Abstract
The effective treatment of depression has been reported to reduce the severity of alcohol use, potentially reflecting improvements in common brain reward circuits. We hypothesized that augmentation therapy of escitalopram with aripiprazole would improve depressive symptoms as well as reduce craving for alcohol and cue-induced brain activity in patients with co-morbid alcohol dependence and major depressive disorder, compared with treatment with escitalopram alone. Thirty-five subjects with major depressive disorder and alcohol dependence were recruited and randomly assigned into 17 aripiprazole + escitalopram and 18 escitalopram only groups. At baseline and following six weeks of treatment, symptoms of depression, craving for alcohol and brain activity were evaluated. During the six week treatment period, Beck Depression Inventory and clinical global index-severity (CGI-S) scores decreased in both the aripiprazole + escitalopram and escitalopram only groups. In addition, following the treatment period, the Korean alcohol urge questionnaire scores in the aripiprazole + escitalopram group were reduced from 23.3±8.4 to 14.3±4.9, compared with those of the escitalopram group of from 21.6±8.4 to 19.3±7.1 (F=13.1, p<0.01). The activity within the anterior cingulate was increased in response to the presentation of alcohol drinking scenes following treatment in the aripiprazole + escitalopram group. The change of brain activity within the left anterior cingulate gyrus in all patients with co-morbid alcohol dependence and major depressive disorder was negatively correlated with the change in craving for alcohol. These findings suggest that the effects of aripiprazole on anterior cingulate cortex might mediate the successful treatment of alcohol dependence in patients with major depressive disorder.
Keywords: Aripiprazole, escitalopram, major depressive disorder, alcohol dependence, functional magnetic resonance imaging, craving
Introduction
Alcohol dependence and major depressive disorder
Major depressive disorder (MDD) and substance dependence are prevalent and costly disorders that are frequently co-morbid (Kessler et al., 1997; Regier et al., 1990). Treatment outcomes in patients who are dually diagnosed with MDD and alcohol use disorders are worse than in patients with MDD or alcohol dependence only (Lynskey, 1998; Ostacher, 2007). In a meta-analysis of treatment for depression in patients with alcohol dependence, Ostacher (2007) suggested that effective treatment of depression might also reduce the severity of alcohol use, as successful treatment of co-morbid MDD and alcohol dependence may reflect improvements in common brain reward circuits (Baumann et al., 2000; Comings and Blum, 2000; Stein, 2008; Tremblay et al., 2005) and neurotransmitter function (Berman et al., 2009; Huang et al., 2006; Paterson and Markou, 2007). In 102 patients with alcohol dependence, anhedonia can be commonly observed in alcohol withdrawal (Martinotti et al., 2008).
Corticostriatal–limbic circuitry in alcohol dependence and MDD
The balance within corticostriatal–limbic circuitry, including prefrontal cortex, anterior cingulate, hippocampus, amygdala and striatum, is thought to be important in contributing to the pathophysiology of patients with co-occurring MDD and alcohol dependence (Stein, 2008). Reduced rates of serotonin synthesis have been suggested to trigger depression (Rosa-Neto et al., 2004) and disrupted striatal dopamine in patients with MDD has been reported to increase the salience of mild negative stimuli (Nestler and Carlezon, 2006), which contributes to recurrent depressive episodes (Frank and Thase, 1999). Alcohol dependence has been associated with reward deficiency, which is a risk factor for abuse and addiction (Comings and Blum, 2000). Interestingly, Tremblay et al. (2005) have suggested that patients with MDD might also have deficits in reward circuitry in the prefrontal cortex with evidence of altered brain activation in the ventrolateral prefrontal and orbitofrontal cortices in response to dextroamphetamine administration. Recent neuroimaging studies have indicated that disruption of corticostriatal–limbic circuitry may be centrally associated with alcohol cue-related craving (Filbey et al., 2008; Lopez et al., 1999). Filbey et al. (2008) reported that the mesocorticolimbic structures, including orbitofrontal cortex, striatum and ventral tegmental area, were positively correlated with craving for alcohol. In response to alcohol drinking cues, patients with alcohol dependence showed increased brain activity in prefrontal cortex and anterior limbic regions compared with social drinkers (Myrick et al., 2004). Grusser et al. (2004) have suggested that anterior cingulate, medial prefrontal cortex and striatum were closely associated with the motivational value and attentional processing of alcohol cues.
In the pathogenesis of alcohol dependence, dysfunction of the dopamine and serotonin systems is also well established (Ross and Peselow, 2009). Dopamine is thought to play a crucial role in initiation and reinstatement in addiction (Baler and Volkow, 2006; Kalivas and Volkow, 2005). Initiation has been associated with increased dopamine levels in the striatum (Baler and Volkow, 2006). Reinstatement, including compulsiveness and unrestrained use, has been associated with the tract from the ventral tegmental area to the prefrontal cortex (Kalivas and Volkow, 2005). The association between decreased serotonin level and alcohol preference has consistently been reported in animal and human studies (Katner and Weiss, 2001; Mantere et al., 2002; Nishikawa et al., 2009; Smith and Weiss, 1999; Storvik et al., 2006, 2007). In rodent studies, ethanol exposure has been reported to decrease brain serotonin levels and serotonin neurons in alcohol-preferring rodents (Katner and Weiss, 2001; Smith and Weiss, 1999). In post-mortem studies, patients with alcohol dependence showed decreased serotonin transporter levels in hippocampus, anterior cingulate, striatum, amygdala and hypothalamus (Mantere et al., 2002; Storvik et al., 2006, 2007). Using positron emission tomography, Nishikawa et al. (2009) noted that serotonin synthesis was lower in the bilateral orbitofrontal cortices of patients with alcohol dependence compared with healthy control subjects.
Aripiprazole
Aripiprazole is a partial agonist at both presynaptic autoreceptor and postsynaptic dopamine D2 receptors, as well as at 5-HT1A receptors, and an antagonist at 5-HT2A receptors. There have been three large studies documenting the therapeutic effect of aripiprazole for the treatment of depression (Berman et al., 2007, 2009; Marcus et al., 2008). A first multicenter, randomized, double-blind, placebo-controlled study of 178 patients with MDD who showed incomplete response to one prospective and one to three past treatments within the current episode showed that adjunctive aripiprazole treatment was efficacious and safe in patients with MDD (Berman et al., 2007). A second multicenter, randomized, double-blind, placebo-controlled study of 190 patients with MDD who did not respond to at least one and up to three past and one possibly further prospective antidepressant therapy (Marcus et al., 2008) showed that adjunctive aripiprazole was more efficacious and better tolerated compared with adjunctive placebo. In a similar study, Berman et al. (2009) have also reported that aripiprazole augmentation of antidepressants was efficacious and well-tolerated in patients with MDD who do not respond adequately to standard antidepressant monotherapy. Recent studies have suggested that the putative activity of aripiprazole on frontal-subcortical circuits might also be associated with successful treatment of alcohol dependence (Martinotti et al., 2007, 2009b; Vergne and Anton, 2010). Martinotti et al. (2009b) observed that six of 13 detoxified patients with alcohol dependence remained alcohol free state during a 16-week aripiprazole monotherapy period. In a double-blind monotherapy comparison between aripiprazole and naltrexone in patients with alcohol dependence, aripiprazole was as effective at reducing alcohol use and craving for alcohol as naltrexone (Martinotti et al., 2009b),
Hypothesis
Based on the shared role of corticostriatal circuitry and the pharmacodynamic properties of aripiprazole, we hypothesized that augmentation therapy of escitalopram with aripiprazole would improve depressive symptoms as well as reduce craving for alcohol and cue-induced brain activity in patients with alcohol dependence compared with treatment with escitalopram alone. In addition, we expected that the effective treatment of depression and alcohol dependence would be associated with increased activity of the brain areas in corticostriatal circuitry in response to alcohol drinking cues.
