Abstract
We evaluated whether a brief 3-week family therapy intervention would change patterns of brain activation in response to affection and gaming cues in adolescents from dysfunctional families who met criteria for on-line game addiction. Fifteen adolescents with on-line game addiction and fifteen adolescents without problematic on-line game play and an intact family structure were recruited. Over 3 weeks, families were asked to carry out homework assignments focused on increasing family cohesion for more than 1 hour/day and 4 days/week. Before therapy, adolescents with on-line game addiction demonstrated decreased activity as measured by functional magnetic resonance imaging (fMRI) within the caudate, middle temporal gyrus, and occipital lobe in response to images depicting parental affection and increased activity of the middle frontal and inferior parietal in response scenes from on-line games, relative to healthy comparison subjects. Improvement in perceived family cohesion following 3 weeks of treatment was associated with an increase in the activity of the caudate nucleus in response to affection stimuli and was inversely correlated with changes in on-line game playing time. With evidence of brain activation changes in response to on-line game playing cues and images depicting parental love, the present findings suggest that family cohesion may be an important factor in the treatment of problematic on-line game playing.
Keywords: Family therapy, On-line game addiction, Functional magnetic resonance imaging, Caudate, Dorsolateral prefrontal cortex
1. Introduction
1.1. Family structure and family therapy in adolescents with addiction
An association between dysfunctional family structure and adolescent substance use has been suggested by several public health studies (Frojd et al., 2007; Roustit et al., 2007). In a smoking survey involving 32,961 youth, smoking, alcohol, and drug use were associated with non-intact families (Mak et al., 2010). Moreover, a perceived lack of family closeness and love has been suggested to increase the risk of health threatening behavior, such as drug taking in adolescents (Reynolds and Rob, 1988). In a study of family factors contributing to internet addiction, Yen et al. (2007) reported that higher levels of parent-adolescent conflict and lower family function were associated with internet addiction. China’s “left behind children,” due to parental migration from rural to urban areas for work, have been reported to be at increased risk of physical inactivity, internet addiction, and smoking (Gao et al., 2010). In a study of 1369 university students, Tsai et al. (2009) reported that deficient social support was a significant risk factor for internet addiction. Further, loneliness and familial discord have also been reported to lead to internet addiction (Young, 1996; Nalwa and Anand, 2003).
Although there is some controversy in terms of the feasibility of providing treatment, family therapy has been suggested for patients with substance dependence (Crane, 2007; Morgan and Crane, 2010). Compared to a psychoeducational drug treatment intervention, integrated family and cognitive behavior therapy has been reported to reduce rates of marijuana use and improve problem solving and learning strategy skills in adolescents with substance dependence (Latimer et al., 2003). Parental monitoring and interest in their children has also been reported to be important for the treatment and management of adolescents with internet addiction (Lin et al., 2009). Young (2009) have emphasized that parental efforts such as limit setting with respect to playing time and switching computer usage from game playing to doing homework are important elements for the treatment of adolescent internet addiction. In addition, family therapy modified by short-term Brief Strategic Family Therapy for substance addiction is useful in reducing compulsive gaming in adolescents (Robbins et al., 2011).
1.2. Similar brain activity in response to affection and addiction
In spite of results demonstrating that family therapy is effective for the treatment of adolescents with addiction, there are few published studies demonstrating brain changes induced by family focused therapy in patients with substance dependence. In contrast, there have been several studies of brain activity in response to romantic or parental love and affection. These studies have noted a correlation between stimuli depicting love or interpersonal attachment and brain activity in frontal cortex and striatum (Bartels and Zeki, 2004; Taylor et al., 2009; Frascella et al., 2010). Notably, these same brain regions also respond to drug cues in cohorts of drug dependent subjects and several investigators have noted that love and addiction share similar characteristics (Fisher et al., 2005; Frascella et al., 2010). For example, a lover’s intense interest in a preferred individual, unstable mood, craving, obsession, compulsion, distorted reality, and loss of self-control parallel similar findings in drug users (Griffin-Shelley, 1991; Mellody et al., 1992). Based on the observation of increased activation in the right ventral tegmental area and right caudate nucleus in response to images of beloved others, Fisher et al. (Fisher et al., 2005) suggested that dopaminergic reward pathways may be linked to the recognition of romantic love. Compared to unfamiliar faces, increased activation in the parahippocampal gyrus, middle superior temporal gyri and middle frontal gyrus were observed in response to images of partner faces (Taylor et al., 2009). Bartels and Zeki (2004) have reported activation of the caudate nucleus in response to images depicting maternal love and romantic love in healthy subjects.
Interestingly, prefrontal cortex and subcortical areas may also mediate responses to video game play (Koepp and Silver, 1998; Matsuda and Hiraki, 2006). In a near infrared spectroscopy (NIRS) study of thirteen children and adolescents (7–14 years old), a sustained decrease of oxygenated hemoglobin in the bilateral dorsal prefrontal cortex was observed during video game play (Matsuda and Hiraki, 2006). Koepp and Silver (Koepp and Silver, 1998) have noted a release of dopamine in the thalamus during game play. Recent fMRI studies of on-line game play have suggested that the brain activation observed in response to on-line game cues may be similar to that observed in patients with substance dependence who are exposed to drug cues (Ko et al., 2009; Han et al., 2011). Ko et al. (2009) have reported that patients with on-line game addiction show increased activity in dorsolateral prefrontal cortex, orbitofrontal cortex, anterior cingulate, nucleus accumbens, and caudate nucleus, in response to on-line game cues compared with activation patterns observed in healthy volunteers. Our previous fMRI study (Han et al., 2011) also reported that the craving for on-line game play in response to on-line game cues was associated with the beta values for clusters of activation within the left inferior frontal gyrus, left parahippocampal gyrus, and right thalamus in response to on-line game cues in healthy volunteers.
