Abstract
Objective:
To determine whether increased elimination of gadobenate ion via the hepatobiliary pathway might compensate for reduced/absent elimination via the urinary pathway in the event of compromised renal function, as a possible protective mechanism against nephrogenic systemic fibrosis (NSF).
Methods:
15 male Crl:CD® R(SD)Br rats (Charles River Italia, Como, Italy) randomized to three treatment groups: (1) animals with occluded bile ducts, (2) animals with occluded renal vessels and (3) control animals, each received 0.25 mmol kg−1 of bodyweight of gadobenate dimeglumine (MultiHance®; Bracco Imaging SpA, Milan, Italy). Urine and bile were collected from 0−30, 30−60, 60−120, 120−240 and 240−480 min after gadobenate dimeglumine administration prior to exsanguination. Determinations of gadobenate ion in blood, bile and urine were performed by high-performance liquid chromatography. Gadolinium (Gd3+) levels in excised liver and kidneys were determined by X-ray fluorescence.
Results:
The recovery of gadobenate ion in the urine of rats with bile duct occlusion was significantly higher than that in the urine of normal rats (89.1 ± 4.2% vs 60.6 ± 2.8%; p < 0.0001). Conversely, mean recovery in the bile of rats with renal vessel occlusion was significantly higher than that in the bile of normal rats (96.16 ± 0.55% vs 33.5 ± 4.7%; p < 0.0001). Gadobenate ion was not quantifiable in any group 8 h post-injection.
Conclusion:
Compensatory elimination may be an effective means to overcome compromised renal or hepatobiliary elimination.
Advances in knowledge:
The absence of NSF in at-risk patients administered with gadobenate dimeglumine may in part reflect greater Gd3+ elimination via the hepatobiliary route.
Nephrogenic systemic fibrosis (NSF) is a rare, systemic fibrosing disorder characterized by thickening and induration of the skin, flexion contractures and impaired mobility of the nearby joints, as well as fibrosing changes in connective tissues of internal organs.1,2 Although the first cases of NSF were identified in 1997 and the first published report of 14 cases appeared in 2000,3 it was not until 2006 that a possible association with exposure to gadolinium (Gd3+)-based contrast agents (GBCAs) became apparent.4 By December 2012, 815 distinct cases of NSF had been reported in 200 articles in the peer-reviewed literature, the vast majority of which [595/815 (73.0%)] were observed in the USA.5
Most theories on the mechanism behind the development of NSF have focused on GBCA molecular structure and stability as factors determining an increased risk with some agents relative to others.6,7 Thus, the non-ionic, open-chain (linear) GBCAs, gadodiamide and gadoversetamide, have the lowest kinetic stability and highest propensity to release Gd3+ and, as a group, have been associated with the greatest number of unconfounded cases of NSF (approximately 78% with gadodiamide and 1.3% with gadoversetamide).5 Conversely, the macrocyclic GBCAs, gadoterate meglumine, gadobutrol and gadoteridol, have the highest kinetic stability and least propensity to release free Gd3+ and, as a group, have been associated with very few unconfounded cases [none with gadoteridol, very few (0.7%) with gadobutrol or gadoterate meglumine].5 Based on these observations, the European Medicines Agency (EMA) and UK Medicines and Healthcare Products Regulatory Agency (MHRA) introduced a classification scheme for GBCAs based on observed and perceived risk for NSF.8,9 Thus, the macrocyclic GBCAs are categorized as low risk for NSF while the non-ionic, open-chain (linear) GBCAs are categorized as high risk. Also included in the category of high-risk agents is gadopentetate dimeglumine because of a comparatively high number of unconfounded NSF cases associated with this agent (approximately 20% of published unconfounded cases5).
Of particular interest, however, are gadobenate dimeglumine (MultiHance®; Bracco Imaging SpA, Milan, Italy), gadofosveset trisodium and gadoxetate disodium that are categorized as having intermediate risk for NSF.8,9 Although these agents are ionic, open-chain GBCAs like gadopentetate dimeglumine, no unconfounded cases of NSF have yet been reported for any of these agents.5 The principal molecular difference between these agents and gadopentetate dimeglumine is that each possesses an aromatic group on the contrast-effective molecule, whereas gadopentetate dimeglumine does not.10 Among the unique features conferred by this aromatic moiety is that each of these three GBCAs are taken up by functioning hepatocytes to a greater or lesser extent and excreted via the hepatobiliary route into the bile and, ultimately, the faeces.11–20 The degree to which these agents are eliminated via the hepatobiliary route is species dependent. Thus, in human subjects with normal renal and liver function, between 2% and 4% of the injected dose of gadobenate dimeglumine is eliminated by this route, while the remainder is eliminated into the urine via the kidneys.19,20 Conversely, hepatobiliary elimination of gadobenate dimeglumine in animals has been shown to range between approximately 25% and 55% of the injected dose depending on the species, with rats demonstrating the greatest biliary excretion followed by dogs, rabbits and monkeys.21 The possibility to eliminate Gd3+ via the hepatobiliary pathway is clearly potentially highly advantageous in patients with severe chronic kidney disease [CKD; Stage 4 or 5 according to the CKD classification of the US National Kidney Foundation;22 glomerular filtration rate (GFR) <30 ml min−1 1.73 m−2 or renal failure] or end-stage renal disease who are at risk of developing NSF but who nevertheless require a contrast-enhanced MRI examination for diagnostic purposes.
Compensatory elimination of Gd3+ has previously been demonstrated in rats with severely impaired liver and kidney function after administration of gadoxetate disodium.23 The aim of our study was to determine whether compensatory elimination of Gd3+ occurs similarly in rats with impaired hepatic or renal function after administration of gadobenate dimeglumine.
METHODS AND MATERIALS
Animals
15 male Crl:CD® R(SD)Br rats (weight at treatment, 240–320 g; Charles River Italia, Como, Italy) were used for the study. Animals were quarantined for at least 4 days prior to treatment (three animals per cage; each cage: 59 × 38 × 20 cm) and were maintained ad libitum on 4Rf21-GLP pellets (Mucedola, Milan, Italy) certified as being without oestrogenic activity and as having contaminant levels within acceptable limits. Tap water sterilized by ultraviolet irradiation and filtered through 1.0- and 0.2-m filters was similarly available ad libitum throughout the study. Environmental conditions were controlled and monitored throughout the quarantine period (temperature, 21.7 °C; relative humidity, 53.3%; air change, 15–20 h−1; lighting automatically controlled to give a 12-h photoperiod per day).
Following the quarantine period, the 15 animals were randomly assigned to three treatment groups (5 animals per group): (1) animals to undergo bile duct occlusion and assessment of urinary excretion; (2) animals to undergo renal vessel occlusion and assessment of biliary excretion; and (3) control (normal) animals for assessment of normal urinary and biliary excretion. The treatments applied to animals in Groups 1 and 2 resemble the human pathological condition of biliary occlusion and of end-stage renal failure or bilateral nephrectomy, respectively.
All animals were fasted for 16–18 h prior to testing but were not deprived of drinking water. All animal procedures were conducted according to national and international guidelines (Italian D.L. No. 116 of 27 January 1992 and Directive 2010/63/EU) on the use of animals for experimental purposes. No validated non-animal alternatives are known that would meet the objectives of the study.
