Abstract
Objective:
To assess the accuracy of a 4-month post-(chemo)radiotherapy 18-fludeoxyglucose (18F-FDG) positron emission tomography (PET)-CT for head and neck squamous cell carcinoma (HNSCC).
Methods:
105 patients who underwent a baseline and response assessment 18F-FDG PET-CT scan between 2008 and April 2013 were identified. 18F-FDG PET-CT outcomes were analysed with reference to clinicopathological outcomes.
Results:
79 of 105 (75%) 18F-FDG PET-CT scans demonstrated a complete metabolic response; 19 of 101 (19%) for assessable primary tumours were positive; and 19 of 93 (20%) for patients with nodal disease were equivocal (n = 10) or positive (n = 9). The sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) for primary and nodal disease were 90%, 89%, 47%, 99% and 91%, 89%, 53% and 99%, respectively. Eight of nine patients with a positive nodal response scan had clinicopathological evidence of residual nodal disease (PPV, 89%). 2 of 10 patients with equivocal nodal responses had clinicopathological evidence of residual nodal disease (PPV, 20%).
Conclusion:
18F-FDG PET-CT 4 months post treatment has a very high NPV. A positive 18F-FDG PET-CT has a high PPV for residual nodal disease. By contrast, patients who have an equivocal nodal response have a low PPV.
Advances in knowledge:
Response assessment 18F-FDG PET-CT is a valuable tool in guiding the selective use of neck dissection following (chemo)radiotherapy for HNSCC. An equivocal lymph node response has a limited predictive value for persistent disease, and optimal management remains a clinical challenge.
INTRODUCTION
Non-surgical treatment in the form of concurrent chemoradiotherapy has now been established as a standard of care in the management of locally advanced head and neck squamous cell carcinoma (HNSCC), for both unresectable disease1 and organ preservation.2 However, the optimal management of nodal disease in the modern era remains a highly controversial area.3 The practice of a planned neck dissection following (chemo)radiotherapy was based upon the low sensitivity of clinical assessment and radiology for accurately detecting residual lymph node disease.3–5 However, neck dissection has an impact upon long-term toxicity.6,7 The desire to avoid the morbidity of multimodality treatment has driven a shift in practice towards the use of neck dissection only for patients failing to achieve a clinical and/or radiological complete lymph node response post treatment.3,5,8–10 Within this approach, accurate radiological assessment of the nodal response is required to avoid unnecessary neck dissection.
Cross-sectional imaging with CT or MRI is inherently limited when assessing the status of a post-treatment lymph node residuum. Classical radiological size criteria11 do not necessarily apply in the post-treatment setting. Combined functional and anatomical imaging with 18-fludeoxyglucose (18F-FDG) positron emission tomography (PET)-CT offers the potential to improve the accuracy of response assessment following completion of (chemo)radiotherapy for HSNCC. The utility of PET-CT in identifying which patients would not benefit from surgical intervention depends upon the negative predictive value (NPV) of the scan. Conversely, the ability of PET-CT-based response assessment to correctly identify patients in whom surgery is required is critically dependent upon the positive predictive value (PPV); this is a challenging area with regard to images with an “equivocal” or uncertain response interpretation. These test characteristics are particularly important with regard to lymph node response and the ongoing debate regarding the role of a “planned” neck dissection; a high NPV for persistent neck disease would provide a justification to omit neck dissection, whereas the PPV would determine how useful PET-CT is in correctly selecting patients who do require neck dissection. Reported PPVs and NPVs have varied across studies10,12–15 and are likely to be affected by multiple factors, including patients, cancer and treatment characteristics, method of interpretation and timing of post-treatment imaging.
In our centre, we have adopted a policy of performing 18F-FDG PET-CT at baseline, and response assessment delayed until approximately 4 months following completion of radiotherapy for locally advanced HNSCC. We have previously reported our initial experience of 44 patients with early clinical follow-up (median, 14 months) with a high NPV but only limited PPV with “positive” and “equivocal” scan results grouped together.16 The purpose of this present study was to review long-term outcomes of a larger cohort of patients using qualitative image interpretation, to further inform upon the critical issues of NPV, PPV, the outcome of “equivocal” imaging and the optimal timing of response assessment imaging.
METHODS AND MATERIALS
This retrospective study was approved by the institutional review board of Leeds Cancer Centre, UK.
