Abstract
Background
Atrial fibrillation (AF) is a highly prevalent condition associated with high morbidity and mortality that can cause or exacerbate heart failure and is an important risk factor for stroke. AF is the disorganized propagation of electrical activity in the atrium, which prevents organized contractions. However, the effect of microRNAs and the patterns of the regulatory network of AF remain vague.
Material/Methods
The mRNA expression data of atrial tissue splices from 3 conditions – permanent atrial fibrillation (AF), sinus rhythm (SR), and human left ventricular non-failing myocardium (LV) – were downloaded from GSE2240 and the differentially expressed genes (DEGs) between the 3 kinds of samples were calculated. Then we constructed 3 miRNA-DEGs networks and these networks were integrated to construct the final merged AF-related microRNA regulatory network. Finally, we constructed the miRNA-inflammation networks to detect the roles of miRNAs in inflammation development of AF.
Results
This network included 108 DEGs, and 27 microRNAs and DEGs are regulated by both microRNAs. We found that a sub-network composed by miR-124, miR-183, miR-215, miR-192, and a DEG of EGR1 were all represents in these 3 networks. Based on functional enrichment analysis, some biological process, such as energy and glucan metabolic process and heart and blood vessel development, were found to be regulated by miRNAs in AF. Some miRNAs, such as miR-26b and miR-355p, were involved in inflammation in AF.
Conclusions
In conclusion, the microRNA regulatory network sheds new light on the molecular mechanism of AF with this non-coding regulated model.
MeSH Keywords: Atrial Fibrillation, MicroRNAs, Neurogenic Inflammation
Background
Atrial fibrillation (AF) is a highly prevalent condition associated with high morbidity and mortality, which can cause or exacerbate heart failure and is an important risk factor for stroke. AF is the disorganized propagation of electrical activity in the atrium, which prevents organized heart contractions. As a result, the atrial depolarization wave front, the P-wave, measured during sinus rhythm (SR), devolves into a series of fibrillatory waves in the surface electrocardiogram (ECG). AF is known to be progressive in nature [1,2].
Recently, microRNA has become a focus of many researchers. The microRNAs, which are 21–25-nucleotides-long non-coding RNA molecules, function in transcriptional and post-transcriptional regulation of gene expression and they are involved in various diseases. Recent studies have uncovered an important role of microRNAs (miRNAs) in regulating cardiac excitability and arrhythmogenesis in various cardiac diseases, including myocardial infarction [3], cardiac hypertrophy [4], diabetic cardiomyopathy [5], and atrial fibrillation (AF) [6,7]. The above studies have indicated the important roles of microRNAs in AF, but most of their underlying mechanisms are still unknown. Furthermore, the regulatory action of microRNAs in development from AF to SR has not been clearly defined.
In this study, we downloaded the mRNA expression data of atrial tissue splices from 3 conditions – permanent atrial fibrillation (AF), sinus rhythm (SR), and human left ventricular non-failing myocardium (LV). Then we calculated the differential expressed genes between theses 3 kinds of samples and constructed 3 miRNA-DEGs networks. Finally, we constructed the miRNA-inflammation networks to detect the roles of miRNAs in development of inflammation in AF.
Material and Methods
Microarray data and differentially expressed genes analysis
The mRNA expression data of atrial tissue was obtained from Gene Expression Omnibus (GEO, http://www.ncbi.nlm.nih.gov/geo) with GEO Series accession number GSE2240. This profile was based on human atrial tissue splices from 3 conditions – permanent atrial fibrillation (AF), sinus rhythm (SR), and human left ventricular non-failing myocardium (LV) [8]. Ten atrial fibrillation samples, 20 sinus rhythm samples, and 5 left ventricular non-failing myocardium samples were profiled using Affymetrix Human Genome U133A Array and Affymetrix Human Genome U133B Array, respectively [8]. For these 2 platforms, only genes contained in both platforms were retained for further study, and there are 4317 genes involved in this study. The fold change values were calculated for each gene in 3 comparisons (AF vs. SR, AF vs. LV, and SR vs. LV) in each platform. In each comparison, genes with absolute values of log2 (fold change value) greater than 1 in either platform were reported as differential expressed genes (DEGs). Finally, we obtained 14 DEGs from AF vs. SR, 118 DEGs from AF vs. LV, and 131 DEGs from SR vs. LV.