1.3. Hypothesis
In the current study, based on published findings, we evaluated whether a brief 3-week family therapy intervention would change patterns of brain activation in response to affection and gaming cues in adolescents from dysfunctional families who met criteria for on-line game addiction.
2. Method
2.1. Subjects
From adolescents and their parents who visited the Department of Psychiatry of Chung Ang University Medical Center for evaluation and treatment of possible on-line game addiction, fifteen families with moderate to severe family dysfunction agreed to participate in this study. Dysfunctional families were defined as having Family Adaptability, Partnership, Growth, Affection, and Resolve (FAPGAR) scores (Smilkstein, 1978, 1980) of less than 3; an adaptability score on the Family Adaptability and Cohesion Evaluation scale (FACES III) (Olson, 1986, 1991) of less than 24; and a cohesion score on FACES III of less than 40. In addition, the criteria for problematic on-line game play were 1) game playing time greater than four hours per day and 30 hours per week (Ko et al., 2009; Han et al., 2010): 2) Young Internet Addiction Scale (YIAS) scores (Young, 1996; Yoo et al., 2004; Ha et al., 2006) greater than 50. In an epidemiology study of Korean school students, Yoo et al. (2004) reported that 14% of students met the criteria of problematic internet addiction using a standard of IAD>50. ; and 3) impaired behaviors or distress due to excessive on-line game play which are modified from DSM-IV criteria for substance abuse (American_Psychiatric_Association, 2000). For the screening of other psychiatric problems, the Structured Clinical Interview for DSM-IV and the Beck Depression Inventory (BDI) were administered (Beck et al., 1961). Exclusion criteria included: (1) adolescents with a history or current episode of psychiatric disease; (2) adolescents with a history of substance abuse or dependence including alcohol and tobacco; (3) adolescents with neurological or medical disorders; and (4) adolescents with a contraindication to MRI scanning such as claustrophobia and metal implants. Fifteen healthy comparison families were recruited by word of mouth and using flyers posted within Chung Ang University seeking healthy families with adolescent children. The research protocol was approved by the Chung Ang University Hospital Institutional Review Board. Written informed consent was provided by all adolescents and their parents.
There were no differences in terms of age (the adolescents with problematic online game play: 14.2±1.5 years; healthy comparison subjects: 14.0±1.3 years, z=0.61, p=0.54) and years of education (the adolescents with problematic on-line game play: 7.5±1.8 years, healthy comparison subjects: 7.0±1.3 years, z=1.08, p=0.28). Between the two groups, there were significant differences in terms of YIAS scores (z=4.12, p<0.01), total game playing time (z=3.98, p<0.01), and FAPGAR scores (z=3.21, p<0.01). The mean time of video game play in 15 adolescents with on-line game addiction (34.5±9.6 hours/week) and in 15 healthy comparison (3.1±1.7 hours/week) were recorded by patients themselves and confirmed by parental report. The mean YIAS scores of adolescents with on-line game addiction and healthy comparison subjects at baseline were 75.1±11.2 and 34.5±9.6, respectively. The mean FAPGAR scores for adolescents with on-line game addiction and healthy comparison subjects at baseline were 2.5±1.5 and 5.8±1.8, respectively.
2.2. Family therapy
The severity of problematic on-line game playing was evaluated by the amount of on-line game play and Young Internet Addiction Scale (YIAS) scores (Young, 1996). Family function was assessed using Family Adaptability, Partnership, Growth, Affection, and Resolve (APGAR) scores (Smilkstein, 1978, 1980).
The adolescents with problematic on-line game play and dysfunctional families and their parents visited seven times (two for baseline evaluations of clinical status and brain activity, and five sessions for family therapy). At the first visit and the final evaluation visit, the families visited Chung Ang University Hospital for assessment of brain activity and the severity of problematic on-line game play. During the first day of family therapy, all the families underwent an assessment of family function and cohesion. During the second day of family therapy, a child psychiatrist and family members shared an interaction focused on the reduction of problem behaviors and enhancing family cohesion (Gorell, 1998), In addition, each dysfunctional family, with the help of a child psychiatrist, created exercises designed to improve family cohesion. Three families chose shared sports such as jumping rope, swimming, and badminton. Three families played board games. Two families cooked food together. Two families studied a foreign language together. One family (adolescent and mother) attended an art institution for lessons on drawing animated characters. Three families watched famous dramas together. During the 3 weeks of treatment, all families were asked to work on their new interactions for more than 1 hour/day and 4 days/week. Each family was asked to take a picture of themselves engaged in these new, shared activities. On days 3, 4, and 5 of family therapy, adolescents and parents described their new activities and the adolescents were rewarded with a small gift and stickers. In addition, the psychiatrist and family members met to continue focusing on steps that could be taken to continue the improvement in family dynamics (Gorell, 1998). Of 15 families, one family dropped out without any notification following the first visit. There was no other treatment for subjects and families except family therapy during the course of study. There was no follow-up or treatment provided to members of the healthy comparison group.
