Abstract
Objective:
To evaluate the diagnostic performance of ultrasound, MRI and fluorine-18 fludeoxyglucose positron emission tomography (18F-FDG PET)/CT for the diagnosis of metastatic axillary lymph node (ALN) after neoadjuvant chemotherapy (NAC) and to find out histopathological factors affecting the diagnostic performance of these imaging modalities.
Methods:
From January 2012 to November 2014, 191 consecutive patients with breast cancer who underwent NAC before surgery were retrospectively reviewed. We included 139 patients with ALN metastasis that was confirmed on fine needle aspiration or core needle biopsy at initial diagnosis.
Results:
After NAC, 39 (28%) patients showed negative conversion of ALN on surgical specimens of sentinel lymph node (LN) or ALN. The sensitivity of ultrasound, MRI and PET/CT was 50% (48/96), 72% (70/97) and 22% (16/73), respectively. The specificity of ultrasound, MRI and PET/CT was 77% (30/39), 54% (21/39) and 85% (22/26), respectively. The Az value of combination of ultrasound and PET/CT was the highest (0.634) followed by ultrasound (0.626) and combination of ultrasound, MRI and PET/CT (0.617). The size of tumour deposit in LN and oestrogen receptor was significantly associated with the diagnostic performance of ultrasound (p < 0.001 and p = 0.009, respectively) and MRI (p = 0.045 and p = 0.036, respectively). The percentage diameter decrease, size of tumour deposit in LN, progesterone receptor, HER2 and histological grade were significantly associated with the diagnostic performance of PET/CT (p = 0.023, p = 0.002, p = 0.036, p = 0.044 and p = 0.008, respectively). On multivariate logistic regression analysis, size of tumour deposit within LN was identified as being independently associated with diagnostic performance of ultrasound [odds ratio, 13.07; 95% confidence interval (CI), 2.95–57.96] and PET/CT (odds ratio, 6.47; 95% CI, 1.407–29.737).
Conclusion:
Combination of three imaging modalities showed the highest sensitivity, and PET/CT showed the highest specificity for the evaluation of ALN metastasis after NAC. Ultrasound alone or combination of ultrasound and PET/CT showed the highest positive-predictive value. The size of tumour deposit within ALN was significantly associated with diagnostic performance of ultrasound and PET/CT.
Advances in knowledge:
This study is about the diagnostic performance of ultrasound, MRI, PET/CT and combination of each imaging modality for the evaluation of metastatic ALN after NAC. Of many histopathological factors, only the size of tumour deposit within ALN was an independent factor associated with the diagnostic performance of ultrasound and PET/CT.
Axillary lymph node (ALN) metastasis is one of the most significant prognostic factors in patients with breast cancer. As the management of axillary lesions has been diverse, the detection of axillary nodal lesion has been more important. The diagnostic accuracy of ultrasound and MRI for the detection of metastatic ALNs has been studied by many researchers. Sensitivity and specificity of ultrasound for the detection of metastatic ALNs have been reported as 41.2–70.8% and 54.5–93.7%.1–4 Sensitivity and specificity of MRI have been reported as 36–79% and 93–100%, respectively.4–6
Neoadjuvant chemotherapy (NAC) has become the standard treatment not only in patients with locally advanced breast cancer but also in early invasive breast cancer in an attempt to downstage the primary cancer and to reduce micrometastasis. If the ALN metastasis is confirmed on fine needle aspiration biopsy (FNAB) or core needle biopsy (CNB) at initial diagnosis, ALN dissection (ALND) is usually performed, regardless of the responsiveness of ALN. Residual metastatic lesion of ALNs after NAC is an important prognostic factor of disease-free survival.7,8
In ACOSOG Z1071 trial, in patients with breast cancer with clinical N1 stage receiving NAC, if two or more sentinel lymph nodes (SLNs) were removed, the false-negative rate of SLN biopsy (SLNB) was relatively low, 12.6%. Therefore, the role of axillary imaging in NAC setting should be to find out metastatic lymph nodes (LNs) for surgeons to proceed directly to ALND. Another role could be to correctly diagnose negative LN to safely omit SLNB. Despite the importance of restaging of nodal status, there have been few studies about diagnostic accuracy of imaging modalities for detection of metastatic ALNs after NAC.
The purpose of our study was to evaluate the diagnostic performance of ultrasound, MRI and fluorine-18 fludeoxyglucose positron emission tomography (18F-FDG PET)/CT for the diagnosis of metastatic ALNs after NAC and to find out histopathological factors affecting the diagnostic accuracy of these imaging modalities.
METHODS AND MATERIALS
Patients
The institutional review board of Ajou University approved this retrospective study. From January 2012 to November 2014, 191 consecutive patients with breast cancer who underwent ultrasound and MRI with/without PET/CT for assessment of tumour response after NAC were retrospectively reviewed. We included 139 patients with ALN metastases that were confirmed on FNAB or CNB at initial diagnosis. Of 139 patients, 4 patients did not undergo follow-up ultrasound, 3 patients did not undergo follow-up MRI and 40 patients did not undergo follow-up PET/CT. Therefore, 135 patients were included for the ultrasound analysis, 136 patients were included for the MRI analysis and 99 patients were included for the PET-CT analysis. Patient characteristics are summarized in Table 1.
Table 1.
