Abstract
Objective:
To evaluate the feasibility of measuring the stiffness of corpus cavernosum penis (CCP) with ShearWave™ Elastography (SWE; SuperSonic Imagine, Aix-en-Provence, France).
Methods:
40 healthy volunteers with ages ranging from 19 to 81 years (mean, 36 years; standard deviation, 17 years) were selected in this study. The ultrafast ultrasound device Aixplorer® (SuperSonic Imagine) was used for the research and the probe selected was SuperLinear™ SL15-4 (SuperSonic Imagine). The shear wave stiffness (SWS) of CCP was measured using SWE images. The measurement indexes of SWS included (1) SWS of CCP measured in the transverse section (SWS-T), (2) SWS of CCP measured in the longitudinal section (SWS-L) and (3) mean of SWS-T and SWS-L (SWS-M). The interval between hormone test and SWE examination of each subject was less than 7 days. The paired t-test was used to analyse the differences between SWS-T and SWS-L. The Pearson correlation was used to analyse the correlation of SWS of CCP with age as well as with sex hormone levels.
Results:
There was no significant difference between SWS-T and SWS-L (p > 0.05). SWS (SWS-T, SWS-L, SWS-M) was negatively correlated with age and oestradiol value, and SWS (SWS-T, SWS-L, SWS-M) was positively correlated with testosterone value.
Conclusion:
SWE could serve as a new non-invasive method of evaluating the stiffness of CCP.
Advances in knowledge:
It is the first time that we have discussed the feasibility of measuring the stiffness of CCP with SWE and analysed the correlation of SWS of CCP with age as well as with sex hormone levels.
ShearWave™ Elastography (SWE; SuperSonic Imagine, Aix-en-Provence, France) is a new ultrasound technology that could be used to assess tissue stiffness in clinic and is currently the only elastography technology certified by the US Food and Drug Administration. This technology makes it possible to quantify the tissue stiffness accurately. Unlike early elastography methods, which rely on manual compression and measuring tissue displacement, SWE requires no manual compression, and it uses ultrasonic pulse to make the tissue generate shear waves. A real-time SWE map is produced by detection of shear wave velocity and colour coding. Since the propagation speed of the shear wave is determined by the stiffness of tissue, the real-time SWE map could be used to measure the overall and local stiffness of tissue exactly. Studies have shown that this technology could be used to evaluate the stiffness of intravital tissue accurately.1–3
Furthermore, SWE is a safe, non-ionizing and non-invasive technique that has the same thermal and mechanical energy as conventional Doppler imaging. It is applicable to the examination of human reproductive organs. The tissue structure of corpus cavernosum penis (CCP) has important impact on the stiffness of CCP. Therefore, the stiffness of CCP could be used as an index for assessing the tissue structure of CCP. At the same time, the tissue structure of CCP has important impact on the erectile function. So, it is of great clinical significance to measure the stiffness of CCP.
The stiffness of CCP is closely associated with age.4 Previous pathological studies have indicated that, with increase in age, sinusoids in CCP gradually decrease, occlusion and fibrosis occur, the smooth muscle cells also atrophy and decrease gradually. These changes in tissue structure and composition of CCP can directly lead to the change of its stiffness. The stiffness of CCP is also associated with sex hormone levels. Testosterone can promote the generation of smooth muscle cells and enhance cell vitality in CCP. Cell vitality and generation of smooth muscle cells in CCP decline with testosterone decrease, which can lead to cell atrophy and reduction in their number and consequently a change of stiffness of CCP.5–7
However, at present, no accurate and non-invasive method can be used to assess stiffness of CCP in clinic. In this study, we evaluated the feasibility of measuring stiffness of CCP with SWE by analysing the correlation of the stiffness value of CCP with age as well as sex hormone levels.
METHODS AND MATERIALS
Subjects
The study received the Human Subject Ethic Subcommitees of Shanghai First People's Hospital approval and informed written consents were obtained from all the subjects. 40 volunteers with ages ranging from 19 to 81 years (mean, 36 years; standard deviation, 17 years) were selected, whose blood pressure, blood glucose and blood lipid were all within the normal range (the range of systolic blood pressure is from 90 to 140 mmHg and diastolic blood pressure is from 60 to 90 mmHg; the range of fasting plasma glucose is from 3.9 to 6.9 mmol l−1 and the range of triglyceride is from 0.56 to 1.70 mmol l−1). Peyronie's disease, penis trauma and congenital deformity were all excluded.
