Abstract
Context
Since 1995, the International Classification of Rhabdomyosarcoma has provided prognostically relevant classification for rhabdomyosarcoma (RMS) and allowed risk stratification for children with RMS. The International Classification of Rhabdomyosarcoma includes botryoid and spindle cell RMS as superior-risk groups, embryonal RMS as an intermediate-risk group, and alveolar RMS as an unfavorable-risk group. The 2013 World Health Organization (WHO) classification of skeletal muscle tumors modified the histologic classification of RMS to include sclerosing RMS as a type of spindle cell RMS separate from embryonal RMS. The current WHO classification includes embryonal, alveolar, spindle cell/sclerosing, and pleomorphic subtypes of RMS and does not separate the botryoid subtype.
Objective
To determine if the WHO classification applies to pediatric RMS.
Design
To accomplish this goal, we reviewed 9 consecutive Children’s Oncology Group clinical trials to compare the WHO and International Classification of Rhabdomyosarcoma classifications with outcome and site of disease.
Results
Except for a subset of low-risk RMS, the outcome for botryoid was not significantly different from typical embryonal RMS when analyzed by primary site. Similarly, pediatric spindle cell and sclerosing patterns of RMS did not appear significantly different from typical embryonal RMS, with one exception: spindle cell RMS in the parameningeal region had an inferior outcome with 28% event-free survival.
Conclusion
Our data support use of the WHO RMS classification in the pediatric population, with the caveat that histologic diagnosis does not necessarily confer the same prognostic information in children as in adults.
The International Classification of Rhabdomyosarcoma (ICR) provided a prognostically relevant classification system that has been in use since 1995.1 This system included histologic subtypes with a superior prognosis (botryoid and spindle cell rhabdomyosarcoma [RMS]), an intermediate prognosis (typical embryonal RMS) and a poor prognosis (alveolar RMS). Our concepts of RMS classification have undergone several modifications since publication of the ICR. The ICR indicated that any amount of an alveolar pattern was diagnostic of alveolar RMS, but this resulted in an increase in the incidence of alveolar RMS from a range of 20% to 25% to a range of 40% to 45% of all RMS as compared with earlier studies.2 Additionally, newer subtypes of RMS, such as sclerosing RMS, have been described since publication of the ICR.3,4 The relationship of sclerosing RMS to embryonal RMS has not been clear, although lack of a PAX gene fusion appears to separate it from alveolar RMS.
A recent rereview of alveolar RMS in patients enrolled in Children’s Oncology Group (COG) studies from 1999 to 2005 examined shifts in histologic classification as a result of the ICR. This study highlighted many histologic mimics of alveolar RMS. In particular, the dense pattern of embryonal RMS may closely mimic solid alveolar RMS, and sclerosing RMS is often confused for classic alveolar RMS. Rereview resulted in the reclassification of approximately 30% of tumors originally classified as alveolar, the most common reason for reclassification being the presence of at least focal dense or sclerosing patterns of RMS.2
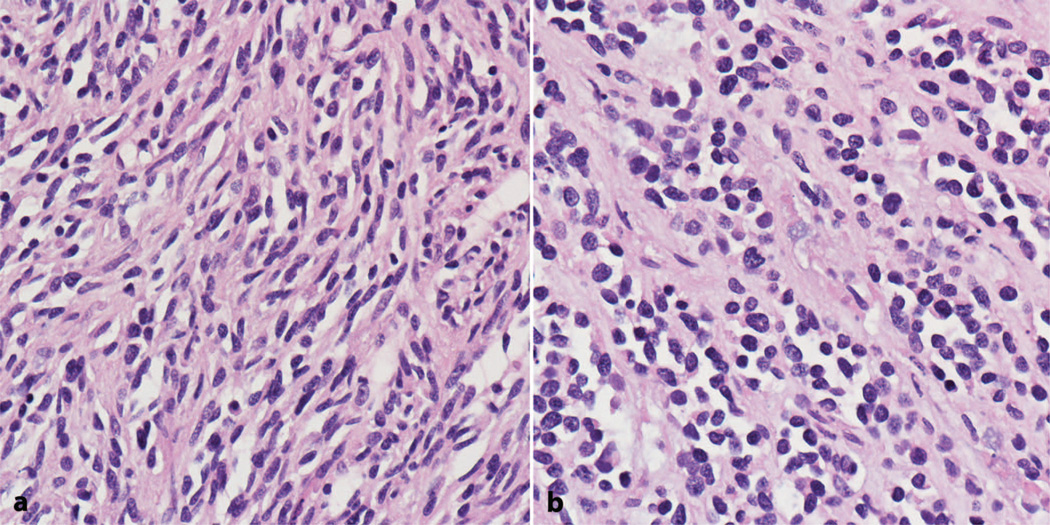
The fourth edition of the World Health Organization (WHO) Classification of Tumours of Soft Tissue and Bone modified the classification system of RMS.5 These changes included the elimination of botryoid RMS as an independent diagnosis, instead classifying it under typical embryonal RMS. Spindle cell/sclerosing RMS was also established as a histologic type separate from embryonal (Figure 1, a and b). Alveolar and pleomorphic RMS, the other 2 subtypes in the WHO system, were not substantially modified.