Inclusion criteria
Since August 2008, patients treated under the care of two oncologists (MS and RJDP) with definitive non-surgical treatment for locally advanced HNSCC underwent 18F-FDG PET-CT at baseline with the intention of performing a response assessment 18F-FDG PET-CT 16 weeks following the final fraction of radiotherapy according to an institutional protocol. For the purposes of this study, consecutive patients who underwent baseline and response assessment 18F-FDG PET-CT for head and neck cancer between August 2008 and April 2013 were retrospectively identified from an institutional PET-CT database. Electronic case notes were used to identify patients who fulfilled the eligibility criteria for the study. Disease staging was performed according to the 2002 classification of the American Joint Committee on Cancer Staging. Human papillomavirus (HPV) status was not routinely determined during this time period.
Eligible patients to be included in retrospective analysis fulfilled all of the following criteria:
(1) Histologically confirmed squamous cell carcinoma of the oropharynx, oral cavity, hypopharynx, larynx, paranasal sinuses or unknown site, and presumed mucosal.
(2) Received radical non-surgical treatment (radiotherapy alone or chemoradiotherapy).
(3) 18F-FDG PET-CT performed as a baseline prior to treatment.
(4) 18F-FDG PET-CT performed as response assessment post (chemo)radiotherapy.
(5) No evidence of distant metastatic disease.
Exclusion criteria included:
(1) Nasopharynx cancer.
(2) Previous therapeutic resection of primary or nodal disease.
(3) History of radiotherapy.
(4) 18F-FDG PET-CT performed only following response assessment with CT and/or MRI.
Radiotherapy
Patients were treated with either three-dimensional (3D)-conformal radiotherapy or intensity-modulated radiotherapy (IMRT), which was gradually introduced into routine clinical practice from December 2008. 3D-conformal radiotherapy consisted of a parallel opposed pair or 5- to 7-field conformal technique, as previously described.17 IMRT was delivered using a compartmental approach to target volume delineation and a 5- to 7-angle step-and-shoot technique. Institutional protocols were followed with a radical treatment dose of 70 Gy in 35 fractions over 7 weeks, with lower doses to prophylactic dose regions (54–63 Gy in 35 fractions over 7 weeks). One other dose fractionation of 65 Gy in 30 fractions with a prophylactic neck dose of 54 Gy in 30 fractions was used for one patient in the analysed group.
Chemotherapy
Induction chemotherapy with docetaxel, cisplatin and 5-fluorouracil was delivered to a proportion of patients as previously described.18 Concurrent chemotherapy routinely consisted of cisplatin 100 mg m−2 at Days 1 and 29. Carboplatin area under the curve 4 was substituted for cisplatin if creatinine clearance was <55 ml min−1.
Response assessment and follow-up
Tumour response was routinely assessed at approximately 16 weeks following the final fraction of radiotherapy by clinical examination, nasoendoscopy where appropriate and 18F-FDG PET-CT. Examination under anaesthetic and biopsies were performed at clinical discretion following response assessment. In general, patients who achieved a complete metabolic PET-CT response did not undergo biopsy. Patients with less than a complete response were managed on an individual basis based upon opinion of the specialist multidisciplinary team. Subsequently, patients were followed up with physical examination and flexible endoscopy every 6–8 weeks in the first year after treatment, every 3 months for an additional 2 years and every 6 months until discharge at 5 years.
18-Fludeoxyglucose positron emission tomography-CT protocol
18F-FDG PET-CT examinations prior to June 2010 were performed on a 16-slice Discovery STE PET-CT scanner (GE Healthcare, Amersham, UK) and from June 2010 on a 64-slice Philips Gemini TF64 scanner (Philips Healthcare, Best, Netherlands). PET acquisition from skull vertex to upper thighs was performed 60 min after a 400-MBq dose of intravenous 18F-FDG. A silence protocol was employed in the uptake period following tracer injection to minimize physiological tracer activity within the head and neck region. The CT component was performed according to a standardized protocol (without the use of iodinated contrast medium) with the following settings: 140 kV; 80 mAs; tube rotation time, 0.5 s per rotation; pitch, 6; section thickness, 3.75 mm (to match the PET section thickness). Patients maintained normal shallow respiration during the CT acquisition. Images were reconstructed using a standard ordered subset expectation maximization algorithm with CT for attenuation correction. Both non-attenuation-corrected and attenuation-corrected datasets were reconstructed.