Patient characteristics
| Characteristics | All (n = 139) | Patients for ultrasound analysis (n = 135) | Patients for MRI analysis (n = 136) | Patients for positron emission tomography/CT analysis (n = 99) |
|---|---|---|---|---|
| Age (years) (mean ± standard deviation) | 46.4 ± 10.0 | 46.3 ± 9.1 | 46.3 ± 9.2 | 45.7 ± 8.9 |
| Tumour size (cm) (mean ± standard deviation) | 3.8 ± 2.2 | 3.8 ± 2.2 | 3.8 ± 2.2 | 4.0 ± 2.1 |
| T stage | ||||
| T1 | 23 | 23 | 23 | 9 |
| T2 | 79 | 76 | 78 | 62 |
| T3 | 16 | 16 | 14 | 13 |
| T4 | 21 | 20 | 21 | 15 |
| Tumour histology | ||||
| Infiltrating ductal | 131 | 127 | 128 | 91 |
| Infiltrating lobular | 3 | 3 | 3 | 3 |
| Other | 5 | 5 | 5 | 5 |
| Nuclear grade | ||||
| Low | 56 | 54 | 55 | 37 |
| High | 57 | 55 | 55 | 44 |
| Histological grade | ||||
| Low | 72 | 70 | 71 | 50 |
| High | 42 | 40 | 40 | 32 |
| Oestrogen receptor | ||||
| Negative | 41 | 40 | 40 | 30 |
| Positive | 98 | 95 | 96 | 69 |
| Progesterone receptor | ||||
| Negative | 68 | 66 | 67 | 48 |
| Positive | 71 | 69 | 69 | 51 |
| HER2 | ||||
| Negative | 98 | 96 | 97 | 72 |
| Positive | 41 | 39 | 39 | 27 |
Ultrasound technique and interpretation
Ultrasound examinations had been performed in all patients by one of two radiologists with 7 years' and 12 years' experience in breast imaging. Each patient was evaluated with real-time ultrasound using a Siemens Acuson S2000 Ultrasound system (Siemens Healthcare, Mountain View, CA) or Hi Vision Ascendus system (Hitachi Medical, Tokyo, Japan) with a 15-MHz linear array transducer.
ALN metastasis was suspected if the LN had any of the following morphologic characteristics: eccentric or concentric cortical thickening >3 mm, absent fatty hilum, a transverse axis-to-longitudinal axis ratio more than two or increased blood flow in the thickened cortex on Doppler image.
FNAB was performed without local anaesthesia. The needle was inserted into the area to be sampled and repeatedly redirected within the cortex and subcapsular area while suction was applied.
MRI technique and interpretation
All MR examinations were performed using a 1.5-T system (Signa HDxt; GE Healthcare, Milwaukee, WI) with a dedicated breast coil (8-channel HD breast array, GE Healthcare). Gadobutrol (Gadovist®; Bayer Schering Pharma, Berlin, Germany) was injected into an antecubital vein at a dose of 0.1 mmol kg–1 of body weight and at a rate of 3 ml s–1, followed by a 20-ml saline flush for all patients.
The imaging protocol of a 1.5-T scanner consisted of fat suppressed axial fast spin echo T2 weighted images (repetition time/echo time, 4000/74 ms; slice thickness, 3 mm) and dynamic unenhanced and contrast-enhanced fat-saturated three-dimensional (3D) gradient-echo T1 weighted imaging (5.1/2.4; flip angle, 10°; image matrix, 300 × 300 pixels; field of view, 300 ×300 mm; section thickness, 1.5 mm; and section gap, 0 mm). Sagittal and coronal reformatted images were obtained using raw data. Standard subtraction images were obtained by subtracting the pre-contrast images from the early peak post-contrast image (obtained at 80 s after contrast injection) on a pixel-by-pixel basis. In addition, maximum intensity projection reconstructions were applied to the subtraction images. ALN metastasis was suspected if the LN had any of the following morphologic characteristics: eccentric or concentric cortical thickening >3 mm, absent fatty hilum, a transverse axis-to-longitudinal axis ratio more than two on T2 weighted image.
Positron emission tomography CT technique and interpretation
After fasting for least 6 h, patients were administered 370 MBq of 18F-FDG intravenously. All patients were instructed to rest comfortably for 60 min and to urinate before scanning. Whole-body PET-CT images were obtained with a Discovery ST scanner (GE Healthcare, Milwaukee, WI). Seven to eight frames (3 min per frame) of emission PET data were acquired in 3D mode after a non-contrast CT scan from the base of the skull to the upper thigh (tube rotation time of 1 s per revolution, 120 kV, 60 mA, 7.5 mm per rotation and an acquisition time of 60.9 s for a scan length of 867 mm). Emission PET images were reconstructed using an iterative method (ordered-subsets expectation maximization with 2 iterations and 30 subsets; field of view, 600 mm; slice thickness, 3.27 mm) and attenuation corrected with non-contrast CT. Two nuclear medicine physicians reviewed 18F-FDG PET-CT images on an AW workstation (v. 4.4; GE Healthcare, Milwaukee, WI) by consensus. Focal 18F-FDG uptake with intensity higher than that of normal background soft tissue on PET image was considered to be positive for metastasis. CT images were used for anatomic localization, but not for decision making. For the semi-quantitative analysis of 18F-FDG uptake, standardized uptake values (SUVs) were calculated based on injected dose and body weight. Circular regions of interest were placed on the ALN and maximum SUVs (SUVmax) were recorded. When the lesion could not be visualized owing to a lack of 18F-FDG uptake, an SUV of zero was recorded.
