Abstract
Intra-arterial therapies (IATs) play a pivotal role in the management of patients with primary and secondary liver malignancies. The unique advantages of these treatments are their ability to selectively deliver a high dose of anticancer treatment while preserving healthy liver tissue. The proven efficacy of these catheter-based locoregional therapies in a highly systemic chemoresistant cancer such as hepatocellular carcinoma (HCC), along with the minimally invasive nature of these treatments, quickly yielded wide acceptance in the medical community and revolutionized the field of Interventional Oncology. In this article, we describe the clinical rationale and background of catheter-based IATs. We provide an overview of clinical achievements of these treatments alone and in combination with sorafenib in patients with HCC.
Hepatocellular carcinoma (HCC) is the fifth most common malignancy in males and the seventh in females.1 This disease carries a dismal prognosis, corresponding to the third most common cancer-related death worldwide.2 The incidence of HCC is rising worldwide and has almost tripled in the last three decades in the USA.3 About 80% of patients with HCC are diagnosed with disease that is not amenable to liver transplantation or surgical resection.4 Thus, a majority of patients with unresectable HCC are referred for image-guided locoregional therapies. These interventional treatments include catheter-based techniques [transarterial chemoembolization (TACE), drug-eluting beads TACE (DEBs-TACE), transarterial embolization (TAE), radioembolization using yttrium-90 (Y90)] and percutaneous thermal (radiofrequency, microwave and laser ablation or cryoablation) and chemical (such as percutaneous ethanol injection) techniques. More recently, techniques such as irreversible electroporation and high-intensity focused ultrasound have been developed.
Although being part of the armamentarium of Interventional Oncology, percutaneous locoregional ablative therapies and their combination with catheter-based intra-arterial therapies (IATs), as well as the role of the latter in the downstaging and bridging of patients with HCC to surgical resection and liver transplantation, will not be discussed because of space limitations. The aim of this article is to describe the clinical rationale and background of catheter-based IATs in patients with HCC. An overview of clinical achievements of these treatments alone and in combination with sorafenib will be discussed.
CLINICAL RATIONALE
As opposed to most other organs, the liver has a dual vascular supply via the portal vein and the hepatic artery. Interestingly, liver malignancies are predominantly vascularized by the hepatic artery, while non-tumoral liver parenchyma is supplied mostly by the portal vein.5 This pathophysiological particularity provides a unique advantage for catheter-based therapies. In TACE, the embolization of the vascular supply to the tumour causes ischaemic tumour necrosis and prevents rapid washout of the chemotherapeutic drug allowing for better locoregional diffusion of the payload into targeted tumour tissues. This allows the locoregional infusion to reach a therapeutic drug concentration that could otherwise not be achieved by a systemic delivery, thus maximizing anticancer drug efficacy while minimizing systemic toxicity. In TAE, only bland embolization is performed (i.e. no chemotherapeutic drug is delivered). In radioembolization, liver-directed catheter-infused Y90-loaded microspheres can deliver tumoricidal radiation doses while sparing healthy liver tissue and surrounding structures.
PATIENT SELECTION
The best available treatment for every HCC patient should always be decided in consensus by a multidisciplinary team. IATs can be used to downstage lesions to surgical treatment, as a bridge to surgical tumour resection or liver transplantation, and for palliative treatment of patients with unresectable HCC. Specifically, TACE has been included in the official treatment guidelines for the management of HCC.6 According to the Barcelona Clinic Liver Cancer (BCLC) staging system, the most widely used treatment algorithm for HCC in Western countries, TACE can be applied as a palliative therapy in patients with intermediate-stage disease.6 Not all patients with unresectable HCC can benefit from TACE. The best candidates have preserved liver function, adequate performance status [according to Eastern Cooperative Oncology Group (ECOG) performance status], asymptomatic and liver-limited multinodular disease without tumour vascular invasion.6–8 Liver function, assessed by the Child–Pugh classification, plays a pivotal role in patient selection. Ideal candidates are Child–Pugh A, but patients with up to Child–Pugh B7 without ascites can be considered for therapy.6 The presence of main or branch portal vein thrombosis (PVT) with or without tumour invasion decreasing significantly the blood flow (e.g. hepatofugal blood flow on ultrasound) is a contraindication to TACE due to the risk of ischaemic liver necrosis.9 However, TACE can be performed safely in selected patients with PVT.10–12 Selection criteria for TAE are similar to TACE. Until recently, there were no defined recommendations for the use of radioembolization in patients with HCC. Potential indications for radioembolization include patients with intermediate-stage disease who are poor candidates for TACE, patients with large solitary tumours invading segmental or lobar branches of the portal vein and patients with progressive disease after TACE.13 The most important contraindications to all IATs are similar and are summarized in Table 1.
Table 1.
Main contraindications to TACE and radioembolization
| Relative contraindications | Absolute contraindications |
|---|---|
| • Diffuse tumour burden involving >50% of the liver | • Eastern Cooperative Oncology Group performance status >2 |
| • Segmental or branch PVTa | • Severely reduced portal flow by branch or main PVT (e.g. hepatofugal blood flow)a |
| • Extrahepatic metastases | • Active systemic infection |
| • Ascites | • Uncorrectable bleeding disorder |
| • Serum bilirubin >3 mg dl–1 | • Uncorrectable contrast media sensitivity |
| • High serum levels of lactate dehydrogenase (>425 U l–1) | • Leukopenia (white blood cell count <1000 μl–1) |
| • High serum levels of aspartate aminotransferase and alanine aminotransferase (>5 × upper limit of normal) | • Renal insufficiency (serum creatinine >2 mg dl–1, glomerular filtration rate <30 ml min–1) |
| • Biliary obstruction | • Hepatic encephalopathy |
| • Severe thrombocytopaenia (<50,000 μl–1) | • Excessive hepatopulmonary shuntingb |
| • Recent variceal bleeding | • Tc99m-MAA scan showing gastrointestinal deposition technically not correctableb |
| • Intractable arteriovenous fistula | |
| • Right-to-left cardiopulmonary shunting | |
| • Prior hepatic radiotherapyb |
PVT, portal venous thrombosis; TACE, transarterial embolization; Tc99m-MAA, technetium-99m—macroaggregate albumin.
Specific to TACE.
Specific to radioembolization.
BACKGROUND AND CLINICAL EVIDENCE
Transarterial chemoembolization
The 1970s was the decade that brought the advent of catheter-based arterial embolization techniques. Indications such as control of haemorrhage and palliation of local symptoms like pain in cancer patients quickly expanded to include bridge therapy to surgery and cancer treatment, notably renal cancer, using embolic agents such as Gelfoam® and coils.14–18 In the late 1970s–early 1980s, studies began to evaluate the combination of an anticancer drug (mitomycin C or doxorubicin) followed by an embolic agent (gelatin-based agent; Gelfoam) in unresectable HCC.19,20 Hence, the concept of “chemoembolization” was introduced. Nakamura et al21 compared this combination therapy to the infusion of an emulsion composed of a chemotherapeutic drug and an oily contrast medium (Lipiodol®; Laboratoire Guerbet, Aulnay-sous-Bois, France) followed by Gelfoam embolization and demonstrated a survival benefit for the latter. This work along with others established the use of Lipiodol in TACE [known as conventional TACE (cTACE)]. Lipiodol combines unique properties of a drug carrier and an embolic agent. Moreover, this poppy seed oil-based chemical is a radio-opaque contrast agent that accumulates preferentially in hepatic malignancies and persists in tumour nodules for weeks.22–24 Lipiodol has been widely used as a suspension medium for chemotherapeutic agents in cTACE. Because no universally accepted protocol exists, several regimens of cTACE have been used. Doxorubicin is the most commonly administered drug worldwide. In the USA, a combination of doxorubicin, mitomycin C and cisplatin (currently not administered due to shortage) is utilized (Figure 1).25 This combination has been recently shown to have a higher response rate and less tumour progression compared to doxorubicin alone.26 There is evidence showing that chemoembolization followed by bland embolization achieves better local response and survival compared to chemoembolization alone.27,28 Several different types of embolization material delivered after the infusion of the Lipiodol/anticancer drug emulsion have been used and they include temporary materials such as absorbable gelatin (Gelfoam; Pfizer Inc., New York, NY) and degradable starch microspheres (Spherex; Pharmacia AB, Stockholm, Sweden) or permanent materials such as polyvinyl alcohol (PVA) particles (Contour SE; Boston Scientific, Marlborough, MA) PVA hydrogel beads (Bead Block™; Biocompatibles UK Ltd, Farnham, UK), polyphosphazene-coated polyacrylate microspheres (Embozene® Microspheres; CelaNova Bio-Sciences, Peachtree, GA) and trisacryl gelatin microspheres (Embosphere® Microspheres; Merit Medical Systems, Inc., South Jordan, UT).29,30 cTACE is performed on demand allowing for a personalized patient-centred approach and better safety profile compared to fixed interval treatment. cTACE is safe and well tolerated with a favourable long-term toxicity profile.31 While no consensus exists about the number of cTACE procedures to achieve satisfactory target lesion treatment, at least two sessions of chemoembolization should be performed before further treatment is abandoned or alternative therapies are considered.32 Patients are typically followed up 6 weeks after the procedure for clinical, blood work and cross-sectional imaging evaluation.
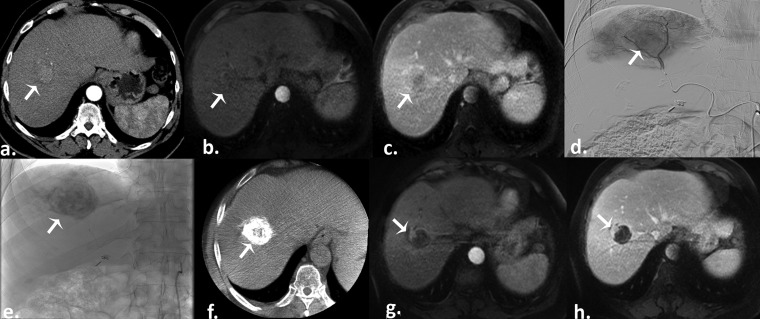
Figure 1.