Categorization of 18-fludeoxyglucose positron emission tomography-CT response assessment
To evaluate the application of 18F-FDG PET-CT to clinical decision-making, the categorization of the 18F-FDG PET-CT response for the purposes of this analysis was based upon formal radiology reports. All 18F-FDG PET-CT scans were reported by a team of three highly experienced clinicians dual certified in radiology and nuclear medicine (range of PET-CT experience, 6–10 years). 18F-FDG PET-CT images were assessed qualitatively (by comparison of tumour or nodal tracer activity with background physiological uptake). Semi-quantitative assessment [maximum standardized uptake value (SUVmax)] of residual tumour or nodal uptake was also documented, but this was not fundamental to the qualitative interpretation of response. Primary tumour and nodal SUVmax values were documented. Results of post-treatment 18F-FDG PET-CT were categorized into “positive”, “equivocal” or “negative” for the primary site and nodal sites separately, as previously described.16 Areas of 18F-FDG uptake were classified as positive if uptake was focal, corresponding to a structural abnormality and of greater intensity than background liver activity. Scans were classed as equivocal if focal 18F-FDG uptake was reduced from baseline or was below liver background but above that of surrounding normal tissues. Scans were classed as negative in the absence of any abnormal focal 18F-FDG uptake or diffuse 18F-FDG uptake in the absence of corresponding anatomical abnormality on the CT which was considered to be radiotherapy related. The presence or absence of residual tissue on the CT component of the post-treatment 18F-FDG PET-CT was recorded.
Analysis and statistics
Follow-up duration was defined as from the last day of radiotherapy treatment. Pathology from either a biopsy or surgical procedure was defined as the gold standard for determining the presence of persistent or recurrent locoregional disease. In patients who did not receive a biopsy, serial negative physical examinations over the follow-up period and any relevant imaging investigations were used as the confirmation of disease-free status. Sensitivity, specificity, PPV and NPV were calculated using 2 × 2 tables constructed using clinicopathological outcomes.
RESULTS
105 patients fulfilled the criteria for analysis; median age was 57 years (range, 25–75 years). 87 of 105 (83%) patients received concurrent chemoradiation, and 14 of 105 (13%) had radiation therapy alone. Patient demographics, tumour site, subsite, tumour–node–metastasis stage, histology and treatment details are summarized in Table 1. No patients had definite evidence of metastatic disease on baseline 18F-FDG PET-CT.
Table 1.
Demographics, tumour and treatment details (n = 105)
| Characteristics | n = 105 | % |
|---|---|---|
| Gender | ||
| Male | 78 | 74 |
| Female | 27 | 26 |
| Tumour localization | ||
| Oropharynx | 76 | 72 |
| Larynx | 8 | 8 |
| Hypopharynx | 16 | 15 |
| Paranasal sinus | 1 | 1 |
| Unknown primary | 4 | 4 |
| T stage | ||
| TX | 4 | 4 |
| T1 | 20 | 19 |
| T2 | 33 | 31 |
| T3 | 25 | 24 |
| T4 | 23 | 22 |
| N stage | ||
| N0 | 12 | 11 |
| N1 | 6 | 6 |
| N2 | 84 | 80 |
| N2a | 6 | 6 |
| N2b | 58 | 55 |
| N2c | 20 | 19 |
| N3 | 3 | 3 |
| Stage group (AJCC) | ||
| I | 0 | 0 |
| II | 4 | 4 |
| III | 8 | 8 |
| IV | 93 | 88 |
| Histopathology | ||
| Squamous cell carcinoma | 105 | 100 |
| Treatment | ||
| Radical RT | 18 | 17 |
| Cisplatin RT | 65 | 62 |
| Carboplatin RT | 1 | 1 |
| TPF + cisplatin RT | 15 | 14 |
| PF + cisplatin RT | 1 | 1 |
| Cetuximab RT | 5 | 5 |
| Radiotherapy dose | ||
| 70 Gy in 35 fractions | 104 | 99 |
| 65 Gy in 30 fractions | 1 | 1 |
| Radiotherapy technique | ||
| Three-dimensional-conformal radiotherapy | 23 | 22 |
| Intensity-modulated radiotherapy | 82 | 78 |
AJCC, American Joint Committee on Cancer; PF, cisplatin and 5-fluorouracil; RT, radiotherapy; TPF, docetaxel, cisplatin and 5-fluorouracil.
Response assessment 18F-FDG PET-CT was performed at a median of 17.4 weeks following completion of (chemo)radiation (interquartile range, 16.3–18.4 weeks; range 9–24 months). Median follow-up from the completion of treatment was 25 months (range, 7–73 months). 79 of 105 (75%) response assessment 18F-FDG PET-CT scans demonstrated a complete metabolic response. 19 of 101 (19%) for patients with assessable primary tumours were positive. 19 of 93 (20%) for patients with pre-treatment nodal disease were equivocal or positive. Based upon clinicopathological outcome and follow-up, the sensitivity, specificity, PPV and NPV for response assessment 18F-FDG PET-CT are summarized for the primary tumour and lymph node disease in Table 2; for the purposes of this analysis, scans in which a complete metabolic response was not achieved were grouped together. Distant metastases were detected on response assessment 18F-FDG PET-CT in 9 of 105 patients (9%); in 6 of these 9 cases, there was a complete metabolic locoregional response, 2 had residual nodal disease and 1 had residual primary disease.
