After 3, 6, 9, or 12 h of incubation at 37 °C with 5% CO2, the endothelial cell tubular structure formation was quantified by calculating the tube length under a high-powered field (HPF; 200×) with an Olympus inverted microscope, and the inhibition percentage was calculated by setting the untreated wells to 100%26.
Matrigel plug assay
All animal experiments were conducted at Tongji Medical College of Huazhong University with Hubei Legislation for Animal Care. Briefly, 6-week-old C57/BL6 mice (n=3 each group) were injected subcutaneously in the midventral abdominal region with 500 μL of Matrigel containing 50 μg/plug MM-MVs or PBS as a control. After 6 d, the mice were sacrificed, the plugs were removed, and analysis was performed as previously described26.
Conventional PCR and ELISA assay
EA.hy926 cells (5×104 cells/ml) were treated with MM-MVs as in the Transwell migration assay. The following primer sequences were used: VEGF, sense 5′-CTGCTGTCTTGGGTGCATTG-3′ and antisense 5′-TTCACATTTGTTGTGCTGTAG-3′ (378 bp); IL-6, sense 5′-CCTTAAAGCTGCGCAGAATG-3′ and antisense 5′-ATTCAATGAGGAGACTTGCC-3′ (284 bp). ELISA Kits (Boster, Wuhan, China) were used to analyze the VEGF and IL-6 present in the supernatants.
Statistical analysis
The data are expressed as the mean±SEM of at least three experiments. Statistical analyses were performed using a paired Student's t test, and the differences were considered statistically significant when P<0.05.
Results
Validation of RPMI8226 cell-derived microvesicles
The presence of RPMI 8226 cell-derived MM-MVs was validated using TEM, FM and flow cytometry to examine the morphological and immunophenotyping characteristics. As shown in Figure 1A, a representative TEM shows the characteristic cup-shaped morphology of the MM-MVs, and the scale bar shows that these MM-MVs have a diameter range of 100–1000 nm. Exposure of phosphatidylserine (PS) and CD138 are typical markers for MVs27 and myeloma cells28, respectively. We used Annexin-V and an anti-CD138 antibody to mark the MVs that were released from the myeloma cells. Most of the MM-MVs from the RPMI 8226 cells expressed PS (94.5%±3.6%) and CD138 (82.1%± 5.3%) (Figure 1B), confirming that these vesicles were derived from myeloma cells. Exosomes are stored as intraluminal vesicles within multivesicular bodies in the late-endosome; as such, they share biochemical characteristics with the internal vesicles of multivesicle bodies. The vesicles isolated from the RPMI 8226 cells mostly expressed PS and CD138 with a size of 100 to 1000 nm and were, therefore, considered MVs, not exosomes.
Figure 1.
Validation of MM-MVs. (A) Morphology of the MM-MVs from RPMI8226 cells observed by TEM. The arrows show microvesicles with sphericity, and the scale bar represents 0.5 μm (×25 600). (B) The markers of the MM-MVs were confirmed by flow cytometry. Standard microbeads with a diameter of 1 μm were used to set the upper size limit for the MVs and were used to gate the MVs. MVs stained with Annexin-V and anti-CD138 were analyzed by flow cytometry. Panels 1, 3, and 2, 4 show the microbead gating (1 μm) for the PS and CD138 expression analyses, respectively. Q2 of panels 2 and 4 show the PS and CD138 expression, respectively. The data represent the mean±SEM of three experiments.
