Abstract
Objective
To describe the attitudes of patients and their mental health providers regarding participation in a controlled trial of directly monitored naltrexone (NTX) treatment for alcohol dependence in schizophrenia.
Method
Ninety participants with schizophrenia and their providers were asked to report opinions of treatment with oral NTX or placebo 3 times per week for 12 weeks, motivational counseling (MI), and voucher-based incentives (VBI) for attendance
Results
Seventy-nine percent of participants “liked the study a lot,” and 94% reported that it was helpful. Study components rated as helpful by participants were: VBI (95% of participants), meeting with staff 3 times per week (84%), reporting alcohol use (82%), MI (82%), reporting psychiatric symptoms (73%), breath alcohol testing (72%), and study medication (57%). Benefits reported by patients were: feeling better mentally (67%), drinking less (52%), feeling better physically (49%), and stopping drinking (27%). Seventy percent of providers reported that the study was helpful. Benefits noted by providers included: reduced drinking (33%), better treatment adherence (32%), stopping drinking (23%), and reduced psychiatric symptoms (22%). Patient/provider responses agreed on helpfulness with stopping or reducing drinking.
Conclusions
Most participants with schizophrenia liked participating in a clinical trial of directly observed naltrexone treatment for alcohol dependence, and found incentives for attendance, frequent staff contact and monitoring of drinking, and motivational counseling to be the most helpful. Most participants reported improvement in mental health and reduced drinking. Mental health providers also reported that the study was helpful, but they did not describe the same degree of benefit as did patients.
Keywords: Alcohol dependence, attitudes of patients and clinical providers, naltrexone treatment, schizophrenia
INTRODUCTION
Comorbid substance use disorders (SUDs) occur more frequently in patients with schizophrenia-spectrum disorders than in the population at large (1). Comorbid SUDs and serious mental illness present complex treatment challenges and are associated with increased problems including violence (2), crime (3), suicide (4), and occupational, housing and economic problems (5). Recent meta-analyses of clinical trials in individuals with severe and persistent mental illness such as schizophrenia have found poor retention in treatment, with pooled dropout rates ranging from 28 to 55% (e.g., 6). Comorbid alcohol and other substance abuse further impair treatment engagement, retention, and adherence (7, 8).
Naltrexone is an effective pharmacotherapy for alcohol dependence in the general population (9), and studies have found that it may be effective in the treatment of comorbid alcohol dependence in patients with schizophrenia (10–12). Adherence is essential to naltrexone's effectiveness. Recent studies have shown significantly better outcomes in patients who demonstrated adherence to more than 80% of possible naltrexone doses (e.g., 13). Given these findings, and the observed problems with adherence in patients with comorbid SMI and SUDs, it is important to identify ways to maximize adherence to naltrexone. Patient reports regarding the acceptability of naltrexone treatment in a clinical research setting can be reasonably expected to correlate with the likelihood of treatment adherence to medication. Learning about patient attitudes concerning various aspects of naltrexone treatment may contribute to the development of strategies for maximizing adherence to medications and counseling for alcohol dependence patients with serious mental illness. It could also help inform future research involving patients with these comorbid conditions improve research design and maximize retention and adherence to study procedures. To our knowledge, there are no published reports on the attitudes of patients with schizophrenia toward clinical trials in the treatment of alcohol or other substance dependence. The aim of this report is to describe the perceptions of these patients following participation in a trial of naltrexone treatment.
METHOD
This report analyzes data on 90 participants who completed a NIAAA-funded controlled clinical trial of directly observed treatment with oral naltrexone or placebo for alcohol use disorders in schizophrenia (12). Data were collected from November 2003 to June 2008. This report provides analysis on the attitudes of participants and their clinical providers regarding the clinical trial. Participants were recruited from community mental health clinics in Syracuse, New York, and provided written informed consent approved by the SUNY Upstate Medical University Institutional Review Board. All participants were prescribed antipsychotic medications by their clinical treatment providers. Baseline demographic and clinical characteristics of participants are outlined in Tables 1 and 2, respectively. Overall, the sample was primarily male, single, Caucasian, unemployed, with low-income, most often receiving disability payments. The clinical trial required participants to attend three visits per week for directly-observed treatment with oral naltrexone or placebo over 12 weeks. All participants received weekly motivational counseling sessions and were seen in research offices located at their respective outpatient clinical sites. If needed, participants were provided assistance with transportation (e.g., bus tokens, bus passes, or taxis).