Conventional transarterial chemoembolization in a 68-year-old male with a history of hepatitis C cirrhosis recently diagnosed with hepatocellular carcinoma (HCC). (a) Pretreatment arterial phase CT scan reveals a hyperenhancing right hepatic lobe mass consistent with HCC (arrow). (b) Pretreatment arterial phase T1 weighted gradient-echo MR scan demonstrates the lesion, with subsequent washout on the portal venous phase (c) (arrows). (d) Selective digital subtraction angiography demonstrates tumour blush (arrow). Postembolization single-snap shot (e) and cone beam CT (f) show an excellent Lipiodol® deposition into the tumour (arrows). Arterial (g) and portal venous (h) phases T1 weighted gradient-echo MR images obtained 1 month after therapy demonstrate a good result (partial response) with small residual viable tumour (arrows).
The lack of consensus and uniformly adopted protocols together with heterogeneous study cohorts casted doubt on the benefit of cTACE in patients with unresectable HCC. Four randomized controlled trials (RCTs) failed to show any survival benefit of cTACE vs symptomatic treatment33–35 or tamoxifen,36 and two systematic reviews37,38 highlighted discrepant data. However, in 2002, two RCTs clearly demonstrated the survival benefit of cTACE over symptomatic treatment in selected HCC patients who were not eligible for surgical therapy.7,8 These two landmark studies along with a systematic review of RCTs39 led to the inclusion of cTACE into the official treatment guidelines for HCC.6,40,41 cTACE is endorsed by the American Association for the Study of the Liver Diseases (AASLD) and the European Association for the Study of the Liver (EASL).6,40,41 Upon the introduction of cTACE, the median overall survival (OS) for intermediate stage HCC patients (according to the BCLC classification) increased from approximately 16 to 20 months, propelling this treatment modality as the standard of care.6,39
The presence of portal invasion is a common feature in HCC patients. This classifies a patient as advanced stage according to the BCLC classification and no IAT is recommended. Selected patients with PVT treated with cTACE have shown a potential benefit in survival compared to best supportive care.10–12 However, it should be noted that this evidence is only based on non-controlled studies.
Key points of conventional TACE
cTACE is safe and effective in properly selected patients
Preserved liver function is essential as survival benefit has only been shown for patients with Child–Pugh A or B7 without ascites
Ideal patient profile: preserved liver function, adequate ECOG performance status, asymptomatic multinodular liver disease without macrovascular tumour invasion and extrahepatic disease
cTACE constitutes the standard of care for intermediate-stage (BCLC B) HCC patient (Level I evidence)
Drug-eluting beads transarterial chemoembolization
Microparticulate drug delivery systems have come a long way. Ehrlich's “magic bullet” concept dating from the beginning of the 20th century,42 with the goal of improved drug efficacy while reducing related side effects, has led to important investigations in the field of drug delivery. In 1987, the ideal characteristics of drug-eluting microspheres were described:43 appropriate-sized beads containing a variety of anticancer drugs are used as vehicles for the locoregional delivery via the arterial system of an organ harboring cancer, to cause tumour infarction through an embolic effect and providing an ideal setting for a controlled and sustained drug release with decreased systemic exposure. The following two decades saw the refinement and further development of DEBs which resulted in a promising alternative to conventional Lipiodol-based regimens. Several DEB systems have been tested such as DC Bead (Biocompatibles UK Ltd) and HepaSphere™/QuadraSphere® microspheres (Merit Medical Systems, Inc.). The DC Beads are PVA-based microspheres and range from 75 to 900 µm in size, while the HepaSphere microspheres are superabsorbent polymer based and range from 120 to 800 µm in size. Both systems can be efficiently loaded with doxorubicin (and irinotecan),44 and preclinical studies have shown safe pharmacokinetic profiles with sustained drug release and antitumour efficacy.45–47 In clinical practice, DEB-TACE has the same indications and contraindications as cTACE. The best candidates for DEB-TACE have a disease that can be selectively targeted. To date, most of the clinical data about DEB-TACE have been generated with DC Bead. Therefore, doxorubicin-loaded HepaSphere microspheres have less clinical validation although similar results to DC Bead have been reported.48,49 Major studies with DC Bead are presented here.
In 2007, Varela et al50 published seminal data on the safety, pharmacokinetics and efficacy of DC Bead loaded with doxorubicin (DEBDOX; 500–700 µm) in selected patients (n = 27) with HCC (Child–Pugh A, BCLC B). The procedure was well tolerated with postembolization syndrome observed in 37% of patients after the first DEB-TACE, which dropped to 18% after the second. Two patients developed liver abscess. Importantly, peak plasma concentrations of doxorubicin were significantly lower compared to those measured in cTACE. Objective response was seen in two-thirds of the patients according to the EASL guidelines [i.e. tumour response is based on the assessment of enhancing portion of the target tumours (the product of bidimensional diameter of enhancing tissue)].51 The same year, the results of a combined Phase I/II study in patients with HCC (n = 15/n = 20, respectively; 100% Child–Pugh A) were reported.52 The Phase I trial, a dose-escalating study starting from 25 to 150 mg of doxorubicin, showed no dose-limiting toxicity. The Phase II trial showed an objective response in 70% of the patients according to the modified response evaluation criteria in solid tumours (mRECIST; tumour response is based on the assessment of the longest enhancing diameter of the target tumours). Six patients had treatment-related complications. In 2008, similar results were reported in an open-label, single-centre, single-arm study including 62 patients with unresectable HCC.53 Patients received up to three sessions of DEBDOX (300–500 µm). At 9-month follow-up, the objective response was 80.7%. All patients reported postembolization symptoms, although severe procedure-related complications were observed in only 3.2%. In the United States, the first prospective Phase II pilot study evaluating the safety and efficacy of DEBDOX (100–300 or 300–500 µm) was reported in 2009.54 20 patients (Child–Pugh A, 75%; BCLC C, 60%) underwent 34 DEB-TACE sessions. The safety profile was good with only 10% of grade III toxicities. Two patients died within 30 days after DEB-TACE but neither death could be attributed to the procedure. Objective response at 1 month was 60% (EASL) and disease control at 6 months was 95% (RECIST; tumour response is based on the assessment of the longest diameter of the target tumours). More importantly, this study reported encouraging outcomes in patients with more advanced disease with a median progression-free survival (PFS) of 13 months and OS of 26 months (Figure 2). Recently, unparalleled outcomes were achieved in almost 300 HCC patients with early and intermediate stage disease [mean OS of 43.8 months55 and median OS of 48.6 months (BCLC A: 54.2 months and BCLC B: 47.7 months)56]. Of note, these two works highlight the pivotal role and challenge of patient selection in HCC.
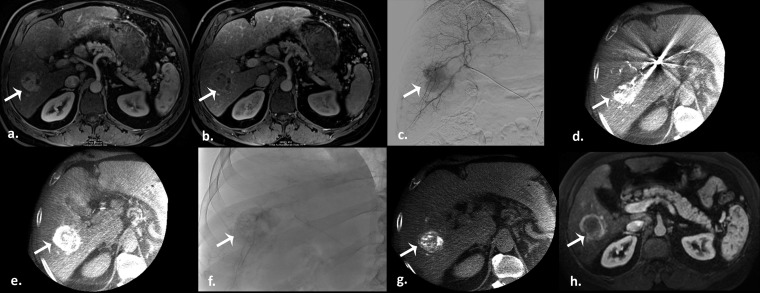
Figure 2.
Drug-eluting beads transarterial chemoembolization in a 55-year-old male with a history of nonalcoholic steatohepatitis. (a) Pretreatment arterial phase T1 weighted gradient-echo MR scan demonstrates a hyperenhancing hepatocellular carcinoma in the right hepatic lobe, with subsequent washout on the portal venous phase (b) (arrows). (c) Selective digital subtraction angiography clearly demonstrates tumour blush (arrow). Intraprocedural pretreatment dual-phase cone beam CT (CBCT) in early (d) and late (e) arterial phases evidence the lesion (arrows). Postembolization single-snap shot (f) and CBCT (g) show an excellent contrast staining of the tumour (arrows). (h) Arterial phase T1 weighted gradient-echo MR scan obtained 1 month after therapy demonstrate a good result (partial response) (arrow).
The safety and survival outcomes of DEB-TACE in patients with advanced stage HCC have recently been evaluated. Two retrospective works combining 201 patients (Child–Pugh A/B: 123/78, BCLC C: 100%, ECOG 0/1/2: 22/139/40) reported 19 patients with grade 3 toxicities.57,58 Neither grade 4 toxicities nor 30-day mortality were observed. Similar median OS was obtained with 13.357 and 13.5 months,58 respectively. These studies underscore the favourable toxicity profile of DEB-TACE with advanced disease and the reported encouraging outcomes need to be validated in well-conducted prospective trials.
The question about the safety and the size of available DEBDOX was addressed in a recent publication.59 The use of small caliber beads (100–300 µm) in tumours of <6 cm was not associated with an increase in liver toxicity or complications when compared to larger beads (300–500 µm).59 An ongoing prospective trial is evaluating the feasibility and safety of using 70–150 µm beads (LC-Bead M1) loaded with doxorubicin in patients with HCC and will certainly bring more data about the use of small-caliber microspheres (Figure 3).60
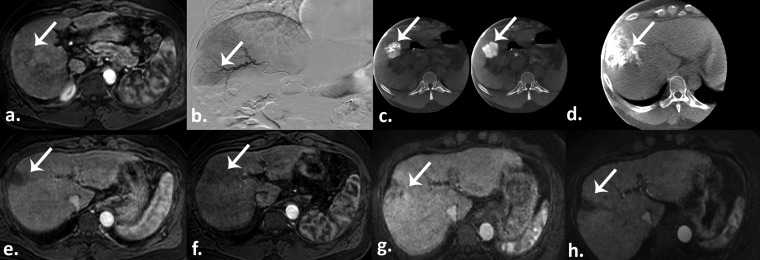
Figure 3.
Drug-eluting beads transarterial chemoembolization in a 48-year-old male with a history of alcohol abuse-related liver cirrhosis and hepatocellular carcinoma (HCC). (a) Pretreatment arterial phase T1 weighted gradient-echo MR scan demonstrates a hyperenhancing HCC in segment six (arrow). (b) Selective digital subtraction angiography shows tumour blush (arrow). (c) Intraprocedural pretreatment dual-phase cone beam CT (CBCT) shows the hypervascularized lesion (arrows). (d) Postembolization intraprocedural CBCT shows an excellent contrast staining of the segment containing the tumour (arrow). Arterial phase T1 weighted gradient-echo MR scans obtained 1 month (e), 3 months (f), 6 months (g), and 9 months (h) after therapy demonstrate chemoembosegmentectomy with complete response and no residual viable tumour (arrows).
