Abstract
Objective:
To evaluate the dosimetry of compensator intensity modulation-based stereotactic body radiotherapy (SBRT) [non-coplanar intensity-modulated radiotherapy (ncIMRT)], its use was compared with that of three-dimensional conformation-based SBRT, for patients with Stage I non-small-cell lung cancer (NSCLC).
Methods:
21 consecutive patients with Stage I NSCLC were treated with ncIMRT or SBRT at Tokyo Medical University. To compare the two techniques, ncIMRT and SBRT plans for each patient were generated, where the planning target volume (PTV) coverages were adjusted to be equivalent to each other. The prescribed dose was set as 75 Gy in 30 fractions. PTV coverage, conformity index, conformation number (CN) and homogeneity index (HI) were used to compare the two strategies.
Results:
There was no statistically significant difference between PTV coverage for the 100%, 95% and 90% dose levels in the SBRT plan and those in the ncIMRT plan. The CN values were 0.53 ± 0.13 in the SBRT plan and 0.72 ± 0.10 in the ncIMRT plan. These values were significantly better than those of the SBRT plan (p < 0.001). The HI in the ncIMRT plan was 1.04 ± 0.03%, which was also significantly better than that of SBRT.
Conclusion:
The ncIMRT plan provided superior conformity and reduced the doses to the lung for patients with Stage I NSCLC.
Advances in knowledge:
The delivery technique with compensator intensity modulation-based SBRT was evaluated. Concerning target motion, this is thought to be more robust and safer than SBRT for early-stage NSCLC.
Population-based studies have shown that approximately half of patients with radically treatable Stage I to III non-small-cell lung cancer (NSCLC) have been diagnosed as Stage I.1,2 Stereotactic body radiotherapy (SBRT) was considered to be a treatment option for patients with Stage I NSCLC who were unsuitable for surgery. In most studies, the SBRT outcomes were comparable with surgery in terms of local control and survival.3,4 Therefore, the use of SBRT for patients with Stage I NSCLC has gradually increased in number.5
Videtic et al6 first reported excellent local control for Stage I NSCLC when using SBRT based on intensity-modulated radiotherapy (IMRT). Recently, a new type of IMRT named volumetric modulated arc therapy (VMAT) has also been introduced into clinical use. However, the IMRT dose delivery obtained by moving multileaf collimators was not consistent for a moving target.7–10 By contrast, IMRT using compensated filter was capable of providing constant beams to a moving target and was consistent in the delivered dose distribution.8,11,12 Furthermore, adjustment of respiratory-induced tumour motion is difficult13,14 when multileaf collimators were used. We think gated irradiation using IMRT-compensated filter is an ideal method for moving targets. However, when using a compensator intensity modulation-based SBRT [non-coplanar IMRT (ncIMRT)] plan, the dosimetric benefit remains unknown for Stage I NSCLC. Thus, we investigated the benefits of the dose distribution of the ncIMRT plan for Stage I NSCLC via a comparison of the dosimetric parameters.
METHODS AND MATERIALS
From February 2010 to January 2012, 21 consecutive patients with Stage I NSCLC were treated with three-dimensional conformal radiotherapy (3D-CRT)-based non-coplanar SBRT (SBRT plan) or compensator-based ncIMRT plan at Tokyo Medical University, Tokyo, Japan.
At the time of treatment planning, patients were immobilized in a supine position using a body-fixed shell system (Pelvicast; Orfit Industries n.v., Wijnegem, Belgium) to press against the abdomen in order to restrict tumour motion. All tumour and diaphragm motions were checked by fluoroscopy during a few cycles of free breathing. Tumour motions that exceed 1.0 cm and rapid growth tumour were excluded from ncIMRT eligibility. Among the 21 patients in this study, 13 patients were treated with the ncIMRT procedure. Of the remaining eight patients, three were considered to be ineligible for ncIMRT owing to the large target motion. Five patients were immediately treated by 3D-CRT-based SBRT because of rapid tumour growth. Clinical target volume (CTV) was defined as the gross tumour volume plus an added uniform margin of 0.5 cm in all directions, accounting for the microscopic spread of tumour cells. Planning target volume (PTV) was defined as the CTV plus a margin of 0.5 cm in the axial direction and 1.5 cm in the craniocaudal direction to the CTV, which accounted for tumour motion and set-up margin. In the treatment planning, the five corresponding non-coplanar directions were used for both ncIMRT and SBRT plans. No more than two beams were set to enter the contralateral lung, and each beam was adjusted to avoid critical organs such as the spinal cord and oesophagus. For both plans, the dose distributions were calculated with the superposition algorithm of a treatment planning system (Xio v. 4.6; Elekta AB, Stockholm, Sweden).
