Abstract
Background
An increasing body of evidence from neuropsychological and neuroimaging studies suggests that exposure to marijuana throughout adolescence disrupts key cortical maturation processes occurring during this developmental phase. GABA-modulating pharmacologic treatments that elevate brain GABA concentration recently have been shown to decrease withdrawal symptoms and improve executive functioning in marijuana-dependent adult subjects. The goal of this study was to investigate whether the lower ACC glutamate previously reported in adolescent chronic marijuana smokers is associated with lower ACC GABA levels.
Methods
Standard and metabolite-edited proton MRS data were acquired from adolescent marijuana users (N = 13) and similarly aged non-using controls (N = 16) using a clinical 3T MRI system.
Results
The adolescent marijuana-using cohort showed significantly lower ACC GABA levels (−22%, p = 0.03), which paralleled significantly lower ACC glutamate levels (−14%, p = 0.01). Importantly, the lower ACC GABA and glutamate levels detected in the adolescent cohort remained significant after controlling for age and sex.
Conclusions
The present spectroscopic findings support functional neuroimaging data documenting cingulate dysfunction in marijuana-dependent adolescents. Glutamatergic and GABAergic abnormalities potentially underlie cingulate dysfunction in adolescent chronic marijuana users, and the opportunity for testing suitable pharmacologic treatments with a non-invasive pharmacodynamic evaluation exists.
Keywords: Adolescent, Marijuana, Proton magnetic resonance spectroscopy, Anterior cingulate cortex (ACC), γ-Amino butyric acid (GABA), Glutamate, Cannabis
1. Introduction
Marijuana is the most commonly used illicit drug among adolescents in the United States, and the annual prevalence of marijuana use is estimated at 11, 24 and 32% for 8th, 10th and 12th graders, respectively (Johnston et al., 2009). Chronic exposure to marijuana throughout adolescence is thought to disturb prefrontal cortical maturation that occurs during this critical developmental phase, which could ultimately give rise to neurobiological impairments propagating through to adult brain circuits (Rubino and Parolaro, 2008). There exists, therefore, the potential for marijuana-induced long-term deficits in decision-making capabilities, emotional processing and cognitive performance (Realini et al., 2009).
Neuropsychological studies in animal models suggests that chronic exposure to D9-tetrahydrocannabinol (THC), the primary psychoactive cannabinoid present within marijuana (Mechoulam and Gaoni, 1965), causes more irreversible residual behavioral effects in immature rats when compared to adult THC-treated rats (Stiglick and Kalant, 1985). Recent animal studies have shown that chronic exposure to synthetic cannabinoids during adolescence induces persistent behavioral and working memory deficits, changes that are not observed in adult cannabinoid-treated rats (Schneider and Koch, 2003; O’Shea et al., 2004). Human neuropsychological studies have reported attentional dysfunction (Ehrenreich et al., 1999) and cognitive deficits (Pope et al., 2003) in early-onset marijuana users. Functional magnetic resonance imaging (fMRI) studies in adult chronic marijuana smokers have demonstrated that cue-induced changes in anterior cingulate cortex (ACC) activation are associated with altered affective response (Gruber et al., 2009) and inhibitory processing (Gruber and Yurgelun-Todd, 2005). Neuroimaging studies in younger subjects suggest that prefrontal cortical functional and structural alterations are detectable in adolescent chronic marijuana users compared to matched controls (Schweinsburg et al., 2008; Hester et al., 2009; Jager et al., 2010; Churchwell et al., 2010).
The prefrontal cortical functional and structural changes occurring within adolescent chronic marijuana users are likely to be accompanied by changes in neurochemistry and metabolism. We recently used proton (1H) magnetic resonance spectroscopy (MRS) to demonstrate that glutamate (Glu; the primary excitatory amino acid neurotransmitter within the mammalian central nervous system) levels were significantly lower within the anterior cingulate cortex (ACC; a brain region that plays a central role in a variety of important executive functions) of adolescent chronic marijuana smokers when compared to control subjects (Prescot et al., 2011). Glu and γ-amino butyric acid (GABA; the primary inhibitory amino acid neurotransmitter), and their respective receptor systems, are known to play critical roles in cortical remodeling throughout adolescence (Crews et al., 2007). A recent clinical proof-of-concept study investigated the use of a calcium channel/GABA-modulating drug, gabapentin, demonstrating significantly decreased withdrawal symptoms and improved overall executive functioning in marijuana-dependent adult subjects (Mason et al., 2012). Although the mechanisms of gabapentin action are largely unknown, gabapentin challenges have been shown to significantly increase cortical GABA levels in healthy subjects using 1H MRS (Cai et al., 2012).
A key question is whether chronic exposure to exogenous cannabinoids during adolescence results in detectable alterations in GABA metabolism as well as Glu metabolism. The objective of the present study was to utilize standard 1H MRS techniques in addition to metabolite-editing 1H MRS methods for selectively measuring ACC Glu and GABA levels, respectively, in adolescent chronic marijuana users and control subjects. Based on our Glu 1H MRS findings (Prescot et al., 2011) and precedent clinical evidence demonstrating the efficacy of GABA-modulating pharmacotherapies in adult marijuana-dependent populations (Mason et al., 2012), we hypothesized that the lower ACC Glu levels detected in adolescent chronic marijuana using individuals would be paralleled by lower ACC GABA concentration.
2. Methods and materials
2.1. Subject selection
The local Institutional Review Board at the University of Utah approved this investigation. Seventeen adolescent marijuana (MJ) and seventeen similarly aged healthy control (HC) subjects between the ages 13 and 19 years initially were recruited from the greater Salt Lake area using local advertisements. Ultimately, data from sixteen HC and thirteen MJ subjects were included in the final analysis (see Section 3.1).
At enrollment, all MJ subjects were unaware of inclusion criteria into specific participant groups, such as lifetime use, or the purpose of the study beyond a better understanding of brain development with and without marijuana exposure. Subjects were enrolled if they reported having smoked at least 100 times in the previous year as determined by an initial phone screen. In the context of this study the term “smoke” does not refer to “puffs” but the incidence of use or the number of separate occasions a joint, blunt or bowl was used. Seven of the thirteen MJ subjects (54%) reported having used marijuana within the previous 24 h and six of the thirteen participants (46%) reported having used marijuana more than 24 h before scanning.