Drug-eluting beads transarterial chemoembolization vs conventional transarterial chemoembolization
A prospective randomized multicentre trial of 212 patients across Europe compared efficacy and safety of DEB-TACE using DEBDOX to cTACE.61 Although response rates were higher in the DEB-TACE group, this study failed to show any statistically significant difference in efficacy compared to cTACE in the entire study population. However, patients with more advanced (ECOG 1, BCLC B, bilobar lesions) and recurrent disease showed better objective response when treated with DEB-TACE. With a significant decrease of liver toxicity and doxorubicin-related adverse events, this trial confirmed the better tolerability profile of DEB-TACE over cTACE. In a retrospective study, Song et al62 reported a better treatment response in patients who received DEBDOX vs cTACE with no differences in treatment-related liver toxicity. Longer time to progression (TTP) and better OS were also seen with DEBDOX.62 These promising results, however, need to be confirmed in a well-designed prospective RCT of properly selected HCC patients to fully establish the survival benefit of DEB-TACE over cTACE.
Key points of DEB-TACE
DEB-TACE is safe and effective in properly selected patients
Ideal patient profile: preserved liver function, adequate ECOG performance status, asymptomatic and selectively targetable liver disease (as opposed to lobar treatment), without macrovascular tumour invasion and extrahepatic disease
DEB-TACE has a better toxicity profile compared to cTACE (Level I evidence)
To date, DEB-TACE has not shown better survival compared to cTACE
Transarterial embolization
Unlike TACE, TAE relies solely on the occlusion of the vascular supply to the tumour. Proponents of TAE believe that the ischaemic insult induced by embolization is sufficient to cause tumour cell death and that the adjunction of a chemotherapeutic agent contributes to unnecessary toxicity. In 1998, Bruix et al63 reported the results of a prospective single centre RCT comparing TAE (n = 40) vs symptomatic treatment (n = 40) in patients with unresectable HCC. Distal TAE was performed using Gelfoam; in patients with unilobar disease, distal TAE was combined with proximal coiling (n = 18). Despite a marked antitumoral effect (partial response was observed in 55% of the patients in the TAE group), there was no benefit in survival compared to untreated patients. In 2008, the outcomes of 322 patients treated with 766 TAEs were reported in a single-armed single-institution retrospective study.64 The median OS was 21 months (16–26 months). The 1-, 2-, and 3-year survival rates were 66%, 46%, and 33%, respectively. In the absence of PVT or extrahepatic disease, the median survival was 40 months (31–52 months) and the 1-, 2-, and 3-year survival rates were 84%, 66% and 51%, respectively. These promising results escalated the need for an RCT. In 2010, an RCT comparing TAE (Bead Block, 100–300 and 300–500 µm) vs DEB-TACE (DC Bead, 100–300 and 300–500 µm) in 84 (43/41) patients with intermediate-stage HCC was published.65 Patients were randomized by tumour size and treated every 2 months, up to three procedures. Complications were similar in both groups. DEB-TACE yielded a better local response with higher response rates at every time point of the study (6, 9 and 12 months) reaching statistical significance at 9 months. DEB-TACE had fewer recurrences at 9 and 12 months and longer TTP compared to TAE (10.6 ± 2.7 vs 9 ± 2.3 months, respectively).65 Unfortunately, the short follow-up (12 months) precluded any definite conclusion about survival. The results of a Phase II study comparing TAE (Bead Block) vs DEB-TACE (LC Beads—150 mg doxorubicin) were recently presented (not yet published).66 Study arms were composed of 51 and 50 patients (TAE and DEB-TACE, respectively). No difference in adverse events, response or disease control rate, PFS or OS [6.8 vs 8.9 months (p = 0.59) and 14 vs 16 months (p = 0.7) for TAE and DEB-TACE, respectively] were found between both groups.
Taken together, these results demonstrate that TAE has a clear antitumour effect. These findings suggest that the main trigger of cell death in TACE can also be attributed to the ischaemic insult provided by the embolization. However, the addition of cytotoxic drug is beneficial until proven otherwise based on the current clinical evidence. Thus, further investigations are needed to dispute the dominance of cytotoxic catheter-based therapies over bland embolization. TAE is currently not endorsed by the EASL or the AASLD.6
Key points of TAE
TAE is safe and effective in properly selected patients
Ideal patient profile: preserved liver function, adequate ECOG performance status, asymptomatic and selectively targetable liver disease (as opposed to lobar treatment), without macrovascular tumour invasion and extrahepatic disease
Further studies are needed to evaluate TAE in comparison to other catheter-based therapies
TAE is currently not included in the official treatment guidelines for HCC
Radioembolization with yttrium-90
Radioembolization is the catheter-based intra-arterial infusion of microspheres labelled with a radioactive isotope. To date, Y90, a pure beta-emitter, is the most widely used isotope in the locoregional treatment of liver malignancies. In contrast to TACE, the antitumour effect is mainly caused by the radiation while embolization is only a minor contributor.67 Two types of microspheres are currently used. The first are resin-based (SIR-Spheres®; Sirtex Medical Ltd, North Sydney, Australia) with a diameter of 20–60 µm and an activity of 50 Bq per microsphere. The number of spheres per vial is 40–80 × 106. The second are glass-based (TheraSphere®; MDS Nordion, Ottawa, Canada) with a diameter of 20–30 µm and an activity of 2500 Bq per microsphere. The number of spheres per vial is 1.2–8 × 106.68 Despite these particularities, both microsphere types have shown similar efficacy and outcomes.69
This technology, although already evaluated in the late 1950s–60s,70–72 has matured into a palliative treatment option for patients with HCC over the last two decades. Salem and Thurston published a detailed and comprehensive literature review of seminal works of Y90 radioembolization in HCC patients, covering the late 1980s and mid 2000s.73 In substance, these studies established the safety of this technique, the optimal tumoricidal dose calculation based on hepatic and tumour volumes, the role of the shunt study and the management of collaterals responsible for liver shunting. Many of the more recent and influential works are highlighted here.
In 2004–05, two important series established the safety and efficacy of Y90 radioembolization in HCC patients.74,75 These landmark works have since been confirmed in two of the largest studies reported to date.76,77 In 2010, Salem et al76 published a single-centre, prospective study in 291 patients (Child–Pugh A/B/C, 131/152/8; BCLC A/B/C/D, 48/83/152/8; PVT, 125). Response rates according to EASL were 57%. The median follow-up was 30.9 months. Overall median TTP was 7.9 months. Median survival time for Child–Pugh/BCLC A compared to Child–Pugh/BCLC B patients was 17.2/26.9 and 7.7/17.2 months, respectively. Child–Pugh A patients with or without PVT had a median survival of 10.4 and 22.1 months, whereas Child–Pugh B patients with or without PVT had a median survival of 5.6 and 14.8 months, respectively. Sangro et al77 reported findings on a large multicentre retrospective study conducted in Europe. Here, 325 patients (Child–Pugh A/B, 268/57; BCLC A/B/C/D, 52/87/183/3; PVT, 76) were included. The median OS of the cohort was 12.8 months and depended on the BCLC class (BCLC A, 24.4 months; BCLC B, 16.9 months; BCLC C, 10.0 months) reflecting liver function and tumour burden. The presence of PVT was identified as a negative predictive factor of survival (10 months with PVT vs 15.3 months without PVT).
Patients with HCC invading the portal vein constitute a common indication for Y90 radioembolization. Indeed, the apprehensiveness of decreasing the arterial blood supply by TACE in the setting of portal vein invasion provides an appealing rationale for the use of Y90 radioembolization. In 2008, Kulik et al78 evaluated the safety and efficacy of Y90 in the presence (n = 37) and absence (n = 71) of PVT. Cirrhosis and PVT had higher adverse event rates. However, there were no clinically significant differences in bilirubin toxicities when stratifying by PVT status. Moreover, the risk of hepatic encephalopathy and liver failure was not increased by the presence of PVT. Objective response was reported in 70%. Survival data were hampered by a short follow-up (6 months). Importantly, this study established the safety profile of Y90 radioembolization in PVT and further supported the notion of the microembolic (compared to macroembolic for TACE) component of this liver-directed brachytherapy (Figure 4). In 2010, Hilgard et al79 reported the results of a prospective, single-centre observational study designed to validate safety and antitumour effect of Y90 in advanced stage HCC patients who were not candidates for TACE or other locoregional treatments. 108 patients (Child–Pugh A/B, 84/24; BCLC A/B/C, 2/51/55; PVT, 33) underwent 159 Y90 sessions. The most frequent adverse events were transient fatigue and abdominal pain. Objective response at 90 days was 40%. For the entire cohort, the median TTP was 10 months and median survival time was 16.4 months (Child–Pugh A/B, 17.2/6 months; no PVT/PVT, 16.4/10 months).
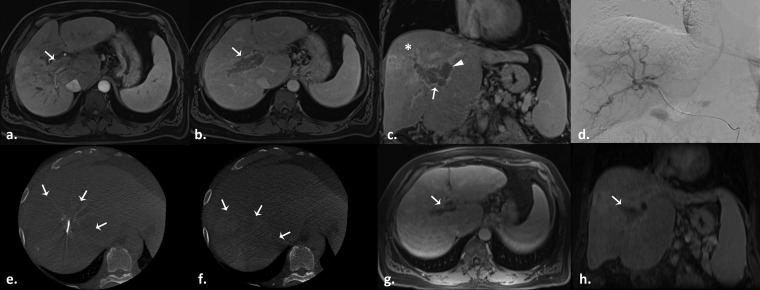
Figure 4.
Radioembolization with glass-based Y90 microspheres in a 62-year-old male with a history of haemachromatosis and right lobe infiltrative hepatocellular carcinoma with portal vein invasion. (a) Pretreatment arterial phase T1 weighted gradient-echo MR image demonstrates an enhancing mass in the right portal vein consistent with tumour thrombus (arrow). Portal vein tumour thrombus washout is visible on the subsequent portal venous on axial (b) and coronal (c) views (arrows), as well as its extension into the left portal vein (c, arrowhead). The infiltrative tumour of the right hemi-liver is better appreciated on the coronal view (star). (d) Selective digital subtraction angiography fails to clearly demonstrate tumour blush. Intraprocedural pretreatment dual-phase cone beam CT in early (e) and late (f) arterial phases better evidence the lesion extension (arrows). Axial (g) and coronal (h) venous phase T1 weighted gradient-echo MR images obtained 3 months after therapy demonstrate portal vein thrombus shrinkage (arrows).