First, the ncIMRT plans were generated under prescribed conditions calculated by the treatment planning system. These plans were converted into dose intensity maps and computed compensator filters, which were made of solid brass material custom-made by the .decimal, Inc. (Sanford, FL). In the SBRT plan, a 100% dose was prescribed to the isocentre. In the ncIMRT plan, a 100% dose was determined to cover 95% of the PTV volume and to cover 100% of the CTV volume. The maximal PTV dose was set at 107%. Concerning dose constraints, the percentage of the lung volume receiving a dose of 20 Gy or more was minimized, with a maximum of 35% of the total lung volume minus the CTV volume. The mean lung dose received no more than 20 Gy. The maximal dose to the spinal cord was kept at <50 Gy. The maximal dose to the oesophagus was kept at <66 Gy. The percentage of the heart volume receiving >60 Gy was <33%, 45 Gy <66% and 40 Gy <100%. To compare the two planning techniques, the PTV coverages (PTV enclosed volume/PTV volume) in the ncIMRT plans were matched with those in the SBRT plans. All 21 patients were diagnosed with medically inoperable Stage I NSCLC, and 75 Gy in 30 fractions was prescribed.
To support an analytical comparison of the two plans, the following parameters for tumour coverage or normal organs at risk were defined:
- (1) The PTV coverage was defined as:
where PTV enclosed was defined as the PTV volume enclosed by the prescribed isodose line. - (3) The healthy tissue CI16 was defined as:
- (4) The conformation number (CN)17 was defined as:
- (5) The homogeneity index (HI) was defined as:
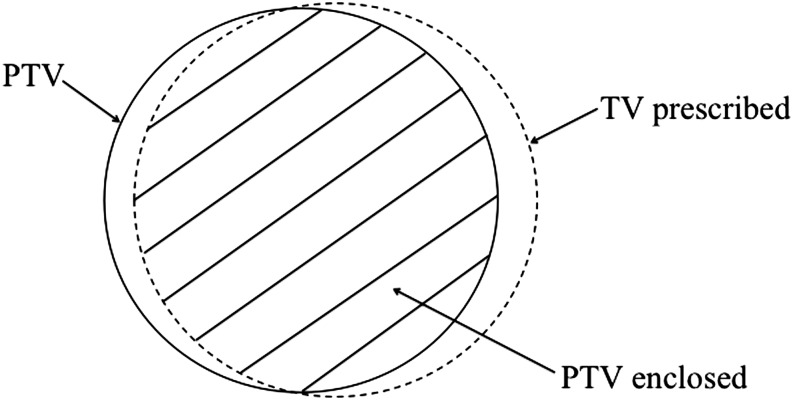
Figure 1.
Definition of the volumes used in parameters for tumour coverage and conformity. The solid line shows the planning target volume (PTV). The broken line depicts the total volume receiving the prescribed dose or greater (TV prescribed). The hatched area is the volume within the target that receives the prescribed dose or greater (PTV enclosed).
The risk of radiation pneumonitis was evaluated via the volume of the lung exposed to at least 5 Gy (V5Gy), 10 Gy (V10Gy), 15 Gy (V15Gy) and 20 Gy (V20Gy). Risks at the spinal cord and oesophagus attributed to excessive dose were measured via the maximum value of the dosage to which they were exposed. The risk of the heart owing to radiotherapy was assessed via the mean and maximum values of the irradiated dose.
STATISTICAL ANALYSIS
All continuous variables between the two groups were compared via the Wilcoxon signed-rank test. All statistical tests were performed with a two-sided assumption. A p-value of <0.05 was considered statistically significant, using the R software v. 3.1.0 (R Foundation, Vienna, Austria; http://www.r-project.org/).
RESULTS
21 patients were evaluated in this study. Individual tumour characteristics are shown in Table 1. All patients were diagnosed with medically inoperable conditions owing to poor respiratory function and/or presence of a cardiovascular disease. 13 patients were male and eight were female, and the median age was 75 years (range, 66–90 years). 4 patients were diagnosed with T1a, 5 with T1b and 12 with T2a tumour stage according to the Union for International Cancer Control (UICC) classification. 16 (76%) and 5 (24%) tumours were located in peripheral and central areas, respectively, according to the RTOG 0236 criteria. 10 (48%) and 11 (52%) tumours were located in the right and left lung, respectively. The median volume was 9.99 ml (range, 1.16–42.82 ml) for CTV and 34.84 ml (range, 6.59–88.85 ml) for PTV.