Table 1 presents the demographic data and relevant clinical variable information for both cohorts. The Hamilton rating scale for depression (HAM-D) and Hamilton rating scale for anxiety (HAM-A) data also are provided in Table 1. HC participants had no DSM-IV Axis I diagnosis based on structured and clinical interviews and had no first-degree family history of bipolar disorder or psychosis. Exclusion criteria for all subjects included major sensorimotor handicaps (e.g., deafness, blindness, paralysis); full scale IQ <70; history of claustrophobia, autism, schizophrenia, anorexia nervosa or bulimia, other drug dependence/abuse or alcohol dependence/abuse (during 3 months prior to scan), active medical or neurological disease, history of ECT; metal fragments or implants; and current pregnancy or lactation. All subjects provided written assent, and their parents (or legal guardians) provided written informed consent for their adolescent’s participation. Subjects under the age of 18 completed the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Episode (K-SADS-PL; Kaufman et al., 1997) whereas subjects over the age of 18 completed the Structured Clinical Interview for DSM-IV Patient Version (SCID-P). All HC and MJ subjects underwent a one step urine drug test (iScreen, San Diego, CA) on the day of scanning to test for the presence of cocaine, methamphetamine, THC, opiates and benzodiazepines. Subjects who tested positive for any illicit substance other than THC were excluded from the study. Following successful enrollment into the study a urine sample was retained for quantitative 9-carboxy-tetrahydrocannabinol analysis (ARUP Laboratories, Salt Lake City, UT; detection range: 4–1000 ng/mL). Information regarding age of first MJ use, age of regular use, and frequency of use was obtained on all participants, and total lifetime MJ use was calculated by averaging the number of smokes per week multiplied by duration of use.
Table 1.
Demographics of the HC and MJ subject groups including clinical variable information for the MJ cohort.
| HC, N = 16 | MJ, N = 13 | |
|---|---|---|
| Gender: male/female | 7/9 | 11/2 |
| †Age (years): mean ± SD/range | 16.0 ±2.2/13–19 | 17.9 ±1.0/16–19 |
| ‡HAM-D: mean ± SD/range | 0.1 ±0.3/0–1 | 2.3 ±3.6/0–11 |
| HAM-A: mean ± SD/range | 1.6 ±2.5/0–7 | 2.2 ± 2.3/0–7 |
| Age of first use (years) mean ± SD/range | 15.2 ±1.4/13–17 | |
| Age of regular use (years) mean ± SD/range | 16.0 ±0.9/14–17 | |
| Total use (number of smokes) mean ± SD/range | 1124 ±1314/235–5250 | |
| Cannabinoid count (ng/mL) mean ± SD/range | 359 ± 365/16- 1000a |
Two subjects registered a negative (−ve) urine cannabinoid count immediately prior to scanning, and two subjects showed cannabinoid counts greaterthan the maximum detectable level of the test procedure (>1000 ng/mL).
Signficant differences existed between the HC and MJ populations for age (p = 0.01).
Signficant differences existed between the HC and MJ populations for HAM-D (p = 0.02).
Abbreviations: HC, healthy control cohort; MJ, marijuana using cohort; N, number of subjects per cohort; SD, standard deviation; HAM-A, Hamilton rating scale for anxiety; HAM-D, Hamilton rating scale for depression; and ng/mL, nanogram per milliliter.
While all subjects screened negative for psychiatric history based on a phone screen, direct diagnostic interviews indicated that two MJ subjects reported a history (>3 months prior to MRS) of alcohol abuse but not dependence, and one MJ subject had a history (>3 months prior to MRS) of alcohol abuse and dependence. One MJ subject reported current nicotine at 1.5 cigarettes per day (7 month duration), and a second MJ subject reported current nicotine at 1 cigarettes per week (2 month duration). A third MJ subject reported a history of nicotine use 1 year prior to clinical interview (2 cigarettes per day; 2 month duration). No subjects within the HC cohort reported current nicotine use or a history of nicotine use and/or dependence. In addition, two MJ subjects reported a history of depression and were taking antidepressants at the time of MRS.
2.2. Data acquisition
1H MRS scans were performed on a 3.0 Tesla Siemens (Erlangen, Germany) MAGNETOM Trio whole-body MRI/MRS system. A manufacturer-supplied circularly polarized body radiofrequency (RF) coil and a 12-channel phased array receive-only head coil were used for RF transmission and signal reception, respectively. Three-dimensional, high-resolution, magnetization-prepared, rapid gradient echo (MP-RAGE) MR images (TR/TE/TI = 2000/3.53/1100 ms; FOV = 256 × 256 × 224 mm; 1 mm isotropic resolution) were obtained to facilitate the bilateral positioning of a 3.0 cm × 2.5 cm × 2.0 cm MRS voxel within predominantly gray matter of the ACC. The MRS voxel was obliqued along the sagital plane with its smallest dimension spanning the anterior–posterior axis. Localized shimming was performed to yield water signal linewidths of ≤10 Hz.
Conventional 1H MRS data were acquired using the methodology previously described (Prescot et al., 2011). GABA-edited 1H MRS spectra were acquired using a variant of the J-difference edited MEGAPRESS method (Mescher et al., 1998) with elimination of the four-compartment artifact (Kaiser et al., 2007). Outer volume suppression (OVS) was applied for all MRS acquisitions using hyperbolic secant adiabatic full passage RF pulses to excite saturation bands positioned at least 2-cm away from the MRS voxel faces. A three-pulse water elimination through T1-effects (Ogg et al., 1994) was used for global water suppression. Unsuppressed water data (TR = 2000 ms, NEX = 4) were acquired from all subjects for both sequence types and used for eddy current correction and normalization of metabolite signal integrals.
2.3. Data processing
Brain extraction and tissue segmentation was applied to all MP-RAGE images using the BET (Smith, 2002) and FAST (Zhang et al., 2001) tools provided with the FMRIB software library (FSL; Smith et al., 2004). MATLAB (The MathWorks, Natick, MA) was used to extract the 3D volume corresponding to the positioned MRS voxel and calculate within-voxel gray matter (GM), white matter (WM) and cerebrospinal fluid (CSF) tissue content for each subject. The within-voxel gray matter percentage was calculated as the ratio to total brain matter, i.e., 100 × GM/(WM + GM).
All free induction decay (FID) data were collected individually without signal averaging. MATLAB functions were used to process coil channel data as follows. Exponential apodization (line broadening = 2 Hz) was applied to each individual FID followed by fast Fourier transformation (FFT). Automated frequency–drift correction was then performed in the frequency domain based on the position of the 2.0 ppm NAA methyl resonance. Note that for MEGAPRESS data, the NAA signal is eliminated for the ‘on’ condition and those data were frequency-corrected using the directly preceding ‘off’ scan parameter. The required frequency-correction was performed in the time domain by applying a linear phase to the target FID. All frequency-corrected FIDs then were averaged for each coil channel and subsequently eddy current corrected using the corresponding unsuppressed water data and a previously reported time domain method (Klose, 1990). Individual coil channel-specific weighting function coefficients were applied to the metabolite and unsuppressed water FIDs prior to coil channel (Natt et al., 2005). These procedures resulted in a single FID for PRESS and two FIDs for MEGAPRESS corresponding to the ‘on’ and ‘off’ editing conditions. The MEGAPRESS GABA difference-edited data were calculated by subtracting the ‘off’ from the ‘on’ FID.