Taken together, these encouraging outcomes underline the increasing role of Y90 radioembolization in the treatment of HCC, especially in patients with locally advanced tumour with or without PVT with preserved liver function (Child–Pugh A) or impaired liver function (Child–Pugh B) without PVT.
Y90 vs conventional transarterial chemoembolization
In 2011, Salem et al80 published a comparative study of the efficacy of Y90 radioembolization vs cTACE. Patients were balanced for Child–Pugh class, BCLC and United Network for Organ Sharing systems. Both therapies showed a similar response rate. Y90 radioembolization demonstrated a longer TTP compared to cTACE (13.3 vs 8.4 months, respectively, p = 0.046). However, no difference in median OS was observed (20.5 months for Y90 vs 17.4 months for cTACE; p = 0.232). Patients with intermediate-stage disease had a similar survival whether treated by cTACE (17.5 months) or Y90 radioembolization (17.2 months; p = 0.42).
Despite convincing and compelling data supporting the safety and efficacy of Y90, the evidence, to date, is derived solely from retrospective and prospective non-controlled studies. As such, the AASLD and EASL have not endorsed radioembolization as a standard of care. A head-to-head comparison of Y90 to cTACE is a real challenge because similarities in survival outcomes require a large patient cohort (>1000) to demonstrate equivalence.80 However, many clinical studies are under way. Among them, a multicentre randomized Phase II trial (PREMIERE: Prospective Randomized Trial of Radioembolization and Chemoembolization in Hepatocellular Carcinoma81) comparing Y90 to cTACE is very much expected.
Key points of Y90 radioembolization
Y90 radioembolization is safe, particularly in the setting of portal venous invasion, in properly selected patients
Patients with intermediate-stage disease (BCLC B) who are poor candidates for TACE, patients with large solitary tumours invading the portal vein or patients with progressive disease after TACE are considered good candidates
Y90 radioembolization achieved strong anticancer efficacy but did not demonstrate survival benefit over TACE
There are no RCTs comparing Y90 radioembolization with other IATs
Y90 radioembolization is currently not endorsed by the AASLD or EASL
ANTIANGIOGENIC THERAPY
Unequivocal evidence has demonstrated that hypoxia present in the tumour microenvironment triggers the accumulation of hypoxia-inducible factor one and the subsequent overexpression of the pro-angiogenic vascular endothelial growth factor (VEGF) gene.82,83 Thus, targeting molecular mechanisms of angiogenesis has been the subject of intense research. However, no antiangiogenic drug that entered Phase III trials showed significant benefits with one exception, sorafenib, an oral multikinase inhibitor of VEGF receptors (1–3), Raf-1, platelet-derived growth factor receptor β, B-Raf and c-Kit. Indeed, between 2005 and 2007, two Phase III, multicentre, double-blinded, placebo-controlled trials independently demonstrated a survival benefit of sorafenib over supportive treatment.84,85 In the Western series,84 the median OS was 10.7 months in the sorafenib group and 7.9 months in the placebo group (p < 0.001), whereas in the Asian series,85 the median OS was 6.5 months for the sorafenib group and 4.2 months for the placebo group (p = 0.014). In a notoriously systemic chemoresistant cancer such as HCC, these positive results, although modest, earned wide acceptance. Sorafenib became the standard of care and changed the landscape of advanced disease in HCC. Major works with sorafenib combined various IATs are presented here.
Conventional transarterial chemoembolization and sorafenib
The interim results of the START (Study in Asia of the Combination of TACE with sorafenib in HCC Patients) trial were recently published.86 This prospective Phase II, open label study evaluates the safety and efficacy of the combination of cTACE (Lipiodol/doxorubicin followed by Gelfoam embolization) and sorafenib in patients from the Asia-Pacific region with intermediate-stage HCC. Sorafenib (400 mg twice daily) was given on an interrupted treatment schedule starting 4–7 days after cTACE, with cTACE sessions being performed on demand. Patients who were not candidates for additional cTACE procedures continued on sorafenib monotherapy until unacceptable toxicity or disease progression occurred. A majority of the 147 patients experienced gastrointestinal (62.6%) or skin (57.8%) adverse events, most being mild to moderate. Objective response according to mRECIST was seen in 52.4% of patients. PFS and TTP were 9 and 9.3 months, respectively. This study confirmed the safety profile of the combination therapy using an interrupted sorafenib protocol.87
In 2013, a propensity score analysis was performed to investigate outcomes of the combination of cTACE (Lipiodol/Cisplatin + Gelfoam) with sorafenib (n = 164) and sorafenib alone (n = 191) in a retrospective cohort study with advanced-stage HCC (BCLC C).88 All patients received sorafenib therapy for at least 5 weeks. The median TTP in the combination group was significantly longer compared to the sorafenib group (2.7 vs 2.1 months, respectively; p = 0.011). Importantly, however, there was no benefit in survival (9.1 vs 6.7 months; p = 0.21).
Drug-eluting bead-transarterial chemoembolization and sorafenib
In 2011, a Phase II prospective single-centre study evaluated DEB-TACE (LC Beads, 100–300 µm; doxorubicin) in combination with sorafenib in 35 patients (Child–Pugh A/B, 31/4; BCLC B/C, 12/23; PVT, 11).89 Patients were treated on a 6-week cycle regimen, in which one cycle consisted of 400 mg of sorafenib twice daily, initiated 1 week before DEB-TACE and administered continuously to obtain synergistic effects. The primary end points were safety and toxicity, while efficacy was the secondary end point. The median number of cycles per patient was two (range, 1–5). Most patients experienced at least one grade 3–4 toxicity; however, most toxicities were grade minor, 83% grade 1–2 and 17% grade 3–4. Objective response was observed in 58% (EASL). This study not only demonstrated the safety profile of sorafenib combined with DEB-TACE but also established the feasibility and tolerability of the continuous administration of sorafenib.
A Phase II, multicentre, international, randomized, double-blinded trial [the sorafenib or placebo in combination with TACE with doxorubicin-eluting beads (DEBDOX) for intermediate-stage HCC—(SPACE)—trial]90 was conducted in patients with intermediate-stage HCC to evaluate whether sorafenib with DEB-TACE has an impact on disease progression compared to DEB-TACE alone (completed, unpublished trial). 307 patients were randomized to receive sorafenib 400 mg twice daily or matching placebo continuously until progression. All patients underwent DEB-TACE 3–7 days after the first dose of the study drug, on Day 1 (±4 days) of months 3, 7 and 13, and every 6 months thereafter. The median TTP was 169 days for the sorafenib group vs 166 days for the placebo group. Although the hazard ratios seemed to favour the combination treatment over the placebo arm, there was no statistically significant benefit in TTP or survival for the combination therapy compared to DEB-TACE alone.
Overall, the combination of sorafenib and cTACE or DEB-TACE has shown to be safe and well tolerated. However, results regarding efficacy and benefit in OS have yet to be confirmed. As such, multiple studies have been designed to investigate the combination of cTACE or DEB-TACE with systemic antiangiogenic therapy; of particular importance are two Phase III randomized, double-blinded trials.91,92
Y90 and sorafenib
Y90 radioembolization is gaining acceptance in patients with more advanced intermediate-stage and early advanced-stage HCC patients, in part due to fewer procedure-related toxicities and better quality of life compared to TACE.93 Given this favourable profile, the combination of Y90 with sorafenib is appealing. Although data about this combination therapy are scarce, most trials being at an early stage or have yet to start, some results were made available recently.
An open-label, multicentre, single-arm, Phase II study conducted in the Asia–Pacific region evaluated the safety and efficacy of sequential treatment with radioembolization and sorafenib.94 Sorafenib (400 mg twice daily) was initiated 14 days after Y90 and given continuously until tumour progression or drug-related adverse events in 29 patients (Child–Pugh A/B, 20/9; BCLC B/C, 11/18; PVT, 8). The median daily dose of sorafenib was 600 mg (range, 127–791 mg). A majority of patients (28/29) experienced ≥grade 1 toxicity, while ≥grade 3 toxicity was observed in 15 patients (52%). Objective response (RECIST) was seen in 25%. Median TTP was 15.2 and 9.0 months for BCLC B and C patients, respectively. Median OS for BCLC B and C patients was 20.3 and 8.6 months, respectively. It was concluded that the sequential treatment of Y90 and sorafenib is safe and potentially effective.
The safety interim analysis of the combination of Y90 radioembolization and sorafenib compared with sorafenib alone in patients with advanced-stage disease was recently published.95 These preliminary results are part of a prospective, multicentre RCT currently being conducted in Europe.96 Patients with good performance status and preserved liver function, who were not candidates for TACE or progressed after TACE, were included in the analysis. Eligible candidates (n = 40; 20 each arm) were further stratified by the presence or absence of PVT. Patients received sorafenib on Day 3 after the last Y90 procedure. Both treatment arms received sorafenib 200 mg twice daily for 1 week, then the dose was increased to 400 mg twice daily. The regimen was continuous until disease progression or the advent of intolerable drug-related toxicity. Dose and duration of sorafenib was similar between groups (median daily dose 614 mg over 8.5 months and 557 mg over 9.6 months in the combination and sorafenib groups, respectively). Two patients died, one in each arm, but neither event was considered to be treatment related. The incidence of total (196 vs 222) and grade ≥3 (43 vs 47) adverse events was similar in the combination vs sorafenib groups, respectively. It was concluded that radioembolization followed by sorafenib is as safe and well tolerated as sorafenib alone.
Taken together, these results underline the potential of combining Y90 radioembolization and sorafenib in patients with more advanced disease. Ongoing studies will certainly shed more light on the potential benefit of this combination treatment,96,97 while other trials challenge the hegemony of sorafenib comparing Y90 radioembolization to the antiangiogenic therapy.98–100
FUTURE DIRECTIONS
Catheter-based IATs have achieved unparalleled results in the treatment of patients with HCC. Together with other treatment modalities in the field of Interventional Radiology, these minimally invasive therapies have led to the establishment of Interventional Oncology as the fourth pillar of cancer therapy. The future of catheter-based therapies is promising.