Table 1.
Details of 21 patients' characteristics and tumours
| Case | Sex | Age | Tumour stage | Locationa | Distribution | Gross tumour volume (cm3) | Planning target volume (cm3) |
|---|---|---|---|---|---|---|---|
| 1 | F | 85 | 1b | P | L/U | 9.99 | 27.92 |
| 2 | M | 71 | 2a | P | L/U | 7.28 | 26.98 |
| 3 | M | 75 | 1b | C | L/U | 8.81 | 34.84 |
| 4 | F | 79 | 2a | P | R/U | 4.23 | 11.00 |
| 5 | F | 90 | 2a | C | L/Low | 18.69 | 61.27 |
| 6 | F | 74 | 1a | P | R/U | 1.16 | 6.59 |
| 7 | F | 89 | 2a | P | R/Low | 28.86 | 81.68 |
| 8 | M | 72 | 2a | C | R/Low | 2.57 | 12.38 |
| 9 | F | 68 | 2a | C | R/U | 5.33 | 23.27 |
| 10 | M | 71 | 2a | C | L/Low | 10.20 | 47.02 |
| 11 | M | 85 | 1b | P | R/Mid | 7.45 | 57.20 |
| 12 | M | 74 | 1b | P | L/Low | 10.10 | 34.78 |
| 13 | M | 78 | 2a | P | R/U | 30.41 | 67.86 |
| 14 | F | 79 | 2a | P | R/U | 26.74 | 76.74 |
| 15 | M | 85 | 2a | P | R/Low | 42.82 | 88.85 |
| 16 | M | 86 | 2a | P | L/U | 14.53 | 42.07 |
| 17 | M | 75 | 1a | P | L/U | 8.42 | 30.51 |
| 18 | M | 73 | 2a | P | L/U | 5.51 | 18.84 |
| 19 | M | 67 | 1b | C | L/U | 20.75 | 57.01 |
| 20 | F | 79 | 1a | P | L/Low | 16.91 | 50.62 |
| 21 | M | 66 | 1a | P | R/Mid | 6.27 | 32.14 |
C, central; F, female; L, left; Low, lower; M, male; Mid, middle; P, peripheral; R, right; U, upper.
According to Radiation Therapy Oncology Group 0236.
Table 2 shows a comparison of the two plan strategies by tumour coverage, several CIs and HI. PTV coverage rates for V100%, V95% and V90% were 86.7 ± 15.5%, 99.3 ± 0.9% and 99.9 ± 0.2% in the SBRT plans and 86.9 ± 12.0%, 98.9 ± 2.8% and 99.7 ± .9% in the ncIMRT plans, respectively, which were not significantly different. RTOG CI values in the ncIMRT and SBRT plans were 1.05 ± 0.21 and 1.47 ± 0.21, which were significantly different (p < 0.001). Healthy tissue CI values in the ncIMRT and SBRT plans were 0.84 ± 0.09 and 0.63 ± 0.14, respectively, which were also significantly different (p < 0.001). CN values for the 100, 75 and 50 percentage levels were 0.53 ± 0.13, 0.27 ± 0.06 and 0.15 ± 0.04 in the SBRT plans and 0.72 ± 0.10, 0.33 ± 0.08 and 0.18 ± 0.04 in the ncIMRT plans, respectively. The ncIMRT plans were significantly better for each CN percentage level compared with those of the SBRT plans (p < 0.001). The HI of the ncIMRT plans (1.04 ± 0.03) was also significantly better than that of the SBRT plans. Figure 2 showed dose distributions and their dose–volume histograms in the SBRT plan and in the ncIMRT plan.
Table 2.