Surgical procedures
Urinary excretion in rats with bile duct occlusion
Animals were anesthetized by intraperitoneal injection of sodium pentobarbital at 30 mg kg−1; if necessary further aliquots of anaesthetic were injected to maintain anaesthesia throughout the experimental period. After laparotomy, the common bile duct was isolated and a silk wire ligation made just near the mouth of the common bile duct into the duodenum. An Intramedic® PE 50 polyethylene catheter (Becton, Dickinson and Co., Parsipanny, NJ) was inserted into the urinary bladder. Two ligatures were made, the first to fasten the catheter to the wall of the bladder and the second to reduce the volume between the mouth of the ureter and the catheter. The abdominal cavity was closed with sutures, and the animal placed on a surgical table warmed to 37 °C to keep the body temperature within physiological limits. The right femoral vein was exposed by making a cut through the skin in the region overlying the joint of the hind limb to the trunk of the body. This was where gadobenate dimeglumine was to be injected.
Biliary excretion in rats with renal vessel occlusion
Animals were similarly anesthetized by intraperitoneal injection of sodium pentobarbital at 30 mg kg−1 with top-up injections of anaesthetic administered as and when necessary to maintain anaesthesia throughout the experimental period. Laparotomy was performed and the common bile duct cannulated with an Intramedic PE 50 polyethylene catheter. The renal arteries and veins of both kidneys were isolated and ligated with silk wire. Thereafter, closure of the abdominal cavity with sutures, maintenance of body temperature at 37 °C and exposure of the right femoral vein for injection of gadobenate dimeglumine was performed as described above.
Biliary and urinary excretion in normal rats
Similar surgical procedures were performed as described above except that Intramedic PE 50 polyethylene catheters were inserted into both the common bile duct and urinary bladder.
Dosing and sampling procedures
Gadobenate dimeglumine (0.5 M) was injected at a dose of 0.25 mmol kg−1 into the right femoral vein of all animals at a rate of 6 ml min−1. Injection volumes were calculated on the basis of dose and animal weight. All injections were performed 30 min after surgical treatment, once healing had occurred and the animals had stabilized. The timings for contrast injection following surgical intervention were consistent across animals and treatment groups.
Bile was collected for 30 min before the injection of gadobenate dimeglumine. Thereafter, both urine and bile were collected during the following periods: 0–30, 30–60, 60–120, 120–240 and 240–480 min after gadobenate dimeglumine administration. After the sampling period, i.e. at 480 min after gadobenate dimeglumine administration, the animal was exsanguinated through the abdominal aorta and blood collected for the assay of gadobenate ion by high-performance liquid chromatography (HPLC) and for determination of total plasma bilirubin. The liver and kidneys were excised for the assay of Gd3+ by X-ray fluorescence (XRF).
High-performance liquid chromatography analysis of gadobenate ion in bile, urine and plasma
All bile, urine and plasma samples collected during the study were stored at −20 °C until the day of analysis. HPLC analysis of gadobenate ion was performed as described elsewhere.24 Quantification was performed in duplicate by interpolation from calibration curves. The calibration curve was determined by assaying standard solutions of gadobenate dimeglumine over a range of concentrations from 10 to 1000 µg gadobenate ion per millilitre.
The working standard solutions of gadobenate dimeglumine were prepared with the same batch that was used in the treatments. A check of the precision and accuracy of the HPLC method was performed during the study with standard control samples containing 26.2, 262, 450 and 900 µg gadobenate ion per millilitre in bile, plasma and urine. The method detection limits for gadobenate ion in bile, urine and plasma were 1.1, 5.1 and 0.73 µg gadobenate ion per millilitre, respectively.
X-ray fluorescence analysis of gadolinium in liver and kidneys
The amounts of gadobenate ion in the liver and kidneys were calculated in terms of Gd3+ concentration since it is well established that neither in vivo dissociation nor metabolism of gadobenate ion occurs.16
The excised livers and kidneys of all animals were stored at −20 °C until the day of analysis. All samples were weighed, lyophilized and digested in a microwave oven by suspending the sample in nitric acid. Gd3+ was then assayed by XRF according to standard procedures.25 Quantification was performed in duplicate by interpolation from calibration curves. The calibration curve was determined by assaying standard solutions of gadobenate dimeglumine over the range of concentrations from 8 to 1591 µg Gd3+ ml−1. The working standard solutions of gadobenate dimeglumine were prepared with the same batch that was used in the treatments. An internal standard solution of 0.2 M manganese(II) chloride (E. Merck, Darmstadt, Germany) was prepared. A check of the precision and accuracy of the XRF method was performed during the study with standard control samples containing 39.31 and 393.1 µg Gd3+ ml−1 in bile and urine. The limit of quantification of the XRF method was 5 µg Gd3+ ml−1.
Assay of bilirubin
Blood samples were centrifuged (15 min; 4000 rpm) and the plasma supernatant used for the assay of free, bound and conjugated bilirubin. The assay was performed using a standardized colourimetric method based on the reaction of bilirubin with 2,5-dichlorophenyl diazonium salt and absorbance measurement at 550 nm. The assay was performed using a Cobas® Miras AutoAnalyser (Hoffmann-La Roche, Basel, Switzerland) and kit no. 19717 for assay of total bilirubin (E. Merck).
Statistical analysis
The cumulative biliary and urinary excretion (expressed as percent of the administered dose), the biliary and urinary concentration and excretion rates of gadobenate ion and the biliary flows were calculated. All values were expressed as mean ± standard deviation.
Analysis of variance for repeated measures was applied to verify that (1) there was no difference between the effects of gadobenate dimeglumine in normal animals and animals with renal vessel or bile duct occlusion; (2) that there was no difference between the effects of the times; and (3) that there was no effect of the time × animal condition interaction. The chosen significance level was α = 0.05.
To verify the hypothesis that, at a certain time, the effects of the compound on the two groups of animals are the same, the least square means were compared with a series of post hoc t-tests. Again, the chosen significance level for each pairwise comparison was α = 0.05.
Finally, in order to normalize the distribution and homogenize the group variances, data for percentage biliary excretion and percentage urinary excretion were transformed with the function 2arcsin√. Similarly, data (χ) for biliary flow and urinary excretion rate were transformed with the function ln(χ) and biliary excretion rate and urinary concentration with the function √(χ).
RESULTS
Analysis of variance for repeated measures revealed no significant differences between the effects of gadobenate dimeglumine in normal animals and in animals with renal vessel or bile duct occlusion (p ≤ 0.01), between the effects of the times (p ≤ 0.01) or for the effects of the time × animal condition interaction (p ≤ 0.006) for any determination.
Analytical procedures
The precision and accuracy of the HPLC method was ±2% and ±1%, respectively, for the quantification of gadobenate ion in plasma, and ±1.3% and ±2%, respectively, for the quantification of gadobenate ion in urine and bile. Similarly, the precision and accuracy of the XRF method was in both cases ±2% for the quantification of Gd3+ in the liver and kidneys.