ShearWave Elastography imaging
The SWE imaging of CCP was carried out using the ultrafast ultrasound device Aixplorer® (SuperSonic Imagine) and the probe selected was SuperLinear™ SL15-4 (SuperSonic Imagine). SWE mode was started after CCP was shown clearly by two-dimensional ultrasound. When SWE imaging was carried out for each patient, we first selected each of the three modes (“Res”, “Std” and “Pen”) for SWE imaging, according to the image quality to determine the best mode. Then SWE imaging and measurement was carried out again under this mode. In this study, the best mode of all the patients was “Pen”. The mid segment of CCP was chosen for imaging in transverse and longitudinal sections, respectively. When scanning transversely, the probe was perpendicular to the long axis of penis and the SWE imaging box contained both left CCP (Left-CCP) and right CCP (Right-CCP). When scanning longitudinally, the probe was parallel to the long axis of each CCP and the maximum longitudinal section was selected. The standard of a qualified SWE image was that each area of the region of interest (ROI) was filled with colour, the image was like an oil painting and there were no mosaic-like coloured points. All the SWE images were obtained with the penis in the flaccid state. All patients kept breathing regularly during the whole examination.
Measurement of stiffness
The shear wave stiffness (SWS) of Left-CCP and Right-CCP was measured, respectively, using SWE images. Kilopascal (kPa) was selected as the unit. ROI was a circle depicted with tunica albuginea as the boundary. Measurement indexes of SWS included (1) SWS of CCP measured in the transverse section (SWS-T), SWS-T was equal to the average of Left-CCP and Right-CCP; (2) SWS of CCP measured in the longitudinal section (SWS-L), SWS-T was equal to the average of Left-CCP and Right-CCP; (3) mean of SWS-T and SWS-L (SWS-M), SWS-M was equal to the average of SWS-T and SWS-L.
Hormone test
The interval between hormone test and SWE examination of each subject was less than 7 days. The measurement indexes included testosterone and oestradiol.
Statistical analysis
The paired t-test was used to analyse the differences between SWS-T and SWS-L. The Pearson correlation was used to analyse the correlation of SWS of CCP with age as well as sex hormone levels. The partial correlation was used to analyse the correlation of testosterone with age. The statistical analyses were performed using SPSS® software v. 18.0 for Windows (SPSS Inc., Chicago, IL). p < 0.01 was considered statistically significant.
RESULTS
The SWE imaging of CCP of all the subjects was finished successfully, and the images were all qualified (Figure 1). The SWS-T and SWS-L of CCP of all the subjects were measured exactly using SWE images (Figure 2). The examination time ranged from 5 to 10 min. After the examination, all the subjects indicated that there was not any physical or psychological discomfort during the examination, and they would like to accept this examination.
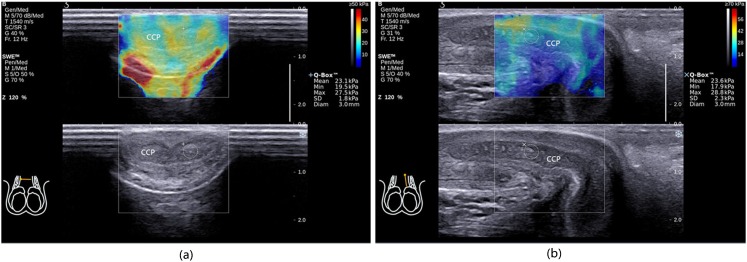
Figure 1.
Case 17. Qualified ShearWave™ Elastography (SWE) images of the corpus cavernosum penis (CCP): each area of the CCP was filled with colour, the image was like an oil painting and there were no mosaic-like coloured points. (a) The transverse section and (b) the longitudinal section. Diam, diameter; Max, maximum; Min, minimum; Q-Box™, measurement results of shear wave stiffness; SD, standard deviation.