Patient selection
Published outcomes are predominantly impacted by major prognostic factors such as liver function, portal vein invasion, performance status, tumour burden and response to therapy. Thus, patient selection for IATs is fundamental. Intermediate-stage HCC encompasses a very heterogeneous group, which led to many difficulties when comparing trials.101 Highly selected intermediate-stage HCC patients have been shown to reach exceptional outcomes.56 Further studies implementing a discernible subpopulation of patients will certainly lead to a better identification of the ideal catheter-based therapy.
Many staging systems for patients with HCC have been developed.102 While the BCLC staging system is the most widely used treatment algorithm in Western countries, no standard system has been uniformly adopted worldwide. A new classification was recently proposed, the Hong Kong Liver Cancer (HKLC) staging system.103 This prognostic classification was retrospectively developed from 3856 patients with HCC. Four prognostic factors served as the backbone of the new system: ECOG performance status, Child–Pugh class, liver tumour status and presence of extrahepatic vascular invasion/metastasis. Importantly, the new system identified subsets of intermediate- and advanced-stage BCLC patients for whom a more aggressive treatment approach was recommended. This staging system may more accurately reflect contemporary treatment approaches that are typically seen in daily clinical practice. Indeed, whereas the BCLC system would preclude certain patients from some IATs, the HKLC system would not only allow but recommend such treatments. It is very clear that the number of IATs would dramatically increase should the HKLC staging system be universally adopted. With reported better outcomes compared to BCLC, this new system will certainly make headlines and challenge BCLC. The validity of HKLC should be tested, notably in Europe and North America where hepatitis C is the main trigger for HCC compared to hepatitis B in Asia (about 80% of the HKLC cohort had hepatitis B).
Sorafenib
The advent of sorafenib as the standard of care in advanced-stage HCC patients changed the landscape of BCLC C. Ongoing research evaluating this therapy alone or in combination with catheter-based treatment will certainly change the paradigm for this category of patients. Future investigations should focus on the ideal timing and type (sequential, interrupted vs continuous) of sorafenib administration. Moreover, the ideal subset of patients for the combination therapy with sorafenib remains to be elucidated. While the toxicity profile of Y90 radioembolization and DEB-TACE has been shown to be more favourable than cTACE, and thus appears more attractive for a combination therapy with sorafenib, the ideal catheter-based therapy has yet to be determined.
Evaluation of treatment response
Evaluation of treatment response after IAT is a key component for patient management and is performed on a daily basis in clinical practice. The survival-based end points traditionally used in clinical studies have largely been replaced by radiologic objective response, which has been widely used and accepted as a surrogate end point. Baseline imaging is typically performed within 2–3 weeks prior to therapy, and follow-up imaging is performed 4–6 weeks after IAT. Most liver tumours exhibit heterogeneous pattern of necrosis after catheter-based treatments that challenge tumour response evaluation.104 While conventional response criteria assessing anatomic (i.e. size-based) changes in the tumour (World Health Organization response criteria and RECIST) have shown their limitations compared to contrast enhancement-based criteria (EASL and mRECIST),51,105,106 new response criteria using three dimensional (3D) quantitative approaches are already being evaluated and will certainly improve upon established guidelines.107,108
From bench to bedside
The field of catheter-based therapies will continue to grow. Understanding the molecular biology of cancer is crucial in the development of therapies. Thus, continued experimental research is fundamental and every effort should be carried out to translate basic scientific findings into therapeutic options for patients. Dynamic multiphase contrast-enhanced CT and MRI have achieved unparalleled accuracy in the diagnosis of HCC in the presence of a cirrhotic liver, relegating the role of the biopsy to a second level. However, collection of tissue samples should be favoured in future research to better understand liver cancer molecular biology and identify new molecular targets. A personalized medicine approach is likely to develop in the near future. Development of novel drugs such as agents targeting tumour metabolism or hypoxia, new drug delivery systems and interventional equipment is part of some of the exciting ongoing research. Innovative concepts such as imageable (i.e. radio-opaque) beads109 or beads loaded with antiangiogenic agents110 are currently being investigated. Imaging modalities such as cone beam CT will be further refined allowing for a more comprehensive utilization of 3D imaging technology in the procedure room. Moreover, image fusion techniques and software identifying tumour-feeding arteries111 will undoubtedly help treatment guidance and expand indications for therapy.
In conclusion, catheter-based IATs have revolutionized the treatment of HCC. Level I evidence of the benefit in survival of cTACE led to the recognition of catheter-based therapies in the management of patient with unresectable HCC. cTACE remains the standard of care in HCC patients with intermediate-stage disease (BCLC B). DEB-TACE has a decreased toxicity profile compared to cTACE (Level I evidence) with similar tumour response rates. However, no survival benefit of DEB-TACE over cTACE has been shown to date. Y90 is maturing as a serious treatment option for patients situated between intermediate and advanced stages. Sorafenib has revolutionized the systemic therapy of HCC and is indicated in advanced-stage disease with preserved liver function and in patients with progressing lesions despite locoregional treatments (Level I evidence).
CONFLICTS OF INTEREST
Jean-François Geschwind, MD, is a consultant for Biocompatibles/BTG, Bayer HealthCare, Guerbet, Nordion/BTG, Philips Healthcare and Jennerex.
Acknowledgments
ACKNOWLEDGEMENTS
The authors thank Sonia Sahu, B.A., and Arun Chockalingam for their help in editing the manuscript and figures.
Contributor Information
R Duran, Email: rduran4@jhmi.edu, rafaelduran.md@gmail.com.
J Chapiro, Email: jchapir1@jhmi.edu.
R E Schernthaner, Email: rschern1@jhmi.edu.
J-F H Geschwind, Email: jfg@jhmi.edu.
FUNDING
Jean-François Geschwind received funding from Biocompatibles/BTG, Bayer HealthCare, Philips Medical, Nordion/BTG, Threshold, Guerbet, DOD, NCI-ECOG and NIH-R01. He is also the Founder and CEO of PreScience Labs, LLC.
REFERENCES
- 1.El-Serag HB. Hepatocellular carcinoma. N Engl J Med 2011; 365: 1118–27. doi: 10.1056/NEJMra1001683 [DOI] [PubMed] [Google Scholar]
- 2.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet 2012; 379: 1245–55. doi: 10.1016/S0140-6736(11)61347-0 [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB, Kanwal F. Epidemiology of hepatocellular carcinoma in the United States: where are we? Where do we go? Hepatology 2014; 60: 1767–75. doi: 10.1002/hep.27222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arciero CA, Sigurdson ER. Liver-directed therapies for hepatocellular carcinoma. J Natl Compr Canc Netw 2006; 4: 768–74. [DOI] [PubMed] [Google Scholar]
- 5.Breedis C, Young G. The blood supply of neoplasms in the liver. Am J Pathol 1954; 30: 969–77. [PMC free article] [PubMed] [Google Scholar]
- 6.European Association for the Study of the Liver; European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012; 56: 908–43. doi: 10.1016/j.jhep.2011.12.001 [DOI] [PubMed] [Google Scholar]
- 7.Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet 2002; 359: 1734–9. doi: 10.1016/S0140-6736(02)08649-X [DOI] [PubMed] [Google Scholar]
- 8.Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 2002; 35: 1164–71. doi: 10.1053/jhep.2002.33156 [DOI] [PubMed] [Google Scholar]
- 9.Raoul JL, Sangro B, Forner A, Mazzaferro V, Piscaglia F, Bolondi L, et al. Evolving strategies for the management of intermediate-stage hepatocellular carcinoma: available evidence and expert opinion on the use of transarterial chemoembolization. Cancer Treat Rev 2011; 37: 212–20. doi: 10.1016/j.ctrv.2010.07.006 [DOI] [PubMed] [Google Scholar]
- 10.Kim KM, Kim JH, Park IS, Ko GY, Yoon HK, Sung KB, et al. Reappraisal of repeated transarterial chemoembolization in the treatment of hepatocellular carcinoma with portal vein invasion. J Gastroenterol Hepatol 2009; 24: 806–14. doi: 10.1111/j.1440-1746.2008.05728.x [DOI] [PubMed] [Google Scholar]
- 11.Luo J, Guo RP, Lai EC, Zhang YJ, Lau WY, Chen MS, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma with portal vein tumor thrombosis: a prospective comparative study. Ann Surg Oncol 2011; 18: 413–20. doi: 10.1245/s10434-010-1321-8 [DOI] [PubMed] [Google Scholar]
- 12.Chung GE, Lee JH, Kim HY, Hwang SY, Kim JS, Chung JW, et al. Transarterial chemoembolization can be safely performed in patients with hepatocellular carcinoma invading the main portal vein and may improve the overall survival. Radiology 2011; 258: 627–34. doi: 10.1148/radiol.10101058 [DOI] [PubMed] [Google Scholar]