Then we calculated the expression correlation values between DEGs and other genes for each condition, using Pearson correlation coefficient method. In each comparison, if the correlation value of 1 pair of genes (1 DEG and another gene) was less than 0.5 in one condition and more than 0.8 in another conditions or vice versa, this pair was identified as a differential co-expression pair.
Heapmap.2 in gplots package was used to describe the expression landscape of all samples in each platform.
Functional enrichment analysis
To implement functional annotation with different regulatory networks, we used the database for annotation, visualization, and integrated discovery (DAVID) tool [9]. DAVID is a high-throughput and integrated data-mining environment and can analyses a given gene list derived from genomic experiments [9]. DAVID was used for pathway enrichment analysis based on hypergeometric distribution. P-value less than 0.05 was chosen as the cutoff criterion for statistically significant GO terms related to AF and SR.
Construction the microRNA-DEGs network
To construct microRNA-DEGs networks, we downloaded the experimentally verified associations between human microRNAs and their targets from miRTarBase, which has accumulated more than 50 000 miRNA-target interactions collected by manually surveying pertinent literature after systematic data mining of the text [10]. This dataset contained miRNA-target interactions consisting of 963 miRNAs and 12518 mRNAs. We mapped the DEGs between the 3 kinds of samples into the above interactions and extracted the corresponding miRNA-DEG interactions. Finally, we constructed 3 miRNA-DEGs networks.
Construction of the microRNA-inflammation network
To construct the microRNA-inflammation networks, a set of inflammation genes was obtained from the Gene Ontology categories “inflammatory response” (GO: 0006954) and “regulation of inflammatory response” (GO: 0050727), namely the human inflammation gene set containing 231 genes. Then the 231 genes were intersected with the DEGs of AF vs. SR, AF vs. LV, and SR vs. LV. Finally, the miRNAs which regulated 3 sets of overlapped genes were extracted and the microRNA-inflammation networks were constructed. These 3 networks were visualized by Cytoscape.
Results
Differentially expressed genes analysis between 3 samples
To identify the differentially expressed genes (DEGs) of AF vs. SR, AF vs. LV, and SR vs. LV, we obtained the microarray dataset GSE2240 of 3 samples from the GEO database (http://www.ncbi.nlm.nih.gov/geo/). We then used fold change method to identify DEGs of AF vs. SR, AF vs. LV and SR vs. LV. A total of 14, 118, and 131 genes were considered differentially expressed in AF vs. SR, AF vs. LV and SR vs. LV, respectively (see Material and Methods).