Automated evaluations regarding spectral data quality also were applied to individual subject MEGAPRESS data prior to inclusion in statistical analysis. The first of these was based on determining the NAA amplitude for all 256 ‘off’ scans during a given acquisition to determine the coefficient of variation (CV; calculated as standard deviation ± mean) for NAA peak height. Data with NAA amplitude CV values ≤15% were included for statistical analysis. A second evaluation considered the residual water peak amplitude in the MEGAPRESS GABA data. The MEGAPRESS sequence employed should yield data characterized by almost no residual water owing to the WET water suppression scheme coupled with further elimination imposed by MEGA-editing. Data were excluded if the absolute amplitude of residual water was two times greater than the edited GABA 3.0 ppm resonance.
2.4. Spectral quantification
PRESS unsuppressed water signal area was calculated after fitting a Voigt line-shape to the real component of the phased frequency-domain data (Marshall et al., 1997). PRESS metabolite data were fitted using the methods previously described (Prescot et al., 2011), whereas all MEGAPRESS GABA data were fitted as detailed elsewhere (Boy et al., 2010). Metabolite peak areas were corrected for the CSF-fraction using within-voxel segmented MRI data, and normalized using the PRESS TE = 30 ms unsuppressed water signal integral scaled by 10−5 and 10−9 for MEGAPRESS and PRESS, respectively. Metabolite/water ratios thus are expressed as institutional units (i.u) and presented as the mean ± standard deviation (SD).
One way analysis of variance (ANOVA) was used for comparing group mean metabolite levels using OriginPro (OriginLab Corporation, Northampton, MA), which also was used for running Pearson’s correlation analyses. We tested the relationship between ACC metabolite levels and age of first use, age of regular use, total use and urine cannabinoid count. Both total marijuana use and cannabinoid count were found to be represented by non-normal distributions. Prior to running correlation analysis, both of these variables were adjusted to be normally distributed using a logarithmic transformation. In addition, potential confounding variables were evaluated using analysis of covariance (ANCOVA) for two independent samples using http://faculty.vassar.edu/lowry/ancova2L.html (last accessed 05/24/2011). Statistical analysis was performed using all HC and MJ subject data, and also following the exclusion of specific subject data or controlling for the effects of age, sex, within-voxel tissue content, alcohol, nicotine, and depression/medication.
3. Results
3.1. Data inclusion
One HC subject showed a NAA peak amplitude CV value of 22% whereas three MJ subjects had values of greater than 20%. The resulting mean (±SD) NAA peak amplitude CV values were 9 ± 2% and 10 ± 3% for the HC and MJ cohorts, respectively. MRS spectra from one further MJ subject was excluded from the analysis as the data did not satisfy the residual water amplitude criterion. Further visual inspection of the MEGAPRESS data from that individual revealed a broad residual water component propagating throughout the baseline and into the Glu/Gln (3.75 ppm) and GABA (3.0 ppm) chemical shift region. Therefore, MRS data acquired from sixteen HC subjects and thirteen MJ subjects were included in the statistical analysis.
3.2. Tissue segmentation
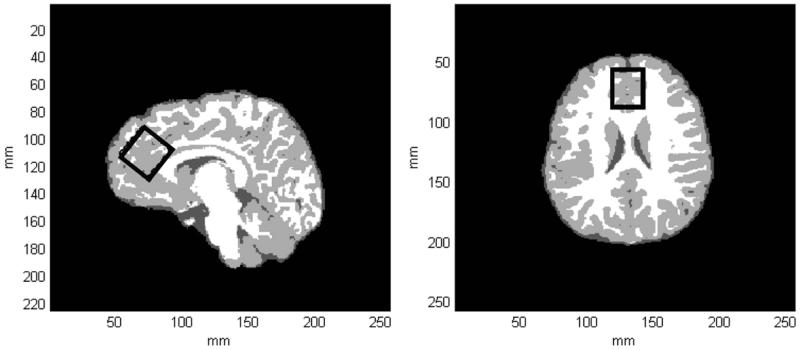
Fig. 1 displays tissue-segmented axial and sagital images extracted from a 3D MP-RAGE dataset recorded from a single HC subject. The black rectangle shown on both images depicts the MRS voxel positioning within the ACC (see Figure legend for more details). Table 2 displays the within-voxel GM and CSF content for both subject cohorts and statistical analysis did not reveal any significant differences between the two groups. There was no significant difference in the ACC water reference signal integral between the two groups (MJ 1.41 ± 0.07; HC 1.37 ± 0.05, p = 0.11).
Fig. 1.
(a) A tissue-segmented axial slice extracted from a 3D MPRAGE dataset recorded from a 17-year-old female HC subject. WM, GM and CSF are represented by white, light gray and dark gray pixels, respectively. (b) A tissue-segmented sagital slice extracted from the same MPRAGE dataset. The black rectangle depicts the positioning of the MRS voxel within the ACC, which was obliqued along the sagital dimension. For this subject, GM, WM and CSF tissue fractions were estimated to be 74, 24 and 2%, respectively.
Table 2.
MRS voxel GM (see text for details) and CSF content expressed as group mean % fraction ± SD.
| Tissue type | HC | MJ | Statistics |
|---|---|---|---|
| GM | 70 ± 5 | 72 ± 4 | F(1,27) = 0.7, p = 0.4 |
| CSF | 8 ± 4 | 9± 2 | F(1,27) = 0.1, p = 0.7 |
Abbreviations: MRS, magnetic resonance spectroscopy; GM, gray matter; CSF, cerebrospinal fluid; HC, healthy control cohort; and MJ, marijuana using cohort.
3.3. Spectral and statistical analysis
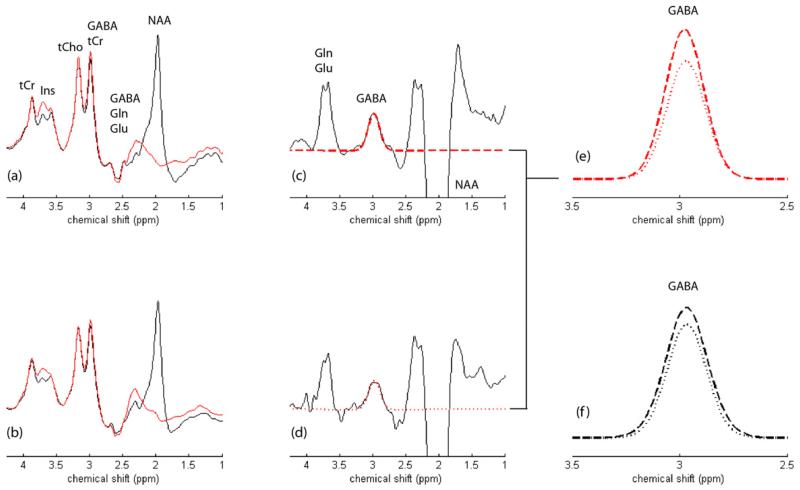
GABA-edited MEGAPRESS 1H MRS data recorded from the ACC of an 17-year-old female HC and an 18-year-old female MJ subject are presented in Fig. 2.
Fig. 2.
