Abstract
Objective:
Arthroscopy is “the gold standard” for the diagnosis of knee cartilage lesions. However, it is invasive and expensive, and displays all the potential complications of an open surgical procedure. Ultra-high-field MRI now offers good opportunities for the indirect assessment of the integrity and structural changes of joint cartilage of the knee. The goal of the present study is to determine the site of early cartilaginous lesions in adults with non-traumatic knee pain.
Methods:
3-T MRI examinations of 200 asymptomatic knees with standard and three-dimensional double-echo steady-state (3D-DESS) cartilage-specific sequences were prospectively studied for early degenerative lesions of the tibiofemoral joint. Lesions were classified and mapped using the modified Outerbridge and modified International Cartilage Repair Society classifications.
Results:
A total of 1437 lesions were detected: 56.1% grade I, 33.5% grade II, 7.2% grade III and 3.3% grade IV. Cartographically, grade I lesions were most common in the anteromedial tibial areas; grade II lesions in the anteromedial L5 femoral areas; and grade III in the centromedial M2 femoral areas.
Conclusion:
3-T MRI with standard and 3D-DESS cartilage-specific sequences demonstrated that areas predisposed to early osteoarthritis are the central, lateral and ventromedial tibial plateau, as well as the central and medial femoral condyle.
Advances in knowledge:
In contrast with previous studies reporting early cartilaginous lesions in the medial tibial compartment and/or in the medial femoral condyle, this study demonstrates that, regardless of grade, lesions preferentially occur at the L5 and M4 tibial and L5 and L2 femoral areas of the knee joint.
INTRODUCTION
Cartilage defects display poor healing ability and favour the development of early osteoarthritis. These lesions are associated with significant morbidity and frequently require surgical treatment.1 Although arthroscopy is considered as “the gold standard” for diagnosis of knee cartilage lesions, having accuracy as high as 95–98%, it is invasive and expensive, and displays all the potential complications of an open surgical procedure.2,3
Cartilage lesions are difficult to evaluate by standard MRI.4–7 3-T MRI displays a distinct signal gain that may be transferred into a higher spatial resolution as well as a shorter acquisition time, thus facilitating the detection of cartilaginous lesions.4,8 The use of three-dimensional double-echo steady-state (3D-DESS) sequences combined with the standard sequences on a 3-T MRI scanner has provided good diagnostic results and therefore has served as a standard of reference in cartilage imaging in many major preceding studies.9–11
Various classifications of cartilaginous changes of the knee have been applied in the current literature.12–14 The Outerbridge15 classification remains the gold standard for macroscopic cartilage evaluation. A modification of this classification is used for MRI diagnosis.16 However, it does not provide information on the localization and extent of the defect. For this reason, the International Cartilage Repair Society (ICRS) Standard Workshop adopted a different classification for the localization of knee cartilage lesions.17
The goal of the present study is to determine the site of early cartilaginous lesions in adults with non-traumatic knee pain.
METHODS AND MATERIALS
Following appropriate institutional review board approval, 200 MRI knee examinations were prospectively performed in 177 asymptomatic patients (75 females, 102 males; mean age, 42 years; range, 18–81 years). All 177 patients were sampled consecutively from our outpatient clinic (Department of Orthopaedic Surgery, Inselspital, University of Bern, Switzerland). Cartilage degeneration was evaluated with the modified Outerbridge classification while cartographic analysis was performed using the ICRS scheme. Patients aged less than 18 years at the time of MRI examination and those with intra-articular fractures or varus–valgus axis deviation >10° on orthoradiograms were excluded from the study group. To obtain age-specific measurements, patients were divided into two subgroups: (A) comprising subjects aged 18–50 years and (B) subjects aged 51–81 years.18
All MRI examinations were performed with an advanced 3-T MRI scanner (MAGNETOM® Verio, TIM software v. VB 17; Siemens Healthcare, Erlangen, Germany) using a dedicated 15-channel phased array knee coil. Knee joint cartilage was evaluated using standard sequences as well as an isotropic multiplanar reconstructible 3D-DESS sequence (DESS gradient recalled echo) with selective water excitation. The repetition time was 5 ms, and the echo time was 14.2 ms. The flip angle was 25°. An isotropic voxel size with a volume of 0.33 mm3 was applied for cartilage imaging. For localization of the cartilage lesions, multiplanar reconstructions in axial, sagittal and coronal planes optimized for the anatomy of the joint were used. Cartilage lesions were classified based on their extent and depth using the modified Outerbridge15 classification (Figures 1–4).
