Abstract
Here I present the R package 'plantecophys', a toolkit to analyse and model leaf gas exchange data. Measurements of leaf photosynthesis and transpiration are routinely collected with portable gas exchange instruments, and analysed with a few key models. These models include the Farquhar-von Caemmerer-Berry (FvCB) model of leaf photosynthesis, the Ball-Berry models of stomatal conductance, and the coupled leaf gas exchange model which combines the supply and demand functions for CO2 in the leaf. The 'plantecophys' R package includes functions for fitting these models to measurements, as well as simulating from the fitted models to aid in interpreting experimental data. Here I describe the functionality and implementation of the new package, and give some examples of its use. I briefly describe functions for fitting the FvCB model of photosynthesis to measurements of photosynthesis-CO2 response curves ('A-Ci curves'), fitting Ball-Berry type models, modelling C3 photosynthesis with the coupled photosynthesis-stomatal conductance model, modelling C4 photosynthesis, numerical solution of optimal stomatal behaviour, and energy balance calculations using the Penman-Monteith equation. This open-source package makes technically challenging calculations easily accessible for many users and is freely available on CRAN.
Introduction
Since the advent of portable gas exchange instruments [1,2], a wealth of data on leaf gas exchange of CO2 and H2O has been collected [3]. These data play a central role in physiological plant ecology [4], to better understand and quantify inter-specific differences in photosynthesis and transpiration, and to quantify and model the rapid response to changes in environmental drivers such as light, humidity and temperature. Not only do leaf gas exchange data allow detailed studies of the underlying plant physiology, they are also used to parameterize an important component of process-based models of vegetation function used to predict global water and carbon cycling [5,6].
The photosynthesis model of Farquhar, von Caemmerer and Berry [7] (the 'FvCB model') is widely used in interpreting and modelling leaf gas exchange, by providing comparable metrics of the photosynthetic capacity, and predicting the response of photosynthesis to changes in the CO2 concentration inside the leaf air space (Ci). This widely cited model is embedded in many process-based models of vegetation function [5,8]. The key prediction of the model is the response of photosynthesis to [CO2] inside the leaf (either chloroplastic [CO2], Cc, or intercellular [CO2], Ci). It can also account for changes in leaf temperature if the various temperature sensitivities are parameterized [9–11]. To employ the model, it is generally fit to observations of net photosynthesis along a range of [CO2] concentrations, yielding well-known measures of photosynthetic capacity (Vcmax and Jmax, and optionally Rd) [12].
I do not repeat a detailed description of the FvCB model here, as it has been described many times [10,11]. But generally it is of the form,
| (1) |
where An is the net rate of CO2 assimulation, Ac is the gross photosynthesis rate when Rubisco activity is limiting, Aj when RuBP-regeneration is limiting, and Rd the rate of dark respiration (see Fig 1A). Ac and Aj are non-linear functions of the chloroplastic CO2 concentration (Cc), both of the form k1 (CcΓ*)/(k2+Cc), where Γ* is the CO2 compensation point without Rd, and k1 and k2 are different parameter combinations for Ac and Aj. The details of these functions and the temperature dependence of the various parameters are described elsewhere [11].
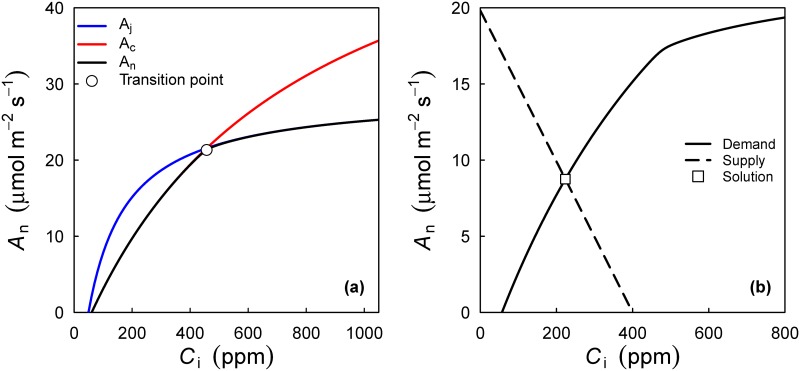
Fig 1. Supply and demand functions for leaf photosynthesis.
(a) Leaf photosynthesis—CO2 response curve as modelled with the FvCB model. (b) The intersection of the supply and demand curves of photosynthesis. The Photosyn function solves for Ci if gs, Vcmax, Jmax and Rd (and other parameters to the FvCB model) are known.
In the practical application of the FvCB model, when leaf gas exchange is measured with a portable gas exchange instrument, estimates of Cc are difficult to obtain because they require an estimate of the mesophyll conductance (gm). In this case, it is customary to us the intercellular [CO2] concentration (Ci) as the driver of photosynthesis. This approach is useful because Ci can be estimated from concurrent measurements of CO2 and H2O flux [13]. In the remainder of this article I will use Cc as the driver of photosynthesis, but point out that this can be replaced by Ci if the user does not have an estimate of gm. When gm is known, Cc is calculated from Ci with Eq 2.
| (2) |
where gm is the mesophyll conductance (mol m-2 s-1). Although this method assumes that gm is constant for a given leaf, it is well known that gm responds dynamically to fluctuations in environmental drivers [14] although some of the variation in gm may due to artefacts related to (photo-)respiratory effects on measured gm with standard methods [15]. Because no model has been developed to date that adequately captures the variation in gm, it is a constant parameter in the implementation presented here. However, it can still be used to study the effects of non-constant gm on rates of photosynthesis and its response to environmental drivers, as the parameter can be varied in model simulations.