(a) and (b) show the MEGAPRESS editing pulse ‘on’ (red spectra) and ‘off’ (black spectra) condition 1H MRS spectra recorded from a HC and MJ subject, respectively. The main signals tentatively assigned in (a) and can be directly translated to (b). The resulting MEGAPRESS difference spectra are presented for the (c) HC and (d) MJ subject, which are characterized by an inverted NAA resonance at 2.0 ppm, the edited GABA peak (plus macromolecule) at 3.0 ppm and a co-edited composite Gln/Glu resonance at 3.75 ppm. The estimated GABA fits are overlaid in both MEGAPRESS GABA-edited datasets (red dashed spectra) with the direct overlay of the HC and MJ GABA fits presented in (e). This particular MJ subject showed a 17% lower CSF-corrected GABA:water ratio when compared to the HC data. The group averaged GABA fits are presented in panel (f) for both the HC (dashed line) and MJ (dotted line) cohorts. Note that the vertical scaling was increased fivefold for the MEGAPRESS-edited data in (c) and (d) whereas the vertical scaling for plots (e) and (f) was enhanced by a factor of twenty.
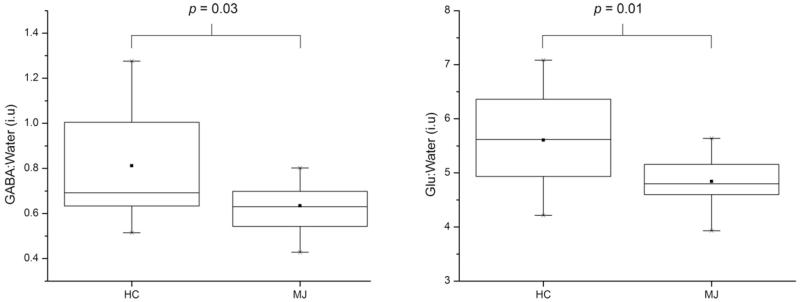
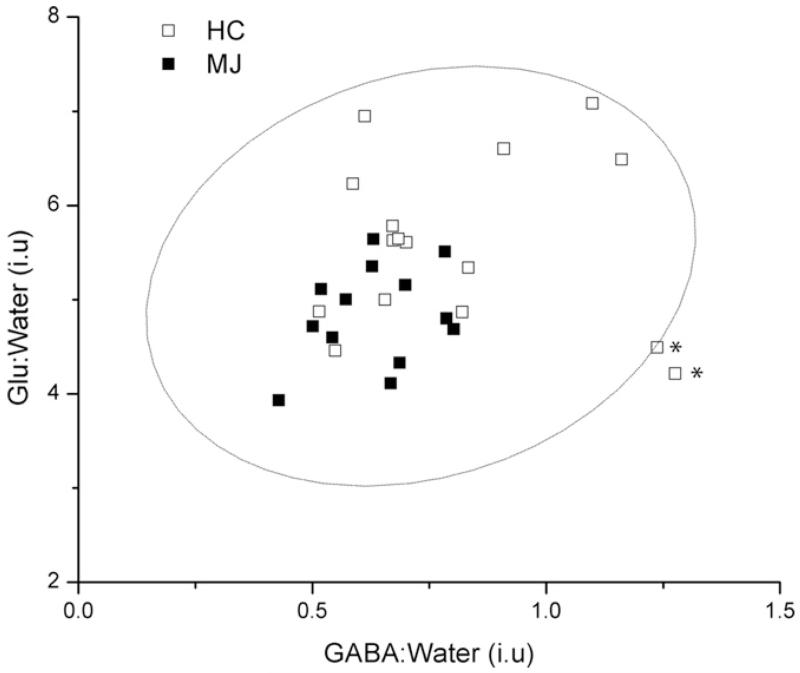
The figure shows the GABA-edited ‘on’ and ‘off’ conditions, the difference-edited spectra and GABA spectral fits for both subjects (see figure legend for details). LC Model-fitted PRESS (TE = 30 ms) 1H MRS data recorded from these populations have been presented in a recent publication (Prescot et al., 2011).Fig. 3 displays box plots comparing the group mean CSF-corrected GABA:water and Glu:water ratios (see Fig. 3 legend for details). The MJ cohort showed significantly lower ACC GABA (MJ 0.63 ± 0.12; HC 0.81 ± 0.25, F(1,27) = 5.4, p = 0.03) and ACC Glu (MJ 4.84 ± 0.52; HC 5.61 ± 0.88, F(1,27) = 7.5, p = 0.01) levels. A plot showing the water-normalized Glu versus GABA levels is presented in Fig. 4. No significant relationship existed when the analysis incorporated all of the subject data (r = 0.20, p = 0.3), although a significant positive correlation was observed following the removal of two potential HC outliers from the analysis (r = 0.56, p = 0.002).
Fig. 3.
Box plots showing the mean water-normalized GABA (left) and Glu (right) levels measured in the HC and MJ cohorts. The ■ symbol and within-box horizontal lines represent the mean and median values, respectively. The box extremities correspond to the 25th and 75th percentiles and the ‘×’ symbol represents the full data range.
Fig. 4.
A plot of the water-normalized Glu versus GABA levels for all HC (unfilled squares) and MJ (filled squares) subjects. The overlaid confidence ellipse (dashed line) was computed using a 95% confidence level. The * symbols denote the two potential outliers from the HC population (see text for statistical results).
The conventional 1H MRS data from MJ subjects also revealed significantly lower N-acetyl aspartate (NAA: MJ 2.28 ± 0.42; HC 2.60 ± 0.44, F(1,27) = 4.0, p = 0.05), total creatine (tCr: MJ 2.06 ± 0.22; HC 2.26 ± 0.19, F(1,27) = 6.6, p = 0.02) and myo-inositol (Ins: MJ 4.84 ± 0.52; HC 5.61 ± 0.88, F(1,27) = 4.2, p = 0.01) levels, findings that are consistent with our previous observations (Prescot et al., 2011).
Lower ACC GABA and Glu levels remained statistically significant after controlling for subject age (GABA: F(1,26) = 4.62, p = 0.04; Glu: F(1,26) = 4.62, p = 0.04), HAM-D scores (GABA: F(1,26) = 5.11, p = 0.03; Glu: F(1,26) = 4.92, p = 0.04), within-voxel GM percentage (GABA: F(1,26) = 5.76, p = 0.02; Glu: F(1,26) = 8.37, p < 0.01), alcohol history (GABA: F(1,26) = 5.10, p = 0.03; Glu: F(1,26) = 5.60, p = 0.03), nicotine use (GABA: F(1,26) = 4.30, p = 0.05; Glu: F(1,26) = 5.98, p = 0.02), and antidepressant medication (GABA: F(1,26) = 4.99, p = 0.03; Glu: F(1,26) = 6.66, p = 0.02). Further (ANOVA) analysis showed that lower ACC GABA and Glu levels remained significant after removing the three subjects with a history of alcohol use/dependence (GABA: F(1,24) = 4.85, p = 0.04; Glu: F(1,26) = 5.30, p = 0.03), three subjects with a history of nicotine use (GABA: F(1,24) = 4.25, p = 0.05; Glu: F(1,26) = 5.98, p = 0.02), and two subjects who were taking antidepressant medication at the time of MRS scanning (GABA: F(1,25) = 4.80, p = 0.04; Glu: F(1,26) = 6.56, p = 0.02).