TABLE 1.
Sociodemographic characteristics of study participants (N = 90)
| Characteristic | N | % |
|---|---|---|
| Gender | 64 | 71 |
| Male | 26 | 29 |
| Race | ||
| Caucasian | 38 | 42 |
| African-American | 37 | 41 |
| Other | 15 | 17 |
| Ethnicity | ||
| Non-Hispanic | 85 | 94 |
| Hispanic | 5 | 6 |
| Married | 6 | 7 |
| Living independently | 64 | 71 |
| Income source | ||
| Disability | 59 | 66 |
| Public Assistance/Welfare | 17 | 19 |
| Other | 10 | 11 |
| Employment | 4 | 4 |
| Age (M ± SD) | 42 ± 9 | |
| Education (M ± SD) | 12 ± 2 | |
| Monthly income (M ± SD), Median, IQR | $659 ± $347, $666 | [$495, $756] |
TABLE 2.
Clinical characteristics of study participants (N = 90)
| N | % | Range | Percentile and interpretation | ||
|---|---|---|---|---|---|
| Diagnosis | |||||
| Schizophrenia | 45 | 50 | |||
| Schizoaffective Disorder | 45 | 50 | |||
| Alcohol Abuse | 4 | 4 | |||
| Alcohol Dependence | 86 | 96 | |||
| Mean | SD | ||||
| PANSSa | |||||
| Positive | 15.4 | 5.2 | 7.0–31.0 | 21, Low | |
| Negative | 13.5 | 4.9 | 7.0–30.0 | 12, Low | |
| General | 32.3 | 7.1 | 20.0–49.0 | 24, Low | |
| Composite | 1.9 | 7.4 | –16.0–+20.0 | 66, Average | |
| CDSSb | 5.2 | 4.0 | .0–15.0 | 74% specificity, 100% sensitivity for depression | |
| ASIc | Mean | Median | SD | Range | |
| Alcohol Composite | .53 | .54 | .21 | .12–.92 | |
| TLFBd | |||||
| Number of drinking days | 3.0 | 3.0 | 2.4 | 0–7 | |
| Drinks per week | 36.5 | 21.4 | 46.3 | 0–268.8 | |
| Number of heavy drinking days | 2.2 | 1.0 | 2.3 | 0–7 | |
Positive and Negative Syndrome Scale. Possible range of scores:
Positive 7–49; Negative 7–49, General 16–112; Composite –42–+42.
PANSS “Low” and “Average” refer to inpatient sample of individuals with schizophrenia on which PANSS was normed.
Calgary Depression Scale for Schizophrenia. Possible range of scores: 0–27.
ASI = Alcohol Severity Index, last 30 days. Possible range of scores 0–1, Higher number indicates higher severity for Alcohol Composite.
TLFB = Timeline Followback, baseline week.