- 13.Sangro B, Iñarrairaegui M, Bilbao JI. Radioembolization for hepatocellular carcinoma. J Hepatol 2012; 56: 464–73. doi: 10.1016/j.jhep.2011.07.012 [DOI] [PubMed] [Google Scholar]
- 14.Doyon D, Mouzon A, Jourde AM, Regensberg C, Frileux C. Hepatic, arterial embolization in patients with malignant liver tumours (author's transl). [In French.] Ann Radiol (Paris) 1974; 17: 593–603. [PubMed] [Google Scholar]
- 15.Goldstein HM, Medellin H, Beydoun MT, Wallace S, Ben-Menachem Y, Bracken RB, et al. Transcatheter embolization of renal cell carcinoma. Am J Roentgenol Radium Ther Nucl Med 1975; 123: 557–62. doi: 10.2214/ajr.123.3.557 [DOI] [PubMed] [Google Scholar]
- 16.Goldstein HM, Wallace S, Anderson JH, Bree RL, Gianturco C. Transcatheter occlusion of abdominal tumors. Radiology 1976; 120: 539–45. doi: 10.1148/120.3.539 [DOI] [PubMed] [Google Scholar]
- 17.Allison DJ, Modlin IM, Jenkins WJ. Treatment of carcinoid liver metastases by hepatic-artery embolisation. Lancet 1977; 2: 1323–5. doi: 10.1016/S0140-6736(77)90369-5 [DOI] [PubMed] [Google Scholar]
- 18.Wheeler PG, Melia W, Dubbins P, Jones B, Nunnerley H, Johnson P, et al. Non-operative arterial embolisation in primary liver tumours. Br Med J 1979; 2: 242–4. doi: 10.1136/bmj.2.6184.242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamada R, Nakatsuka H, Nakamura K, Sato M, Itami M, Kobayashi N, et al. Hepatic artery embolization in 32 patients with unresectable hepatoma. Osaka City Med J 1980; 26: 81–96. [PubMed] [Google Scholar]
- 20.Yamada R, Sato M, Kawabata M, Nakatsuka H, Nakamura K, Takashima S. Hepatic artery embolization in 120 patients with unresectable hepatoma. Radiology 1983; 148: 397–401. doi: 10.1148/radiology.148.2.6306721 [DOI] [PubMed] [Google Scholar]
- 21.Nakamura H, Hashimoto T, Oi H, Sawada S. Transcatheter oily chemoembolization of hepatocellular carcinoma. Radiology 1989; 170: 783–6. doi: 10.1148/radiology.170.3.2536946 [DOI] [PubMed] [Google Scholar]
- 22.Konno T, Maeda H, Iwai K, Tashiro S, Maki S, Morinaga T, et al. Effect of arterial administration of high-molecular-weight anticancer agent SMANCS with lipid lymphographic agent on hepatoma: a preliminary report. Eur J Cancer Clin Oncol 1983; 19: 1053–65. doi: 10.1016/0277-5379(83)90028-7 [DOI] [PubMed] [Google Scholar]
- 23.Nakakuma K, Tashiro S, Hiraoka T, Uemura K, Konno T, Miyauchi Y, et al. Studies on anticancer treatment with an oily anticancer drug injected into the ligated feeding hepatic artery for liver cancer. Cancer 1983; 52: 2193–200. doi: 10.1002/1097-0142(19831215)52:123.0.CO;2-R [DOI] [PubMed] [Google Scholar]
- 24.Yumoto Y, Jinno K, Tokuyama K, Araki Y, Ishimitsu T, Maeda H, et al. Hepatocellular carcinoma detected by iodized oil. Radiology 1985; 154: 19–24. doi: 10.1148/radiology.154.1.2981112 [DOI] [PubMed] [Google Scholar]
- 25.Lewandowski RJ, Geschwind JF, Liapi E, Salem R. Transcatheter intraarterial therapies: rationale and overview. Radiology 2011; 259: 641–57. doi: 10.1148/radiol.11081489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petruzzi NJ, Frangos AJ, Fenkel JM, Herrine SK, Hann HW, Rossi S, et al. Single-center comparison of three chemoembolization regimens for hepatocellular carcinoma. J Vasc Interv Radiol 2013; 24: 266–73. doi: 10.1016/j.jvir.2012.10.025 [DOI] [PubMed] [Google Scholar]
- 27.Takayasu K, Arii S, Ikai I, Kudo M, Matsuyama Y, Kojiro M, et al. ; Liver Cancer Study Group of Japan. Overall survival after transarterial lipiodol infusion chemotherapy with or without embolization for unresectable hepatocellular carcinoma: propensity score analysis. AJR Am J Roentgenol 2010; 194: 830–7. doi: 10.2214/AJR.09.3308 [DOI] [PubMed] [Google Scholar]
- 28.Takayasu K, Shima Y, Muramatsu Y, Moriyama N, Yamada T, Makuuchi M, et al. Hepatocellular carcinoma: treatment with intraarterial iodized oil with and without chemotherapeutic agents. Radiology 1987; 163: 345–51. doi: 10.1148/radiology.163.2.3031724 [DOI] [PubMed] [Google Scholar]
- 29.Brown DB, Cardella JF, Sacks D, Goldberg SN, Gervais DA, Rajan D, et al. Quality improvement guidelines for transhepatic arterial chemoembolization, embolization, and chemotherapeutic infusion for hepatic malignancy. J Vasc Interv Radiol 2006; 17: 225–32. doi: 10.1097/01.RVI.0000195330.47954.48 [DOI] [PubMed] [Google Scholar]
- 30.Lewis AL, Holden RR. DC Bead embolic drug-eluting bead: clinical application in the locoregional treatment of tumours. Expert Opin Drug Deliv 2011; 8: 153–69. doi: 10.1517/17425247.2011.545388 [DOI] [PubMed] [Google Scholar]
- 31.Buijs M, Vossen JA, Frangakis C, Hong K, Georgiades CS, Chen Y, et al. Nonresectable hepatocellular carcinoma: long-term toxicity in patients treated with transarterial chemoembolization—single-center experience. Radiology 2008; 249: 346–54. doi: 10.1148/radiol.2483071902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Georgiades C, Geschwind JF, Harrison N, Hines-Peralta A, Liapi E, Hong K, et al. Lack of response after initial chemoembolization for hepatocellular carcinoma: does it predict failure of subsequent treatment? Radiology 2012; 265: 115–23. doi: 10.1148/radiol.12112264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pelletier G, Roche A, Ink O, Anciaux ML, Derhy S, Rougier P, et al. A randomized trial of hepatic arterial chemoembolization in patients with unresectable hepatocellular carcinoma. J Hepatol 1990; 11: 181–4. doi: 10.1016/0168-8278(90)90110-D [DOI] [PubMed] [Google Scholar]
- 34.Madden MV, Krige JE, Bailey S, Beningfield SJ, Geddes C, Werner ID, et al. Randomised trial of targeted chemotherapy with lipiodol and 5-epidoxorubicin compared with symptomatic treatment for hepatoma. Gut 1993; 34: 1598–600. doi: 10.1136/gut.34.11.1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.A comparison of lipiodol chemoembolization and conservative treatment for unresectable hepatocellular carcinoma. Groupe d'Etude et de Traitement du Carcinome Hepatocellulaire. N Engl J Med 1995; 332: 1256–61. doi: 10.1056/NEJM199505113321903 [DOI] [PubMed] [Google Scholar]
- 36.Pelletier G, Ducreux M, Gay F, Luboinski M, Hagège H, Dao T, et al. Treatment of unresectable hepatocellular carcinoma with lipiodol chemoembolization: a multicenter randomized trial. Groupe CHC. J Hepatol 1998; 29: 129–34. doi: 10.1016/S0168-8278(98)80187-6 [DOI] [PubMed] [Google Scholar]
- 37.Simonetti RG, Liberati A, Angiolini C, Pagliaro L. Treatment of hepatocellular carcinoma: a systematic review of randomized controlled trials. Ann Oncol 1997; 8: 117–36. doi: 10.1023/A:1008285123736 [DOI] [PubMed] [Google Scholar]
- 38.Mathurin P, Rixe O, Carbonell N, Bernard B, Cluzel P, Bellin MF, et al. Review article: overview of medical treatments in unresectable hepatocellular carcinoma—an impossible meta-analysis? Aliment Pharmacol Ther 1998; 12: 111–26. doi: 10.1046/j.1365-2036.1998.00286.x [DOI] [PubMed] [Google Scholar]
- 39.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology 2003; 37: 429–42. doi: 10.1053/jhep.2003.50047 [DOI] [PubMed] [Google Scholar]
- 40.Bruix J, Sherman M; Practice Guidelines Committee; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology 2005; 42: 1208–36. doi: 10.1002/hep.20933 [DOI] [PubMed] [Google Scholar]
- 41.Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology 2011; 53: 1020–2. doi: 10.1002/hep.24199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ehrlich P. Collected studies on immunity. New York: John Wiley & Sons; 1906.
- 43.Kerr DJ. Microparticulate drug delivery systems as an adjunct to cancer treatment. Cancer Drug Deliv 1987; 4: 55–61. doi: 10.1089/cdd.1987.4.55 [DOI] [PubMed] [Google Scholar]
- 44.Jordan O, Denys A, De Baere T, Boulens N, Doelker E. Comparative study of chemoembolization loadable beads: in vitro drug release and physical properties of DC bead and hepasphere loaded with doxorubicin and irinotecan. J Vasc Interv Radiol 2010; 21: 1084–90. doi: 10.1016/j.jvir.2010.02.042 [DOI] [PubMed] [Google Scholar]
- 45.Hong K, Khwaja A, Liapi E, Torbenson MS, Georgiades CS, Geschwind JF. New intra-arterial drug delivery system for the treatment of liver cancer: preclinical assessment in a rabbit model of liver cancer. Clin Cancer Res 2006; 12: 2563–7. doi: 10.1158/1078-0432.CCR-05-2225 [DOI] [PubMed] [Google Scholar]
- 46.Lee KH, Liapi EA, Cornell C, Reb P, Buijs M, Vossen JA, et al. Doxorubicin-loaded QuadraSphere microspheres: plasma pharmacokinetics and intratumoral drug concentration in an animal model of liver cancer. Cardiovasc Intervent Radiol 2010; 33: 576–82. doi: 10.1007/s00270-010-9794-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gupta S, Wright KC, Ensor J, Van Pelt CS, Dixon KA, Kundra V. Hepatic arterial embolization with doxorubicin-loaded superabsorbent polymer microspheres in a rabbit liver tumor model. Cardiovasc Intervent Radiol 2011; 34: 1021–30. doi: 10.1007/s00270-011-0154-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grosso M, Vignali C, Quaretti P, Nicolini A, Melchiorre F, Gallarato G, et al. Transarterial chemoembolization for hepatocellular carcinoma with drug-eluting microspheres: preliminary results from an Italian multicentre study. Cardiovasc Intervent Radiol 2008; 31: 1141–9. doi: 10.1007/s00270-008-9409-2 [DOI] [PubMed] [Google Scholar]