Including sex as a covariate shifted the findings to marginal and high significance for ACC GABA (F(1,26) = 3.86, p = 0.06) and Glu (F(1,26) = 14.67, p < 0.001) levels, respectively. Further statistical analysis comparing only male HC and MJ subjects revealed significantly lower ACC GABA (MJ 0.61 ± 0.12; HC 0.82 ± 0.22, F(1,16) = 7.05, p = 0.02) and Glu (MJ 4.90 ± 0.54; HC 6.10 ± 0.72, F(1,16) = 16.4, p < 0.001) levels.
We observed a trend toward a negative relationship between GABA levels and total marijuana use (r = −0.43, p = 0.10). No significant relationship between age and GABA and Glu levels were detected for the sample as a whole or for the individual cohorts.
4. Discussion
Maturation of the prefrontal cortex is a primary feature of adolescent neurodevelopment (Huttenlocher, 1979; Slotkin, 2002). A reduction in the overall number of synaptic connections is driven by neuroregulatory processes including synaptic pruning and elimination, which act to retain more efficient neuronal networks (Cohen-Cory, 2002). The neurobiological changes occurring throughout adolescence present a critical vulnerable period susceptible to the effects of drugs of abuse (Spear, 2007) including marijuana. Recent neuroimaging investigations have demonstrated abnormal cortical activation patterns (Schweinsburg et al., 2008) and prefrontal cortical volume differences (Churchwell et al., 2010) in adolescent chronic marijuana users compared to controls. It is well established that changes in the Glu and GABA receptor systems play a critical underlying role in cortical remodeling throughout adolescence (Crews et al., 2007).
The main aim of the present study was to utilize standard and metabolite-editing MRS methods for evaluating ACC Glu and GABA levels in adolescent MJ subjects and control subjects. The primary outcome was that significantly lower MJ GABA levels (−22%) paralleled lower MJ Glu (−14%), tCr (−9%), Ins (−14%), and NAA (−12%) levels. The GABA findings are particularly striking when considering (i) clinical evidence that demonstrates significantly decreased withdrawal symptoms and improved overall executive functioning in marijuana-dependent adult subjects (Mason et al., 2012), and (ii) brain GABA increases measured using 1H MRS (Cai et al., 2012), following gabapentin challenges. The correlation between ACC GABA and Glu levels was statistically insignificant, although a significant positive relationship was calculated after excluding two HC subject data points. Although further studies are necessary, the trend toward this correlation contrasts a recent study of depressed adolescent subjects showing significantly lower ACC GABA levels in the absence of ACC Glx (glutamate + glutamine) alterations (Gabbay et al., 2012). The lower ACC NAA level (−12%) observed in the MJ population is supported by a previous 1H MRS study that documented significantly lower (−11%) dorsal lateral prefrontal cortical NAA levels in recreational marijuana users compared to matched control subjects (Hermann et al., 2007). We also observed a trend toward lower ACC GABA levels with total marijuana use, suggesting that greater MJ use is associated with lower cingulate GABA concentration. However, the MJ cohort for the present study was relatively small (N = 13) and a single individual had much higher total marijuana use than the other subjects. We currently are enrolling additional MJ subjects with higher total use and lower ages for first and regular marijuana use to further investigate this observation.
The lower ACC MJ Glu level (−14%) reported here is in close agreement with the lower basal ganglia Glu level (−12%) previously observed in adult chronic marijuana users (Chang et al., 2006), although similar investigations documenting GABA levels in adult users have not been reported to date. Nevertheless, direct comparison of Glu and GABA levels in adolescent and adult chronic marijuana users remains difficult due to the lack of 1H MRS data that spans childhood → adolescence → adulthood. Based on age related Glu changes reported for adult studies (Sailasuta et al., 2008; Chang et al., 2009), we controlled for subject age, and continued to detect significantly lower ACC MJ GABA and Glu levels. Regardless, the execution of comprehensive longitudinal 1H MRS studies designed to track Glu and GABA levels in non-using subjects throughout childhood → adolescence → adulthood would help put the present findings into context. The ACC GABA and Glu alterations detected in our MJ cohort also should be considered alongside previous functional neuroimaging investigations reporting frontal lobe impairments in similar adolescent populations (Schweinsburg et al., 2008; Hester et al., 2009). Future work might include the correlation of brain chemistry and ACC GABA levels with regional functional activity, as has been reported for emotional processing in healthy volunteers (Northoff et al., 2007).
THC activates the cannabinoid type-1 (CB1) receptor (Devane et al., 1988), the most abundant Gi/Go-protein-coupled receptor in the mammalian brain found to be enriched in the cortex, hippocampus, basal ganglia and cerebellum (Herkenham et al., 1991; Glass et al., 1997). CB1 receptors are located on presynaptic GABA and Glu neurons where they are activated by the endocannabinoids anandamide (Devane et al., 1992) and 2-arachidonoyl glycerol (Sugiura et al., 1995). Endocannabinoids are thought to play a crucial role in synaptic regulation, where once released from postsynaptic neurons they diffuse in a retrograde fashion across the synaptic cleft to activate the CB1 receptor (Wilson and Nicoll, 2002). Activation of the CB1 receptor inhibits presynaptic Ca2+ influx which in turn decreases the probability of neurotransmitter release. Retrograde inhibition of neurotransmission has been reported for GABA and Glu neurons throughout the whole brain (Schlicker and Kathmann, 2001) and individual brain structures (Hajos and Freund, 2002; Hoffman et al., 2007; Laaris et al., 2010). Glu and GABA are implicated in range of psychiatric and substance abuse disorders (Mason and Krystal, 2006), and it is tempting to suggest that the lower ACC GABA and Glu levels detected in the present study are associated with a chronic activation and modification of the endocannabinoid system. However, GABA and Glu levels as measured using MRS do not directly reflect glutamatergic and GABAergic activity. Following its release from a glutamatergic neuron, Glu is rapidly transported from the synaptic cleft into astroglia where it is converted into glutamine (Gln). Astroglial Gln is then shuttled into glutamatergic neurons where it is reconverted to Glu, thereby completing the Glu–Gln cycle. GABA on the other hand is synthesized from Glu within GABAergic neurons. After its release and inhibitory signal transduction at postsynaptic receptors, GABA is transported into astroglia, converted to Gln (via Glu), and transported back into GABAergic neurons to complete the GABA–Gln cycle. The complex multi-compartmental nature of Glu and GABA metabolism means that the Glu and GABA signals detected using ‘static’ MRS measures could mask the dynamic changes associated with neurotransmitter synthesis, release and metabolism. Therefore, the lower ACC GABA and Glu levels reported here remains inconclusive, as the findings may represent altered neuronal and/or glial function. Clearly, additional spectroscopic information regarding brain Gln would provide key information regarding glutamatergic and GABAergic turnover and we currently are applying two-dimensional (2D) 1H MRS methods (Schulte and Boesiger, 2006) for the concomitant detection of GABA, Gln, and Glu, in adolescent MJ and HC populations. Furthermore, future work in adolescent MJ and HC populations might involve the acquisition of quantitative ‘dynamic’ information regarding Glu and GABA metabolism using carbon-13 (13C) MRS with 13C-labeled substrate infusion (de Graaf et al., 2011).