Comparison of two strategies by various parameters
| Variable | Non-coplanar intensity-modulated radiotherapy plan | Stereotactic body radiotherapy plan | p-value |
|---|---|---|---|
| Clinical target volume (ml) | 9.99 (range, 1.16–42.82) |
||
| PTV volume (ml) | 34.84 (range, 6.59–88.85) |
||
| PTV coverage (%) | |||
| V100% | 86.9 ± 12.0 | 86.7 ± 15.5 | 0.59 |
| V95% | 98.9 ± 2.8 | 99.3 ± 0.9 | 0.20 |
| V90% | 99.7 ± 0.9 | 99.9 ± 0.2 | 0.34 |
| Radiation Therapy Oncology Group CI | 1.05 ± 0.21 | 1.47 ± 0.21 | <0.001 |
| Healthy tissue CI | 0.84 ± 0.09 | 0.63 ± 0.14 | <0.001 |
| CN | |||
| CN 100 | 0.72 ± 0.10 | 0.53 ± 0.13 | <0.001 |
| CN 75 | 0.33 ± 0.08 | 0.27 ± 0.06 | <0.001 |
| CN 50 | 0.18 ± 0.04 | 0.15 ± 0.04 | <0.001 |
| Homogeneity index | 1.04 ± 0.03 | 1.11 ± 0.03 | <0.001 |
CI, conformity index; CN, conformal number; PTV, planning target volume; Vx%, the volume receiving ≥x% of the prescribed dose.
Parameters are calculated by Wilcoxon signed-rank sum test.
Figure 2.
A case of dose distribution for Stage I non-small-cell lung cancer in the non-coplanar intensity-modulated radiotherapy (ncIMRT) plan (right) and the stereotactic body radiotherapy (SBRT) plan (left) (a). The dose–volume histogram shows that the conformity in the ncIMRT plan is found to be better than that in the SBRT plan (b). CTV, clinical target volume; PTV, planning target volume.
Table 3 shows differences in the dosimetric parameters for organs at risk. The mean dose to the lung was 6.9 ± 2.4 Gy in the SBRT plan and was significantly lower, at 5.6 ± 2.1 Gy, in the ncIMRT plan. The V5Gy, V10Gy, V15Gy and V20Gy values of the lung were 28.8 ± 9.6%, 18.7 ± 6.6%, 13.5 ± 5.1% and 10.8 ± 4.6% in the SBRT plan and 24.3 ± 9.0%, 16.2 ± 6.4%, 11.6 ± 4.8% and 8.8 ± 3.8% in the ncIMRT plan, respectively. The percentage of the volume irradiated by each dose level in the ncIMRT plans was also significantly better than that of the SBRT plans. The maximum doses to the spinal cord were 10.8 ± 7.4 Gy in the ncIMRT plans and 15.3 ± 8.6 Gy in the SBRT plans (p = 0.002). The maximum doses to the oesophagus were 18.1 ± 17.2 Gy in the ncIMRT plans and 22.3 ± 17.4 Gy in the SBRT plans (p < 0.001). The maximum doses to the heart in the ncIMRT plans were significantly lower than that in the SBRT plans (35.1 ± 29.1 Gy in ncIMRT vs 39.9 ± 30.1 Gy in SBRT; p < 0.001). For the ncIMRT plans, a strong relationship between maximum dose and the target distance from the heart was also found (correlation coefficient = −0.76). The mean heart doses in the ncIMRT plans (3.6 ± 4.6 Gy) were significantly lower than that in the SBRT plans (5.2 ± 5.7 Gy; p = 0.007).
Table 3.
Dosimetric parameters of organs at risk
| Variable | Non-coplanar intensity-modulated radiotherapy plan | Stereotactic body radiotherapy plan | p-value |
|---|---|---|---|
| Lung | |||
| Mean lung dose (Gy) | 5.6 ± 2.1a | 6.9 ± 2.4 | <0.001 |
| V5 (%) | 24.3 ± 9.0a | 28.8 ± 9.6 | <0.001 |
| V10 (%) | 16.2 ± 6.4a | 18.7 ± 6.6 | <0.001 |
| V15 (%) | 11.6 ± 4.8a | 13.5 ± 5.1 | 0.001 |
| V20 (%) | 8.8 ± 3.8a | 10.8 ± 4.6 | <0.001 |
| Spinal cord Dmax (Gy) | 10.8 ± 7.4a | 15.3 ± 8.6 | 0.002 |
| Oesophageal Dmax (Gy) | 18.1 ± 17.2a | 22.3 ± 17.4 | <0.001 |
| Heart Dmax (Gy) | 35.1 ± 29.1a | 39.9 ± 30.1 | <0.001 |
| Heart Dmean (Gy) | 3.6 ± 4.6a | 5.2 ± 5.7 | 0.007 |
Dmax, maximal dose; Dmean, mean dose; V5, V10, V15, V20, lung receiving a dose >5, 10, 15 and 20 Gy.
Parameters are calculated by Wilcoxon single-rank test.
Significant difference.
