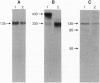
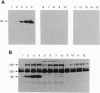
Abstract
To assess the function of the cytoplasmic domain of the insulin receptor (IR) beta subunit, we have studied a mutant IR truncated by 365 aa (HIR delta 978), thereby deleting > 90% of the cytoplasmic domain. HIR delta 978 receptors were processed normally to homodimers that were expressed at the cell surface where they bind insulin with normal affinity. Although these truncated IRs were inactive with respect to ligand-induced internalization and autophosphorylation, insulin stimulated endogenous substrate (pp185) phosphorylation significantly more in HIR delta 978 cells than in untransfected Rat1 cells. Importantly, despite absence of the beta-subunit cytoplasmic domain, fibroblasts expressing HIR delta 978 receptors displayed enhanced sensitivity to insulin for stimulation of glucose incorporation into glycogen, alpha-aminoisobutyric acid uptake, thymidine incorporation, and S6 kinase activity compared with parental fibroblasts. Insulin also induced the expression of the protooncogene c-fos and the early growth response gene Egr-1 in HIR delta 978 cells far greater than in parental Rat1 fibroblasts. Furthermore, an agonistic monoclonal antibody specific for the human IR stimulated insulin action in fibroblasts expressing wild-type human IR but had no effect on HIR delta 978 cells. In conclusion, the HIR delta 978 truncated IRs appear to confer enhanced insulin sensitivity by augmenting the signaling properties of the endogenous rodent IRs.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman J. P., Hayflick J. S., Vasser M., Seeburg P. H. In vitro deletional mutagenesis for bacterial production of the 20,000-dalton form of human pituitary growth hormone. DNA. 1983;2(3):183–193. doi: 10.1089/dna.1983.2.183. [DOI] [PubMed] [Google Scholar]
- Backer J. M., Kahn C. R., White M. F. Tyrosine phosphorylation of the insulin receptor during insulin-stimulated internalization in rat hepatoma cells. J Biol Chem. 1989 Jan 25;264(3):1694–1701. [PubMed] [Google Scholar]
- Belyavsky A., Vinogradova T., Rajewsky K. PCR-based cDNA library construction: general cDNA libraries at the level of a few cells. Nucleic Acids Res. 1989 Apr 25;17(8):2919–2932. doi: 10.1093/nar/17.8.2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cama A., Quon M. J., de la Luz Sierra M., Taylor S. I. Substitution of isoleucine for methionine at position 1153 in the beta-subunit of the human insulin receptor. A mutation that impairs receptor tyrosine kinase activity, receptor endocytosis, and insulin action. J Biol Chem. 1992 Apr 25;267(12):8383–8389. [PubMed] [Google Scholar]
- Carpentier J. L., Paccaud J. P., Gorden P., Rutter W. J., Orci L. Insulin-induced surface redistribution regulates internalization of the insulin receptor and requires its autophosphorylation. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):162–166. doi: 10.1073/pnas.89.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin J. E., Tavaré J. M., Ellis L., Roth R. A. Evidence for hybrid rodent and human insulin receptors in transfected cells. J Biol Chem. 1991 Aug 25;266(24):15587–15590. [PubMed] [Google Scholar]
- Chou C. K., Dull T. J., Russell D. S., Gherzi R., Lebwohl D., Ullrich A., Rosen O. M. Human insulin receptors mutated at the ATP-binding site lack protein tyrosine kinase activity and fail to mediate postreceptor effects of insulin. J Biol Chem. 1987 Feb 5;262(4):1842–1847. [PubMed] [Google Scholar]
- Debant A., Clauser E., Ponzio G., Filloux C., Auzan C., Contreres J. O., Rossi B. Replacement of insulin receptor tyrosine residues 1162 and 1163 does not alter the mitogenic effect of the hormone. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8032–8036. doi: 10.1073/pnas.85.21.8032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebina Y., Araki E., Taira M., Shimada F., Mori M., Craik C. S., Siddle K., Pierce S. B., Roth R. A., Rutter W. J. Replacement of lysine residue 1030 in the putative ATP-binding region of the insulin receptor abolishes insulin- and antibody-stimulated glucose uptake and receptor kinase activity. Proc Natl Acad Sci U S A. 1987 Feb;84(3):704–708. doi: 10.1073/pnas.84.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis L., Clauser E., Morgan D. O., Edery M., Roth R. A., Rutter W. J. Replacement of insulin receptor tyrosine residues 1162 and 1163 compromises insulin-stimulated kinase activity and uptake of 2-deoxyglucose. Cell. 1986 Jun 6;45(5):721–732. doi: 10.1016/0092-8674(86)90786-5. [DOI] [PubMed] [Google Scholar]