A more rigorous understanding of the effects of adolescent chronic cannabinoid exposure could be gained using established preclinical rodent models, which have been shown to induce dose-dependent decreases in CB1 receptor binding (Dalton and Zavitsanou, 2010), long-lasting memory and anxiety impairments (O’Shea et al., 2004), and persisting cognitive deficits (Rubino and Parolaro, 2008). These models present an opportunity for serial 1H and 13C MRS measures prior-to and following cannabinoid exposure (adolescence), and to monitor neurochemistry after cannabinoid cessation (adulthood). Furthermore, these types of longitudinal studies also will help establish if there exists a correlation between adolescent cannabinoid exposure, CB1 receptor density, and alterations in Glu and GABA levels/cycling as measured using 1H and 13C MRS.
4.1. Study strengths and limitations
Lubman et al. (2007) reported high prevalence of co-morbid psychiatric disorders in one hundred substance-abusing adolescents and young adults, with 49% meeting criteria for current mood or anxiety disorder, and 68% reporting a lifetime history. This is consistent with a study evaluating 90 cannabis users where current mood disorder was present in 48% in the last 12 months (Guillem et al., 2009). In the present study, one MJ subject was taking a selective serotonin reuptake inhibitor (SSRI; citalopram) whereas a second was taking an atypical antidepressant (bupropion). Several MRS studies have documented increased cortical GABA levels following administration of SSRI drugs in healthy controls (Bhagwagar et al., 2004) and depressed patients (Sanacora et al., 2002). Modulation of cortical Glu could not be detected in healthy volunteers receiving citalopram (Taylor et al., 2010), and GABA and Glu modulation in response to atypical antidepressants has not been reported. In light of this, we compared unmedicated individuals with controls and continued to observe significantly lower GABA and Glu levels in MJ subjects.
Cortical GABA (Epperson et al., 2002) and Glu (Batra et al., 2008) levels are known to fluctuate as a function of menstrual cycle phase. Menstrual cycle and phase details were not recorded from the 11 female subjects scanned in the present study. It was thus critical to investigate sex effects and rerun statistical analysis (ANOVA) with the exclusion of the female subject data. The apparent lower MJ ACC GABA and Glu levels remained statistically significant after comparing the male-only populations. Note that the influence of gender on brain metabolite concentration is also likely to be independent of menstrual hormonal effects, and for the present study co-varying for sex shifted the lower ACC GABA finding to marginal significance (p = 0.06). Based on these observations, studies from larger sample sizes with sex-matched populations are warranted, and those studies would benefit from multiple MRS time points obtained throughout the menstrual cycle phase in female populations.
Currently, due to the cross-sectional study design, we cannot conclude whether the detected Glu and GABA differences represent marijuana-induced neurotoxicity or a premorbid neurochemical state. Longitudinal MRS investigations in adolescent MJ populations might help evaluate neurochemical changes and normalization following marijuana cessation. Preclinical MRS measurements performed prior-to and during chronic cannabinoid exposure in suitable adolescent rodent models will be invaluable to help address the notion of a premorbid condition.
This study proposed, a priori, to test for differences in GABA concentration that might be associated with adolescence marijuana abuse. This hypothesis was based on (i) our previous MRS study (Prescot et al., 2011), and (ii) preclinical observations of changes in GABAergic neurotransmission that have been associated with marijuana exposure. Although the present GABA finding does not require a correction for multiple comparisons, future studies are warranted making use of larger sample sizes and multiple testing procedures. A related caveat associated with the present study concerns ANCOVA and the testing of potential confounding variables on an individual basis. The present study results thus remain subject to Type 1 error and studies in larger populations will allow for the application of statistical models that enable a more rigorous testing for correlations between multiple independent variables.
Partial volume effects are likely to result from the large ACC voxel size, which was necessary due to the relative insensitivity of GABA-editing MRS. Controlling for GM fraction was crucial considering documented GM/WM differences in GABA concentration (Jensen et al., 2005; Geramita et al., 2011). It currently is unclear whether the GABA and Glu differences are specific to the ACC region examined. Our ongoing 2D 1H MRS measurements are focused on the ACC, the parietal–occipital cortex, and the hippocampus, to establish whether the detected neurochemical differences are exclusive to the ACC, or manifested across multiple functionally distinct brain regions. In conclusion, the present spectroscopic findings support functional neuroimaging data documenting cingulate dysfunction in marijuana-dependent individuals. Glutamatergic and GABAergic abnormalities potentially underlie cingulate dysfunction in adolescent chronic marijuana users, and the opportunity for testing new or currently available pharmacologic treatments (e.g., gabapentin) with a non-invasive pharmacodynamic evaluation (MRS) exists.
Acknowledgement
The authors wish to Ms. Allison Locatelli for her assistance with subject recruitment and screening.
Role of funding source
This study was supported by National Institute on Drug Abuse (NIDA) funding awarded to Dr. Yurgelun-Todd (NIDA 1R01 DA020269), Dr. Renshaw (NIDA K24 DA015116), and Dr. Prescot (NIDA R03DA031321). As the sponsor of the project, NIDA did not have a role in the data collection, data analysis or interpretation, writing of the manuscript, or the decision to submit such for publication.
Footnotes
Contributors
Dr. Prescot designed the study, implemented the spectroscopy protocols, acquired the data, performed data processing and statistical analysis, and wrote the manuscript. Dr. Renshaw interpreted data analyses and reviewed and edited all versions of the manuscript. Dr. Yurgelun-Todd created the overall study design and protocol, directed subject recruitment, interpreted data analyses and reviewed and edited all versions of the manuscript.
Conflict of interest
Dr. Yurgelun-Todd is a consultant to Kyowa Hakko, Eli Lilly and Janssen Pharmaceutica. Dr. Renshaw is a consultant to Kyowa Hakko, Novartis and Roche and receives research support from Roche and GlaxoSmithKline. However, the authors report that there are no conflicts of interest relevant to this research study.