MM-MVs integrate into EA.hy926 cells
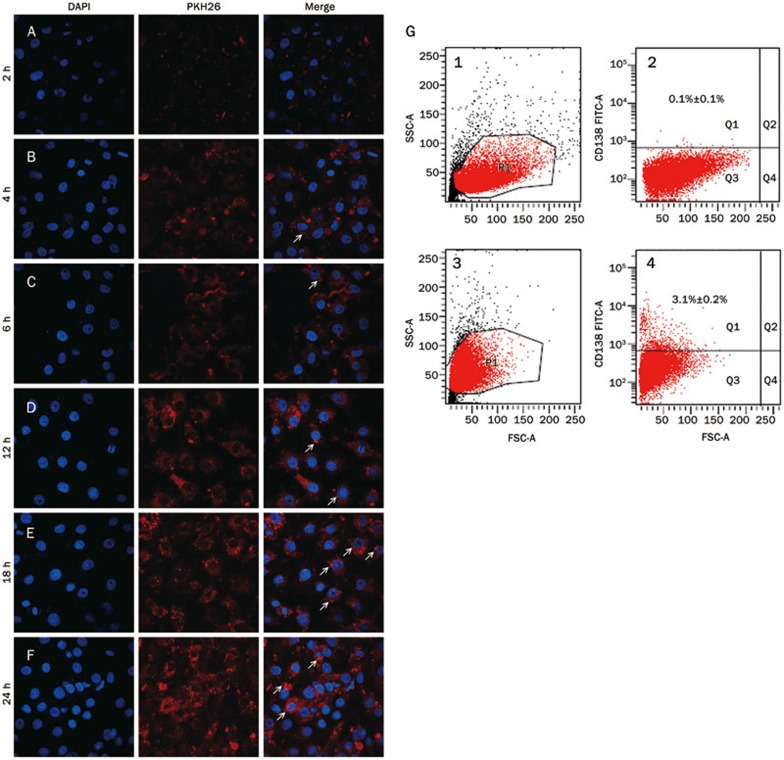
MVs can be internalized by the target cells as previously reported29, and we posited that MM-MVs from RPMI 8226 cells could analogously interact with endothelial cells. After incubation with EA.hy926 cells for various times, fluorescent microscopy analysis confirmed that the MM-MVs were incorporated into the EA.hy926 cells, and a time-dependent transfer of the fluorescent MM-MVs (labeled with PKH-26) was observed (Figure 2A–2F). After 24 h at 37 °C, most of the MM-MVs fluorescence was located around the DAPI-stained nuclei of the EA.hy926 cells, suggesting an almost complete transfer from the MM-MVs to the target cells. Because EA.hy926 cells do not express the CD138 surface marker, we further investigated CD138 expression on EA.hy926 cells to verify the interaction of the MM-MVs with the EA.hy926 cells. As shown in Figure 2G, after incubation for 24 h, 3% of the EA.hy926 cells expressed CD138. This successful transfer of CD138 from the MM-MVs to the endothelial cells also confirmed the interaction between these bodies.
Figure 2.
Incorporation of the MM-MVs into the EA.hy926 cells. (A–F) show the incorporation at 2, 4, 6, 12, 18, and 24 h, respectively. The arrows indicate the MM-MVs that incorporated into the EA.hy926 endothelial cells around the nuclei. (G) After 24 h of incubation with the MM-MVs, the expression of CD138 in EA.hy926 cells was detected using a FACScan flow cytometer. Panels 2 and 4 of G indicate CD138 expression before and after incubation, whereas panels 1 and 3 represent the standard microbead gating, respectively. Q1s of panels 2 and 4 show the CD138+ events of the EA.hy926 cells. The data represent the mean±SEM of three experiments.
MM-MVs promote the proliferation of EA.hy926 cells
Proliferation of endothelial cells is one step in the multi-step process of angiogenesis. To investigate the effect of MM-MVs on this key step, the MM-MVs were incubated with EA.hy926 cells for various amounts of time, and the proliferation of the EA.hy926 cells was assessed using an MTT test. As shown in Figure 3, MM-MVs significantly induced the proliferation of the EA.hy926 in a time-dependent manner, and the proliferative effect was significant after 24 h of incubation. Notably, after 48 h, the EA.hy926 cells co-cultured with MM-MVs continued to proliferate, whereas the proliferation of the cells cultured without MM-MVs began to decrease. This effect indicates that the MM-MVs accelerated the growth of EA.hy926 cells and helped maintain their proliferative properties. Therefore, it is not surprising that MM-MVs have the potential to promote blood vessel formation and angiogenesis of endothelial cells.