Figure 1.
(a) Grade I lesion in a 25-years-old female patient. (b) Grade I lesion in a 65-year-old male patient.
Figure 4.
(a) Grade IV lesion in a 28-year-old female patient. (b) Grade IV lesion in a 64-year-old male patient.
Figure 2.
(a) Grade II lesion in a 22-year-old male patient. (b) Grade II lesion in a 67-year-old male patient.
Figure 3.
(a) Grade III lesion in a 44-year-old female patient. (b) Grade III lesion in a 68-year-old male patient.
Two independent readers, an orthopaedic surgeon with experience in musculoskeletal imaging and a radiologist, performed the MRI analysis. If a lesion was observed by at least one of the two readers, then a positive lesion was documented. Agreements were examined using the coefficient for interrater agreement (Cohen kappa). The interpretation was translated into five scales: poor (<0.20), fair (0.21–0.40), moderate (0.41–0.60), good (0.61–0.80) and very good (0.81–1.00).19
The interclass correlation coefficient (ICC) was calculated amongst the two independent readers for each grade of lesion identified on MRI evaluations.
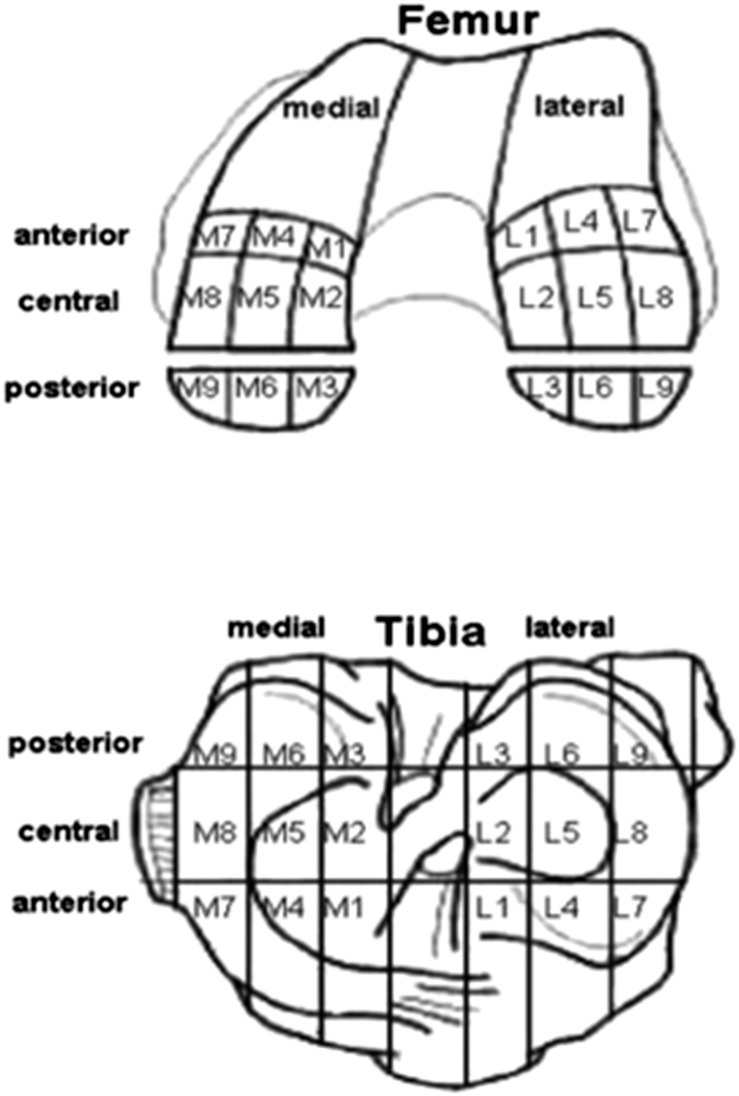
To document the localization of cartilage degeneration, the modified ICRS classification was applied.17 Areas were consecutively numbered from one to nine, while medial and lateral compartments were marked with an “M” and “L”, respectively (Figure 5).