Figure 1.
Rhabdomyosarcoma with spindle cell (a) and sclerosing (b) features (hematoxylin-eosin, original magnification ×400).
Although the ICR is no longer adequate, whether these changes put forth by the WHO classification should be applied to pediatric RMS is unclear. Although studies of RMS in adults suggest that sclerosing patterns of RMS are not seen in embryonal tumors, we observed sclerosis in combination with dense or typical patterns of embryonal RMS.2 Anecdotal experience suggests that a subset of spindle cell/sclerosing RMS in children may behave more like adult tumors, but there are few published data on the outcome of this new subtype in children.6,7 It is unclear if the favorable prognosis of both botryoid and spindle cell RMS in children relates to tumor site or a unique biology. We thus analyzed a very large group of patients from consecutive COG studies to determine if botryoid and spindle cell/sclerosing patterns of RMS should be classified as distinct subtypes of RMS in children. Our analysis supports the use of the WHO classification system for risk stratification of pediatric RMS, although these diagnostic groups do not necessarily confer the same prognostic information in children as in adults.
MATERIALS AND METHODS
For our analysis, we combined data from 9 consecutive Intergroup Rhabdomyosarcoma Study Group/COG clinical trials (IRS-IVP, IRS-IV, IRS-V [Topotecan], D9602, D9802, D9803, ARST0331, ARST0431, and ARST0531), which enrolled patients from 1987 to 2012. Patients were included in this analysis if they had a diagnosis of embryonal histology (including botryoid and spindle cell variants) and were eligible for and treated in one of these studies (N = 2192).8–17 Outcome for patients with RMS can be predicted by characteristics of the disease and its presentation at diagnosis. Thus, the analyses that follow were performed separately for patients in the low, intermediate, and high prognostic risk strata. The definitions of prognostic risk stratification have been previously described.18 In brief, the definitions of risk strata were as follows: low-risk, nonmetastatic tumors arising at a favorable primary site (Table 1) or an unfavorable site with resection prior to chemotherapy; intermediate risk, nonmetastatic tumors arising at an unfavorable primary site and incompletely resected prior to chemotherapy; and high risk, metastatic tumors.
Table 1.
Favorable Primary Sites of Origin for Pediatric Rhabdomyosarcoma
| Anatomic Site of Origin |
|---|
| Orbit |
| Paratestis |
| Superficial head/neck |
| Female genitourinary |
For most of our analyses, histologic subtype was based on the ICR diagnosis rendered at the time of trial enrollment by the central review pathologists. The WHO entity pleomorphic RMS is not considered a separate diagnosis in pediatric patients, instead being incorporated in cases with diffuse anaplasia. Patients with alveolar RMS, undifferentiated sarcoma, or unclassifiable RMS were excluded from the current analysis. Because the sclerosing pattern of RMS is not recognized by the ICR, analysis of sclerosing RMS could not be conducted for the entire group of COG studies. Instead, our analysis is restricted to tumors identified by histologic rereview of RMS cases enrolled in COG D-series studies, which enrolled patients from 1997 to 2005.2 Rereview is anticipated to have captured most if not all sclerosing RMS for 3 reasons. First, the ICR required only focal alveolar features, resulting in the overdiagnosis of alveolar RMS during this period. Second, the sclerosing (microalveolar) pattern mimics alveolar RMS. And third, rereview included all cases enrolled with an original diagnosis of alveolar RMS.
Event-free survival (EFS) was defined as the time from study enrollment to the first occurrence of progression, relapse after response, or death from any cause. Patients not experiencing an event were censored at their last follow-up time. Estimates of time-to-event distributions were calculated using the Kaplan-Meier method, and distributions were compared using log-rank tests. The effect of histologic subtype on outcome after adjusting for the effect of risk strata was assessed using the Cox proportional hazards model.