Method
Subjects
Among patients who were evaluated by the Department of Psychiatry of Chung Ang University Medical Center and Eunpyeong Hospital for co-morbid alcohol problems and MDD, 35 subjects agreed to participate in this research study. Before and after detoxification, a psychiatrist (DHH) assessed and diagnosed patients as having co-morbid MDD and alcohol dependence based on the Structured Clinical Interview for DSM-IV. The inclusion criteria include: (1) first onset comorbid major depression and alcohol dependence or recurrent psychotropic medication naïve patients with MDD and alcohol dependence; (2) Michigan alcohol screening test (MAST) score >19 for alcohol problems; (3) Beck Depression Inventory (BDI) (Beck et al., 1961) > 19; (4) impaired behaviors or distress due to maladaptive patterns which are consistent with DSM-IV criteria for MDD. Exclusion criteria include: (1) patients with history or current episode of other Axis I psychiatric diseases; (2) patients with other substance abuse history (except for tobacco); (3) patients with medical illness; (4) patients with claustrophobia. The inclusion criteria for healthy control subjects include: (1) no history of present psychiatric disorders including mood changes; (2) MAST <19; (3) BDI < 10; (4) no history of trauma; (5) no history of drug or alcohol abuse/dependence. The Chung Ang University Hospital Institutional Review Board and the national regulatory authorities in accordance with local requirements approved the research protocol for this study. The current study was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki (1964) and subsequent revision. Before starting detoxification, written informed consent was provided by all participants.
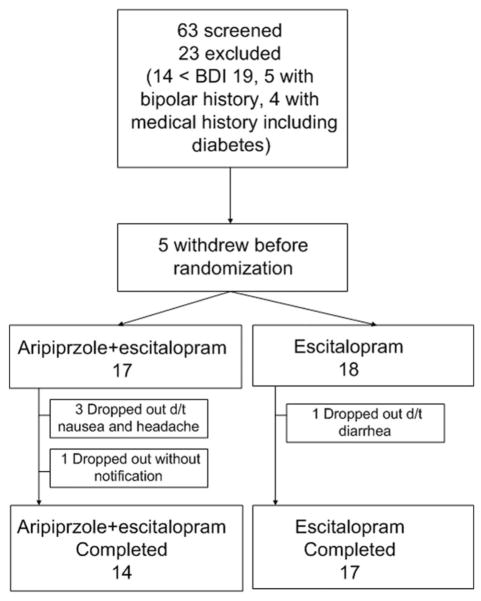
Among 40 patients with MDD and alcohol dependence, five patients who could not complete detoxification were excluded from enrollment before randomization. There were no changes in BDI (z=0.52, p=0.6) and MAST (z= 1.7, p=0.9) scores. Thirty-five subjects were randomly assigned to receive either aripiprazole + escitalopram or escitalopram only in a 1:1 ratio. Seventeen patients treated with aripiprazole + escitalopram and 18 patients treated with escitalopram only entered into a six-week treatment period (Figure 1). Three subjects in the aripiprazole + escitalopram group discontinued treatment due to nausea and headache. One subject in the aripiprazole + escitalopram group did not respond to follow-up without any notification. One subject in the escitalopram group stopped treatment due to diarrhea. Finally, 31 subjects (14 aripiprazole + escitalopram and 17 escitalopram) completed the protocol.
Figure 1.
Consort diagram of recruitment.
BDI: Beck Depression Inventory scale score; d/t: due to
Study procedure
Over a period of 5–10 days, all subjects were detoxified with lorazepam (1–4 mg/day), thiamine (100mg/day orally) and multiple vitamin (containing folate) injection, used according to validated protocols (Asplund et al., 2004; Lejoyeux et al., 1998). After this detoxification period, patients with co-morbid alcohol dependence and MDD were asked to assess baseline clinical scales and started treatment with either aripiprazole + escitalopram or escitalopram only. The subjects in aripiprazole + escitalopram group were asked to take a flexible dose of aripiprazole (Abilify™, Otsuka, Korea) 5–15mg (Janiri et al., 2007) and escitalopram (Lexapro®, Lundbeck, Korea) 10–20mg daily for six weeks. The subjects in the escitalopram group were asked to take only escitalopram 10–20mg daily for six weeks. The treatment period was decided as six weeks in accordance with previous studies of antidepressants and aripiprazole adjuvant treatment (Taneja et al., 2012; Weisler et al., 2011). Aripiprazole was started at 5 mg/day during the first week and then increased to 15 mg/day thereafter. Escitalopram was started at 10 mg/day during the first week and then increased to 20 mg/day thereafter. Three-session education regarding the nature and health consequences of alcohol dependence (conducted by a doctor and social worker) and three-session individual supportive psychotherapy were provided to all patients during the study period. Lorazepam, zolpidem and propranolol as necessary were used for managing tremor, anxiety and insomnia. Anti-craving medications for alcohol such as acamprosate and naltrexone were not used in this study.
At baseline and following six weeks of treatment, symptoms of depression, craving for alcohol and alcohol use were evaluated using the BDI (Beck et al., 1961), the Korean alcohol urge questionnaire (AUQ-K) and outcome measure questionnaires, respectively. The use of alcohol was verified based on the reports of patients and family members as well as assessment of alcohol hepatic indices aspartate aminotransferase (AST), alanine aminotransferase (ALT) and γ-glutamyl transpeptidase (GGT). In the case of disagreement between patients and family members, the reports of the family members were adopted.
Definitions of outcomes are defined as: (1) relapse into alcohol dependence was defined as either five or more standard drinks (standard dosage = 50mg/day) on a drinking occasion or drinking on more than five days per week (Snyder and Bowers, 2008) and (2) response to antidepressant treatment was defined as reduction in follow-up BDI scores to less than 50% of initial BDI scores. Alcohol drinking behavior was checked at two, four and six weeks following detoxification.