Figure 5.
Modified International Cartilage Repair Society classification showing the different areas.7
For each femoral and tibial compartment, the occurrence of grade 1–4 arthritis at each position was tabulated by frequency tables. Combined pie charts and bubble plots were used to display the results. Within each block, univariate logistic regression models, with the position of the defect as predictor, were used to explore the occurrence of arthritis of any grade. Pairwise comparisons of all possible locations of cartilaginous lesions were performed. To account for multiple testing, the Bonferroni–Holm adjustment of p-values was used within each compartment, where p-values <0.05 were classified as statistically significant. Multiple logistic regression models were performed to analyse the influence of different parameters, including age, leg axis, knee joint side and sex, on the occurrence of osteoarthritis at each position in each joint compartment. Calculations were performed with SAS® v. 9.2 (SAS Institute Inc., Cary, NC) and S-Plus v. 8.1 (TIBCO Software Inc., Palo Alto, CA) software.
RESULTS
The detailed results are shown in Tables 1–4. All 200 knees exhibited cartilage lesions. Interobserver agreement between the two readers was rated very good for the localization (k = 0.86) and good (k = 0.78) for the grading of the lesions. The ICC for all lesions identified on MRI evaluations was 0.72 (p < 0.001), showing consistency between the two independent reviewers for all grades of lesion.
Table 1.
Distribution of the cartilaginous lesions of the lateral femur
| Position | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Total |
|---|---|---|---|---|---|
| L1 | 16 | 9 | 0 | 0 | 25 |
| L4 | 16 | 11 | 1 | 0 | 28 |
| L7 | 2 | 3 | 1 | 0 | 6 |
| L2 | 40 | 49 | 3 | 2 | 94 |
| L5 | 55 | 52 | 8 | 4 | 119 |
| L8 | 3 | 5 | 3 | 1 | 12 |
| L3 | 43 | 19 | 2 | 2 | 66 |
| L6 | 30 | 21 | 5 | 5 | 61 |
| L9 | 8 | 5 | 2 | 1 | 16 |
| Total | 213 | 174 | 25 | 15 | 427 |
Table 4.
Distribution of the cartilaginous lesions in the medial tibia
| Position | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Total |
|---|---|---|---|---|---|
| M9 | 2 | 1 | 1 | 1 | 5 |
| M6 | 1 | 1 | 1 | 1 | 4 |
| M3 | 7 | 3 | 1 | 0 | 11 |
| M8 | 3 | 2 | 2 | 2 | 9 |
| M5 | 51 | 33 | 3 | 2 | 89 |
| M2 | 36 | 8 | 0 | 1 | 45 |
| M7 | 4 | 2 | 2 | 1 | 9 |
| M4 | 59 | 28 | 3 | 2 | 92 |
| M1 | 32 | 15 | 1 | 0 | 48 |
| Total | 195 | 93 | 14 | 10 | 312 |
Table 2.
Distribution of the cartilaginous lesions of the medial femur
| Position | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Total |
|---|---|---|---|---|---|
| M7 | 8 | 1 | 1 | 1 | 11 |
| M4 | 60 | 30 | 6 | 1 | 97 |
| M1 | 25 | 16 | 7 | 0 | 48 |
| M8 | 4 | 2 | 1 | 1 | 8 |
| M5 | 41 | 31 | 7 | 4 | 83 |
| M2 | 30 | 18 | 11 | 5 | 64 |
| M9 | 4 | 0 | 2 | 0 | 6 |
| M6 | 7 | 3 | 3 | 0 | 13 |
| M3 | 9 | 9 | 5 | 0 | 23 |
| Total | 188 | 110 | 43 | 12 | 353 |
Table 3.