Urinary excretion in rats with bile duct occlusion
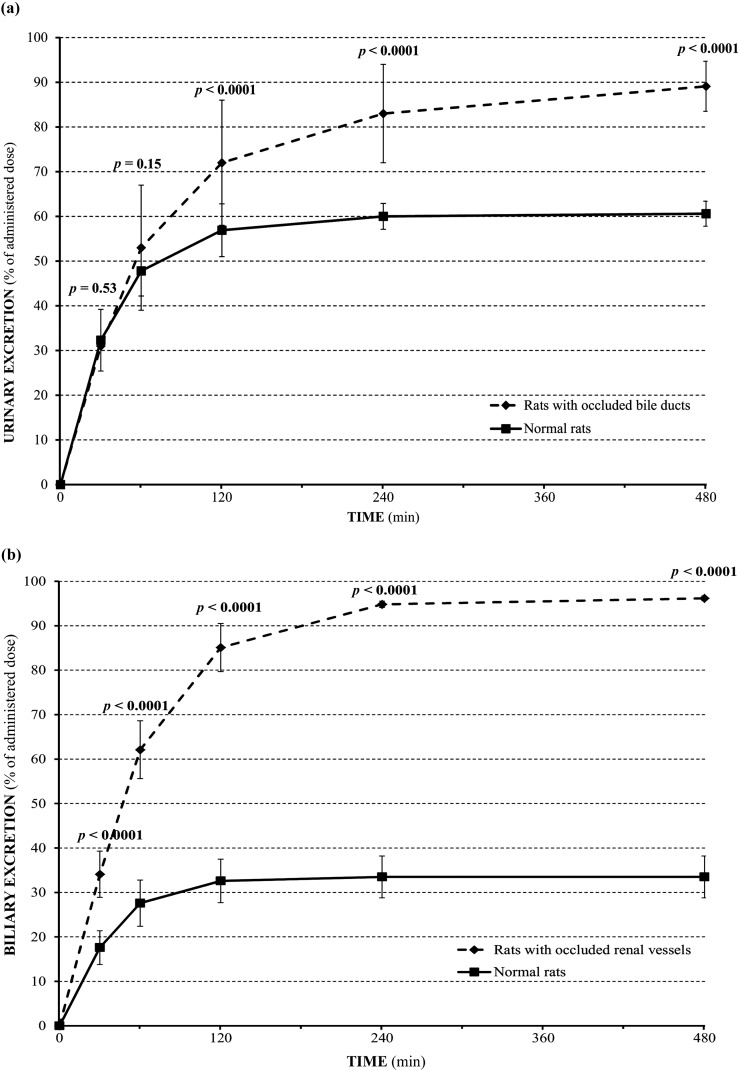
The urinary excretion of gadobenate ion was significantly (p < 0.0001) higher in rats with bile duct occlusion than in normal rats at all time points during the period between 60 and 480 min after injection. The mean recovery (0–480 min) of gadobenate ion in the urine of rats with bile duct occlusion was 89.1 ± 4.2%, while in the urine of normal rats it was 60.6 ± 2.8%. A comparison of the mean cumulative urinary excretion (percentage of administered dose) over time is shown in Figure 1a.
Figure 1.
Cumulative urinary (a) and biliary (b) excretion of gadobenate2− (percentage of injected dose) after intravenous injection of 0.25 mmol kg−1 gadobenate dimeglumine to rats (n = 5 per group) with bile duct occlusion (a) or urinary vessel occlusion (b).
In normal animals, the urinary concentration of gadobenate ion peaked at 128 ± 27 µmol ml−1 between 0 and 30 min after injection (Table 1). Conversely, in animals with bile duct occlusion, the urinary concentration peaked at 146 ± 12 µmol ml−1 between 30 and 60 min after injection. The urinary concentration of gadobenate ion was significantly (p ≤ 0.002) higher in rats with bile duct occlusion than in normal animals at time points between 30 and 120 min after injection (Table 1).
Table 1.
Urinary concentration and excretion rate of gadobenate ion after intravenous administration of 0.25 mmol kg−1 of bodyweight of gadobenate dimeglumine to normal rats and rats with bile duct occlusion (n = 5)
| Time period (min) | Urinary concentration (µmol ml−1) |
Urinary excretion rate (µmol min−1 kg−1) |
||
|---|---|---|---|---|
| Normal rats | Rats with bile duct occlusion | Normal rats | Rats with bile duct occlusion | |
| 0–30 | 128 ± 27 | 98 ± 24 | 2.69 ± 0.58 | 2.60 ± 1.1 |
|
p = 0.032 |
p = 0.74 |
|||
| 30–60 | 95 ± 18 | 146 ± 12 | 1.29 ± 0.60 | 1.9 ± 0.5 |
|
p = 0.001 |
p = 0.246 |
|||
| 60–120 | 41.3 ± 5.9 | 75 ± 20 | 0.38 ± 0.12 | 0.79 ± 0.14 |
|
p = 0.002 |
p = 0.052 |
|||
| 120–240 | 8.4 ± 9.7 | 14.7 ± 8.4 | 0.066 ± 0.074 | 0.23 ± 0.14 |
|
p = 0.088 |
p = 0.001 |
|||
| 240–480 | 0.54 ± 0.45 | 3.1 ± 2.5 | 0.006 ± 0.006 | 0.063 ± 0.052 |
| p = 0.138 | p < 0.0001 | |||
The urinary excretion rates for gadobenate ion were maximal for both groups between 0 and 30 min after injection (2.69 ± 0.58 and 2.6 ± 1.1 µmol min−1 kg−1 for control rats and rats with bile duct occlusion, respectively). The urinary excretion rate for gadobenate ion was significantly (p ≤ 0.001) higher in rats with bile duct occlusion between 120 and 480 min after injection (Table 1).
Biliary excretion in rats with renal vessel occlusion
The biliary excretion of gadobenate ion was significantly (p < 0.0001) higher in rats with renal vessel occlusion than in normal rats at all time points after gadobenate dimeglumine injection. The overall mean recovery (0–480 min) of gadobenate ion was 96.16 ± 0.55% in the bile of rats with renal vessel occlusion compared with 33.5 ± 4.7% in the bile of normal rats. A comparison of the mean cumulative biliary excretion (percentage of administered dose) over time is shown in Figure 1b.
Peak values for gadobenate ion concentration occurred for both groups between 0 and 30 min after gadobenate dimeglumine administration (16.6 ± 1.9 µmol ml−1 for control animals; 20.6 ± 2.4 µmol ml−1 for animals with renal vessel occlusion) (Table 2). Likewise, the biliary excretion rate of gadobenate ion peaked for both groups between 0 and 30 min after administration (1.47 ± 0.31 µmol min−1 kg−1 for control animals; 2.84 ± 0.44 µmol min−1 kg−1 for animals with renal vessel occlusion). Significantly (p ≤ 0.008) higher values were noted at all time points up to 240 min post-injection for biliary gadobenate ion concentration and at all time points up to 480 min post-injection for biliary excretion rate (Table 2). Similar findings were noted for biliary flow, which peaked for both groups between 0 and 30 min after administration (88 ± 12 µmol min−1 kg−1 for control animals; 137.2 ± 5.4 µmol min−1 kg−1 for animals with renal vessel occlusion; p ≤ 0.002 at all time points up to 240 min post-injection).