Histopathological evaluation
All patients underwent surgical resection for breast cancer with SLNB and/or ALND. The routinely formalin-fixed, paraffin-embedded tissue blocks of tumours and ALNs were sectioned to 4-μm thickness and stained with haematoxylin–eosin. The specimens of tumour and ALNs were evaluated according to the following histopathological features: tumour size, histological type of carcinoma, perinodal extension of tumour in ALN, size of tumour deposit in ALN, Black nuclear grade (nuclear grade 1, poorly differentiated; grade 2, moderately differentiated; and grade 3, well differentiated) and modified Bloom–Richardson histological grade (histological grade 1, well differentiated; grade 2, moderately differentiated; and grade 3, poorly differentiated). For dichotomous-dependent variables, nuclear grade was classified as high (grade 1) vs low (grades 2 and 3) and histological grade as low (grades 1 and 2) vs high (grade 3).
If no metastasis was detected in SLN or ALN, immunohistochemical (IHC) staining was performed. Each node was recorded as benign (stage pN0), isolated tumour cells [stage pN0 (i+); ≤0.2 mm], micrometastasis (stage pN1mi; 0.2 ≤ 2.0 mm) or macrometastasis (stage pN1–3; >2.0 mm). We classified micrometastasis as positive LN. There was no case of isolated tumour cells in our study.
Expression of estrogen receptor (ER), progesterone receptor (PR) and HER2 was evaluated in the surgically removed specimens using standard avidin–biotin complex IHC staining methods. The ER and PR status was assessed using the Allred score, which was expressed as the sum of the proportion score and the intensity score of positively stained tumour cells. Tumours with an Allred score of at least three were regarded as positive. The intensity of HER2 staining was scored as 0, 1+, 2+ or 3+. Tumours with a 3+ score were classified as HER2 positive, and tumours with a 0 or 1+ score were classified as negative. In tumours with a 2+ score, gene amplification by using fluorescence in situ hybridization was used to determine HER2 status. All specimens were reviewed by a pathologist with 16 years' experience.
Statistical analysis
The diagnostic performance of ultrasound, MRI and PET/CT for the evaluation of ALN after NAC was evaluated with receiver operating characteristic (ROC) curve analysis. The diagnostic accuracy was estimated by calculating the area under the ROC curve (Az value).
Univariate analysis was performed using χ2 test for the evaluation of relationships between the rate of correct diagnosis of ultrasound, MRI and PET/CT with histopathological factors (initial tumour size, tumour size after NAC, percentage diameter decrease, size of tumour deposit in LN, perinodal tumour extension, oestrogen receptor, progesterone receptor, HER2, nuclear grade and histological grade). The definition of correct diagnosis was that the imaging findings and final pathological results were concordant, and the definition of incorrect diagnosis was that they were discordant.
Multivariate analysis was performed using logistical regression of the variables that were found to be statistically significant on univariate analyses. Analyses were performed using the SPSS® v. 19.0 statistical software package (SPSS Inc., Chicago, IL), with a value of p < 0.05 considered to be significant.
RESULTS
For 139 patients with ALN metastasis at the time of initial diagnosis, the sensitivity of ultrasound, MRI and PET/CT was 88% (123/139), 93% (128/137) and 89% (118/133), respectively.
After NAC, 39 (28%) patients showed negative conversion of ALN on surgical specimens of sentinel LN or ALN. The sensitivity of ultrasound, MRI and PET/CT was 50% (48/96), 72% (70/97) and 22% (16/73), respectively. The specificity of ultrasound, MRI and PET-CT was 77% (30/39), 54% (21/39) and 85% (22/26), respectively. The positive-predictive value (PPV) was 84% (48/57), 80% (70/88) and 80% (16/20) and the negative-predictive value (NPV) was 38% (30/78), 44% (21/48) and 28% (22/79), respectively (Table 2).
Table 2.
Sensitivity, specificity, positive-predictive value (PPV) and negative-predictive value (NPV) of ultrasound, MRI and positron emission tomography (PET)/CT after neoadjuvant chemotherapy
| Diagnostic performance | Ultrasound | MRI | PET/CT | Ultrasound + MRI | Ultrasound + PET/CT | MRI + PET/CT | Ultrasound + MRI + PET/CT |
|---|---|---|---|---|---|---|---|
| Sensitivity | 50% (48/96) | 72% (70/97) | 22% (16/73) | 77% (72/94) | 54% (38/71) | 77% (54/70) | 81% (56/69) |
| Specificity | 77% (30/39) | 54% (21/39) | 85% (22/26) | 51% (20/39) | 73% (19/26) | 42% (11/26) | 42% (11/26) |
| PPV | 84% (48/57) | 80% (70/88) | 80% (16/20) | 79% (72/91) | 84% (38/45) | 78% (54/69) | 79% (56/71) |
| NPV | 38% (30/78) | 44% (21/48) | 28% (22/79) | 48% (20/42) | 37% (19/52) | 41% (11/27) | 46% (11/24) |
According to the combined imaging modalities, the sensitivity, specificity, PPV and NPV for detecting axillary nodal metastasis were 77%, 51%, 79% and 48% for ultrasound combined with MRI; 54%, 73%, 84% and 37% for ultrasound combined with PET/CT; 77%, 42%, 78% and 41% for MRI combined with PET/CT; and 81%, 42%, 79% and 46% for all three imaging modalities combined, respectively (Table 2).