MiRNA-DEGs network
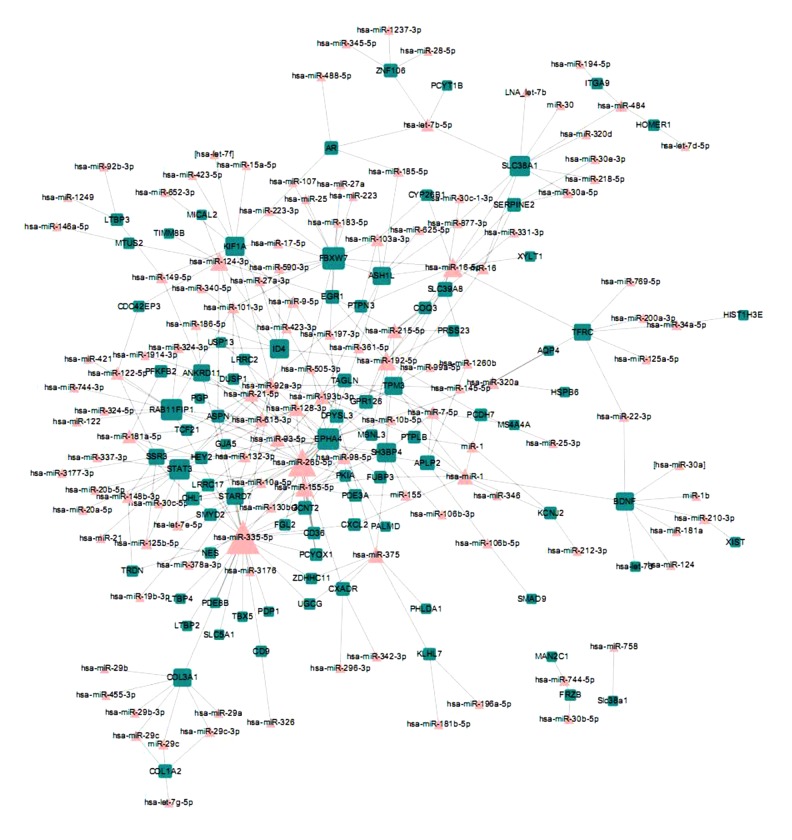
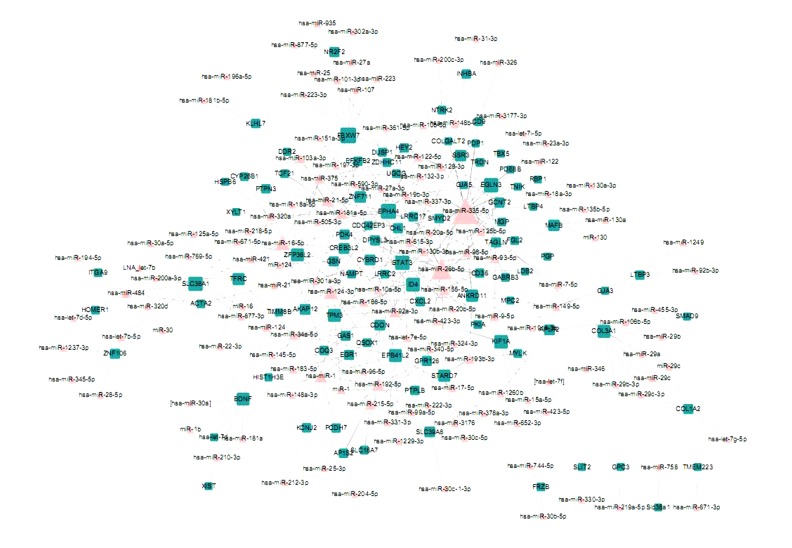
To obtain this network, we first constructed the experimental verified interactions between miRNA and mRNAs. After mapping the DEGs between the 3 kinds of samples into the above interactions, we obtained 3 miRNA-DEGs networks: AF vs. SR, AF vs. LV, and SR vs. LV (Figures 1–3). In these networks, triangle nodes represent miRNAs and rectangle nodes represent DEGs. In the AF vs. SR network, there were 45 nodes and 44 edges. In the AF vs. LV network, there were 212 nodes and 304 edges. The SR vs. LV network consisted of 235 nodes and 345 edges. Finally, the 3 networks were integrated to detect the miRNAs, which involved the development from non-failing myocardium to atrial fibrillation (AF) and sinus rhythm (SR) (Figure 4). We found that a sub-network composed by miR-124, miR-183, miR-215, miR-192, and a DEG of EGR1 were all represents in the above 3 networks.
Figure 1.
MiRNA-DEGs network of AF and LV. The rectangle nodes represent DEGs and the triangle nodes represent miRNAs.
Figure 2.

MiRNA-DEGs network of AF and SR. The rectangle nodes represent DEGs and the triangle nodes represent miRNAs.
Figure 3.
MiRNA-DEGs network of LV and SR. The rectangle nodes represent DEGs and the triangle nodes represent miRNAs.
Figure 4.
The merged miRNA-DEGs network.
Functional enrichment analysis
To explore the biological functions of different sets of DEGs, we conducted GO functional enrichment analysis to the 3 sets of DEGs. Tables 1–3 showed the significant enriched GO terms of AF vs. SR, AF vs. LV, and SR vs. LVy. Some biological terms, such as regulation of heart growth (GO: 0060420), were related to both atrial fibrillation and sinus rhythm.
Table 1.
The significantly enriched Go terms of DEGs between AF and LV.
| Term | P value | Genes |
|---|---|---|
| GO: 0034404~nucleobase, nucleoside and nucleotide biosynthetic process | 0.017073 | NAMPT, ATP1B4, NME7 |
| GO: 0034654~nucleobase, nucleoside, nucleotide and nucleic acid biosynthetic process | 0.017073 | NAMPT, ATP1B4, NME7 |
| GO: 0006753~nucleoside phosphate metabolic process | 0.037143 | NAMPT, ATP1B4, NME7 |
| GO: 0006073~cellular glucan metabolic process | 0.03743 | PPP1R1A, PPP1CB |
| GO: 0044042~glucan metabolic process | 0.03743 | PPP1R1A, PPP1CB |
| GO: 0006112~energy reserve metabolic process | 0.044556 | PPP1R1A, PPP1CB |
Table 2.