Fitting the FvCB model to data requires some finesse because net photosynthesis is modelled as a minimum function of two non-linear equations that is sometimes difficult to fit. Moreover, sample sizes collected are often small due to time constraints in the field. A widely used published method requires the user to specify the transition of Vcmax to Jmax limitation [16], a process that is both arbitrary and prevents batch analysis. Another method [17] requires online submission of data and fits the model without much control or knowledge of the fitting process (following [18]), and does not report standard errors of the estimated parameters. Undoubtedly many more implementations of the fitting process have been developed over the years, but few of these are made publicly available (but see available online tools [19,20]). What is missing is an open-source tool that can be used for reproducible and transparent analysis of A-Ci curves.
Through Eq 1, we have a dependency of photosynthesis on the availability of the substrate, Cc. To estimate Cc itself, we need Ci, which can be estimated when with stomatal conductance to CO2. From Fick's law, we can relate An to gs and Ci as,
| (3) |
where gs is the conductance to H2O (the factor 1.6 converts to conductance to CO2). We now have two equations for An: the 'demand function' (Eq 1), and the 'supply function' (Eq 3). At steady state these two equations should be equal, which can be graphically shown as in Fig 1B (cf. [21]).
Because gs itself responds to environmental drivers, another expression is needed to end up with a fully coupled model of leaf gas exchange. The most widely-used, though empirical, gs model is the Ball-Berry [22] class of models. This model posits an entirely empirical equation that describes the response of gs to air humidity, CO2 and An. This way, effects of leaf temperature and PPFD—both of which are known to affect gs—are modelled through the dependency of An on these drivers. A general form of the Ball-Berry model is,
| (4) |
where D is the vapour pressure deficit (kPa), g0 and g1 are empirical parameters, and f(D) can be one of many functions that describe the response to the vapour pressure deficit (D, [23,24]) or relative humidity [22]. An alternative approach to modelling gs is through the hypothesis that stomata act optimally in the sense that they maximize photosynthesis while minimizing water loss. This hypothesis was first developed by Cowan and Farquhar [25] and has seen many applications. Medlyn et al. [24] showed that the optimality hypothesis, when coupled to the FvCB model, leads to an expression analogous to the Ball-Berry type models (Eq 4), but with a different D response function (f(D) in Eq 3) compared to the original Ball-Berry model.
Finally we can combine the biochemical demand function of photosynthesis (Eq 1) with the supply function (Eq 2) and an expression for the dependency of gs on environmental drivers (Eq 3). This 'coupled' leaf gas exchange model [23,26,27] is implemented in many process-based ecosystem and global land surface models [5,8,28,29]. This model allows prediction of An, gs and leaf transpiration rate in response to all major environmental drivers (except soil water limitation), and incorporates key leaf traits (g1, Vcmax, Jmax, Rd, and their temperature dependencies).
Despite the widespread use of the FvCB model and the coupled leaf gas exchange model, tools to analyse data and perform simulations are scattered and subject to little standardization. Fitting the FvCB model to CO2 response curves is a standard procedure but different methods can yield different parameter values, making comparisons difficult. The coupled leaf gas exchange model is not straightforward to implement, and I do not know of any standalone open-source implementations. I here describe the plantecophys package, implemented in the R language [30]. The code is freely available (without restrictions), and managed with a version control system. The package is the result of our work on leaf and canopy modelling of photosynthesis and stomatal conductance [24,31–36], with many additions based on user requests.
Design and Implementation
The main functions
The main tools included in the plantecophys package are to a) fit A-Ci curves to estimate Vcmax, Jmax and Rd, b) fit Ball-Berry type models, c) simulate from the coupled leaf gas exchange model and d) calculate the optimal stomatal conductance. The key functions in the package are summarized in Table 1.
Table 1. Main functions in the plantecophys package.
| Function | Description |
|---|---|
| fitaci | Fit, summarize, plot and simulate photosynthesis-[CO2] response curves (A-Ci curves) |
| fitBB | Fit Ball-Berry type models of stomatal conductance |
| FARAO | Estimate optimal stomatal conductance with a numerical implementation of the Cowan-Farquhar hypothesis |
| Photosyn | Simulate C3 photosynthesis and transpiration with the coupled leaf gas exchange model. Also simulates the FvCB model when either Ci or gs is given as input. |
| PhotosynEB | Estimate leaf temperature from energy balance, when a significant leaf boundary layer is present |
| AciC4 | Simulates the dependence of C4 photosynthesis on the intercellular CO2 concentration |
| RHtoVPD etc. | Convert between commonly used units (relative humidity, vapour pressure deficit, dewpoint temperature) |
Language
The 'plantecophys' package is implemented in R, has no dependencies on other packages, and does not require compilation (i.e. it is written in native R only). As such it builds easily, and is highly portable. The source code is maintained with git version control, and is hosted in an online repository (http://www.bitbucket.org/remkoduursma/plantecophys), from which a development version of the package can easily be installed. The repository includes an issue tracker, where users can suggest changes or report bugs. This paper describes version 0.6.6 (git SHA b9a18c9).