Table 2.
Response assessment 18-fludeoxyglucose (18F-FDG) positron emission tomography (PET)-CT in 105 patients with locally advanced head and neck squamous cell carcinoma after (chemo)radiation
| Scan outcome | Primary site (n = 101) | Neck nodes (n = 93) | Overall (primary and neck) (n = 105) |
|---|---|---|---|
| 18F-FDG PET-CT positive | 19 | 19 | 28 |
| 18F-FDG PET-CT negative | 82 | 74 | 77 |
| True positive | 9 | 10 | 15 |
| True negative | 81 | 73 | 76 |
| False positive | 10 | 9 | 12 |
| False negative | 1 | 1 | 2 |
| Sensitivity (%) | 90 | 91 | 88 |
| Specificity (%) | 89 | 89 | 86 |
| Positive predictive value (%) | 47 | 53 | 56 |
| Negative predictive value (%) | 99 | 99 | 97 |
Lymph node 18-fludeoxyglucose positron emission tomography-CT response
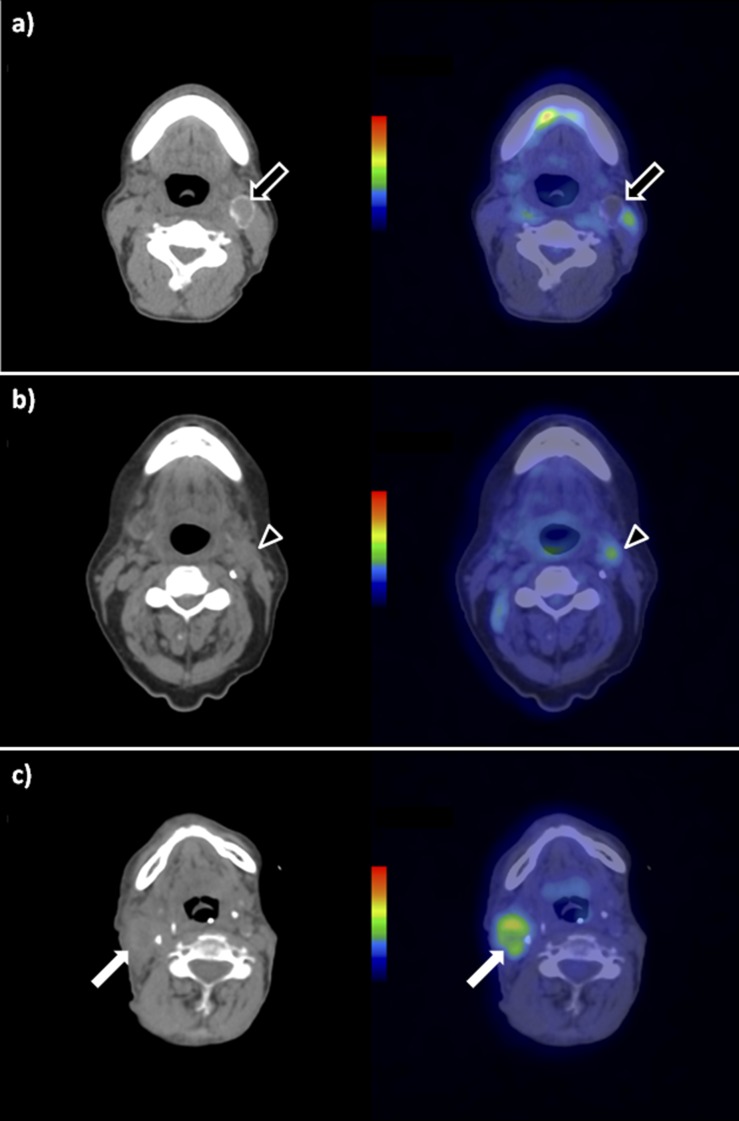
93 of 105 patients (89%) had nodal disease at baseline [87 of 93 (94%) N2 or N3]. Out of these 93 patients, 74 (80%) response assessment 18F-FDG PET-CT scans were categorized as showing a complete metabolic response, 9 (10%) as positive and 10 (11%) as equivocal in lymph nodes. Examples are shown in Figure 1.
Figure 1.