2.3. fMRI scanning
Adolescents with problematic game play and dysfunctional families visited Chung Ang University Hospital (CAUH) for fMRI scanning at baseline and following 3 weeks of treatment. At each visit, brain activity was assessed during the presentation of two sets of stimuli: one included scenes demonstrating affection and the other included scenes of on-line game play. All functional imaging was performed using a 3 T blood oxygen level dependent (BOLD) functional magnetic resonance imaging sequence (fMRI, Achieva 3.0, Philips, Eindhoven, the Netherlands). In both scanning sessions, the stimulus was presented using an IFIS-SATM system (MRI Device Corporation, Waukesha, WI, USA). Acquisition parameters for the fMRI session included: 180 echo planar images (EPI, 33 transverse slices, 4.0 mm thickness, voxel size of 1.8×1.8×4.0 mm, echo time TE=30 ms, repetition time (TR)=3000 ms, flip angle=90°, in-plane resolution=128×128 pixels, field of view (FOV)=230×230 mm) were recorded at 3-s intervals. For anatomical imaging, 3D T1-weighted magnetization-prepared rapid gradient echo (MPRAGE) data were gathered with these parameters: TR=2000 ms, TE=4.00 ms, FOV=256×256 mm, 340 slices, 0.9×0.9×1.0 mm voxel size, flip angle=30°.
2.4. fMRI paradigm
Both adolescents with on-line game addiction and healthy comparison subjects were asked to view affection and game scenes without responding. The affection scenes consisted of pictures showing a mother and father hugging their child, a mother kissing her child, a family birthday party, a child playing soccer with their father, and a happy family with smiling faces. Game scenes consisted of a separate set of stimuli showing images from the game that each adolescent usually played: four adolescents played Lineage® (http://lineage.plaync.co.kr/), four played Sudden Attack® (http://suddenattack.netmarble.net/), three played World of WarCraft® (http://kr.battle.net/), two played AION® (http://aion.plaync.co.kr/), one played Dungeon and Fighter® (http://df.nexon.com/), and one played StarCraft® (http://kr.battle.net/ko/int/).
The affection stimulus was 450 s long and consisted of five continuous 90-s segments. Each 90-s segment consisted of three 30-s sub-segments. A white cross on a black background (B), a neutral scene (N; tree, flow, book, chair), and stimulation (S) were included in these 90-s segments. The five segments were ordered as follows: B-N-S, B-S-N, S-B-N, N-B-S, and S-N-B. In the scanning protocol, the game stimulus was presented in the same manner as the affection stimulus: a neutral scene (N, animation) and stimulation (S; on-line game scene).
2.5. fMRI data analysis
The Brain Voyager software package (BVQX 1.9, Brain Innovation, Maastricht, The Netherlands) was used for analyzing acquired fMRI data, as detailed in Han et al. (Han et al., 2010). Briefly, each fMRI time series was registered to the MPRAGE 3D data set using a multi-scale algorithm. The anatomic images were spatially normalized to standard Talairach space (Talairach and Tournoux, 1988). The same nonlinear transformation was subsequently applied to the fMRI time series data. Preprocessing steps included slice scan time correction and motion correction. The functional data were spatially smoothed using a 6-mm full width at half-maximum (FWHM) Gaussian kernel and temporally smoothed using a 4-s Gaussian kernel.
2.6. Statistical analysis
For the analysis of fMRI signal time-courses, the general linear model (GLM) and random effects analysis (RFX) were applied to construct individual and group statistical parametric maps of brain activation. Differences in demographic data including FAPGAR scores, YIAS scores, and on-line game play time between adolescents with on-line game addiction and healthy comparison subjects were compared using the Mann–Whitney U test. As a second-level analysis in adolescents with on-line game addiction, the changes of mean β value in clusters during the 3 weeks of treatment and the change of YIAS scores, FAPGAR scores and playing time were analyzed using Spearman correlations.
3. Results
3.1. Changes in severity of on-line game play and family cohesion during three weeks of family therapy in adolescents with on-line game addiction
Following 3 weeks of treatment, the mean YIAS scores (baseline (B): 75.1±11.2, 3 weeks (3 W): 54.2±12.1, F=98.4, p<0.01) and the mean on-line game playing time (B: 34.5±9.6 hours/weeks, 3 W: 12.4±9.1 hours/weeks, F=85.2, p<0.01) were decreased. In contrast, the mean FAPGAR scores increased (B: 2.5±1.5, 3 W: 5.8± 1.8, F=42.3, p<0.01). The change of FAPGAR scores was negatively correlated with the change of YIAS scores (r=−0.59, p=0.03) and on-line game playing time (r=−0.70, p=0.01).
3.2. Brain activity in response to affection stimuli and game stimuli at baseline and 3 weeks
In response to affection stimuli at baseline, adolescents with online game addiction showed no significant clusters at FDR <0.05 value. However, two clusters of activity in adolescents with on-line game addiction were identified at uncorrected p<0.001 value: right occipital lobe fusiform gyrus (Brodmann area (BA) 19), left middle frontal gyrus (BA9). After the 3-week treatment period, in response to affection stimuli at baseline, adolescents with on-line game addiction showed no significant clusters at FDR <0.05 value. However, four clusters of activity in healthy comparisons were identified at uncorrected p<0.001 value; right temporal fusiform gyrus (BA37), right thalamus, right parietal lobe, precuneus (BA7), left occipital lingual gyrus (BA18). In response to affection stimuli at baseline, healthy comparison subjects showed no significant clusters at FDR <0.05 value. However, five clusters of activity in healthy comparison subjects were identified at uncorrected p<0.001 value: right superior temporal gyrus (BA39), right cerebellum posterior lobe, right caudate, right occipital fusiform gyrus (BA18), left temporal fusiform gyrus (BA37), and left middle temporal gyrus (BA37) (Table 1).