All code used in this article (including the code to generate the article written in markdown, all figures and full example code), can be downloaded from Ref. [37]. The repository also includes code to demonstrates how to extract additional statistics from fitted A-Ci curves.
Results and Discussion
Fitting A-Ci curves
The fitaci function fits the FvCB model, yielding estimates of Vcmax, Jmax and Rd and their standard errors. Instead of fitting the minimum function (Eq 1), fitaci fits the hyperbolic minimum of Ac and Aj, which avoids a discontinuity (Eq 5).
| (5) |
where θ is a shape parameter, set to 0.9999, and Am is the hyperbolic minimum of Ac and Aj. The fit of the FvCB model to data is achieved with non-linear least squares, and standard errors of the parameters are estimated with standard methods (nls function in base R, see [38]). The fitaci function includes methods to estimate appropriate starting values from the data, and attempts the fits along a wide range of possible starting values. Optionally, Rd can be provided as a known value, otherwise it is estimated from the A-Ci curve. The user does not have to provide the transition point (see Fig 1), as this is estimated by fitaci automatically. It is however an option to fix the transition point (via the citransition argument), which may be helpful to check whether the best fit was achieved. Finally, the user can provide an estimate of mesophyll conductance (gm) (following [39]), in which case the fitted values of Vcmax and Jmax can be interpreted as chloroplastic rates.
Because the fitting uses non-linear least squares, standard methods can be employed to estimate standard errors (SE), confidence intervals, and correlation of the fitted parameters. The fitaci function returns by default the SE and confidence intervals, and the built-in help page for the fitaci function shows how the nlstools package can be used to provide a detailed overview of the statistics of the non-linear least squares fit.
Required inputs are measurements of An and Ci, and optionally leaf temperature (Tleaf), and photosynthetically active radiation (PAR). Also required are estimates of Michaelis-Menten constants (Kc, Ko or the combination Km) and Γ*. In the FvCB model, Jmax, Vcmax and leaf respiration (Rd) (and other parameters like Γ*, Kc and Ko) all depend non-linearly on Tleaf. The Photosyn function incorporates standard temperature sensitivities for all parameters of the FvCB model (following [11]). Optionally, measured (or otherwise modelled) Km and Γ* can be provided as input.
The function takes a dataframe as input, which includes measurements of An, Ci and optionally Tleaf and PAR, and is easily used like this:
# Fit FvCB model
f <- fitaci(mydata)
# Print a summary with coefficients and more
f
# Make standard plot
lot(f)
The output of the above example is shown in Fig 2. Additionally, the batch utility fitacis can be used to fit many curves at once, for example one for each species or site in a dataset. I show this functionality in the example application further below.
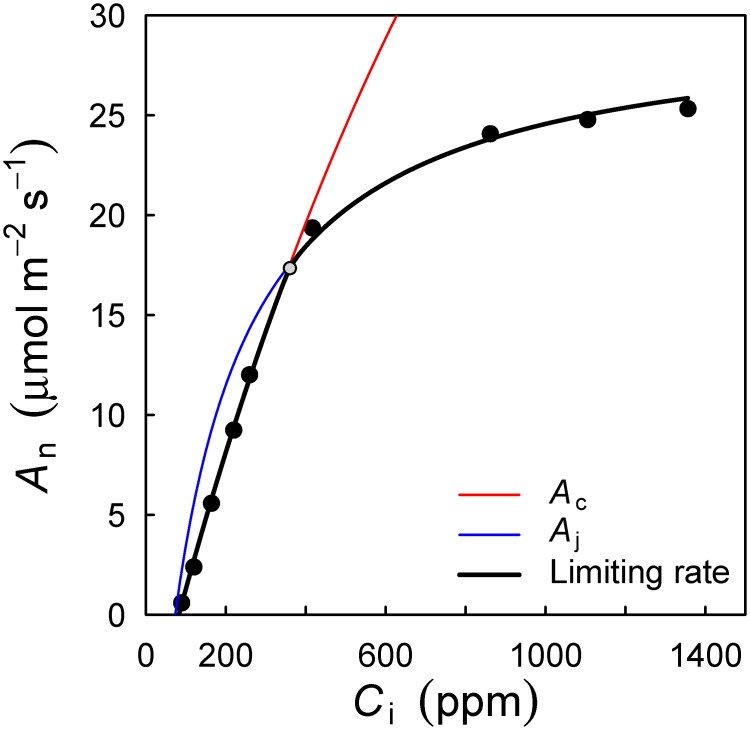
Fig 2. Standard output from the fitaci function.