Lymph node appearances on 18-fludeoxyglucose positron emission tomography (PET)-CT post-chemoradiotherapy. Nodal appearances following chemoradiotherapy on axial unenhanced CT (left column) and axial fused PET-CT (right column). (a) Negative node: 2 cm photopenic residual left Level II node. The presence of calcification post treatment is consistent with a healing response. Arrows, left Level II lymph node. (b) Equivocal node: 1.5 cm residual left Level II node displaying low-grade uptake [maximum standardized uptake value (SUVmax), 2.7]. Arrowheads, left Level II lymph node. (c) Positive node: 3 cm residual right Level II node displaying moderate-grade uptake (SUVmax, 5.3). Arrows, right Level II lymph node.
Complete metabolic nodal response
1 patient of 74 (1.4%) with a complete metabolic response in lymph node disease subsequently suffered a pathologically proven isolated nodal relapse 14 months following the completion of chemoradiotherapy for a T3N2b tonsil cancer and was managed with neck dissection with no evidence of relapse in the primary site.
12 patients had residual nodal masses that were non-avid on 18F-FDG PET-CT; 11 of 12 also had a complete metabolic response in the primary site and 1 patient had residual 18F-FDG avidity in the primary site with subsequent negative biopsies. All of these 12 patients were managed without neck dissection. After a median follow-up of 40 months (range, 12–63 months), none of these 12 patients has had locoregional failure, although 1 has died following the development of brain metastases.
Positive metabolic nodal response
Of the nine scans with 18F-FDG-positive residual lymph nodes, median nodal SUVmax was 5.2 (range, 2.9–9.6); eight of nine of these patients had clinicopathological evidence of residual lymph node disease. The PPV of a nodal “positive” 18F-FDG PET-CT response scan is 89%. Four of nine patients had a complete metabolic response at the site of primary disease. Two of nine patients had developed lung metastases on the response assessment 18F-FDG PET-CT and subsequently progressed clinically in the lymph node region. Four of nine patients had evidence of residual disease in both the primary site and lymph nodes on response assessment 18F-FDG PET-CT with subsequent clinical progression. Three of nine patients had residual 18F-FDG avidity classified as positive in the neck nodes alone with a complete primary response; two were inoperable owing to the extent of nodal disease and subsequently progressed, and one was observed with no evidence of clinical progression in the lymph nodes but developed lung metastases and died with no evidence of lymph node progression 9 months following the response assessment 18F-FDG PET-CT.
Equivocal metabolic nodal response
The 10 patients with an “equivocal” nodal response by 18F-FDG PET-CT assessment are summarized in Table 3. All of these patients had received chemoradiotherapy treatment. 2 of 10 (20%) patients developed subsequent nodal progression with synchronous lung metastases. Of the remaining eight patients, none have any evidence of disease recurrence in neck lymph nodes. One patient underwent a repeat 18F-FDG PET-CT scan to reassess nodal activity after a further interval of 2 months; this 18F-FDG PET-CT was unchanged and neck dissection was performed with no evidence of pathological disease. Three of these patients had undergone neck dissections in which there was no pathological evidence of residual disease. The PPV of an “equivocal” nodal response for subsequent nodal recurrence is 20%.
Table 3.
Summary of outcomes of patients with an equivocal lymph node response assessment by positron emission tomography (PET)-CT
| Primary | Stage | Treatment | Baseline LN size (mm) | Baseline LN SUVmax | Response in primary | Response LN size (mm) | Response LN SUVmax | F/U (months) | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Oropharynx, base of tongue | T4N2b | Cisplatin RT | 19 | 5.4 | PR | 9 | 2.6 | 41 | EUA-CR in primary; DF |
| Oropharynx, tonsil | T2N2b | Cisplatin RT | 12 | 3.0 | CR | 7 | 2.4 | 27 | DF |
| Unknown primary | TxN2b | TPF + cisplatin RT | 28 | 9.0 | N/A | 8 | 2.7 | 60 | Negative neck dissection; DF |
| Oropharynx, tonsil | T3N2b | Cisplatin RT | 35 | 8.7 | CR | 15 | 2.7 | 37 | Rpt PET unchanged 2/12; negative neck dissection; DF |
| Oropharynx, base of tongue | T1N2b | Cisplatin RT | 30 | 9.7 | CR | 9 | 2.3 | 32 | DF |
| Oropharynx, base of tongue | T1N2c | Cisplatin RT | 24 | 10.8 | CR | 8 | 2.4 | 12 | DF, equivocal lung nodules on F/U |
| Oropharynx, base of tongue | T3N2b | Cisplatin RT | 29 | 12.6 | CR | 7 | 2.1 | 44 | Lung metastases developed at 12/12 F/U; no nodal progression; died after 36 months |
| Hypopharynx | T3N2c | Cisplatin RT | 14 | 11.3 | CR | 9 | 2.8 | 13 | FNA neck: squamous cell carcinoma. Subsequent nodal progression with new lung metastases |
| Oropharynx, tonsil | T2N1 | Cisplatin RT | 10 | 3.0 | No response | 6 | 2.0 | 16 | Salvage surgery to primary/neck; no pathological LN in neck |
| Oropharynx, tonsil | T1N2b | Cisplatin RT | 50 | 16.4 | CR | 7 | 2.4 | 57 | Regional neck recurrence with synchronous lung metastases |
CR, complete response; DF, disease free; EUA, examination under anaesthetic; FNA, fine-needle aspiration; F/U, follow-up; LN, lymph node; N/A, not applicable; PR, partial response; Rpt, repeat; RT, radiotherapy; SUVmax, maximum standardized uptake value; TPF, docetaxel, cisplatin and 5-fluorouracil.
