Abstract
Objective:
Synchronous malignancy in both breasts is a rare incidence. The present study aims at dosimetric comparison of conventional bitangential radiotherapy (RT) technique with conventional [field-in-field (FIF)] and rotational [Helical TomoTherapy® and TomoDirect™ (TD); Accuray Inc., Sunnyvale, CA] intensity-modulated RT for patients with synchronous bilateral breast cancer (SBBC).
Methods:
CT data sets of 10 patients with SBBC were selected for the present study. RT was planned for all patients on both sides to whole breast and/or chest wall using the above-mentioned techniques. Six females with breast conservation on at least one side also had a composite plan along with tumour bed (TB) boost using sequential electrons for bitangential and FIF techniques or sequential helical tomotherapy (HT) boost (for TD) or simultaneous integrated boost (SIB) for HT.
Results:
All techniques produced acceptable target coverage. The hotspot was significantly lower with FIF technique and HT but higher with TD. For the organs at risk doses, HT resulted in significant reduction of the higher dose volumes. Similarly, TD resulted in significant reduction of the mean dose to the heart and total lung by reducing the lower dose volumes. All techniques of delivering boost to the TB were comparable in terms of target coverage. HT-SIB markedly reduced mean doses to the total lung and heart by specifically lowering the higher dose volumes.
Conclusion:
This study demonstrates the cardiac and pulmonary sparing ability of tomotherapy in the setting of SBBC.
Advances in knowledge:
This is the first study demonstrating feasibility of treatment of SBBC using tomotherapy.
Breast cancer is the most common malignancy amongst females in the world, including Indian females.1 Cancer in both breasts is an uncommon presentation. Incidence of bilateral breast cancer (BBC) has been reported in the range of 1.4–11.8% with the majority being metachronous cancer.2,3 Depending upon various definitions adopted by authors, synchronous BBC (SBBC) accounts for approximately 0.4–2.8% of all breast cancers.4,5 Whether bilaterality confers worse prognosis or similar prognosis is yet to be conclusively determined. Some studies have indicated that there is no difference in survival between the unilateral vs BBC patient groups, while other studies claim that bilateral carcinoma significantly reduces survival.6,7 Treatment in patients with BBC is similar to that in patients with unilateral breast cancer wherein adjuvant radiotherapy (RT) forms an integral part of the breast conservation algorithm. The safety of breast conservation surgery (BCS) for SBBC has been documented in literature.8,9 Adjuvant RT for breast cancer typically includes whole breast irradiation after lumpectomy or chest wall irradiation after mastectomy with or without regional nodal irradiation. This is accomplished using conventional bitangential portals that include part of the anterior chest wall adjacent to the RT target.10–12 RT delivery in cases of SBBC is even more complex owing to multiple field junctions, which results in heterogeneous dose distributions as well as significantly higher irradiation volume of organs at risk (OARs) such as the lungs and heart.
The reported incidence of radiation pneumonitis (RP) varies from 0% to 80% depending upon the radiation technique, length of follow-up, imaging modality used and the end point chosen.13–16 Although symptomatic RP is a rare clinical complication for unilateral breast cancer, it has a potential detrimental effect of reducing the normal functional reserve and should be taken into consideration given the long life expectancy of patients and higher volume of irradiation owing to bilaterality in patients with SBBC. The risk and severity of RP is influenced by various therapy-related (volume of incidentally irradiated lung, region of irradiated lung, radiation dose, fractionation and concomitant use of systemic agents, particularly paclitaxel) and patient-related factors (age, pre-existing lung disease, poor pulmonary function, smoking habits, genetic predisposition). The most significant amongst these include patient age, locoregional RT, reduced pre-RT pulmonary reserve and concomitant tamoxifen use with adjuvant RT.17–19 These factors correlate with various dosimetric indices [V20, D25, mean lung dose (MLD)] that predict the risk of RP.20
Similarly, the toxic effect of radiation on the heart has been well documented. The long-term risk of ischaemic heart disease following breast RT has been correlated with the mean heart dose, maximum heart distance and various dosimetric parameters (V20, V30 and V40). Moreover, several patient-related risk factors (body mass index, diabetes mellitus, dyslipidaemia, tobacco/alcohol consumption, prior heart disease) and systemic agents (anthracyclines, trastuzumab, tamoxifen) modify the risk of radiation-induced cardiac toxicity.21,22 Patients with BBC receive a higher radiation dose to the heart (owing to scatter radiation from the right side) and would be at increased risk of radiation-induced cardiac toxicity.23
Although techniques of delivering RT have improved considerably for various sites in past two decades, the technique of delivering RT to the breast or chest wall, unilateral or bilateral, has not changed much. Various other methods have been used to deliver RT to the breast and/or the chest wall for SBBC across the world, such as electron arc therapy, or static or rotational intensity-modulated RT (IMRT), but none has been compared with conventional bitangential RT.24,25
Helical TomoTherapy® (HT) (Accuray Inc., Sunnyvale CA) is a radiation delivery modality that delivers an intensity-modulated fan beam using a 6-MV linear accelerator mounted on a ring gantry that rotates around the patient as he/she advances slowly through the gantry bore.26 Dosimetric data regarding the use of HT in breast cancer treatment have resulted in equivocal results, not only in the context of target coverage and homogeneity but in the sparing of the heart and lungs as well. Although HT has been studied in the context of partial breast irradiation, whole breast irradiation and locoregional nodal irradiation,27–30 fewer data are available on the dosimetry and feasibility of HT in the context of SBBC requiring bilateral adjuvant radiation with or without simultaneous integrated boost (SIB) of the tumour bed (TB).
TomoDirect™ (TD) (Accuray Inc.) is a static or non-rotational extension of HT, which is also referred to as TomoTherapy®. In this application of TomoTherapy, the patient is translated craniocaudally through fixed gantry positions with simultaneous beam modulation. Up to 12 coplanar fixed beams can be used for dose optimization and target coverage. Similar to HT, dosimetric and clinical data are also available with TD in both, three-dimensional conformal RT (3DCRT) and/or IMRT mode for treatment of unilateral breast cancer treatment.28,31–33 However, no data are available on the dosimetry and feasibility of TD in the context of SBBC.
In our study, we aimed to compare conventional bitangential RT with conventional IMRT and two techniques of tomotherapy, namely HT and TD dosimetrically in the context of SBBC.
METHODS AND MATERIALS
In this pilot study, those patients who were diagnosed with SBBC and received conventional bitangential RT to the breast and/or chest wall were identified during January 2009 to November 2013. CT planning data sets of these patients were retrieved for the dosimetric comparison. This constituted a total of 10 patients inclusive of 4 patients who had BCS on both sides, while 4 had modified radical mastectomy on both sides. Two females had mastectomy on one side (left side for both) and breast conservation on another side. All cases had a histopathological diagnosis of infiltrating ductal carcinoma (IDC) in both breasts.
Conventional planning
Conventional plans were made for each side respecting principles of conventional bitangential treatment planning consisting of two opposed tangential beams of 6- or 10-MV energies for unilateral breast RT with at least 0.7 to 1.0-cm gap between medial tangential portals of both sides. All plans were made on the treatment planning system ARIA™ (Eclipse™ v. 8.6.0; Varian® Medical Systems, Palo Alto, CA) for treatment on a Varian Trilogy®, with a 120-leaf multileaf collimator (MLC; Varian Medical Systems). 15° and 30° physical wedges in superoinferior direction were used when indicated. The heart was spared by using the MLC whenever possible without compromising target volume coverage. Bolus was not used for any chest wall planning. For five patients (including one chest wall), the 6- and 15-MV combinations were used, while for the rest, only the 6-MV combination was used. Plans were made with acceptable central lung distance (CLD; mean CLD was 1.66 cm for the right side and 1.87 cm for the left side) and maximum heart distance (MHD; mean MHD, 1.45 cm). Each side plan was summated and the sum plans were evaluated respecting the International Committee on Radiation Units and Measurements (ICRU) criteria and disregarding contours. For TB boost, electron plans of appropriate energy were generated in the same planning CT scan. Single electron boost fields were added using rectangular apertures. The gantry was angled to be perpendicular to the skin surface, and the collimator was rotated for the best fit to the planning target volume (PTV) shape. Electron energies from 6 to 20 MeV were used, with the aim to put the 90% isodose at the posterior limit of the PTV boost. The dose was prescribed to the 90% isodose. Plans were calculated using the electron Monte-Carlo algorithm.
A composite plan was produced by summing the boost plan and the whole breast plan of both sides (whenever applicable).
Contouring
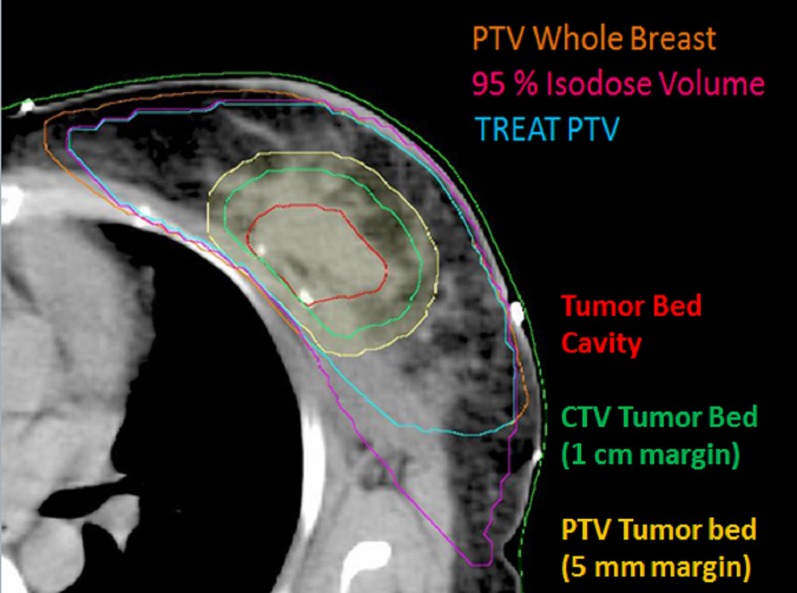
The clinical target volume (CTV) for the breast and/or chest wall was contoured with the help of a wire placed on the patient during planning CT scan. A 5-mm margin was grown to this volume to form the PTV. The PTV was cropped from the skin by 5 and 3 mm in case of post-breast conservation surgery and post-mastectomy RT, respectively. OARs such as the lung on each side and the heart were contoured. Both lungs were delineated with automatic segmentation. The heart was contoured from the level of the pulmonary trunk to the apex and included the pericardium but not the major vessels. In cases of BCS, the TB was delineated with the help of seroma or surgical clips, if present, or with a metal wire placed over the scar. A margin of 1 cm was given in all directions to form CTV boost. Volume of CTV boost was modified so as to be confined within the breast volume. Furthermore, a 5-mm margin was given to make PTV boost, to be confined within PTV breast volume (Figure 1).