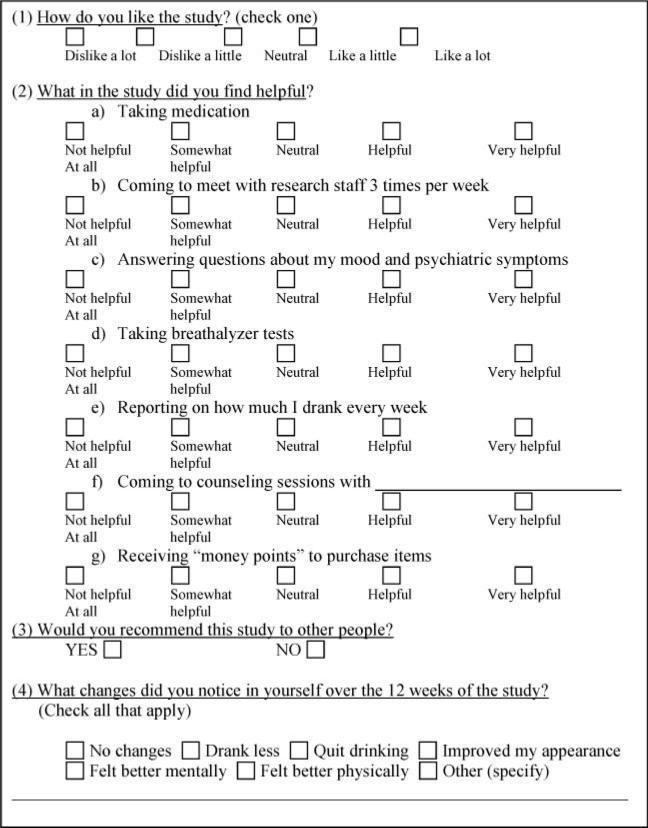
A voucher-based incentive system with monetary value was employed to reinforce attendance and to reimburse participants for their time. The following measures were administered at baseline and periodically over the course of the study: Positive and Negative Syndrome Scale [PANSS] (14); Calgary Depression Scale for Schizophrenia [CDSS] (15); Addiction Severity Index [ASI] (16); Time Line Follow Back [TLFB] (17); and breath alcohol testing. At the end of treatment, participants completed an End-of-Study Questionnaire (constructed by the study team) to obtain self-report of perceptions and attitudes regarding the helpfulness of various components of the study. Participants’ primary clinical mental health providers (N = 90) were also mailed a questionnaire about the helpfulness of the clinical trial for their respective patients. End-of-Study Questionnaires for participants and providers are displayed in Figs. 1 and 2, respectively. We measured the frequencies of participants’ and providers’ responses to the questionnaires. McNemar test of agreement with exact tests of significance was used to analyze agreement between participants and providers on certain outcome points. Data was analyzed using SPSS (v14.1) for Windows.
FIG. 1.
End-of-Study Questionnaire (participants).
FIG. 2.
End-of-Study Questionnaire (providers).
RESULTS
Seventy-nine (88%) of study participants completed the End-of-Study Questionnaire. Eleven (12%) did not complete the questionnaire because they had dropped out of the study and could not be reached. Seventy-nine percent of those completing the questionnaire reported that they liked the study “a lot,” 9% indicated that they liked the study “a little,” 10% indicated that they felt “neutral” about the study, and only 1% indicated that they “dislike the study a little.” Table 3 displays components of the treatment study that participants marked as “helpful” and “very helpful.” VBI was the study component endorsed as being helpful by nearly all (95%) participants, and over 80% reported that it was helpful for them to meet with research staff three times per week, to be asked to report how much alcohol they consumed each week, and to attend counseling sessions. The majority also reported that they found it helpful to be asked questions about mood and psychiatric symptoms, to undergo breath alcohol testing, and to take study medication. Ninety-six percent of participants indicated that they would recommend the study to others. The following is a summary of the proportion of participants who perceived various changes as a result of study involvement: feeling better mentally (67%) drinking less (52%), feeling better physically (49%), quitting drinking (27%), and having improved their appearance (35%). Only 6% of participants perceived no changes in themselves during the study. Patients’ responses to a request for “other comments,” an open-ended item on the questionnaire, could be categorized into the following themes of how they perceived the study to be helpful: (a) helped the participant to save money that would have been used for alcohol; (b) helped the participant to express feelings, and become more aware of drinking; (c) helped the participant to improve health; (d) helped the participant to cut down on the use of other drugs.
TABLE 3.