The significantly enriched Go terms of DEGs between AF and SR.
| Term | P value | Genes |
|---|---|---|
| GO: 0060043~regulation of cardiac muscle cell proliferation | 0.002554 | TBX5, HEY2, CXADR |
| GO: 0055021~regulation of cardiac muscle growth | 0.002554 | TBX5, HEY2, CXADR |
| GO: 0055024~regulation of cardiac muscle tissue development | 0.002554 | TBX5, HEY2, CXADR |
| GO: 0007167~enzyme linked receptor protein signaling pathway | 0.002662 | EPHA4, FGF18, SMAD9, LTBP2, LTBP3, USP9Y, COL3A1, COL1A2, STAT3 |
| GO: 0060420~regulation of heart growth | 0.003051 | TBX5, HEY2, CXADR |
| GO: 0016202~regulation of striated muscle tissue development | 0.005113 | TBX5, HEY2, MBNL3, CXADR |
| GO: 0009888~tissue development | 0.017406 | TCF21, FGF18, PGP, TBX5, UGCG, COL3A1, COL1A2, DHRS9, ENO3, CXADR, HOMER1 |
| GO: 0001944~vasculature development | 0.031215 | TCF21, FGF18, HEY2, COL3A1, COL1A2, GJA5 |
| GO: 0060044~negative regulation of cardiac muscle cell proliferation | 0.034502 | TBX5, CXADR |
| GO: 0001656~metanephros development | 0.03786 | TCF21, BDNF, SLC5A1 |
| GO: 0009887~organ morphogenesis | 0.043332 | TCF21, FGF18, PGP, TBX5, HEY2, ANKRD11, COL1A2, TMEM176B, STAT3 |
Table 3.
The significantly enriched Go terms of DEGs between SR and LV.
| Term | P value | Genes |
|---|---|---|
| GO: 0009887~organ morphogenesis | 3.74E-05 | FGF18, MAFB, TBX5, MGP, GAS1, STAT3, SLIT2, TCF21, PGP, GPC3, SMARCD3, HEY2, ANKRD11, NTRK2, COL1A2, TMEM176B |
| GO: 0009888~tissue development | 2.36E-04 | FGF18, TBX5, COL3A1, UGCG, DHRS9, MGP, HOMER1, SLIT2, TCF21, PGP, GPC3, GSN, COL1A2, MYH11, ENO3, NR2F2 |
| GO: 0001944~vasculature development | 8.74E-04 | TCF21, FGF18, HEY2, COL3A1, NTRK2, COL1A2, NR2F2, GJA5, SLIT2 |
| GO: 0007167~enzyme linked receptor protein signaling pathway | 0.001607 | EPHA4, FGF18, SMAD9, LTBP3, USP9Y, COL3A1, NTRK2, COL1A2, DDR2, STAT3 |
| GO: 0001568~blood vessel development | 0.003407 | FGF18, HEY2, COL3A1, NTRK2, COL1A2, NR2F2, GJA5, SLIT2 |
| GO: 0001656~metanephros development | 0.005137 | TCF21, BDNF, GPC3, SLIT2 |
| GO: 0022008~neurogenesis | 0.008224 | EPHA4, BDNF, GNAO1, MCOLN3, GSN, NTRK2, ID4, GAS1, NR2F2, NTM, STAT3, SLIT2 |
| GO: 0048699~generation of neurons | 0.013489 | EPHA4, BDNF, GNAO1, MCOLN3, NTRK2, ID4, GAS1, NR2F2, NTM, STAT3, SLIT2 |
| GO: 0006776~vitamin A metabolic process | 0.014275 | RBP1, CYP26B1, DHRS9 |
| GO: 0001523~retinoid metabolic process | 0.014275 | RBP1, CYP26B1, DHRS9 |
| GO: 0048729~tissue morphogenesis | 0.014479 | TCF21, PGP, GPC3, TBX5, COL1A2, SLIT2 |
| GO: 0006721~terpenoid metabolic process | 0.016753 | RBP1, CYP26B1, DHRS9 |
| GO: 0007166~cell surface receptor linked signal transduction | 0.018533 | FGF18, SMAD9, GNAO1, GPR126, GABRA4, ADAM23, LTBP3, USP9Y, CXCL2, COL3A1, AKAP12, NPY6R, HOMER1, FRZB, DDR2, STAT3, APLP2, INHBA, EPHA4, ITGA9, CDON, NTRK2, HEY2, COL1A2 |