Distribution of the cartilaginous lesions in the lateral tibia
| Position | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Total |
|---|---|---|---|---|---|
| L3 | 29 | 20 | 6 | 2 | 57 |
| L6 | 47 | 23 | 3 | 2 | 75 |
| L7 | 7 | 3 | 2 | 0 | 12 |
| L2 | 46 | 21 | 3 | 3 | 73 |
| L5 | 64 | 30 | 4 | 2 | 100 |
| L8 | 7 | 0 | 2 | 1 | 10 |
| L1 | 1 | 0 | 0 | 0 | 1 |
| L4 | 6 | 6 | 0 | 0 | 12 |
| L7 | 3 | 1 | 1 | 0 | 5 |
| Total | 210 | 104 | 21 | 10 | 345 |
A total of 1437 lesions were detected: 806 grade I lesions (56.1%), 481 grade II lesions (33.5%), 103 grade III lesions (7.2%) and 47 grade IV lesions (3.3%). The area most commonly affected was the lateral femoral condyle with 427 (29.7%) lesions, followed by the medial femoral condyle with 353 (24.6%) lesions, the lateral tibial plateau with 345 (24.0%) lesions and the medial tibial plateau with 312 (21.7%) lesions.
Grade I lesions were preferentially located L5 tibial lesions (64 lesions), followed by M4 tibial lesions (59 lesions). Grade II lesions were most often located L5 femoral lesions (52 lesions) and L2 femoral lesions (49 lesions). The only clustering of grade 3 lesions was found in M2 femoral lesions (11 lesions). Grade 4 lesions were rare (n = 5).
Group A patients (mean age, 33.4 years; 57 females; 78 males) had a total of 808 lesions, of which 521 (65%) were grade I, 232 (29%) were grade II, 36 (4%) were grade III and 19 (2%) were grade IV. Of Group A lesions, 30% were located in the lateral femoral condyle, 23% in the lateral tibial plateau, 24% in the medial femoral condyle and 24% in the medial tibial plateau.
In Group B (mean age, 59.7 years; 26 females; 38 males), a total of 629 lesions were detected, 285 (45%) of which were grade I, 249 (40%) were grade II, 67 (11%) were grade III and 28 (4%) were grade IV. Of Group B lesions, 30% were found in the lateral femoral condyle, 26% in the lateral tibial plateau, 25% in the medial femoral condyle and 19% in the medial tibial plateau.
DISCUSSION
The goal of the present study was to detect the site of onset of cartilage degeneration in asymptomatic patients. Detailed clinical classification of cartilaginous lesions through the modified Outerbridge classification14 proved to be easily applicable. The modified cartographic ICRS classification17 also enabled precise and simple documentation of the localization of cartilaginous lesions of the knee joint. The results of this study demonstrate that even grade IV cartilage lesions may be asymptomatic. Although a single site for the onset of gonarthrosis could not be detected, a clear predilection for central areas of the lateral compartment and for anterior areas of the medial compartment was revealed. These findings are consistent with the study of Iwaki et al on the analysis of tibiofemoral movement of the knee joint.20
Various classifications are currently applied predominantly for arthroscopic evaluation of cartilage defects.21 New classification systems focus mainly on objective parameters, such as lesion depth and extent, and less on surface/superficial properties.14,17,22,23 Although the modified Outerbridge classification15 is widely accepted for arthroscopic grading of cartilage lesions, in the cadaver study of Cameron et al,24 it demonstrated only moderate validity, depending on the localization and severity of the lesions.15,25 Precise data on a lesion's characteristics, such as location, depth and surface area, as well as the status of the contiguous cartilage, are essential for both diagnosis and optimal treatment choice.1