DISCUSSION
This study showed that ncIMRT plans produced significantly better CI and CN and lower lung dosage compared with values obtained in the SBRT plans. In addition, the maximum doses to the spinal cord, oesophagus and heart in the ncIMRT plans were significantly lower than those in the SBRT plans. Some authors have demonstrated the superiority of dosimetric parameters in IMRT plans compared with those in 3D-CRT plans.18–20 Liu et al20 reported a treatment planning study of ten patients with lung cancer, showing that the CI and HI values in the IMRT plan were significantly superior to those in the 3D-CRT plan. In addition, an IMRT plan is able to reduce the irradiated dose of lung normal tissue compared with a 3D-CRT plan. Zhang et al18 and Ong et al19 reported better conformity for tumours and lower doses for risk organ in VMAT compared with those in 3D-CRT. These results indicated the improved value of dosimetric parameters adding intensity modulation to SBRT for early-stage NSCLC.
Concerning the optimal field number, Christian et al21 compared various fields for up to nine-field IMRT plans in 10 patients with NSCLC with 3D-CRT plans. Five and nine-field coplanar and six-field ncIMRT plans produced significantly better conformity than six-field non-coplanar 3D-CRT plans but not three-field IMRT. Therefore, five-field IMRT appears reasonable for achieving optimal conformity. Thus, in this study, the conformity of five-field IMRT was used to compare dosimetric parameters between SBRT and ncIMRT plans.
There are several different parameters for assessing the geometrical fitting of the prescription dose to the target volume (Table 4). It is necessary to analyse the dose conformity using the above parameters. The RTOG CI is widely used owing to its simplicity.15 Videtic et al6 reported that the median value of RTOG CI for 26 patients with 28 tumours of Stage I NSCLC treated with IMRT was 1.38 (range, 1.12–1.80). Seco et al22 also reported an RTOG CI that was modified to refer to the 95% prescription isodose. The average CI was 1.74 (range, 1.49–2.10) for the VMAT plan. Our plans' average RTOG CI of 1.05 (range, 0.44–1.42) appeared superior to these reports.
Table 4.
Comparison of the conformal radiotherapy of previous reports
| Study | Modality | Patients | PTV (cm3) | Size (cm) | Dose/fr. | Conformity index | Homogeneity index | Lung V20 (%) | Lung mean (Gy) |
|---|---|---|---|---|---|---|---|---|---|
| Ong et al19 | VMAT | 18 | 33 | NA | 55 Gy/5 fr. (three cases) 60 Gy/8 fr. (six cases) 54 Gy/3 fr. (nine cases) |
1.1a | NA | 5.4 | NA |
| Dynamic conformal arc therapy | 1.3a | NA | 5.4 | NA | |||||
| Stereotactic body radiotherapy | 1.18a | NA | 4.9 | NA | |||||
| IMRT (sliding window) | 9 | NA | NA | 1.07a | NA | 4.2 | NA | ||
| Zhang et al18 | 3D-CRT | 15 | 61 | NA | 50 Gy/5 fr. | 0.67b | NA | 6.8 | 4.97 |
| Non-coplanar-flattening filter-free VMAT | 0.81b | NA | 5.6 | 4.49 | |||||
| Coplanar VMAT | 0.79b | NA | 5.8 | 4.59 | |||||
| Navarria25 | VMAT | 46 | 46.7 | 26 | 48 Gy/4 fr. | NA | NA | 7.3 (ipsi) | 7.9 (ipsi) |
| 3D-CRT | 86 | 59.9 | 21 | NA | NA | 11.8 (ipsi) | 7.2 (ipsi) | ||
| Holt et al23 | VMAT | 27 | 44.5 | NA | 56 Gy/3 fr. | 1.13c | NA | 5.4 | 4.1 |
| Non-coplanar IMRT | 1.11c | NA | 5 | 4.1 | |||||
| Coplanar IMRT | 1.12c | NA | 5.7 | 4.2 | |||||
| Videtic et al6 | IMRT | 26 | 26.2 (median) | NA | 50 Gy/5 fr. | 1.38d | 1.08e | 3.36 | 0.54 |
| Liu et al20 | Dynamic sliding window IMRT | 10 | 403.1 (include Stage III) | NA | 63 Gy/35 fr. | 1.37f | 1.15g | NA | NA |
| 3D-CRT | 2.35f | 1.14g | NA | NA |
3D-CRT, three-dimensional conformal radiotherapy; Fr., fraction; IMRT, intensity-modulated radiotherapy; ipsi, ipsilateral; NA, not available; PTV, planning target volume; Vx%, the volume receiving ≥x% of the prescribed dose; VMAT, volumetric modulated arc therapy.