Table 1.
Brain areas in response to affection/game stimuli.
| Cluster | Coordinate
|
Number of voxels | P value | |||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Brain activity in response to affection in adolescents with problematic on-line game play at baseline | ||||||
| 1 | 29 | −84 | −14 | 210 | Uncorrected p<0.001 | Right occipital lobe, fusiform gyrus, BA19 |
| 2 | −45 | 6 | 36 | 52 | Uncorrected p<0.001 | Left middle frontal gyrus, BA 9 |
| Brain activity in response to affection in adolescents with problematic on-line game play at 3 weeks | ||||||
| 1 | 48 | −47 | −18 | 111 | Uncorrected p<0.001 | Right temporal lobe, fusiform gyrus, BA 37 |
| 2 | 24 | −26 | −1 | 48 | Uncorrected p<0.001 | Right thalamus |
| 3 | 10 | −62 | 33 | 85 | Uncorrected p<0.001 | Right parietal lobe, precuneus, BA 7 |
| 4 | −14 | −85 | −13 | 26 | Uncorrected p<0.001 | Left occipital lobe, lingual gyrus, BA 18 |
| Brain activity in response to affection in healthy comparison subjects at baseline | ||||||
| 1 | 51 | −52 | 5 | 105 | Uncorrected p<0.001 | Right superior temporal gyrus, BA 39 |
| 2 | 27 | −69 | −21 | 282 | Uncorrected p<0.001 | Right cerebellum, posterior lobe |
| 3 | 14 | −2 | 16 | 25 | Uncorrected p<0.001 | Right caudate body |
| 4 | −20 | −83 | −18 | 197 | Uncorrected p<0.001 | Left occipital lobe, fusiform gyrus, BA 18 |
| 5 | −42 | −60 | −23 | 45 | Uncorrected p<0.001 | Left temporal lobe, fusiform gyrus, BA 37 |
| 6 | −48 | −59 | 3 | 110 | Uncorrected p<0.001 | Left middle temporal gyrus, BA 37 |
| Brain activity in response to game in adolescents with problematic on-line game play at baseline | ||||||
| 1 | 55 | −36 | 19 | 26 | Uncorrected p<0.001 | Right insula, BA 13 |
| 2 | −31 | 49 | 7 | 35 | Uncorrected p<0.001 | Left middle frontal gyrus, BA 10 |
| Brain activity in response to game in adolescents with problematic on-line game play at 3 weeks | ||||||
| 1 | 25 | −79 | −13 | 93 | FDR <0.05, p<0.0002 | Right occipital lobe, fusiform gyrus, BA 19 |
| 2 | −11 | −89 | −7 | 25 | FDR <0.05, p<0.0002 | Left inferior occipital gyrus, BA 17 |
| Brain activity in response to game in healthy comparison subjects at baseline | ||||||
| 1 | 25 | −75 | −13 | 320 | FDR<0.05, p<0.0007 | Right occipital lobe, fusiform gyrus, BA 19 |
| 2 | −24 | −83 | −13 | 284 | FDR<0.05, p<0.0007 | Left occipital lobe, fusiform gyrus, BA 19 |
| Interaction between group (subjects vs. controls) and stimuli (affection vs. neutral) in response to affection at baseline | ||||||
| 1 | 7 | 2 | 15 | 83 | FDR<0.05, p<0.005 | Right caudate body |
| 2 | −39 | −67 | 4 | 320 | FDR<0.05, p<0.005 | Left middle occipital gyrus, BA 37 |
| 3 | 29 | −66 | −2 | 290 | FDR<0.05, p<0.005 | Right lingual gyrus, BA 19 |
| Interaction between group (subjects vs. controls) and game stimuli (game vs. neutral) in response to game at baseline | ||||||
| 1 | 44 | 6 | −10 | 52 | FDR<0.05, p<0.003 | Right superior temporal gyrus, BA 38 |
| 2 | 16 | −42 | −34 | 37 | FDR<0.05, p<0.003 | Right cerebellum, posterior lobe |
| 3 | −10 | −24 | 10 | 55 | FDR<0.05, p<0.003 | Left thalamus |
| 4 | −27 | 44 | 9 | 63 | FDR<0.05, p<0.003 | Left middle frontal gyrus, BA 10 |
| 5 | −36 | 18 | −6 | 116 | FDR<0.05, p<0.003 | Left inferior frontal gyrus, BA 47 |
In response to game stimuli at baseline, adolescents with on-line game addiction showed no significant clusters at FDR <0.05 value. However, two clusters of activity in adolescents with on-line game addiction were identified at uncorrected p<0.001 value: right insular (BA13), left middle frontal gyrus (BA10). After the 3-week treatment period, in response to game stimuli at baseline, adolescents with online game addiction group showed significant two clusters at FDR <0.05, p<0.0002 value: right occipital lobe fusiform gyrus and left middle frontal gyrus. In response to game stimuli at baseline, two clusters of activity in healthy comparisons were identified at FDR<0.05, p<0.0007 value; right occipital fusiform gyrus (BA19) and left occipital fusiform gyrus (BA19) (Table 1).