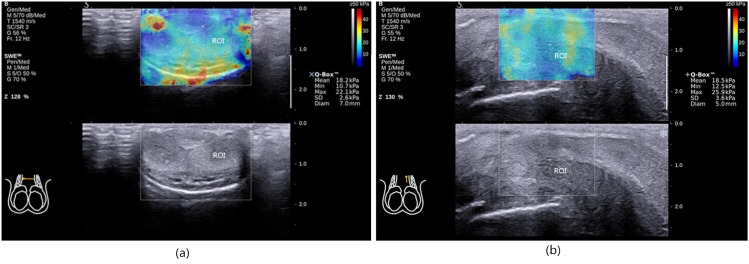
Figure 2.
Case 28. (a) Shear wave stiffness (SWS)-measured in the transverse section (SWS-T) measurement, region of interest (ROI) was a circle depicted with tunica albuginea as the boundary. (b) SWS measure in the longitudinal section (SWS-L) measurement, ROI was a circle depicted with tunica albuginea as the boundary. Diam, diameter; Max, maximum; Min, minimum; Q-Box™, measurement results of SWS; SD, standard deviation; SWE, ShearWave™ Elastography.
The results of SWS are shown in Table 1. There was no significant difference between SWS-T of Left-CCP and Right-CCP (p = 0.476), and there was no significant difference between SWS-L of Left-CCP and Right-CCP (p = 0.885). There was no significant difference between SWS-T and SWS-L (p > 0.05) (Table 2).
Table 1.
ShearWave™ Elastography measurement results of the stiffness of corpus cavernosum penis (CCP)
| Case | Age (years) | SWS-T (kPa) | SWS-T-R (kPa) | SWS-T-L (kPa) | SWS-L (kPa) | SWS-L-R (kPa) | SWS-L-L (kPa) | SWS-M (kPa) |
|---|---|---|---|---|---|---|---|---|
| 1 | 19 | 28.8 | 29 | 28.5 | 28.3 | 28.9 | 27.7 | 28.5 |
| 2 | 25 | 23.0 | 22.8 | 23.2 | 23.6 | 23.5 | 23.7 | 23.3 |
| 3 | 26 | 22.7 | 22 | 23.3 | 22.2 | 21.9 | 22.5 | 22.4 |
| 4 | 26 | 28.5 | 29 | 28 | 29.0 | 31 | 27 | 28.8 |
| 5 | 26 | 24.5 | 24.4 | 24.5 | 25.5 | 26 | 25 | 25.0 |
| 6 | 26 | 22.6 | 20.3 | 24.8 | 22.5 | 24.2 | 20.8 | 22.5 |
| 7 | 26 | 24.3 | 24.5 | 24 | 24.4 | 24.5 | 24.3 | 24.3 |
| 8 | 27 | 21.1 | 21.52 | 20.7 | 21.4 | 21.48 | 21.35 | 21.3 |
| 9 | 27 | 24.9 | 24.5 | 25.3 | 25.4 | 24.7 | 26 | 25.1 |
| 10 | 27 | 23.5 | 23.7 | 23.2 | 23.3 | 22.5 | 24 | 23.4 |
| 11 | 28 | 20.8 | 20 | 21.5 | 21.5 | 21.7 | 21.3 | 21.1 |
| 12 | 29 | 24.9 | 24.9 | 24.88 | 25.1 | 25.05 | 25.14 | 25.0 |
| 13 | 29 | 24.4 | 24 | 24.8 | 23.3 | 22.7 | 23.8 | 23.8 |
| 14 | 29 | 23.1 | 23.7 | 22.5 | 22.8 | 22.4 | 23.1 | 22.9 |
| 15 | 30 | 23.2 | 24.4 | 22 | 22.9 | 23.5 | 22.2 | 23.0 |
| 16 | 30 | 24.4 | 25.2 | 23.5 | 25.2 | 25.7 | 24.7 | 24.8 |
| 17 | 30 | 23.8 | 24.4 | 23.1 | 23.5 | 23.4 | 23.6 | 23.6 |
| 18 | 31 | 21.8 | 21.4 | 22.2 | 23.0 | 23.2 | 22.8 | 22.4 |