Assessment of brain activity and craving for internet video game play
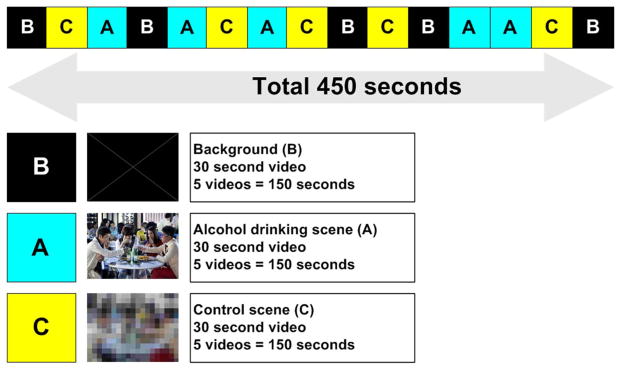
At baseline and following six weeks of treatment, brain activity in response to alcohol drinking cue presentation was assessed by 1.5 Tesla functional magnetic resonance imaging (fMRI). All MR imaging was performed on a 1.5 Tesla Espree MRI scanner (SIEMENS, Erlangen, Germany). The silent 450 s videotape consisted of five continuous 90 s segments. Each 90 s segment consisted of three 30 s sub-segments. A white cross on a black background (B), a control (C, mosaic modification of the alcohol drinking video) and the alcohol drinking video (A) were included in these 90-second segments. The alcohol drinking scene consisted of a video showing several people encouraging alcohol consumption in a bar. The mosaic control scene is a mosaic modified alcohol drinking video which was originally identical to the video presented in the alcohol drinking stimulation (A) (Ko et al., 2009). The five segments were ordered as follows: B-C-A, B-AC, A-C-B, C-B-A and A-C-B (Figure 2). This video was presented using an IFIS-SA™ system (MRI Device Corporation, Waukesha, WI, USA) during a single fMRI scanning session. For the fMRI session, gradient-recalled echo planar images (EPIs) (37 transverse slices, 5.0 mm thickness, a voxel size of 3.5 mm × 3.5 mm × 5.0 mm, TE=30ms, TR=3000ms, in-plane resolution=64×64 pixels, field of view (FOV)=230 mm × 230 mm) were recorded at 3-s intervals. For anatomical imaging, 3D T1-weighted magnetization-prepared rapid gradient echo (MPRAGE) data were collected with the following parameters: TR=1500 ms, TE=3.00 ms, FOV= 256 mm × 256 mm, 128 slices, 1.0 mm × 1.0 mm × 1.33 mm voxel size.
Figure 2.
The design of the fMRI block paradigm.
fMRI data analysis
Functional images were assessed using Brain Voyager software (BVQX 1.9, Brain Innovation, Maastricht, The Netherlands). Data analysis methods have been detailed in a previous publication (Han et al., 2011). Briefly, the fMRI time series data was co-registered to the anatomical 3D data sets for each subject using the multi-scale algorithm provided. Individual 3D structural images were spatially normalized to standard Talairach space (Talairach and Tournoux, 1988). A nonlinear transformation was subsequently applied to the T2*-weighted fMRI time series data. Slice scan time and 3D motion correction were applied and the functional data were spatially smoothed using a Gaussian kernel with a FWHM of 6mm and temporally smoothed using a Gaussian kernel of 4 s.
Statistical analysis
The analyses were only performed in completers. Between-group differences in terms of age, education, alcohol and smoking habits were analyzed with ANOVA or the chi-square test. Changes in depressive symptoms and craving for alcohol between baseline and week 6 were analyzed using a Wilcoxon’s signed rank test. Changes in alcohol use between baseline and week 6 were analyzed using a chi-square test. As a covariate of baseline BDI score, dependent variable for response and independent variable for medication groups, logistic regression analysis was performed for response rates. Controlling for the change of BDI scores, changes in AUQ-K scores between baseline and week 6 were also analyzed with repeated measures ANCOVA. Correlation between depressive symptoms and the craving for alcohol were analyzed with Pearson correlations. For all statistical analyses, the α level for significance was set at 0.05 and all analyses were performed using Statistica 6.0.
The general linear model (GLM) and random effects analysis (RFX) were applied to analyze the fMRI signal time-courses on a voxel by voxel basis and to generate individual and group statistical parametric maps of brain activation. For all analyses, we regarded the associations as significant when the False Discovery Rate (FDR) was less than or equal to 0.05 in 100 adjacent voxels. As a second-level analysis in the aripiprazole + escitalopram and escitalopram only groups, the changes in craving for alcohol, mood and the activity of clusters during the six weeks of treatment were analyzed by repeated measures ANOVA. Spearman correlations were used to evaluate relationships between the change of mean β value in clusters, craving for alcohol and mood.
Results
Clinical characteristics
There were no significant differences in terms of age, sex, education years and smoking habits between patients treated with aripiprazole + escitalopram, patients treated with escitalopram only and healthy control subjects (Table 1). There were statistically significant baseline differences in BDI, clinical global index-severity (CGI-S), MAST scores and AUQ-K scores between these three groups. However, there were no significant differences in BDI, CGI-S, MAST, and AUQ-K scores between the aripiprazole + escitalopram and escitalopram only groups at baseline (Table 1). The BDI scores were positively correlated with AUQ-K scores in both patient groups at baseline (r=0.43, p=0.02). The AUQ-K scores were positively correlated with MAST scores in both patient groups at baseline (r=0.66, p<0.01). Comparing alcohol hepatic indices before and after medication treatment, there were significant decreases in AST (<0.01), ALT (<0.01) and GGT (<0.01) in both groups.
Table 1.
Demographic characteristics.