Figure 1.
Target volume delineation [note: metal wires are used to mark extent of breast clinically. Planning target volume (PTV) whole breast; volume covered by 95% isodose using conventional bitangential technique; TREAT PTV; tumour bed cavity; clinical target volume (CTV) tumour bed; PTV tumour bed]. TREAT PTV, term for volume common to reference volume and PTV.
PTV modification
PTVs were modified according to the volume covered in conventional portals so as to keep comparable targets for all treatment plans. For the breast and chest wall RT, the volume covered by 95% and 90% of the prescription dose was taken as the reference volume created with the help of the tool “convert isodose to contour” in ARIA. In three instances with breast conservation <95% isodose (90% isodose in two instances, 93% in one instance) was taken as the reference volume to achieve adequate coverage of boost volume. The volume common to the reference volume and PTV was created and termed as “TREAT PTV”. These contours were used for planning other treatment techniques (Figure 2).
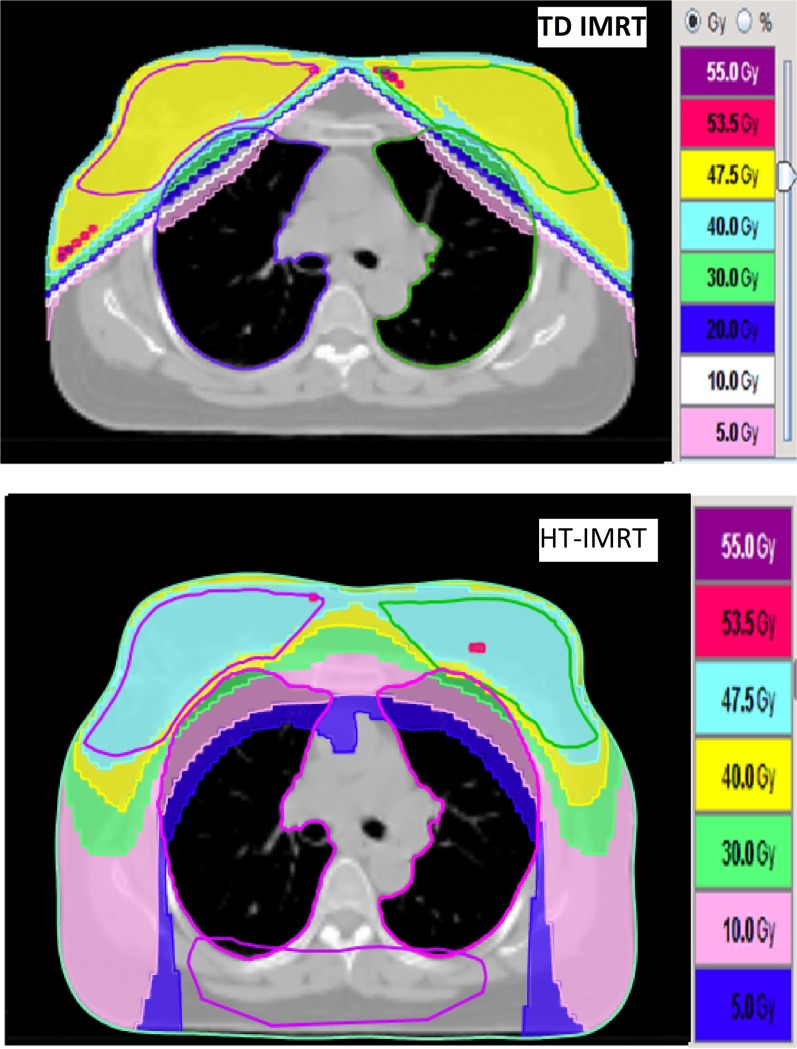
Figure 2.
Dose distribution with TomoDirect™ (TD) (Accuray Inc., Sunnyvale, CA) intensity-modulated radiotherapy (IMRT) and helical tomotherapy (HT) IMRT (whole breast only). Dose volumes are given with reference to prescription dose. 55 Gy, i.e. 110% of 50 Gy prescription dose; 53.5 Gy, i.e. 107%; 47.5 Gy i.e. 95% of 50 Gy prescription dose. (note the helping structure, a horizontal dummy along the posterior part of the body was used for blocking the entry of the beamlets).
Field-in-field intensity-modulated radiotherapy planning
Field-in-field (FIF) plans were made for each TREAT PTV on treatment planning system (TPS) (Eclipse). In the FIF technique, an initial calculation was performed first with two open tangential photon beams without any beam modifiers. After achieving dose distribution, hotspot volumes (volume receiving >107%) were created as structures by TPS, and for these hot spot volumes, blocking subfields (average three subfields for each side) were then determined in order to improve the dose homogeneity for the PTV in a stepwise manner. Precaution was taken that none of the subfields should contribute <5 cGy or >20 cGy to the final plan. The heart was spared by using the MLC whenever possible without compromising target volume coverage. The main field and the subfields were merged into one portal, including several MLC segments for sequential irradiation. Boost plans were generated same as those for conventional plan.
Tomotherapy planning
Each TREAT PTV was planned on Helical TomoTherapy Hi-Art® system (TomoTherapy Inc., Madison, WI), TomoPlan (TomoTherapy planning system) v. 4.2.0 for HT-IMRT and TD with 3DCRT (TD-3DCRT) and IMRT (TD-IMRT) separately. Helping structures were created within the body volume outside the PTV where significant hotspots were likely to occur. In addition, a horizontal dummy along the posterior part of the body was used for blocking the entry of the beamlets (Figure 2).
For each plan, the treatment field width (FW), pitch (defined as the ratio of the distance travelled per rotation to the axial FW used for treatment) and modulation factor (MF) need to be selected. Then, the dose distribution for each beamlet that passes through the target is calculated by a convolution/superposition algorithm. Once the beamlet calculation step is completed, the optimization process begins and an iterative least-squares minimization method is used to optimize the objective function. During the final dose computation, the optimized sinogram is converted to the delivery sinogram, taking into account the leaf fluence output factors and latency data. A fine calculation grid (256 × 256 pixels) was used both in the optimization and calculation processes. The TPS was driven by dose-based objectives, their associated penalties and ROI-based weighting factors (for HT-IMRT and TD-IMRT). For target volumes, the minimum and maximum dose values and their respective penalties were used in addition to a dose–volume histogram (DVH)-based prescription point. OAR objectives were described by a maximum dose, a DVH-based constraint and their respective penalties.
TomoDirect planning
TomoDirect three-dimensional conformal radiotherapy for whole breast/chest wall:
The TD-3DCRT mode represents a simpler planning mode in which the DVH-based prescription was selected for target structures, which in turn influences the optimization process by only setting two parameters: the tissue compensation and the normal tissue homogeneity. All plans were performed with the normal tissue homogeneity option and setting the tissue compensation to high, in order to allow for a more meaningful comparison with other techniques. In general, two to four beams were selected for each side such that it covers the PTV adequately and avoids entry through couch whenever possible, otherwise the conflict message was ignored. An entrance beam block was placed on the heart to minimize dose to the heart.
TomoDirect intensity-modulated radiotherapy for whole breast/chest wall:
Two or four tangential beams for each side with a jaw width of 2.5 cm were used. Beam angles were selected in order to minimize the dose to OARs taking into consideration the CLD and MHD. Only those MLC leaves required to treat the target were used. Three MLC leaves were kept open on the anterior edge of the beams. A “fine” calculation grid was used for both optimization and calculation. In the TD-IMRT mode, a 30-mm ring around the PTV was used to help reduce skin overdosage. Helping structures were created within the body volume outside the PTV where significant hotspots were likely to occur. A directional block was placed on the heart to minimize dose to the heart. OARs were used as avoidance structures for the optimization process. A MF of 2.0–3.0 was used in all IMRT plans.
Tumour bed boost planning:
Boost to the TB was planned using HT with parameters described as below.
Sum plan
TD-3DCRT and TD-IMRT plans were summated with the boost plan to simulate complete treatment plan.
Helical planning
HT plan parameters consisted of a 2.5-cm FW, 0.3 pitch and 3.0 MF. The heart was directionally blocked to prevent beamlets from entering through those organs. Avoidance structures were used as needed in order to decrease high dose spill over into the non-delineated normal tissues. SIB planning was carried out in a similar manner keeping different priorities for boost PTV and TREAT PTV.
Dose prescription
For all plans, the intended dose prescription was to deliver 50 Gy in 25 fractions to TREAT PTV (in whole breast irradiation/chest wall irradiation alone) and sequential boost to the TB to a dose of 15 Gy in 6 fractions [for α/β = 3, biologically effective dose (BED) = 110.83 Gy]. In HT, 61 Gy in 25 fractions were delivered to boost PTV in HT-SIB plan (for α/β = 3, BED = 110.61 Gy). The boost prescription for the rest of the plans was 15 Gy in six fractions (Figure 2). The plans were evaluated with reference to the ICRU criteria of 95% of the target volume getting covered with 95% of the prescribed dose with minimum spillage to the surrounding normal tissue. In order to achieve this, the dose prescription was modified in most patients. Dose prescriptions in most patients were such that 95% of the PTV will receive 47.5 Gy dose, i.e. 95% of the prescribed dose 50 Gy. Various permutations and combinations were used to ensure adequate coverage of PTV without any hotspots and maximum OAR sparing.
