Abstract
Donor-derived bacterial infection is a recognized complication of solid organ transplantation (SOT). The present report describes the clinical details and successful outcome in a liver transplant recipient despite transmission of methicillin-resistant Staphylococcus aureus (MRSA) from a deceased donor with MRSA endocarditis and bacteremia. We further describe whole genome sequencing (WGS) and complete de novo assembly of the donor and recipient MRSA isolate genomes, which confirms that both isolates are genetically 100% identical. We propose that similar application of WGS techniques to future investigations of donor bacterial transmission would strengthen the definition of proven bacterial transmission in SOT, particularly in the presence of highly clonal bacteria such as MRSA. WGS will further improve our understanding of the epidemiology of bacterial transmission in SOT and the risk of adverse patient outcomes when it occurs.
Introduction
Donor-derived infections are a rare complication of solid organ transplantation (SOT) (1). In retrospective database studies, no transmitted bacterial infections were described despite 5% of donors having bacteremia (2,3). In contrast, the US Organ Procurement and Transplantation Network (OPTN) reported 145 recipients with confirmed donor-transmitted infections from 2005 to 2011, including 34 recipients with bacterial transmissions that resulted in nine deaths (1). Although current guidelines recommend that active bacterial infection in the donor should ideally be treated and resolved prior to transplantation, these guidelines acknowledge that bacteremic donors may be considered (1). However, specific evidence-based criteria for accepting bacteremic donors are not available at this time, largely due to the lack of empirical data from confirmed transmissions.
Transmission in the transplantation literature is typically based on clinical and epidemiologic data and currently loosely defined as clear evidence of the same infection in the donor and at least one of the recipients (4). However, this definition may be insufficient for highly clonal pathogens commonly acquired in the hospital setting such as methicil-lin-resistant Staphylococcus aureus (MRSA). Although whole genome sequencing (WGS) has been used to investigate nosocomial bacterial outbreaks (5), there are no previous reports where it has been employed to confirm donor transmission of bacterial infection to an organ recipient. We report a patient who underwent liver transplantation (LT) from a deceased donor with known MRSA endocarditis and bacteremia. We assembled whole genome sequences of the donor and recipient isolates to complete closure to confirm genetic identity and confirm donor transmission of MRSA via LT.
Case Report
Donor
The donor was a 40-year-old woman who presented to another hospital with a drug overdose, multifocal embolic cerebrovascular accident and MRSA mitral valve endocarditis. Donor blood cultures obtained on hospital days 1–6 grew MRSA (vancomycin minimum inhibitory concentration [MIC] 1–2 μmg/mL). She was treated with vancomycin from hospital day 3 until organ donation. An echocardio-gram revealed multiple large mobile densities on the mitral valve compatible with vegetations. On hospital day 7, organ donation was performed. Donor blood cultures obtained by the New York Organ Donor Network (NYODN) on this day were negative although this information was not available when the decision was made to proceed with LT. All other organs were declined.
Recipient
The LT recipient was a 64-year-old man with cirrhosis from hepatitis C virus and hepatocellular carcinoma and was hospitalized with decompensated cirrhosis and acute kidney injury. The patient’s Model for End-Stage Liver Disease (MELD) score was 29. Blood, urine and ascitic fluid cultures drawn prior to deceased donor liver transplantation (DDLT) were all negative. The recipient had no prosthetic devices or history of MRSA colonization or infection. After informed consent that included discussion of the specific donor transmission risk, the patient underwent DDLT without complication. Vancomycin and ertapenem were given for perioperative prophylaxis. No adjustments in standard immunosuppression were implemented. Blood cultures were drawn 6 h after surgical closure to assess for acquisition of MRSA from the donor. These cultures grew Gram-positive cocci in clusters in the aerobic and anaerobic bottles after 17 and 21 h, respectively; and the isolates were subsequently confirmed as MRSA with a susceptibility pattern identical to the most recent donor isolates (oxacillin MIC >4 μg/mL, vancomycin MIC 2 μg/mL). Susceptibilities were obtained by Vitek®2 (bioMerieux, Durham, NC), and screening for heterogeneous vancomy-cin-intermediate S. aureus (h-VISA) by E-test GRD was negative (6). The NYODN and the United Network for Organ Sharing were informed of a likely donor transmission. Ertapenem was discontinued and vancomycin was continued (1 g every 12 h). Repeat blood cultures drawn 27 h later were negative. Subsequent blood cultures were also negative. The patient recovered and was discharged 13 days after DDLT. Vancomycin was discontinued approximately 4 weeks after DDLT. The patient remains well with good graft function and no evidence of infection 12 months after DDLT.
