Abstract
Objective:
To compare image quality of different reconstruction techniques in submillisievert ultralow-dose CT colonography (CTC) and to correlate colonic findings with subsequent optical colonoscopy.
Methods:
58 patients underwent ultralow-dose CTC. The images were reconstructed with filtered back projection (FBP), hybrid iterative reconstruction (HIR) or model-based iterative reconstruction (MBIR) techniques. In each segment, endoluminal noise (expressed as standard deviation of endoluminal density) was measured and image quality was rated on a five-point Likert scale by two independent readers. Colonic lesions were evaluated in consensus and correlated with subsequent optical colonoscopy where possible.
Results:
The estimated radiation dose was 0.41 ± 0.05 mSv for the supine and 0.42 ± 0.04 mSv for the prone acquisitions. In the endoluminal view, the image quality was rated better in HIR, whereas better scores were obtained in MBIR in the cross-sectional view, where the endoluminal noise was the lowest (p < 0.0001). Five (26%) polyps were not identified using both computer-aided detection and endoluminal inspection in FBP images vs only one (5%) in MBIR and none in HIR images.
Conclusion:
This study showed that in submillisievert ultralow-dose CTC, the image quality for the endoluminal view is better when HIR is used, whereas MBIR yields superior images for the cross-sectional view. The inferior quality of images reconstructed with FBP may result in decreased detection of colonic lesions.
Advances in knowledge:
Radiation dose from CTC can be safely reduced <1 mSv for both positions when iterative reconstruction is used. MBIR provides better image quality in the cross-sectional view and HIR in the endoluminal view.
CT colonography (CTC) has a comparable sensitivity and specificity to optical colonoscopy (OC) in diagnosing relevant colonic lesions.1,2 Compared with OC, its major disadvantages are the radiation dose and the inability to biopsy or remove polyps.3,4 Although the true risk of stochastic effects from a CTC examination in adults is very low, its routine large-scale use must be responsibly weighted against its benefits.5 Fortunately, high contrast among colonic wall, intraluminal air and tagged stool, as well as the widely accepted minimal size of a polyp to be reported (which relates to the required spatial resolution), allow reduction of the time–current product to 50–30 mAs in a 120-kV protocol without sacrificing diagnostic acceptability.6 For CTC, the estimated benefit–risk ratio of 24–35 : 1 per 7–8 mSv can be increased in direct proportion to the decrease of the radiation dose, provided that the image quality is maintained.4 Further reduction of the radiation dose while maintaining diagnostic acceptability requires special considerations regarding acquisition and image-processing techniques.7–9 In particular, new developments in the field of iterative reconstruction offer further reduction of the radiation dose with preserved image quality.7,10
The objective of this study was to compare image quality of different reconstruction techniques in a submillisievert ultralow-dose CTC in order to assess image quality and recommend the most applicable technique. Furthermore, we evaluated colonic findings and, where possible, correlated the findings with subsequent OC.
METHODS AND MATERIALS
The study was performed in accordance with the Declaration of Helsinki, and the study protocol was approved by the local institutional review board. All patients included in this study signed an informed consent.
58 consecutive patients who were scheduled for CTC between January and April 2013 were included in this prospective study. Patients with requests for restaging and simultaneous evaluation of colonic and extracolonic structures or patients without stool tagging were not considered for the study. The patients were 64 ± 16 years old, and 17 (29%) patients were males.
The indications for CTC included a combination of the following: incomplete colonoscopy (37 patients), positive faecal occult blood test (7), constipation (5), previous diverticulitis (4), abdominal pain (4), weight loss (3), diverticulosis (3), adhesions (2), screening, diarrhoea, polyp follow-up, positive family history, anaemia, melaena, elevated oncomarkers, irritable bowel syndrome etc (one each).
1 day before the examination, all patients underwent full cathartic bowel preparation (200 ml of 40% magnesium sulfate) with stool tagging with 250 ml 2.1% barium (Micropaque® CT; Guerbet, Roissy, France). Prior to scanning, all patients received an intravenous bolus of 20 mg hyoscine butylbromide (Buscopan®, Boehringer Ingelheim, Germany). The colon was inflated manually with room air via a rectal tube.