Table 2.
Biliary concentration and excretion rate of gadobenate ion and biliary flow after intravenous administration of 0.25 mmol kg−1 of bodyweight gadobenate dimeglumine to normal rats and rats with renal vessel occlusion (n = 5)
| Time period (min) | Biliary concentration (µmol ml−1) |
Biliary excretion rate (µmol min−1 kg−1) |
Biliary flow (µl min−1 kg−1) |
|||
|---|---|---|---|---|---|---|
| Normal rats | Rats with renal vessel occlusion | Normal rats | Rats with renal vessel occlusion | Normal rats | Rats with renal vessel occlusion | |
| 0–30 | 16.6 ± 1.9 | 20.6 ± 2.4 | 1.47 ± 0.32 | 2.84 ± 0.44 | 88 ± 12 | 137.2 ± 5.4 |
|
p < 0.0001 |
p < 0.0001 |
p < 0.0001 |
||||
| 30–60 | 11.8 ± 2.0 | 19.89 ± 0.92 | 0.83 ± 0.13 | 2.34 ± 0.11 | 71 ± 11 | 117.5 ± 4.2 |
|
p < 0.0001 |
p < 0.0001 |
p < 0.0001 |
||||
| 60–120 | 3.4 ± 0.72 | 11.7 ± 1.0 | 0.208 ± 0.032 | 0.96 ± 0.10 | 62 ± 10 | 82.2 ± 7.4 |
|
p < 0.0001 |
p < 0.0001 |
p < 0.0001 |
||||
| 120–240 | 0.34 ± 0.13 | 2.8 ± 1.3 | 0.019 ± 0.006 | 0.20 ± 0.10 | 58.7 ± 7.4 | 70.4 ± 5.3 |
|
p = 0.008 |
p < 0.0001 |
p = 0.002 |
||||
| 240–480 | 0 ± 0 | 0.25 ± 0.15 | 0 ± 0 | 0.014 ± 0.007 | 53.5 ± 5.5 | 57.3 ± 6.8 |
| p = 0.784 | p = 0.037 | p = 0.243 | ||||
Gadobenate ion retention
Gadobenate ion was not quantifiable in plasma (<5 µg gadobenate ion per millilitre) in any group 8 h after gadobenate dimeglumine administration. Assuming a plasma volume in rats of 40.4 ml kg−1,26 the residual content of gadobenate ion in plasma at 8 h post-injection was therefore <0.12% of the injected dose for both normal animals and for animals with bile duct occlusion and renal vessel occlusion.
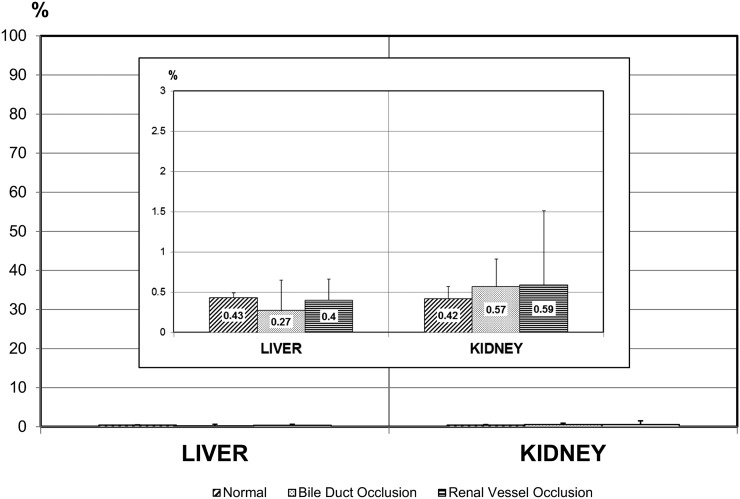
Gd3+ retention in the liver and kidneys at 8 h after gadobenate dimeglumine administration accounted, respectively, for 0.27 ± 0.38% and 0.57 ± 0.34% of the injected dose for rats with bile duct occlusion; 0.40 ± 0.26% and 0.59 ± 0.92% of the injected dose for rats with renal vessel occlusion; and for 0.43 ± 0.06% and 0.42 ± 0.15% of the injected dose for normal rats (Figure 2).
Figure 2.
Residual gadolinium (Gd3+) levels in liver and kidney at 8 h post-injection of 0.25 mmol kg−1 gadobenate dimeglumine to normal rats and to rats with bile duct occlusion or urinary vessel occlusion (n = 5 per group). The residual Gd3+ levels after 8 h accounted for <0.6% of the injected dose in all groups and were similar across groups (see inset).
The total plasma bilirubin level was higher in rats with bile duct occlusion (30.0 ± 5.8 µmol l−1) than in rats with renal vessel occlusion (7.5 ± 2.1 µmol l−1) and in normal rats (4.0 ± 2.9 µmol l−1).
DISCUSSION
This study shows that compensatory elimination of gadobenate ion through either the urinary or hepatobiliary pathway occurs from rats whose normal excretory function has been compromised by ligation of either the common bile duct or renal vessels. Specifically, our study shows that at 8 h after injection almost 90% of the injected dose of gadobenate dimeglumine is excreted via the kidneys in animals with occluded bile ducts compared with approximately 60% in normal animals. Conversely, approximately 96% of the injected dose is excreted via the hepatobiliary pathway in animals with occluded renal vessels compared with approximately 34% in normal animals. Importantly, the residual content of gadobenate ion in plasma at 8 h post-injection was in all cases <0.12% of the injected dose, while assessments of Gd3+ retention in the liver and kidneys revealed minimal differences between rats with occluded bile ducts/renal vessels and normal rats. These data are in agreement with those of Mühler et al23 and suggest that compensatory elimination may be a physiological mechanism to overcome compromised biliary/renal excretion. In support of this conclusion are previous data that reveal almost identical values for the plasma half-life of elimination of gadobenate ion in normal rats with ligated biliary duct (31.1 ± 1.2 min) or renal vessels (31.2 ± 1.2 min).27 Taken together, these data suggest that compensatory elimination may be a realistic means to eliminate GBCAs that are taken up by functioning hepatocytes and excreted in the bile.
That a similar mechanism might exist in humans is suggested by the results of Swan et al28 who evaluated the safety and pharmacokinetics of gadobenate dimeglumine in subjects with moderate or severe renal impairment (defined as creatinine clearance of 31–60 and 10–30 ml min−1, respectively). They demonstrated that at 216 h after administration of 0.2 mmol kg−1 of bodyweight of gadobenate dimeglumine, the mean Gd3+ recovery in the urine and faeces of subjects with moderate renal impairment accounted for 74% and 6% of the injected dose, respectively, while in subjects with severe renal impairment, the mean Gd3+ recovery at 216 h post-injection accounted for 69% and 8% of the injected dose, respectively. Clearly, the level of hepatobiliary elimination in both cases was up from the 2–4% determined for healthy volunteers with normal renal function19,20 and increased with increasing degree of renal impairment. Studies on patients with impaired liver function have shown no deleterious effects on the elimination of gadobenate dimeglumine,29 possibly reflecting the fact that urinary elimination accounts for approximately 96% of the injected dose in patients with normal renal function.