RESULTS
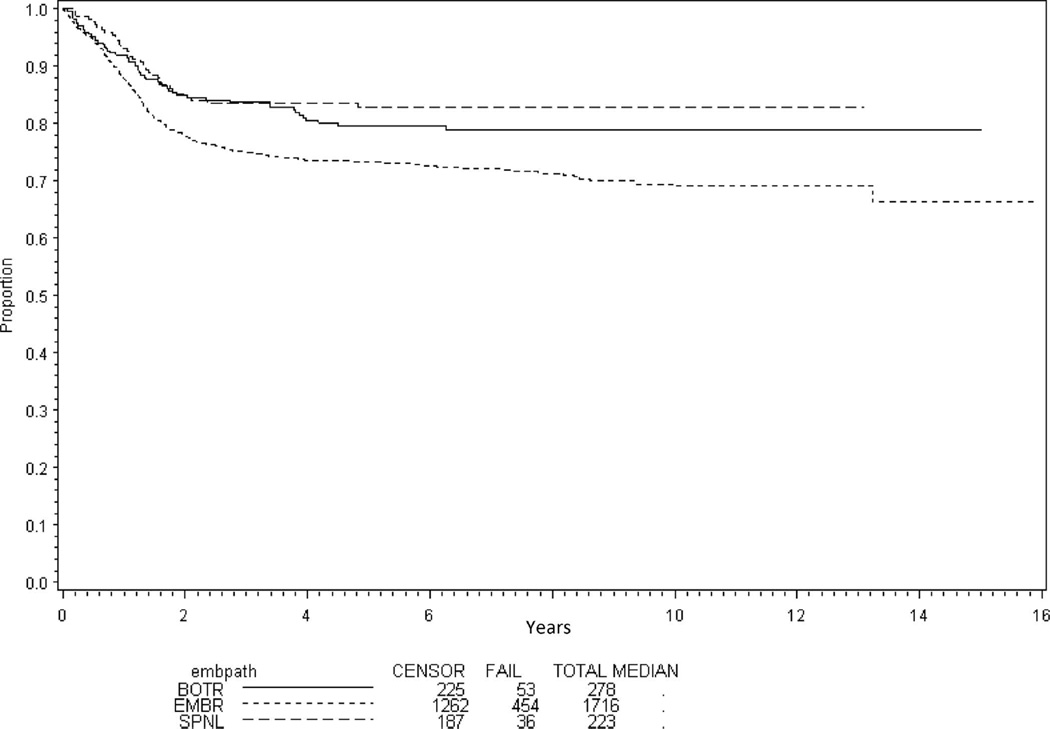
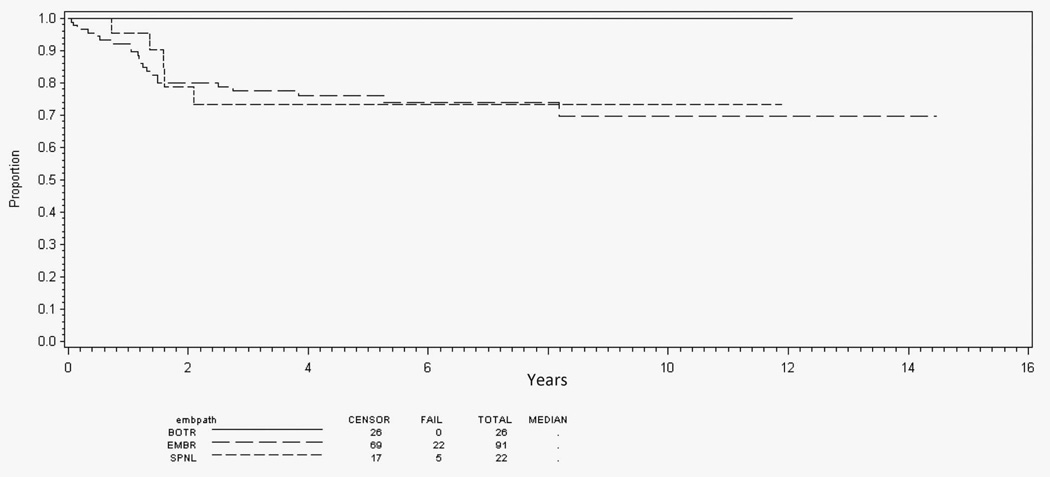
Analysis of outcome by histologic type for all patients with localized disease (n = 1674) shows improved EFS for patients with botryoid (80%; 95% confidence interval [CI], 74%–84%) and spindle cell (83%; 95% CI, 77%–87%) RMS compared with typical embryonal RMS (73%; 95% CI, 71%–75%) (P < .001; Figure 2). However, after adjusting for risk, there was no difference in EFS by histologic subtype (P = .66).
Figure 2.
Event-free survival for all localized disease patients by embryonal histology subtype. Abbreviations: BOTR, botryoid; EMBR, typical embryonal; SPNL, spindle cell.
High Risk
Among patients with high-risk embryonal RMS (n = 234), only 4 were classified as having a botryoid pattern and 5 as having a spindle cell pattern. Because of small population size, no analysis of patients with high-risk features was performed.