Figure 3.
MM-MVs promoted the proliferation of EA.hy926 cells. EA.hy926 cells (5×104 cells/mL) were treated with 5 μg/mL MM-MVs or PBS (control) for 12, 24, 36, or 48 h, and cell proliferation was assessed with an MTT assay. Mean±SEM. n=3. aP>0.05, cP<0.01.
MM-MVs induce the invasion of EA.hy926 cells
Cell migration and invasion are critical for endothelial cells to form blood vessels during tumor angiogenesis; therefore, these processes are necessary for tumor growth and metastasis. We further investigated the effects of MM-MVs on the chemotactic motility of EA.hy926 cells, which was determined using the transwell cell invasion assay. As shown in Figure 4, after incubation with 5 μg/mL MM-MVs for 12, 24, or 36 h, the number of hexamethyl pararosaniline-stained EA.hy926 cells on the bottom of the membrane was significantly increased compared to the PBS control. This indicated that MM-MVs could significantly promote the invasion of EA.hy926 cells (Figure 4A and 4B). However, this effect was not observed after 48 h incubation with MM-MVs; this indicated that the ability of the MM-MVs to promote invasion of endothelial cells had reached its peak.
Figure 4.
Induced invasion of EA.hy926 cells by MM-MVs. Cell migration was assessed by manually counting the invasive stained cells on the bottom of the membrane. (A) Stained cells on the membrane. (B) Invasive cell counts. Mean±SEM. n=3. aP>0.05, cP<0.01 vs the PBS control.
MM-MVs induce capillary structure formation in EA.hy926 cells in vitro and vascularization in vivo
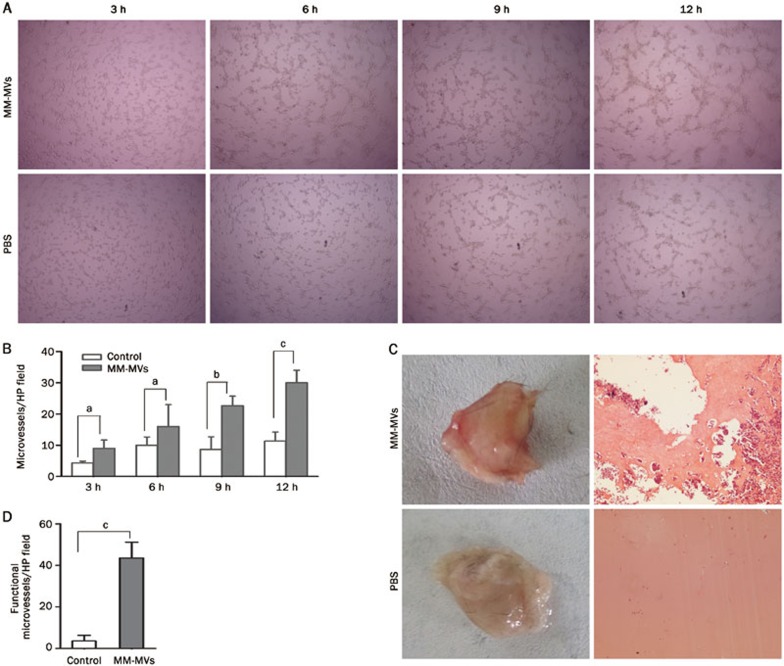
Tube formation of endothelial cells is another key step in the very complex process of angiogenesis. The two-dimensional Matrigel assay was used to investigate whether MM-MVs can induce tube formation in endothelial cells. The ability of the endothelial cells to form tubular structures was assessed in the presence or absence of MM-MVs by calculating the number of microvessels in each HP field with a microscope. EA.hy926 cells plated on Matrigel in a limited medium with 5 μg/mL MM-MVs formed more tubes in a time-dependent manner (Figure 5A). Although the MM-MVs appeared to stimulate tubular structure formation in the EA.hy926 cells, the effects were not statistically significant, compared to the PBS control group, after 3 or 6 h of incubation. However, after 9 h of incubation, the MM-MVs significantly promoted tube formation of the endothelial cells in a time-dependent manner (Figure 5A, 5B).