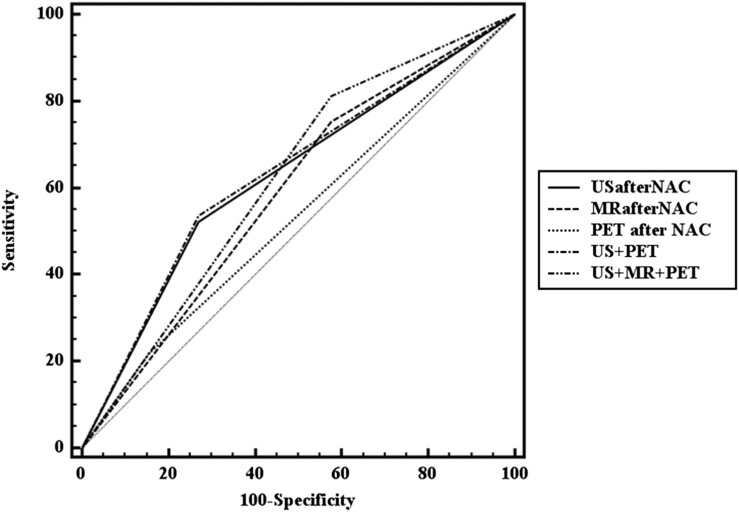
The area under the ROC curve (Az) of each imaging modality and comparison of ROC curves between imaging modalities are summarized in Table 3 and Figure 1. The Az value of combination of ultrasound and PET/CT was the highest (0.634) followed by ultrasound (0.626), combination of ultrasound, MRI and PET/CT (0.617) and combination of ultrasound and MRI (0.610).
Table 3.
Comparison of diagnostic abilities among imaging modalities
| Imaging | Az value |
p-value* vs |
||||||
|---|---|---|---|---|---|---|---|---|
| Ultrasound | MRI | PET/CT | Ultrasound + MRI | Ultrasound + PET/CT | MRI + PET/CT | Ultrasound + MRI + PET/CT | ||
| Ultrasound | 0.626 | – | 0.496 | 0.03* | 0.763 | 0.317 | 0.584 | 0.868 |
| MRI | 0.588 | 0.496 | – | 0.336 | 0.079 | 0.424 | 0.317 | 0.041* |
| PET/CT | 0.532 | 0.030* | 0.336 | – | 0.182 | 0.017* | 0.27 | 0.138 |
| Ultrasound + MRI | 0.610 | 0.763 | 0.079 | 0.182 | – | 0.666 | 0.317 | 0.317 |
| Ultrasound + PET/CT | 0.634 | 0.317 | 0.424 | 0.017* | 0.666 | – | 0.496 | 0.763 |
| MRI + PET/CT | 0.596 | 0.584 | 0.317 | 0.27 | 0.317 | 0.496 | – | 0.079 |
| Ultrasound + MRI + PET/CT | 0.617 | 0.868 | 0.041* | 0.138 | 0.317 | 0.763 | 0.079 | – |
PET, positron emission tomography.
*p < 0.05.
Figure 1.
Receiver operating characteristic analysis curves for the diagnosis of axillary lymph node metastasis after neoadjuvant chemotherapy (NAC). PET, positron emission tomography; US, ultrasound.
The Az value of ultrasound was significantly greater than that of PET/CT (p = 0.03), the Az value of combination of ultrasound and PET/CT was greater than that of PET/CT alone (p = 0.017) and the Az value of combination of all imaging modalities was greater than that of MRI alone (p = 0.041).
Univariate analysis was performed for the evaluation of relationships between the rate of correct diagnosis of ultrasound, MRI and PET/CT with histopathological factors (Table 4). The size of tumour deposit in LN and oestrogen receptor was significantly associated with the rate of correct diagnosis of ultrasound (p < 0.001 and p = 0.009, respectively) and MRI (p = 0.045 and 0.036, respectively). The percentage diameter decrease, size of tumour deposit in LN, progesterone receptor, HER2 and histological grade were significantly associated with the rate of correct diagnosis of PET/CT (p = 0.023, p = 0.002, p = 0.036, p = 0.044 and p = 0.008, respectively).
Table 4.