Study components that participants indicated as “helpful”/“very helpful”
| Component | N | % |
|---|---|---|
| Receiving “money points” (incentives) to purchase items | 75 | 95 |
| Meeting with research staff 3 times per week | 66 | 84 |
| Reporting at each visit on how much they drank | 65 | 82 |
| Participating in counseling sessions | 65 | 82 |
| Answering questions about mood and psychiatric symptoms | 58 | 73 |
| Taking breathalyzer tests | 57 | 72 |
| Taking study medication | 45 | 57 |
Seventy (78%) of providers completed the End-of Study Questionnaire. A majority of providers (64%) indicated that they had worked with their patients for more than 6 months. Seventy percent of responding providers reported that the study was helpful to their patients. Thirty-three percent of the providers indicated that their patients reduced drinking during the study, and 23% reported that their patients had stopped drinking. Thirty-two percent of providers reported improvement in their patients’ adherence to their primary mental health treatment while in the study. Twenty-two percent of providers reported a reduction in their patients’ psychiatric symptoms, and 10% reported that their patients improved their appearance. Providers’ comments on the open-section of the questionnaire could be summarized into the following categories of how the study helped patients: (a) helped patients to increase awareness of drinking and knowledge about alcohol; (b) provided support and meaningful activity; (c) improved attendance to the clinical treatment program; (d) helped to reduce other substance use.
A McNemar test of agreement, with exact tests of significance between patient and provider responses, revealed that patients and providers agreed in their ratings of the study's helpfulness in terms of quitting and reducing drinking, but disagreed on the areas of improved appearance (p = .002), and reduction of psychiatric symptoms (p < .001), with more participants than providers reporting positive outcomes.
DISCUSSION
This report analyzes the attitudes and perceptions of research participants and their clinical providers regarding participation in a 12-week, double blind, placebo-controlled study of naltrexone treatment for alcohol use disorders in schizophrenia. To our knowledge, this is the first report that describes the opinions of patients with schizophrenia/schizoaffective disorders and comorbid AUDs regarding participation in a clinical trial of alcohol or substance abuse treatment. Since the study of naltrexone treatment involved multiple components (e.g., medication, counseling, incentives, frequent attendance, close and frequent monitoring, and the administration of psychiatric and substance use measures), it was important to attempt to identify which components were the most salient for participants, in order to inform the design of clinical programs and research trials for this patient population and assist clinicians and clinical investigators to maximize adherence to naltrexone or other treatments.
Based on the results of this study, the most helpful component for participants was the provision of voucher-based incentives for attendance. This finding is consistent with previous literature on the successful use of incentives to reinforce behavior change in patients with schizophrenia (10, 18, 19). The next most helpful aspects of the study for participants were the close monitoring provided by attending research visits three times per week and their report on drinking behavior to research staff. Prior to implementing the study, we were concerned that three times per week study visits may be too time-consuming, place unacceptable demands on patients, and be perceived as too burdensome by them. However, our findings indicate that frequent visits were well-accepted by patients, possibly related to the provision of incentives for attendance and the high level of attention from research staff associated with study visits. A majority of participants also found it helpful to provide an account of their drinking behavior at each study visit. Frequent monitoring of the amount of alcohol consumed between visits may be perceived by patients as an indication of concern regarding patients’ well-being as well as their drinking behaviors. Close monitoring may work synergistically with taking medication and receiving motivational counseling to help patients change their drinking behavior.
Motivational counseling was rated as helpful by 82% of our patients. This type of counseling is typically used to explore the benefits and costs of drinking, and to help patients build motivation for change (20). Motivational counseling with patients diagnosed with schizophrenia and substance use disorders may be helpful to patients as a tool to highlight strengths and build a sense of self-efficacy.
The study components described above as being rated as the most helpful by participants—incentives, frequent study visits, reporting alcohol use, and motivational counseling—may be appropriate not only to improve the treatment of substance use disorders in patients with schizophrenia but could also be applied to the design of clinical research trials to maximize attendance and adherence to the study intervention.
Reporting on psychiatric symptoms, breath alcohol testing, and taking study medication were also reported to be helpful by a majority of participants. This finding appears to suggest that patients with schizophrenia find the monitoring of symptoms and breath alcohol highly acceptable to them, countering possible concerns that these activities may be perceived as intrusive or unpleasant. These findings may provide encouragement to clinicians and investigators who wish to closely examine substance use behavior among patients with serious mental illness.