80% isodose volume/PTV encompassed by 80% isodose.
(PTV encompassed by 100% isodose/PTV) × (PTV encompassed by 100% isodose/100% isodose volume).
Prescription isodose volume/PTV encompassed by prescription isodose.
Prescription isodose volume/PTV.
Maximum dose/prescription dose.
Prescription isodose volume/PTV.
D5%/D95%: D5% and D95% correspond to the dose delivered to 5% and 95% of the PTV.
Lomax and Scheib16 defined another parameter of healthy tissue CI as the ratio of PTV enclosed by the prescribed isodose line to the total volume of the prescribed dose. Inverse numbers for healthy tissue CI used by Ong et al19 were 1.10 in VMAT, 1.18 in SBRT and 1.07 in IMRT for 18 actually treated peripheral lung tumours. Holt et al23 reported similar results from 27 patients with lung tumours, with inverse numbers for healthy tissue CI of 1.13 in VMAT, 1.11 in ncIMRT and 1.12 in coplanar IMRT. In our results, the inverse value of healthy tissue CI was calculated as 1.19, which was comparable with those in their reports. Although the ncIMRT appears better than others in terms of CI, careful interpretation is necessary because there are several different definitions of CI (Table 4).
Each of the conformity parameters described above has an essential pitfall in translating its values. Although both target coverage and avoidance of the prescribed dose from adjacent normal healthy tissue should be evaluated simultaneously, each parameter is capable of evaluating only one or neither of these points. The CN proposed by van't Riet et al,17 which is defined by multiplying the two above conformity indices, can combine these two concepts and explain both at the same time. In this article, the CN parameter was therefore used to assess dose conformity. The CN 100 in coplanar VMAT and non-coplanar flattening filter-free VMAT are 0.79 and 0.81, respectively.18 Chi et al24 showed relatively lower CN 100 values of 0.61–0.64, calculated for the helical tomotherapy and VMAT plans. The CN 100 value of 0.72 in this report was comparable with the results of those studies.
Chang et al11 placed an emphasis on the reduced treatment time of compensator-based IMRT relative to of segmented multileaf collimator-based SBRT. In the ncIMRT plan, preparation time for making compensated filters is necessary, which delays the starting time. Another advantage of using compensator-based IMRT is consistency in the inherent static intensity map, which is beneficial for organ motion. An interplay effect is the variation of the dose distribution difference between the interfractional motion of the anatomy and the movement of the multileaf collimators. Ehler et al8 demonstrated that compensator-based IMRT overcomes this interplay effect and showed that the dose difference between “step and shoot” and “sliding window” and compensator-based IMRT was up to 45.10% and 47.92%, respectively. They concluded that compensator-based IMRT provided the most uniform dose distribution to a moving target compared with segmented multileaf collimators and dynamic multileaf collimators. Bortfeld et al7 also reported that compensator-based IMRT achieved a smaller standard deviation for the dose distribution in a moving target than that of a segmental multileaf collimator. Rao et al12 demonstrated that the deviation of the distribution would be washed out over a conventional 30 fractioned course, owing to averaging of the variation by random delivery timing at different fractions. This means that compensator-based IMRT has an advantage for delivery to a moving target, especially in a hypofraction course. Therefore, the intrinsic features of compensator-based IMRT, where a relatively homogeneous dose map and static distribution over the fractions were delivered, could provide a more robust treatment, especially for moving tumours such as early-stage NSCLC. Concerning patient selection, all patients were surgically inaccessible owing to medical reasons, including deteriorated cardiopulmonary conditions. Therefore, a conventional fraction dose was delivered to reduce toxicities even in the SBRT plan.
CONCLUSION
We found that ncIMRT plans produced better conformity and homogeneity than SBRT plans and could spare lung and surrounding healthy organs in this planning study for patients with Stage I NSCLC. A phase II clinical trial using ncIMRT for Stage I NSCLC is now under way to clarify the clinical outcomes at our institution.
Contributor Information
Y Tajima, Email: cacaobean77@gmail.com.
H Nakayama, Email: hnakayam@tokyo-med.ac.jp.
T Itonaga, Email: ti19850704@yahoo.co.jp.
S Shiraishi, Email: s-nogi@tokyo-med.ac.jp.
M Okubo, Email: okubo@tokyo-med.ac.jp.
R Mikami, Email: mikami_tmu@yahoo.co.jp.