The potential clinical impact of a compensatory mechanism of elimination in patients with severe renal impairment at risk of NSF is enormous. As noted elsewhere,30,31 NSF is primarily observed in patients exhibiting fulminant renal impairment; among 732 (90%) of 815 published unconfounded cases reported between January 2000 and December 2012 in which the type and degree of renal impairment prior to NSF onset was reported, patients with Stage 5 kidney disease (GFR: <15 ml min−1 1.73 m−2) accounted for 644 (88%) cases, while patients with acute renal failure accounted for a further 72 (10%) cases.5 Just 15 (2%) cases were reported in patients with Stage 4 kidney disease (GFR: 15–29 ml min−1 1.73 m−2) and just 1 (0.1%) in a patient purported to have Stage 3 CKD (GFR: 30–59 ml min−1 1.73 m−2). The comparatively few cases of NSF among patients with estimated GFR (eGFR) >15 ml min−1 1.73 m−2 suggest that an eGFR above this level (i.e. approximately 10% residual kidney function) is sufficient to protect against NSF in the vast majority of cases.30,31 As recently suggested by Heverhagen et al,32 a 5% elimination via the hepatobiliary pathway, converted, corresponds to an eGFR of approximately 6 ml min−11.73 m−2 via the kidney. By analogy, based on the findings of Swan et al,28 a hepatobiliary elimination of approximately 8% in patients with severe renal insufficiency (eGFR: <15 ml min−11.73 m−2) would correspond to an eGFR of approximately 10 ml min−1 1.73 m−2. Although not much for a healthy subject with normal renal function, this level of elimination via the hepatobiliary pathway would already account for approximately two-thirds of the Gd3+ excretion needed to markedly reduce the risk of NSF in at-risk patients. Assuming that liver function and hepatobiliary elimination are not compromised in such patients, these data suggest that a residual eGFR of little more than 5 ml min−1 1.73 m−2 may be sufficient to safeguard against NSF in most patients administered gadobenate dimeglumine, particularly if haemodialysis is undertaken immediately after the MRI procedure.
Although the above considerations are valid for gadobenate dimeglumine given its level of hepatobiliary elimination,19,20,28 they are perhaps less relevant for gadoxetate disodium that already undergoes 50% elimination by the hepatobiliary pathway even in subjects with normal renal function.13–15 Likewise, such considerations are not valid for other GBCAs that are not taken up by functioning hepatocytes to any appreciable extent and are not eliminated via the hepatobiliary pathway. Apart from stability issues, this might in part explain why unconfounded cases of NSF have been reported for gadopentetate dimeglumine5 despite a fundamentally similar molecular structure (ionic, linear, missing only the aromatic group) to that of gadobenate dimeglumine.33,34 Similarly, it might also partly explain why unconfounded cases of NSF have been reported for one of the more kinetically stable macrocyclic GBCAs, gadobutrol,35,36 although it should be pointed out that these cases have been the subject of some debate.37,38 Notably, in the case of gadobutrol its two-fold higher concentration in the vial (commercially available as a 1.0 M formulation compared with the 0.5 M formulations of all other GBCAs) is potentially problematic in patients at risk of NSF because of the possibility of excess dosage (a two-fold higher dose of Gd3+ if equal volumes are injected) if care is not taken to lower the volume administered.32,39
A second advantage conferred by the aromatic group on gadobenate dimeglumine, apart from a capacity for uptake by functioning hepatocytes, is increased r1-relaxivity relative to that of other GBCAs.40,41 This increased r1-relaxivity is the result of weak, transient interaction of the gadobenate2− molecule with serum albumin,42,43 mediated by the aromatic (benzyloxymethyl) group, which slows the tumbling rate of the complex, leading to stronger relaxation enhancement effects and hence greater signal intensity enhancement on T1 weighted images.44 Numerous intra-individual crossover studies across a range of clinical applications have demonstrated improved image quality and better diagnostic performance for gadobenate dimeglumine relative to comparator GBCAs when both agents are administered at an equivalent approved dose of 0.1 mmol kg−1 of body weight.45–51 Other studies, particularly for MR angiography (MRA) applications, have demonstrated equivalent or even superior image quality and diagnostic performance for a single dose of gadobenate dimeglumine compared with a double dose of gadopentetate dimeglumine.52–56 This is potentially very important for patients at risk of NSF who require a contrast-enhanced MRA examination for diagnostic purposes. Higher doses of GBCAs have frequently been used in these patients because of the risk of insufficient contrast enhancement for accurate visualization and diagnosis if the GBCA dose is too low.57–60 This has particularly been the case for patients undergoing MRA of the peripheral run-off vasculature because of the larger field of view, smaller size of the vessels and greater susceptibility to flow alterations if vessels are heavily diseased. Unfortunately, such patients are frequently elderly and have associated renal insufficiency or end-stage renal disease.61,62 To administer high GBCA doses to these patients would be inadvisable because of the established greater risk of NSF with high GBCA doses.5–7,30–32,63–66 In these patients, the opportunity to obtain diagnostic images with a lower dose of a GBCA that is excreted in part by the hepatobiliary pathway would be potentially highly beneficial. Preliminary studies have looked to assess the potential of lower doses of gadobenate dimeglumine for MRI procedures in patients at risk of NSF.67
A final consideration concerns the classification of GBCAs by the EMA and MHRA.8,9 It is ironic that there should be fewer (zero) unconfounded cases of NSF among the group of GBCAs considered at intermediate risk of NSF than among the group considered at low risk of NSF, and it is worth bearing in mind that other regulatory authorities include gadobenate dimeglumine with the macrocyclic GBCAs in the group considered at low risk of NSF.68,69
In summary, this study shows that compensatory elimination of gadobenate ion through either the urinary or hepatobiliary pathway occurs from rats whose normal excretory function has been compromised. Although there is evidence of a similar mechanism in humans,28 it should be borne in mind that the normal level of biliary excretion is much greater in rats than in humans21 and that further studies in humans are needed before firm conclusions can be drawn. Nevertheless, a similar compensatory mechanism in humans combined with the possibility to administer a lower overall dose to patients at heightened risk of NSF and greater awareness and care by the radiological community in general may explain in part why no unconfounded cases of NSF have yet been reported for this agent, despite many millions of administrations32 across a wide range of approved indications.16
CONFLICTS OF INTEREST
Authors Miles Andrew Kirchin and Gianpaolo Pirovano are employees of Bracco, the manufacturers of gadobenate dimeglumine.
FUNDING
This work was supported by Bracco.