DISCUSSION
In the meta-analysis of response assessment PET-CT in 2011 by Gupta et al12 of 51 studies with 2335 patients, a high NPV was reported for the primary and nodal sites of 95.1% and 94.5%, respectively. In this analysis, there appeared to be no significant difference in test characteristics between the use of combined PET-CT in more recent series compared with earlier single modality PET imaging in earlier series. In the meta-analysis, an improvement in sensitivity and specificity was found when comparing PET performed more than 12 weeks post treatment. Although several large series10,13–15 have been published subsequent to the meta-analysis by Gupta et al,12 the reported NPV results are broadly similar to the meta-analysis with response assessment commonly performed at around 3 months post treatment. For example, Marcus et al13 reported 214 patients with PET-CT performed at a median of 12 weeks post treatment with an NPV of 91%. In a series of 101 patients with HPV-associated oropharyngeal cancer, Vainshtein et al14 reported the NPV for the primary and nodal disease to be 97–98% and 91–95%, respectively, depending upon the method of scan interpretation with PET-CT performed at a median of 13 weeks post treatment.
By contrast, in our series, we have found a higher NPV of 99% for both the primary and nodal disease. It is interesting to speculate on the reasons for the very high NPV in our series. A major difference in our series is that response assessment was performed at a median of 17.4 weeks post treatment, which is considerably later than in most published reports.10,13–15 To the best of our knowledge, only one other group has reported the use of a “delayed” response assessment PET-CT at 4–6 months post treatment in a series of 52 patients;19 in this study, the NPV of PET-CT for both the primary and nodal disease was 100%. These data raise the possibility that increasing the interval from completion of treatment maximizes the NPV of PET-CT, increasing confidence in avoiding surgical intervention. Timing of response assessment is a balance between the competing interests of allowing sufficient time for tumour response and allowing early radiation reactions to subside, with the need to avoid missing interim disease progression and the onset of radiation-induced tissue fibrosis, which may complicate neck dissection. In this regard, there are no clear comparative data regarding the morbidity of neck dissection performed early or late post treatment.
The PPV of PET-CT response assessment is generally considered to be limited; in their meta-analysis, Gupta et al12 reported a PPV for primary and nodal sites of 58.6% and 52.1%, respectively. This issue is complicated by the lack of a widely accepted method for interpreting PET-CT imaging post treatment. The method of categorizing response is likely to have a major bearing on the PPV, with “equivocal” and “positive” responses often grouped together for analysis.16 In our series, PET-CT scans were interpreted qualitatively into positive, equivocal and negative categories; this methodology is similar to that reported by Porceddu et al.15 The nodal PPV of “positive” response imaging was 89% and of “equivocal” imaging was 20%. These data suggest that with regard to nodal disease, an equivocal metabolic response implies a low likelihood of harbouring residual disease, whereas a true metabolically positive response scan has a high PPV. These data are consistent with Porceddu et al.15 In their prospective series, 11 patients had equivocal PET response assessment findings and were managed with a repeat PET scan after an interval 4–6 weeks when repeat imaging in 10 of 11 cases showed a complete metabolic response with no subsequent nodal failures. Similarly, in a retrospective cohort of 101 patients with HPV-positive oropharyngeal cancer reported by Vainshtein et al,14 regional nodal failure occurred in 2 of 9 patients with what was reported as a “near-complete response” on 18F-FDG PET-CT. Interestingly, Marcus et al13 have recently proposed the “Hopkins criteria” for interpreting response assessment scans, based upon a five-point qualitative scale; the category of “equivocal” responses utilized by ourselves and Porceddu et al15 would be categorized as negative scans according to this classification. Although these data suggest a low PPV, the clinical management of “equivocal” nodal responses remains unclear;20 options include repeat interval imaging, clinical follow-up and neck dissection. A Phase III study in the UK National Cancer Research Institute portfolio (PET Neck) completed recruitment in late 2012, examining the ability of 18F-FDG PET-CT to avoid the need for a planned neck dissection; analysis of these data may shed further light on this area.