The examinations were performed on a 256-slice scanner (Brilliance iCT 256; Philips Healthcare, Best, Netherlands) with the following parameters: peak tube voltage, 120 kV; planned tube time–current product 10 mAs with current modulation (DoseRight™); detector collimation, 128 × 0.625 mm; rotation time, 0.5 s; pitch, 0.601; matrix size, 512 × 512 pixels; and standard resolution. The first acquisition was carried out in the supine position, the second in the prone position after additional insufflation of the colon. In the prone position, the patient's chest was supported with a cushion to reduce compression of the transverse colon. Both acquisitions were performed at the end of inspiration.
Both the supine and prone scans were reconstructed in 1.0-mm sections with 50% overlap. Three reconstruction techniques were used for each scan. The first reconstruction was carried out with filtered back projection (FBP) and a soft reconstruction filter A (synonym to “kernel”). The second reconstruction was performed with the same filter A and hybrid iterative reconstruction (HIR; iDose4; Philips Healthcare) set at the highest level (Level 6, maximal noise reduction). The first and second reconstruction types were carried out on an original reconstructor that was supplied with the CT scanner. For the third reconstruction, a specific model-based iterative reconstruction (MBIR) using a reconstructor prototype (IMR; Philips Healthcare) with “body routine level 2” setting was used.11 The reconstruction settings were based on our experience and small-scale experiments with different reconstruction settings that we tested before this study. MBIR also became recently commercially available.
Reconstruction times were recorded in FBP and HIR as the time between the output of the first and last images. For MBIR series reconstructed on a prototype, estimation was based on the progress of the reconstruction queue.
Images were evaluated in a dedicated environment (CT virtual colonoscopy) on Intellispace Portal (Philips Healthcare). The radiation dose was estimated from the dose–length product using a weighting factor 0.015 Sv Gy−1. The adequacy of this calculation was verified in seven patients using ImPACT CT patient dosimetry calculator (ImPACT, London, UK).
Two readers (LL and JJ) with experience in reading CTC (>1000 and >700 cases) evaluated all examinations and reconstructions in the endoluminal view for image acceptability for CTC on a five-point Likert scale (1, excellent; 5, unacceptable) in each of the six standard colonic segments, where the lumen was sufficiently distended (distension ≤3; 1, excellent distension; 5, collapsed). Both readers were allowed to change the density-rendering threshold (below which a voxel becomes transparent and represents intraluminal gas) to adjust the appearance of the endoluminal view.12 Each colonic segment was evaluated in axial thin sections and 900/100-HU window setting for image quality on the same five-point Likert scale. Endoluminal density and noise expressed as standard deviation was measured in an air-filled lumen in a circular region of interest of approximately 200 mm2 in each colonic segment. The series was anonymized and reviewed in a random order.
Both readers evaluated colonic findings in both patients' positions simultaneously and corresponding reconstructions in consensus, and all accepted lesions were measured automatically (volume) or automatically with manual adjustment (greatest diameter) in the endoluminal view in the “CT Virtual Colonoscopy” environment with default endoluminal-rendering threshold (−744 HU). If a patient had OC within the next 2 months, the findings were correlated. Difficulty of constructing the fly path, which is mostly calculated automatically but has to be reworked manually in some cases, was expressed on a five-point scale (1, automatic; 5, impossible), and the number of computer-aided detection (CAD) strikes was recorded.12
Statistical analysis
Statistical tests were performed using Prism v. 5.0 (GraphPad Software, San Diego, CA) and IBM SPSS® Statistics v. 19 (SPSS Inc., Chicago, IL). To test for statistical significance, either analysis of variance (ANOVA) with Bonferroni post hoc tests or Wilcoxon signed rank test was used. Contingency tables were evaluated using Fischer's exact test. Interobserver agreement was expressed as Goodman and Kruskal's gamma statistics, as the grading scale was ordinal. A p-value <0.05 was considered significant.