| 19 | 31 | 22.4 | 23 | 21.8 | 21.8 | 22.1 | 21.4 | 22.1 |
| 20 | 32 | 22.6 | 21.7 | 23.5 | 21.0 | 20 | 22 | 21.8 |
| 21 | 32 | 18.9 | 18.6 | 19.2 | 19.0 | 18.5 | 19.5 | 19.0 |
| 22 | 36 | 22.0 | 21.2 | 22.7 | 22.1 | 19.7 | 24.4 | 22.0 |
| 23 | 36 | 22.5 | 21.2 | 23.7 | 22.1 | 21.7 | 22.5 | 22.3 |
| 24 | 36 | 20.2 | 19.6 | 20.8 | 18.7 | 18.3 | 19.1 | 19.5 |
| 25 | 37 | 17.4 | 16.3 | 18.5 | 16.8 | 15.8 | 17.7 | 17.1 |
| 26 | 55 | 20.6 | 19.4 | 21.8 | 20.7 | 20.8 | 20.5 | 20.6 |
| 27 | 58 | 18.3 | 18.6 | 17.9 | 18.5 | 18.7 | 18.2 | 18.4 |
| 28 | 61 | 17.8 | 17.4 | 18.2 | 18.6 | 18.7 | 18.5 | 18.2 |
| 29 | 72 | 15.9 | 15 | 16.7 | 13.4 | 13.1 | 13.6 | 14.6 |
| 30 | 78 | 16.7 | 16.9 | 16.5 | 16.5 | 15.8 | 17.2 | 16.6 |
| 31 | 81 | 14.7 | 15.9 | 13.4 | 13.2 | 13.8 | 12.6 | 13.9 |
| 32 | 81 | 17.7 | 16.9 | 18.5 | 20.0 | 20.6 | 19.4 | 18.9 |
| 33 | 20 | 22.9 | 22.9 | 22.8 | 23.0 | 22.9 | 23 | 22.9 |
| 34 | 20 | 24.2 | 24.4 | 24 | 25.3 | 24.8 | 25.7 | 24.7 |
| 35 | 20 | 26.9 | 27.2 | 26.6 | 27.2 | 27.8 | 26.6 | 27.1 |
| 36 | 25 | 22.8 | 23.1 | 22.4 | 22.8 | 23.6 | 22 | 22.8 |
| 37 | 25 | 22.3 | 22.4 | 22.1 | 22.7 | 22.8 | 22.5 | 22.5 |
| 38 | 35 | 26.7 | 27.1 | 26.3 | 24.9 | 24.9 | 24.8 | 25.8 |
| 39 | 35 | 26.2 | 27.4 | 24.9 | 28.0 | 28.2 | 27.8 | 27.1 |
| 40 | 44 | 20.6 | 20.4 | 20.8 | 20.0 | 20.2 | 19.8 | 20.3 |
| Mean, standard deviation | 36 ± 17 | 22.2 ± 3.2 | 22.2 ± 3.5 | 22.3 ± 3.1 | 22.2 ± 3.5 | 22.2 ± 3.8 | 22.2 ± 3.4 | 22.2 ± 3.3 |
SWS-L, shear wave stiffness of CCP measured in the longitudinal section; SWS-L-L, SWS-L of left CCP; SWS-L-R, SWS-L of right CCP; SWS-M, mean of SWS-T and SWS-L; SWS-T, shear wave stiffness of CCP measured in the transverse section; SWS-T-L, SWS-T of left CCP; SWS-T-R, SWS-T of right CCP.
Table 2.
Comparison of shear wave stiffness (SWS) of corpus cavernosum penis (CCP) measured in different sections (mean ± standard deviation)
| Section | SWS of CCP (kPa) |
|---|---|
| SWS-T | 22.2 ± 3.2 |
| SWS-L | 22.2 ± 3.5 |
| p-value | 0.817 |
SWS-L, SWS of CCP measured in the longitudinal section; SWS-T, SWS of CCP measured in the transverse section.