Figure 5.
Induced angiogenesis of EA.hy926 cells by MM-MVs. (A) EA.hy926 cells (2×104 cells/mL, 100 μL) were combined with 10 μL of MM-MVs (at a final concentration of 5 μg/mL) or PBS (10 μL) in a 96-well plate. After 3, 6, 9, or 12 h, tube formation was analyzed in vitro. (B) Comparisons of the MM-MVs vs the control in (A). Mean±SEM. n=3. aP>0.05, bP<0.05, cP<0.01. (C) Six-week-old C57/BL6 mice (n=3 each group) were injected subcutaneously with 500 μL of Matrigel containing 50 μg/plug MM-MVs or PBS. After 6 d, the mice were sacrificed, and the plugs were removed. The degree of vascularization was evaluated. (D) Comparisons of the MM-MVs vs the control in (C).
To further confirm the effects of MM-MVs on the vascularization of EA.hy926 cells in vivo, the mouse Matrigel plug assay was performed in the presence or absence of 5 μg/mL MM-MVs using six-week-old C57/BL6 mice. The Matrigel plugs were removed after 6 days. As shown in Figure 5C, the plugs containing the MM-MVs were dark red after fixation, and the vessels were abundantly filled with intact red blood cells. In contrast, the plugs containing only PBS were significantly paler, indicating less vessel formation in vivo. After H&E staining, the number of neovessels were analyzed and quantified (Figure 5D). The results indicated that 5 μg/mL MM-MVs could significantly induce the vascularization of EA.hy926 cells in vivo.
MM-MVs promote autocrine IL-6 and VEGF production in EA.hy926 cells
The angiogenic switch in MM is driven by various angiogenic cytokines, which are secreted in the bone marrow microenvironment. IL-6 and VEGF, the two important cytokines, are critically important for myeloma cell proliferation, angiogenesis and disease progression30,31. In a paracrine loop, myeloma cells can stimulate stromal cells or endothelial cells to secret IL-6 and VEGF. To determine the effects of MM-MVs on IL-6 and VEGF secretion on endothelial cells, we analyzed the levels of these proteins in EA.hy926 cells or in the conditioned medium. After incubation with or without MM-MVs for 12, 24, 36, or 48 h, we examined the levels of IL-6 and VEGF mRNA in the EA.hy926 cells, and the results demonstrated that both IL-6 and VEGF mRNA levels were higher in the MM-MVs-treated EA.hy926 cells than in the PBS group, as shown in Figure 6A. Moreover, the ELISA results indicated that the levels of IL-6 and VEGF in the medium from EA.hy926 cells were significantly higher in the MM-MVs-treated EA.hy926 cells compared to the PBS group (Figure 6B and 6C). Taken together, these results indicated that MM-MVs could promote IL-6 and VEGF expression and secretion in EA.hy926 cells.
Figure 6.
Enhanced autocrine IL-6 and VEGF in EA.hy926 cells by MM-MVs. Compared to the PBS group, the expression of IL-6 and VEGF mRNA was elevated in the EA.hy926 cells treated with MM-MVs (A). Increased levels of VEGF (B) and IL-6 (C) were found in the media of EA.hy926 cells treated with MM-MVs. Mean±SEM. n=3. aP>0.05, cP<0.01.
Discussion
It is known that cells communicate and exchange information by employing classical mechanisms, such as secreting soluble factors or cell-to-cell adhesion contacts32. Although these classical events have been commonly thought of as the predominant modes of cell-to-cell communication, a novel type of vesicle secretion has recently received a great deal of attention8,9,15,33. Though the secretion of exosomes by myeloma cells and the cells in the tumor microenvironment was recently discovered21,34, there have been no previous studies about MM-MVs. The current study identified MM-MVs as a potential mediator of angiogenesis.