| APZ + Esctp (17)
|
Esctp only (18)
|
Healthy controls (15) | Statistics | ||||
|---|---|---|---|---|---|---|---|
| Entire (N=17) | Completers (N=14) | Entire (N=18) | Completers (N=17) | ||||
| Age (years) | 39.1±8.8 | 39.6±8.4 | 40.0±6.4 | 41.6±5.9 | 42.6±8.2 | F=0.59, p=0.55 | F=1.18, p=0.32 |
| Sex (male/female) | 10/7 | 9/5 | 13/5 | 12/5 | 15/4 | χ2=1.7, p=0.41 | χ2=0.89, p=0.64 |
| Education (years) | 11.7±1.6 | 11.2±2.0 | 11.6±3.1 | 11.9±3.1 | 12.1±3.4 | F=0.89, p=0.41 | F=0.34, p=0.71 |
| Smoking (non-smoker/smoker) | 9/8 | 8/6 | 10/8 | 9/8 | 10/5 | χ2=0.6, p=0.71 | χ2=0.64, p=0.73 |
| Medication (mg) | |||||||
| Aripiprazole | 7.0±3.5 | 7.1±3.6 | – | – | – | – | – |
| Escitalopram | 13.2±6.1 | 13.2±6.4 | 14.0±7.2 | 14.1±7.3 | – | – | – |
| Lorazepam | 1.4±0.8 | 1.4±0.6 | 1.5±0.6 | 1.5±0.7 | – | – | – |
| BDI baseline | 32.0±13.1 | 32.1±13.1 | 29.5±10.0 | 29.6±9.9 | 2.9±2.3 | F=36.1, p<0.01* | F=32.8, p<0.01* |
| Six weeks | 16.0±14.9 | 16.9±8.9 | |||||
| CGI-S baseline | 4.5±0.7 | 4.6±0.8 | 4.2±0.8 | 4.2±0.7 | 1.1±0.3 | F=105.6, p<0.01* | F=99.8, p<0.01* |
| Six weeks | 2.7±1.1 | 2.8±0.8 | |||||
| MAST | 27.2±12.0 | 25.6±10.4 | 25.6±13.5 | 26.9±14.5 | 7.4±4.4 | F=11.4, p<0.01* | F=13.6, p<0.01* |
| AUQ-K baseline | 23.3±8.2 | 23.3±8.3 | 21.6±7.1 | 21.6±7.1 | 6.2±2.7 | F=19.8, p<0.01* | F=24.6, p<0.01* |
| Six weeks | 14.8±6.1 | 18.2±7.2 | |||||
Post hoc test*: aripiprazole (APZ) + escitalopram (Esctp) = escitalopram only ≫ healthy controls.
During the six week treatment period: BDI scores, CGI-S (z=2.4, p=0.01) and scores (z=3.3, p<0.01) in aripiprazole + escitalopram group, BDI (z=3.4, p<0.01) and CGI-S (z=3.5, p<0.01) in escitalopram only group. During the six week treatment period the AUQ-K scores (z=2.3, p=0.02) in the aripiprazole + escitalopram, the AUQ-K scores (z=1.0, p=0.33) in the escitalopram only group.
BDI: Beck Depression Inventory; CGI-S: clinical global index-severity; MAST: Michigan alcohol screening test; AUQ-K: Korean alcohol urge questionnaire.
Change of AUQ-K and BDI scores between the aripiprazole + escitalopram and escitalopram only groups during the six week treatment period
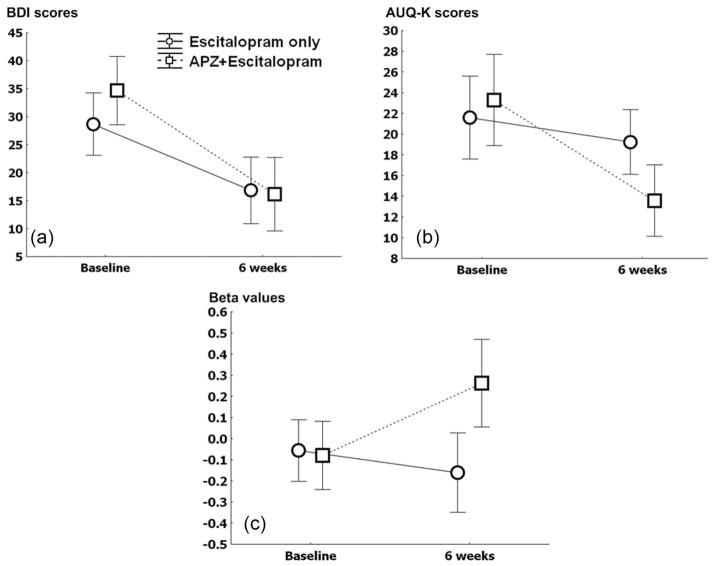
During the six week treatment period, the BDI scores and CGI-S scores decreased in both the aripiprazole + escitalopram (BDI: baseline (B): 32.1±13.1, six weeks (6 wk): 16.0±14.9, z=2.4, p=0.01; CGI-S: B: 4.6±0.8, 6 wk: 2.7±1.1, z=3.3, p<0.01) and escitalopram only groups (BDI: B: 29.6±2.3, z=3.4, p<0.01, 6 wk: 16.9±8.9; CGI-S: B: 4.2±0.7, 6 wk: 2.8±0.8, z=3.5, p<0.01) (Figure 3). However, there were no significant differences in the number of patients responding to depression treatment between the aripiprazole + escitalopram (responder 10 and non-responder four) and escitalopram only (responder 11 and non-responder six) groups (β =0.27, SEM=0.17, t=1.5, p=0.15). The AUQ-K scores were decreased in the aripiprazole + escitalopram group (B: 23.3±8.3, 6 wk: 14.8±6.1, z=2.3, p=0.02). However, the AUQ-K scores in the escitalopram only group were not changed (B: 21.6±7.1, 6 wk: 18.2±7.2, z=1.0, p=0.33). There were no significant differences in the number of patients who remained alcohol free between aripiprazole + escitalopram (abstinent 15 and relapse two) and escitalopram only (abstinent 14 and relapse four) groups (χ2=0.68, p=0.66).
Figure 3.
The changes in BDI scores, AUQ-K scores and beta values during six weeks. (a) The changes in Beck Depression Inventory (BDI) scores between the aripiprazole (APZ) + escitalopram and escitalopram only groups, repeated measures ANOVA, F=2.3, p=0.13. (b) The changes in Korean alcohol urge questionnaire (AUQ-K) scores between the aripiprazole + escitalopram and escitalopram only groups, repeated measures ANOVA, F=4.9, p=0.03. (c) The changes in beta values of left anterior cingulate gyrus between the aripiprazole + escitalopram and escitalopram only groups, repeated measures ANOVA, F=6.3, p=0.02.
During the medication period, AUQ-K scores in the aripiprazole + escitalopram group were reduced compared with those of the escitalopram group (F=4.9, p=0.03) (Figure 3). The BDI scores (F=2.3, p=0.13) and CGI-S scores (F=1.1, p=0.30) in the aripiprazole + escitalopram group were reduced compared with those of the escitalopram group at a weak trend level. There was a marginally significant correlation between the changes in AUQ-K scores and BDI scores (r=0.35, p=0.051) in all patients with co-morbid alcohol dependence and depressive symptoms.
In both patient groups, subjects who remained alcohol free for the six week treatment period did not differ in terms of the response to depression treatment within aripiprazole + escitalopram (responder eight and non-responder four) and escitalopram only (responder nine and non-responder six) groups (χ2=0.13, p=0.72). The AUQ-K scores were decreased in both the aripiprazole + escitalopram (F=29.3, p<0.01) and escitalopram (F=4.8, p=0.04) groups. However, the AUQ-K scores in the aripiprazole + escitalopram group decreased at a trend level compared with those observed in the escitalopram group (F=3.22, p=0.08).