An is the net photosynthetic rate, Ci the intercellular CO2 concentration. Symbols are measurements, the black line the fitted FvCB model of photosynthesis. Colored lines indicate the two photosynthesis rates in the FvCB model. In the default mode, the fitaci function estimates Vcmax, Jmax and Rd from the fitted curve. Optionally, Rd is provided as an input, for example when it was measured separately. In this example, Vcmax was estimated as 46.8 (SE 1.47), Jmax was 105.2 (SE 1.36) and Rd was 1.3 (SE 0.24). Assumed parameters were Km = 1460 and Γ* = 64.8 (all in units of μmol m-2 s-1). The R2 of a regression of measured vs. fitted was 0.99.
A C4 model of leaf photosynthesis [40] is also implemented (in AciC4), but at the moment it is only possible to fit the C3 model of leaf photosynthesis to A-Ci curves.
Fitting stomatal conductance models
The straightforward fitBB function provides an interface to non-linear or linear regression to fit one of three stomatal conductance models [22–24]. This yields estimates of g1 and (optionally) g0, which are necessary inputs to the coupled leaf gas exchange model. Note that the user must provide stomatal conductance to H2O (not CO2) as input to the fitting process, which is the standard output of portable gas exchange instruments. This function is demonstrated in the example application further below.
Coupled leaf gas exchange model
The intersection of the supply and demand curves of photosynthesis (Fig 1B) gives the steady-state intercellular CO2 concentration (Ci). This is solved by the Photosyn function. This flexible interface can be used to either 1) estimate An when Ci is known (Photosyn(Ci = …); equivalent to Aci(…)), 2) estimate An when gs is known (Photosyn(GS = …)) (cf. Fig 1B) or c) solve for Ci from the coupled leaf gas exchange model (Eqs 1,3 and 4).
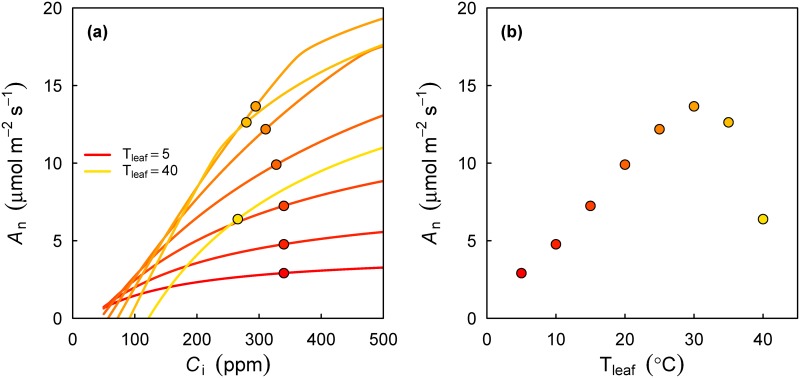
To demonstrate the use of the coupled gas exchange model, I visualize the temperature response of An when both Tleaf and D are varying. In field conditions, D is always strongly positively related to Tleaf. The consequence is that when studying D or Tleaf responses in the field, both drivers have to be accounted for simultaneously [35,41]. Fig 3B shows simulated A-Ci curves and the solutions of the coupled leaf gas exchange models at a range of Tleaf and corresponding D (calculated following [35]). Both Vcmax and Jmax have a peaked response to Tleaf, so that at a given Ci, An first increases with Tleaf and then decreases (lines, Fig 3A). As a result of increasing D, the modelled Ci decreases (symbols, Fig 3A, as a consequence of Eq 4). The net result is a peaked response of An as a function of D (Fig 3B).
Fig 3. Response of An and Ci to combined changes in Tleaf and D.
(A) Lines are A-Ci curves simulated at a range of values for Tleaf. Symbols are the solutions of the coupled leaf gas exchange model, while also taking into account the correlation between D and Tleaf (based on an empirical relationship [35]: D = 0.000605*Tair 2.39). Note that as Tleaf and D increase, Ci decreases. (B) The corresponding temperature optimum of An. Symbols are the same as in panel (A) but plotted against Tleaf.
The simplified code to produce Fig 3B, using the Photosyn function, is given below. Note that in this example the default values of many parameters (e.g. Jmax, g1) are used in the call to Photosyn, but all of these can be set by the user.
# Set range of leaf temperature
tleafs <- seq(5, 40, by = 5)
# Define D as a function of Tleaf
vpdfun <- function(tair)0.000605*tair^2.39
# Simulate.
run1 <- Photosyn(Tleaf = tleafs, VPD = vpdfun(tleafs))
# Plot (produces Fig 3b minus the special formatting)
with(run1, plot(Tleaf, ALEAF))
The Photosyn function assumes that the boundary layer conductance (gbl) is high compared to gs, so that Tleaf is close to Tair. As an alternative, the PhotosynEB function calculates Tleaf from the leaf energy balance. Transpiration is calculated with the Penman-Monteith equation [42], which accounts for boundary layer effects. The details of PhotosynEB are not described here (see the built in help file for more information), because it is very similar to other implementations [28,43].
Numerical solution of optimal stomatal conductance
The FARAO function (FARquhar And Optimality) calculates optimal stomatal conductance based on the Cowan-Farquhar [25] hypothesis that stomata respond to environmental drivers in order to maximize photosynthesis while minimizing water loss. This implementation was used by Medlyn et al. [24] to compare a simplified model of optimal stomatal conductance to the full numerical solution.