RESULTS
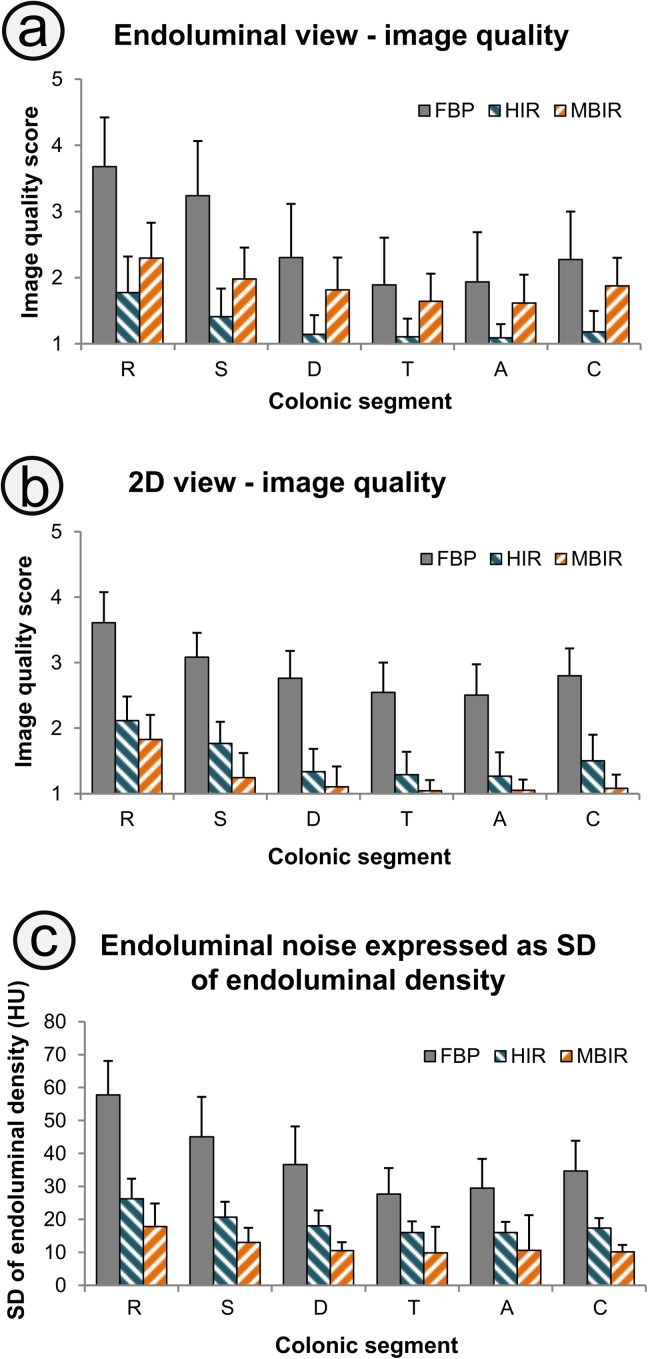
The estimated mean radiation dose was 0.41 ± 0.05 mSv for the supine and 0.42 ± 0.04 mSv for the prone acquisitions. The tube time–current product was modulated by the current modulation component to 8.1 ± 1.0 mAs in the supine acquisition and to 8.0 ± 0.7 mAs in the prone acquisition. Image quality scores in the endoluminal and cross-sectional view and the intraluminal noise expressed as standard deviation of endoluminal density are shown in Figure 1. A comparison of the endoluminal and cross-sectional view among FBP, HIR and MBIR is shown in Figure 2.
Figure 1.
Image quality in the endoluminal (a) and cross-sectional (b) views graded on a five-point Likert scale (1, excellent; 5, unacceptable) shows that in the endoluminal view, better scores are obtained with hybrid iterative reconstruction (HIR), whereas model-based iterative reconstruction (MBIR) yields superior images for the cross-sectional view. MBIR also provides the greatest reduction of image noise expressed as standard deviation (SD) of the endoluminal density (c). All differences between the reconstruction techniques are significant ( p < 0.0001). 2D, two dimensional; A, ascending; C, caecum; D, descending; FBP, filtered back projection; R, rectum; S, sigmoid; T, transverse.