Vesicles are usually classified into two different types, exosomes and MVs, which are also called shedding vesicles, shedding microvesicles, or microparticles6. Irrespectively of their origin, MVs are circular membrane fragments that retain the characteristics of the parental cell. In contrast, exosomes are smaller and more homogenous; these vesicles originate in the endosome and range from 30 to 120 nm in size35,36. In this study, isolated TMVs from RPMI 8226 cells had a large size distribution that was distinct from the exosomes of myeloma cells20. Furthermore, these MM-MVs expressed the membrane components PS and CD138. These data demonstrated that the vesicles were not exosomes but were instead MVs from the MM cells.
It has been reported that microparticle-mediated transfer of viral receptors confers new functions to the target cells37. The current study confirmed the transferred expression of syndecan-1 to the endothelial cells. MM is characterized by high expression of syndecan-1 (CD138), which is present on the surface of myeloma cells and can be shed into the tumor microenvironment4,38,39,40. Recently, exosome secretion by myeloma cells and the protein cargo of sydecan-1 were reported to dramatically increase when myeloma cells were exposed to exogenous heparanase or when the expression of heparanase was enhanced20. Furthermore, we showed that MM-MV-treated endothelial cells had enhanced levels of VEGF and IL-6, the key growth factors involved in myeloma41. These results support the role of MM-MVs in angiogenesis, consistent with findings in other cancers12,14,21. Of note, the origin of MM-endothelial cells is actually unknown42,43. The present study supports the idea that these cells are malignantly transformed endothelial cells, perhaps partly due to MM-MVs; this idea is highly consistent with the observation that TMVs can form niches that favor tumor development22,44.
It is well known that MVs are important for the intercellular exchange of molecular information9,17,18,19,29,45,46,47. While the antigens on the surface of MVs can resemble those of their parental cells48, many studies have shown that MVs contain a more unique cargo than the parental cells8,10,11,17,49. The microparticles shed from drug-resistant cancer cells are able to spread the cell surface P-glycoprotein (P-gp) to the drug-sensitive recipient cells11, and cancer cells harboring oncogenic EGFR can export this receptor in MVs, leading to transformation-like changes in the adjacent tumor cells and endothelial cells9. Importantly, secreted miRNAs can be packaged into MVs and delivered into the recipient cells; these miRNAs can then act as physiologically functional molecules to exert gene silencing through the same mechanism as endogenous miRNAs16. In the present study, the biologically active molecules horizontally transferred to endothelial cells via MM-MVs may include more than sydecan-1 (CD138), and further studies are needed to explore their contents. Angiogenic cytokines, such as VEGF, have been discovered in exosomes from myeloma cells21. It is reasonable to believe that the enriched clusters of molecules in the MM-MVs, as a whole, may possess the ability to transform the target endothelial cells.
Collectively, our data showed that myeloma RPMI 8226 cells can secrete MM-MVs harboring oncogenic CD138, which is a distinct type of angiogenic regulator, and the incorporation of the MM-MVs into endothelial cells lead to the reprogramming of the endothelial cells. We postulate that these events could contribute to the understanding of the complex pathogenesis and progression of myeloma and the distinct nature of the MM-endothelial cells and may help provide new therapeutic targets to treat MM. Because the origins of MVs in patients are highly heterogeneous and are hard to define or study, we chose the RPMI 8226 cell line as the myeloma cell model. The deficiencies are obvious, and further clinical investigations are urgently needed.
Author contribution
Qiu-bai LI and Zhi-chao CHEN designed the study; Yan LIU, Xiao-jian ZHU, Chen ZENG, Pin-hui WU, and Hong-xiang WANG performed the work; Chen ZENG helped in the construction of the figures and performed the statistical analyses; Qiu-bai LI, Yan LIU, and Pin-hui WU wrote the paper.