S Sugahara, Email: ssuga@tokyo-med.ac.jp.
K Tokuuye, Email: ktokuue@tokyo-med.ac.jp.
REFERENCES
- 1.Ou SH, Zell JA. Carcinoma NOS is a common histologic diagnosis and is increasing in proportion among non-small cell lung cancer histologies. J Thorac Oncol 2009; 4: 1202–11. doi: 10.1097/JTO.0b013e3181b28fb9 [DOI] [PubMed] [Google Scholar]
- 2.Kaergaard Starr L, Osler M, Steding-Jessen M, Lidegaard Frederiksen B, Jakobsen E, Østerlind K, et al. Socioeconomic position and surgery for early-stage non-small-cell lung cancer: a population-based study in Denmark. Lung Cancer 2013; 79: 262–9. doi: 10.1016/j.lungcan.2012.11.023 [DOI] [PubMed] [Google Scholar]
- 3.Palma D, Visser O, Lagerwaard FJ, Belderbos J, Slotman B, Senan S. Treatment of stage I NSCLC in elderly patients: a population-based matched-pair comparison of stereotactic radiotherapy versus surgery. Radiother Oncol 2011; 101: 240–4. doi: 10.1016/j.radonc.2011.06.029 [DOI] [PubMed] [Google Scholar]
- 4.Soldà F, Lodge M, Ashley S, Whitington A, Goldstraw P, Brada M. Stereotactic radiotherapy (SABR) for the treatment of primary non-small cell lung cancer; systematic review and comparison with a surgical cohort. Radiother Oncol 2013; 109: 1–7. doi: 10.1016/j.radonc.2013.09.006 [DOI] [PubMed] [Google Scholar]
- 5.Vest MT, Herrin J, Soulos PR, Decker RH, Tanoue L, Michaud G, et al. Use of new treatment modalities for non-small cell lung cancer care in the Medicare population. Chest 2013; 143: 429–35. doi: 10.1378/chest.12-1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Videtic GM, Stephans K, Reddy C, Gajdos S, Kolar M, Clouser E, et al. Intensity-modulated radiotherapy-based stereotactic body radiotherapy for medically inoperable early-stage lung cancer: excellent local control. Int J Radiat Oncol Biol Phys 2010; 77: 344–9. doi: 10.1016/j.ijrobp.2009.05.004 [DOI] [PubMed] [Google Scholar]
- 7.Bortfeld T, Jokivarsi K, Goitein M, Kung J, Jiang SB. Effects of intra-fraction motion on IMRT dose delivery: statistical analysis and simulation. Phys Med Biol 2002; 47: 2203–20. doi: 10.1088/0031-9155/47/13/302 [DOI] [PubMed] [Google Scholar]
- 8.Ehler ED, Nelms BE, Tomé WA. On the dose to a moving target while employing different IMRT delivery mechanisms. Radiother Oncol 2007; 83: 49–56. doi: 10.1016/j.radonc.2007.02.007 [DOI] [PubMed] [Google Scholar]
- 9.Kim B, Chen J, Kron T, Battista J. Motion-induced dose artifacts in helical tomotherapy. Phys Med Biol 2009; 54: 5707–34. doi: 10.1088/0031-9155/54/19/004 [DOI] [PubMed] [Google Scholar]
- 10.Seppala J, Suilamo S, Kulmala J, Mali P, Minn H. A dosimetric phantom study of dose accuracy and build-up effects using IMRT and RapidArc in stereotactic irradiation of lung tumours. Radiat Oncol 2012; 7: 79. doi: 10.1186/1748-717X-7-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang SX, Cullip TJ, Deschesne KM, Miller EP, Rosenman JG. Compensators: an alternative IMRT delivery technique. J Appl Clin Med Phys 2004; 5: 15–36. doi: 10.1120/jacmp.2021.25274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rao M, Wu J, Cao D, Wong T, Mehta V, Shepard D, et al. Dosimetric impact of breathing motion in lung stereotactic body radiotherapy treatment using intensity modulated radiotherapy and volumetric modulated arc therapy [corrected]. Int J Radiat Oncol Biol Phys 2012; 83: e251–6. doi: 10.1016/j.ijrobp.2011.12.001 [DOI] [PubMed] [Google Scholar]
- 13.Mageras GS, Pevsner A, Yorke ED, Rosenzweig KE, Ford EC, Hertanto A, et al. Measurement of lung tumor motion using respiration-correlated CT. Int J Radiat Oncol Biol Phys 2004; 60: 933–41. doi: 10.1016/j.ijrobp.2004.06.021 [DOI] [PubMed] [Google Scholar]