REFERENCES
- 1.Cowper SE, Su LD, Bhawan J, Robin HS, LeBoit PE. Nephrogenic fibrosing dermopathy. Am J Dermatopathol 2001; 23: 383–93. [DOI] [PubMed] [Google Scholar]
- 2.Girardi M, Kay J, Elston DM, Leboit PE, Abu-Alfa A, Cowper SE. Nephrogenic systemic fibrosis: clinicopathological definition and workup recommendations. J Am Acad Dermatol 2011; 65: 1095–106. doi: 10.1016/j.jaad.2010.08.041 [DOI] [PubMed] [Google Scholar]
- 3.Cowper SE, Robin HS, Steinberg SM, Su LD, Gupta S, LeBoit PE. Scleromyxoedema-like cutaneous diseases in renal-dialysis patients. Lancet 2000; 356: 1000–1. [DOI] [PubMed] [Google Scholar]
- 4.Grobner T. Gadolinium—a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant 2006; 21: 1104–8. Erratum in: Nephrol Dial Transplant 2006; 21: 1745. [DOI] [PubMed] [Google Scholar]
- 5.Spinazzi A. MRI contrast agents and nephrogenic systemic fibrosis. In: Shellock FG, Crues JV, eds. MRI bioeffects, safety, and patient management. Los Angeles, CA: Biomedical Research Publishing Group; 2014. pp. 256–81. [Google Scholar]
- 6.Dawson P. Nephrogenic systemic fibrosis: possible mechanisms and imaging management strategies. J Magn Reson Imaging 2008; 28: 797–804. doi: 10.1002/jmri.21521 [DOI] [PubMed] [Google Scholar]
- 7.van der Molen AJ. Nephrogenic systemic fibrosis and the role of gadolinium contrast media. J Med Imaging Radiat Oncol 2008; 52: 339–50. doi: 10.1111/j.1440-1673.2008.01965.x [DOI] [PubMed] [Google Scholar]
- 8.Thomsen HS, Morcos SK, Almén T, Bellin MF, Bertolotto M, Bongartz G, et al. ; ESUR Contrast Medium Safety Committee. Nephrogenic systemic fibrosis and gadolinium-based contrast media: updated ESUR Contrast Medium Safety Committee guidelines. Eur Radiol 2013; 23: 307–18. doi: 10.1007/s00330-012-2597-9 [DOI] [PubMed] [Google Scholar]
- 9.Gadolinium-containing contrast agents: new advice to minimise the risk of nephrogenic systemic fibrosis. [Updated 11 January 2010; accessed 12 February 2015.]. Available from: https://www.gov.uk/drug-safety-update/gadolinium-containing-contrast-agents-new-advice-to-minimise-the-risk-of-nephrogenic-systemic-fibrosis
- 10.Hao D, Ai T, Goerner F, Hu X, Runge VM, Tweedle M. MRI contrast agents: basic chemistry and safety. J Magn Reson Imaging 2012; 36: 1060–71. doi: 10.1002/jmri.23725 [DOI] [PubMed] [Google Scholar]
- 11.Aime S, Caravan P. Biodistribution of gadolinium-based contrast agents, including gadolinium deposition. J Magn Reson Imaging 2009; 30: 1259–67. doi: 10.1002/jmri.21969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parmelee DJ, Walovitch RC, Ouellet HS, Lauffer RB. Preclinical evaluation of the pharmacokinetics, biodistribution, and elimination of MS-325, a blood pool agent for magnetic resonance imaging. Invest Radiol 1997; 32: 741–7. [DOI] [PubMed] [Google Scholar]
- 13.Hamm B, Staks T, Mühler A, Bollow M, Taupitz M, Frenzel T, et al. Phase I clinical evaluation of Gd-EOB-DTPA as a hepatobiliary MR contrast agent: safety, pharmacokinetics, and MR imaging. Radiology 1995; 195: 785–92. [DOI] [PubMed] [Google Scholar]
- 14.Schuhmann-Giampieri G, Mahler M, Röll G, Maibauer R, Schmitz S. Pharmacokinetics of the liver-specific contrast agent Gd-EOB-DTPA in relation to contrast-enhanced liver imaging in humans. J Clin Pharmacol 1997; 37: 587–96. [DOI] [PubMed] [Google Scholar]
- 15.Gschwend S, Ebert W, Schultze-Mosgau M, Breuer J. Pharmacokinetics and imaging properties of Gd-EOB-DTPA in patients with hepatic and renal impairment. Invest Radiol 2011; 46: 556–66. doi: 10.1097/RLI.0b013e31821a218a [DOI] [PubMed] [Google Scholar]
- 16.MultiHance® Summary of Product Characteristics (SPC). Bracco UK Ltd. High Wycombe, UK. [Accessed 12 February 2015.] Available from: https://www.medicines.org.uk/emc/medicine/6132
- 17.Ablavar® [package insert]. North Billerica, MA: Lantheus Medical Imaging, Inc. [Accessed 12 February 2015.] Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021711s003lbl.pdf [Google Scholar]
- 18.Primovist® Summary of Product Characteristics (SPC). Bayer plc. Newbury, UK. [Accessed 12 February 2015.] Available from: https://www.medicines.org.uk/emc/medicine/15927
- 19.Kirchin MA, Pirovano GP, Spinazzi A. Gadobenate dimeglumine (Gd-BOPTA). An overview. Invest Radiol 1998; 33: 798–809. [DOI] [PubMed] [Google Scholar]
- 20.Spinazzi A, Lorusso V, Pirovano GP, Kirchin M. Safety, tolerance, biodistribution and MR imaging enhancement of the liver with gadobenate dimeglumine: results of clinical pharmacologic and pilot imaging studies in nonpatient and patient volunteers. Acad Radiol 1999; 6: 282–91. [DOI] [PubMed] [Google Scholar]
- 21.Lorusso V, Arbughi T, Tirone P, de Haën C. Pharmacokinetics and tissue distribution in animals of gadobenate ion, the magnetic resonance imaging contrast enhancing component of gadobenate dimeglumine 0.5 M solution for injection (MultiHance). J Comput Assist Tomogr 1999; 23(Suppl. 1): S181–94. [DOI] [PubMed] [Google Scholar]
- 22.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39(Suppl. 1): S1–266. [PubMed] [Google Scholar]
- 23.Mühler A, Heinzelmann I, Weinmann HJ. Elimination of gadolinium-ethoxybenzyl-DTPA in a rat model of severely impaired liver and kidney excretory function. An experimental study in rats. Invest Radiol 1994; 29: 213–16. [DOI] [PubMed] [Google Scholar]
- 24.Lorusso V, Poggesi I, Arbughi T, Dal Fiume D, Tirone P. High-performance liquid chromatographic assay of the magnetic resonance imaging contrast agent gadobenate in plasma, urine and bile. J Chromatogr B Biomed Appl 1994; 656: 415–22. [DOI] [PubMed] [Google Scholar]
- 25.Arbughi T, Bartolomeo MP, Costa G, Lorusso V, Tirone P. X-ray fluorescence spectrometric determination of gadolinium in biological tissues. XXI National Congress of Analytical Chemistry. Book of abstracts Nr. SP21. Cagliari, Italy: Società Chimica Italiana; 1994. [Google Scholar]
- 26.Baker HJ, Lindsey JR, Weisbroth SH, eds. Appendix 1. The laboratory, rat. volume 1. New York, NY: Academic Press; 1979. pp. 411–12. [Google Scholar]