Intermediate Risk
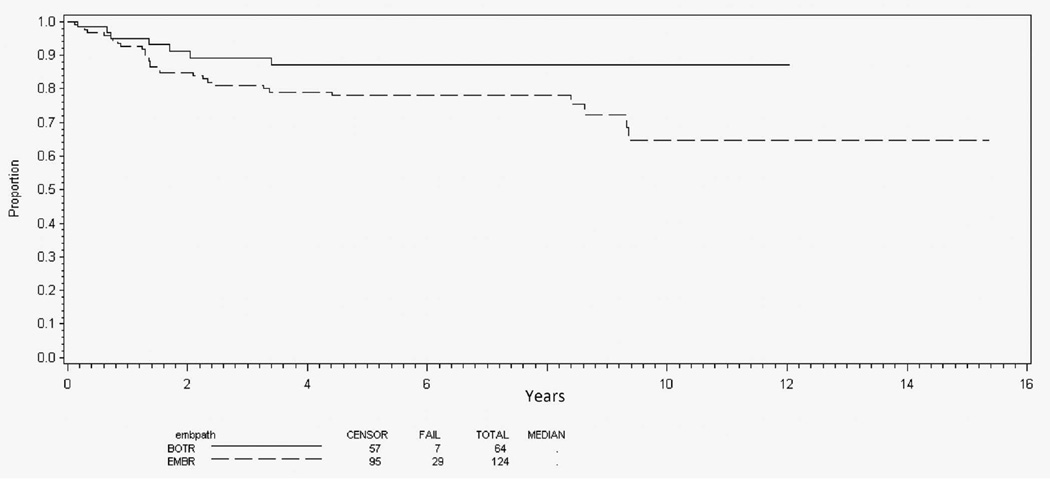
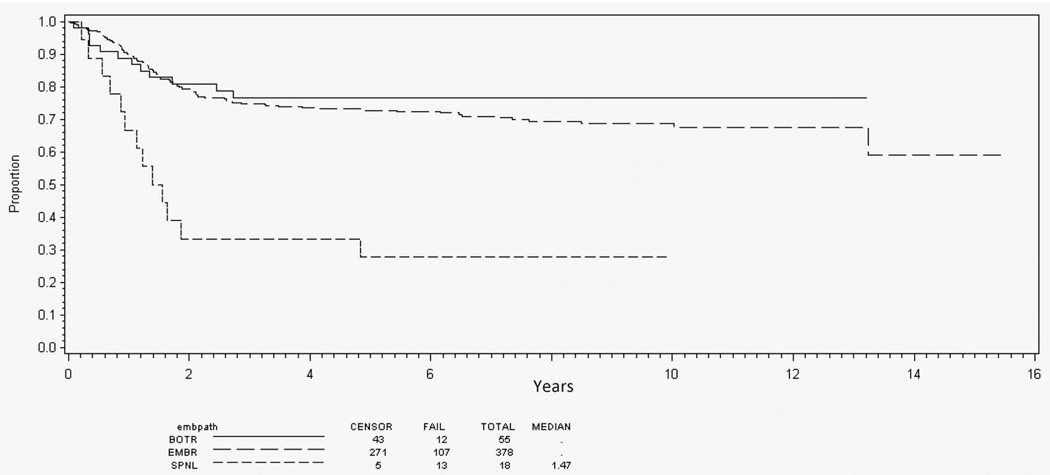
Among patients with nonalveolar RMS and intermediate-risk features (n = 869), 125 (14%) had botryoid-pattern RMS and 34 (4%) spindle cell–pattern RMS. Botryoid disease was preferentially seen in patients with bladder/prostate and parameningeal primary sites (Table 2). Spindle cell disease occurred in a distribution similar to that of typical embryonal RMS. The 5-year EFS for patients with bladder/prostate primary sites was slightly better for patients with botryoid-pattern RMS (87%) than for those with typical embryonal RMS (78%), but this did not reach statistical significance (P = .06; Figure 3). Spindle cell–pattern RMS was not analyzed because of its rarity at this primary site. Among patients with parameningeal primary site, however, spindle cell pattern portended much poorer EFS (28%) than botryoid or typical patterns (76% and 73%, respectively) (P < .001; Figure 4). Because of the small population of patients with botryoid (n = 6) or spindle cell (n = 13) RMS at other primary sites, no further analysis was performed.
Table 2.
Histologic Type by Primary Site for Intermediate-Risk Rhabdomyosarcoma
| Primary Site | Botryoid, No. (%) |
Embryonal, No. (%) |
Spindle Cell, No. (%) |
|---|---|---|---|
| Bladder/prostate | 64 (51) | 124 (17) | 3 (9) |
| Parameningeal | 55 (44) | 378 (53) | 18 (53) |
| Other sites | 6 (5) | 208 (29) | 13 (38) |
| Total | 125 | 710 | 34 |
Figure 3.