3.3. Interaction between group (adolescents with on-line game addiction vs. HC) and stimuli (affection/game vs. neutral) at baseline
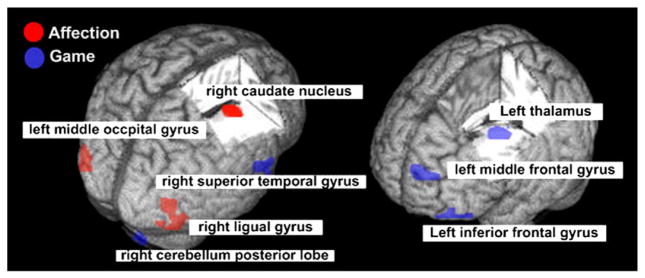
On an interaction between group (adolescents with on-line game addiction vs. healthy comparison subjects) and stimuli (affection vs. neutral) at baseline, three clusters of activity were identified at FDR<0.05, p<0.005 value; right caudate nucleus (caudate-region of interest (ROI)), left middle occipital gyrus (BA37), and right lingual gyrus (BA19) (Table 1, Fig. 1).
Fig. 1.
Interaction between group (subjects vs. controls) and stimuli (affection/game vs. neutral) in response to affection/game at baselineI. Affection: Interaction between group (adolescents with on-line game addiction vs. controls) and stimuli (affection vs. neutral) in response to affection at baseline, FDR<0.05, p<0.005; right caudate body: Talairach x, y, z; 7, 2, 15; right occipital lingual gyrus: 29, −66, −2, Brodmann area (BA) 19; left middle occipital gyrus: −39, −67, 4, BA37. Game: Interaction between group (adolescents with on-line game addiction vs. controls) and game stimuli (game vs. neutral) in response to game at baseline, FDR<0.005, p<0.003; left thalamus: −10, −24, 10; left middle frontal gyrus: −27, 44, 9, BA 10; left inferior frontal gyrus: −36, 18, −6, BA 47.
On an interaction between group (adolescents with on-line game addiction vs. healthy comparison subjects) and stimuli (game vs. neutral) at baseline, five clusters of activity were identified at FDR< 0.05, p<0.003 value; right superior temporal gyrus (BA38), right cerebellum posterior lobe, left thalamus, left middle frontal gyrus (MFG-ROI, BA10), left inferior frontal gyrus (BA47) (Table 1, Fig. 1).
The mean β value of right caudate body (caudate-ROI) in total subjects was positively correlated with FAPGAR scores (r=0.44, p=0.02). The mean β value of left middle frontal gyrus (MFG-ROI) in total subjects was positively correlated with YIAS scores (r=0.74, p<0.01) and on-line game playing time (r=0.79, p<0.01). In addition, the mean β value of left middle frontal gyrus in total subjects was negatively correlated with FAPGAR scores (r=−0.61, p<0.01).
3.3.1. Changes in brain activity during three weeks of family therapy in adolescents with on-line game addiction
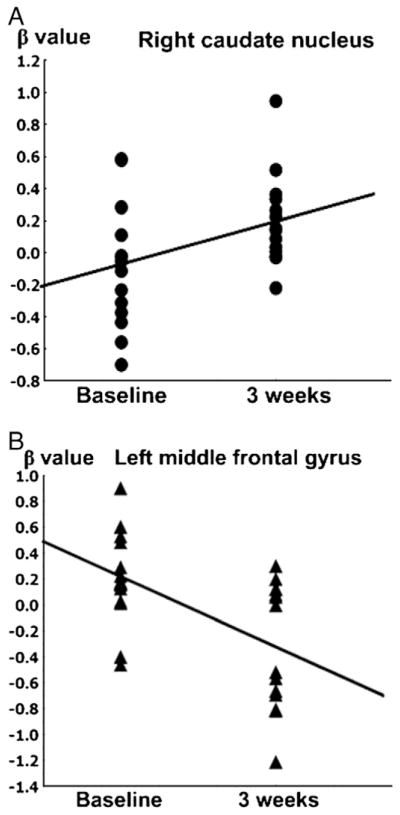
During treatment, the mean β value of caudate body in adolescents with on-line game addiction in response to affection cues was increased (F=5.0, p=0.04) and the mean β value of left middle frontal gyrus (F=5.4, p=0.04) in adolescents with on-line game addiction in response to game stimulation were decreased. However, there was no significant change of mean β values of other clusters in response to affection or game stimuli. The change of mean β value for caudate body in adolescents with on-line game addiction was positively correlated with the change of FAPGAR scores (r=0.64, p<0.01) (Fig. 2).
Fig. 2.
Changes of beta values during 3 weeks of treatment. A: the changes of beta values in caudate in response to affection stimulation (F=5.0, p=0.04), B: the changes of beta values in left middle frontal gyrus clusters in response to game stimulation (F=5.4, p=0.04).
4. Discussion
The current findings suggest that adolescents with problematic on-line game play and dysfunctional family cohesion show decreased activity of caudate, middle temporal gyrus, and occipital lobe in response to affection stimulation and increased activity of middle frontal, inferior parietal, and cerebellum in response to game stimulation, compared to healthy adolescents with functional family structures. In addition, improvement in perceived family cohesion is associated with an increase in the activity of the caudate nucleus in response to affection stimuli and is inversely correlated with changes in on-line game playing time and the activity of the dorsolateral prefrontal cortex in response to on-line game stimuli. Although there are few published studies (Schiepek et al., 2009) showing neurobiological changes associated with family therapy, the present study suggests that brain changes may be influenced by changes in the demonstrated parental interest in a child or family cohesion.