Acknowledgments
This research was supported by grants from the National Natural Science Foundation of China (81272624 and 81071943). We would like to thank the Flow Cytometry Lab of Tongji Medical College for the detection and the analysis of the surface markers of the MM-MVs.
References
- 1Strobeck M. Multiple myeloma therapies. Nat Rev Drug Discov 2007; 6: 181–2. [DOI] [PubMed] [Google Scholar]
- 2Singh AV, Bandi M, Raje N, Richardson P, Palladino MA, Chauhan D, et al. A novel vascular disrupting agent plinabulin triggers JNK-mediated apoptosis and inhibits angiogenesis in multiple myeloma cells. Blood 2011; 117: 5692–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3Podar K, Chauhan D, Anderson KC. Bone marrow microenvironment and the identification of new targets for myeloma therapy. Leukemia 2008; 23: 10–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4Purushothaman A, Uyama T, Kobayashi F, Yamada S, Sugahara K, Rapraeger AC, et al. Heparanase-enhanced shedding of syndecan-1 by myeloma cells promotes endothelial invasion and angiogenesis. Blood 2010; 115: 2449–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5Hose D, Moreaux J, Meissner T, Seckinger A, Goldschmidt H, Benner A, et al. Induction of angiogenesis by normal and malignant plasma cells. Blood 2009; 114: 128–43. [DOI] [PubMed] [Google Scholar]
- 6Van der Pol E, Boing AN, Harrison P, Sturk A, Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev 2012; 64: 676–705. [DOI] [PubMed] [Google Scholar]
- 7Szczepanski MJ, Szajnik M, Welsh A, Whiteside TL, Boyiadzis M. Blast-derived microvesicles in sera from patients with acute myeloid leukemia suppress natural killer cell function via membrane-associated transforming growth factor-1. Haematologica 2011; 96: 1302–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8Al-Nedawi K, Meehan B, Rak J. Microvesicles: Messengers and mediators of tumor progression. Cell Cycle 2009; 8: 2014–8. [DOI] [PubMed] [Google Scholar]
- 9Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol 2008; 10: 619–24. [DOI] [PubMed] [Google Scholar]
- 10Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P, et al. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia 2006; 20: 847–56. [DOI] [PubMed] [Google Scholar]
- 11Bebawy M, Combes V, Lee E, Jaiswal R, Gong J, Bonhoure A, et al. Membrane microparticles mediate transfer of P-glycoprotein to drug sensitive cancer cells. Leukemia 2009; 23: 1643–9. [DOI] [PubMed] [Google Scholar]
- 12Wysoczynski M, Ratajczak MZ. Lung cancer secreted microvesicles: Underappreciated modulators of microenvironment in expanding tumors. Int J Cancer 2009; 125: 1595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13Ghosh AK, Secreto CR, Knox TR, Ding W, Mukhopadhyay D, Kay NE. Circulating microvesicles in B-cell chronic lymphocytic leukemia can stimulate marrow stromal cells: implications for disease progression. Blood 2010; 115: 1755–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14Kawamoto T, Ohga N, Akiyama K, Hirata N, Kitahara S, Maishi N, et al. Tumor-derived microvesicles induce proangiogenic phenotype in endothelial cells via endocytosis. PLoS One 2012; 7: e34045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15Antonyak MA, Li B, Boroughs LK, Johnson JL, Druso JE, Bryant KL, et al. Cancer cell-derived microvesicles induce transformation by transferring tissue transglutaminase and fibronectin to recipient cells. Proc Natl Acad Sci U S A 2011; 108: 4852–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16Stoorvogel W. Functional transfer of microRNA by exosomes. Blood 2012; 119: 646–8. [DOI] [PubMed] [Google Scholar]