Event-free survival for intermediate-risk genitourinary/bladder-prostate primary site by embryonal histology subtype. Abbreviations: BOTR, botryoid; EMBR, typical embryonal.
Figure 4.
Event-free survival for intermediate-risk parameningeal primary site by embryonal histology subtype. Abbreviations: BOTR, botryoid; EMBR, typical embryonal; SPNL, spindle cell.
Low Risk
Among patients with low-risk features (n = 1089), 166 (15%) had botryoid-pattern RMS and 189 (17%) had spindle cell–pattern RMS. Botryoid and spindle cell patterns of RMS were preferentially seen in non–bladder/prostate genitourinary and paratestis primary sites, respectively (Table 3). Patients with head and neck botryoid disease (n = 26) had better EFS (100%; P = .02; Figure 5) than patients with typical embryonal RMS head and neck primaries. No differences in EFS were seen by histologic subtype for orbit, paratestis, non–bladder/prostate genitourinary, or “other” primaries (data not shown).
Table 3.
Histologic Type by Primary Site for Low-Risk Rhabdomyosarcoma
| Primary Site | Botryoid, No. (%) |
Embryonal, No. (%) |
Spindle cell, No. (%) |
|---|---|---|---|
| Head and neck | 26 (16) | 91 (12) | 22 (12) |
| Orbit | 20 (12) | 219 (30) | 16 (8) |
| Paratestis | ⋯ | 269 (37) | 125 (66) |
| Genitourinary (non–bladder/prostate) | 92 (55) | 47 (6) | ⋯ |
| Other sites | 28 (17) | 108 (15) | 26 (14) |
| Total | 166 | 734 | 189 |
Figure 5.
Event-free survival for low-risk head and neck primary site by embryonal histology subtype. Abbreviations: BOTR, botryoid; EMBR, typical embryonal; SPNL, spindle cell.
Sclerosing RMS
Although not recognized in the ICR, the sclerosing pattern of RMS was included as a diagnostic category on recent histologic rereviews of predominantly alveolar RMS enrolled in COG D-series studies. Review of the low-risk study (D9602) identified 2 cases with a predominantly sclerosing pattern of RMS from 35 patients originally diagnosed with alveolar RMS. No cases with a sclerosing pattern were identified in the 86 RMS cases reviewed in the high-risk study (D9802). Because of the low frequency of sclerosing RMS in these studies, no further analysis of these patients was performed.
Children’s Oncology Group pathologists recently rereviewed 255 alveolar RMS and 38 embryonal RMS enrolled in intermediate-risk trial D9803. Of cases originally called alveolar RMS, rereview identified 17 with a predominantly sclerosing pattern, 6 with a combined sclerosing/dense pattern, and 5 with a combined spindled/sclerosing pattern.2 Because the WHO definition of sclerosing/spindle cell RMS excludes cases with typical embryonal histology, the 6 cases showing a combination of sclerosing and dense patterns of embryonal RMS were excluded from outcome analysis. Of the 22 remaining cases, 7 (32%) arose in the parameningeal region; 5 (23%) arose in the extremities; 5 (23%) arose in genitourinary, non–bladder/prostate sites; 2 were in the perineum; and 1 each arose in the orbit, head and neck, and trunk. Thirteen patients were younger than 10 years and 9 patients were aged 10 years or older. The 5-year EFS for these patients was 81% (95% CI, 57%–92%) and the 5-year overall survival was 86% (95% CI, 62%–95%). The small number of these patients precludes statistical comparison of histology and site.
COMMENT
Risk stratification in RMS is accomplished through a complex algorithm incorporating a clinical group (based on disease extent and surgical results) and tumor stage (based on clinical factors including site of disease, tumor size, and presence or absence of regional or distant metastatic disease).13,18,19 Historically, risk stratification has also taken ICR subtype into account. Recent studies confirming the prognostic significance of fusion status in RMS suggested that histologic subtype (ie, alveolar versus embryonal RMS) might be replaced by fusion status for final risk stratification.20 Fusion status may represent the most robust prognostic marker for alveolar RMS, but there has not been a recent analysis of the prognostic significance of the various patterns of nonalveolar RMS in children. This is of particular relevance given the recent modifications proposed in the WHO classification of RMS and new data suggesting that spindle cell/sclerosing RMS is an aggressive tumor in the adult population.5 To address these modifications, we analyzed a series of consecutive COG studies to determine the effect of histologic type and tumor site on patient outcomes.