4.1. The effect of family therapy on on-line game addiction
Although internet addiction is known to be difficult to treat (Young, 1996; Ha et al., 2006), family therapy over seven sessions showed positive effects in reducing on-line game addiction severity in the current research. Previous studies have already reported that parental interest is an important factor for improving the symptoms in adolescents with internet addiction (Lin et al., 2009; Young, 2009). In addition, Young (Young, 2009) reported that eight sessions of cognitive behavioral therapy could control the internet addiction symptoms in 114 adults with internet addiction. The reason for dramatic improvement of on-line game addiction in the present study may be due to subject selection. In other words, the current research recruited adolescents with on-line game addiction as well as dysfunctional families. In addition, we applied family therapy with a primary focus on family cohesion.
4.2. The caudate nucleus in response to affection stimulation
In the present study, adolescents with on-line game addiction at baseline demonstrated decreased activity of the right caudate nucleus in response to affection stimuli, compared to healthy adolescents with functional family structures. In addition, improvement of perceived family cohesion increased the activity of caudate nucleus in adolescents with on-line game addiction. Romantic and maternal love are thought to share activation of the same brain region (striatum) as a part of reward circuitry (Zeki, 2007). The reward pathway consisting of the ventral tegmental area and the caudate nucleus has been reported to mediate the response to stimuli depicting romantic affection (Bartels and Zeki, 2004; Fisher et al., 2005). Aron et al. (2005) have reported increased activity in the right caudate in response to images of lovers in the early stage of romantic passion. Further, Vrticka et al. (2008) have reported that parent and child attachment style, assessed with Adult Attachment Questionnaire (Kaitz et al., 2004), can modulate activation of striatum and ventral tegmental area in response to social relationships.
Interestingly, this reward system (striatum and ventral tegmental area) that has been implicated in depicting love, interpersonal attachment, and on-line game addiction has been linked with dopamine release (Gunn et al., 1997; Koepp and Silver, 1998; Bartels and Zeki, 2004; Pruessner et al., 2004; Aron et al., 2005). The characteristics of love, such as preferred interest in someone, energy elevation, euphoric mood, craving for attachment with the preferred individual, and intense motivation to be a good partner have been associated with dopamine release in this system (Bartels and Zeki, 2004; Aron et al., 2005). The college student with poor parental care during early life demonstrates increased dopamine release in ventral striatum in response to psychosocial stress, compared to college students with good early life parental care (Pruessner et al., 2004). An association between dopamine release in striatum and on-line game play has also reported (Gunn et al., 1997; Koepp and Silver, 1998).
Overall, we suggest that the adolescent with problematic on-line game playing and dysfunctional family cohesion tends to pay less attention to the parent child attachment relationship. In addition, the adolescent with poor family cohesion may play on-line games as a compensation for striatal dopamine deficits resulting from poor parental love.
4.3. The dorsolateral prefrontal cortex in response to game stimulation
The activity of the left dorsolateral prefrontal cortex (DLPFC) in response to game stimulation in adolescents with on-line game addiction was higher than in the healthy adolescents. In addition, the activity of the DLPFC decreased following three weeks of family therapy and a reduction in on-line game play. Previous studies of on-line game play have noted increased activity of the DLPFC in patients with on-line game addiction (Ko et al., 2009). Moreover, the decreased activity of the DLPFC in patients with on-line game addiction during treatment has also been observed in adolescents with on-line game addiction following 6 weeks of bupropion therapy (Han et al., 2010). As reported in patients with alcohol or cocaine dependence (Grant et al., 1996; Maas et al., 1998; George et al., 2001), the DLPFC in adolescents with problematic on-line game play and dysfunctional family cohesion activates in response to on-line game stimuli. In a positron emission tomographic study of patients with cocaine dependence, increased glucose metabolism was found in DLPFC when cocaine dependent subjects were exposed to cocaine related cues (Grant et al., 1996). The DLPFC is thought to be associated with the salience for attention and reward expectancy in patients with alcohol dependence or pathologic gambling (George et al., 2001; Crockford et al., 2005). We hypothesize that the changes in DLPFC cortex activity in adolescents with on-line game addiction is due to a decrease in on-line game playing as seen in other substance addictions.
5. Limitations
There are several limitations to the current study. First, the number of subjects was relatively small. Second, variations in family structure such as a single parent household, variable sibling numbers, and different child rearing styles were not considered. Finally, because the activation of healthy adolescents was not evaluated following three weeks of family therapy and compared to changes observed in the adolescents with problematic game play, the change of brain activation in adolescents with on-line game addiction might be associated with re-exposure to the same picture which could decrease the response. A larger sample, a longer duration of treatment, and consideration of treatment effects in comparison subjects will be needed in future studies.
6. Conclusion
With evidence of brain activation changes in response to on-line game playing cues and images depicting parental love, the present findings suggest that family cohesion may be important factor in the treatment of problematic on-line game playing. The caudate nucleus, in particular, activates to a much greater extent following three weeks of family therapy. In contrast, the activity of the dorsolateral prefrontal cortex in response to on-line game cues was reduced after treatment of adolescents with poor parental support.
Acknowledgments
This work was supported by Korean Game Culture Foundation.