Owing to paucity of data in SBBC, OAR doses achieved with the conventional bitangential plan were used as the reference and other techniques were optimized against these values.
Plan evaluation
Evaluation of plans was based on DVH analysis. For the PTV, the values of mean, minimum and maximum doses with V90%, V95%, V107% and V110% (the volumes receiving at least 90%, 95%, 107% or 110% of the prescribed dose) were reported.
The homogeneity of the dose distribution was measured by:
Minimum dose received by 2% and 98% of the PTV (D2% and D98%) served as the maximum and the minimum doses, respectively. Therefore, a lower HI is indicative of a more homogeneous dose distribution across the PTV.
A conformity index (CI) was defined as:
V100N and V100PTV signify the volume of normal tissue outside the PTV and the volume of the PTV receiving 100% of the prescribed dose, respectively.
The CI measures the degree of isodose conformity to the PTV, and it is crucial in the treatment efficiency. A plan with a lower CI value was more conformal.
For OARs, the analysis included the mean dose, the maximum dose and the minimum dose and a set of V × Gy (OAR volume receiving at least ×Gy) such as V5, V10, V20, V30 and V40 for the lung and heart along with CLD and MHD for the lung and heart, respectively.
Statistical analysis
As the data were non-parametric, the Kruskal–Wallis one-way analysis of variance was used to compare dosimetric differences among the plans. For pairwise comparisons among the five techniques, a Bonferroni correction was applied and an adjusted p-value of 0.0125 was considered to be significant to meet the overall significance level of <0.05. A less rigid test would be to report uncorrected p-values, i.e. considering a p-value of <0.05 as significant. This would account for significant p-values for few a dosimetric parameters that are of borderline significance with the correction applied. However, as multiple pairwise comparisons were made, the final results have been presented with the corrected p-values. Other techniques were compared with conventional techniques as standard technique with Mann–Whitney U tests. All statistical tests were performed using SPSS® statistics software v. 22.0.0.0 (SPSS Inc., Chicago, IL).
RESULTS
The mean volume of PTV was 486.72 cm3 (range, 389.50–607.00 cm3) and 562.04 cc (range, 930.1–313.2 cm3) for the right and left PTVs, respectively. The mean volume of PTV was 596.77 cm3 (range, 453.7–930.1 cm3) and 451.99 cm3 (range, 313.2–597.3 cm3) for the breast conservation side and post-mastectomy side. The mean value of PTV for TB boost was 115.31 cc (range, 77.9–149.9 cm3).
The mean volume of the total lung, right lung, left lung and heart were 1808.44 cm3 (range, 1388.0–2456.3 cm3), 1006.53 cm3 (range, 813.7–1349.2 cm3), 807.43 cm3 (range, 569.3–1115.3 cm3) and 468.02 cm3 (range, 317.9–693.1 cm3), respectively.
Analysis of treatment parameters for whole breast/chest wall radiation compared with conventional technique
Planning target volume coverage
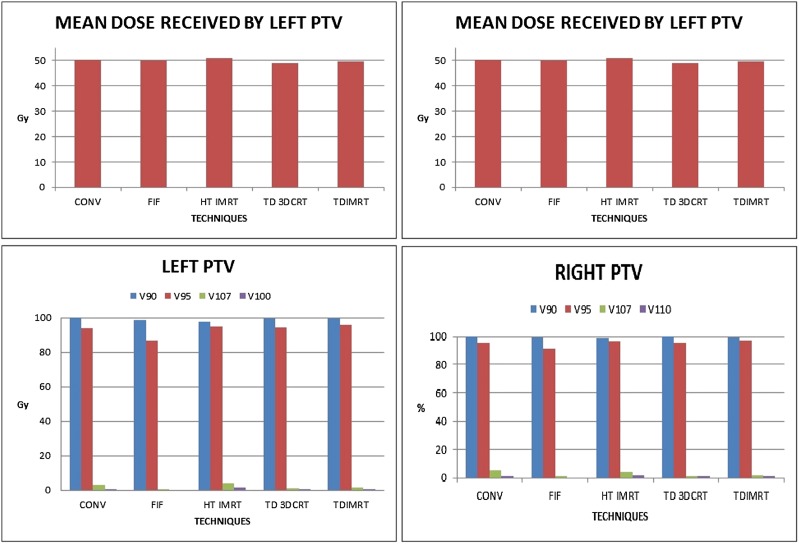
PTV coverage on both sides with 95% isodose was slightly better with tomotherapy techniques though with marginal decrease in coverage with 90% isodose. FIF technique reduced hot spot (volume receiving >107% of prescribed dose) in the PTV on both sides while tomotherapy reduced the same for right-sided PTV only. When the entire body is concerned, volume of hot spot was markedly reduced with FIF technique (1.58 vs 47.43 cm3; p < 0.01) as expected and reduced marginally with HT-IMRT technique (37.54 vs 47.43 cm3; p = non-significant). However, the TD-3DCRT plans not only resulted in higher volume of hotspot within the body but also lower homogeneity index (10.51 for left PTV and 9.37 for right PTV). CI for HT-IMRT (1.27) was better than for all other techniques (Tables 1 and 2, Figures 2 and 3).
Table 1.
Dosimetric parameters for TREAT planning target volume (PTV) coverage (without boost)
| Variable | Technique |
||||
|---|---|---|---|---|---|
| Conv | FIF-IMRT | HT-IMRT | TD-3DCRT | TD-IMRT | |
| Left PTV | |||||
| Max. (Gy) | 54.44 (0.90) | 53.13 (0.46) | 55.13 (2.87) | 57.84 (2.05) | 59.78 (3.03) |
| Min. (Gy) | 44.32 (2.04) | 39.48 (8.21) | 37.06 (4.38) | 33.23 (4.62) | 32.09 (6.61) |
| Mean (Gy) | 50.17 (0.60) | 49.77 (0.65) | 50.99 (0.78) | 48.77 (0.21) | 49.52 (0.64) |
| V90 (%) | 99.92 (0.11) | 98.77 (0.83) | 97.86 (2.05) | 99.82 (0.15) | 99.65 (0.29) |
| V95 (%) | 93.69 (6.78) | 92.23 (3.30) | 94.86 (3.75) | 94.46 (2.75) | 95.93 (3.21) |
| V107 (%) | 3.05 (3.06) | 0.17 (0.43) | 1.10 (1.43) | 1.20 (0.59) | 1.69 (1.14) |
| V110 (%) | 0.03 (0.78) | 0.00 (0.12) | 0.42 (0.13) | 0.43 (0.30) | 0.66(0.57) |
| HI | 12.98 (2.70) | 13.58 (5.16) | 15.49 (7.74) | 10.51 (2.29) | 10.32 (2.47) |
| Right PTV | |||||
| Max. (Gy) | 55.10 (0.72) | 53.17 (0.55) | 54.90 (2.85) | 57.94 (1.87) | 58.89 (2.56) |
| Min. (Gy) | 45.56 (3.28) | 40.37 (8.33) | 38.20 (3.45) | 36.01 (7.32) | 30.21 (8.96) |
| Mean (Gy) | 50.72 (0.71) | 49.88 (0.65) | 51.07 (0.90) | 48.70 (0.29) | 48.53 (3.43) |
| V90 (%) | 99.98 (0.01) | 99.40 (1.55) | 98.67 (1.36) | 99.88 (0.87) | 99.74 (0.20) |
| V95 (%) | 95.32 (6.14) | 94.20 (3.13) | 96.35 (2.85) | 95.03 (3.37) | 96.99 (1.95) |
| V107 (%) | 5.06 (4.10) | 0.15 (0.43) | 0.64 (1.10) | 0.97 (0.81) | 1.56 (1.67) |
| V110 (%) | 0.24 (0.34) | 0.00 (0.00) | 0.04 (0.11) | 0.21 (0.16) | 0.51 (0.45) |
| HI | 12.26 (2.26) | 13.46 (4.58) | 13.77 (6.53) | 9.37 (2.49) | 10.1 (2.35) |
| Conformity index | 1.36 (0.15) | 1.55 (0.42) | 1.27 (0.38) | 5.24 (2.46) | 2.11 (0.74) |
| Volume of body receiving 107% of prescribed dose (cm3) | 47.44 (28.79) | 1.58 (1.75) | 37.54 (100.59) | 80.38 (39.81) | 48.59 (38.30) |
3DCRT, three-dimensional conformal radiotherapy; conv., conventional; FIF, field in field; HI, homogeneity index; HT, helical tomotherapy; IMRT, intensity-modulated radiotherapy; max., maximum; min., minimum; SIB, simultaneous integrated boost; TD, TomoDirect™ (Accuray Inc., Sunnyvale, CA); TREAT PTV, term for volume common to reference volume and PTV; Vx, target volume receiving at least X% of prescribed dose.
The values in the table are expressed as mean (standard deviation).
Table 2.
Significant p-values using Mann–Whitney U test [comparing planning target volume (PTV) TREAT coverage parameters as given in Table 1]
| Variables | Comparisons |
|||
|---|---|---|---|---|
| Conv vs FIF | Conv vs HT-IMRT | Conv vs TD-3DCRT | Conv vs TD-IMRT | |
| Left PTV | ||||
| Mean | 0.019 | 0.035 | 0.000 | 0.023 |
| V90 | 0.023 | 0.000 | 0.089 | 0.002 |
| V107 | 0.009 | 0.218 | 0.529 | 0.631 |
| V110 | 0.481 | 0.529 | 0.001 | 0.001 |
| HI | 0.971 | 0.631 | 0.043 | 0.035 |
| Right PTV | ||||
| Mean | 0.001 | 0.280 | 0.000 | 0.001 |
| V90 | 0.035 | 0.004 | 0.000 | 0.000 |
| V107 | 0.000 | 0.000 | 0.004 | 0.023 |
| V110 | 0.063 | 0.193 | 0.529 | 0.089 |
| HI | 0.971 | 0.971 | 0.002 | 0.029 |
| Conformity index | 0.353 | 0.005 | 0.000 | 0.015 |
| Volume of body receiving >107% of prescribed dose | 0.001 | 0.015 | 0.063 | 0.863 |
3DCRT, three-dimensional conformal radiotherapy; conv, conventional; FIF, field in field; HI, homogeneity index; HT, helical tomotherapy; IMRT, intensity-modulated radiotherapy; TD, TomoDirect™; Vx, target volume receiving at least X% of prescribed dose.