The botryoid pattern of RMS is defined by its location beneath a mucosal surface (such as the bladder) with formation of a cambium layer. In the ICR, it is associated with a superior prognosis. However, for most primary sites, after considering the clinical risk stratification mentioned above, botryoid subtype did not further influence EFS in children with embryonal RMS. Patients with intermediate-risk botryoid tumors involving the bladder/prostate showed a trend towards improved EFS compared with those with typical embryonal RMS, but this failed to reach significance. The botryoid pattern of RMS retained prognostic significance only for a small group of patients with low-risk head and neck tumors when compared with embryonal or spindle cell RMS subtypes at the same primary site. The potential relationship of this observation to surgical resectability was not analyzed. Further, children with low-risk RMS have an excellent outcome in general, and further reductions in the standard chemotherapy for this subset are unlikely. Although in our analysis the botryoid subtype of RMS retained prognostic significance in this clinical setting, its continued use as a specific subtype of RMS appears to have limited clinical utility. Therefore, subsuming the botryoid subtype into embryonal RMS as per the WHO classification appears justified.
Spindle cell RMS was originally described as a paratesticular pediatric tumor associated with a superior prognosis.21 However, with a single exception, our analysis indicates that spindle cell RMS does not confer a prognosis different than that found in children with typical embryonal RMS when analyzed by tumor site. Children with intermediate-risk parameningeal spindle cell tumors had much poorer EFS than patients with embryonal RMS subtypes in this location. This is quite interesting because spindle cell RMS in adult patients most often arises in the head and neck region, and it carries a poor prognosis.
There may be a molecular basis for the poorer prognosis in spindle cell RMS occurring at head and neck sites. Application of next-generation sequencing approaches recently revealed a recurrent myogenic differentiation 1 (MYOD1) mutation in a subset of predominantly adult patients with spindle cell RMS, most frequently in tumors arising from the head and neck or extremity.6,22,23 This mutation—leading to a single amino acid change (L122R) in the DNA-binding element of the MyoD transcription factor—leads the mutant protein to act more like the MYC oncogene than a promyogenic differentiation factor.23,24 Patients with RMS harboring this mutation had a poor outcome; none of the 7 older RMS patients with MYOD1 mutation survived 9 years past diagnosis in one series,23 and none of 4 pediatric RMS patients with MYOD1 mutation survived in another series.6 Comprehensive genomic analysis of 2 large series of pediatric RMS tumors identified only one case with a MYOD1 mutation, suggesting that MYOD1 mutation is relatively rare in this population.25,26 We plan to examine a larger cohort of embryonal RMS with targeted sequencing including MYOD1 to determine its frequency and the associated clinical characteristics in a less-selected study population, as well as within the spindle cell parameningeal tumors identified in our analysis.
In the WHO classification, sclerosing RMS is considered a variant pattern of spindle cell RMS, as descriptions note increasing degrees of hyalinization and matrix formation in spindle cell tumors.27 Sclerosing RMS is more common in adults, arises in the extremities and head and neck region, and has a more aggressive course.4 Agaram et al6 also identified recurrent MyoD1 mutations in sclerosing RMS. Data on the outcome of sclerosing RMS in the pediatric population are limited, however. The largest prior study of sclerosing RMS in children had a follow-up of 0.01 to 3.58 years with 3 of 13 patients relapsing and 1 death from disease.7
Although limited to D9803 intermediate-risk study patients, our outcome analysis of sclerosing RMS represents the largest published cohort of pediatric patients with sclerosing or sclerosing/spindle cell patterns of RMS. The outcome for this select group of D9803 patients was similar to that of those with typical embryonal RMS, with an overall survival of 86% for sclerosing RMS versus 81% for typical embryonal RMS.2 Although a subset of spindle cell or sclerosing RMS cases occurring in children may be biologically similar to their adult counterparts, this histologic pattern does not automatically confer a poor prognosis in the pediatric population.
In summary, the WHO classification of skeletal muscle tumors can be applied to the pediatric population for direct risk stratification and treatment and for the design of future studies. Our data support eliminating the botryoid pattern of RMS as a specific histologic subtype, as in only limited clinical scenarios is it associated with a superior outcome. Because there are currently no treatment differences between botryoid and typical embryonal RMS, and no further therapeutic modifications are proposed for low-risk RMS, the diagnosis of botryoid RMS may be safely subsumed within typical embryonal RMS. Similarly, spindle cell and sclerosing RMS have an outcome similar to that of typical embryonal RMS, with the exception of parameningeal spindle cell tumors. We plan to analyze cases of pediatric spindle cell/sclerosing RMS for the presence of MYOD1 mutations, and to determine the frequency and significance of MYOD1 mutation in a larger cohort of patients. If the inferior outcome of spindle cell/sclerosing RMS with MYOD1 mutation is confirmed, future risk stratification in children with RMS could include MYOD1 mutation status in addition to FOXO1 fusion. Indeed, because published, large-scale RMS sequencing efforts have not been undertaken to correlate clinical outcome with any specific mutation,25,26 it is possible that MYOD1 mutation is just the first of other molecular features that could continue to provide prognostic information beyond that provided by histologic subtype.