Materials and Methods
Donor and recipient blood culture isolates were obtained and genotyped by a combination of standard methods including spa (S. aureus protein A) and SCCmec (staphylococcal cassette chromosome mec) typing (7,8). spa polymerase chain reaction (PCR) products were compared and clonal complexes assigned using http://spaserver2.ridom.de (7,8). Screening for presence of Panton-Valentine leukocidin (PVL) toxin was also performed (9). DNA extraction was performed after bacterial lysis using lysozyme/lysostaphin treatment or mechanical disruption, followed by column purification and ethanol precipitation. Completed DNA preparations were sequenced on the PacBio RSII platform and sequence was assembled using a custom pipeline based on HGAP version 1.4 (Pacific Biosciences, Menlo Park, CA) (10). Full details are provided in the Supporting Information.
Results
Initial genotyping revealed that both the donor and recipient isolates were spa type 1, t008 (CC-8), SCCmec type IV and PVL positive. This is compatible with a USA300 clonal origin, the most prevalent community-acquired MRSA in the United States (11). Each isolate was sequenced in duplicate on the PacBio RSII platform before and after size selection of DNA fragments >10 kb to both allow for detection of small plasmids and to optimize for long-read chromosomal sequencing. The finished assemblies for each isolate consisted of a complete, circularized 2.9 Mb chromosome and two plasmids of 27 and 3 kb. Whole genome comparison between the two isolates showed that they were identical except for six single-base insertions/deletions (indels) in homopolymer regions on the chromosomal DNA. Further assessment by Sanger sequencing identified these indels as assembly artifacts (Figure S1), and confirmed that the donor and recipient isolates were genetically identical. The transplant isolate genome is available from GenBank (accessions: CP007176-CP007178).
To further classify and characterize our transplant isolate, we first constructed a phylogenetic tree based on single nucleotide variant (SNV) differences within core genomic regions shared with nine other fully sequenced S. aureus genomes (Figure 1A), confirming a USA300 origin. A second tree was constructed based only on USA300 isolates, using 806 core-genomic SNVs identified across 11 strains by Uhlemann et al (12) (Figure 1B). Both trees indicate that the transplant isolate forms a distinct clade with FPR3757 and TCH1516 reference strains, even among closely related clinical isolates.
Figure 1. Comparison of the transplant isolate to related strains.
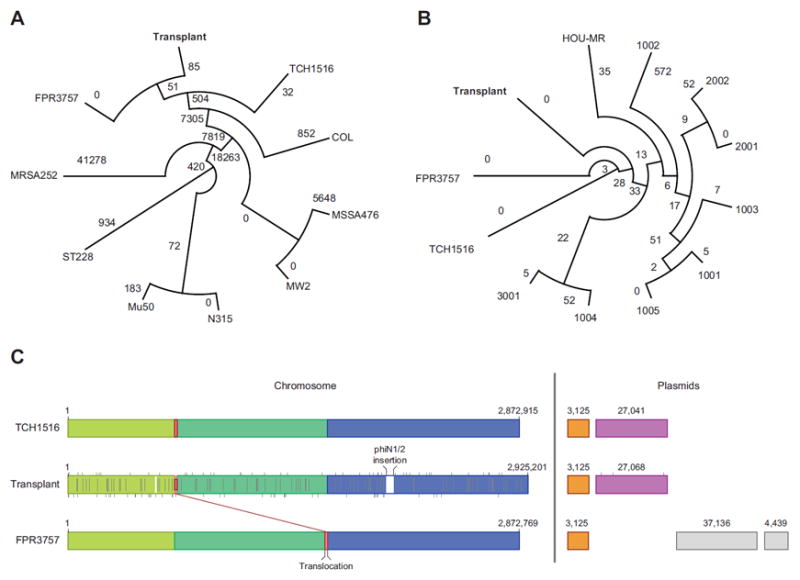
(A) Phylogeny of transplant isolate compared to nine fully sequenced staphylococci genomes obtained from GenBank. Trees were constructed by inferring ancestral states using RAxML-8.0.4 (18); branch lengths correspond to single nucleotide variant (SNV) distances from branch points, and drawn using Dendroscope 3 (19). (B) Same as (A), but including 11 USA300 isolates only (12). (C) Detailed comparison of the transplant isolate to the closely related TCH1516 and FPR3757 strains. Mauve 2.3.1 (20) was used to perform a comparison of the three genomes. Colored blocks correspond to contiguous chunks of sequence in the same order—highlighting a single rearrangement relative to FPR3757. Uncolored blocks correspond to novel sequence in the transplant genome. Vertical gray lines correspond to SNVs in the transplant patient relative to TCH1516 (top), FPR3757 (bottom) or both (center). TCH1516 had 50 unique SNVs relative to the transplant isolate, compared to 68 for FPR3757, with 93 SNVs shared.