Association of diagnostic performance of ultrasound, MRI and positron emission tomography (PET)/CT with histopathological factors
| Histological factor | Ultrasound |
p-value | MRI |
p-value | PET/CT |
p-value | |||
|---|---|---|---|---|---|---|---|---|---|
| Correct diagnosis | Incorrect diagnosis | Correct diagnosis | Incorrect diagnosis | Correct diagnosis | Incorrect diagnosis | ||||
| Initial tumour size | 3.75 ± 2.19 | 3.86 ± 2.27 | 0.792 | 3.6 ± 2.24 | 4.18 ± 2.17 | 0.153 | 4.3 ± 2.3 | 3.89 ± 2.15 | 0.371 |
| Tumour size after neoadjuvant chemotherapy | 1.65 ± 1.81 | 2.17 ± 2.21 | 0.133 | 1.74 ± 1.83 | 2.17 ± 2.3 | 0.24 | 1.95 ± 2.35 | 2.37 ± 2.04 | 0.343 |
| Percentage diameter decrease | 53.9 ± 39.48 | 45.8 ± 35.35 | 0.224 | 50.28 ± 38.96 | 49.12 ± 37.44 | 0.869 | 57.16 ± 42.92 | 37.85 ± 38.79 | 0.023* |
| Size of tumour deposit in lymph node | 0.97 ± 0.63 | 0.5 ± 0.31 | <0.001* | 0.84 ± 0.61 | 0.57 ± 0.42 | 0.045* | 1.09 ± 0.6 | 0.61 ± 0.49 | 0.002* |
| Perinodal tumour extension | 0.709 | 0.329 | 0.904 | ||||||
| Negative | 16 (35.6%) | 14 (31.8%) | 19 (30.2%) | 11 (40.7%) | 7 (36.8%) | 18 (35.3%) | |||
| Positive | 29 (64.4%) | 30 (68.2%) | 44 (69.8%) | 16 (59.3%) | 12 (63.2%) | 33 (64.7%) | |||
| Oestrogen receptor | 0.009* | 0.036* | 0.061 | ||||||
| Negative | 30 (38.5%) | 10 (17.5%) | 32 (35.2%) | 8 (17.8%) | 16 (41%) | 14 (23.3%) | |||
| Positive | 48 (61.5%) | 47 (82.5%) | 59 (64.8%) | 37 (82.2%) | 23 (59%) | 46 (76.7%) | |||
| Progesterone receptor | 0.318 | 0.248 | 0.036* | ||||||
| Negative | 41 (52.6%) | 25 (43.9%) | 48 (52.7%) | 19 (42.2%) | 24 (61.5%) | 24 (40%) | |||
| Positive | 37 (47.4%) | 32 (56.1%) | 43 (47.3%) | 26 (57.8%) | 15 (38.5%) | 36 (60%) | |||
| HER2 | 0.183 | 0.716 | 0.044* | ||||||
| Negative | 52 (66.7%) | 44 (77.2%) | 64 (70.3%) | 33 (73.3%) | 24 (61.5%) | 48 (80%) | |||
| Positive | 26 (33.3%) | 13 (22.8%) | 27 (29.7%) | 12 (26.7%) | 15 (38.5%) | 12 (20%) | |||
| Nuclear grade | 0.778 | 0.313 | 0.37 | ||||||
| Low | 28 (48.3%) | 26 (51%) | 34 (46.6%) | 21 (56.8%) | 10 (38.5%) | 27 (49.1%) | |||
| High | 30 (51.7%) | 25 (49%) | 39 (53.4%) | 16 (43.2%) | 16 (61.5%) | 28 (50.9%) | |||
| Histological grade | 0.071 | 0.262 | 0.008* | ||||||
| Low | 33 (55.9%) | 37 (72.5%) | 44 (60.3%) | 27 (71.1%) | 11 (40.7%) | 39 (70.9%) | |||
| High | 26 (44.1%) | 14 (27.5%) | 29 (39.7%) | 11 (28.9%) | 16 (59.3%) | 16 (29.1%) | |||
*p < 0.05.
Multivariate logistic regression analysis was performed with the variables associated with diagnostic performance of imaging modalities through univariate analysis (Table 5). Size of tumour deposit within LN was identified as being independently associated with diagnostic performance of ultrasound [odds ratio, 13.07; 95% confidence interval (CI), 2.95–57.96] and PET/CT [odds ratio, 6.47; 95% CI, 1.407–29.737]. Oestrogen receptor, percentage diameter decrease, progesterone receptor, HER2 and histological grade were not significant independent factors for diagnostic performance. A representative case is shown in Figure 2.
Table 5.
Logistic regression analysis for variables associated with diagnostic performance of ultrasound, MRI and positron emission tomography (PET)/CT
| Variables | β | Standard error | Odds ratio | 95% confidence interval | p-value |
|---|---|---|---|---|---|
| Ultrasound | |||||
| Size of tumour deposition within LN | 2.571 | 0.760 | 13.073 | 2.949–57.964 | 0.001* |
| Oestrogen receptor | −0.981 | 0.599 | 0.375 | 0.116–1.213 | 0.101 |
| MRI | |||||
| Size of tumour deposition within LN | 1.127 | 0.579 | 3.085 | 0.992–9.592 | 0.052 |
| Oestrogen receptor | −0.333 | 0.599 | 0.717 | 0.221–2.320 | 0.578 |
| PET/CT | |||||
| Size of tumour deposition within LN | 1.867 | 0.778 | 6.468 | 1.407–29.737 | 0.016* |
| Percentage diameter decrease | −0.002 | 0.010 | 0.998 | 0.978–1.018 | 0.812 |
| Progesterone receptor | −1.363 | 0.879 | 0.256 | 0.046–1.434 | 0.121 |
| HER2 | −0.342 | 1.036 | 0.710 | 0.093–5.411 | 0.741 |
| Histological grade | 1.808 | 0.926 | 6.101 | 0.994–37.46 | 0.051 |
LN, lymph node.