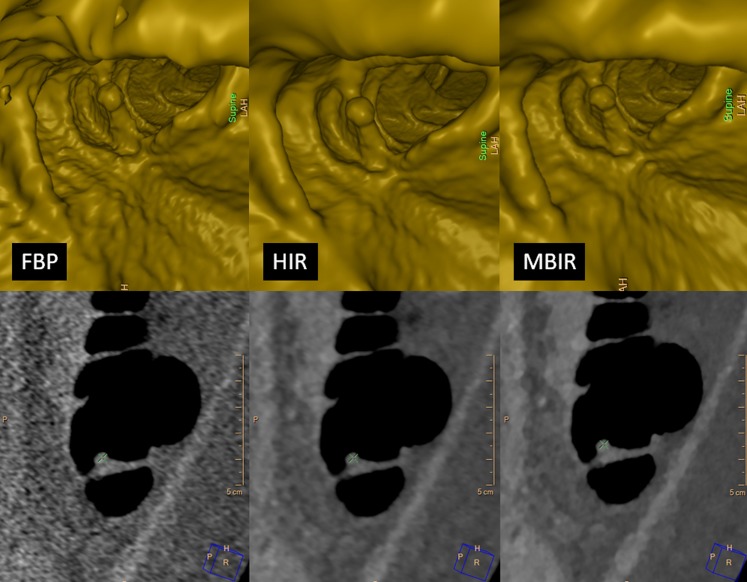
Figure 2.
Comparison of the endoluminal (rendering threshold = −700 HU) and cross-sectional view (900/100 HU window) of a small pedunculated polyp (8 mm) in the ascending colon among filtered back projection (FBP), hybrid iterative reconstruction (HIR) and model-based iterative reconstruction (MBIR) shows that whilst HIR produces the best images if viewed in the endoluminal view, images reconstructed with MBIR have better quality in the cross-sectional view. The images were acquired with the following parameters: peak tube voltage, 120 kVp; time–current product, 8 mAs; collimation, 128 × 0.625 mm; rotation time, 0.5 s; pitch, 0.601; matrix size, 512 × 512 pixels; standard resolution; dose–length product (DLR), 26 mGy cm; estimated dose, 0.39 mSv; slice thickness, 1 mm; increment, 0.5 mm (50% overlap).
The average time needed for reconstruction of 810 ± 79 images per acquisition was 44 ± 8 s with FBP and 94 ± 13 s with HIR (p < 0.01). Reconstruction times of MBIR series were about 10 min per acquisition (reconstructed on a prototype).
A total of 19 polyps were found in 15 patients: 16 were sessile and 3 pedunculated; 14 small (6–9 mm) and 5 large (>9 mm). The polyps were located in the rectum (1), sigmoid (7), descending (3), transverse (3) or ascending colon (2), or the caecum (3). Eight polyps were found in one position only. Seven polyps were verified by a subsequent OC (true positive) and one polyp was not found (likely a false positive). The rest of polyps were scheduled for a follow-up or disregarded as clinically unimportant in the view of the patient's age and general health status.
Combining the prone and supine scans for the diagnosis, five polyps (one large) were not identified using CAD and endoluminal inspection in images reconstructed with FBP, whereas only one small polyp in MBIR was missed and none in HIR images. The CAD alone would miss six polyps (one large) in FBP images, two small polyps in MBIR and none in HIR images. Altogether, if each acquisition was evaluated separately, the CAD would miss 13 polyps (3 large) in FBP, 1 small polyp in HIR and 4 small polyps in MBIR images from all 30 evaluable locations (8 polyps could be visualized in one position only). The performance of CAD is shown in Table 1.
Table 1.
The performance of computer-aided detection (CAD) and the reader according to different reconstruction techniques—filtered back projection (FBP), hybrid iterative reconstruction (HIR) and model-based iterative reconstruction (MBIR)—expressed as per scan or per patient
| Reconstruction type | CAD strikes per patient, median (25th–75th percentile) | CAD true positive (%) | Polyp missed rate (per scan)a (%) |
Polyp missed rate (per patient)b (%) |
||
|---|---|---|---|---|---|---|
| CAD | CAD + inspection | CAD | CAD + inspection | |||
| FBP | 3 (2–7)c | 6.3 | 11.2d | 9.5d | 10.3e | 8.6e |
| HIR | 5 (2–8) | 8.6 | 0.9 | 0 | 0 | 0 |
| MBIR | 5 (3–7) | 7.6 | 3.4 | 0.9 | 3.4 | 1.7 |
Only in visible locations, where the lumen was sufficiently distended (distension ≤3; 1, excellent distension; 5, collapsed). There were 15 polyps identified in supine scans and 15 polyps in prone scans. Eight polyps were visible in one position only.