- 14.Seppenwoolde Y, Shirato H, Kitamura K, Shimizu S, van Herk M, Lebesque JV, et al. Precise and real-time measurement of 3D tumor motion in lung due to breathing and heartbeat, measured during radiotherapy. Int J Radiat Oncol Biol Phys 2002; 53: 822–34. doi: 10.1016/S0360-3016(02)02803-1 [DOI] [PubMed] [Google Scholar]
- 15.Shaw E, Kline R, Gillin M, Souhami L, Hirschfeld A, Dinapoli R, et al. Radiation Therapy Oncology Group: radiosurgery quality assurance guidelines. Int J Radiat Oncol Biol Phys 1993; 27: 1231–9. doi: 10.1016/0360-3016(93)90548-A [DOI] [PubMed] [Google Scholar]
- 16.Lomax NJ, Scheib SG. Quantifying the degree of conformity in radiosurgery treatment planning. Int J Radiat Oncol Biol Phys 2003; 55: 1409–19. doi: 10.1016/S0360-3016(02)04599-6 [DOI] [PubMed] [Google Scholar]
- 17.van't Riet A, Mak AC, Moerland MA, Elders LH, van der Zee W. A conformation number to quantify the degree of conformality in brachytherapy and external beam irradiation: application to the prostate. Int J Radiat Oncol Biol Phys 1997; 37: 731–6. [DOI] [PubMed] [Google Scholar]
- 18.Zhang GG, Ku L, Dilling TJ, Stevens CW, Zhang RR, Li W, et al. Volumetric modulated arc planning for lung stereotactic body radiotherapy using conventional and unflattened photon beams: a dosimetric comparison with 3D technique. Radiat Oncol 2011; 6: 152. doi: 10.1186/1748-717X-6-152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ong CL, Verbakel WF, Cuijpers JP, Slotman BJ, Lagerwaard FJ, Senan S. Stereotactic radiotherapy for peripheral lung tumors: a comparison of volumetric modulated arc therapy with 3 other delivery techniques. Radiother Oncol 2010; 97: 437–42. doi: 10.1016/j.radonc.2010.09.027 [DOI] [PubMed] [Google Scholar]
- 20.Liu HH, Wang X, Dong L, Wu Q, Liao Z, Stevens CW, et al. Feasibility of sparing lung and other thoracic structures with intensity-modulated radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2004; 58: 1268–79. doi: 10.1016/j.ijrobp.2003.09.085 [DOI] [PubMed] [Google Scholar]
- 21.Christian JA, Bedford JL, Webb S, Brada M. Comparison of inverse-planned three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2007; 67: 735–41. doi: 10.1016/j.ijrobp.2006.09.047 [DOI] [PubMed] [Google Scholar]
- 22.Seco J, Gu G, Marcelos T, Kooy H, Willers H. Proton arc reduces range uncertainty effects and improves conformality compared with photon volumetric modulated arc therapy in stereotactic body radiation therapy for non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2013; 87: 188–94. doi: 10.1016/j.ijrobp.2013.04.048 [DOI] [PubMed] [Google Scholar]
- 23.Holt A, van Vliet-Vroegindeweij C, Mans A, Belderbos JS, Damen EM. Volumetric-modulated arc therapy for stereotactic body radiotherapy of lung tumors: a comparison with intensity-modulated radiotherapy techniques. Int J Radiat Oncol Biol Phys 2011; 81: 1560–7. doi: 10.1016/j.ijrobp.2010.09.014 [DOI] [PubMed] [Google Scholar]
- 24.Chi A, Ma P, Fu G, Hobbs G, Welsh JS, Nguyen NP, et al. Critical structure sparing in stereotactic ablative radiotherapy for central lung lesions: helical tomotherapy vs. volumetric modulated arc therapy. PLoS One 2013; 8: e59729. doi: 10.1371/journal.pone.0059729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Navarria P, Ascolese AM, Mancosu P, Alongi F, Clerici E, Tozz A, et al. Volumetric modulated arc therapy with flattening filter free (FFF) beams for stereotactic body radiation therapy (SBRT) in patients with medically inoperable early-stage non small cell lung cancer (NSCLC). Radiother Oncol 2013; 107: 414–18. doi: 10.1016/j.radonc.2013.04.016 [DOI] [PubMed] [Google Scholar]