Significant p-values are shown in bold.
Figure 3.
Dose received by TREAT planning target volume (PTV). 3DCRT, three-dimensional conformal radiotherapy; conv, conventional; FIF, field in field; HT, helical tomotherapy; IMRT, intensity-modulated radiotherapy; SIB, simultaneous integrated boost; TD, TomoDirect™ (Accuray Inc., Sunnyvale, CA); TREAT PTV, term for volume common to reference volume and PTV; Vx, volume receiving at least x% of prescribed dose.
Organ at risk sparing (total lung and heart)
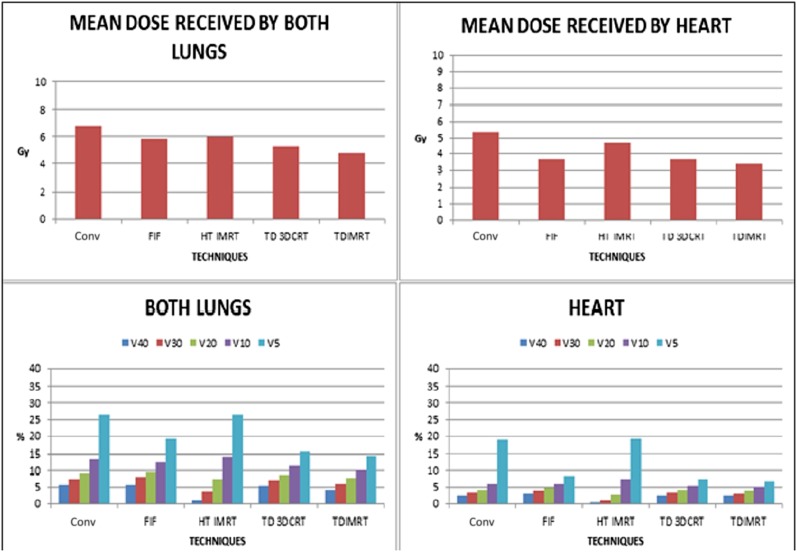
On comparing doses to OARs, not only were higher dose volumes significantly less with HT-IMRT but HT-IMRT also had comparable low dose volumes. The V40 and V30 for the total lung was reduced from 5.51% to 0.97% (p < 0.01) and from 7.36% to 3.67% (p < 0.01), respectively. Similarly, for the heart, V40 was reduced from 2.39% to 0.20% (p < 0.01) (Tables 3 and 4, Figure 4).
Table 3.
Dosimetric parameters for organ at risk sparing (without boost)
| Variable | Technique |
||||
|---|---|---|---|---|---|
| Conv | FIF | HT-IMRT | TD-3DCRT | TD-IMRT | |
| Heart | |||||
| Max. (Gy) | 49.99 (1.57) | 47.09 (6.01) | 42.88 (6.61) | 50.19 (12.13) | 48.11 (13.50) |
| Min. (Gy) | 2.51 (2.60) | 0.61 (0.21) | 1.57 (0.32) | 0.78 (0.12) | 0.70 (0.11) |
| Mean (Gy) | 5.33 (1.67) | 3.67 (2.59) | 4.68 (0.96) | 3.65 (1.96) | 3.38 (2.04) |
| V40 | 2.39 (2.95) | 2.99 (4.62) | 0.20 (0.32) | 2.49 (2.89) | 2.31 (3.18) |
| V30 | 3.25 (3.37) | 3.84 (5.25) | 1.04 (1.10) | 3.27 (3.36) | 3.08 (3.71) |
| V20 | 4.15 (3.75) | 4.65 (5.74) | 2.77 (1.90) | 4.10 (3.86) | 3.93 (4.26) |
| V10 | 5.79 (4.36) | 5.89 (6.35) | 7.44 (3.57) | 5.41 (4.68) | 5.16 (5.07) |
| V5 | 19.04 (8.51) | 8.17 (7.21) | 19.33 (7.24) | 7.25 (5.90) | 6.81 (6.04) |
| Total lung | |||||
| Max. (Gy) | 51.24 (1.23) | 49.68 (1.5) | 50.16 (2.07) | 53.35 (1.49) | 52.72 (1.26) |
| Min. (Gy) | 0.391 (0.30) | 0.17 (0.13) | 0.93 (0.348) | 0.38 (0.89) | 0.35 (0.07) |
| Mean (Gy) | 6.75 (1.21) | 5.86 (1.81) | 5.99 (0.981) | 5.30 (0.76) | 4.76 (1.06) |
| V40 | 5.51 (1.87) | 5.73 (2.97) | 0.97 (0.72) | 5.18 (1.19) | 4.17 (1.75) |
| V30 | 7.36 (2.02) | 7.98 (3.35) | 3.67 (1.18) | 6.93 (1.33) | 5.91 (1.91) |
| V20 | 9.03 (2.23) | 9.63 (3.66) | 7.25 (1.65) | 8.57 (1.42) | 7.56 (2.02) |
| V10 | 13.52 (3.28) | 12.64 (4.36) | 14.13 (2.44) | 11.33 (1.69) | 10.28 (2.38) |
| V5 | 26.55 (6.93) | 19.09 (6.11) | 26.37 (7.70) | 15.80 (2.20) | 14.34 (2.94) |
3DCRT, three-dimensional conformal radiotherapy; conv, conventional; FIF, field in field; HT, helical tomotherapy; IMRT, intensity-modulated radiotherapy; max., maximum; min., minimum; TD, TomoDirect™ (Accuray Inc., Sunnyvale, CA); Vx, volume of OAR receiving at least x Gy.
The values in the table are expressed as mean (standard deviation).
Table 4.
Significant p-values using Mann–Whitney U test (comparing parameters for organ at risk sparing as given in Table 3)
| Variables | Comparisons |
|||
|---|---|---|---|---|
| Conv vs FIF | Conv vs HT-IMRT | Conv vs TD-3DCRT | Conv vs TD-IMRT | |
| Total lung | ||||
| Mean | 0.280 | 0.190 | 0.007 | 0.001 |
| V40 | 0.853 | 0.000 | 0.684 | 0.105 |
| V30 | 1.000 | 0.000 | 0.631 | 0.218 |
| V10 | 0.631 | 0.579 | 0.089 | 0.035 |
| V5 | 0.029 | 0.684 | 0.000 | 0.000 |
| Heart | ||||
| Mean | 0.007 | 0.579 | 0.029 | 0.004 |
| V40 | 0.631 | 0.000 | 0.793 | 0.853 |
| V5 | 0.005 | 0.853 | 0.003 | 0.002 |
3DCRT, three-dimensional conformal radiotherapy; conv, conventional; FIF, field in field; HT, helical tomotherapy; IMRT, intensity-modulated radiotherapy; TD, TomoDirect™ (Accuray Inc., Sunnyvale, CA); Vx, volume of OAR receiving at least x Gy.
Significant p-values are shown in bold.
Figure 4.
Dose received by lungs and heart (comparing plans without boost). 3DCRT, three-dimensional conformal radiotherapy; conv, conventional; FIF, field in field; HT, helical tomotherapy; IMRT, intensity-modulated radiotherapy; TD, TomoDirect™ (Accuray Inc., Sunnyvale, CA).
Low dose volume (V5) was significantly reduced using TD-3DCRT and TD-IMRT, both for the total lung as well as the heart (p < 0.01 for both total lung and heart). A similar finding is reflected in the mean doses of the total lung and heart with TD-IMRT (p < 0.01) but only for the total lung with TD-3DCRT (p < 0.01). However, FIF technique reduced the mean dose to the heart only, by lowering the V5 volume (p < 0.01 for both V5 and mean heart dose). Overall, TD-IMRT seemed to be both pulmonary and cardiac sparing for bilateral irradiation of the breast/chest wall alone, whereas FIF-IMRT seemed to be only cardiac sparing.
Analysis of treatment parameters for adjuvant whole breast with boost compared with conventional technique
Planning target volume coverage
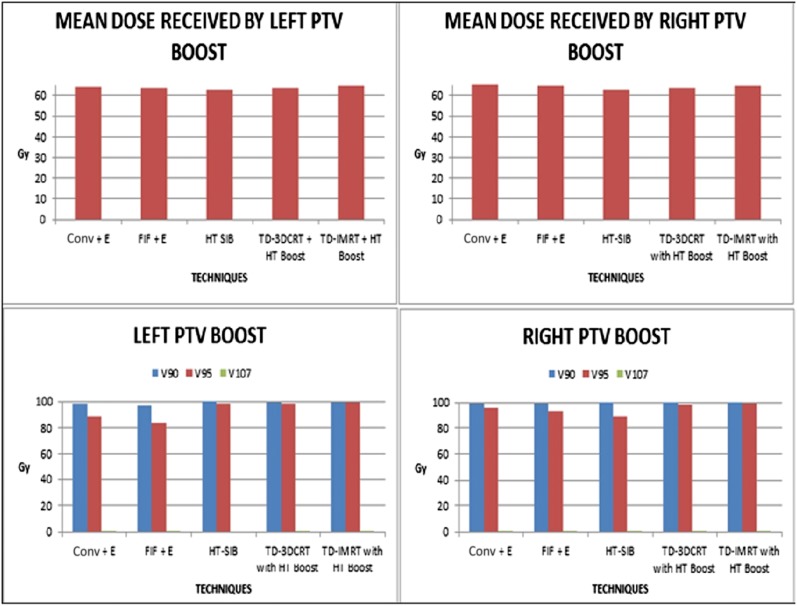
All the three techniques (sequential electron boost with bitangential or FIF technique, sequential HT boost with TD and SIB using HT) of delivering boost to the TB were comparable with each other in terms of PTV coverage (Tables 5 and 6, Figure 5).