Non-invasive procedures such as MRI are being vigorously discussed regarding their sensitivity and specificity and have so far produced good results. Literature data report that MRI is a sensitive and accurate tool for evaluating the morphology and structure of joint cartilage, as well as the accompanying pathological changes.16,26,27 Whether or not MRI underestimates the number and size of cartilage lesions of the knee joint is a point of controversy.28 There is general agreement that cartilage damage evaluation on standard MRI examination remains problematic. However, recently developed cartilage-specific MRI sequences were able to demonstrate, depending on the severity of the lesion, a sensitivity of 43–87% and a specificity of 89–97%.9,25,29–32 In the present study, joint cartilage was examined using the 3D-DESS sequence on a 3-T MRI scanner, which various studies have shown to be very well suited for this purpose and, therefore, has served as a standard of reference in cartilage imaging in many major preceeding studies.9–11 In addition to visualizing the actual cartilage thickness plus adequate contrasting of the cartilage–bone border, this sequence has the advantage of enabling multiplanar 3D reconstruction, thus allowing detailed evaluation of joint cartilage lesions.10,11,33 Based on the current development of various cartilage-specific sequences for improving sensitivity, the status of 3-T MRI as an accurate diagnostic tool, especially for grade III and IV cartilage defects, can be confirmed.25,30,34–36
von Engelhardt et al30 reported a uniform (medial, lateral) distribution of grade I/II defects in 3-T MRI examinations. High-grade lesions (grade III/IV) showed a predilection for medial localization. Their results correspond closely with those of the present study. Overall, 54.5% of the detected lesions were located in the medial compartment and 45.3% in the lateral compartment. In our cohort, higher grade lesions were located in the medial knee joint compartment. However, several studies report cartilaginous lesions occurring preferentially in the medial compartment and/or in the medial femoral condyle.1,12,28,31,37–40
To recognize age-specific differences, patients were divided into two age subgroups: (A) subjects aged 18–50 years and (B) subjects aged 51–81 years. The majority of grade I lesions occurred in Group A (65%). Grade II lesions were almost equally distributed between the two groups. Group B had 65% of all grade III lesions and 60% of all grade IV lesions, clearly more than Group A. Similar to Curl et al,38 age-specific changes were examined only in grade IV lesions. The results show a similar trend: 72% of grade IV lesions were found in patients aged over 40 years.
Despite our efforts to ensure the validity of the present study, certain limitations are observed. Although our results indicated a clear predilection for central areas of the lateral compartment and for anterior areas of the medial compartment, this patient cohort is too small to allow permanent conclusions on the model of cartilage degeneration and consequent bone attrition. Additionally, since radiological evaluation included only MRI scanning, no correlation could be established between MRI findings and those of conventional X-rays. Moreover, it should always be borne in mind that MRI scans can underestimate cartilage thickness owing to minimal subchondral calcification.
CONCLUSIONS
Our results demonstrate that even grade IV cartilage lesions may be asymptomatic. In contrast with previous studies reporting early cartilaginous lesions in the medial tibial compartment and/or in the medial femoral condyle, the results of the present study showed that, regardless of grade, lesions preferentially occur at the L5 and M4 tibial and L5 and L2 femoral areas of the knee joint.
Contributor Information
D S Evangelopoulos, Email: ds.evangelopoulos@gmail.com.
M Huesler, Email: manuela.huesler@bluewin.ch.
S S Ahmad, Email: sufiansamy@gmail.com.
E Aghayev, Email: emin.aghayev@memcenter.unibe.ch.
M Neukamp, Email: michal.neukamp@memcenter.unibe.ch.
C Röder, Email: christoph.roeder@memcenter.unibe.ch.
A Exadaktylos, Email: aristomenis.exadaktylos@insel.ch.
H Bonel, Email: harald.bonel@insel.ch.
S Kohl, Email: sandro.kohl@insel.ch.