| GO: 0007417~central nervous system development | 0.020067 | SMAD9, GNAO1, ADAM23, GSN, MAFB, ID4, GAS1, NR2F2, APLP2 |
| GO: 0042490~mechanoreceptor differentiation | 0.022211 | BDNF, MCOLN3, NTRK2 |
| GO: 0007420~brain development | 0.027914 | SMAD9, GNAO1, MAFB, ID4, GAS1, NR2F2, APLP2 |
| GO: 0035113~embryonic appendage morphogenesis | 0.032187 | TBX5, CYP26B1, GAS1, GJA5 |
| GO: 0030326~embryonic limb morphogenesis | 0.032187 | TBX5, CYP26B1, GAS1, GJA5 |
| GO: 0051146~striated muscle cell differentiation | 0.033135 | TBX5, CDON, MYH11, HOMER1 |
| GO: 0060429~epithelium development | 0.035001 | TCF21, PGP, GPC3, TBX5, DHRS9, NR2F2 |
| GO: 0007423~sensory organ development | 0.03614 | BDNF, MCOLN3, MAFB, NTRK2, GAS1, STAT3 |
| GO: 0045597~positive regulation of cell differentiation | 0.03614 | INHBA, BDNF, CD36, SMAD9, TBX5, SLIT2 |
| GO: 0043583~ear development | 0.040179 | BDNF, MCOLN3, MAFB, GAS1 |
| GO: 0001822~kidney development | 0.041242 | TCF21, BDNF, GPC3, SLIT2 |
| GO: 0035108~limb morphogenesis | 0.044517 | TBX5, CYP26B1, GAS1, GJA5 |
| GO: 0030324~lung development | 0.044517 | TCF21, FGF18, TBX5, MGP |
| GO: 0002009~morphogenesis of an epithelium | 0.04677 | TCF21, PGP, GPC3, TBX5 |
| GO: 0014047~glutamate secretion | 0.046902 | BDNF, NTRK2 |
| GO: 0030323~respiratory tube development | 0.047918 | TCF21, FGF18, TBX5, MGP |
| GO: 0006720~isoprenoid metabolic process | 0.047932 | RBP1, CYP26B1, DHRS9 |
MicroRNA-inflammation network
To explore the inflammation mechanism in the development of atrial fibrillation and sinus rhythm, we constructed a miRNA-inflammation network, in which the nodes represent inflammation-related DEGs and miRNAs and miR-355 and miR-26b emerged as hubs (Figure 5).
Figure 5.

The miRNA-inflammation network. The triangle nodes represent miRNAs and the rectangle nodes represent DEGs which were also related to inflammation.
Discussion
In this study, based on the GSE2240 from GEO database, and microRNA-targets relationships from the miRTarBase database, 3 microRNA regulatory networks were constructed. Then the 3 networks were merged to a final microRNA regulatory network including 27 microRNAs and 108 DEGs.
At first, DEGs between atrial fibrillation (AF), sinus rhythm (SR), and human left ventricular non-failing myocardium (LV) were obtained. We found that a gene-EGR1 was continuously differentially expressed among the 3 kinds of samples. The early growth response transcription factor Egr-1 controls cell-specific responses to proliferation, differentiation, and apoptosis [11]. Although we did not find directed association between AF and EGR1, EGR-1 can up-regulate Siva-1 expression and induces cardiac fibroblast apoptosis. Furthermore, EGR-1 works as a master regulator that plays a key role in triggering inflammation-induced tissue injury after ischemia and reperfusion [12].