Table 5.
Dosimetric parameters for planning target volume (PTV) boost coverage (with boost)
| Variable | Technique |
||||
|---|---|---|---|---|---|
| Conv + E | FIF + E | HT-SIB | TD-3DCRT with HT Boost | TD-IMRT with HT Boost | |
| Left PTV | |||||
| Max. (Gy) | 69.16 (2.15) | 68.79 (2.17) |
64.73 (0.49) | 68.45 (2.87) | 68.89 (2.25) |
| Min. (Gy) | 48.41 (16.93) | 47.50 (18.33) |
54.23 (2.73) | 49.76 (20.85) | 45.21 (19.88) |
| Mean (Gy) | 64.33 (0.83) | 63.61 (1.17) |
62.45 (0.76) | 63.53 (0.36) | 64.48 (0.51) |
| V90 (%) | 98.72 (1.32) | 98.00 (2.13) |
99.79 (0.30) | 99.52 (0.95) | 99.52 (0.95) |
| V95 (%) | 89.59 (3.13) | 91.10 (4.54) |
98.93 (0.92) | 98.33 (1.75) | 99.34 (1.03) |
| V107 (%) | 0.01 (0.02) | 0.15 (0.30) |
0.00 (0.00) | 0.52 (1.05) | 0.27 (0.54) |
| V110 (%) | 0.00 (0.00) | 0.00 (0.00) |
0.00 (0.00) | 0.10 (0.20) | 0.02 (0.03) |
| Right PTV | |||||
| Max. (Gy) | 69.62 (0.89) | 69.00 (1.39) |
64.66 (0.98) | 67.94 (2.99) | 68.02 (1.67) |
| Min. (Gy) | 52.19 (16.27) | 51.19 (15.68) |
52.39 (5.28) | 56.18 (4.77) | 56.28 (5.36) |
| Mean (Gy) | 65.13 (1.12) | 64.65 (0.74) |
62.41 (0.63) | 63.60 (0.26) | 64.46 (0.42) |
| V90 (%) | 99.59 (0.64) | 99.27 (0.97) |
99.78 (0.23) | 99.13 (0.16) | 99.79 (0.28) |
| V95 (%) | 96.47 (3.70) | 93.86 (4.70) |
98.16 (1.78) | 98.87 (0.24) | 99.20 (0.55) |
| V107 (%) | 0.02 (0.04) | 0.002 (0.004) |
0.12 (0.28) | 0.24 (0.48) | 0.16 (0.41) |
| V110 (%) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.08 (0.20) | 0.00 (0.00) |
3DCRT, three-dimensional conformal radiotherapy; conv, conventional; E, electron boost to tumour bed; FIF, field in field; HT, helical tomotherapy; IMRT, intensity-modulated radiotherapy; max., maximum; min., minimum; SIB, simultaneous integrated boost; TD, TomoDirect™ (Accuray Inc., Sunnnyvale, CA); Vx, target volume receiving at least X% of prescription dose.
The values in the table are expressed as mean (standard deviation).
Table 6.
Significant p-values using Mann–Whitney U test [comparing planning target volume (PTV) boost coverage parameters as given in Table 5]
| Variables | Techniques |
|||
|---|---|---|---|---|
| Conv + E vs FIF + E | Conv + E vs HT-SIB | Conv + E vs TD-3DCRT with HT Boost | Conv vs TD-IMRT with HT Boost | |
| Left PTV | ||||
| Mean | 0.486 | 0.029 | 0.114 | 0.686 |
| V95 | 1.000 | 0.029 | 0.029 | 0.029 |
| Right PTV | ||||
| Mean | 0.485 | 0.002 | 0.026 | 0.310 |
3DCRT, three-dimensional conformal radiotherapy; conv, conventional; E, electron boost to tumour bed; FIF, field in field; HT, helical tomotherapy; IMRT, intensity-modulated radiotherapy; SIB, simultaneous integrated boost; TD, TomoDirect™ (Accuray Inc., Sunnnyvale, CA); V95, target volume receiving at least 95% prescription dose.
The significant p-value is shown in bold.
Figure 5.
Dose received by planning target volume (PTV) boost. 3DCRT, three-dimensional conformal radiotherapy; conv, conventional; E, electron boost to tumour bed; FIF, field in field; HT, helical tomotherapy; IMRT, intensity-modulated radiotherapy; SIB, simultaneous integrated boost; TD, TomoDirect™ (Accuray Inc., Sunnyvale, CA).
Organ at risk sparing (total lung and heart)
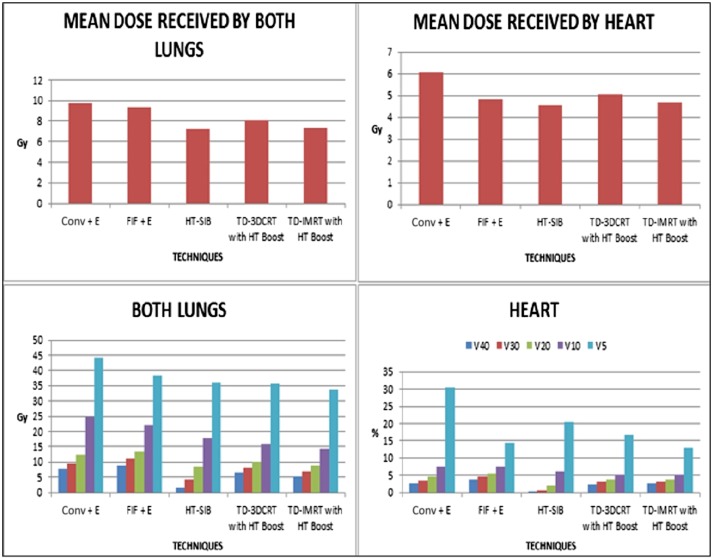
On comparing OAR sparing, results similar to that seen with the whole breast and/or chest wall irradiation alone were noted. HT with SIB was significantly better for high dose volume with comparable low dose volume, especially for the total lung. On the other hand, TD-3DCRT and TD-IMRT with HT boost were significantly better for low dose volumes (Tables 7 and 8, Figure 6).
Table 7.
Dosimetric parameters for organs at risk sparing (with boost)
| Variable | Technique |
||||
|---|---|---|---|---|---|
| Conv + E | FIF + E | HT-SIB | TD-3DCRT with HT boost | TD-IMRT with HT boost | |
| Heart | |||||
| Max. (Gy) | 51.35 (3.25) | 47.59 (8.47) | 36.97 (12.06) | 53.01 (7.45) | 46.07 (16.68) |
| Min. (Gy) | 1.97 (0.34) | 0.79 (0.42) | 1.67 (0.36) | 1.43 (0.50) | 1.34 (0.47) |
| Mean (Gy) | 6.07 (1.87) | 4.83 (3.17) | 4.56 (1.07) | 5.06 (2.39) | 4.70 (2.67) |
| V40 | 2.62 (3.84) | 3.74 (5.94) | 0.13 (0.24) | 2.42 (3.80) | 2.46 (4.31) |
| V30 | 3.50 (4.41) | 4.59 (6.85) | 0.73 (1.12) | 3.07 (4.38) | 3.10 (4.97) |
| V20 | 4.47 (4.90) | 5.46 (6.52) | 1.90 (2.40) | 3.76 (4.89) | 3.89 (5.71) |
| V10 | 7.40 (5.25) | 7.41 (8.13) | 5.95 (3.99) | 5.05 (5.78) | 5.16 (6.66) |
| V5 | 30.51 (6.35) | 14.25 (8.78) | 20.33 (6.57) | 16.76 (7.17) | 12.82 (6.66) |
| Total lung | |||||
| Max. (Gy) | 63.09 (3.95) | 62.40 (4.06) | 57.40(3.83) | 64.92 (3.63) | 64.20 (2.48) |
| Min. (Gy) | 0.59 (0.29) | 0.30 (0.16) | 0.83 (0.24) | 0.55 (0.10) | 0.50 (0.09) |
| Mean (Gy) | 9.76 (1.26) | 9.34 (1.34) | 7.24 (0.91) | 8.07 (0.56) | 7.36 (0.96) |
| V40 | 7.73 (1.22) | 8.94 (2.19) | 1.59 (0.88) | 6.43 (1.26) | 5.17 (2.12) |
| V30 | 9.53 (1.22) | 10.98 (2.56) | 4.37 (1.29) | 8.07 (1.31) | 6.80 (2.26) |
| V20 | 12.36 (2.06) | 13.32 (2.89) | 8.38 (1.70) | 10.05 (1.30) | 8.67 (2.29) |
| V10 | 24.60 (5.80) | 22.25 (4.47) | 18.02 (3.51) | 15.97 (1.58) | 14.40 (2.19) |
| V5 | 44.15 (7.97) | 38.48 (6.74) | 36.00 (5.03) | 35.89 (4.74) | 33.91 (4.13) |
3DCRT, three-dimensional conformal radiotherapy; conv, conventional; E, electron boost to tumour bed; FIF, field in field; HT, helical tomotherapy; IMRT, intensity-modulated radiotherapy; max., maximum; min., minimum; SIB, simultaneous integrated boost; TD, TomoDirect™ (Accuray Inc., Sunnyvale, CA); Vx, volume of OAR receiving at least x Gy.
The values in the table are expressed as mean (standard deviation).
Table 8.