Further comparative analysis within the clade showed that all chromosomal genes from FPR3757 and TCH1516 are present in the transplant isolate with major structural differences limited to a 43 001 bp prophage insertion in the transplant isolate, and a 13 368 bp translocation compared to FPR3757 (Figure 1C). BLAST comparison to the GenBank nucleotide database indicated that the insertion is closely related to the phiNM1 and phiNM2 prophages described in S. aureus Newman, which were shown to affect its virulence in a murine model of abscess formation (13). The prophage insert carries homologs of four virulence genes (14); three of these genes (SAV0876, SAV1978 and SAV1986) are also present in at least one of three other prophages present on the chromosome, but one gene (SAV0855) is unique to the prophage insert and the transplant isolate. Overall, SNV-, structural- and plasmid-level comparisons indicated that the transplant isolate is most closely related to TCH1516 (Figure 1C), while possessing distinct features that may affect its virulence.
Discussion
We report the use of long-read WGS and de novo assembly of the chromosome and plasmids to confirm transmission of MRSA via DDLT. Although the clinical and epidemiological details of this case already provided substantial evidence of donor transmission, WGS confirmed complete genetic identity of donor and recipient isolates. Furthermore, since the isolates are genetically distinct from even closely related sequenced clinical isolates, we consider posttransplant hospital acquisition extremely unlikely and believe that our results strongly support transmission from donor to recipient. We further expect that WGS will be particularly useful when suspected donor-transmitted bacterial infections occur later after transplant when it is more difficult to distinguish donor-derived infection from posttransplant hospital-acquired infection.
To date, there have been only two reports of suspected donor-transmitted S. aureus infections in SOT recipients that included efforts to confirm the relatedness of the donor and recipient strains (15,16). In one MRSA transmission cluster involving recipients of the kidneys and liver, evidence of identity was based on restriction fragment length polymorhphism (RFLP) analysis and PCR typing (15). More recently, donor transmission of methicillin-susceptible S. aureus (MSSA) to two DDLT recipients was described and relatedness was demonstrated by pulsed-field gel electrophoresis (PFGE) (16). Each of these studies relied on a subset of genetic information to assign clonality; however, most MRSA infections are highly related with only a handful of clones dominating the globe. As such, these typing methods cannot provide conclusive evidence of MRSA transmission (11). In our case of clinically presumed transmission, conventional typing revealed that both isolates originated from the USA300 clone. Since nearly all community-acquired MRSA strains in the United States originate from this clone (17), WGS was essential to prove genetic identity at both chromosome and extra-chromosomal elements. Although genome sequencing without complete assembly has been used to differentiate hospital and community-acquired infections and study transmission in general (5), only completely finished genomes give absolute certainty about the genetics of a given isolate.