- 17Jaiswal R, Gong J, Sambasivam S, Combes V, Mathys JM, Davey R, et al. Microparticle-associated nucleic acids mediate trait dominance in cancer. FASEB J 2012; 26: 420–29. [DOI] [PubMed] [Google Scholar]
- 18D'Souza-Schorey C, Clancy JW. Tumor-derived microvesicles: shedding light on novel microenvironment modulators and prospective cancer biomarkers. Genes Dev 2012; 26: 1287–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19Umezu T, Ohyashiki K, Kuroda M, Ohyashiki JH. Leukemia cell to endothelial cell communication via exosomal miRNAs. Oncogene 2013; 32: 2747–55. [DOI] [PubMed] [Google Scholar]
- 20Thompson CA, Purushothaman A, Ramani VC, Vlodavsky I, Sanderson RD. Heparanase regulates secretion, composition, and function of tumor cell-derived exosomes. J Biol Chem 2013; 288: 10093–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21Mineo M, Garfield SH, Taverna S, Flugy A, De Leo G, Alessandro R, et al. Exosomes released by K562 chronic myeloid leukemia cells promote angiogenesis in a src-dependent fashion. Angiogenesis 2011; 15: 33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22Grange C, Tapparo M, Collino F, Vitillo L, Damasco C, Deregibus MC, et al. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res 2011; 71: 5346–56. [DOI] [PubMed] [Google Scholar]
- 23Millimaggi D, Mari M, D'Ascenzo S, Carosa E, Jannini EA, Zucker S, et al. Tumor vesicle-associated CD147 modulates the angiogenic capability of endothelial cells. Neoplasia 2007; 9: 349–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24Castellana D, Zobairi F, Martinez MC, Panaro MA, Mitolo V, Freyssinet JM, et al. Membrane microvesicles as actors in the establishment of a favorable prostatic tumoral niche: a role for activated fibroblasts and CX3CL1-CX3CR1 axis. Cancer Res 2009; 69: 785–93. [DOI] [PubMed] [Google Scholar]
- 25Chai C, Zheng S, Feng J, Wu X, Yang J, Wei M. A novel method for establishment and characterization of extrahepatic bile duct epithelial cells from mice. In Vitro Cell Dev Biol Anim 2010; 46: 820–3. [DOI] [PubMed] [Google Scholar]
- 26Pang X, Yi T, Yi Z, Cho SG, Qu W, Pinkaew D, et al. Morelloflavone, a biflavonoid, inhibits tumor angiogenesis by targeting Rho GTPases and extracellular signal-regulated kinase signaling pathways. Cancer Res 2009; 69: 518–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27Beyer C, Pisetsky DS. The role of microparticles in the pathogenesis of rheumatic diseases. Nat Rev Rheumatol 2010; 6: 21–9. [DOI] [PubMed] [Google Scholar]
- 28Matsui W, Huff CA, Wang Q, Malehorn MT, Barber J, Tanhehco Y, et al. Characterization of clonogenic multiple myeloma cells. Blood 2004; 103: 2332–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29Pap E, Pállinger É, Falus A. The role of membrane vesicles in tumorigenesis. Crit Rev Oncol Hematol 2011; 79: 213–23. [DOI] [PubMed] [Google Scholar]
- 30Dankbar B, Padró T, Leo R, Feldmann B, Kropff M, Mesters RM, et al. Vascular endothelial growth factor and interleukin-6 in paracrine tumor-stromal cell interactions in multiple myeloma. Blood 2000; 95: 2630–6. [PubMed] [Google Scholar]