Significant p-values using Mann–Whitney U test (comparing parameters for organs at risk sparing as given in Table 7)
| Variables/comparisons | Comparisons |
|||
|---|---|---|---|---|
| Conv vs FIF | Conv vs HT-IMRT | Conv vs TD-3DCRT | Conv vs TD-IMRT | |
| Total lung | ||||
| Mean | 1.000 | 0.004 | 0.009 | 0.004 |
| V40 | 0.394 | 0.002 | 0.240 | 0.093 |
| V30 | 0.310 | 0.002 | 0.132 | 0.065 |
| V20 | 0.286 | 0.004 | 0.041 | 0.015 |
| V10 | 0.699 | 0.041 | 0.002 | 0.002 |
| Heart | ||||
| V5 | 0.009 | 0.015 | 0.015 | 0.009 |
3DCRT, three-dimensional conformal radiotherapy; conv, conventional; FIF, field in field; HT, helical tomotherapy; IMRT, intensity-modulated radiotherapy; TD, TomoDirect™ (Accuray Inc., Sunnyvale, CA); Vx, volume of OAR receiving at least x Gy.
Significant p-values are shown in bold.
Figure 6.
Dose received by lungs and heart (comparing plans with boost). 3DCRT, three-dimensional conformal radiotherapy; conv, conventional; FIF, field in field; HT, helical tomotherapy; IMRT, intensity-modulated radiotherapy; SIB, simultaneous integrated boost; TD, TomoDirect™ (Accuray Inc., Sunnyvale, CA).
For HT-SIB, on comparison with conventional techniques, the percentage decrease in heart dose was 1915.38%, 379.45%, 135.26%, 24.36% and 50.07% for V40, V30, V20, V10 and V5, respectively. Similarly, the decrease in lung dose was 386.16%, 118.07%, 47.49%, 36.51% and 22.63%, respectively, for V40, V30, V20, V10 and V5. Similar changes were also seen with other techniques but not as marked as that seen in HT-SIB. Overall, HT-SIB seemed to be the best cardiac- and pulmonary-sparing technique in the setting of SBBC.
DISCUSSION
Although breast cancer is one of the common malignancies, synchronous presentation is rare and accounts for only 0.4–2.8% of all newly diagnosed breast cancer cases. Unlike treatment of unilateral breast cancer, RT planning and treatment of SBBC is challenging. The goal of this pilot study was to assess the dosimetric feasibility and the pros and cons of various RT techniques for SBBC, including FIF-IMRT, HT and TD (both 3DCRT mode and IMRT mode) in comparison with the conventional bitangential technique.
Although the Radiation Therapy Oncology group34 and the Danish group35 have published guidelines for delineating the whole breast or chest wall as well as the TB, these guidelines are not used universally. Clinical marking to define borders of RT portals is the most commonly used method.36,37 In the present study, we also used a similar method for defining target volume, which was subsequently modified using isodose volume (95% isodose volume of conventional treatment plan) as described earlier. This method has also been explored by others to maintain similarity in target volume when comparing modern techniques with conventional methods.38,39 Similar problems were also encountered while delineating TB.40 The margins given to grow the CTV for TB is not standardized, which can be one of the reasons for the large boost volume seen in the present study, apart from the large volume of TB itself.37 In one study,37 only a 5-mm margin was given to create CTV TB. In another study,41 the average boost volume was 36 cm3, which is less than one-third of that in the present study. Hence, the heterogeneity of target volume delineation has to be kept in mind while comparing the dosimetric results of different techniques across all studies.
FIF-IMRT is routinely used to reduce the volume of hotspots (volume receiving >107% of the prescription dose). This was also confirmed in the present study as the mean value of the body receiving >107% of the prescribed dose was only 1.58 cm3 with FIF compared with 47.44 cm3 with conventional technique with equivalent coverage of TREAT PTV. Moreover, 107% isodose volume with FIF was also much lower than with the other techniques (HT-IMRT, 37.54 cm3; TD-3DCRT, 80.38 cm3; TD-IMRT, 48.59 cm3). Considering OAR sparing, mean doses to the lungs and heart were less than those achieved with conventional techniques, although not statistically significant for the total lung (5.86 vs 6.75 Gy; p-value not significant, and 3.67 vs 5.33 Gy; p < 0.01). Thus, FIF-IMRT was cardiac sparing but not lung sparing. These results were similar to some other reports in the literature for unilateral breast cancer. In one study of 30 females treated for adjuvant whole breast irradiation, the PTV coverage with FIF was 96.7% (vs 93.2% of the present study), but the mean lung and heart doses were 8.42% and 3.07% (vs 5.73% and 3.67% of the present study).36 Similarly, Pili et al42 have shown improved OAR sparing with the use of FIF in unilateral breast cancer treatment, and a similar trend was also seen in the present study.
HT is advantageous in terms of better coverage of PTV and OAR sparing across most of the sites.43 This potential of HT was explored for SBBC and consistent results were seen. The mean dose to PTV and V95 coverage was comparable with the conventional plan with significantly better CI (1.27 vs 1.36). Major advantage was noted in decrease in high dose volumes (V40 and V30) in OARs without increase in low dose volumes to OARs with HT-IMRT (for both plans with and without SIB). However, the major dosimetric advantage that was reflected in a significant reduction in MLD (and non-significant reduction in mean heart dose) was observed for plans with SIB. This finding defines a sound position of HT for adjuvant breast radiation for SBBC. Moreover, considering the concern of second cancers owing to low dose spill, it was also reassuring to observe that the dosimetric advantages of HT were achieved without increase in low dose spill. In the literature, the role of HT in SBBC is sparse. Few case reports suggested a possible role of HT in BBC owing to complex target volume but with contrasting results. In one case report, lung doses (V20 > 5% and V5 > 20%) were much less than those in the present study (V20, 7.25% and V5, 26.37%)44, but another report suggested V5 as high as 87% where SIB plan was used for treatment.45
The role of HT is explored much more in unilateral breast cancer. Although indirect data are available from the dosimetric studies carried out for unilateral breast cancer, the results from this study are slightly different from a few of the studies reported earlier.46,47 Schubert et al47 in a similar dosimetric comparison for left-sided whole breast irradiation (without boost) not only reported higher low dose spillage for the ipsilateral lung and heart but also higher mean doses to the contralateral lung and breast. In the present study, dosimetric advantages of HT have been achieved without an increase in the low dose spill both for whole breast irradiation alone as well as for plans with SIB. As both sides are being treated, the only OARs in case of SBBC are the total lung and heart. Hence, tomotherapy planning for SBBC becomes somewhat simpler compared with unilateral cases where the contralateral lung and breast also have to be optimized. Moreover, the low dose spill is of particularly concern in left-sided disease, as achieving heart constraints receives more priority. The present data are consistent with the large volume of studies examining the role of HT in unilateral breast cancer.
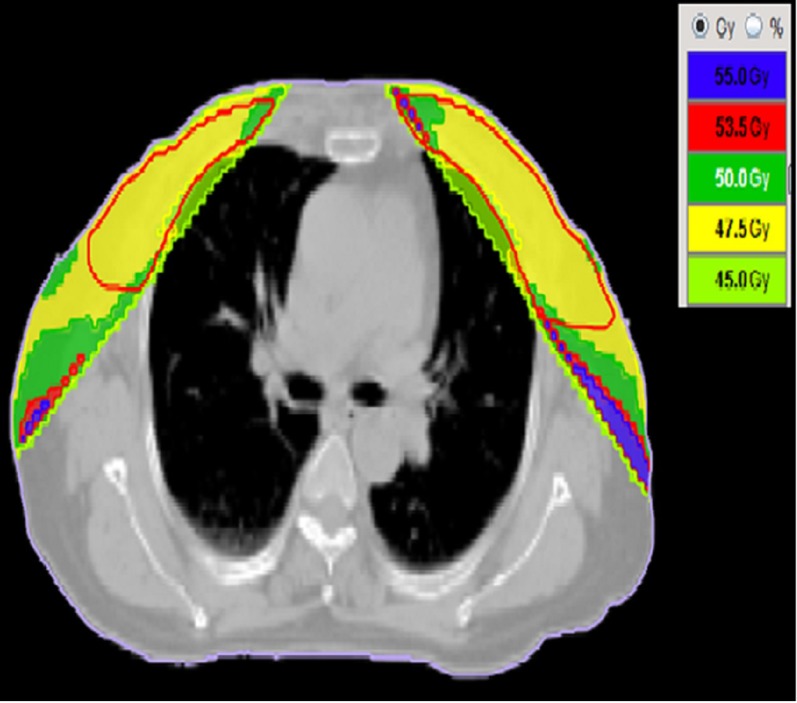
In the present study, TD has been evaluated probably for the first time for treatment of SBBC. With multiple limitations encountered in planning of TD, it cannot be concluded that TD can be safely used in treatment of SBBC. One such limitation is the limited number (i.e. 12) of fields that can be used for planning, especially when the supraclavicular fossa is also included in the treatment volume. This also limits the number of fields that can be used for delivering SIB while using TD-IMRT. In the present study, TD-3DCRT and TD-IMRT were comparable to conventional techniques in PTV coverage but a high volume of hotspots in PTV or outside PTV was seen, mainly with TD-3DCRT (Figure 7). This was one reason for significantly worse CI with TD-3DCRT technique. Compared with HT-IMRT, the plans were more homogeneous than the TD one. This is likely owing to the availability of a greater number of possible irradiation angles in the helical plans. The use of fixed gantry angles allows less shaping of the prescription dose to the target volume. The major advantage of TD was seen with sparing of the heart and lung tissue. It reduced low dose volume significantly. For the total lung, mean dose was significantly reduced from 6.75 Gy for conventional technique to 5.30 Gy for TD-3DCRT and 4.76 Gy for TD-IMRT (without SIB plans). A similar decline was seen for all volumetric parameters, but more marked for V5 and V10. The mean dose to the heart was also significantly reduced from 5.33 Gy for conventional technique to 3.65 Gy for TD-3DCRT and 3.38 Gy for TD-IMRT technique (without SIB plans). As seen in the lung, a statistically significant difference was also seen for heart volume receiving low doses such as V5 and V10. However, for plans with SIB, non-significant reduction in OAR doses was observed with both the techniques. Trends similar to those in the present study were seen by Schubert et al47 while comparing 3DCRT technique with forward planning IMRT, inverse planning IMRT, HT-IMRT and TD-IMRT.