With a limited donor pool and an expanding transplant waiting list, consideration of organs from infected donors is necessary. Overall, the safety and favorable outcomes associated with donor-transmitted bacterial infections are suggested by the available literature (1,2). However, further research is needed to better understand the risk factors, epidemiology, and outcomes associated with transmission, particularly with resistant bacteria. We acknowledge that our decision to accept the organ and our approach to recipient management cannot be considered the standard of care until future studies validate the favorable outcome we observed. Unresolved issues include whether certain organs or specific pathogens are more likely to be associated with transmission (16). Prospective studies of donors and recipients in suspected bacterial transmissions should be performed to enhance our understanding of transmission risk and recipient outcomes. WGS could eliminate falsely suspected transmissions and strengthen the case definition of bacterial transmission in such studies.
In summary, our case highlights that MRSA can be transmitted via DDLT from a donor with recent bacteremia, and clinicians should be aware that negative blood cultures on the day of organ donation does not always predict that transmission will not occur. However, a favorable outcome can still be achieved with perioperative vancomycin followed by an extended course of therapy, particularly when transmission is confirmed. In the current era of organ shortage, transplant clinicians should consider the use of organs from donors with bacteremia involving pathogens susceptible to standard antibacterial therapy. Finally, we demonstrate the use of WGS to confirm transmission and propose its use in future investigations of suspected transplant transmissions of bacterial infection. WGS should result in a more accurate estimate of transmission rates, provide an opportunity to better understand factors associated with transmission risk and outcome, and yield insight into strain characteristics that are not obtained from standard assays currently in use.
Supplementary Material
Supplementary Methods
Six homopolymer regions containing indels between donor and recipient isolates were amplified by PCR and Sanger sequenced. Each panel shows the traces obtained in duplicate (forward and reverse strands) for from each isolate. The positions of the indels in the original assemblies are highlighted in gray.
Acknowledgments
This research was supported in part by NIAID-supported NRSA Institutional Research Training Grant (5 T32 AI 7647-13) for Global Health Research (DRA). Sequencing was funded by the Icahn Institute for Genomics and Multiscale Biology, and analyses were supported in part through the computational resources and staff expertise provided by the Department of Scientific Computing at the Icahn School of Medicine at Mount Sinai. We thank Anne-Catrin Uhlemann, MD, PhD, from Columbia University Medical Center for contributing Staphylococcus aureus phylogenies, and Zulyema Peralta, BS, Martha Lewis, BS, Matthew Pendleton, MS and Fran Wallach, MD for their experimental contributions and helpful discussions.
Abbreviations
- DDLT
deceased donor liver transplantation
- h-VISA
heterogeneous vancomycin-intermediate S. aureus
- LT
liver transplantation
- MELD
Model for End-Stage Liver Disease
- MIC
minimum inhibitory concentration
- MRSA
methicillin-resistant Staphylococcus aureus
- NYODN
New York Organ Donor Network
- OPTN
Organ Procurement and Transplantation Network
- PCR
polymerase chain reaction
- PFGE
pulsed-field gel electrophoresis
- PVL
Panton-Valentine leukocidin
- RFLP
restriction fragment length polymorhphism
- SCCmec
staphylococcal cassette chromosome mec
- SNV
single nucleotide variant
- SOT
solid organ transplantation
- spa
S. aureus protein A
- WGS
whole genome sequencing
Footnotes
Supporting Information
Additional Supporting Information may be found in the online version of this article.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Ison MG, Grossi P. Practice ASTIDCo. Donor-derived infections in solid organ transplantation. Am J Transplant. 2013;13:22–30. doi: 10.1111/ajt.12095. [DOI] [PubMed] [Google Scholar]
- 2.Freeman RB, Giatras I, Falagas ME, et al. Outcome of transplantation of organs procured from bacteremic donors. Transplantation. 1999;68:1107–1111. doi: 10.1097/00007890-199910270-00008. [DOI] [PubMed] [Google Scholar]