- 49.Malagari K, Pomoni M, Moschouris H, Kelekis A, Charokopakis A, Bouma E, et al. Chemoembolization of hepatocellular carcinoma with HepaSphere 30-60 µm. Safety and efficacy study. Cardiovasc Intervent Radiol 2014; 37: 165–75. doi: 10.1007/s00270-013-0777-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Varela M, Real MI, Burrel M, Forner A, Sala M, Brunet M, et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol 2007; 46: 474–81. doi: 10.1016/j.jhep.2006.10.020 [DOI] [PubMed] [Google Scholar]
- 51.Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. ; EASL Panel of Experts on HCC. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol 2001; 35: 421–30. doi: 10.1016/S0168-8278(01)00130-1 [DOI] [PubMed] [Google Scholar]
- 52.Poon RT, Tso WK, Pang RW, Ng KK, Woo R, Tai KS, et al. A phase I/II trial of chemoembolization for hepatocellular carcinoma using a novel intra-arterial drug-eluting bead. Clin Gastroenterol Hepatol 2007; 5: 1100–8. doi: 10.1016/j.cgh.2007.04.021 [DOI] [PubMed] [Google Scholar]
- 53.Malagari K, Chatzimichael K, Alexopoulou E, Kelekis A, Hall B, Dourakis S, et al. Transarterial chemoembolization of unresectable hepatocellular carcinoma with drug eluting beads: results of an open-label study of 62 patients. Cardiovasc Intervent Radiol 2008; 31: 269–80. doi: 10.1007/s00270-007-9226-z [DOI] [PubMed] [Google Scholar]
- 54.Reyes DK, Vossen JA, Kamel IR, Azad NS, Wahlin TA, Torbenson MS, et al. Single-center phase II trial of transarterial chemoembolization with drug-eluting beads for patients with unresectable hepatocellular carcinoma: initial experience in the United States. Cancer J 2009; 15: 526–32. doi: 10.1097/PPO.0b013e3181c5214b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Malagari K, Pomoni M, Moschouris H, Bouma E, Koskinas J, Stefaniotou A, et al. Chemoembolization with doxorubicin-eluting beads for unresectable hepatocellular carcinoma: five-year survival analysis. Cardiovasc Intervent Radiol 2012; 35: 1119–28. doi: 10.1007/s00270-012-0394-0 [DOI] [PubMed] [Google Scholar]
- 56.Burrel M, Reig M, Forner A, Barrufet M, de Lope CR, Tremosini S, et al. Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolisation (TACE) using Drug Eluting Beads. Implications for clinical practice and trial design. J Hepatol 2012; 56: 1330–5. doi: 10.1016/j.jhep.2012.01.008 [DOI] [PubMed] [Google Scholar]
- 57.Kalva SP, Pectasides M, Liu R, Rachamreddy N, Surakanti S, Yeddula K, et al. Safety and effectiveness of chemoembolization with drug-eluting beads for advanced-stage hepatocellular carcinoma. Cardiovasc Intervent Radiol 2014; 37: 381–7. doi: 10.1007/s00270-013-0654-7 [DOI] [PubMed] [Google Scholar]
- 58.Prajapati HJ, Dhanasekaran R, El-Rayes BF, Kauh JS, Maithel SK, Chen Z, et al. Safety and efficacy of doxorubicin drug-eluting bead transarterial chemoembolization in patients with advanced hepatocellular carcinoma. J Vasc Interv Radiol 2013; 24: 307–15. doi: 10.1016/j.jvir.2012.11.026 [DOI] [PubMed] [Google Scholar]
- 59.Malagari K, Pomoni M, Spyridopoulos TN, Moschouris H, Kelekis A, Dourakis S, et al. Safety profile of sequential transcatheter chemoembolization with DC Bead™: results of 237 hepatocellular carcinoma (HCC) patients. Cardiovasc Intervent Radiol 2011; 34: 774–85. doi: 10.1007/s00270-010-0044-3 [DOI] [PubMed] [Google Scholar]
- 60.Johns Hopkins Medical Institutions. Doxorubicin-eluting LC Bead M1 for Patients with Hepatocellular Carcinoma (DEBDOX). In: ClinicalTrials.gov NLM Identifier: NCT02007954. Bethesda, MD: National Library of Medicine (US); 2000. [Cited 24 June 2014]. Available from: http://clinicaltrials.gov/show/NCT02007954
- 61.Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, et al. ; PRECISION V Investigators. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol 2010; 33: 41–52. doi: 10.1007/s00270-009-9711-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Song MJ, Chun HJ, Song do S, Kim HY, Yoo SH, Park CH, et al. Comparative study between doxorubicin-eluting beads and conventional transarterial chemoembolization for treatment of hepatocellular carcinoma. J Hepatol 2012; 57: 1244–50. doi: 10.1016/j.jhep.2012.07.017 [DOI] [PubMed] [Google Scholar]
- 63.Bruix J, Llovet JM, Castells A, Montaña X, Brú C, Ayuso MC, et al. Transarterial embolization versus symptomatic treatment in patients with advanced hepatocellular carcinoma: results of a randomized, controlled trial in a single institution. Hepatology 1998; 27: 1578–83. doi: 10.1002/hep.510270617 [DOI] [PubMed] [Google Scholar]
- 64.Maluccio MA, Covey AM, Porat LB, Schubert J, Brody LA, Sofocleous CT, et al. Transcatheter arterial embolization with only particles for the treatment of unresectable hepatocellular carcinoma. J Vasc Interv Radiol 2008; 19: 862–9. doi: 10.1016/j.jvir.2008.02.013 [DOI] [PubMed] [Google Scholar]
- 65.Malagari K, Pomoni M, Kelekis A, Pomoni A, Dourakis S, Spyridopoulos T, et al. Prospective randomized comparison of chemoembolization with doxorubicin-eluting beads and bland embolization with BeadBlock for hepatocellular carcinoma. Cardiovasc Intervent Radiol 2010; 33: 541–51. doi: 10.1007/s00270-009-9750-0 [DOI] [PubMed] [Google Scholar]
- 66.Brown KT, Gonen M, Do K, Covey AM, Getrajdman G, Zhao B, et al. Randomized phase II study of hepatic arterial embolization of hepatocellular carcinoma (HCC) with micospheres alone (bead block (BB)) versus doxorubicin loaded microspheres (LC bead (LCB)). J Vasc Interv Radiol 2014; 25: S24. doi: 10.1016/j.jvir.2013.12.058 [DOI] [Google Scholar]
- 67.Sato K, Lewandowski RJ, Bui JT, Omary R, Hunter RD, Kulik L, et al. Treatment of unresectable primary and metastatic liver cancer with yttrium-90 microspheres (TheraSphere): assessment of hepatic arterial embolization. Cardiovasc Intervent Radiol 2006; 29: 522–9. doi: 10.1007/s00270-005-0171-4 [DOI] [PubMed] [Google Scholar]
- 68.Dezarn WA, Cessna JT, DeWerd LA, Feng W, Gates VL, Halama J, et al. ; American Association of Physicists in Medicine. Recommendations of the American Association of Physicists in Medicine on dosimetry, imaging, and quality assurance procedures for 90Y microsphere brachytherapy in the treatment of hepatic malignancies. Med Phys 2011; 38: 4824–45. doi: 10.1118/1.3608909 [DOI] [PubMed] [Google Scholar]
- 69.Sangro B, Salem R, Kennedy A, Coldwell D, Wasan H. Radioembolization for hepatocellular carcinoma: a review of the evidence and treatment recommendations. Am J Clin Oncol 2011; 34: 422–31. doi: 10.1097/COC.0b013e3181df0a50 [DOI] [PubMed] [Google Scholar]
- 70.Ya PM, Guzman T, Loken MK, Perry JF, Jr. Isotope localization with tagged microspheres. Surgery 1961; 49: 644–50. [PubMed] [Google Scholar]
- 71.Kim YS, Lafave JW, Maclean LD. The use of radiating microspheres in the treatment of experimental and human malignancy. Surgery 1962; 52: 220–31. [PubMed] [Google Scholar]
- 72.Ariel IM. Treatment of inoperable primary pancreatic and liver cancer by the intra-arterial administration of radioactive isotopes (Y90 radiating microspheres). Ann Surg 1965; 162: 267–78. doi: 10.1097/00000658-196508000-00018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Salem R, Thurston KG. Radioembolization with yttrium-90 microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies: part 3: comprehensive literature review and future direction. J Vasc Interv Radiol 2006; 17: 1571–93. doi: 10.1097/01.RVI.0000236744.34720.73 [DOI] [PubMed] [Google Scholar]
- 74.Geschwind JF, Salem R, Carr BI, Soulen MC, Thurston KG, Goin KA, et al. Yttrium-90 microspheres for the treatment of hepatocellular carcinoma. Gastroenterology 2004; 127(5 Suppl. 1): S194–205. doi: 10.1053/j.gastro.2004.09.034 [DOI] [PubMed] [Google Scholar]
- 75.Salem R, Lewandowski RJ, Atassi B, Gordon SC, Gates VL, Barakat O, et al. Treatment of unresectable hepatocellular carcinoma with use of 90Y microspheres (TheraSphere): safety, tumor response, and survival. J Vasc Interv Radiol 2005; 16: 1627–39. doi: 10.1097/01.RVI.0000184594.01661.81 [DOI] [PubMed] [Google Scholar]
- 76.Salem R, Lewandowski RJ, Mulcahy MF, Riaz A, Ryu RK, Ibrahim S, et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology 2010; 138: 52–64. doi: 10.1053/j.gastro.2009.09.006 [DOI] [PubMed] [Google Scholar]
- 77.Sangro B, Carpanese L, Cianni R, Golfieri R, Gasparini D, Ezziddin S, et al. ; European Network on Radioembolization with Yttrium-90 Resin Microspheres (ENRY). Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluation. Hepatology 2011; 54: 868–78. doi: 10.1002/hep.24451 [DOI] [PubMed] [Google Scholar]
- 78.Kulik LM, Carr BI, Mulcahy MF, Lewandowski RJ, Atassi B, Ryu RK, et al. Safety and efficacy of 90Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology 2008; 47: 71–81. doi: 10.1002/hep.21980 [DOI] [PubMed] [Google Scholar]
- 79.Hilgard P, Hamami M, Fouly AE, Scherag A, Müller S, Ertle J, et al. Radioembolization with yttrium-90 glass microspheres in hepatocellular carcinoma: European experience on safety and long-term survival. Hepatology 2010; 52: 1741–9. doi: 10.1002/hep.23944 [DOI] [PubMed] [Google Scholar]
- 80.Salem R, Lewandowski RJ, Kulik L, Wang E, Riaz A, Ryu RK, et al. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology 2011; 140: 497–507.e2. doi: 10.1053/j.gastro.2010.10.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Northwestern University/National Cancer Institute (NCI). Prospective Randomized Trial of Radioembolization and Chemoembolization in Hepatocellular Carcinoma (PREMIERE). In: ClinicalTrials.gov. NLM Identifier: NCT00956930. Bethesda, MD: National Library of Medicine (US); 2000. [Cited 1 July 2014]. Available from: http://clinicaltrials.gov/show/NCT00956930