Footnotes
The authors have no relevant financial interest in the products or companies described in this article.
References
- 1.Newton WA, Gehan EA, Webber BL, et al. Classification of rhabdomyosarcomas and related sarcomas: pathologic aspects and proposal for a new classification—an intergroup rhabdomyosarcoma study. Cancer. 1995;76(6):1073–1085. doi: 10.1002/1097-0142(19950915)76:6<1073::aid-cncr2820760624>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 2.Rudzinski ER, Teot LA, Anderson JR, et al. Dense pattern of embryonal rhabdomyosarcoma, a lesion easily confused with alveolar rhabdomyosarcoma: a report from the Soft Tissue Sarcoma Committee of the Children’s Oncology Group. Am J Clin Pathol. 2013;140(1):82–90. doi: 10.1309/AJCPA1WN7ARPCMKQ. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mentzel T, Katenkamp D. Sclerosing, pseudovascular rhabdomyosarcoma in adults: clinicopathological and immunohistochemical analysis of three cases. Virchows Arch. 2000;436(4):305–311. doi: 10.1007/s004280050451. [DOI] [PubMed] [Google Scholar]
- 4.Folpe AL, McKenney JK, Bridge JA, Weiss SW. Sclerosing rhabdomyosarcoma in adults: report of four cases of a hyalinizing, matrix-rich variant of rhabdomyosarcoma that may be confused with osteosarcoma, chondrosarcoma, or angiosarcoma. Am J Surg Pathol. 2002;26(9):1175–1183. doi: 10.1097/00000478-200209000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F, editors. WHO Classification of Tumours of Soft Tissue and Bone. 4th ed. Lyon, France: International Agency for Research on Cancer; 2013. World Health Organization Classification of Tumours; vol 5. [Google Scholar]
- 6.Agaram NP, Chen CL, Zhang L, LaQuaglia MP, Wexler L, Antonescu CR. Recurrent MYOD1 mutations in pediatric and adult sclerosing and spindle cell rhabdomyosarcomas: evidence for a common pathogenesis. Genes Chromosomes Cancer. 2014;53(9):779–787. doi: 10.1002/gcc.22187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiles MC, Parham DM, Qualman SJ, et al. Sclerosing rhabdomyosarcomas in children and adolescents: a clinicopathologic review of 13 cases from the Intergroup Rhabdomyosarcoma Study Group and Children’s Oncology Group. Pediatr Dev Pathol. 2004;7(6):583–594. doi: 10.1007/s10024-004-5058-x. [DOI] [PubMed] [Google Scholar]
- 8.Arndt C, Tefft M, Gehan E, et al. A feasibility, toxicity, and early response study of etoposide, ifosfamide, and vincristine for the treatment of children with rhabdomyosarcoma: a report from the Intergroup Rhabdomyosarcoma Study (IRS) IV pilot study. J Pediatr Hematol Oncol. 1997;19(2):124–129. doi: 10.1097/00043426-199703000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Crist WM, Anderson JR, Meza JL, et al. Intergroup rhabdomyosarcoma study-IV: results for patients with nonmetastatic disease. J Clin Oncol. 2001;19(12):3091–3102. doi: 10.1200/JCO.2001.19.12.3091. [DOI] [PubMed] [Google Scholar]
- 10.Raney RB, Walterhouse DO, Meza JL, et al. Results of the Intergroup Rhabdomyosarcoma Study Group D9602 protocol, using vincristine and dactinomycin with or without cyclophosphamide and radiation therapy, for newly diagnosed patients with low-risk embryonal rhabdomyosarcoma: a report from the Soft Tissue Sarcoma Committee of the Children’s Oncology Group. J Clin Oncol. 2011;29(10):1312–1318. doi: 10.1200/JCO.2010.30.4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arndt CAS, Stoner JA, Hawkins DS, et al. Vincristine, actinomycin, and cyclophosphamide compared with vincristine, actinomycin, and cyclophosphamide alternating with vincristine, topotecan, and cyclophosphamide for intermediate-risk rhabdomyosarcoma: Children’s Oncology Group study D9803. J Clin Oncol. 2009;27(31):5182–5188. doi: 10.1200/JCO.2009.22.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pappo AS, Lyden E, Breitfeld P, et al. Two consecutive phase II window trials of irinotecan alone or in combination with vincristine for the treatment of metastatic rhabdomyosarcoma: the Children’s Oncology Group. J Clin Oncol. 2007;25(4):362–369. doi: 10.1200/JCO.2006.07.1720. [DOI] [PubMed] [Google Scholar]