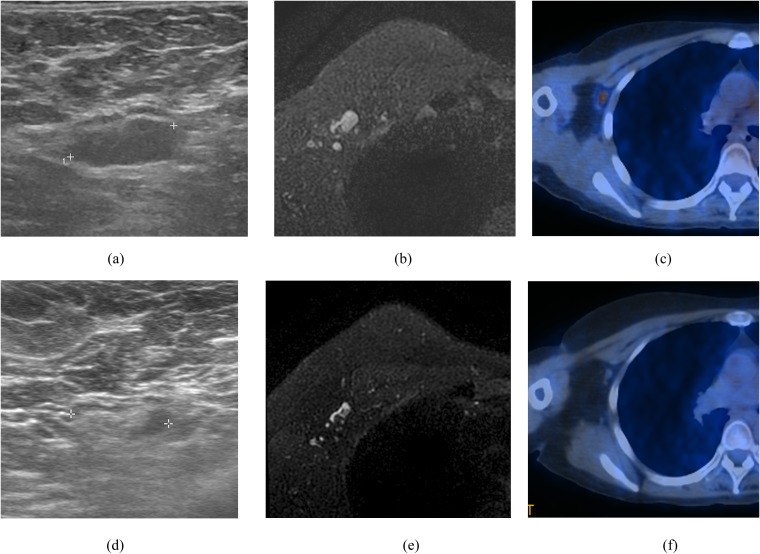
Figure 2.
A 44-year-old female who had invasive ductal carcinoma with negative estrogen receptor and positive HER2 in the right breast and biopsy confirmed metastatic lymph node in right axilla. Initial ultrasound (a) and MRI (b) showed the axillary lymph node (ALN) showing eccentric cortical thickening >3 mm. Initial positron emission tomography (PET)-CT (c) also showed increased fluorine-18 fludeoxyglucose uptake in the right ALN (peak standardized uptake value = 2.8). After neoadjuvant chemotherapy (NAC) treatment, ultrasound (d) and PET-CT (f) showed no evidence of metastasis in right ALN. However, MRI (e) after NAC showed ALN showing 3-mm eccentric cortical thickening suggesting remaining metastasis.
DISCUSSION
About 20–40% of patients underwent negative node conversion after NAC, and the best surgical management of ALN is unclear.9,10 In our results, 39 (28%) patients showed negative conversion of ALN on surgical specimens of sentinel LN or ALN after NAC. A previous study reported that involvement of LNs at the time of surgery, not the initial axillary node stage, was significantly associated with distant disease-free survival.11 In ACOSOG Z1071 trial,12 in patients with breast cancer with clinical N1 stage receiving NAC, if two or more sentinel LNs were removed, the false-negative rate of sentinel node biopsy was 12.6%. The false-negative rate was significantly higher in patients with only one sentinel node dissection or when SLNs were mapped with a single agent (dye or isotope) than dual agents. In another prospective multicentre trial SENTINA,13 the detection rate of SLNB before NAC was 99.1%. However, in patients with LN conversion after NAC, the detection rate of SLNB was 80.1% and the false-negative rate was 14.2%. Thus, the accuracy of SLNB was less favourable in patients after NAC than in patients who underwent SLNB without NAC. However, >50% of patients who had negative conversion after NAC could be spared from further ALND or regional treatment such as radiation therapy.13
A previous study reported that sensitivity, specificity, PPV and NPV of MRI for ALN evaluation after NAC were 85.7%, 89%, 92% and 80.9%, respectively.14 In a recent study analysing the diagnostic performance of ultrasound, MRI and PET-CT for metastatic LN after NAC,15 the sensitivity was 70%, 61% and 63% and the specificity was 58%, 59% and 85%, respectively. In a recent review article,16 the sensitivity of ultrasound, MRI and PET-CT was 58–86%, 59% and 48–85%, respectively, for the detection of pathological complete remission of ALN.
In our study, the sensitivity of ultrasound, MRI and PET/CT was 50%, 72% and 22% and the specificity was 77%, 54% and 85%, respectively. We think that the reason why MRI showed the highest sensitivity compared with ultrasound and PET-CT is that we could perform side-by-side analyses of the individual pairs of MR studies before and after NAC treatment concentrating on the changes of initially metastatic LNs. We could detect more remaining metastatic LNs with subtle eccentric cortical thickening of 3 mm on MRI than on ultrasound. Our results also revealed that the combination of ultrasound, MRI and PET/CT showed the highest sensitivity (81%) and PET/CT alone showed the highest specificity (85%).
The PPV of imaging modalities in our study ranged from 78% to 84% with the highest PPV in ultrasound and combination of ultrasound and PET/CT. However, NPV was relatively low ranging from 28% to 48%. Combination of ultrasound and MRI showed the highest NPV (48%). Therefore, it cannot be considered a substitute for the SLNB procedure for the diagnosis of negative conversion of ALN. Relatively high PPV of ultrasound and combination of ultrasound and PET/CT could help surgeons to proceed directly to ALND without prior SLNB.