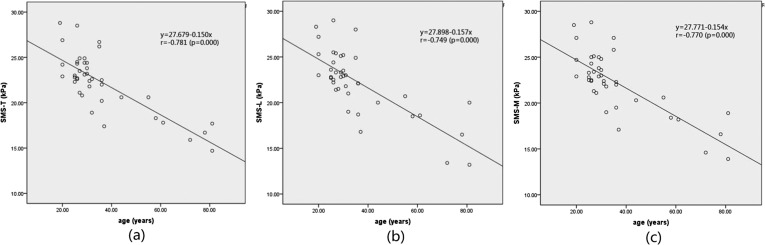
The results of the analysis of correlation of the SWS of CCP with age showed that SWS-T, SWS-L and SWS-M were all highly correlated with age (p < 0.01) (Figure 3). The correlation coefficient of SWS-T and age was −0.781; the correlation coefficient of SWS-L and age was −0.749; and the correlation coefficient of SWS-M and age was −0.770 (Table 3).
Figure 3.
(a) Shear wave stiffness (SWS) of corpus cavernosum penis (CCP) measured in the transverse section (SWS-T) plotted against age. (b) SWS of CCP measured in the longitudinal section (SWS-L) plotted against age. (c) Mean of SWS-T and SWS-L (SWS-M) plotted against age.
Table 3.
Linear correlation analysis of shear wave stiffness (SWS) of corpus cavernosum penis (CCP) and age (n = 40)
| SWS of CCP | Correlation coefficient (r-value) | p-value |
|---|---|---|
| SWS-T | −0.781 | 0.000 |
| SWS-L | −0.749 | 0.000 |
| SWS-M | −0.770 | 0.000 |
SWS-L, SWS of CCP measured in the longitudinal section; SWS-M, mean of SWS-T and SWS-L; SWS-T, SWS of CCP measured in the transverse section.
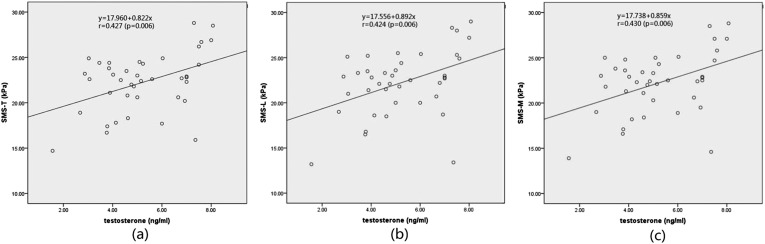
The results of the analysis of correlation of the SWS of CCP with testosterone showed that SWS-T, SWS-L and SWS-M were all positively correlated with testosterone (p < 0.01) (Figure 4). The correlation coefficient of SWS-T and testosterone was 0.427; the correlation coefficient of SWS-L and testosterone was 0.424; and the correlation coefficient of SWS-M and testosterone was 0.430 (Table 4).
Figure 4.
(a) Shear wave stiffness (SWS) of corpus cavernosum penis (CCP) measured in the transverse section (SWS-T) plotted against testosterone. (b) SWS of CCP measured in the longitudinal section (SWS-L) plotted against testosterone. (c) Mean of SWS-T and SWS-L (SWS-M) plotted against testosterone.
Table 4.
Linear correlation analysis of shear wave stiffness (SWS) of corpus cavernosum penis (CCP) and testosterone (n = 40)
| SWS of CCP | Correlation coefficient (r-value) | p-value |
|---|---|---|
| SWS-T | 0.427 | 0.006 |
| SWS-L | 0.424 | 0.006 |
| SWS-M | 0.430 | 0.006 |
SWS-L, SWS of CCP measured in the longitudinal section; SWS-M, mean of SWS-T and SWS-L; SWS-T, SWS of CCP measured in the transverse section.
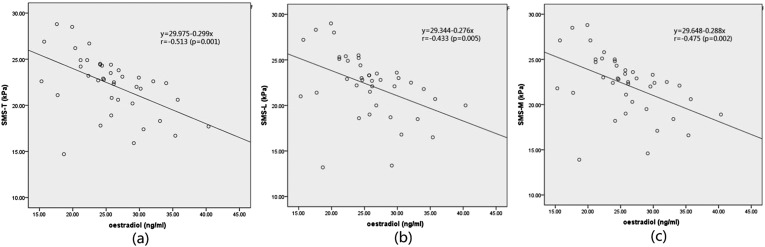
The results of the analysis of correlation of the SWS of CCP with oestradiol showed that SWS-T, SWS-L and SWS-M were all negatively correlated with oestradiol (p < 0.01) (Figure 5). The correlation coefficient of SWS-T and oestradiol was −0.513; the correlation coefficient of SWS-L and oestradiol was −0.433; and the correlation coefficient of SWS-M and oestradiol was −0.475 (Table 5).