- 13.Malempati S, Hawkins DS. Rhabdomyosarcoma: review of the Children’s Oncology Group (COG) Soft-Tissue Sarcoma Committee experience and rationale for current COG studies. Pediatr Blood Cancer. 2012;59(1):5–10. doi: 10.1002/pbc.24118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pappo AS, Lyden E, Breneman J, et al. Up-front window trial of topotecan in previously untreated children and adolescents with metastatic rhabdomyosarcoma: an intergroup rhabdomyosarcoma study. J Clin Oncol. 2001;19(1):213–219. doi: 10.1200/JCO.2001.19.1.213. [DOI] [PubMed] [Google Scholar]
- 15.Walterhouse DO, Lyden ER, Breitfeld PP, Qualman SJ, Wharam MD, Meyer WH. Efficacy of topotecan and cyclophosphamide given in a phase II window trial in children with newly diagnosed metastatic rhabdomyosarcoma: a Children’s Oncology Group study. J Clin Oncol. 2004;22(8):1398–1403. doi: 10.1200/JCO.2004.05.184. [DOI] [PubMed] [Google Scholar]
- 16.Hawkins DS, Anderson JR, Mascarenhas L, et al. Vincristine, dactinomycin, cyclophosphamide (VAC) versus VAC/V plus irinotecan (VI) for intermediate-risk rhabdomyosarcoma (IRRMS): a report from the Children’s Oncology Group Soft Tissue Sarcoma Committee [abstract] J Clin Oncol. 2014;32(15s):10004. [Google Scholar]
- 17.Weigel B, Lyden E, Anderson JR, et al. Early results from Children’s Oncology Group (COG) ARST0431: intensive multidrug therapy for patients with metastatic rhabdomyosarcoma (RMS) [abstract] J Clin Oncol. 2010;28(15s):9503. [Google Scholar]
- 18.Hawkins DS, Spunt SL, Skapek SX. Children’s Oncology Group’s 2013 blueprint for research: soft tissue sarcomas. Pediatr Blood Cancer. 2013;60(6):1001–1008. doi: 10.1002/pbc.24435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hawkins DS, Gupta AA, Rudzinski ER. What is new in the biology and treatment of pediatric rhabdomyosarcoma? Curr Opin Pediatr. 2014;26(1):50–56. doi: 10.1097/MOP.0000000000000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skapek SX, Anderson J, Barr FG, et al. PAX-FOXO1 fusion status drives unfavorable outcome for children with rhabdomyosarcoma: a Children’s Oncology Group report. Pediatr Blood Cancer. 2013;60(9):1411–1417. doi: 10.1002/pbc.24532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leuschner I, Newton WA, Schmidt D, et al. Spindle cell variants of embryonal rhabdomyosarcoma in the paratesticular region: a report of the Intergroup Rhabdomyosarcoma Study. Am J Surg Pathol. 1993;17(3):221–230. doi: 10.1097/00000478-199303000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Szuhai K, de Jong D, Leung WY, Fletcher CD, Hogendoorn PC. Transactivating mutation of the MYOD1 gene is a frequent event in adult spindle cell rhabdomyosarcoma. J Pathol. 2014;232(3):300–307. doi: 10.1002/path.4307. [DOI] [PubMed] [Google Scholar]
- 23.Kohsaka S, Shukla N, Ameur N, et al. A recurrent neomorphic mutation in MYOD1 defines a clinically aggressive subset of embryonal rhabdomyosarcoma associated with PI3K-AKT pathway mutations. Nat Genet. 2014;46(6):595–600. doi: 10.1038/ng.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Antwerp ME, Chen DG, Chang C, Prochownik EV. A point mutation in the MyoD basic domain imparts c-Myc-like properties. Proc Natl Acad Sci U S A. 1992;89(19):9010–9014. doi: 10.1073/pnas.89.19.9010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shern JF, Chen L, Chmielecki J, et al. Comprehensive genomic analysis of rhabdomyosarcoma reveals a landscape of alterations affecting a common genetic axis in fusion-positive and fusion-negative tumors. Cancer Discov. 2014;4(2):216–231. doi: 10.1158/2159-8290.CD-13-0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen X, Stewart E, Shelat AA, et al. Targeting oxidative stress in embryonal rhabdomyosarcoma. Cancer Cell. 2013;24(6):710–724. doi: 10.1016/j.ccr.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nascimento AF, Fletcher CD. Spindle cell rhabdomyosarcoma in adults. Am J Surg Pathol. 2005;29(8):1106–1113. [PubMed] [Google Scholar]