In the last observation carried forward (LOCF) population, two subjects treated with aripiprazole + escitalopram for seven days and who did not complete the study showed no significant change in BDI (z=0.71, p=0.48), CGI-S (z=0.42, p=0.82) or AUQ-K scores (z=0.41, p=0.47). One subject treated with aripiprazole + escitalopram did not return after baseline assessment. One subject treated with escitalopram for 14 days and who did not complete the study showed decreased BDI (from 45 to 35) and CGI-S (from 4 to 3) scores. However, there was no change in AUQ-K scores.
Brain activity in response to alcohol drinking scene at baseline
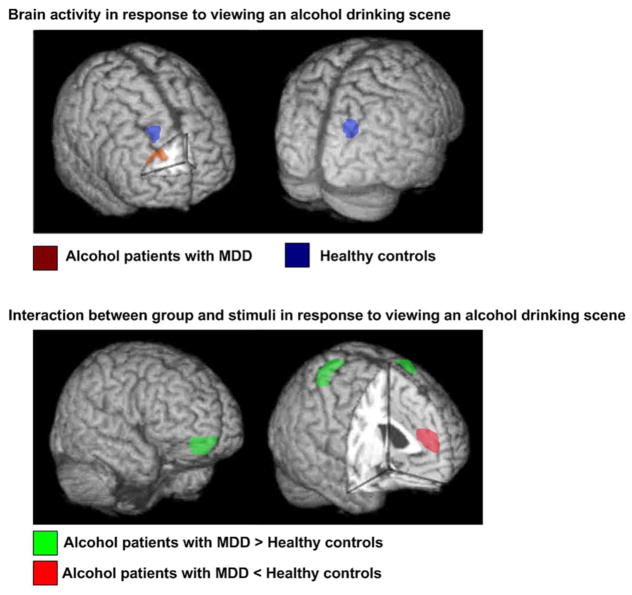
In response to viewing alcohol drinking scenes in the mosaicalcohol drinking scene contrast, patients with co-morbid alcohol dependence and MDD showed one significant cluster (CL) including the right middle frontal gyrus (CL1, BA10) at FDR <0.05, p=0.002 value (Figure 4 and Table 2).
Figure 4.
Brain areas in response to alcohol drinking scene stimuli.
Table 2.
Brain areas in response to alcohol drinking scene stimuli at baseline.
| CLs | Coordinate
|
No. of voxels | p value | Brain areas | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Brain activity in response to alcohol drinking scene in patients with co-morbid alcohol dependence and MDD | ||||||
| 1 | 27 | 45 | 10 | 40 | pFDR<0.05=0.002 | Right middle frontal gyrus, BA 10 |
| Brain activity in response to alcohol drinking scene in healthy comparison subjects | ||||||
| 2 | 23 | 48 | 30 | 40 | puncorrected<0.001 | Right superior frontal gyrus, BA 9 |
| 3 | −38 | −77 | 33 | 65 | puncorrected<0.001 | Left superior occipital gyrus, BA 19 |
| Interaction between group (patients > HC) and stimuli (alcohol versus mosaic) in response to alcohol drinking scene | ||||||
| 4 | 2 | 46 | −6 | 200 | pFDR<0.05=0.004 | Right medial frontal gyrus, BA 10 |
| 5 | 14 | −17 | 62 | 250 | pFDR<0.05=0.004 | Right frontal, precuneus BA 6 |
| 6 | −15 | −60 | 0 | 150 | pFDR<0.05=0.004 | Left occipital lingual gyrus BA 19 |
| 7 | −14 | −60 | 48 | 500 | pFDR<0.05=0.004 | Left parietal, precuneus BA 7 |
| Interaction between group (patients < HC) and stimuli (alcohol versus mosaic) in response to alcohol drinking scene | ||||||
| 8 | −9 | 36 | 23 | 250 | pFDR<0.01=0.0002 | Left anterior cingulate gyrus, BA 32 |
CL: cluster; MDD: major depressive disorder, HC: healthy controls; BA: Brodmann area.
In response to viewing alcohol drinking scenes in the mosaicalcohol drinking scene contrast, healthy control subjects showed no significant clusters at FDR <0.05 value. However, two clusters of activity in healthy control subjects were identified at uncorrected p <0.001 value: right superior frontal gyrus (CL2, Brodmann area (BA) 9) and left parietal precuneus (CL3, BA 19) (Figure 4).
Interaction between group (patients versus HC) and stimuli (alcohol versus mosaic) at baseline
On an interaction between group (patients co-morbid for alcohol dependence and MDD > healthy comparison subjects) and stimuli (alcohol versus mosaic) at baseline, four clusters of activity were identified at FDR<0.05, p=0.004 value; right medial frontal gyrus (CL4, BA 10), right frontal, precuneus (CL5, BA 6), left occipital lingual gyrus (CL6, BA19) and left parietal, precuneus (CL7, BA 7) (Figure 4).
On an interaction between group (patients co-morbid for alcohol dependence and MDD < healthy comparison subjects) and stimuli (alcohol versus mosaic) at baseline, one cluster of activity was identified at FDR<0.01, p=0.0002 value; left anterior cingulate gyrus (CL8, BA 32) (Figure 4).
The mean β value of left anterior cingulate gyrus (CL8) in all patients with co-morbid alcohol dependence and MDD was negatively correlated with BDI scores (r= −0.48, p<0.01), MAST scores (r= −0.49, p<0.01), and AUQ-K scores (r= −0.56, p<0.01). The mean β value of right medial frontal gyrus (CL4) in both patient groups was positively correlated with BDI scores (r=0.78, p<0.01). There was no correlation between other clusters, BDI, MAST and AUQ-K scores.
Changes in brain activity during six week therapy in patients with co-morbid alcohol dependence and MDD
During treatment, the mean β values within the left anterior cingulate gyrus (CL8) in the aripiprazole + escitalopram group in response to the alcohol drinking scenes increased compared with values observed in the escitalopram only group (F=6.3, p=0.02) (Figure 3). However, there was no significant difference in the change of mean β values for the right medial frontal gyrus (CL4) in response to viewing alcohol drinking scenes between the aripiprazole + escitalopram and escitalopram only groups. The change of mean β value for left anterior cingulate gyrus (CL8) in all patients with co-morbid alcohol dependence and MDD was negatively correlated with the change of AUQ-K scores (r= −0.65, p<0.01).
Discussion
During the six week treatment period, depressive symptoms and CGI-S scores were significantly reduced in both the aripiprazole + escitalopram and escitalopram only groups. However, there was no significant difference in the response rate between the two groups. There was also no difference in the amount of alcohol use between the two groups. Craving for alcohol was decreased in the aripiprazole + escitalopram group compared with the escitalopram only group. The activity within the anterior cingulate was increased in response to the presentation of alcohol drinking scenes following treatment in the aripiprazole + escitalopram group. The change of brain activity within the left anterior cingulate gyrus in all patients with co-morbid alcohol dependence and MDD was negatively correlated with the change in craving for alcohol.