References
- American_Psychiatric_Association. Diagnostic and Statistical Manual of Mental Disorders. American Psychiatry Association; Washington, DC: 2000. [Google Scholar]
- Aron A, Fisher H, Mashek DJ, Strong G, Li H, Brown LL. Reward, motivation, and emotion systems associated with early-stage intense romantic love. Journal of Neurophysiology. 2005;94(1):327–337. doi: 10.1152/jn.00838.2004. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The neural correlates of maternal and romantic love. NeuroImage. 2004;21(3):1155–1166. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4(6):561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Crane DR. Research on the cost of providing family therapy: a summary and progress report. Clinical Child Psychology and Psychiatry. 2007;12(2):313–320. doi: 10.1177/1359104507075940. [DOI] [PubMed] [Google Scholar]
- Crockford DN, Goodyear B, Edwards J, Quickfall J, el-Guebaly N. Cue-induced brain activity in pathological gamblers. Biological Psychiatry. 2005;58(10):787–795. doi: 10.1016/j.biopsych.2005.04.037. [DOI] [PubMed] [Google Scholar]
- Fisher H, Aron A, Brown LL. Romantic love: an fMRI study of a neural mechanism for mate choice. The Journal of Comparative Neurology. 2005;493(1):58–62. doi: 10.1002/cne.20772. [DOI] [PubMed] [Google Scholar]
- Frascella J, Potenza MN, Brown LL, Childress AR. Shared brain vulnerabilities open the way for nonsubstance addictions: carving addiction at a new joint? Annals of the New York Academy of Sciences. 2010;1187:294–315. doi: 10.1111/j.1749-6632.2009.05420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frojd S, Kaltiala-Heino R, Rimpela M. The association of parental monitoring and family structure with diverse maladjustment outcomes in middle adolescent boys and girls. Nordic Journal of Psychiatry. 2007;61(4):296–303. doi: 10.1080/08039480701415277. [DOI] [PubMed] [Google Scholar]
- Gao Y, Li LP, Kim JH, Congdon N, Lau J, Griffiths S. The impact of parental migration on health status and health behaviours among left behind adolescent school children in China. BMC Public Health. 2010;10:56. doi: 10.1186/1471-2458-10-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George MS, Anton RF, Bloomer C, Teneback C, Drobes DJ, Lorberbaum JP, Nahas Z, Vicent DJ. Activation of prefrontal cortex and anterior thalamus in alcoholic subjects on exposure to alcohol-specific cues. Archives of General Psychiatry. 2001;58(4):345–352. doi: 10.1001/archpsyc.58.4.345. [DOI] [PubMed] [Google Scholar]
- Gorell BG. Family Therapy in Changing Times. Mc Millan Press; London: 1998. [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A. Activation of memory circuits during cue-elicited cocaine craving. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(21):12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin-Shelley E. Sex and Love: Addiction, Treatment and Recovery. Praeger; Westport, CT: 1991. [Google Scholar]
- Gunn RN, Lammertsma AA, Hume SP, Cunningham VJ. Parametric imaging of ligand-receptor binding in PET using a simplified reference region model. NeuroImage. 1997;6(4):279–287. doi: 10.1006/nimg.1997.0303. [DOI] [PubMed] [Google Scholar]
- Ha JH, Yoo HJ, Cho IH, Chin B, Shin D, Kim JH. Psychiatric comorbidity assessed in Korean children and adolescents who screen positive for Internet addiction. The Journal of Clinical Psychiatry. 2006;67(5):821–826. doi: 10.4088/jcp.v67n0517. [DOI] [PubMed] [Google Scholar]
- Han DH, Hwang JW, Renshaw PF. Bupropion sustained release treatment decreases craving for video games and cue-induced brain activity in patients with Internet video game addiction. Experimental and clinical psychopharmacology. 2010;18(4):297–304. doi: 10.1037/a0020023. [DOI] [PubMed] [Google Scholar]
- Han DH, Bolo N, Daniels AM, Arenella L, Lyoo IK, Renshaw PF. Brain activity and desire for Internet video game play. Comprehensive Psychiatry. 2011;52(1):88–95. doi: 10.1016/j.comppsych.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaitz M, Bar-Haim Y, Lehrer M, Grossman E. Adult attachment style and interpersonal distance. Attachment & Human Development. 2004;6:285–304. doi: 10.1080/14616730412331281520. [DOI] [PubMed] [Google Scholar]
- Ko CH, Liu GC, Hsiao S, Yen JY, Yang MJ, Lin WC, Yen CF, Hen CCS. Brain activities associated with gaming urge of online gaming addiction. Journal of Psychiatry Research. 2009;43(7):739–747. doi: 10.1016/j.jpsychires.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Koepp DM, Silver PA. Nucleocytoplasmic transport and cell proliferation. Biochimica et Biophysica Acta. 1998;1377(2):M39–M47. doi: 10.1016/s0304-419x(97)00036-x. [DOI] [PubMed] [Google Scholar]
- Latimer WW, Winters KC, D’Zurilla T, Nichols M. Integrated family and cognitive-behavioral therapy for adolescent substance abusers: a stage I efficacy study. Drug and Alcohol Dependence. 2003;71(3):303–317. doi: 10.1016/s0376-8716(03)00171-6. [DOI] [PubMed] [Google Scholar]
- Lin CH, Lin SL, Wu CP. The effects of parental monitoring and leisure boredom on adolescents’ Internet addiction. Adolescence. 2009;44(176):993–1004. [PubMed] [Google Scholar]
- Maas LC, Lukas SE, Kaufman MJ, Weiss RD, Daniels SL, Rogers VW, Kukes TJ, Renshaw PF. Functional magnetic resonance imaging of human brain activation during cue-induced cocaine craving. The American Journal of Psychiatry. 1998;155(1):124–126. doi: 10.1176/ajp.155.1.124. [DOI] [PubMed] [Google Scholar]