In the study of Park et al,17 higher T stage (≥T2) and lymphovascular invasion were significantly associated with false-negative ALN on ultrasound and ultrasound-guided FNAB in patients who underwent primary surgery without NAC. In the study of Kim et al,18 SUVmax on 18F-FDG PET/CT was effective for the ALN evaluation in ER-positive/HER2-negative subtypes and in HER2-positive subtypes. However, it was not effective in triple-negative subtypes. However, these studies are about the cases of primary surgery without NAC. Breast cancer is a heterogeneous disease with biologically different phenotypes. Molecular subtype classification by gene expression profiling has led to a better understanding of tumour biology and behaviour. However, because of the high cost and technical complexity, IHC classification based on the expression of ER, PR and HER2 has been investigated as a substitute for the molecular gene profiling. This IHC classification showed good correlation with intrinsic molecular subtypes of luminal, HER2-positive and basal-like forms.19
Univariate analysis of our study demonstrated that the positive oestrogen receptor and positive progesterone receptor were associated with incorrect diagnosis rate of ultrasound, MRI and PET/CT. This result is somewhat different from the previous study that demonstrated PET/CT was effective in ER-positive/HER2-negative subtype.18 There have been many studies about the variable chemoresponsiveness according to the subtypes of breast cancers. Luminal type has been shown to be less sensitive to NAC and had the least likelihood of pathological complete remission, whereas HER2-positive and triple-negative subtypes showed better chemosensitivity to NAC.20–23 Our univariate analysis showed that breast cancers with positive hormone receptor were associated with higher rate of incorrect diagnosis of ALN statue after NAC. Because of worse responsiveness to NAC in hormone receptor-positive cancers, there may be higher probability of residual cancer cells in the ALN compared with hormone receptor-negative subtypes, regardless of negative imaging findings.
However, multivariate logistic analysis showed that hormone receptor status and HER2 status did not affect the diagnostic performance of all imaging modalities. Only the size of tumour deposit in LN was a significant independent factor associated with diagnostic performance of ultrasound and PET/CT.
Our result demonstrated that ultrasound alone and combination of ultrasound and PET/CT showed the highest PPV (84%). Therefore, if there is suspicious ALN on ultrasound, we could help surgeons to proceed directly to ALND.
There are several limitations in our study. First, total number of patients was relatively small. Further prospective larger multicentre studies are needed to validate our results. Second, this study was performed retrospectively from a single centre, and it could cause selection bias.
In conclusion, combination of three imaging modalities showed the highest sensitivity and PET/CT showed the highest specificity for the evaluation of ALN metastasis after NAC. Ultrasound alone or combination of ultrasound and PET/CT showed the highest PPV. The size of tumour deposit within ALN was significantly associated with diagnostic performance of ultrasound and PET/CT.
Contributor Information
S You, Email: seulgi88322@gmail.com.
D K Kang, Email: kdklsm@ajou.ac.kr.
Y S Jung, Email: smartblade@ajou.ac.kr.
Y-S An, Email: aysays77@naver.com.
T H Kim, Email: medhand@ajou.ac.kr.
REFERENCES
- 1.Lee MC, Eatrides J, Chau A, Han G, Kiluk JV, Khakpour N, et al. Consequences of axillary ultrasound in patients with T2 or greater invasive breast cancers. Ann Surg Oncol 2011; 18: 72–7. doi: 10.1245/s10434-010-1171-4 [DOI] [PubMed] [Google Scholar]
- 2.Nori J, Vanzi E, Bazzocchi M, Bufalini FN, Distante V, Branconi F, et al. Role of axillary ultrasound examination in the selection of breast cancer patients for sentinel node biopsy. Am J Surg 2007; 193: 16–20. doi: 10.1016/j.amjsurg.2006.02.021 [DOI] [PubMed] [Google Scholar]
- 3.Park SH, Kim MJ, Park BW, Moon HJ, Kwak JY, Kim EK. Impact of preoperative ultrasonography and fine-needle aspiration of axillary lymph nodes on surgical management of primary breast cancer. Ann Surg Oncol 2011; 18: 738–44. doi: 10.1245/s10434-010-1347-y [DOI] [PubMed] [Google Scholar]
- 4.Choi YJ, Shin YD, Kang YH, Lee MS, Lee MK, Cho BS, et al. The effects of preoperative (18)F-FDG PET/CT in breast cancer patients in comparison to the conventional imaging study. J Breast Cancer 2012; 15: 441–8. doi: 10.4048/jbc.2012.15.4.441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kvistad KA, Rydland J, Smethurst HB, Lundgren S, Fjøsne HE, Haraldseth O. Axillary lymph node metastases in breast cancer: preoperative detection with dynamic contrast-enhanced MRI. Eur Radiol 2000; 10: 1464–71. doi: 10.1007/s003300000370 [DOI] [PubMed] [Google Scholar]