Simultaneous inspection of supine and prone scans. There were 19 polyps altogether.
p < 0.01, Wilcoxon signed rank test.
p < 0.001 for FBP–HIR; p < 0.01 for FBP–MBIR, Fischer's exact test.
p < 0.05 for FBP–HIR, Fischer's exact test.
The average polyp size was 8.6 ± 1.9 mm in FBP, 8.8 ± 2.2 mm in HIR and 8.7 ± 2.1 mm in MBIR [not significant (n.s.)]. The polyp volume was 99 ± 84 mm3 in FBP, 101 ± 81 mm3 in HIR and 95 ± 69 mm3 in MBIR (n.s.).
Scores of difficulty of the fly path construction among the reconstructions were similar for all reconstruction techniques (1.5 ± 0.8 for each reconstruction technique, n.s.).
The preferred threshold for reading in the endoluminal view was increased from the default −744 HU to −625 ± 16 HU in the FBP series, −680 ± 19 HU in the HIR series and −652 ± 24 HU in the MBIR series (p < 0.0001 for all differences).
Interobserver agreement expressed as Goodman and Kruskal's gamma statistics was 0.85 for image quality in the endoluminal three-dimensional view and 0.95 in the cross-sectional two-dimensional view.
DISCUSSION
In this study, we decided to use a standard recommended 120 kV peak tube voltage.13 Our initial attempts to use low-energy scanning at 100 kV peak tube voltage that may otherwise be used to decrease the radiation dose by 20% were discouraging, because in a submillisievert region, it contributed to increased image noise to a greater extent than what could be compensated by increasing the tube current while maintaining low radiation dose.8,14 A 140-kV protocol used with a 10-mAs time–current product and FBP results in a 1.8- to 2.4-mSv radiation dose and ensures excellent sensitivity for detection of relevant (≥6 mm) lesions.6 However, in our specific scanner, the reference time–current product cannot be set to <10 mAs and therefore the 140-kV protocol was not used. It was already shown that low-dose images produced at 120 kV with an approximately 1 mSv radiation dose in the supine or prone position have significantly decreased quality in the endoluminal view, but reportedly, the perception of the relevant polyps is not significantly impaired even with FBP.13,15 In this study, the average cumulative radiation dose for both acquisitions was 0.83 mSv, which would imply roughly a 9-fold increase of the benefit-to-risk ratio (cancers prevented to induced) for CTC screening (every 5 years between 50 and 80 years of age) estimated by Berrington de González et al4 (24 : 1 to 35 : 1 with 7–8 mSv per screen) provided that the detection of the relevant polyps would remain unchanged.15
Apart from iterative reconstruction, further dose-reducing and image-optimizing techniques were used.3 Dynamic z-collimator reduces overscanning at the ends of the acquisition. Choosing an appropriate section thickness and soft reconstruction filter (kernel) further reduces noise in the endoluminal view.16 Initial images reconstructed with the smallest section thickness (0.67 mm) were of inferior quality and were discouraging. On the other hand, no improvement of image quality was seen beyond 1 mm.17 Given the great intrinsic contrast among air, tagged stool, polyps or colonic wall and the surrounding fat tissue, and the generally accepted minimal size of a clinically relevant polyp to be reported of 6 mm, decreased spatial resolution of the image is not a major concern.3,14,15 Further techniques included tube current modulation and carefully adjusted scan length to scan only the colon with a safety margin of approximately 3 cm.
Reconstruction times of CTC acquisitions with HIR were not substantially (albeit significantly) prolonged compared with FBP and did not slow the workflow in any aspect, as was shown previously even in an emergency setting.18 MBIR reconstruction carried out on a prototype took considerably longer, but the advertised reconstruction times for a commercially available MBIR manufactured by the same vendor claim to be approximately one and a half times longer than that for HIR.