- Mak KK, Ho SY, Thomas GN, Schooling CM, McGhee SM, Lam TH. Family structure, parent–child conversation time and substance use among Chinese adolescents. BMC Public Health. 2010;10:503. doi: 10.1186/1471-2458-10-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda G, Hiraki K. Sustained decrease in oxygenated hemoglobin during video games in the dorsal prefrontal cortex: a NIRS study of children. NeuroImage. 2006;29(3):706–711. doi: 10.1016/j.neuroimage.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Mellody P, Miller AW, Miller K. Facing Love Addiction. Harper; San Francisco, New York: 1992. [Google Scholar]
- Morgan TB, Crane DR. Cost-effectiveness of family-based substance abuse treatment. Journal of Marital and Family Therapy. 2010;36(4):486–498. doi: 10.1111/j.1752-0606.2010.00195.x. [DOI] [PubMed] [Google Scholar]
- Nalwa K, Anad Ap. Internet addiction in students: a cause of concern. Cyberpsychology. Behavior & Network. 2003;6(6):653–656. doi: 10.1089/109493103322725441. [DOI] [PubMed] [Google Scholar]
- Olson DH. Circumplex Model VII: validation studies and FACES III. Family Process. 1986;25(3):337–351. doi: 10.1111/j.1545-5300.1986.00337.x. [DOI] [PubMed] [Google Scholar]
- Olson DH. Three-dimensional (3-D) Circumplex Model and revised scoring of FACES III. Family Process. 1991;30(1):74–79. doi: 10.1111/j.1545-5300.1991.00074.x. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Champagne F, Meaney MJ, Dagher A. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [11 C]raclopride. Journal of Neuroscience. 2004;24(11):2825–2831. doi: 10.1523/JNEUROSCI.3422-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds I, Rob MI. The role of family difficulties in adolescent depression, drug-taking and other problem behaviours. Medical Journal of Australia. 1988;149(5):250–256. doi: 10.5694/j.1326-5377.1988.tb120597.x. [DOI] [PubMed] [Google Scholar]
- Robbins MS, Feaster DJ, Horigian VE, Puccinelli MJ, Henderson C, Szapocznik J. Therapist adherence in brief strategic family therapy for adolescent drug abusers. Journal of Consulting and Clinical Psychology. 2011;79(1):43–53. doi: 10.1037/a0022146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roustit C, Chaix B, Chauvin P. Family breakup and adolescents’ psychosocial maladjustment: public health implications of family disruptions. Pediatrics. 2007;120(4):e984–e991. doi: 10.1542/peds.2006-3172. [DOI] [PubMed] [Google Scholar]
- Schiepek G, Tominschek I, Karch S, Lutz J, Mulert C, Meindl T, Pogarell O. A controlled single case study with repeated fMRI measurements during the treatment of a patient with obsessive-compulsive disorder: testing the nonlinear dynamics approach to psychotherapy. World Journal of Biological Psychiatry. 2009;10(4 pt2):658–668. doi: 10.1080/15622970802311829. [DOI] [PubMed] [Google Scholar]
- Smilkstein G. The family APGAR: a proposal for a family function test and its use by physicians. Journal of Family Practice. 1978;6(6):1231–1239. [PubMed] [Google Scholar]
- Smilkstein G. The cycle of family function: a conceptual model for family medicine. Journal of Family Practice. 1980;11(2):223–232. [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotactic Atlas of the Human Brain. Thieme Medical Publishers, Inc; New York: 1988. [Google Scholar]
- Taylor MJ, Arsalidou M, Bayless SJ, Morris D, Evans JW, Barbeau EJ. Neural correlates of personally familiar faces: parents, partner and own faces. Human Brain Mapping. 2009;30(7):2008–2020. doi: 10.1002/hbm.20646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HF, Cheng SH, Yeh TL, Shih CC, Chen KC, Yang YC, Yang YK. The risk factors of Internet addiction–a survey of university freshmen. Psychiatry Research. 2009;167(3):294–299. doi: 10.1016/j.psychres.2008.01.015. [DOI] [PubMed] [Google Scholar]
- Vrticka P, Andersson F, Grandjean D, Sander D, Vuilleumier P. Individual attachment style modulates human amygdala and striatum activation during social appraisal. PLoS One. 2008;3:e2868. doi: 10.1371/journal.pone.0002868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen JY, Yen CF, Chen CC, Chen SH, Ko CH. Family factors of internet addiction and substance use experience in Taiwanese adolescents. Cyberpsychology, Behavior, and Social Networking. 2007;10(3):323–329. doi: 10.1089/cpb.2006.9948. [DOI] [PubMed] [Google Scholar]
- Yoo HJ, Cho SC, Ha JH, Yune SK, Kim SJ, Hwang J, Chung A, Sung YH, Lyoo IK. Attention deficit hyperactivity symptoms and Internet addiction. Psychiatry and Clinical Neuroscience. 2004;58(5):487–494. doi: 10.1111/j.1440-1819.2004.01290.x. [DOI] [PubMed] [Google Scholar]
- Young KS. Psychology of computer use: XL. Addictive use of the Internet: a case that breaks the stereotype. Psychological Reports. 1996;79:899–902. doi: 10.2466/pr0.1996.79.3.899. [DOI] [PubMed] [Google Scholar]
- Young KS. Understanding online gaming addiction and treatment issues for adolescents. American Journal of Family Therapy. 2009;37:355–372. [Google Scholar]
- Zeki S. The neurobiology of love. Federation of European Biochemical Societies Letters. 2007;581(14):2575–2579. doi: 10.1016/j.febslet.2007.03.094. [DOI] [PubMed] [Google Scholar]