- 3.Lumbreras C, Sanz F, Gonzalez A, et al. Clinical significance of donor-unrecognized bacteremia in the outcome of solid-organ transplant recipients. Clin Infect Dis. 2001;33:722–726. doi: 10.1086/322599. [DOI] [PubMed] [Google Scholar]
- 4.Garzoni C, Ison MG. Uniform definitions for donor-derived infectious disease transmissions in solid organ transplantation. Transplantation. 2011;92:1297–1300. doi: 10.1097/TP.0b013e318236cd02. [DOI] [PubMed] [Google Scholar]
- 5.Eyre DW, Golubchik T, Gordon NC, et al. A pilot study of rapid benchtop sequencing of Staphylococcus aureus and Clostridium difficile for outbreak detection and surveillance. BMJ Open. 2012;2 doi: 10.1136/bmjopen-2012-001124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yusof A, Engelhardt A, Karlsson A, et al. Evaluation of a new Etest vancomycin-teicoplanin strip for detection of glycopeptide-intermediate Staphylococcus aureus (GISA), in particular, heterogeneous GISA. J Clin Microbiol. 2008;46:3042–3047. doi: 10.1128/JCM.00265-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shopsin B, Gomez M, Montgomery SO, et al. Evaluation of protein A gene polymorphic region DNA sequencing for typing of Staphylococcus aureus strains. J Clin Microbiol. 1999;37:3556–3563. doi: 10.1128/jcm.37.11.3556-3563.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Milheirico C, Oliveira DC, de Lencastre H. Update to the multiplex PCR strategy for assignment of mec element types in Staphylococcus aureus. Antimicrob Agents Chemother. 2007;51:3374–3377. doi: 10.1128/AAC.00275-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mendes RE, Sader HS, Deshpande LM, Diep BA, Chambers HF, Jones RN. Characterization of baseline methicillin-resistant Staphylococcus aureus isolates recovered from Phase IV clinical trial for linezolid. J Clin Microbiol. 2010;48:568–574. doi: 10.1128/JCM.01384-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chin CS, Alexander DH, Marks P, et al. Nonhybrid, finished microbial genome assemblies from long-read SMRT sequencing data. Nat Methods. 2013;10:563–569. doi: 10.1038/nmeth.2474. [DOI] [PubMed] [Google Scholar]
- 11.Chambers HF, Deleo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol. 2009;7:629–641. doi: 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uhlemann AC, Kennedy AD, Martens C, Porcella SF, Deleo FR, Lowy FD. Toward an understanding of the evolution of Staphylococcus aureus strain USA300 during colonization in community households. Genome Biol Evol. 2012;4:1275–1285. doi: 10.1093/gbe/evs094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bae T, Baba T, Hiramatsu K, Schneewind O. Prophages of Staphylococcus aureus Newman and their contribution to virulence. Mol Microbiol. 2006;62:1035–1047. doi: 10.1111/j.1365-2958.2006.05441.x. [DOI] [PubMed] [Google Scholar]
- 14.Bae T, Banger AK, Wallace A, et al. Staphylococcus aureus virulence genes identified by bursa aurealis mutagenesis and nematode killing. Proc Natl Acad Sci USA. 2004;101:12312–12317. doi: 10.1073/pnas.0404728101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnston L, Chui L, Chang N, et al. Cross-Canada spread of methicillin-resistant Staphylococcus aureus via transplant organs. Clin Infect Dis. 1999;29:819–823. doi: 10.1086/520442. [DOI] [PubMed] [Google Scholar]
- 16.Doucette KE, Al-Saif M, Kneteman N, et al. Donor-derived bacteremia in liver transplant recipients despite antibiotic prophylaxis. Am J Transplant. 2013;13:1080–1083. doi: 10.1111/ajt.12133. [DOI] [PubMed] [Google Scholar]
- 17.DeLeo FR, Otto M, Kreiswirth BN, Chambers HF. Community-associated methicillin-resistant Staphylococcus aureus. Lancet. 2010;375:1557–1568. doi: 10.1016/S0140-6736(09)61999-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stamatakis A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huson DH, Scornavacca C. Dendroscope 3: An interactive tool for rooted phylogenetic trees and networks. Syst Biol. 2012;61:1061–1067. doi: 10.1093/sysbio/sys062. [DOI] [PubMed] [Google Scholar]
- 20.Darling AE, Mau B, Perna NT. progressiveMauve: Multiple genome alignment with gene gain, loss and rearrangement. PLoS ONE. 2010;5:e11147. doi: 10.1371/journal.pone.0011147. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Methods
Six homopolymer regions containing indels between donor and recipient isolates were amplified by PCR and Sanger sequenced. Each panel shows the traces obtained in duplicate (forward and reverse strands) for from each isolate. The positions of the indels in the original assemblies are highlighted in gray.