Changes in clinical symptoms
There have been several reports that the augmentation of selective serotonin reuptake inhibitors (SSRIs) with antipsychotics is effective for the treatment of refractory MDD (Nelson et al., 2010; Sheffrin et al., 2009). Nelson et al. (2010) reported that aripiprazole augmentation of standard antidepressant treatment was more effective in reducing the core symptoms of depression including mood, anxiety and insomnia. Moreover, aripiprazole has been reported to result in a lower burden of adverse effects relative to other antipsychotics including amisulpride, aripiprazole, clozapine, olanzapine, quetiapine, risperidone, sertindole, ziprasidone and zotepine (Bersani et al., 2005; Vohora, 2007). In older depressive patients (mean age=73.9 years) with incomplete response to SSRIs or serotonin and norepinephrine reuptake inhibitor (SNRI) treatment, aripiprazole was reported to be effective in improving depressive symptoms (Sheffrin et al., 2009).
In the present study, aripiprazole + escitalopram treatment reduced craving for alcohol in patients with co-morbid alcohol dependence and MDD compared with escitalopram only treatment. The effectiveness of aripiprazole for the treatment of alcohol dependence has been previously reported (Janiri et al., 2007; Voronin et al., 2008). In a 16 week follow-up study, Janiri et al. (2007) reported that 46% of alcohol dependent patients with aripiprazole treatment remained abstinent and showed reduced craving for alcohol. Voronin et al. (2008) also found that aripiprazole reduced craving and alcohol consumption and improved impulse control.
However, the present results did not show aripiprazole + escitalopram to be more effective in improving depressive symptoms compared with escitalopram only treatment. In addition, there was no difference between groups in the amount of alcohol use during the treatment period. The different results between previous studies and ours may be due to: 1) the small number of subjects, 2) co-morbidity of alcohol dependence, and 3) the definition of subjects and short observation period. Nelson et al. (2010) observed 373 patients with refractory MDD for six weeks and Sheffrin et al. (2009) observed 24 patients with refractory MDD for 12 weeks, while we assessed 35 first onset or drug naïve patients with co-morbid alcohol dependence and MDD for six weeks.
Changes in brain activity
Compared with healthy subjects, patients with co-morbid alcohol dependence and MDD showed decreased activity in the anterior cingulate cortex in response to alcohol drinking scenes. In addition, the activity of anterior cingulate in the aripiprazole + escitalopram group increased compared with the escitalopram only group following the six week treatment period. The change in activity of the left anterior cingulate gyrus in all patients with co-morbid alcohol dependence and MDD was negatively correlated with the change in craving for alcohol.
The anterior cingulate is regarded as an important hub mediating depressive symptoms and the experience of negative mood (Mayberg, 2003). In addition, decreased metabolism within the dorsal frontal and anterior cingulate cortices has been associated with disruption of attention and concentration in patients with MDD (Liotti and Mayberg, 2001). There have been several studies that have evaluated the activity of the anterior cingulate in patients with MDD using various methods (Kennedy et al., 2001; Mayberg et al., 2005). In a positron emission tomography with F-18 Fluorodeoxyglucose positron emission tomography study, Kennedy et al. (2001) noted increased glucose metabolism in the prefrontal cortex including the dorsal anterior cingulate following six weeks of paroxetine treatment. Mayberg et al. (2005) reported that chronic deep brain stimulation of the subgenual anterior cingulate improved depressive symptoms in patients with treatment-resistant depression (Mayberg et al., 2005). In response to the presentation of alcohol related words, young women (18–24 years old) were reported to demonstrate increased brain activity in subcallosal, anterior cingulate, left prefrontal and bilateral insular regions (Tapert et al., 2004). In response to alcohol beverage pictures, brain activity in the nucleus accumbens, anterior cingulate and left orbitofrontal cortex was positively correlated with craving for alcohol in patients with alcohol dependence (Myrick et al., 2004). In the relapse of alcohol dependent patients, atrophy of the anterior cingulate was reported comparing healthy control subjects with alcohol patients who remain abstinent (Beck et al., 2012). The decreased activity of anterior cingulate in response to alcohol cues in our study was different from the results of Myrick et al. (2004) and Beck et al. (2012) (increased activity of anterior cingulate in response to alcohol cues). We think that these differences may be due to the co-morbidity of major depression as the studies of Liotti and Mayberg (2001), Kennedy et al. (2001) and Mayberg et al. (2003) have consistently reported decreased activity and metabolism in the anterior cingulate of patients with MDD.
In a review of novel mechanisms of aripiprazole in the treatment of alcohol dependence, aripiprazole was suggested to target frontosubcortical circuits which have been associated with dys-regulation of reward and impulsivity in patients with alcohol dependence (Vergne and Anton, 2010). Voronin et al. (2008) suggested that aripiprazole would improve impulse control (self-control for alcohol) by enhancing the function of the frontal cortex in patients with alcohol dependence. Schlagenhauf et al. (2010) have already reported that aripiprazole activated the anterior cingulate in patients with schizophrenia. During a working memory task in a BOLD fMRI study, hypoactivation in the dorsal anterior cingulate of schizophrenic patients with conventional antipsychotics was observed to normalize after a switch to aripiprazole (Schlagenhauf et al., 2010). In an animal model, aripiprazole was reported to release dopamine in the medial prefrontal cortex (Li et al., 2004). The dopamine in the frontal cortex is thought to modulate BOLD responses during performance of working memory tasks (Dixon et al., 2005).
Taken together, these findings suggest that dopamine release induced by aripiprazole might be associated with increased activation of the anterior cingulate, which may control craving for alcohol during alcohol-cue stimulation in patients with MDD.
Limitations
There are several limitations in the present study. First, the small number of subjects and the relatively short treatment period may not fully reflect the effects of medication. The short research period may not be long enough for observing the relapse into excessive drinking. Although there were differences in the changes of BDI and AUQ-K scores between the aripiprazole + escitalopram and escitalopram groups, the numbers of responders and relapsers were not different between treatment groups. Further, the co-morbidity of alcohol dependence could affect possible changes in depressive symptoms. Second, because the current research is focused on the neuroimaging correlates of treatment, the changes of clinical variables were not systemically noted over the period of treatment. Finally, we did not include patients with multiple substance dependence. Future studies should assess the clinical and brain characteristics of patients with multiple substance dependence. The characteristics of polyabuse have been reported to be different from those of single substance dependence in terms of social factors, childhood trauma, personality, suicidal behavior and comorbid Axis I diagnosis (Martinotti et al., 2009a).
Conclusion
In this pilot study, adjunctive aripiprazole treatment reduced craving for alcohol in MDD patients with alcohol dependence and increased brain activity in the anterior cingulate. Increased activity in the anterior cingulate was associated with decreased craving. These results suggest that adjunctive aripiprazole treatment may be a useful intervention in MDD patients with alcohol dependence and that the anterior cingulate may play a role in mediating these effects.
Acknowledgments
Funding
This research was supported by Korea Otsuka Pharmaceuticals (K-O-2010-3).