Figure 7.
Hot spot with TomoDirect™ (Accuray Inc., Sunnyvale, CA) three-dimensional conformal radiotherapy (strip of high dose volume seen at lateral end outside planning target volume). Dose volumes are given with reference to prescription dose. 55 Gy, i.e. 110% of 50 Gy prescription dose; 53.5 Gy, i.e. 107%; 47.5 Gy, i.e. 95% of 50 Gy prescription dose.
Here, three different methods of delivering boost to TB were evaluated in combination with whole breast irradiation. It can be safely concluded that the most effective method of delivering TB boost was using SIB with HT. HT-SIB had better OAR sparing than did the other methods (sequential boost with electron or HT) compared here. A part of it is because of lower dose in HT-SIB (61 Gy in HT-SIB plan vs 65 Gy in other plans). It should be noted that plans using TD-IMRT with dynamic jaw, i.e. TomoEDGE™ (TomoTherapy Inc.), were not considered in this study. Low physical dose (61 Gy with HT-SIB vs 65 Gy for other methods) may also contribute to the decrease in dosimetric parameters for OAR but still cannot completely explain the approximately 90% reduction in V40 doses of the heart and lung when compared with conventional techniques with electron boost. Similar trends were obtained by Hijal et al48 comparing HT-SIB with conventional 3DCRT. Another study by Franco et al49 with TD-3DCRT combined with HT boost confirms similar results. It is less pronounced with conventional plans and sequential electron boost compared with sequential HT boost after TD-3DCRT or TD-IMRT. Here, the decrease in low dose volume to the OAR is more evident than the high dose volume, owing to similar results seen with whole breast RT with TD-3DCRT or TD-IMRT.
Limitations of study
This is a feasibility study carried out on data sets of a heterogeneous patient population of 10 females with SBBC (4 with breast conserved on both side, 4 with post mastectomy on both sides, and 2 with breast conserved on one side and mastectomy on another side); results of this study should be validated by a study with larger sample size and a more homogenous population (only chest wall or SIB alone). In the present study, in order to keep target volumes simpler, irradiation of supraclavicular fossa with conventional plans or tomotherapy was not considered. This is a dosimetric study with no clinical outcome data so the result of this study need to be validated with clinical outcomes. In these patients, the average interfield separation of 22.3 and 22.4 cm for the left side and right side, respectively. This may not be ideal for treatment using 6-MV photons and would contribute to the high volume hotspots as noted in this. One must also acknowledge nuances of inverse planning IMRT for treatment of breast cancer. It leads to an increase in low dose spill over to non-target tissue. Increase in treatment time for delivery may also introduce uncertainty owing to intrafraction movement. Electron boost to TB was planned on the same CT data set taken for whole breast RT in the supine position, which may lead to the use of erroneously high electron energy for target coverage leading to high doses to OARs (although precaution was taken to angulate the gantry perpendicular to the skin surface). Moreover, not all methods of delivering TB boost were explored in this study.
CONCLUSION
RT to bilateral breast or bilateral chest wall is a challenging task in view of large and complex target volume and significant doses that are received by the heart and lungs. The findings of this study suggest that while it is possible to produce acceptable coverage of PTV with 95% isodose using conventional techniques, the radiation doses to the heart and lung is still significant.
This dosimetric study of different techniques demonstrates the ability of these modern techniques to reduce radiation doses, especially volume of the lung and heart receiving high dose of radiation without compromising PTV coverage (specifically HT delivering SIB) in the setting of SBBC.
This study requires further confirmation of dosimetric results in larger number of patients as well as clinical validation to assess clinical feasibility of delivering treatment, and associated acute and late toxicity.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136: 359–86. [DOI] [PubMed] [Google Scholar]
- 2.Michowitz M, Noy S, Lazebnik N, Aladjem D. Bilateral breast cancer. J Surg Oncol 1985; 30: 109–12. [DOI] [PubMed] [Google Scholar]
- 3.Gogas J, Markopoulos C, Skandalakis P, Gogas H. Bilateral breast cancer. Am Surg 1993; 59: 733–5. [PubMed] [Google Scholar]
- 4.Jobsen JJ, van der Palen J, Ong F, Meerwaldt JH. Synchronous, bilateral breast cancer: prognostic value and incidence. Breast 2003; 12: 83–8. [DOI] [PubMed] [Google Scholar]
- 5.Mueller CB, Ames F. Bilateral carcinoma of the breast: frequency and mortality. Can J Surg 1978; 21: 459–65. [PubMed] [Google Scholar]
- 6.Schwentner L, Wolters R, Wischnewsky M, Kreienberg R, Wöckel A. Survival of patients with bilateral versus unilateral breast cancer and impact of guideline adherent adjuvant treatment: a multi-centre cohort study of 5292 patients. Breast 2012; 21: 171–7. doi: 10.1016/j.breast.2011.09.007 [DOI] [PubMed] [Google Scholar]
- 7.Carmichael AR, Bendall S, Lockerbie L, Prescott R, Bates T. The long-term outcome of synchronous bilateral breast cancer is worse than metachronous or unilateral tumours. Eur J Surg Oncol 2002; 28: 388–91. [DOI] [PubMed] [Google Scholar]
- 8.Gollamudi SV, Gelman RS, Peiro G, Schneider LJ, Schnitt SJ, Recht A, et al. Breast-conserving therapy for stage I-II synchronous bilateral breast carcinoma. Cancer 1997; 79: 1362–9. [DOI] [PubMed] [Google Scholar]
- 9.Heaton KM, Peoples GE, Singletary SE, Feig BW, Ross MI, Ames FC, et al. Feasibility of breast conservation therapy in metachronous or synchronous bilateral breast cancer. Ann Surg Oncol 1999; 6: 102–8. [DOI] [PubMed] [Google Scholar]
- 10.Fung MC, Schultz DJ, Solin LJ. Early-stage bilateral breast cancer treated with breast-conserving surgery and definitive irradiation: the University of Pennsylvania experience. Int J Radiat Oncol Biol Phys 1997; 38: 959–67. [DOI] [PubMed] [Google Scholar]
- 11.Yamauchi C, Mitsumori M, Nagata Y, Kokubo M, Inamoto T, Mise K, et al. Bilateral breast-conserving therapy for bilateral breast cancer: results and consideration of radiation technique. Breast Cancer 2005; 12: 135–9. [DOI] [PubMed] [Google Scholar]
- 12.Heron DE, Komarnicky LT, Hyslop T, Schwartz GF, Mansfield CM. Bilateral breast carcinoma: risk factors and outcomes for patients with synchronous and metachronous disease. Cancer 2000; 88: 2739–50. [PubMed] [Google Scholar]
- 13.Hernando ML, Marks LB, Bentel GC, Zhou SM, Hollis D, Das SK, et al. Radiation-induced pulmonary toxicity: a dose-volume histogram analysis in 201 patients with lung cancer. Int J Radiat Oncol Biol Phys 2001; 51: 650–9. [DOI] [PubMed] [Google Scholar]
- 14.Kahan Z, Csenki M, Varga Z, Szil E, Cserháti A, Balogh A, et al. The risk of early and late lung sequelae after conformal radiotherapy in breast cancer patients. Int J Radiat Oncol Biol Phys 2007; 68: 673–81. [DOI] [PubMed] [Google Scholar]
- 15.Nishioka A, Ogawa Y, Hamada N, Terashima M, Inomata T, Yoshida S. Analysis of radiation pneumonitis and radiation-induced lung fibrosis in breast cancer patients after breast conservation treatment. Oncol Rep 1999; 6: 513–17. [DOI] [PubMed] [Google Scholar]
- 16.Ooi GC, Kwong DL, Chan KN, Ngan H, Lock DT, Lam WK, et al. Serial HRCT lung changes after 3-field radiation treatment of breast cancer. Clin Radiol 2000; 55: 817–24. [DOI] [PubMed] [Google Scholar]
- 17.Bentzen SM, Skoczylas JZ, Overgaard M, Overgaard J. Radiotherapy-related lung fibrosis enhanced by tamoxifen. J Natl Cancer Inst 1996; 88: 918–22. [DOI] [PubMed] [Google Scholar]
- 18.Wennberg B, Gagliardi G, Sundbom L, Svane G, Lind P. Early response of lung in breast cancer irradiation: radiologic density changes measured by CT and symptomatic radiation pneumonitis. Int J Radiat Oncol Biol Phys 2002; 52: 1196–206. [DOI] [PubMed] [Google Scholar]
- 19.Yu TK, Whitman GJ, Thames HD, Buzdar AU, Strom EA, Perkins GH, et al. Clinically relevant pneumonitis after sequential paclitaxel-based chemotherapy and radiotherapy in breast cancer patients. J Natl Cancer Inst 2004; 96: 1676–81. [DOI] [PubMed] [Google Scholar]
- 20.Lind PA, Wennberg B, Gagliardi G, Rosfors S, Blom-Goldman U, Lideståhl A, et al. ROC curves and evaluation of radiation-induced pulmonary toxicity in breast cancer. Int J Radiat Oncol Biol Phys 2006; 64: 765–70. [DOI] [PubMed] [Google Scholar]