- Gottschalk W. K. The pathway mediating insulin's effects on pyruvate dehydrogenase bypasses the insulin receptor tyrosine kinase. J Biol Chem. 1991 May 15;266(14):8814–8819. [PubMed] [Google Scholar]
- Heidenreich K. A., Brandenburg D., Berhanu P., Olefsky J. M. Metabolism of photoaffinity-labeled insulin receptors by adipocytes. Role of internalization, degradation, and recycling. J Biol Chem. 1984 May 25;259(10):6511–6515. [PubMed] [Google Scholar]
- Maegawa H., McClain D. A., Freidenberg G., Olefsky J. M., Napier M., Lipari T., Dull T. J., Lee J., Ullrich A. Properties of a human insulin receptor with a COOH-terminal truncation. II. Truncated receptors have normal kinase activity but are defective in signaling metabolic effects. J Biol Chem. 1988 Jun 25;263(18):8912–8917. [PubMed] [Google Scholar]
- Maegawa H., Olefsky J. M., Thies S., Boyd D., Ullrich A., McClain D. A. Insulin receptors with defective tyrosine kinase inhibit normal receptor function at the level of substrate phosphorylation. J Biol Chem. 1988 Sep 5;263(25):12629–12637. [PubMed] [Google Scholar]
- McClain D. A. Endocytosis of insulin receptors is not required for activation or deactivation of the hormone response. J Biol Chem. 1990 Dec 5;265(34):21363–21367. [PubMed] [Google Scholar]
- McClain D. A., Maegawa H., Lee J., Dull T. J., Ulrich A., Olefsky J. M. A mutant insulin receptor with defective tyrosine kinase displays no biologic activity and does not undergo endocytosis. J Biol Chem. 1987 Oct 25;262(30):14663–14671. [PubMed] [Google Scholar]
- Moller D. E., Benecke H., Flier J. S. Biologic activities of naturally occurring human insulin receptor mutations. Evidence that metabolic effects of insulin can be mediated by a kinase-deficient insulin receptor mutant. J Biol Chem. 1991 Jun 15;266(17):10995–11001. [PubMed] [Google Scholar]
- Moller D. E., Flier J. S. Detection of an alteration in the insulin-receptor gene in a patient with insulin resistance, acanthosis nigricans, and the polycystic ovary syndrome (type A insulin resistance). N Engl J Med. 1988 Dec 8;319(23):1526–1529. doi: 10.1056/NEJM198812083192306. [DOI] [PubMed] [Google Scholar]
- Murakami M. S., Rosen O. M. The role of insulin receptor autophosphorylation in signal transduction. J Biol Chem. 1991 Nov 25;266(33):22653–22660. [PubMed] [Google Scholar]
- Odawara M., Kadowaki T., Yamamoto R., Shibasaki Y., Tobe K., Accili D., Bevins C., Mikami Y., Matsuura N., Akanuma Y. Human diabetes associated with a mutation in the tyrosine kinase domain of the insulin receptor. Science. 1989 Jul 7;245(4913):66–68. doi: 10.1126/science.2544998. [DOI] [PubMed] [Google Scholar]
- Ogata K., Terao K. Analytical methods for ribosomal proteins of rat liver 40 S and 60 S subunits by "three-dimensional" acrylamide gel electrophoresis. Methods Enzymol. 1979;59:502–515. doi: 10.1016/0076-6879(79)59110-1. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M. The insulin receptor. A multifunctional protein. Diabetes. 1990 Sep;39(9):1009–1016. doi: 10.2337/diab.39.9.1009. [DOI] [PubMed] [Google Scholar]
- Rajagopalan M., Neidigh J. L., McClain D. A. Amino acid sequences Gly-Pro-Leu-Tyr and Asn-Pro-Glu-Tyr in the submembranous domain of the insulin receptor are required for normal endocytosis. J Biol Chem. 1991 Dec 5;266(34):23068–23073. [PubMed] [Google Scholar]
- Russell D. S., Gherzi R., Johnson E. L., Chou C. K., Rosen O. M. The protein-tyrosine kinase activity of the insulin receptor is necessary for insulin-mediated receptor down-regulation. J Biol Chem. 1987 Aug 25;262(24):11833–11840. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaoka T., Kobayashi M., Takata Y., Ishibashi O., Iwasaki M., Shigeta Y., Goji K., Hisatomi A. Clarification of signaling pathways mediated by insulin and insulin-like growth factor I receptors in fibroblasts from patients with specific defect in insulin receptor. Diabetes. 1988 Nov;37(11):1515–1523. doi: 10.2337/diab.37.11.1515. [DOI] [PubMed] [Google Scholar]