Unsurprisingly, endoluminal noise expressed as standard deviation of the endoluminal density was the greatest with FBP and in the rectum and sigmoid colon, which is a finding in accordance with other authors.7,19 Image quality was rated better with MBIR in the cross-sectional view, whereas HIR delivered better image quality in the endoluminal view. This is probably owing to the fact that, in our experience, although MBIR provides the greatest reduction of image noise, it in fact enhances density boundaries more than HIR, which in turn results in bumpiness of the colonic wall owing to “consolidation” of the residual noise. For this reason, artefacts seen in the endoluminal view (cobblestoning, snow and wall distortion artefacts) seem to be influenced less by the adjustment of the surface-rendering density threshold than in FBP or HIR.15 Whilst the most accurate surface-rendering threshold for accurate polyp measurement is approximately −500 HU, it may not be optimal for polyp detection, because with increased threshold values, the size of a polyp and its conspicuity decreases.20 However, increasing the threshold by 100 HU can be performed to reduce perceived artefacts without loss of diagnostic confidence.13,15 In our small cohort, the size of detected polyps did not vary between the three different reconstruction techniques, which indicates their biometric congruence. This is an important finding because polyp size is the most important biomarker on CTC of its clinical significance, which guides subsequent clinical management, as indicated by CT colonography reporting and data system (C-RADS) follow-up recommendations.21,22
In examinations acquired with ultralow dose, the detection accuracy of CAD in detection of polyps is significantly lower if denoising of images is not performed.23 Similarly, in this study, CAD failed to detect a significant proportion of polyps in FBP images and there were significantly less CAD strikes altogether than in HIR and MBIR. The performance of CAD was the best with HIR images. However, the reported performance of CAD in detection of polyps in ultralow-dose scans varies, mostly owing to the algorithm used, scan settings (attained radiation dose) and the use of image-denoising techniques.23 In this study, the number of CAD strikes per patient was acceptable and in accordance with other studies.21,24,25 We believe that the effect of stool tagging on the performance of CAD (and CTC as a whole) may be more pronounced in ultralow-dose scans, because the heterogeneity of small stool residues may be less conspicuous and small polyps may have heterogeneous structure owing to quantum mottle. On the other hand, large amounts of densely tagged fluid within the colonic lumen may contribute to artefacts much more than in standard dose scans. Therefore, we suppose that ultralow-dose CTC may not perform well in reduced or laxative-free bowel preparation protocols.26
We found significant colonic lesions (≥6 mm in diameter) in 26% of patients, and there were on average 0.33 polyps per patient. This number is higher than those reported in an average-risk screening population in large studies, because a portion of the sample included subjects with an increased risk of colonic neoplasia.1,2 However, the prevalence of a colonic abnormality in various studies has a very wide range between 15% and 72%.27 Because there were significantly fewer polyps detected in FBP images than in HIR and MBIR, we suggest that images reconstructed with FBP in this ultralow-dose setting may result in a decreased detection of polyps, in contrast to images scanned with double the dose.13,15
Study limitations
This pilot study was performed with a limited number of patients, and only less than half of the lesions identified were correlated with a subsequent OC. Even though both readers were blinded to the reconstruction technique, we can assume that the difference in image appearance was revealing. Another reason why our study must be interpreted with some caution is that scoring of the image quality has a subjective bias based on the reader's experience and expectations. Furthermore, image quality does not necessarily correlate with diagnostic performance.28 This is a single vendor study, but similar results may be expected with other systems.29 The results of this study are encouraging and warrant further research with a larger patient group and tandem OC to assess the specificity and sensitivity of the ultralow-dose CTC.
CONCLUSION
This study showed that in a submillisievert ultralow-dose CTC, the image quality for the endoluminal view is better when HIR is used, whereas MBIR yields superior images for the cross-sectional view. The inferior quality of images reconstructed with FBP may result in decreased detection of colonic lesions. CAD performance in ultralow-dose CTC should be interpreted with caution using FBP, in view of the decreased detection rate of abnormalities compared with other reconstruction algorithms. Further research with a larger patient group focused on the specificity and sensitivity of the ultralow-dose CTC compared with optical colonoscopy is warranted.
CONFLICT OF INTEREST
Petr Ourednicek is a clinical scientist at Philips Healthcare.
FUNDING
This study was supported by the First Faculty of Medicine of Charles University in Prague, Prague, Czech Republic (PRVOUK-P27/LF1/1).
Acknowledgments
ACKNOWLEDGMENTS
The authors acknowledge technical support from Lucie Šimáková, Department of Radiology, First Faculty of Medicine in Prague, and from Associate Professor Jarmila Skotáková, Department of Pediatric Radiology, Faculty Hospital in Brno.