Soliciting the opinion of the primary mental health provider creates a bridge between the research and clinical setting, demonstrates respect for the clinician's opinion, and provides an opportunity for researchers to make improvements in subsequent similar trials. Our results showed that a majority of providers had worked with their patients for a substantial amount of time, and they thought the study was helpful to their patients. An important finding was that one-third of the clinicians reported that their patients reduced their drinking and adhered better to their primary clinical treatment. The fact that patients and providers agreed on the helpfulness of the study in terms of helping the patients to quit and/or reduce drinking (79% of patients reported that they quit and/or reduced drinking; 56% of therapists reported that their patients quit and/or reduced drinking) provides further data to support the conclusion that participation in the study helped to change drinking behavior (12). Provider feedback highlighted that a close communication between research staff and the primary therapist is important, and that a patient's participation in a clinical trial may increase the benefits of the primary clinical treatment.
ACKNOWLEDGMENTS
This work was primarily supported by NIAAA grant 5RO1 AA013655-04, Naltrexone Treatment of Alcohol Abuse in Schizophrenia. Dr. Batki also received support from NIDA grant U10 DA015815 and from the Veterans Administration grant for the VISN 21 Center for Integrated Healthcare (CIH). Portions of this manuscript were presented at the 2008 Annual Meeting of the Research Society on Alcoholism.
Footnotes
Declaration of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
Contributor Information
Luba Leontieva, SUNY Upstate Medical University, Psychiatry, Syracuse, New York, USA
Jacqueline Dimmock, SUNY Upstate Medical University, Psychiatry, Syracuse, New York, USA
Michelle Cavallerano, SUNY Upstate Medical University, Psychiatry, Syracuse, New York, USA
Sara DeRycke, SUNY Upstate Medical University, Psychiatry, Syracuse, New York, USA
Zsuzsa Meszaros, SUNY Upstate Medical University, Psychiatry, Syracuse, New York, USA
Kate Carey, Syracuse University, Psychology, Syracuse, New York, USA
Robert Ploutz-Snyder, Universities Space Research Association, NASA JSC, Houston, Texas, USA
Steven L. Batki, University of California, San Francisco, Psychiatry, San Francisco, VA, Medical Center, San Francisco, California, USA
REFERENCES
- 1.Westermeyer J. Comorbid schizophrenia and substance abuse: a review of epidemiology and course. Am J Addict. 2006;15:345–355. doi: 10.1080/10550490600860114. [DOI] [PubMed] [Google Scholar]
- 2.Walsh E, Buchanan A, Fahy T. Violence and schizophrenia: examining the evidence. Br J Psychiatry. 2002;180:490–495. doi: 10.1192/bjp.180.6.490. [DOI] [PubMed] [Google Scholar]
- 3.Wallace C, Mullen PE, Burgess P. Criminal offending in schizophrenia over a 25-year period marked by deinstitutionalization and increasing prevalence of comorbid substance use disorders. Am J Psychiatry. 2004;161(4):716–727. doi: 10.1176/appi.ajp.161.4.716. [DOI] [PubMed] [Google Scholar]
- 4.Hunt IM, Kapur N, Windfuhr K, Robinson J, Bickley H, Flynn S, Parsons R, Burns J, Shaw J, Appleby L. Suicide in schizophrenia: findings from a national clinical survey. J Psychiatr Pract. 2006;12(3):139–147. doi: 10.1097/00131746-200605000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Compton MT, Weiss PS, West JC, Kaslow NJ. The associations between substance use disorders, schizophrenia-spectrum disorders, and Axis IV psychosocial problems. Soc Psychiatry Psychiatr Epidemiol. 2005;40(12):939–946. doi: 10.1007/s00127-005-0964-4. [DOI] [PubMed] [Google Scholar]