- Siddle K., Soos M. A., O'Brien R. M., Ganderton R. H., Taylor R. Monoclonal antibodies as probes of the structure and function of insulin receptors. Biochem Soc Trans. 1987 Feb;15(1):47–51. doi: 10.1042/bst0150047. [DOI] [PubMed] [Google Scholar]
- Smith R. M., Seely B. L., Shah N., Olefsky J. M., Jarett L. Tyrosine kinase-defective insulin receptors undergo insulin-induced microaggregation but do not concentrate in coated pits. J Biol Chem. 1991 Sep 15;266(26):17522–17530. [PubMed] [Google Scholar]
- Soos M. A., Whittaker J., Lammers R., Ullrich A., Siddle K. Receptors for insulin and insulin-like growth factor-I can form hybrid dimers. Characterisation of hybrid receptors in transfected cells. Biochem J. 1990 Sep 1;270(2):383–390. doi: 10.1042/bj2700383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele-Perkins G., Roth R. A. Insulin-mimetic anti-insulin receptor monoclonal antibodies stimulate receptor kinase activity in intact cells. J Biol Chem. 1990 Jun 5;265(16):9458–9463. [PubMed] [Google Scholar]
- Stumpo D. J., Blackshear P. J. Cellular expression of mutant insulin receptors interferes with the rapid transcriptional response to both insulin and insulin-like growth factor I. J Biol Chem. 1991 Jan 5;266(1):455–460. [PubMed] [Google Scholar]
- Stumpo D. J., Blackshear P. J. Insulin and growth factor effects on c-fos expression in normal and protein kinase C-deficient 3T3-L1 fibroblasts and adipocytes. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9453–9457. doi: 10.1073/pnas.83.24.9453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X. J., Rothenberg P., Kahn C. R., Backer J. M., Araki E., Wilden P. A., Cahill D. A., Goldstein B. J., White M. F. Structure of the insulin receptor substrate IRS-1 defines a unique signal transduction protein. Nature. 1991 Jul 4;352(6330):73–77. doi: 10.1038/352073a0. [DOI] [PubMed] [Google Scholar]
- Taira M., Taira M., Hashimoto N., Shimada F., Suzuki Y., Kanatsuka A., Nakamura F., Ebina Y., Tatibana M., Makino H. Human diabetes associated with a deletion of the tyrosine kinase domain of the insulin receptor. Science. 1989 Jul 7;245(4913):63–66. doi: 10.1126/science.2544997. [DOI] [PubMed] [Google Scholar]
- Takata Y., Webster N. J., Olefsky J. M. Intracellular signaling by a mutant human insulin receptor lacking the carboxyl-terminal tyrosine autophosphorylation sites. J Biol Chem. 1992 May 5;267(13):9065–9070. [PubMed] [Google Scholar]
- Takata Y., Webster N. J., Olefsky J. M. Mutation of the two carboxyl-terminal tyrosines results in an insulin receptor with normal metabolic signaling but enhanced mitogenic signaling properties. J Biol Chem. 1991 May 15;266(14):9135–9139. [PubMed] [Google Scholar]
- Taylor R., Soos M. A., Wells A., Argyraki M., Siddle K. Insulin-like and insulin-inhibitory effects of monoclonal antibodies for different epitopes on the human insulin receptor. Biochem J. 1987 Feb 15;242(1):123–129. doi: 10.1042/bj2420123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway J. L., Morrison B. D., Soos M. A., Siddle K., Olefsky J., Ullrich A., McClain D. A., Pessin J. E. Transdominant inhibition of tyrosine kinase activity in mutant insulin/insulin-like growth factor I hybrid receptors. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):214–218. doi: 10.1073/pnas.88.1.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Bell J. R., Chen E. Y., Herrera R., Petruzzelli L. M., Dull T. J., Gray A., Coussens L., Liao Y. C., Tsubokawa M. Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. 1985 Feb 28-Mar 6Nature. 313(6005):756–761. doi: 10.1038/313756a0. [DOI] [PubMed] [Google Scholar]
- Zhang B., Tavaré J. M., Ellis L., Roth R. A. The regulatory role of known tyrosine autophosphorylation sites of the insulin receptor kinase domain. An assessment by replacement with neutral and negatively charged amino acids. J Biol Chem. 1991 Jan 15;266(2):990–996. [PubMed] [Google Scholar]