Footnotes
Conflict of interest
The authors declare that there are no conflict of interest.
References
- Asplund CA, Aaronson JW, Aaronson HE. 3 regimens for alcohol withdrawal and detoxification. J Fam Pract. 2004;53:545–554. [PubMed] [Google Scholar]
- Baler RD, Volkow ND. Drug addiction: The neurobiology of disrupted self-control. Trends Mol Med. 2006;12:559–566. doi: 10.1016/j.molmed.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Dersch CM, et al. Effects of phentermine and fenfluramine on extracellular dopamine and serotonin in rat nucleus accumbens: Therapeutic implications. Synapse. 2000;36:102–113. doi: 10.1002/(SICI)1098-2396(200005)36:2<102::AID-SYN3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Beck A, Wustenberg T, Genauck A, et al. Effect of brain structure, brain function, and brain connectivity on relapse in alcohol-dependent patients. Arch Gen Psychiatry. 2012;69:842–852. doi: 10.1001/archgenpsychiatry.2011.2026. [DOI] [PubMed] [Google Scholar]
- Berman RM, Fava M, Thase ME, et al. Aripiprazole augmentation in major depressive disorder: a double-blind, placebo-controlled study in patients with inadequate response to antidepressants. CNS Spectr. 2009;14:197–206. doi: 10.1017/s1092852900020216. [DOI] [PubMed] [Google Scholar]
- Berman RM, Marcus RN, Swanink R, et al. The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: A multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2007;68:843–853. doi: 10.4088/jcp.v68n0604. [DOI] [PubMed] [Google Scholar]
- Bersani G, Gherardelli S, Clemente R, et al. Neurologic soft signs in schizophrenic patients treated with conventional and atypical antipsychotics. J Clin Psychopharmacol. 2005;25:372–375. doi: 10.1097/01.jcp.0000169268.44421.cf. [DOI] [PubMed] [Google Scholar]
- Comings DE, Blum K. Reward deficiency syndrome: Genetic aspects of behavioral disorders. Prog Brain Res. 2000;126:325–341. doi: 10.1016/S0079-6123(00)26022-6. [DOI] [PubMed] [Google Scholar]
- Dixon AL, Prior M, Morris PM, et al. Dopamine antagonist modulation of amphetamine response as detected using pharmacological MRI. Neuropharmacology. 2005;48:236–245. doi: 10.1016/j.neuropharm.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Filbey FM, Claus E, Audette AR, et al. Exposure to the taste of alcohol elicits activation of the mesocorticolimbic neurocircuitry. Neuropsychopharmacology. 2008;33:1391–1401. doi: 10.1038/sj.npp.1301513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank E, Thase ME. Natural history and preventative treatment of recurrent mood disorders. Annu Rev Med. 1999;50:453–468. doi: 10.1146/annurev.med.50.1.453. [DOI] [PubMed] [Google Scholar]
- Grusser SM, Wrase J, Klein S, et al. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology (Berl) 2004;175:296–302. doi: 10.1007/s00213-004-1828-4. [DOI] [PubMed] [Google Scholar]
- Han DH, Bolo N, Daniels MA, et al. Brain activity and desire for Internet video game play. Compr Psychiatry. 2011;52:88–95. doi: 10.1016/j.comppsych.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M, Ichiwaka J, Li Z, et al. Augmentation by citalopram of risperidone-induced monoamine release in rat prefrontal cortex. Psychopharmacology (Berl) 2006;185:274–281. doi: 10.1007/s00213-005-0206-1. [DOI] [PubMed] [Google Scholar]
- Janiri L, Martinotti G, Di Nicola M. Aripiprazole for relapse prevention and craving in alcohol-dependent subjects: Results from a pilot study. J Clin Psychopharmacol. 2007;27:519–520. doi: 10.1097/JCP.0b013e318150c841. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: A pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Katner SN, Weiss F. Neurochemical characteristics associated with ethanol preference in selected alcohol-preferring and -nonpreferring rats: A quantitative microdialysis study. Alcohol Clin Exp Res. 2001;25:198–205. [PubMed] [Google Scholar]
- Kennedy SH, Evans KR, Kruger S, et al. Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. Am J Psychiatry. 2001;158:899–905. doi: 10.1176/appi.ajp.158.6.899. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Crum RM, Warner LA, et al. Lifetime co-occurrence of DSM-III-R alcohol abuse and dependence with other psychiatric disorders in the National Comorbidity Survey. Arch Gen Psychiatry. 1997;54:313–321. doi: 10.1001/archpsyc.1997.01830160031005. [DOI] [PubMed] [Google Scholar]
- Ko CH, Liu GC, Hsiao S, et al. Brain activities associated with gaming urge of online gaming addiction. J Psychiatr Res. 2009;43:739–747. doi: 10.1016/j.jpsychires.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Lejoyeux M, Solomon J, Ades J. Benzodiazepine treatment for alcohol-dependent patients. Alcohol Alcohol. 1998;33:563–575. doi: 10.1093/alcalc/33.6.563. [DOI] [PubMed] [Google Scholar]
- Li Z, Ichikawa J, Dai J, et al. Aripiprazole, a novel antipsychotic drug, preferentially increases dopamine release in the prefrontal cortex and hippocampus in rat brain. Eur J Pharmacol. 2004;493:75–83. doi: 10.1016/j.ejphar.2004.04.028. [DOI] [PubMed] [Google Scholar]
- Liotti M, Mayberg HS. The role of functional neuroimaging in the neuropsychology of depression. J Clin Exp Neuropsychol. 2001;23:121–136. doi: 10.1076/jcen.23.1.121.1223. [DOI] [PubMed] [Google Scholar]
- Lopez JF, Akil H, Watson SJ. Neural circuits mediating stress. Biol Psychiatry. 1999;46:1461–1471. doi: 10.1016/s0006-3223(99)00266-8. [DOI] [PubMed] [Google Scholar]
- Lynskey MT. The comorbidity of alcohol dependence and affective disorders: Treatment implications. Drug Alcohol Depend. 1998;52:201–209. doi: 10.1016/s0376-8716(98)00095-7. [DOI] [PubMed] [Google Scholar]
- Mantere T, Tupala E, Hall H, et al. Serotonin transporter distribution and density in the cerebral cortex of alcoholic and nonalcoholic comparison subjects: A whole-hemisphere autoradiography study. Am J Psychiatry. 2002;159:599–606. doi: 10.1176/appi.ajp.159.4.599. [DOI] [PubMed] [Google Scholar]
- Marcus RN, McQuade RD, Carson WH, et al. The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: A second multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychopharmacol. 2008;28:156–165. doi: 10.1097/JCP.0b013e31816774f9. [DOI] [PubMed] [Google Scholar]
- Martinotti G, Carli V, Tedeschi D, et al. Mono- and polysubstance dependent subjects differ on social factors, childhood trauma, personality, suicidal behaviour, and comorbid Axis I diagnoses. Addict Behav. 2009a;34:790–793. doi: 10.1016/j.addbeh.2009.04.012. [DOI] [PubMed] [Google Scholar]