REFERENCES
- 1.Pickhardt PJ, Choi JR, Hwang I, Butler JA, Puckett ML, Hildebrandt HA, et al. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N Engl J Med 2003; 349: 2191–200. [DOI] [PubMed] [Google Scholar]
- 2.Johnson CD, Chen MH, Toledano AY, Heiken JP, Dachman A, Kuo MD, et al. Accuracy of CT colonography for detection of large adenomas and cancers. N Engl J Med 2008; 359: 1207–17. doi: 10.1056/NEJMoa0800996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yee J, Weinstein S, Morgan T, Alore P, Aslam R. Advances in CT colonography for colorectal cancer screening and diagnosis. J Cancer 2013; 4: 200–9. doi: 10.7150/jca.5858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berrington de González A, Kim KP, Knudsen AB, Lansdorp-Vogelaar I, Rutter CM, Smith-Bindman R, et al. Radiation-related cancer risks from CT colonography screening: a risk-benefit analysis. AJR Am J Roentgenol 2011; 196: 816–23. doi: 10.2214/AJR.10.4907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brenner DJ, Georgsson MA. Mass screening with CT colonography: should the radiation exposure be of concern? Gastroenterology 2005; 129: 328–37. [DOI] [PubMed] [Google Scholar]
- 6.Iannaccone R, Laghi A, Catalano C, Brink JA, Mangiapane F, Trenna S, et al. Detection of colorectal lesions: lower-dose multi-detector row helical CT colonography compared with conventional colonoscopy. Radiology 2003; 229: 775–81. [DOI] [PubMed] [Google Scholar]
- 7.Flicek KT, Hara AK, Silva AC, Wu Q, Peter MB, Johnson CD. Reducing the radiation dose for CT colonography using adaptive statistical iterative reconstruction: a pilot study. AJR Am J Roentgenol 2010; 195: 126–31. doi: 10.2214/AJR.09.3855 [DOI] [PubMed] [Google Scholar]
- 8.Chang KJ, Caovan DB, Grand DJ, Huda W, Mayo-Smith WW. Reducing radiation dose at CT colonography: decreasing tube voltage to 100 kVp. Radiology 2013; 266: 791–800. doi: 10.1148/radiol.12120134 [DOI] [PubMed] [Google Scholar]
- 9.Chang KJ, Yee J. Dose reduction methods for CT colonography. Abdom Imaging 2013; 38: 224–32. doi: 10.1007/s00261-012-9968-1 [DOI] [PubMed] [Google Scholar]
- 10.Noël PB, Renger B, Fiebich M, Münzel D, Fingerle AA, Rummeny EJ, et al. Does iterative reconstruction lower CT radiation dose: evaluation of 15,000 examinations. PLoS One 2013; 8: e81141. doi: 10.1371/journal.pone.0081141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta D, Thompson R, Morton T, Dhanantwari A, Shefer E. Iterative model reconstruction: simultaneously lowered computed tomography radiation dose and improved image quality. Med Phys Int J 2013; 2: 147–55. [Google Scholar]
- 12.Lambert L, Danes J, Jahoda J, Masek M, Lisy J, Ourednicek P. Submilisievert low-dose CT colonography using iterative reconstruction technique: a feasibility study. Acta Radiol May 2014. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 13.Cohnen M, Vogt C, Beck A, Andersen K, Heinen W, vom Dahl S, et al. Feasibility of MDCT colonography in ultra-low-dose technique in the detection of colorectal lesions: comparison with high-resolution video colonoscopy. AJR Am J Roentgenol 2004; 183: 1355–9. [DOI] [PubMed] [Google Scholar]
- 14.Boellaard TN, de Haan MC, Venema HW, Stoker J. Colon distension and scan protocol for CT-colonography: an overview. Eur J Radiol 2013; 82: 1144–58. doi: 10.1016/j.ejrad.2011.10.030 [DOI] [PubMed] [Google Scholar]
- 15.Fisichella VA, Båth M, Allansdotter Johnsson A, Jäderling F, Bergsten T, Persson U, et al. Evaluation of image quality and lesion perception by human readers on 3D CT colonography: comparison of standard and low radiation dose. Eur Radiol 2010; 20: 630–9. doi: 10.1007/s00330-009-1601-5 [DOI] [PubMed] [Google Scholar]