Then we implemented GO enrichment by the DAVID tool. We found that some terms related to heart and blood vessel development were detected, indicating that the early stage of heart development might be related to AF (Tables 1–3). Furthermore, some biological energy and glucan metabolic process were also found to be related to AF (Table 2). Some studies hypothesized that increased energy requirements in the atria during myocardial fibrillation lead to activation of anaerobic metabolism [13]. Dong et al. found that activation of β3-AR contributes to atrial metabolic remodeling via transcriptional down-regulation of the PGC-1α/NRF-1/Tfam pathway, which is involved in mitochondrial biogenesis and ultimately perturbs mitochondrial function in rapid pacing-induced AF [14].
In the integrated network, we also noticed that in this miRNA synergistic regulatory network some nodes were connected to many other kinds of nodes. For miRNA nodes, miR-355 was the most frequently connected node. miR-355 is over-expressed in human breast cancer and is related to lymph node metastasis and poor patient prognosis. However, to the best of our knowledge, there were no direct reports about miR-355 and AF. This miRNA might be a potential novel regulator in AF. Furthermore, miR-26b also regulated many DEGs and was found to play important roles in cardiovascular diseases. For example, some new miRs were found to modulate physiological cardiac hypertrophy, particularly miR-26b [15]. Furthermore, miR-26b can regulate GATA4 expression, which plays a fundamental role in myocyte growth and survival, via a post-transcriptional mechanism during cardiac hypertrophy [16]. On the other hand, some DEGs were also simultaneously regulated by many miRNAs. For example, CNN3 was regulated by up to 9 miRNAs. Perez-Ilzarbe et al. found that CNN3 was related to paracrine effects of human skeletal myoblasts transplanted in infarcted myocardium [17]. We also noticed that DEG-EGR1 was continuously differentially expressed among the 3 kinds of samples. Four miRNAs – miR-124, miR-192, miR-183, and miR-215 – regulated this DEG. Of these miRNAs, microRNA-124 controls the proliferative, migratory, and inflammatory phenotype of pulmonary vascular fibroblasts [18]. Long-term doxorubicin treatment can induce up-regulation of miR-215 in the rat heart [19].
Finally, we constructed an AF-related miRNA-inflammation network in which DEGs were also related to inflammation. The presence of inflammation in the heart or systemic circulation can predict the onset of AF and recurrence in the general population, as well as in patients after cardiac surgery, cardioversion, and catheter ablation [20]. Inflammation also modulates calcium homeostasis and connexins, which are associated with triggers of AF and heterogeneous atrial conduction [20,21]. Also, the noncoding RNAs seem to participate in vascular homeostasis, inflammation, and platelet function [7]. In our network, some DEGs, such as TPM3, and some miRNAs, such as miR-26b and miR-355p, emerged as hubs, indicating that the miRNA-DEGs regulating these relations also play a key role in inflammation in AF.
Conclusions
We constructed an atrial fibrillation- and sinus rhythm-related microRNA regulatory network and revealed the potential mechanism of development of atrial fibrillation and sinus rhythm on the transcriptional regulation level. Furthermore, we identified some crucial regulators in atrial fibrillation and sinus rhythm. Our research may provide important insights into the inflammation mechanism of AF and SR and potentially serve as a reference for the therapeutic strategies of angiocardiopathy.