- 6.Kemmler G, Hummer M, Widschwendter C, Fleischhacker WW. Dropout rates in placebo-controlled and active-control clinical trials of antipsychotic drugs: A meta-analysis. Archives of General Psychiatry. 2005;62:1305–1312. doi: 10.1001/archpsyc.62.12.1305. [DOI] [PubMed] [Google Scholar]
- 7.Coodin S, Staley D, Cortens B, et al. Patient factors associated with missed appointments in persons with schizophrenia. Canadian Journal of Psychiatry. 2004;49:145–148. doi: 10.1177/070674370404900210. [DOI] [PubMed] [Google Scholar]
- 8.Valenstein M, Blow FC, Copeland LA, McCarthy JF, Zeber JE, Gillon L, Bingham CR, Stavenger T. Poor antipsychotic adherence among patients with schizophrenia: medication and patient factors. Schizophrenia Bulletin. 2004;30:255–264. doi: 10.1093/oxfordjournals.schbul.a007076. [DOI] [PubMed] [Google Scholar]
- 9.Srisurapanont M, Jarusuraisin N. Naltrexone for the treatment of alcoholism: A meta-analysis of randomized controlled trials. International Journal of Neuropsychopharmacology. 2005;8:267–280. doi: 10.1017/S1461145704004997. [DOI] [PubMed] [Google Scholar]
- 10.Petrakis IL, O'Malley S, Rounsaville B, Poling J, McHugh-Strong C, Krystal JH, VA Naltrexone Study Collaboration Group Naltrexone augmentation of neuroleptic treatment in alcohol abusing patients with schizophrenia. [Erratum appears in Psychopharmacology (Berl). 2004; 174(2):300.] Psychopharmacology. 2004;172:291–297. doi: 10.1007/s00213-003-1658-9. [DOI] [PubMed] [Google Scholar]
- 11.Batki SL, Dimmock JA, Wade M, Gately PW, Cornell M, Maisto SA, Carey KB, Ploutz-Snyder R. Monitored naltrexone without counseling for alcohol abuse/dependence in schizophrenia-spectrum disorders. American Journal on Addictions. 2007a;16:253–259. doi: 10.1080/10550490701389732. [DOI] [PubMed] [Google Scholar]
- 12.Batki SL, Dimmock JA, Ploutz-Snyder R, Carey KB, Maisto SA, Cavallerano M, Gallinger L, Leontieva L. Alcoholism Clinical and Experimental Research. 6: suppl. Vol. 31. 255A: 2007b. Naltrexone treatment for patients with schizophrenia and alcohol dependence: The role of adherence—Preliminary findings. (abstract S028) [Google Scholar]
- 13.Chick J, Anton R, Checinski K, Croop R, Drummond DC, Farmer R, Labriola D, Marshall J, Moncrieff J, Morgan MY, Peters T, Ritson B. A multicentre, randomized, double-blind, placebo controlled trial of naltrexone in the treatment of alcohol dependence or abuse. Alcohol Alcoholism. 2000;35:587–93. doi: 10.1093/alcalc/35.6.587. [DOI] [PubMed] [Google Scholar]
- 14.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 15.Addington D, Addington J, Maticka-Tyndale E. Assessing depression in schizophrenia: the Calgary Depression Scale. British Journal of Psychiatry. 1993;(Supplementum):39–44. [PubMed] [Google Scholar]
- 16.McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The fifth edition of the Addiction Severity Index. Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- 17.Sobell LC SM. Timeline Follow-Back. The Humana Press, Inc.; Totowa, NJ: 1992. [Google Scholar]
- 18.Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction. 2006;101:192–203. doi: 10.1111/j.1360-0443.2006.01311.x. [DOI] [PubMed] [Google Scholar]
- 19.Leontieva L, Dimmock JA, Gately PW, Gallinger L, Ploutz-Snyder R, Batki SL. Voucher-based incentives for naltrexone treatment attendance in schizophrenia and alcohol use disorders. Psychiatric Services. 2008;59(3):310–317. doi: 10.1176/ps.2008.59.3.310. [DOI] [PubMed] [Google Scholar]
- 20.Carey KB, Leontieva L, Dimmock J, et al. Adapting motivational interventions for comorbid schizophrenia and alcohol use disorders. Clinical Psychology: Science and Practice. 2007;14(1):39–57. doi: 10.1111/j.1468-2850.2007.00061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]