- 16.Walker MJ, Olszewski ME, Desai MY, Halliburton SS, Flamm SD. New radiation dose saving technologies for 256-slice cardiac computed tomography angiography. Int J Cardiovasc Imaging 2009; 25: 189–99. [Google Scholar]
- 17.Branschofsky M, Vogt C, Aurich V, Beck A, Mödder U, Cohnen M. Feasibility of ultra-low-dose multi-detector-row CT-colonography: detection of artificial endoluminal lesions in an in-vitro-model with optimization of image quality using a noise reduction filter algorithm. Eur J Med Res 2006; 11: 13–19. [PubMed] [Google Scholar]
- 18.Willemink MJ, Schilham AM, Leiner T, Mali WP, de Jong PA, Budde RP. Iterative reconstruction does not substantially delay CT imaging in an emergency setting. Insights Imaging 2013; 4: 391–7. doi: 10.1007/s13244-013-0226-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hara AK, Paden RG, Silva AC, Kujak JL, Lawder HJ, Pavlicek W. Iterative reconstruction technique for reducing body radiation dose at CT: feasibility study. AJR Am J Roentgenol 2009; 193: 764–71. doi: 10.2214/AJR.09.2397 [DOI] [PubMed] [Google Scholar]
- 20.Park SH, Choi EK, Lee SS, Woo JY, Chung SY, Kim YJ, et al. Linear polyp measurement at CT colonography: 3D endoluminal measurement with optimized surface-rendering threshold value and automated measurement. Radiology 2008; 246: 157–67. [DOI] [PubMed] [Google Scholar]
- 21.Summers RM. Polyp size measurement at CT colonography: what do we know and what do we need to know? Radiology 2010; 255: 707–20. doi: 10.1148/radiol.10090877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zalis ME, Barish MA, Choi JR, Dachman AH, Fenlon HM, Ferrucci JT, et al. ; Working Group on Virtual Colonoscopy. CT colonography reporting and data system: a consensus proposal. Radiology 2005; 236: 3–9. [DOI] [PubMed] [Google Scholar]
- 23.Näppi JJ, Imuta M, Yamashita Y, Yoshida H. Computer-aided detection for ultra-low-dose CT colonography. In: Yoshida H, Hawkes D, Vannier MW, eds. Abdominal imaging computational and clinical applications. Berlin, Germany: Springer Berlin Heidelberg; 2012. pp. 40–8. [Google Scholar]
- 24.Mang T, Bogoni L, Salganicoff M, Wolf M, Raykar V, Macari M, et al. Computer-aided detection of colorectal polyps in CT colonography with and without fecal tagging: a stand-alone evaluation. Invest Radiol 2012; 47: 99–108. doi: 10.1097/RLI.0b013e31822b41e1 [DOI] [PubMed] [Google Scholar]
- 25.Robinson C, Halligan S, Iinuma G, Topping W, Punwani S, Honeyfield L, et al. CT colonography: computer-assisted detection of colorectal cancer. Br J Radiol 2011; 84: 435–40. doi: 10.1259/bjr/17848340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Slater A, Betts M, D'Costa H. Laxative-free CT colonography. Br J Radiol 2012; 85: e410–5. doi: 10.1259/bjr/54736800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halligan S, Altman DG, Taylor SA, Mallett S, Deeks JJ, Bartram CI, et al. CT colonography in the detection of colorectal polyps and cancer: systematic review, meta-analysis, and proposed minimum data set for study level reporting. Radiology 2005; 237: 893–904. [DOI] [PubMed] [Google Scholar]
- 28.Pickhardt PJ, Lubner MG, Kim DH, Tang J, Ruma JA, del Rio AM, et al. Abdominal CT with model-based iterative reconstruction (MBIR): initial results of a prospective trial comparing ultralow-dose with standard-dose imaging. Am J Roentgenol 2012; 199: 1266–74. doi: 10.2214/AJR.12.9382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Löve A, Olsson ML, Siemund R, Stålhammar F, Björkman-Burtscher IM, Söderberg M. Six iterative reconstruction algorithms in brain CT: a phantom study on image quality at different radiation dose levels. Br J Radiol 2013; 86: 20130388. doi: 10.1259/bjr.20130388 [DOI] [PMC free article] [PubMed] [Google Scholar]