Footnotes
Source of support: Departmental sources
Reference
- 1.Rostock T, Steven D, Lutomsky B, et al. Atrial fibrillation begets atrial fibrillation in the pulmonary veins on the impact of atrial fibrillation on the electrophysiological properties of the pulmonary veins in humans. J Am Coll Cardiol. 2008;51:2153–60. doi: 10.1016/j.jacc.2008.02.059. [DOI] [PubMed] [Google Scholar]
- 2.Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92:1954–68. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 3.Yang B, Lin H, Xiao J, et al. The muscle-specific microRNA miR-1 regulates cardiac arrhythmogenic potential by targeting GJA1 and KCNJ2. Nat Med. 2007;13:486–91. doi: 10.1038/nm1569. [DOI] [PubMed] [Google Scholar]
- 4.Care A, Catalucci D, Felicetti F, et al. MicroRNA-133 controls cardiac hypertrophy. Nat Med. 2007;13:613–18. doi: 10.1038/nm1582. [DOI] [PubMed] [Google Scholar]
- 5.Feng B, Chen S, George B, et al. miR133a regulates cardiomyocyte hypertrophy in diabetes. Diabetes Metab Res Rev. 2010;26:40–49. doi: 10.1002/dmrr.1054. [DOI] [PubMed] [Google Scholar]
- 6.Luo X, Pan Z, Shan H, et al. MicroRNA-26 governs profibrillatory inward-rectifier potassium current changes in atrial fibrillation. J Clin Invest. 123:1939–51. doi: 10.1172/JCI62185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McManus DD, Freedman JE. MicroRNAs in platelet function and cardiovascular disease. Nat Rev Cardiol. 2015 doi: 10.1038/nrcardio.2015.101. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Barth AS, Merk S, Arnoldi E, et al. Reprogramming of the human atrial transcriptome in permanent atrial fibrillation: expression of a ventricular-like genomic signature. Circ Res. 2005;96:1022–29. doi: 10.1161/01.RES.0000165480.82737.33. [DOI] [PubMed] [Google Scholar]
- 9.Huang da W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsu SD, Tseng YT, Shrestha S, et al. miRTarBase update 2014: an information resource for experimentally validated miRNA-target interactions. Nucleic Acids Res. 2014;42:D78–85. doi: 10.1093/nar/gkt1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang NP, Pang XF, Zhang LH, et al. Attenuation of inflammatory response and reduction in infarct size by postconditioning are associated with downregulation of early growth response 1 during reperfusion in rat heart. Shock. 2014;41:346–54. doi: 10.1097/SHK.0000000000000112. [DOI] [PubMed] [Google Scholar]
- 12.Kimura TE, Duggirala A, Hindmarch CC, et al. Inhibition of Egr1 expression underlies the anti-mitogenic effects of cAMP in vascular smooth muscle cells. J Mol Cell Cardiol. 2014;72:9–19. doi: 10.1016/j.yjmcc.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kondrat’ev BY, Ugdyzhekova DS, Antonchenko IV, et al. Metabolic alterations in rat myocardium in experimental acute atrial fibrillation. Bull Exp Biol Med. 2005;140:397–99. doi: 10.1007/s10517-005-0501-1. [DOI] [PubMed] [Google Scholar]
- 14.Dong J, Zhao J, Zhang M, et al. beta3-adrenoceptor impairs mitochondrial biogenesis and energy metabolism during rapid atrial pacing-induced atrial fibrillation. J Cardiovasc Pharmacol Ther. 2015 doi: 10.1177/1074248415590440. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinelli NC, Cohen CR, Santos KG, et al. An analysis of the global expression of microRNAs in an experimental model of physiological left ventricular hypertrophy. PLoS One. 2014;9:e93271. doi: 10.1371/journal.pone.0093271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han M, Yang Z, Sayed D, et al. GATA4 expression is primarily regulated via a miR-26b-dependent post-transcriptional mechanism during cardiac hypertrophy. Cardiovasc Res. 2012;93:645–54. doi: 10.1093/cvr/cvs001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perez-Ilzarbe M, Agbulut O, Pelacho B, et al. Characterization of the paracrine effects of human skeletal myoblasts transplanted in infarcted myocardium. Eur J Heart Fail. 2008;10:1065–72. doi: 10.1016/j.ejheart.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Wang D, Zhang H, Li M, et al. MicroRNA-124 controls the proliferative, migratory, and inflammatory phenotype of pulmonary vascular fibroblasts. Circ Res. 2014;114:67–78. doi: 10.1161/CIRCRESAHA.114.301633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vacchi-Suzzi C, Bauer Y, Berridge BR, et al. Perturbation of microRNAs in rat heart during chronic doxorubicin treatment. PLoS One. 2012;7:e40395. doi: 10.1371/journal.pone.0040395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu YF, Chen YJ, Lin YJ, Chen SA. Inflammation and the pathogenesis of atrial fibrillation. Nat Rev Cardiol. 2015;12:230–43. doi: 10.1038/nrcardio.2015.2. [DOI] [PubMed] [Google Scholar]
- 21.Wijesurendra RS, Casadei B. Atrial fibrillation: effects beyond the atrium? Cardiovasc Res. 2015;105:238–47. doi: 10.1093/cvr/cvv001. [DOI] [PMC free article] [PubMed] [Google Scholar]