REFERENCES
- 1.Hjelle K, Solheim E, Strand T, Muri R, Brittberg M. Articular cartilage defects in 1,000 knee arthroscopies. Arthroscopy 2002; 18: 730–4. doi: 10.1053/jars.2002.32839 [DOI] [PubMed] [Google Scholar]
- 2.Fischer SP, Fox JM, Del Pizzo W, Friedman MJ, Snyder SJ, Ferkel RD. Accuracy of diagnosis from magnetic resonance imaging of the knee. A multi-center analysis of one thousand and fourteen patients. J Bone Joint Surg Am 1991; 73: 2–10. [PubMed] [Google Scholar]
- 3.Halbrecht JL, Jackson DW. Office arthroscopy: a diagnostic alternative. Arthroscopy 1992; 8: 320–6. doi: 10.1016/0749-8063(92)90062-G [DOI] [PubMed] [Google Scholar]
- 4.Van Dyck P, Kenis C, Vanhoenacker FM, Lambrecht V, Wouters K, Gielen JL, et al. Comparison of 1.5- and 3-T MR imaging for evaluating the articular cartilage of the knee. Knee Surg Sports Traumatol Arthrosc 2014; 22: 1376–84. doi: 10.1007/s00167-013-2704-8 [DOI] [PubMed] [Google Scholar]
- 5.Friemert B, Oberländer Y, Schwarz W, Häberle HJ, Bähren W, Gerngross H, et al. Diagnosis of chondral lesions of the knee joint: can MRI replace arthroscopy? A prospective study. Knee Surg Sports Traumatol Arthrosc 2004; 12: 58–64. doi: 10.1007/s00167-003-0393-4 [DOI] [PubMed] [Google Scholar]
- 6.Ochi M, Sumen Y, Kanda T, Ikuta Y, Itoh K. The diagnostic value and limitation of magnetic resonance imaging on chondral lesions in the knee joint. Arthroscopy 1994; 10: 176–83. doi: 10.1016/S0749-8063(05)80090-8 [DOI] [PubMed] [Google Scholar]
- 7.Handelberg F, Shahabpour M, Casteleyn PP. Chondral lesions of the patella evaluated with computed tomography, magnetic resonance imaging, and arthroscopy. Arthroscopy 1990; 6: 24–9. doi: 10.1016/0749-8063(90)90092-R [DOI] [PubMed] [Google Scholar]
- 8.Weckbach S, Mendlik T, Horger W, Wagner S, Reiser MF, Glaser C. Quantitative assessment of patellar cartilage volume and thickness at 3.0 tesla comparing a 3D-fast low angle shot versus a 3D-true fast imaging with steady-state precession sequence for reproducibility. Invest Radiol 2006; 41: 189–97. [DOI] [PubMed] [Google Scholar]
- 9.Ruehm S, Zanetti M, Romero J, Hodler J. MRI of patellar articular cartilage: evaluation of an optimized gradient echo sequence (3D-DESS). J Magn Reson Imaging 1998; 8: 1246–51. doi: 10.1002/jmri.1880080611 [DOI] [PubMed] [Google Scholar]
- 10.Eckstein F, Hudelmaier M, Wirth W, Kiefer B, Jackson R, Yu J, et al. Double echo steady state magnetic resonance imaging of knee articular cartilage at 3 Tesla: a pilot study for the osteoarthritis initiative. Ann Rheum Dis 2006; 65: 433–41. doi: 10.1136/ard.2005.039370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wirth W, Nevitt M, Hellio Le Graverand MP, Benichou O, Dreher D, Davies RY, et al. ; OAI Investigators. Sensitivity to change of cartilage morphometry using coronal FLASH, sagittal DESS, and coronal MPR DESS protocols—comparative data from the osteoarthritis initiative (OAI). Osteoarthritis Cartilage 2010; 18: 547–54. doi: 10.1016/j.joca.2009.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Acebes C, Roman-Blas JA, Delgado-Baeza E, Palacios I, Herrero-Beaumont G. Correlation between arthroscopic and histopathological grading systems of articular cartilage lesions in knee osteoarthritis. Osteoarthritis Cartilage 2009; 17: 205–12. doi: 10.1016/j.joca.2008.06.010 [DOI] [PubMed] [Google Scholar]