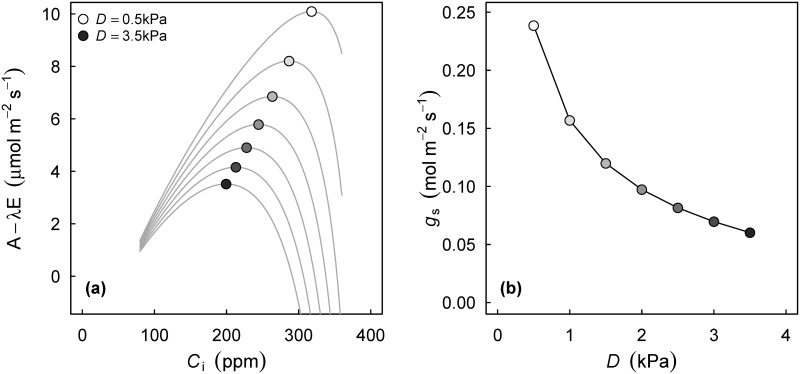
To find optimal stomatal conductance, FARAO finds the Ci for which the quantity An−λE is maximal, where E is the leaf transpiration rate and λ is the marginal cost of water (an empirical parameter related to g1, see [24,25]). An is calculated directly as a function of Ci via the FvCB model (Eq 1), gs is calculated by rearranging Eq 3, and E is calculated assuming perfect coupling (thus E = gsD/Pa, where Pa is atmospheric pressure). This numerical routine does not need specification of an f(D) function as in Eq 4, instead, this function is an emergent property. In Fig 4A, I have calculated An−λE across a range of Ci values, and for different values of D. The FARAO function calculates the optima of these curves, which can for example be used to study the response of stomatal conductance to D (Fig 4B).
Fig 4. Visualization of the optimal model of stomatal conductance.
Provided we have an estimate of the 'cost of water' (λ, mol C mol H2O-1), stomata act to maximize photosynthesis minus transpiration. In (A), individual curves at a range of values for the vapour pressure deficit (D) are plots of A−λE as a function of Ci, demonstrating that an optimum Ci exists. The FARAO function finds this optimum numerically and calculates corresponding An and gs. The corresponding response of gs to D is shown in panel (B).
Optionally, the FARAO function accounts for the presence of a leaf boundary layer (when energybalance = TRUE). In that case it uses PhotosynEB (see description above) to calculate An and E, and solves for Tleaf. A very similar method was employed by Buckley et al. [43], who demonstrated that when a boundary layer is present, frequently an optimal gs cannot be found.
An example application
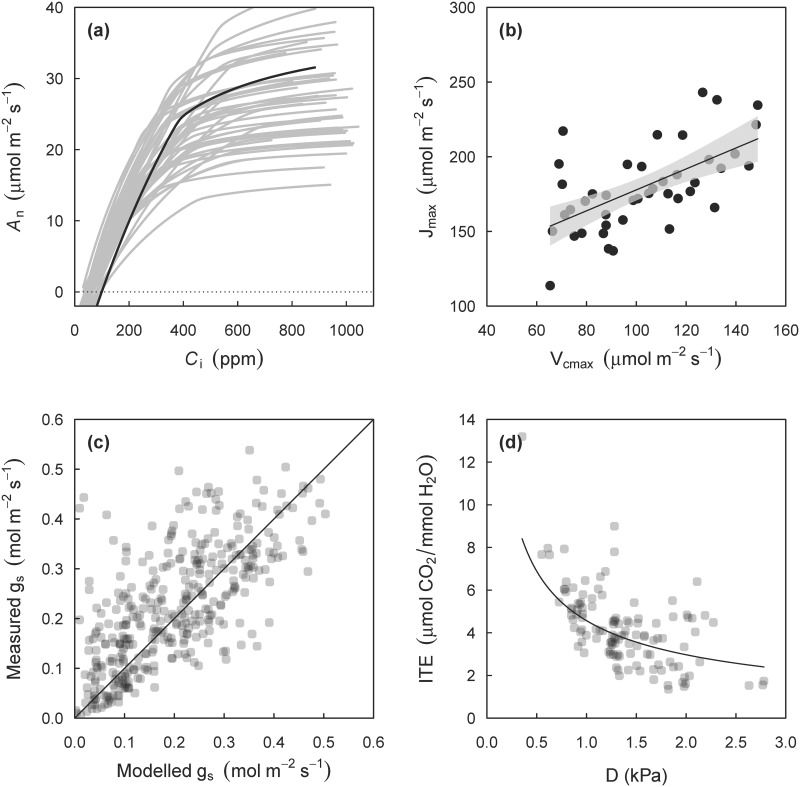
To demonstrate a practical application of the key functions in the package, I use field-collected data from Medlyn et al. [44,45] on Eucalyptus delegatensis. Both A-Ci curves and 'spot gas exchange' data (i.e. leaf gas exchange measurements at prevailing environmental conditions) were collected. Using the fitacis function, it is straightforward to fit all 43 curves to the A-Ci data, and make standard plots of the fitted curves (shown in Fig 5A). The fitted coefficients can be extracted using the coef function, and used to plot a comparison of fitted Vcmax and Jmax values, which show the typical correlation between the two (Fig 5B).