- 21.Bovelli D, Plataniotis G, Roila F; ESMO Guidelines Working Group. Cardiotoxicity of chemotherapeutic agents and radiotherapy-related heart disease: ESMO clinical practice guidelines. Ann Oncol 2010; 21(Suppl. 5): v277–82. doi: 10.1093/annonc/mdq200 [DOI] [PubMed] [Google Scholar]
- 22.Konski A, Li T, Christensen M, Cheng JD, Yu JQ, Crawford K, et al. Symptomatic cardiac toxicity is predicted by dosimetric and patient factors rather than changes in 18F-FDG PET determination of myocardial activity after chemoradiotherapy for esophageal cancer. Radiother Oncol 2012; 104: 72–7. doi: 10.1016/j.radonc.2012.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor CW, Povall JM, McGale P, Nisbet A, Dodwell D, Smith JT, et al. Cardiac dose from tangential breast cancer radiotherapy in the year 2006. Int J Radiat Oncol Biol Phys 2008; 72: 501–7. doi: 10.1016/j.ijrobp.2007.12.058 [DOI] [PubMed] [Google Scholar]
- 24.Nicolini G, Clivio A, Fogliata A, Vanetti E, Cozzi L. Simultaneous integrated boost radiotherapy for bilateral breast: a treatment planning and dosimetric comparison for volumetric modulated arc and fixed field intensity modulated therapy. Radiat Oncol 2009; 4: 27. doi: 10.1186/1748-717X-4-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma PK, Jamema SV, Kaushik K, Budrukkar A, Jalali R, Deshpande DD, et al. Electron arc therapy for bilateral chest wall irradiation: treatment planning and dosimetric study. Clin Oncol (R Coll Radiol) 2011; 23: 216–22. doi: 10.1016/j.clon.2010.09.005 [DOI] [PubMed] [Google Scholar]
- 26.Mackie TR, Holmes T, Swerdloff S, Reckwerdt P, Deasy JO, Yang J, et al. Tomotherapy: a new concept for the delivery of dynamic conformal radiotherapy. Med Phys 1993; 20: 1709–19. [DOI] [PubMed] [Google Scholar]
- 27.Caudrelier JM, Morgan SC, Montgomery L, Lacelle M, Nyiri B, Macpherson M. Helical tomotherapy for locoregional irradiation including the internal mammary chain in left-sided breast cancer: dosimetric evaluation. Radiother Oncol 2009; 90: 99–105. doi: 10.1016/j.radonc.2008.09.028 [DOI] [PubMed] [Google Scholar]
- 28.Jones R, Yang W, Read P, Sheng K. Radiation therapy of post-mastectomy patients with positive nodes using fixed beam tomotherapy. Radiother Oncol 2011; 100: 247–52. doi: 10.1016/j.radonc.2011.05.004 [DOI] [PubMed] [Google Scholar]
- 29.Moon SH, Shin KH, Kim TH, Yoon M, Park S, Lee DH, et al. Dosimetric comparison of four different external beam partial breast irradiation techniques: three-dimensional conformal radiotherapy, intensity-modulated radiotherapy, helical tomotherapy, and proton beam therapy. Radiother Oncol 2009; 90: 66–73. doi: 10.1016/j.radonc.2008.09.027 [DOI] [PubMed] [Google Scholar]
- 30.Van Parijs H, Miedema G, Vinh-Hung V, Verbanck S, Adriaenssens N, Kerkhove D, et al. Short course radiotherapy with simultaneous integrated boost for stage I-II breast cancer, early toxicities of a randomized clinical trial. Radiat Oncol 2012; 7: 80. doi: 10.1186/1748-717X-7-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fields EC, Rabinovitch R, Ryan NE, Miften M, Westerly DC. A detailed evaluation of TomoDirect 3DCRT planning for whole-breast radiation therapy. Med Dosim 2013; 38: 401–6. doi: 10.1016/j.meddos.2013.04.008 [DOI] [PubMed] [Google Scholar]
- 32.Borca VC, Franco P, Catuzzo P, Migliaccio F, Zenone F, Aimonetto S, et al. Does TomoDirect 3DCRT represent a suitable option for post-operative whole breast irradiation? A hypothesis-generating pilot study. Radiat Oncol 2012; 7: 211. doi: 10.1186/1748-717X-7-211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Franco P, Catuzzo P, Cante D, La Porta MR, Sciacero P, Girelli G, et al. TomoDirect: an efficient means to deliver radiation at static angles with tomotherapy. Tumori 2011; 97: 498–502. doi: 10.1700/950.10404 [DOI] [PubMed] [Google Scholar]
- 34.Li XA, Tai A, Arthur DW, Buchholz TA, Macdonald S, Marks LB, et al. ; Radiation Therapy Oncology Group Multi-Institutional and Multiobserver Study. Variability of target and normal structure delineation for breast cancer radiotherapy: an RTOG multi-institutional and multiobserver study. Int J Radiat Oncol Biol Phys 2009; 73: 944–51. doi: 10.1016/j.ijrobp.2008.10.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nielsen MH, Berg M, Pedersen AN, Andersen K, Glavicic V, Jakobsen EH, et al. ; Danish Breast Cancer Cooperative Group Radiotherapy Committee. Delineation of target volumes and organs at risk in adjuvant radiotherapy of early breast cancer: national guidelines and contouring atlas by the Danish Breast Cancer Cooperative Group. Acta Oncol 2013; 52: 703–10. doi: 10.3109/0284186X.2013.765064 [DOI] [PubMed] [Google Scholar]
- 36.Onal C, Sonmez A, Arslan G, Oymak E, Kotek A, Efe E, et al. Dosimetric comparison of the field-in-field technique and tangential wedged beams for breast irradiation. Jpn J Radiol 2012; 30: 218–26. doi: 10.1007/s11604-011-0034-7 [DOI] [PubMed] [Google Scholar]
- 37.Franco P, Zeverino M, Migliaccio F, Cante D, Sciacero P, Casanova Borca V, et al. Intensity-modulated and hypofractionated simultaneous integrated boost adjuvant breast radiation employing statics ports of tomotherapy (TomoDirect): a prospective phase II trial. J Cancer Res Clin Oncol 2014; 140: 167–77. doi: 10.1007/s00432-013-1560-8 [DOI] [PubMed] [Google Scholar]
- 38.Ashenafi M, Boyd RA, Lee TK, Lo KK, Gibbons JP, Rosen II, et al. Feasibility of postmastectomy treatment with helical TomoTherapy. Int J Radiat Oncol Biol Phys 2010; 77: 836–42. doi: 10.1016/j.ijrobp.2009.06.027 [DOI] [PubMed] [Google Scholar]
- 39.Kim SI, Cho SH, Lee JS, Moon HG, Noh WC, Youn HJ, et al. Clinical relevance of lymph node ratio in breast cancer patients with one to three positive lymph nodes. Br J Cancer 2013; 109: 1165–71. doi: 10.1038/bjc.2013.465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coles C, Yarnold J. Localising the tumour bed in breast radiotherapy. Clin Oncol (R Coll Radiol) 2010; 22: 36–8. doi: 10.1016/j.clon.2009.08.014 [DOI] [PubMed] [Google Scholar]
- 41.Donovan EM, Ciurlionis L, Fairfoul J, James H, Mayles H, Manktelow S, et al. Planning with intensity-modulated radiotherapy and tomotherapy to modulate dose across breast to reflect recurrence risk (IMPORT High trial). Int J Radiat Oncol Biol Phys 2011; 79: 1064–72. doi: 10.1016/j.ijrobp.2009.12.052 [DOI] [PubMed] [Google Scholar]
- 42.Pili G, Grimaldi L, Fidanza C, Florio ET, Petruzzelli MF, D’Errico MP, et al. Geometric and dosimetric approach to determine probability of late cardiac mortality in left tangential breast irradiation: comparison between wedged beams and field-in-field technique. Int J Radiat Oncol Biol Phys 2011; 81: 894–900. doi: 10.1016/j.ijrobp.2010.12.021 [DOI] [PubMed] [Google Scholar]
- 43.Bauman G, Yartsev S, Rodrigues G, Lewis C, Venkatesan VM, Yu E, et al. A prospective evaluation of helical tomotherapy. Int J Radiat Oncol Biol Phys 2007; 68: 632–41. [DOI] [PubMed] [Google Scholar]
- 44.O’Donnell H, Cooke K, Walsh N, Plowman PN. Early experience of tomotherapy-based intensity-modulated radiotherapy for breast cancer treatment. Clin Oncol (R Coll Radiol) 2009; 21: 294–301. doi: 10.1016/j.clon.2009.01.010 [DOI] [PubMed] [Google Scholar]
- 45.Lamberth F, Guilbert P, Gaillot-Petit N, Champagne C, Looten-Vieren L, Nguyen TD. Potential indications for helical tomotherapy in breast cancers. [In French.] Cancer Radiother 2014; 18: 7–14. doi: 10.1016/j.canrad.2013.07.148 [DOI] [PubMed] [Google Scholar]
- 46.Goddu SM, Chaudhari S, Mamalui-Hunter M, Pechenaya OL, Pratt D, Mutic S, et al. Helical tomotherapy planning for left-sided breast cancer patients with positive lymph nodes: comparison to conventional multiport breast technique. Int J Radiat Oncol Biol Phys 2009; 73: 1243–51. doi: 10.1016/j.ijrobp.2008.11.004 [DOI] [PubMed] [Google Scholar]
- 47.Schubert LK, Gondi V, Sengbusch E, Westerly DC, Soisson ET, Paliwal BR, et al. Dosimetric comparison of left-sided whole breast irradiation with 3DCRT, forward-planned IMRT, inverse-planned IMRT, helical tomotherapy, and topotherapy. Radiother Oncol 2011; 100: 241–6. doi: 10.1016/j.radonc.2011.01.004 [DOI] [PubMed] [Google Scholar]
- 48.Hijal T, Fournier-Bidoz N, Castro-Pena P, Kirova YM, Zefkili S, Bollet MA, et al. Simultaneous integrated boost in breast conserving treatment of breast cancer: a dosimetric comparison of helical tomotherapy and three-dimensional conformal radiotherapy. Radiother Oncol 2010; 94: 300–6. doi: 10.1016/j.radonc.2009.12.043 [DOI] [PubMed] [Google Scholar]
- 49.Franco P, Zeverino M, Migliaccio F, Sciacero P, Cante D, Casanova Borca V, et al. Intensity-modulated adjuvant whole breast radiation delivered with static angle tomotherapy (TomoDirect): a prospective case series. J Cancer Res Clin Oncol 2013; 139: 1927–36. doi: 10.1007/s00432-013-1515-0 [DOI] [PubMed] [Google Scholar]