- 13.Ficat RP, Philippe J, Hungerford DS. Chondromalacia patellae: a system of classification. Clin Orthop Relat Res 1979; 144: 55–62. [PubMed] [Google Scholar]
- 14.Noyes FR, Stabler CL. A system for grading articular cartilage lesions at arthroscopy. Am J Sports Med 1989; 17: 505–13. doi: 10.1177/036354658901700410 [DOI] [PubMed] [Google Scholar]
- 15.Outerbridge RE. The etiology of chondromalacia patellae. J Bone Joint Surg Br 1961; 43-B: 752–7. [DOI] [PubMed] [Google Scholar]
- 16.Lee KY, Masi JN, Sell CA, Schier R, Link TM, Steinbach LS, et al. Computer-aided quantification of focal cartilage lesions using MRI: accuracy and initial arthroscopic comparison. Osteoarthritis Cartilage 2005; 13: 728–37. doi: 10.1016/j.joca.2005.03.007 [DOI] [PubMed] [Google Scholar]
- 17.Brittberg M, Peterson L. Introduction of an articular cartilage classification. ICRS Newsl 1998; 1: 5–8. [Google Scholar]
- 18.Jinks C, Jordan K, Ong BN, Croft P. A brief screening tool for knee pain in primary care (KNEST). 2. Results from a survey in the general population aged 50 and over. Rheumatology (Oxford) 2004; 43: 55–61. doi: 10.1093/rheumatology/keg438 [DOI] [PubMed] [Google Scholar]
- 19.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33: 159–74. doi: 10.2307/2529310 [DOI] [PubMed] [Google Scholar]
- 20.Iwaki H, Pinskerova V, Freeman MA. Tibiofemoral movement 1: the shapes and relative movements of the femur and tibia in the unloaded cadaver knee. J Bone Joint Surg Br 2000; 82: 1189–95. doi: 10.1302/0301-620X.82B8.10717 [DOI] [PubMed] [Google Scholar]
- 21.Kleemann RU, Krocker D, Cedraro A, Tuischer J, Duda GN. Altered cartilage mechanics and histology in knee osteoarthritis: relation to clinical assessment (ICRS Grade). Osteoarthritis Cartilage 2005; 13: 958–63. doi: 10.1016/j.joca.2005.06.008 [DOI] [PubMed] [Google Scholar]
- 22.Ayral X, Dougados M, Listrat V, Bonvarlet JP, Simonnet J, Poiraudeau S, et al. Chondroscopy: a new method for scoring chondropathy. Semin Arthritis Rheum 1993; 22: 289–97. doi: 10.1016/S0049-0172(05)80008-3 [DOI] [PubMed] [Google Scholar]
- 23.Dougados M, Ayral X, Listrat V, Gueguen A, Bahuaud J, Beaufils P, et al. The SFA system for assessing articular cartilage lesions at arthroscopy of the knee. Arthroscopy 1994; 10: 69–77. doi: 10.1016/S0749-8063(05)80295-6 [DOI] [PubMed] [Google Scholar]
- 24.Cameron ML, Briggs KK, Steadman JR. Reproducibility and reliability of the Outerbridge classification for grading chondral lesions of the knee arthroscopically. Am J Sports Med 2003; 31: 83–6. [DOI] [PubMed] [Google Scholar]
- 25.Potter HG, Linklater JM, Allen AA, Hannafin JA, Haas SB. Magnetic resonance imaging of articular cartilage in the knee. An evaluation with use of fast-spin-echo imaging. J Bone Joint Surg Am 1998; 80: 1276–84. [DOI] [PubMed] [Google Scholar]
- 26.Cicuttini FM, Wluka AE, Wang Y, Stuckey SL. Longitudinal study of changes in tibial and femoral cartilage in knee osteoarthritis. Arthritis Rheum 2004; 50: 94–7. doi: 10.1002/art.11483 [DOI] [PubMed] [Google Scholar]
- 27.Peterfy CG. Imaging of the disease process. Curr Opin Rheumatol 2002; 14: 590–6. doi: 10.1097/00002281-200209000-00020 [DOI] [PubMed] [Google Scholar]