Fig 5. Example application of the plantecophys package to A-Ci curves and spot gas exchange measurements on Eucalyptus delegatensis.
(A) Fitted A-Ci curves with one curve highlighted (B) Estimates of Jmax plotted against Vcmax, obtained from the fitted curves in panel (A). Solid line is a regression line (Jmax = 107.71 + 0.7 Vcmax, R2 = 0.36) with a 95% confidence interval for the mean. (C) Modelled (with the model of Medlyn et al. 2011) versus measured gs (p < 0.0001, R2 = 0.69). Measurements included a wide range of environmental conditions (PAR, Tleaf, D). In this example, only g1 was fit (estimate = 3.31, 95% CI = 3.15–3.47). (D) The predicted response of ITE (An / E) as a function of D from the fitted model in panel (C) (solid line), and the measurements from panel (C) when PAR > 1000.
Next, I fit Eq 4 to the spot gas exchange data, yielding an estimate of g1 (Fig 5C). In this example, I used the model of Medlyn et al. [24], which is given by Eq 6 (in this example, I assumed g0 = 0)
| (6) |
In Fig 5C, modelled gs is compared to measurements. To compare the model prediction of instantaneous transpiration efficiency (An/E) to measurements along the variation in D (Fig 5D), Eq 6 can be rearranged to give (cf. [34], where it is assumed that g0 = 0)
| (7) |
Because fitBB can fit a number of Ball-Berry type variants, the various models can be easily compared in terms of goodness of fit. This simple example application is available in the published repository (see Methods), and simplified code for this example (panels a-c only), omitting special formatting and a few minor settings, is given below.
# Fit A-Ci curves.
# In this case, each separate curve is indexed by a column named 'Curve',
# and the data were already read into a dataframe (tumh)
acifits <- fitacis(tumh, "Curve")
# Plot all A-Ci curves in one panel, highlight one fitted curve.
plot(acifits, "oneplot", highlight = "25")
# Fit Medlyn et al.'s (2011) version of the Ball-Berry model
# Data are already read into a dataframe (tumspot),
# and have standard names (or they can be set).
gfit <- fitBB(tumspot, gsmodel = "BBOpti")
# Plot measured versus modelled, by predicting from the fitted model.
tumspot$GSpred <- predict(gfit$fit, tumspot)
with(tumspot, plot(GSpred, Cond))
Conclusions
We need an open source set of tools to analyse leaf gas exchange data, as these data form a cornerstone of plant physiological ecology. At the moment there are no publicly available tools to fit A-Ci curves or perform simulations with the coupled leaf gas exchange model that can be used as part of a reproducable workflow. The plantecophys R package is implemented in widely used language for data analysis. The package includes a useful set of tools to perform standard, and more advanced, analyses of leaf gas exchange data. The open source framework combined with version control allows further development of the code.
Availability and Requirements
Project name: plantecophys
Project Stable Release: cran.r-project.org/package = plantecophys
Project Home Page: http://www.bitbucket.org/remkoduursma/plantecophys
Project Issue Tracker: http://www.bitbucket.org/remkoduursma/plantecophys/issues
Operating System(s): Platform Independent
Programming Language(s): R
Other Requirements: none
Acknowledgments
David Ellsworth, Belinda Medlyn and Martin De Kauwe are acknowledged for discussions on fitting A-Ci curves. Special thanks to Belinda Medlyn for the code in the MAESTRA model which provided the basis for an early version of the Photosyn function, and for sharing the data used in the example application. Thanks to John Drake for testing and suggesting new features.
Data Availability
The software presented in this paper is available from an online repository (http://www.bitbucket.org/remkoduursma/plantecophys), and the example code in another repository (http://www.github.com/remkoduursma/duursma2015plosone).
Funding Statement
The author has no support or funding to report.