The limitations of the series reported here include the retrospective nature of the analysis. Treatment was heterogeneous in nature with 18 of 105 (17%) receiving radiotherapy alone; it is possible that the accuracy of response assessment 18F-FDG PET-CT may vary between treatment with radiotherapy alone and (chemo)-radiotherapy. An additional limitation is the lack of HPV data in this historical cohort that includes a majority of patients with oropharynx cancer. HPV-positive oropharyngeal cancers are associated with a more favourable prognosis.21 It can be considered that the favourable outcomes without neck dissection by the use of response assessment 18F-FDG PET-CT could be influenced by the inclusion of a significant proportion of patients with HPV-positive oropharyngeal disease. 8 of the 10 patients with equivocal 18F-FDG PET-CT response assessment imaging had oropharyngeal cancer; this may have influenced the finding of a low PPV.
In summary, these data show that a response assessment 18F-FDG PET-CT performed 16 weeks post treatment has a very high NPV for nodal response, allowing the avoidance of neck dissection. A positive 18F-FDG PET-CT has a high PPV for residual nodal disease informing the need for neck dissection. By contrast, patients who have an equivocal nodal response on 18F-FDG PET-CT have a low PPV for residual disease. The optimal management of these patients remains an area requiring further investigation with strategies such as an early repeat PET-CT offering an alternative to an immediate neck dissection.
Contributor Information
F Slevin, Email: finslevin@googlemail.com.
M Subesinghe, Email: manil.subesinghe@gmail.com.
S Ramasamy, Email: van.ramasamy@gmail.com.
M Sen, Email: Mehmet.Sen@leedsth.nhs.uk.
A F Scarsbrook, Email: Andrew.Scarsbrook@leedsth.nhs.uk.
R J D Prestwich, Email: Robin.Prestwich@leedsth.nhs.uk, rprestwich@doctors.org.uk.
REFERENCES
- 1.Adelstein DJ, Li Y, Adams GL, Wagner H, Jr, Kish JA, Ensley JF, et al. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol 2003; 21: 92–8. doi: 10.1200/JCO.2003.01.008 [DOI] [PubMed] [Google Scholar]
- 2.Forastiere AA, Goepfert H, Maor M, Pajak TF, Weber R, Morrison W, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med 2003; 349: 2091–8. doi: 10.1056/NEJMoa031317 [DOI] [PubMed] [Google Scholar]
- 3.Ferlito A, Corry J, Silver CE, Shaha AR, Thomas Robbins K, Rinaldo A. Planned neck dissection for patients with complete response to chemoradiotherapy: a concept approaching obsolescence. Head Neck 2010; 32: 253–61. doi: 10.1002/hed.21173 [DOI] [PubMed] [Google Scholar]
- 4.Brizel DM, Prosnitz RG, Hunter S, Fisher SR, Clough RL, Downey MA, et al. Necessity for adjuvant neck dissection in setting of concurrent chemoradiation for advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys 2004; 58: 1418–23. doi: 10.1016/j.ijrobp.2003.09.004 [DOI] [PubMed] [Google Scholar]
- 5.Hamoir M, Ferlito A, Schmitz S, Hanin FX, Thariat J, Weynand B, et al. The role of neck dissection in the setting of chemoradiation therapy for head and neck squamous cell carcinoma with advanced neck disease. Oral Oncol 2012; 48: 203–10. doi: 10.1016/j.oraloncology.2011.10.015 [DOI] [PubMed] [Google Scholar]
- 6.Machtay M, Moughan J, Trotti A, Garden AS, Weber RS, Cooper JS, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an RTOG analysis. J Clin Oncol 2008; 26: 3582–9. doi: 10.1200/JCO.2007.14.8841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lango MN, Egleston B, Ende K, Feigenberg S, D'Ambrosio DJ, Cohen RB, et al. Impact of neck dissection on long-term feeding tube dependence in patients with head and neck cancer treated with primary radiation or chemoradiation. Head Neck 2010; 32: 341–7. doi: 10.1002/hed.21188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karakaya E, Yetmen O, Oksuz DC, Coyle C, Dyker K, Sen M, et al. Chemoradiotherapy for N2 head and neck squamous cell carcinoma—outcomes without a planned neck dissection: our experience in two hundred and seven patients. Clin Otolaryngol 2013; 38: 347–51. doi: 10.1111/coa.12133 [DOI] [PubMed] [Google Scholar]