Figure 5.
(a) Shear wave stiffness (SWS) of corpus cavernosum penis (CCP) measured in the transverse section (SWS-T) plotted against oestradiol. (b) SWS of CCP measured in the longitudinal section (SWS-L) plotted against oestradiol. (c) Mean of SWS-T and SWS-L (SWS-M) plotted against oestradiol.
Table 5.
Linear correlation analysis of shear wave stiffness (SWS) of the corpus cavernosum penis (CCP) and oestradiol (n = 40)
| SWS of CCP | Correlation coefficient (r-value) | p-value |
|---|---|---|
| SWS-T | −0.513 | 0.001 |
| SWS-L | −0.433 | 0.005 |
| SWS-M | −0.475 | 0.002 |
SWS-L, SWS of CCP measured in the longitudinal section; SWS-M, mean of SWS-T and SWS-L; SWS-T, SWS of CCP measured in the transverse section.
At the same time, the result of partial correlation analysis showed that there was no significant correlation of testosterone with age (p = 0.296) in this population of 40 patients.
DISCUSSION
SWE is a new ultrasound technology that could be used to measure the stiffness of intravital tissue precisely. Studies have found that SWE could be used to identify the difference of stiffness of normal thyroid tissue, Hashimoto's thyroiditis, malignant and benign thyroid nodules.8 The stiffness of invasive ductal carcinoma, mastopathy, intraductal papilloma, fibroadenomas, mammary glands and adipose tissue decreases gradually.9 SWE could also be used to monitor the stiffness of liver in different stages of hepatocirrhosis.10 At the same time, Richards et al11 diagnosed Peyronie's disease with SWE for the first time. The results showed that sonoelastography provides an additional way to characterize, localize and deliver therapy to a lesion in patients with Peyronie's disease and is particularly useful when palpation and B-mode ultrasonography have failed to demonstrate a plaque.
CCP is composed of lots of smooth muscle cells (about 40%), fibre tissues, a tiny amount of endothelial cells and vascular smooth muscle cells etc. The main process of penis erection is as follows: with sexual stimulation, the smooth muscles of CCP and vessels relax, CCP congests and swells and the intracavernous pressure increases gradually. At the same time, the subalbugineous veniplex are compressed, which can lead to decrease of blood reflux. When the intracavernous pressure is high enough, the inflow of penis arteries stops and there is no outflow of subalbugineous veniplex, the penis is under a fully closed condition. Thus, the change of tissue structure of CCP has important effects on penis erection and evaluating the tissue structure of CCP exactly is of great significance in theory and clinic. The current method of evaluating the tissue components of CCP is penis biopsy, by which we can observe the density of smooth muscles and the content of collagen fibres.12,13 But the process is complicated, invasive and liable to cause some severe side effects including penis pain and fibrosis of cavernous tissue; therefore, patients are unlikely to accept this method. So, the clinical application of penis biopsy has been greatly restricted. Since the changes of tissue structure of CCP can directly lead to the change of CCP stiffness, the stiffness of CCP can also be used to evaluate the tissue structure of CCP and measuring the stiffness of CCP has important clinical significance.
So far, there has been no accurate and non-invasive way to adopt to measure the stiffness of CCP in clinic, so it is necessary to explore a non-invasive method of measuring the stiffness of CCP for clinical application. Accordingly, this study is intended to explore the feasibility of measuring the stiffness of CCP with SWE, which is a new method that could be used to measure the stiffness of intravital tissues.