- 27.de Haën C, Lorusso V, Tirone P. Hepatic transport of gadobenate dimeglumine in TR-rats. Acad Radiol 1996; 3(Suppl. 2): S452–4. [DOI] [PubMed] [Google Scholar]
- 28.Swan SK, Lambrecht LJ, Townsend R, Davies BE, McCloud S, Parker JR, et al. Safety and pharmacokinetic profile of gadobenate dimeglumine in subjects with renal impairment. Invest Radiol 1999; 34: 443–8. [DOI] [PubMed] [Google Scholar]
- 29.Davies BE, Kirchin MA, Bensel K, Lorusso V, Davies A, Parker JR, et al. Pharmacokinetics and safety of gadobenate dimeglumine (MultiHance) in subjects with impaired liver function. Invest Radiol 2002; 37: 299–308. [DOI] [PubMed] [Google Scholar]
- 30.Jalandhara N, Arora R, Batuman V. Nephrogenic systemic fibrosis and gadolinium-containing radiological contrast agents: an update. Clin Pharmacol Ther 2011; 89: 920–3. doi: 10.1038/clpt.2010.346 [DOI] [PubMed] [Google Scholar]
- 31.Rydahl C, Thomsen HS, Marckmann P. High prevalence of nephrogenic systemic fibrosis in chronic renal failure patients exposed to gadodiamide, a gadolinium-containing magnetic resonance contrast agent. Invest Radiol 2008; 43: 141–4. doi: 10.1097/RLI.0b013e31815a3407 [DOI] [PubMed] [Google Scholar]
- 32.Heverhagen JT, Krombach GA, Gizewski E. Application of extracellular gadolinium-based MRI contrast agents and the risk of nephrogenic systemic fibrosis. Rofo 2014; 186: 661–9. doi: 10.1055/s-0033-1356403 [DOI] [PubMed] [Google Scholar]
- 33.Laurent S, Elst LV, Muller RN. Comparative study of the physicochemical properties of six clinical low molecular weight gadolinium contrast agents. Contrast Media Mol Imaging 2006; 1: 128–37. [DOI] [PubMed] [Google Scholar]
- 34.Idée JM, Port M, Robic C, Medina C, Sabatou M, Corot C. Role of thermodynamic and kinetic parameters in gadolinium chelate stability. J Magn Reson Imaging 2009; 30: 1249–58. doi: 10.1002/jmri.21967 [DOI] [PubMed] [Google Scholar]
- 35.Wollanka H, Weidenmaier W, Giersig C. NSF after Gadovist exposure: a case report and hypothesis of NSF development. Nephrol Dial Transpl 2009; 24: 3882–4. doi: 10.1093/ndt/gfp494 [DOI] [PubMed] [Google Scholar]
- 36.Elmholdt TR, Jørgensen B, Ramsing M, Pedersen M, Olesen AB. Two cases of nephrogenic systemic fibrosis after exposure to the macrocyclic compound gadobutrol. Nephrol Dial Transpl Plus 2010; 3: 285–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Collidge T, Thomson P, Mark P, Willinek W, Roditi G. Is this really a true case of NSF following Gadovist exposure alone? Nephrol Dial Transpl 2010; 25: 1352–3; author reply 1353–4. doi: 10.1093/ndt/gfq014 [DOI] [PubMed] [Google Scholar]
- 38.Morcos SK, Dawson P. Comments on the case report reported by Elmholdt et al. Nephrol Dial Transpl Plus 2010; 3: 501–2; author reply 502–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Forsting M, Palkowitsch P. Prevalence of acute adverse reactions to gadobutrol—a highly concentrated macrocyclic gadolinium chelate: review of 14,299 patients from observational trials. Eur J Radiol 2010; 74: e186–92. doi: 10.1016/j.ejrad.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 40.Pintaske J, Martirosian P, Graf H, Erb G, Lodemann KP, Claussen CD, et al. Relaxivity of gadopentetate dimeglumine (Magnevist), gadobutrol (Gadovist), and gadobenate dimeglumine (MultiHance) in human blood plasma at 0.2, 1.5, and 3 Tesla. Invest Radiol 2006; 41: 213–21. Erratum in: Invest Radiol 2006; 41: 859. [DOI] [PubMed] [Google Scholar]
- 41.Rohrer M, Bauer H, Mintorovitch J, Requardt M, Weinmann HJ. Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Invest Radiol 2005; 40: 715–24. [DOI] [PubMed] [Google Scholar]
- 42.Cavagna FM, Maggioni F, Castelli PM, Daprà M, Imperatori LG, Lorusso V, et al. Gadolinium chelates with weak binding to serum proteins. A new class of high-efficiency, general purpose contrast agents for magnetic resonance imaging. Invest Radiol 1997; 32: 780–96. [DOI] [PubMed] [Google Scholar]
- 43.Giesel FL, von Tengg-Kobligk H, Wilkinson ID, Siegler P, von der Lieth CW, Frank M, et al. Influence of human serum albumin on longitudinal and transverse relaxation rates (r1 and r2) of magnetic resonance contrast agents. Invest Radiol 2006; 41: 222–8. [DOI] [PubMed] [Google Scholar]
- 44.Kanal E, Maravilla K, Rowley HA. Gadolinium contrast agents for CNS imaging: current concepts and clinical evidence. AJNR Am J Neuroradiol 2014; 35: 2215–26. doi: 10.3174/ajnr.A3917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maravilla KR, Maldjian JA, Schmalfuss IM, Kuhn MJ, Bowen BC, Wippold FJ, 2nd, et al. Contrast enhancement of central nervous system lesions: multicenter intraindividual crossover comparative study of two MR contrast agents. Radiology 2006; 240: 389–400. [DOI] [PubMed] [Google Scholar]
- 46.Rowley HA, Scialfa G, Gao PY, Maldjian JA, Hassell D, Kuhn MJ, et al. Contrast-enhanced MR imaging of brain lesions: a large-scale intraindividual crossover comparison of gadobenate dimeglumine versus gadodiamide. AJNR Am J Neuroradiol 2008; 29: 1684–91. doi: 10.3174/ajnr.A1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seidl Z, Vymazal J, Mechl M, Goyal M, Herman M, Colosimo C, et al. Does higher gadolinium concentration play a role in the morphologic assessment of brain tumors? Results of a multicenter intraindividual crossover comparison of gadobutrol versus gadobenate dimeglumine (the MERIT Study). AJNR Am J Neuroradiol 2012; 33: 1050–8. doi: 10.3174/ajnr.A3033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pediconi F, Catalano C, Occhiato R, Venditti F, Fraioli F, Napoli A, et al. Breast lesion detection and characterization at contrast-enhanced MR mammography: gadobenate dimeglumine versus gadopentetate dimeglumine. Radiology 2005; 237: 45–56. [DOI] [PubMed] [Google Scholar]
- 49.Pediconi F, Catalano C, Padula S, Roselli A, Dominelli V, Cagioli S, et al. Contrast-enhanced MR mammography: improved lesion detection and differentiation with gadobenate dimeglumine. AJR Am J Roentgenol 2008; 191: 1339–46. doi: 10.2214/AJR.07.3533 [DOI] [PubMed] [Google Scholar]