- 9.Karakaya E, Yetmen O, Oksuz DC, Dyker KE, Coyle C, Sen M, et al. Outcomes following chemoradiotherapy for N3 head and neck squamous cell carcinoma without a planned neck dissection. Oral Oncol 2013; 49: 55–9. doi: 10.1016/j.oraloncology.2012.07.010 [DOI] [PubMed] [Google Scholar]
- 10.Goenka A, Morris LG, Rao SS, Wolden SL, Wong RJ, Kraus DH, et al. Long-term regional control in the observed neck following definitive chemoradiation for node-positive oropharyngeal squamous cell cancer. Int J Cancer 2013; 133: 1214–21. doi: 10.1002/ijc.28120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van den Brekel MW, Stel HV, Castelijns JA, Nauta JJ, van der Waal I, Valk J, et al. Cervical lymph node metastasis: assessment of radiologic criteria. Radiology 1990; 177: 379–84. doi: 10.1148/radiology.177.2.2217772 [DOI] [PubMed] [Google Scholar]
- 12.Gupta T, Master Z, Kannan S, Agarwal JP, Ghsoh-Laskar S, Rangarajan V, et al. Diagnostic performance of post-treatment FDG PET or FDG PET/CT imaging in head and neck cancer: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging 2011; 38: 2083–95. doi: 10.1007/s00259-011-1893-y [DOI] [PubMed] [Google Scholar]
- 13.Marcus C, Ciarallo A, Tahari AK, Mena E, Koch W, Wahl RL, et al. Head and neck PET/CT: therapy response interpretation criteria (Hopkins Criteria)-interreader reliability, accuracy, and survival outcomes. J Nucl Med 2014; 55: 1411–16. doi: 10.2967/jnumed.113.136796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vainshtein JM, Spector ME, Stenmark MH, Bradford CR, Wolf GT, Worden FP, et al. Reliability of post-chemoradiotherapy F-18-FDG PET/CT for prediction of locoregional failure in human papillomavirus-associated oropharyngeal cancer. Oral Oncol 2014; 50: 234–9. doi: 10.1016/j.oraloncology.2013.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Porceddu SV, Pryor DI, Burmeister E, Burmeister BH, Poulsen MG, Foote MC, et al. Results of a prospective study of positron emission tomography-directed management of residual nodal abnormalities in node-positive head and neck cancer after definitive radiotherapy with or without systemic therapy. Head Neck 2011; 33: 1675–82. doi: 10.1002/hed.21655 [DOI] [PubMed] [Google Scholar]
- 16.Prestwich RJ, Subesinghe M, Gilbert A, Chowdhury FU, Sen M, Scarsbrook AF. Delayed response assessment with FDG-PET-CT following (chemo)radiotherapy for locally advanced head and neck squamous cell carcinoma. Clin Radiol 2012; 67: 966–75. doi: 10.1016/j.crad.2012.02.016 [DOI] [PubMed] [Google Scholar]
- 17.Prestwich RJ, Kancherla K, Oksuz DC, Williamson D, Dyker KE, Coyle C, et al. A single centre experience with sequential and concomitant chemoradiotherapy in locally advanced stage IV tonsillar cancer. Radiat Oncol 2010; 5: 121. doi: 10.1186/1748-717X-5-121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prestwich RJ, Öksüz DC, Dyker K, Coyle C, Sen M. Feasibility and efficacy of induction docetaxel, cisplatin, and 5-fluorouracil chemotherapy combined with cisplatin concurrent chemoradiotherapy for nonmetastatic Stage IV head-and-neck squamous cell carcinomas. Int J Radiat Oncol Biol Phys 2011; 81: e237–43. doi: 10.1016/j.ijrobp.2011.03.043 [DOI] [PubMed] [Google Scholar]
- 19.Zundel MT, Michel MA, Schultz CJ, Maheshwari M, Wong SJ, Campbell BH, et al. Comparison of physical examination and fluorodeoxyglucose positron emission tomography/computed tomography 4–6 months after radiotherapy to assess residual head-and-neck cancer. Int J Radiat Oncol Biol Phys 2011; 81: e825–32. doi: 10.1016/j.ijrobp.2010.11.072 [DOI] [PubMed] [Google Scholar]
- 20.Prestwich R, Sen M, Scarsbrook A. Qualitative 18F-FDG PET/CT response evaluation after chemotherapy or radiotherapy for head and neck squamous cell carcinoma: is there an equivocal group? J Nucl Med 2014; 55: 2081. doi: 10.2967/jnumed.114.148114 [DOI] [PubMed] [Google Scholar]
- 21.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 2010; 363: 24–35. doi: 10.1056/NEJMoa0912217 [DOI] [PMC free article] [PubMed] [Google Scholar]