Since stiffness of CCP tissue is closely associated with age and sex hormone levels, we analysed the correlation of SWS with age as well as sex hormone levels after excluding other factors that could influence the tissue structure of CCP, such as blood pressure, blood glucose, blood lipid14,15 and organic diseases including penis trauma and Peyronie's diseases.16,17 The results of the analysis showed that the stiffness of CCP was linearly correlated with age and sex hormone levels. That was to say, the SWS of CCP would decrease linearly with increase in age, decrease in testosterone and increase in oestradiol. This finding was completely consistent with the pathological changes of CCP. According to the results of existing research, we found that with increase in age, the CCP tissue would change pathologically as follows: smooth muscle cells would atrophy and decrease gradually, elastic and collagen fibres would reduce gradually, reduction in the number of sinusoids would occur and then occlusion and the sinus gap would become smaller and smaller, finally fibre tissues would proliferate gradually.4 These changes would inevitably lead to the change of shear wave propagation velocity produced in CCP tissue. According to the principles of SWE imaging, SWS is proportional to the square of shear wave velocity, so theoretically SWS of CCP changes with an increase in age. At the same time, studies found that similar pathological changes to CCP tissue would also occur with testosterone decrease,6,18,19 so SWS of CCP would also change with testosterone decreasing.
We also found that there was no significant difference between SWS-T and SWS-L. This suggested that the stiffness of CCP measured with SWE was not affected by section selection. As a non-invasive method, the examination procedure is simple without special preparation, is quick (from 5 to 10 min) and is easily accepted by patients. These features make it a promising method for measuring the stiffness of CCP in clinics.
There are some limitations in the present study. Firstly, we did not compare the new technique with a gold standard. Secondly, we lacked the data from scanning in the state of tumescence. We did not get the data for the patients who would not accept the technique easily, and it was very difficult to ensure the same tumescence degree when SWE examination was carried out for each patient. However, the tumescence degree could affect the SWS values.
CONCLUSION
The use of SWE to measure the stiffness of CCP is not affected by section selection, and the SWE value is highly correlated with age and sex hormone levels. At the same time, the examination time is short, and the success rate is high. There is also no contraindication, and it is easy to be accepted by the patients. In summary, SWE is very promising in clinical application, and it could serve as a new clinical method of measuring the stiffness of CCP.
FUNDING
The financial support from the Science Foundation of School of Medicine, Shanghai Jiao Tong University (14XJ10075) is gratefully acknowledged.
Acknowledgments
ACKNOWLEDGMENTS
We gratefully acknowledge the support from the Science Foundation of School of Medicine and Shanghai Jiao Tong University.
Contributor Information
J-J Zhang, Email: zhjjcool@vip.qq.com.
X-H Qiao, Email: xiaoqiao8274@163.com.
F Gao, Email: gaofeng19870115@163.com.
F Li, Email: medicineli@163.com.
M Bai, Email: baimin101@126.com.
H-P Zhang, Email: zhanggirldan@hotmail.com.
Y Liu, Email: liuyangshsmu@163.com.
L-F Du, Email: dr_dulf@163.com.
J-F Xing, Email: xingshi7018@163.com.
REFERENCES
- 1.Bercoff J, Tanter M, Fink M. Supersonic shear imaging: a new technique for soft tissue elasticity mapping. IEEE Trans Ultrason Ferroelectr Freq Control 2004; 51: 396–409. [DOI] [PubMed] [Google Scholar]
- 2.Tanter M, Bercoff J, Athanasiou A, Deffieux T, Gennisson JL, Montaldo G, et al. Quantitative assessment of breast lesion viscoelasticity: initial clinical results using supersonic shear imaging. Ultrasound Med Biol 2008; 34: 1373–86. doi: 10.1016/j.ultrasmedbio.2008.02.002 [DOI] [PubMed] [Google Scholar]