- 6.Yoshimura G, Sakurai T, Oura S, Suzuma T, Tamaki T, Umemura T, et al. Evaluation of axillary lymph node status in breast cancer with MRI. Breast cancer 1999; 6: 249–58. doi: 10.1007/BF02967179 [DOI] [PubMed] [Google Scholar]
- 7.Kuerer HM, Newman LA, Buzdar AU, Hunt KK, Dhingra K, Buchholz TA, et al. Residual metastatic axillary lymph nodes following neoadjuvant chemotherapy predict disease-free survival in patients with locally advanced breast cancer. Am J Surg 1998; 176: 502–9. doi: 10.1016/S0002-9610(98)00253-0 [DOI] [PubMed] [Google Scholar]
- 8.von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 2012; 30: 1796–804. doi: 10.1200/JCO.2011.38.8595 [DOI] [PubMed] [Google Scholar]
- 9.Bilimoria KY, Bentrem DJ, Hansen NM, Bethke KP, Rademaker AW, Ko CY, et al. Comparison of sentinel lymph node biopsy alone and completion axillary lymph node dissection for node-positive breast cancer. J Clin Oncol 2009; 27: 2946–53. doi: 10.1200/JCO.2008.19.5750 [DOI] [PubMed] [Google Scholar]
- 10.Giuliano AE, Morrow M, Duggal S, Julian TB. Should ACOSOG Z0011 change practice with respect to axillary lymph node dissection for a positive sentinel lymph node biopsy in breast cancer? Clin Exp Metastasis 2012; 29: 687–92. doi: 10.1007/s10585-012-9515-z [DOI] [PubMed] [Google Scholar]
- 11.Rouzier R, Extra JM, Klijanienko J, Falcou MC, Asselain B, Vincent-Salomon A, et al. Incidence and prognostic significance of complete axillary downstaging after primary chemotherapy in breast cancer patients with T1 to T3 tumors and cytologically proven axillary metastatic lymph nodes. J Clin Oncol 2002; 20: 1304–10. doi: 10.1200/JCO.20.5.1304 [DOI] [PubMed] [Google Scholar]
- 12.Boughey JC, Suman VJ, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, et al. ; Alliance for Clinical Trials in Oncology. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA 2013; 310: 1455–61. doi: 10.1001/jama.2013.278932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuehn T, Bauerfeind I, Fehm T, Fleige B, Hausschild M, Helms G, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol 2013; 14: 609–18. doi: 10.1016/S1470-2045(13)70166-9 [DOI] [PubMed] [Google Scholar]
- 14.Javid S, Segara D, Lotfi P, Raza S, Golshan M. Can breast MRI predict axillary lymph node metastasis in women undergoing neoadjuvant chemotherapy. Ann Surg Oncol 2010; 17: 1841–6. doi: 10.1245/s10434-010-0934-2 [DOI] [PubMed] [Google Scholar]
- 15.Hieken TJ, Boughey JC, Jones KN, Shah SS, Glazebrook KN. Imaging response and residual metastatic axillary lymph node disease after neoadjuvant chemotherapy for primary breast cancer. Ann Surg Oncol 2013; 20: 3199–204. doi: 10.1245/s10434-013-3118-z [DOI] [PubMed] [Google Scholar]
- 16.Schipper RJ, Moossdorff M, Beets-Tan RG, Smidt ML, Lobbes MB. Noninvasive nodal restaging in clinically node positive breast cancer patients after neoadjuvant systemic therapy: a systematic review. Eur J Radiol 2015; 84: 41–7. doi: 10.1016/j.ejrad.2014.09.020 [DOI] [PubMed] [Google Scholar]
- 17.Park SH, Kim EK, Park BW, Kim SI, Moon HJ, Kim MJ. False negative results in axillary lymph nodes by ultrasonography and ultrasonography-guided fine-needle aspiration in patients with invasive ductal carcinoma. Ultraschall Med 2013; 34: 559–67. doi: 10.1055/s-0032-1313113 [DOI] [PubMed] [Google Scholar]
- 18.Kim JY, Lee SH, Kim S, Kang T, Bae YT. Tumour 18 F-FDG uptake on preoperative PET/CT may predict axillary lymph node metastasis in ER-positive/HER2-negative and HER2-positive breast cancer subtypes. Eur Radiol 2015; 25: 1172–81. doi: 10.1007/s00330-014-3452-y [DOI] [PubMed] [Google Scholar]
- 19.de Ronde JJ, Hannemann J, Halfwerk H, Mulder L, Straver ME, Vrancken Peeters MJ, et al. Concordance of clinical and molecular breast cancer subtyping in the context of preoperative chemotherapy response. Breast Cancer Res Treat 2010; 119: 119–26. doi: 10.1007/s10549-009-0499-6 [DOI] [PubMed] [Google Scholar]
- 20.Bhargava R, Beriwal S, Dabbs DJ, Ozbek U, Soran A, Johnson RR, et al. Immunohistochemical surrogate markers of breast cancer molecular classes predicts response to neoadjuvant chemotherapy: a single institutional experience with 359 cases. Cancer 2010; 116: 1431–9. doi: 10.1002/cncr.24876 [DOI] [PubMed] [Google Scholar]
- 21.Carey LA, Dees EC, Sawyer L, Gatti L, Moore DT, Collichio F, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res 2007; 13: 2329–34. doi: 10.1158/1078-0432.CCR-06-1109 [DOI] [PubMed] [Google Scholar]
- 22.Rouzier R, Perou CM, Symmans WF, Ibrahim N, Cristofanilli M, Anderson K, et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res 2005; 11: 5678–85. doi: 10.1158/1078-0432.CCR-04-2421 [DOI] [PubMed] [Google Scholar]
- 23.Goldstein NS, Decker D, Severson D, Schell S, Vicini F, Margolis J, et al. Molecular classification system identifies invasive breast carcinoma patients who are most likely and those who are least likely to achieve a complete pathologic response after neoadjuvant chemotherapy. Cancer 2007; 110: 1687–96. doi: 10.1002/cncr.22981 [DOI] [PubMed] [Google Scholar]