- 31Fan F, Schimming A, Jaeger D, Podar K. Targeting the tumor microenvironment: focus on angiogenesis. J Oncol 2012; 2012: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32Nguyen DH, Oketch-Rabah HA, Illa-Bochaca I, Geyer FC, Reis-Filho JS, Mao JH, et al. Radiation acts on the microenvironment to affect breast carcinogenesis by distinct mechanisms that decrease cancer latency and affect tumor type. Cancer Cell 2011; 19: 640–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33Li B, Antonyak MA, Zhang J, Cerione RA. RhoA triggers a specific signaling pathway that generates transforming microvesicles in cancer cells. Oncogene 2012; 31: 4740–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34Roccaro AM, Sacco A, Maiso P, Azab AK, Tai YT, Reagan M, et al. BM mesenchymal stromal cell-derived exosomes facilitate multiple myeloma progression. J Clin Invest 2013; 123: 1542–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35Camussi G, Deregibus MC, Bruno S, Grange C, Fonsato V, Tetta C. Exosome/microvesicle-mediated epigenetic reprogramming of cells. Am J Cancer Res 2011; 1: 98–110. [PMC free article] [PubMed] [Google Scholar]
- 36Heijnen HFG, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood 1999; 94: 3791–9. [PubMed] [Google Scholar]
- 37Gonzalez G, Vituret C, Di Pietro A, Chanson M, Boulanger P, Hong SS. Microparticle-mediated transfer of the viral receptors CAR and CD46, and the CFTR channel in a CHO cell model confers new functions to target cells. PLoS One 2012; 7: e52326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38Khotskaya YB, Dai Y, Ritchie JP, MacLeod V, Yang Y, Zinn K, et al. Syndecan-1 is required for robust growth, vascularization, and metastasis of myeloma tumors in vivo. J Biol Chem 2009; 284: 26085–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39Yang Y, Yaccoby S, Liu W, Langford JK, Pumphrey CY, Theus A, et al. Soluble syndecan-1 promotes growth of myeloma tumors in vivo. Blood 2002; 100: 610–7. [DOI] [PubMed] [Google Scholar]
- 40Ritchie JP, Ramani VC, Ren Y, Naggi A, Torri G, Casu B, et al. SST0001, a chemically modified heparin, inhibits myeloma growth and angiogenesis via disruption of the heparanase/syndecan-1 axis. Clin Cancer Res 2011; 17: 1382–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41Kumar S, Witzig TE, Timm M, Haug J, Wellik L, Fonseca R, et al. Expression of VEGF and its receptors by myeloma cells. Leukemia 2003; 17: 2025–31. [DOI] [PubMed] [Google Scholar]
- 42Ria R, Todoerti K, Berardi S, Coluccia AML, De Luisi A, Mattioli M, et al. Gene expression profiling of bone marrow endothelial cells in patients with multiple myeloma. Clin Cancer Res 2009; 15: 5369–78. [DOI] [PubMed] [Google Scholar]
- 43De Luisi A, Ferrucci A, Coluccia AML, Ria R, Moschetta M, de Luca E, et al. Lenalidomide restrains motility and overangiogenic potential of bone marrow endothelial cells in patients with active multiple myeloma. Clin Cancer Res 2011; 17: 1935–46. [DOI] [PubMed] [Google Scholar]
- 44Peinado H, Lavotshkin S, Lyden D. The secreted factors responsible for pre-metastatic niche formation: old sayings and new thoughts. Semin Cancer Biol 2011; 21: 139–46. [DOI] [PubMed] [Google Scholar]
- 45Hergenreider E, Heydt S, Tréguer K, Boettger T, Horrevoets AJG, Zeiher AM, et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol 2012; 14: 249–56. [DOI] [PubMed] [Google Scholar]
- 46Yang M, Chen J, Su F, Yu B, Su F, Lin L, et al. Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells. Mol Cancer 2011; 10: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47Balaj L, Lessard R, Dai L, Cho YJ, Pomeroy SL, Breakefield XO, et al. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat Commun 2011; 2: 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48Piccin A, Murphy WG, Smith OP. Circulating microparticles: pathophysiology and clinical implications. Blood Rev 2007; 21: 157–71. [DOI] [PubMed] [Google Scholar]
- 49Shao H, Chung J, Balaj L, Charest A, Bigner DD, Carter BS, et al. Protein typing of circulating microvesicles allows real-time monitoring of glioblastoma therapy. Nat Med 2012; 18: 1835–40. [DOI] [PMC free article] [PubMed] [Google Scholar]