- 50.Martincich L, Faivre-Pierret M, Zechmann CM, Corcione S, van den Bosch HC, Peng WJ, et al. Multicenter, double-blind, randomized, intraindividual crossover comparison of gadobenate dimeglumine and gadopentetate dimeglumine for breast MR imaging (DETECT Trial). Radiology 2011; 258: 396–408. doi: 10.1148/radiol.10100968 [DOI] [PubMed] [Google Scholar]
- 51.Gerretsen SC, le Maire TF, Miller S, Thurnher SA, Herborn CU, Michaely HJ, et al. Multicenter, double-blind, randomized, intraindividual crossover comparison of gadobenate dimeglumine and gadopentetate dimeglumine for MR angiography of peripheral arteries. Radiology 2010; 255: 988–1000. doi: 10.1148/radiol.10090357 [DOI] [PubMed] [Google Scholar]
- 52.Pediconi F, Fraioli F, Catalano C, Napoli A, Danti M, Francone M, et al. Gadobenate dimeglumine (Gd-DPTA) vs gadopentetate dimeglumine (Gd-BOPTA) for contrast-enhanced magnetic resonance angiography (MRA): improvement in intravascular signal intensity and contrast to noise ratio. [In English, Italian.] Radiol Med 2003; 106: 87–93. [PubMed] [Google Scholar]
- 53.Prokop M, Schneider G, Vanzulli A, Goyen M, Ruehm SG, Douek P, et al. Contrast-enhanced MR angiography of the renal arteries: blinded multicenter crossover comparison of gadobenate dimeglumine and gadopentetate dimeglumine. Radiology 2005; 234: 399–408. [DOI] [PubMed] [Google Scholar]
- 54.Li Y, Li X, Li D, Lu J, Xing X, Yan F, et al. Multicenter, intraindividual comparison of single-dose gadobenate dimeglumine and double-dose gadopentetate dimeglumine for MR angiography of the supra-aortic arteries (the Supra-Aortic Value Study). AJNR Am J Neuroradiol 2013; 34: 847–54. doi: 10.3174/ajnr.A3298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang J, Yan F, Liu J, Lu J, Li D, Luan J, et al. Multicenter, intra-individual comparison of single dose gadobenate dimeglumine and double dose gadopentetate dimeglumine for MR angiography of the peripheral arteries (the peripheral VALUE study). J Magn Reson Imaging 2013; 38: 926–37. doi: 10.1002/jmri.24040 [DOI] [PubMed] [Google Scholar]
- 56.Woodard PK, Chenevert TL, Sostman HD, Jablonski KA, Stein PD, Goodman LR, et al. Signal quality of single dose gadobenate dimeglumine pulmonary MRA examinations exceeds quality of MRA performed with double dose gadopentetate dimeglumine. Int J Cardiovasc Imaging 2012; 28: 295–301. doi: 10.1007/s10554-011-9821-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thurnher SA, Capelastegui A, Del Olmo FH, Dondelinger RF, Gervás C, Jassoy AG, et al. Safety and effectiveness of single- versus triple-dose gadodiamide injection-enhanced MR angiography of the abdomen: a phase III double-blind multicenter study. Radiology 2001; 219: 137–46. [DOI] [PubMed] [Google Scholar]
- 58.Krause U, Kroencke T, Spielhaupter E, Taupitz M, Kenn W, Hamm B, et al. Contrast-enhanced magnetic resonance angiography of the lower extremities: standard-dose vs. high-dose gadodiamide injection. J Magn Reson Imaging 2005; 21: 449–54. [DOI] [PubMed] [Google Scholar]
- 59.Schaefer PJ, Boudghene FP, Brambs HJ, Bret-Zurita M, Caniego JL, Coulden RA, et al. Abdominal and iliac arterial stenoses: comparative double-blinded randomized study of diagnostic accuracy of 3D MR angiography with gadodiamide or gadopentetate dimeglumine. Radiology 2006; 238: 827–40. [DOI] [PubMed] [Google Scholar]
- 60.Schneider G, Ballarati C, Grazioli L, Manfredi R, Thurnher S, Kroencke TJ, et al. Gadobenate dimeglumine-enhanced MR angiography: diagnostic performance of four doses for detection and grading of carotid, renal, and aorto-iliac stenoses compared to digital subtraction angiography. J Magn Reson Imaging 2007; 26: 1020–32. [DOI] [PubMed] [Google Scholar]
- 61.O'Hare A, Johansen K. Lower-extremity peripheral arterial disease among patients with end-stage renal disease. J Am Soc Nephrol 2001; 12: 2838–47. [DOI] [PubMed] [Google Scholar]
- 62.Matsumae T, Abe Y, Murakami G, Ishihara M, Ueda K, Saito T. Determinants of arterial wall stiffness and peripheral artery occlusive disease in nondiabetic hemodialysis patients. Hypertens Res 2007; 30: 377–85. [DOI] [PubMed] [Google Scholar]
- 63.Broome DR, Girguis MS, Baron PW, Cottrell AC, Kjellin I, Kirk GA. Gadodiamide-associated nephrogenic systemic fibrosis: why radiologists should be concerned. AJR Am J Roentgenol 2007; 188: 586–92. [DOI] [PubMed] [Google Scholar]
- 64.Sadowski EA, Bennett LK, Chan MR, Wentland AL, Garrett AL, Garrett RW, et al. Nephrogenic systemic fibrosis: risk factors and incidence estimation. Radiology 2007; 243: 148–57. [DOI] [PubMed] [Google Scholar]
- 65.Kallen AJ, Jhung MA, Cheng S, Hess T, Turabelidze G, Abramova L, et al. Gadolinium-containing magnetic resonance imaging contrast and nephrogenic systemic fibrosis: a case-control study. Am J Kidney Dis 2008; 51: 966–75. doi: 10.1053/j.ajkd.2007.12.036 [DOI] [PubMed] [Google Scholar]
- 66.Abujudeh HH, Kaewlai R, Kagan A, Chibnik LB, Nazarian RM, High WA, et al. Nephrogenic systemic fibrosis after gadopentetate dimeglumine exposure: case series of 36 patients. Radiology 2009; 253: 81–9. doi: 10.1148/radiol.2531082160 [DOI] [PubMed] [Google Scholar]
- 67.de Campos RO, Heredia V, Ramalho M, De Toni MS, Lugo-Somolinos A, Fuller ER, 3rd, et al. Quarter-dose (0.025 mmol/kg) gadobenate dimeglumine for abdominal MRI in patients at risk for nephrogenic systemic fibrosis: preliminary observations. AJR Am J Roentgenol 2011; 196: 545–52. doi: 10.2214/AJR.10.4500 [DOI] [PubMed] [Google Scholar]
- 68.ACR Manual on Contrast Media. V. 9. 2013. [Accessed 12 February 2015.] Available from: http://www.acr.org/quality-safety/resources/∼/media/37D84428BF1D4E1B9A3A2918DA9E27A3.pdf
- 69.Guideline on the use of gadolinium-containing MRI contrast agents in patients with renal impairment, v. 2. 2013. The Royal Australian and New Zealand College of Radiologists. [Accessed 12 February 2015.] Available from: http://www.ranzcr.edu.au/documents-download/doc_download/553-revised-college-guidelines-for-gadoliniumcontaining-mri-contrast-agents.pdf