- 28.Figueroa D, Calvo R, Vaisman A, Carrasco MA, Moraga C, Delgado I. Knee chondral lesions: incidence and correlation between arthroscopic and magnetic resonance findings. Arthroscopy 2007; 23: 312–15. doi: 10.1016/j.arthro.2006.11.015 [DOI] [PubMed] [Google Scholar]
- 29.Disler DG, McCauley TR, Wirth CR, Fuchs MD. Detection of knee hyaline cartilage defects using fat-suppressed three-dimensional spoiled gradient-echo MR imaging: comparison with standard MR imaging and correlation with arthroscopy. AJR Am J Roentgenol 1995; 165: 377–82. doi: 10.2214/ajr.165.2.7618561 [DOI] [PubMed] [Google Scholar]
- 30.von Engelhardt LV, Kraft CN, Pennekamp PH, Schild HH, Schmitz A, von Falkenhausen M. The evaluation of articular cartilage lesions of the knee with a 3-Tesla magnet. Arthroscopy 2007; 23: 496–502. doi: 10.1016/j.arthro.2006.12.027 [DOI] [PubMed] [Google Scholar]
- 31.Jungius KP, Schmid MR, Zanetti M, Hodler J, Koch P, Pfirrmann CW. Cartilaginous defects of the femorotibial joint: accuracy of coronal short inversion time inversion-recovery MR sequence. Radiology 2006; 240: 482–8. doi: 10.1148/radiol.2401050077 [DOI] [PubMed] [Google Scholar]
- 32.Recht MP, Kramer J, Marcelis S, Pathria MN, Trudell D, Haghighi P, et al. Abnormalities of articular cartilage in the knee: analysis of available MR techniques. Radiology 1993; 187: 473–8. doi: 10.1148/radiology.187.2.8475293 [DOI] [PubMed] [Google Scholar]
- 33.Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage 2008; 16: 1433–41. doi: 10.1016/j.joca.2008.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fischbach F, Bruhn H, Unterhauser F, Ricke J, Wieners G, Felix R, et al. Magnetic resonance imaging of hyaline cartilage defects at 1.5T and 3.0T: comparison of medium T2-weighted fast spin echo, T1-weighted two-dimensional and three-dimensional gradient echo pulse sequences. Acta Radiol 2005; 46: 67–73. doi: 10.1080/02841850510012625 [DOI] [PubMed] [Google Scholar]
- 35.Luhmann SJ, Schootman M, Gordon JE, Wright RW. Magnetic resonance imaging of the knee in children and adolescents. Its role in clinical decision-making. J Bone Joint Surg Am 2005; 87: 497–502. doi: 10.2106/JBJS.C.01630 [DOI] [PubMed] [Google Scholar]
- 36.Masciocchi C, Barile A, Lelli S, Calvisi V. Magnetic resonance imaging (MRI) and arthro-MRI in the evaluation of the chondral pathology of the knee joint. [In Italian.] Radiol Med 2004; 108: 149–58. [PubMed] [Google Scholar]
- 37.Arøen A, Løken S, Heir S, Alvik E, Ekeland A, Granlund OG, et al. Articular cartilage lesions in 993 consecutive knee arthroscopies. Am J Sports Med 2004; 32: 211–15. doi: 10.1177/0363546503259345 [DOI] [PubMed] [Google Scholar]
- 38.Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy 1997; 13: 456–60. doi: 10.1016/S0749-8063(97)90124-9 [DOI] [PubMed] [Google Scholar]
- 39.Niemeyer P, Pestka JM, Erggelet C, Steinwachs M, Salzmann GM, Südkamp NP. Comparison of arthroscopic and open assessment of size and grade of cartilage defects of the knee. Arthroscopy 2011; 27: 46–51. doi: 10.1016/j.arthro.2010.05.024 [DOI] [PubMed] [Google Scholar]
- 40.Widuchowski W, Widuchowski J, Trzaska T. Articular cartilage defects: study of 25,124 knee arthroscopies. Knee 2007; 14: 177–82. doi: 10.1016/j.knee.2007.02.001 [DOI] [PubMed] [Google Scholar]