References
- Batra NA, Seres-Mailo J, Hanstock C, Seres P, Khudabux J, Bellavance F, Baker G, Allen P, Tibbo P, Hui E, Le Melledo JM. Proton magnetic resonance spectroscopy measurement of brain Glu levels in premenstrual dysphoric disorder. Biol. Psychiatry. 2008;63:1178–1184. doi: 10.1016/j.biopsych.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Bhagwagar Z, Wylezinska M, Taylor M, Jezzard P, Matthews PM, Cowen PJ. Increased brain GABA concentrations following acute administration of a selective serotonin reuptake inhibitor. Am. J. Psychiatry. 2004;161:368–370. doi: 10.1176/appi.ajp.161.2.368. [DOI] [PubMed] [Google Scholar]
- Boy F, Evans CJ, Edden RA, Singh KD, Husain M, Sumner P. Individual differences in subconscious motor control predicted by GABA concentration in SMA. Curr. Biol. 2010;20:1779–1785. doi: 10.1016/j.cub.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai K, Nanga RP, Lamprou L, Schinstine C, Elliott M, Hariharan H, Reddy R, Epperson CN. The impact of gabapentin administration on brain GABA and glutamate concentrations: a 7T (1)H MRS study. Neuropsychopharmacology. 2012;37:2764–2771. doi: 10.1038/npp.2012.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Cloak C, Yakupov R, Ernst T. Combined and independent effects of chronic marijuana use and HIV on brain metabolites. J. Neuroimmune Pharmacol. 2006;1:65–76. doi: 10.1007/s11481-005-9005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Jiang CS, Ernst T. Effects of age and sex on brain Glu and other metabolites. Magn. Reson. Imaging. 2009;27:142–145. doi: 10.1016/j.mri.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchwell JC, Lopez-Larson M, Yurgelun-Todd DA. Altered frontal cortical volume and decision making in adolescent cannabis users. Front. Psychol. 2010;1:225. doi: 10.3389/fpsyg.2010.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Cory S. The developing synapse: construction and modulation of synaptic structures and circuits. Science. 2002;298:770–776. doi: 10.1126/science.1075510. [DOI] [PubMed] [Google Scholar]
- Crews F, He J, Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacol. Biochem. Behav. 2007;86:189–199. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Dalton VS, Zavitsanou K. Cannabinoid effects on CB1 receptor density in the adolescent brain: an autoradiographic study using the synthetic cannabinoid HU210. Synapse. 2010;64:845–854. doi: 10.1002/syn.20801. [DOI] [PubMed] [Google Scholar]
- de Graaf RA, Rothman DL, Behar KL. State of the art direct 13C and indirect 1H-[13C] NMR spectroscopy in vivo. A practical guide. NMR Biomed. 2011;24:958–972. doi: 10.1002/nbm.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devane WA, Dysarz FA, 3rd, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol. Pharmacol. 1988;34:605–613. [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Ehrenreich H, Rinn T, Kunert HJ, Moeller MR, Poser W, Schilling L, Gigerenzer G, Hoehe MR. Specific attentional dysfunction in adults following early start of cannabis use. Psychopharmacology (Berl.) 1999;142:295–301. doi: 10.1007/s002130050892. [DOI] [PubMed] [Google Scholar]
- Epperson CN, Haga K, Mason GF, Sellers E, Gueorguieva R, Zhang W, Weiss E, Rothman DL, Krystal JH. Cortical gamma-aminobutyric acid levels across the menstrual cycle in healthy women and those with premenstrual dysphoric disorder: a proton magnetic resonance spectroscopy study. Arch. Gen. Psychiatry. 2002;59:851–858. doi: 10.1001/archpsyc.59.9.851. [DOI] [PubMed] [Google Scholar]
- Gabbay V, Mao X, Klein RG, Ely BA, Babb JS, Panzer AM, Alonso CM, Shungu DC. Anterior cingulate cortex gamma-aminobutyric acid in depressed adolescents: relationship to anhedonia. Arch. Gen. Psychiatry. 2012;69:139–149. doi: 10.1001/archgenpsychiatry.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geramita M, van der Veen JW, Barnett AS, Savostyanova AA, Shen J, Weinberger DR, Marenco S. Reproducibility of prefrontal gamma-aminobutyric acid measurements with J-edited spectroscopy. NMR Biomed. 2011;24:1089–1098. doi: 10.1002/nbm.1662. [DOI] [PubMed] [Google Scholar]
- Glass M, Dragunow M, Faull RL. Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience. 1997;77:299–318. doi: 10.1016/s0306-4522(96)00428-9. [DOI] [PubMed] [Google Scholar]
- Gruber SA, Yurgelun-Todd DA. Neuroimaging of marijuana smokers during inhibitory processing: a pilot investigation. Brain Res. Cogn. Brain Res. 2005;23:107–118. doi: 10.1016/j.cogbrainres.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Gruber SA, Rogowska J, Yurgelun-Todd DA. Altered affective response in marijuana smokers: an FMRI study. Drug Alcohol Depend. 2009;105:139–153. doi: 10.1016/j.drugalcdep.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillem E, Pelissolo A, Vorspan F, Bouchez-Arbabzadeh S, Lepine JP. Sociodemographic profiles, addictive and mental comorbidity in cannabis users in an outpatient specific setting. Encephale. 2009;35:226–233. doi: 10.1016/j.encep.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Hajos N, Freund TF. Pharmacological separation of cannabinoid sensitive receptors on hippocampal excitatory and inhibitory fibers. Neuropharmacology. 2002;43:503–510. doi: 10.1016/s0028-3908(02)00157-0. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J. Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann D, Sartorius H, Welzel H, Walter S, Skopp G, Ende G, Mann K. Dorsolateral prefrontal cortex N-acetylaspartate/total creatine (NAA/tCr) loss in male recreational cannabis users. Biol. Psychiatry. 2007;61:1281–1289. doi: 10.1016/j.biopsych.2006.08.027. [DOI] [PubMed] [Google Scholar]
- Hester R, Nestor L, Garavan H. Impaired error awareness and anterior cingulate cortex hypoactivity in chronic cannabis users. Neuropsychopharmacology. 2009;34:2450–2458. doi: 10.1038/npp.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman AF, Oz M, Yang R, Lichtman AH, Lupica CR. Opposing actions of chronic Delta9-tetrahydrocannabinol and cannabinoid antagonists on hippocampal long-term potentiation. Learn. Mem. 2007;14:63–74. doi: 10.1101/lm.439007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR. Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Jager G, Block RI, Luijten M, Ramsey NF. Cannabis use and memory brain function in adolescent boys: a cross-sectional multicenter functional magnetic resonance imaging study. J. Am. Acad. Child Adolesc. Psychiatry. 2010;49:561–572. doi: 10.1016/j.jaac.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen JE, Frederick Bde B, Renshaw PF. Grey and white matter GABA level differences in the human brain using two-dimensional, J-resolved spectroscopic imaging. NMR Biomed. 2005;18:570–576. doi: 10.1002/nbm.994. [DOI] [PubMed] [Google Scholar]