- 82.Harris AL. Hypoxia—a key regulatory factor in tumour growth. Nat Rev Cancer 2002; 2: 38–47. doi: 10.1038/nrc704 [DOI] [PubMed] [Google Scholar]
- 83.von Marschall Z, Cramer T, Höcker M, Finkenzeller G, Wiedenmann B, Rosewicz S. Dual mechanism of vascular endothelial growth factor upregulation by hypoxia in human hepatocellular carcinoma. Gut 2001; 48: 87–96. doi: 10.1136/gut.48.1.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. ; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359: 378–90. doi: 10.1056/NEJMoa0708857 [DOI] [PubMed] [Google Scholar]
- 85.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009; 10: 25–34. doi: 10.1016/S1470-2045(08)70285-7 [DOI] [PubMed] [Google Scholar]
- 86.Chung YH, Han G, Yoon JH, Yang J, Wang J, Shao GL, et al. Interim analysis of START: study in Asia of the combination of TACE (transcatheter arterial chemoembolization) with sorafenib in patients with hepatocellular carcinoma trial. Int J Cancer 2013; 132: 2448–58. doi: 10.1002/ijc.27925 [DOI] [PubMed] [Google Scholar]
- 87.Park JW, Koh YH, Kim HB, Kim HY, An S, Choi JI, et al. Phase II study of concurrent transarterial chemoembolization and sorafenib in patients with unresectable hepatocellular carcinoma. J Hepatol 2012; 56: 1336–42. doi: 10.1016/j.jhep.2012.01.006 [DOI] [PubMed] [Google Scholar]
- 88.Choi GH, Shim JH, Kim MJ, Ryu MH, Ryoo BY, Kang YK, et al. Sorafenib alone versus sorafenib combined with transarterial chemoembolization for advanced-stage hepatocellular carcinoma: results of propensity score analyses. Radiology 2013; 269: 603–11. doi: 10.1148/radiol.13130150 [DOI] [PubMed] [Google Scholar]
- 89.Pawlik TM, Reyes DK, Cosgrove D, Kamel IR, Bhagat N, Geschwind JF. Phase II trial of sorafenib combined with concurrent transarterial chemoembolization with drug-eluting beads for hepatocellular carcinoma. J Clin Oncol 2011; 29: 3960–7. doi: 10.1200/JCO.2011.37.1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lencioni R, Llovet JM, Han G, Tak WY, Yang J, Leberre MA, et al. Sorafenib or placebo in combination with transarterial chemoembolization (TACE) with doxorubicin-eluting beads (DEBDOX) for intermediate-stage hepatocellular carcinoma (HCC): Phase II, randomized, double-blind SPACE trial. ASCO Annual Meeting Proceedings 2012; 30(Suppl.): LBA154. [Google Scholar]
- 91.National Cancer Institute (NCI); ECOG-ACRIN Cancer Research Group. A Phase III Randomized, Double-Blind Trial of Chemoembolization with or Without Sorafenib in Unresectable Hepatocellular Carcinoma (HCC) in Patients with and Without Vascular Invasion. In: ClinicalTrials.gov. NLM Identifier: NCT01004978. Bethesda, MD: National Library of Medicine (US); 2000. [Cited 1 July 2014]. Available from: http://clinicaltrials.gov/show/NCT01004978
- 92.University College London. TACE-2—a randomised placebo-controlled, double blinded, phase III trial evaluating sorafenib in combination with transarterial chemoembolisation (TACE) in patients with unresectable hepatocellular carcinoma (HCC). In: UK Clinical Research Network Study Portfolio [cited 1 July 2014.] Available from: http://public.ukcrn.org.uk/search/StudyDetail.aspx?StudyID=5347. UKCRN ID: 5347.
- 93.Salem R, Gilbertsen M, Butt Z, Memon K, Vouche M, Hickey R, et al. Increased quality of life among hepatocellular carcinoma patients treated with radioembolization, compared with chemoembolization. Clin Gastroenterol Hepatol 2013; 11: 1358–65.e1. doi: 10.1016/j.cgh.2013.04.028 [DOI] [PubMed] [Google Scholar]
- 94.Chow PK, Poon DY, Khin MW, Singh H, Han HS, Goh AS, et al. ; Asia-Pacific Hepatocellular Carcinoma Trials Group. Multicenter phase II study of sequential radioembolization-sorafenib therapy for inoperable hepatocellular carcinoma. PLoS One 2014; 9: e90909. doi: 10.1371/journal.pone.0090909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ricke J, Bulla K, Kolligs F, Peck-Radosavljevic M, Reimer P, Sangro B, et al. ; SORAMIC study group. Safety and toxicity of radioembolization plus Sorafenib in advanced hepatocellular carcinoma: analysis of the European multicentre trial SORAMIC. Liver Int 2015; 35: 620–6. doi: 10.1111/liv.12622 [DOI] [PubMed] [Google Scholar]
- 96.University of Magdeburg. Sorafenib and Micro-therapy Guided by Primovist Enhanced MRI in Patients with Inoperable Liver Cancer (SORAMIC). In: ClinicalTrials.gov NLM Identifier: NCT01126645. Bethesda, MD: National Library of Medicine (US); 2000. [Cited 1 July 2014]. Available from: http://clinicaltrials.gov/show/NCT01126645
- 97.BTG International Inc. A Phase III Clinical Trial of Intra-arterial TheraSphere® in the Treatment of Patients with Unresectable Hepatocellular Carcinoma (STOP-HCC). In: ClinicalTrials.gov NLM Identifier: NCT01556490. . Bethesda, MD: National Library of Medicine (US); 2000. [Cited 1 July 2014]. Available from: http://clinicaltrials.gov/show/NCT01556490
- 98.Assistance Publique–Hôpitaux de Paris. SorAfenib Versus RADIOEMBOLIZATION in Advanced Hepatocellular Carcinoma (SARAH). In: ClinicalTrials.gov NLM Identifier: NCT01482442. Bethesda, MD: National Library of Medicine (US); 2000. [Cited 1 July 2014]. Available from: http://clinicaltrials.gov/show/NCT01482442
- 99.Singapore General Hospital. Phase III Multi-Centre Open-Label Randomized Controlled Trial of Selective Internal Radiation Therapy (SIRT) Versus Sorafenib in Locally Advanced Hepatocellular Carcinoma (SIRveNIB). In: ClinicalTrials.gov NLM Identifier: NCT01135056. Bethesda, MD: National Library of Medicine (US); 2000. [Cited 1 July 2014]. Available from: http://clinicaltrials.gov/show/NCT01135056
- 100.BTG International Inc. A prospective randomized clinical trial on 90Yttrium trans-arterial radio-embolization (TheraSphere®) vs. standard of care (Sorafenib) for the treatment of advanced hepatocellular carcinoma (HCC) with portal vein thrombosis (PVT). In: ClinicalTrials.gov NLM Identifier: NCT01887717. Bethesda, MD: National Library of Medicine (US); 2000. [Cited 1 July 2014]. Available from: http://clinicaltrials.gov/show/NCT01887717
- 101.Bolondi L, Burroughs A, Dufour JF, Galle PR, Mazzaferro V, Piscaglia F, et al. Heterogeneity of patients with intermediate (BCLC B) hepatocellular carcinoma: proposal for a subclassification to facilitate treatment decisions. Semin Liver Dis 2012; 32: 348–59. doi: 10.1055/s-0032-1329906 [DOI] [PubMed] [Google Scholar]
- 102.Wildi S, Pestalozzi BC, McCormack L, Clavien PA. Critical evaluation of the different staging systems for hepatocellular carcinoma. Br J Surg 2004; 91: 400–8. doi: 10.1002/bjs.4554 [DOI] [PubMed] [Google Scholar]
- 103.Yau T, Tang VY, Yao TJ, Fan ST, Lo CM, Poon RT. Development of Hong Kong Liver Cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology 2014; 146: 1691–700.e3. doi: 10.1053/j.gastro.2014.02.032 [DOI] [PubMed] [Google Scholar]
- 104.Gonzalez-Guindalini FD, Botelho MP, Harmath CB, Sandrasegaran K, Miller FH, Salem R, et al. Assessment of liver tumor response to therapy: role of quantitative imaging. Radiographics 2013; 33: 1781–800. doi: 10.1148/rg.336135511 [DOI] [PubMed] [Google Scholar]
- 105.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010; 30: 52–60. doi: 10.1055/s-0030-1247132 [DOI] [PubMed] [Google Scholar]
- 106.Forner A, Ayuso C, Varela M, Rimola J, Hessheimer AJ, de Lope CR, et al. Evaluation of tumor response after locoregional therapies in hepatocellular carcinoma: are response evaluation criteria in solid tumors reliable? Cancer 2009; 115: 616–23. doi: 10.1002/cncr.24050 [DOI] [PubMed] [Google Scholar]
- 107.Lin M, Pellerin O, Bhagat N, Rao PP, Loffroy R, Ardon R, et al. Quantitative and volumetric European Association for the Study of the Liver and Response Evaluation Criteria in Solid Tumors measurements: feasibility of a semiautomated software method to assess tumor response after transcatheter arterial chemoembolization. J Vasc Interv Radiol 2012; 23: 1629–37. doi: 10.1016/j.jvir.2012.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chapiro J, Wood LD, Lin M, Duran R, Cornish T, Lesage D, et al. Radiologic-pathologic analysis of contrast-enhanced and dffusion-weighted MR imaging in patients with HCC after TACE: diagnostic accuracy of 3D quantitative image analysis. Radiology 2014; 273: 746–58. doi: 10.1148/radiol.14140033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dreher MR, Sharma KV, Woods DL, Reddy G, Tang Y, Pritchard WF, et al. Radiopaque drug-eluting beads for transcatheter embolotherapy: experimental study of drug penetration and coverage in swine. J Vasc Interv Radiol 2012; 23: 257–64.e4. doi: 10.1016/j.jvir.2011.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fuchs K, Bize PE, Dormond O, Denys A, Doelker E, Borchard G, et al. Drug-eluting beads loaded with antiangiogenic agents for chemoembolization: in vitro sunitinib loading and release and in vivo pharmacokinetics in an animal model. J Vasc Interv Radiol 2014; 25: 379–87, 87 e1–2. doi: 10.1016/j.jvir.2013.11.039 [DOI] [PubMed] [Google Scholar]
- 111.Miyayama S, Yamashiro M, Hashimoto M, Hashimoto N, Ikuno M, Okumura K, et al. Identification of small hepatocellular carcinoma and tumor-feeding branches with cone-beam CT guidance technology during transcatheter arterial chemoembolization. J Vasc Interv Radiol 2013; 24: 501–8. doi: 10.1016/j.jvir.2012.12.022 [DOI] [PubMed] [Google Scholar]