References
- 1. McDermitt DK, Norman JM, Davis JT, Ball TM, Arkebauer TJ, Welles JM, et al. CO2 response curves can be measured with a field-portable closed-loop photosynthesis system. Annales des Sciences Forestieres. 1989;46: 416s–420s. 10.1051/forest:19890593 [DOI] [Google Scholar]
- 2. Leuning R, Sands P. Theory and practice of a portable photosynthesis instrument. Plant, Cell & Environment. 1989;12: 669–678. 10.1111/j.1365-3040.1989.tb01236.x [DOI] [Google Scholar]
- 3. Long SP, Bernacchi CJ. Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources of error. Journal of Experimental Botany. 2003;54: 2393–2401. 10.1093/jxb/erg262 [DOI] [PubMed] [Google Scholar]
- 4. Pearcey R, Mooney HA, Rundel P. Plant Physiological Ecology: Field methods and instrumentation. Springer; 1989. [Google Scholar]
- 5. Bonan GB, Williams M, Fisher RA, Oleson KW. Modeling stomatal conductance in the earth system: Linking leaf water-use efficiency and water transport along the soil—plant—atmosphere continuum. Geosci Model Dev. 2014;7: 2193–2222. 10.5194/gmd-7-2193-2014 [DOI] [Google Scholar]
- 6. De Kauwe MG, Kala J, Lin Y-S, Pitman AJ, Medlyn BE, Duursma RA, et al. A test of an optimal stomatal conductance scheme within the CABLE land surface model. Geosci Model Dev. 2015;8: 431–452. 10.5194/gmd-8-431-2015 [DOI] [Google Scholar]
- 7. Farquhar GD, Caemmerer S, Berry JA. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta. 1980;149: 78–90. 10.1007/BF00386231 [DOI] [PubMed] [Google Scholar]
- 8. Sellers PJ, Dickinson RE, Randall DA, Betts AK, Hall FG, Berry JA, et al. Modeling the Exchanges of Energy, Water, and Carbon Between Continents and the Atmosphere. Science. 1997;275: 502–509. 10.1126/science.275.5299.502 [DOI] [PubMed] [Google Scholar]
- 9. von Caemmerer S, Farquhar G. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta. 1981;153: 376–387. 10.1007/BF00384257 [DOI] [PubMed] [Google Scholar]
- 10. Bernacchi CJ, Singsaas EL, Pimentel C, Portis AR Jr, Long SP. Improved temperature response functions for models of Rubisco-limited photosynthesis. Plant, Cell & Environment. 2001;24: 253–259. 10.1111/j.1365-3040.2001.00668.x [DOI] [Google Scholar]
- 11. Medlyn BE, Dreyer E, Ellsworth D, Forstreuter M, Harley PC, Kirschbaum MUF, et al. Temperature response of parameters of a biochemically based model of photosynthesis. II. a review of experimental data. Plant, Cell & Environment. 2002;25: 1167–1179. [Google Scholar]
- 12. Wullschleger SD. Biochemical Limitations to Carbon Assimilation in C3 Plants—A Retrospective Analysis of the A/Ci Curves from 109 Species. Journal of Experimental Botany. 1993;44: 907–920. 10.1093/jxb/44.5.907 [DOI] [Google Scholar]
- 13. Sharkey T, Imai K, Farquhar GD, Cowan I. A direct confirmation of the standard method of estimating intercellular partial pressure of CO2 . Plant physiology. 1982;69: 657–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Flexas J, Ribas-Carbó M, Diaz-Espejo A, Galmés J, Medrano H. Mesophyll conductance to CO2: Current knowledge and future prospects. Plant, Cell & Environment. 2008;31: 602–621. 10.1111/j.1365-3040.2007.01757.x [DOI] [PubMed] [Google Scholar]
- 15. Tholen D, Ethier G, Genty B, Pepin S, Zhu X-G. Variable mesophyll conductance revisited: Theoretical background and experimental implications. Plant, Cell & Environment. 2012;35: 2087–2103. 10.1111/j.1365-3040.2012.02538.x [DOI] [PubMed] [Google Scholar]
- 16. Sharkey TD, Bernacchi CJ, Farquhar GD, Singsaas EL. Fitting photosynthetic carbon dioxide response curves for C3 leaves. Plant, Cell & Environment. 2007;30: 1035–1040. 10.1111/j.1365-3040.2007.01710.x [DOI] [PubMed] [Google Scholar]
- 17.Gu L. LeafWeb. Accessed: 2015-07-09. Available: http://leafweb.ornl.gov
- 18. Gu L, Pallardy SG, Tu K, Law BE, Wullschleger SD. Reliable estimation of biochemical parameters from C3 leaf photosynthesis—intercellular carbon dioxide response curves. Plant, Cell & Environment. 2010;33: 1852–1874. 10.1111/j.1365-3040.2010.02192.x [DOI] [PubMed] [Google Scholar]
- 19.De Kauwe M. FitFarquharModel. Accessed: 2015-07-09. Available: https://github.com/mdekauwe/FitFarquharModel;
- 20.Tu F K.P. LandFlux tools. Accessed: 2015-07-09. Available: http://landflux.org/Tools.php;
- 21. Farquhar G, Sharkey T. Stomatal conductance and photosynthesis. Annual Review of Plant Physiology. 1982;33: 317–345. [Google Scholar]
- 22. Ball JT, Woodrow I, Berry JA. A model predicting stomatal conductance and its contribution to the control of photosynthesis under different environmental conditions In: Biggins J, editor. Progress in photosynthesis research. Dordrecht, the Netherlands; 1987. pp. 221–224. [Google Scholar]
- 23. Leuning R. A critical-appraisal of a combined stomatal-photosynthesis model for C3 plants. Plant, Cell & Environment. 1995;18: 339–355. [Google Scholar]
- 24. Medlyn B, Duursma R, Eamus D, Ellsworth D, Prentice I, Barton C, et al. Reconciling the optimal and empirical approaches to modelling stomatal conductance. Global Change Biology. 2011;17: 2134–2144. 10.1111/j.1365-2486.2010.02375.x [DOI] [Google Scholar]