- Johnston L, O’Malley P, Bachman J, Schulenberg J. Monitoring the Future National Results on Adolescent Drug Use: Overview of Key Findings, 2008. National Institute on Drug Abuse; Bethesda, MD: 2009. NIH Publicaiton No. 09-7401. [Google Scholar]
- Kaiser LG, Young K, Matson GB. Elimination of spatial interference in PRESS-localized editing spectroscopy. Magn. Reson. Med. 2007;58:813–818. doi: 10.1002/mrm.21407. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Klose U. In vivo proton spectroscopy in presence of eddy currents. Magn. Reson. Med. 1990;14:26–30. doi: 10.1002/mrm.1910140104. [DOI] [PubMed] [Google Scholar]
- Laaris N, Good CH, Lupica CR. Delta9-tetrahydrocannabinol is a full agonist at CB1 receptors on GABA neuron axon terminals in the hippocampus. Neuropharmacology. 2010;59:121–127. doi: 10.1016/j.neuropharm.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubman DI, Allen NB, Rogers N, Cementon E, Bonomo Y. The impact of co-occurring mood and anxiety disorders among substance-abusing youth. J. Affect. Disord. 2007;103:105–112. doi: 10.1016/j.jad.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Marshall I, Higinbotham J, Bruce S, Freise A. Use of Voigt lineshape for quantification of in vivo 1H spectra. Magn. Reson. Med. 1997;37:651–657. doi: 10.1002/mrm.1910370504. [DOI] [PubMed] [Google Scholar]
- Mason GF, Krystal JH. MR spectroscopy: its potential role for drug development for the treatment of psychiatric diseases. NMR Biomed. 2006;19:690–701. doi: 10.1002/nbm.1080. [DOI] [PubMed] [Google Scholar]
- Mason BJ, Crean R, Goodell V, Light JM, Quello S, Shadan F, Buffkins K, Kyle M, Adusumalli M, Begovic A, Rao A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychopharmacology. 2012;37:1689–1698. doi: 10.1038/npp.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechoulam R, Gaoni Y. A total synthesis of Dl-Delta-1-tetrahydrocannabinol, the active constituent of hashish. J. Am. Chem. Soc. 1965;87:3273–3275. doi: 10.1021/ja01092a065. [DOI] [PubMed] [Google Scholar]
- Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 1998;11:266–272. doi: 10.1002/(sici)1099-1492(199810)11:6<266::aid-nbm530>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Natt O, Bezkorovaynyy V, Michaelis T, Frahm J. Use of phased array coils for a determination of absolute metabolite concentrations. Magn. Reson. Med. 2005;53:3–8. doi: 10.1002/mrm.20337. [DOI] [PubMed] [Google Scholar]
- Northoff G, Walter M, Schulte RF, Beck J, Dydak U, Henning A, Boeker H, Grimm S, Boesiger P. GABA concentrations in the human anterior cingulate cortex predict negative BOLD responses in fMRI. Nat. Neurosci. 2007;10:1515–1517. doi: 10.1038/nn2001. [DOI] [PubMed] [Google Scholar]
- Ogg RJ, Kingsley PB, Taylor JS. WET, a T1- and B1-insensitive water-suppression method for in vivo localized 1H NMR spectroscopy. J. Magn. Reson. B. 1994;104:1–10. doi: 10.1006/jmrb.1994.1048. [DOI] [PubMed] [Google Scholar]
- O’Shea M, Singh ME, McGregor IS, Mallet PE. Chronic cannabinoid exposure produces lasting memory impairment and increased anxiety in adolescent but not adult rats. J. Psychopharmacol. 2004;18:502–508. doi: 10.1177/026988110401800407. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr., Gruber AJ, Hudson JI, Cohane G, Huestis MA, Yurgelun-Todd D. Early-onset cannabis use and cognitive deficits: what is the nature of the association? Drug Alcohol Depend. 2003;69:303–310. doi: 10.1016/s0376-8716(02)00334-4. [DOI] [PubMed] [Google Scholar]
- Prescot AP, Locatelli AE, Renshaw PF, Yurgelun-Todd DA. Neurochemical alterations in adolescent chronic marijuana smokers: a proton MRS study. Neuroimage. 2011;57:69–75. doi: 10.1016/j.neuroimage.2011.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Realini N, Rubino T, Parolaro D. Neurobiological alterations at adult age triggered by adolescent exposure to cannabinoids. Pharmacol. Res. 2009;60:132–138. doi: 10.1016/j.phrs.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Rubino T, Parolaro D. Long lasting consequences of cannabis exposure in adolescence. Mol. Cell. Endocrinol. 2008;286:S108–S113. doi: 10.1016/j.mce.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Sailasuta N, Ernst T, Chang L. Regional variations and the effects of age and gender on Glu concentrations in the human brain. Magn. Reson. Imaging. 2008;26:667–675. doi: 10.1016/j.mri.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Mason GF, Rothman DL, Krystal JH. Increased occipital cortex GABA concentrations in depressed patients after therapy with selective serotonin reuptake inhibitors. Am. J. Psychiatry. 2002;159:663–665. doi: 10.1176/appi.ajp.159.4.663. [DOI] [PubMed] [Google Scholar]
- Schlicker E, Kathmann M. Modulation of transmitter release via presynaptic cannabinoid receptors. Trends Pharmacol. Sci. 2001;22:565–572. doi: 10.1016/s0165-6147(00)01805-8. [DOI] [PubMed] [Google Scholar]
- Schneider M, Koch M. Chronic pubertal, but not adult chronic cannabinoid treatment impairs sensorimotor gating, recognition memory, and the performance in a progressive ratio task in adult rats. Neuropsychopharmacology. 2003;28:1760–1769. doi: 10.1038/sj.npp.1300225. [DOI] [PubMed] [Google Scholar]
- Schulte RF, Boesiger P. ProFit: two-dimensional prior-knowledge fitting of J-resolved spectra. NMR Biomed. 2006;19:255–263. doi: 10.1002/nbm.1026. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, Brown SA, Tapert SF. The influence of marijuana use on neurocognitive functioning in adolescents. Curr. Drug Abuse Rev. 2008;1:99–111. doi: 10.2174/1874473710801010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA. Nicotine and the adolescent brain: insights from an animal model. Neurotoxicol. Teratol. 2002;24:369–384. doi: 10.1016/s0892-0362(02)00199-x. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum. Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl. 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Spear LP. Assessment of adolescent neurotoxicity: rationale and methodological considerations. Neurotoxicol. Teratol. 2007;29:1–9. doi: 10.1016/j.ntt.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem. Biophys. Res. Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- Stiglick A, Kalant H. Residual effects of chronic cannabis treatment on behavior in mature rats. Psychopharmacology (Berl.) 1985;85:436–439. doi: 10.1007/BF00429660. [DOI] [PubMed] [Google Scholar]
- Taylor MJ, Norbury R, Murphy S, Rudebeck S, Jezzard P, Cowen PJ. Lack of effect of citalopram on magnetic resonance spectroscopy measures of Glu and glutamine in frontal cortex of healthy volunteers. J. Psychopharmacol. 2010;24:1217–1221. doi: 10.1177/0269881109105679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endocannabinoid signaling in the brain. Science. 2002;296:678–682. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans. Med. Imag. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]