- 3.Magri F, Chytiris S, Capelli V, Alessi S, Nalon E, Rotondi M, et al. , Shear wave elastography in the diagnosis of thyroid nodules: feasibility in the case of coexistent chronic autoimmune Hashimoto's thyroiditis. Clin Endocrinol (Oxf) 2012; 76: 137–41. doi: 10.1111/j.1365-2265.2011.04170.x [DOI] [PubMed] [Google Scholar]
- 4.Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Impotence and its medical and psychosocial correlates: results of the Massachusetts male aging study. J Urol 1994; 151: 54–61. [DOI] [PubMed] [Google Scholar]
- 5.Handelsman DJ. Mechanisms of action of testosterone—unravelling a Gordian knot. N Engl J Med 2013; 369: 1058–9. doi: 10.1056/NEJMe1305307 [DOI] [PubMed] [Google Scholar]
- 6.Corona G, Maggi M. The role of testosterone in erectile dysfunction. Nat Rev Urol 2010; 7: 46–56. doi: 10.1038/nrurol.2009.235 [DOI] [PubMed] [Google Scholar]
- 7.Jia JH, Gao GH, Liu B, Qian Z. The histological observation of smooth muscle of spongy body in erectile dysfunction and varied aged normal penis. Chin J Androl 2003; 17: 32–3. [Google Scholar]
- 8.Veyrieres JB, Albarel F, Lombard JV, Berbis J, Sebag F, Oliver C, et al. A threshold value in shear wave elastography to rule out malignant thyroid nodules: a reality? Eur J Radiol 2012; 81: 3965–72. doi: 10.1016/j.ejrad.2012.09.002 [DOI] [PubMed] [Google Scholar]
- 9.Berg WA, Cosgrove DO, Doré CJ, Schäfer FK, Svensson WE, Hooley RJ, et al. ; BE1 Investigators. Shear-wave elastography improves the specificity of breast US: the BE1 multinational study of 939 masses. Radiology 2012; 262: 435–49. doi: 10.1148/radiol.11110640 [DOI] [PubMed] [Google Scholar]
- 10.Guibal A, Boularan C, Bruce M, Vallin M, Pilleul F, Walter T, et al. Evaluation of shearwave elastography for the characterisation of focal liver lesions on ultrasound. Eur Radiol 2013; 23: 1138–49. doi: 10.1007/s00330-012-2692-y [DOI] [PubMed] [Google Scholar]
- 11.Richards G, Goldenberg E, Pek H, Gilbert BR. Penile sonoelastography for the localization of a non-palpable, non-sonographically visualized lesion in a patient with penile curvature from Peyronie's disease. J Sex Med 2014; 11: 516–20. doi: 10.1111/jsm.12396 [DOI] [PubMed] [Google Scholar]
- 12.Falke G, Yoo JJ, Kwon TG, Moreland R, Atala A. Formation of corporal tissue architecture in vivo using human cavernosal muscle and endothelial cells seeded on collagen matrices. Tissue Eng 2003; 9: 871–9. [DOI] [PubMed] [Google Scholar]
- 13.Kershen RT, Yoo JJ, Moreland RB, Krane RJ, Atala A. Reconstitution of human corpus cavernosum smooth muscle in vitro and in vivo. Tissue Eng 2002; 8: 515–24. [DOI] [PubMed] [Google Scholar]
- 14.Mckinlay JB. The worldwide prevalence and epidemiology of erectile dysfunction. Int J Impot Res 2000; 12(Suppl. 4): S6–11. [DOI] [PubMed] [Google Scholar]
- 15.Seftel AD, Sun P, Swindle R. The prevalence of hypertension, hyperlipidemia, diabetes mellitus and depression in men with erectile dysfunction. J Urol 2004; 171: 2341–5. [DOI] [PubMed] [Google Scholar]
- 16.Langston JP, Carson CC, 3rd. Peyronie's disease: review and recent advances. Maturitas 2014; 78: 341–3. doi: 10.1016/j.maturitas.2014.05.024 [DOI] [PubMed] [Google Scholar]
- 17.Gupta MK, Revannasiddaiah S, Susheela SP. A malignant differential diagnosis for Peyronie's disease. BMJ Case Rep 2014. doi: 10.1136/bcr-2013-201446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corona G, Isidori AM, Buvat J, Aversa A, Rastrelli G, Hackett G, et al. Testosterone supplementation and sexual function: a meta-analysis study. J Sex Med 2014; 11: 1577–92. doi: 10.1111/jsm.12536 [DOI] [PubMed] [Google Scholar]
- 19.Liu B, Liu JH, Wang T, Yang J, Wang SG, Lan RZ, et al. Effects of testosterone on the proliferation of rat corpus cavernosum cells in vitro. [In Chinese.] Natl J Androl 2008; 14: 524–6. [PubMed] [Google Scholar]