- 25. Cowan I, Farquhar GD. Stomatal function in relation to leaf metabolism and environment. Symposia of the Society for Experimental Biology. 1977;31: 471–505. [PubMed] [Google Scholar]
- 26. Leuning R. Modelling stomatal behaviour and photosynthesis of Eucalyptus grandis . Australian Journal of Plant Physiology. 1990;17: 159–175. [Google Scholar]
- 27. Collatz G, Ball J, Grivet C, Berry J. Physiological and environmental regulation of stomatal conductance, photosynthesis and transpiration: A model that includes a laminar boundary layer. Agricultural and Forest Meteorology. 1991;54: 107–136. [Google Scholar]
- 28. Wang YP, Leuning R. A two-leaf model for canopy conductance, photosynthesis and partitioning of available energy I: Model description and comparison with a multi-layered model. Agricultural and Forest Meteorology. 1998;91: 89–111. [Google Scholar]
- 29. Duursma RA, Medlyn BE. MAESPA: A model to study interactions between water limitation, environmental drivers and vegetation function at tree and stand levels, with an example application to [CO2] × drought interactions. Geosci Model Dev. 2012;5: 919–940. 10.5194/gmd-5-919-2012 [DOI] [Google Scholar]
- 30. R Core Team. R: A language and environment for statistical computing [Internet]. Vienna, Austria: R Foundation for Statistical Computing; 2015. Available: http://www.R-project.org/ [Google Scholar]
- 31. Barton CVM, Duursma RA, Medlyn BE, Ellsworth DS, Eamus D, Tissue DT, et al. Effects of elevated atmospheric [CO2] on instantaneous transpiration efficiency at leaf and canopy scales in Eucalyptus saligna. Global Change Biology. 2012;18: 585–595. 10.1111/j.1365-2486.2011.02526.x [DOI] [Google Scholar]
- 32. Peltoniemi MS, Duursma RA, Medlyn BE. Co-optimal distribution of leaf nitrogen and hydraulic conductance in plant canopies. Tree Physiology. 2012;32: 510–519. 10.1093/treephys/tps023 [DOI] [PubMed] [Google Scholar]
- 33. Medlyn BE, Duursma RA, De Kauwe MG, Prentice IC. The optimal stomatal response to atmospheric CO2 concentration: Alternative solutions, alternative interpretations. Agricultural and Forest Meteorology. 2013;182–183: 200–203. 10.1016/j.agrformet.2013.04.019 [DOI] [Google Scholar]
- 34. Duursma RA, Payton P, Bange MP, Broughton KJ, Smith RA, Medlyn BE, et al. Near-optimal response of instantaneous transpiration efficiency to vapour pressure deficit, temperature and [CO2] in cotton (Gossypium hirsutum l.). Agricultural and Forest Meteorology. 2013;168: 168–176. 10.1016/j.agrformet.2012.09.005 [DOI] [Google Scholar]
- 35. Duursma RA, Barton CVM, Lin Y-S, Medlyn BE, Eamus D, Tissue DT, et al. The peaked response of transpiration rate to vapour pressure deficit in field conditions can be explained by the temperature optimum of photosynthesis. Agricultural and Forest Meteorology. 2014;189–190: 2–10. 10.1016/j.agrformet.2013.12.007 [DOI] [Google Scholar]
- 36. Lin Y-S, Medlyn BE, Duursma RA, Prentice IC, Wang H, Baig S, et al. Optimal stomatal behaviour around the world. Nature Climate Change. 2015;5: 459–464. 10.1038/nclimate2550 [DOI] [Google Scholar]
- 37.Duursma RA. Code repository to generate figures and manuscript. Database: github [Internet]. Available: https://github.com/RemkoDuursma/duursma2015plosone, 10.5281/zenodo.32026
- 38. Ritz C, Streibig JC. Nonlinear regression with R. Springer; 2008. [Google Scholar]
- 39. Ethier G, Livingston N. On the need to incorporate sensitivity to CO2 transfer conductance into the Farquhar—von Caemmerer—Berry leaf photosynthesis model. Plant, Cell & Environment. 2004;27: 137–153. [Google Scholar]
- 40. von Caemmerer S. Biochemical models of leaf photosynthesis. Csiro Publishing; 2000. [Google Scholar]
- 41. Lin Y-S, Medlyn BE, Ellsworth DS. Temperature responses of leaf net photosynthesis: The role of component processes. Tree Physiology. 2012;32: 219–231. 10.1093/treephys/tpr141 [DOI] [PubMed] [Google Scholar]
- 42. Jones HG. Plants and microclimate: A quantitative approach to environmental plant physiology. 2nd Edition 2nd ed. Cambridge: Cambridge University Press; 1992. [Google Scholar]
- 43. Buckley TN, Martorell S, Diaz-Espejo A, Tomàs M, Medrano H. Is stomatal conductance optimized over both time and space in plant crowns? A field test in grapevine (Vitis vinifera). Plant, Cell & Environment. 2014;37: 2707–2712. 10.1111/pce.12343 [DOI] [PubMed] [Google Scholar]
- 44. Medlyn BE, Pepper DA, O’Grady AP, Keith H. Linking leaf and tree water use with an individual-tree model. Tree Physiology. 2007;27: 1687–1699. [DOI] [PubMed] [Google Scholar]
- 45. Medlyn B, Pepper D, Keith H. Tumbarumba gas exchange. Database: figshare [Internet]. 10.6084/m9.figshare.1538079 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The software presented in this paper is available from an online repository (http://www.bitbucket.org/remkoduursma/plantecophys), and the example code in another repository (http://www.github.com/remkoduursma/duursma2015plosone).