- Martinotti G, Di Nicola M, Di Giannantonio M, et al. Aripiprazole in the treatment of patients with alcohol dependence: A double-blind, comparison trial vs. naltrexone. J Psychopharmacol. 2009b;23:123–129. doi: 10.1177/0269881108089596. [DOI] [PubMed] [Google Scholar]
- Martinotti G, Di Nicola M, Janiri L. Efficacy and safety of aripiprazole in alcohol dependence. Am J Drug Alcohol Abuse. 2007;33:393–401. doi: 10.1080/00952990701313660. [DOI] [PubMed] [Google Scholar]
- Martinotti G, Nicola MD, Reina D, et al. Alcohol protracted withdrawal syndrome: The role of anhedonia. Subst Use Misuse. 2008;43:271–284. doi: 10.1080/10826080701202429. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Modulating dysfunctional limbic–cortical circuits in depression: Towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull. 2003;65:193–207. doi: 10.1093/bmb/65.1.193. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, et al. Differential brain activity in alcoholics and social drinkers to alcohol cues: Relationship to craving. Neuropsychopharmacology. 2004;29:393–402. doi: 10.1038/sj.npp.1300295. [DOI] [PubMed] [Google Scholar]
- Nelson JC, Mankoski R, Baker RA, et al. Effects of aripiprazole adjunctive to standard antidepressant treatment on the core symptoms of depression: A post-hoc, pooled analysis of two large, placebo-controlled studies. J Affect Disord. 2010;120:133–140. doi: 10.1016/j.jad.2009.06.026. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Nishikawa M, Diksic M, Sakai Y, et al. Alterations in brain serotonin synthesis in male alcoholics measured using positron emission tomography. Alcohol Clin Exp Res. 2009;33:233–239. doi: 10.1111/j.1530-0277.2008.00820.x. [DOI] [PubMed] [Google Scholar]
- Ostacher MJ. Comorbid alcohol and substance abuse dependence in depression: Impact on the outcome of antidepressant treatment. Psychiatr Clin North Am. 2007;30:69–76. doi: 10.1016/j.psc.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Markou A. Animal models and treatments for addiction and depression co-morbidity. Neurotox Res. 2007;11:1–32. doi: 10.1007/BF03033479. [DOI] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264:2511–2518. [PubMed] [Google Scholar]
- Rosa-Neto P, Diksic M, Okazawa H, et al. Measurement of brain regional alpha-[11C]methyl-L-tryptophan trapping as a measure of serotonin synthesis in medication-free patients with major depression. Arch Gen Psychiatry. 2004;61:556–563. doi: 10.1001/archpsyc.61.6.556. [DOI] [PubMed] [Google Scholar]
- Ross S, Peselow E. The neurobiology of addictive disorders. Clin Neuropharmacol. 2009;32:269–276. doi: 10.1097/wnf.0b013e3181a9163c. [DOI] [PubMed] [Google Scholar]
- Schlagenhauf F, Dinges M, Beck A, et al. Switching schizophrenia patients from typical neuroleptics to aripiprazole: Effects on working memory dependent functional activation. Schizophr Res. 2010;118:189–200. doi: 10.1016/j.schres.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Sheffrin M, Driscoll HC, Lenze EJ, et al. Pilot study of augmentation with aripiprazole for incomplete response in late-life depression: Getting to remission. J Clin Psychiatry. 2009;70:208–213. doi: 10.4088/jcp.07m03805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AD, Weiss F. Ethanol exposure differentially alters central monoamine neurotransmission in alcohol-preferring versus -nonpreferring rats. J Pharmacol Exp Ther. 1999;288:1223–1228. [PubMed] [Google Scholar]
- Snyder JL, Bowers TG. The efficacy of acamprosate and naltrexone in the treatment of alcohol dependence: A relative benefits analysis of randomized controlled trials. Am J Drug Alcohol Abuse. 2008;34:449–461. doi: 10.1080/00952990802082198. [DOI] [PubMed] [Google Scholar]
- Stein DJ. Depression, anhedonia, and psychomotor symptoms: The role of dopaminergic neurocircuitry. CNS Spectr. 2008;13:561–565. doi: 10.1017/s1092852900016837. [DOI] [PubMed] [Google Scholar]
- Storvik M, Tiihonen J, Haukijarvi T, et al. Lower serotonin transporter binding in caudate in alcoholics. Synapse. 2006;59:144–151. doi: 10.1002/syn.20228. [DOI] [PubMed] [Google Scholar]
- Storvik M, Tiihonen J, Haukijarvi T, et al. Amygdala serotonin transporters in alcoholics measured by whole hemisphere autoradiography. Synapse. 2007;61:629–636. doi: 10.1002/syn.20420. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotactic Atlas of the Human Brain. New York: Thieme Medical Publishers, Inc; 1988. [Google Scholar]
- Taneja C, Papakostas GI, Jing Y, et al. Cost-effectiveness of adjunctive therapy with atypical antipsychotics for acute treatment of major depressive disorder. Ann Pharmacother. 2012;46:642–649. doi: 10.1345/aph.1Q326. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Brown GG, Baratta MV, et al. fMRI BOLD response to alcohol stimuli in alcohol dependent young women. Addict Behav. 2004;29:33–50. doi: 10.1016/j.addbeh.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Tremblay LK, Naranjo CA, Graham SJ, et al. Functional neuroanatomical substrates of altered reward processing in major depressive disorder revealed by a dopaminergic probe. Arch Gen Psychiatry. 2005;62:1228–1236. doi: 10.1001/archpsyc.62.11.1228. [DOI] [PubMed] [Google Scholar]
- Vergne DE, Anton RF. Aripiprazole: A drug with a novel mechanism of action and possible efficacy for alcohol dependence. CNS Neurol Disord Drug Targets. 2010;9:50–54. doi: 10.2174/187152710790966731. [DOI] [PubMed] [Google Scholar]
- Vohora D. Atypical antipsychotic drugs: current issues of safety and efficacy in the management of schizophrenia. Curr Opin Investig Drugs. 2007;8:531–538. [PubMed] [Google Scholar]
- Voronin K, Randall P, Myrick H, et al. Aripiprazole effects on alcohol consumption and subjective reports in a clinical laboratory paradigm – possible influence of self-control. Alcohol Clin Exp Res. 2008;32:1954–1961. doi: 10.1111/j.1530-0277.2008.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisler RH, Khan A, Trivedi MH, et al. Analysis of suicidality in pooled data from 2 double-blind, placebo-controlled aripiprazole adjunctive therapy trials in major depressive disorder. J Clin Psychiatry. 2011;72:548–555. doi: 10.4088/JCP.09m05495gre. [DOI] [PubMed] [Google Scholar]