Abstract
During murine immune development, recurrent B cell clones arise in a predictable fashion. Among these B cells, an archetypical clonotypic set that recognizes phosphorylcholine (PC) antigens and produces anti-PC IgM, first implicated for roles in microbial protection, was later found to become expanded in hyperlipidemic mice and in response to an increased in vivo burden of apoptotic cells. These IgM natural antibodies can enhance clearance of damaged cells and induce intracellular blockade of inflammatory signaling cascades. In clinical populations, raised levels of anti-PC IgM correlate with protection from atherosclerosis and may also down-modulate the severity of autoimmune disease. Human anti-PC-producing clones without hypermutation have been isolated that can similarly discriminate apoptotic from healthy cells. An independent report on unrelated adults has described anti-PC-producing B cells with IgM genes that have conserved CDR3 motifs, similar to stereotypic clonal sets of B cell chronic lymphocytic leukemia (CLL). Taken together, emerging evidence suggests that, despite the capacity to form an effectively limitless range of Ig receptors, the human immune system may often recurrently generate lymphocytes expressing structurally convergent BCRs with protective and homeostatic roles.
Keywords: natural antibody, apoptotic cell, immunoregulation, B cell, apoptotic clearance
Introduction
B lymphocytes produce antibodies that augment host defenses via their capability for recognizing infectious agents, toxins, and other virulence factors. The immune system is remarkable, as a limited repertoire of germ-line precursors can be recombined to produce antibodies with virtually any binding specificity. At birth, humans already have substantial levels of circulating IgM antibodies (i.e., naturally arising antibodies (Nabs)) that are poised to contribute to neonatal host defenses from threats in the external world. Unlike the IgG and IgA antibodies that come from the maternal immune system, IgM antibodies spontaneously arise and are highly expressed in the neonate by B lymphocytes that are clonally selected in the sterile (but not antigen-free) womb. Indeed, some autoreactive clones are common physiologic components of the immune system, with the same clones arising in different members of the species, and these are postulated to contribute to homeostasis through specialized immune functions.1 In recent studies, we have explored the structural as well as in vitro and in vivo functional properties of a class of antibodies that recognize epitopes that arise on damaged and dying cells, with analogues that appear to be conserved across mammalian species.
Distinct subsets of mature B cells, recirculating follicular (B-2), marginal zone (MZ), and B-1 cells, each play discrete but often complementary functional roles in host defenses (reviewed in Ref. 2). Each also has a distinct surface phenotypic profile and cellular activation threshold, and different requirements for second signals after B cell receptor (BCR) stimulation.3 B-1 cells are reported to express a specialized BCR repertoire,2 which in part may be explained because B-1 cell clones have been shown to be positively selected by their cognate self-antigen.4 In contrast, when the precursors of conventional B cells encounter their cognate self-antigen, this instead results in clonal deletion or reactivation of BCR rearrangement machinery that edits out autoreactivity.5 Furthermore, murine B-1 cells are self-replenishing, which is presumed to ensure maintenance of this immune repertoire throughout life. B-1 cells have therefore been implicated as a major source of the high frequency of NAbs that are often autoreactive in mice6 and in humans.7 Rothstein and colleagues have identified a set of circulating B lymphocytes in humans, which are proposed to be human B-1 cells,8 although this topic remains controversial.9
Clonotypic sets within the B-1 cell pool
Studies initiated more than 40 years ago of the prototypic B cell clonotypic set (termed TEPC 15 or T15) have provided a window into many facets of B-cell biology. The first examples of T15 clonotypic B-cell lines were described many decades ago by intraperitoneal delivery of an irritating oil10, 11(and reviewed in Ref. 12). The T15 clonotype is defined by canonical VHS107.1 and Vκ22 antibody gene rearrangements, which display neither somatic hypermutation nor N-insertions at the VH–DH–JH or VL–JL junctions.13 Over the years, B cell clones that express identical or near identical antibody genes have been recurrently isolated in many labs, and the Ig products of these B cells are recognized by clonotype-specific serologic reagents. T15-related clones have also been described with minor variations of the HCDR3 and in the paired L chain usage.14,15
Terminal deoxytransferase (TdT), an enzyme that enhances diversification with non-templated DNA insertions at junctional V–D–J splice sites, is absent in murine fetal immune tissues, which in part explains the limited diversity in the murine early repertoire. There are also biases of the immune system related to early preferential rearrangement of JH-proximal VH genes.13,16 It has been argued that some NAb clones arise without immunization as part of a programmed development of the immune system (and B cell compartment) that may reflect evolutionary selective pressures.17 In mice, with expression (or overexpression) of TdT, B cell development instead yields a broader range of VDJ (and VLJL) rearrangements and potential antigen-binding sites.18
In the absence of TdT, there are rearrangement biases, in part due to primary DNA-directed sequence rearrangements that appear to favor the representation of VHT15-specific genes; but even so, the recurrent canonical VH–VL pairing in T15 clonotypic B cells is unambiguous evidence that there must also be clonal selection based on BCR–antigen interactions. This clonotypic set of structurally homologous antibodies is expressed in diverse immunocompetent murine strains. Adoptive transfer studies support the notion that T15-clonotypic B cells reside predominantly or solely within the B-1 cell pool.19
The predictable recurrence in different individual mice of somatically-generated antibodies, like the T15 clonotypic NAb, has suggested they have features reminiscent of germline-encoded receptors of the innate immune system (discussed in Ref. 20); and hence these NAbs have been described as innate-like.21 In fact, T15 clonotypic antibodies recognize with great specificity antigens containing the phospholipid head group phosphorylcholine (PC).22 Indeed, multiplex antigen microarray analysis has confirmed that T15 NAbs, without hypermutation, recognize diverse PC-containing ligands with little or no cross-reactivity.23 The contribution of the S107.1 VH gene segment produces a BCR with a cavity (or pit) that tightly binds the PC head group, tethered by an aliphatic chain to the surface antibody.24 Hence, non-hypermutated T15 clonotypic antibodies may be unlike many other germ line–encoded NAbs that display a high level of polyreactivity. Mice with targeted deletion of the VHS107.1 gene have a selective functional immunodefect: lack of B cell recognition of ACs due to impaired PC-antigen recognition; other VH gene segments apparently are not functionally equivalent building blocks for generation of PC antibodies.25
T15 clonotypic B cells (and their antibody products) can play central roles in defense from bacterial pathogens, such as Streptococcus pneumoniae.26 Indeed, the prototypic TEPC15 B cell clone is an IgA antibody that recognizes PC-containing antigens present in pneumococcal cell wall polysaccharide.22,27,28 In fact, host defenses from blood-borne pneumococcal infections centrally rely on immune recognition of PC determinants in the pneumococcal cell wall polysaccharide.26 PC binding can often be impaired by somatic mutations.29 PC determinants are also prevalent on many microbes and helminths, and PC antibodies therefore can be cross-reactive with a variety of other microbes,30 including dental plaque bacteria.31
However, even in mice raised under specific pathogen-free conditions, the T15 clonotype–related B cells in BALB/c mice become highly represented in the B cell pool by the end of the first week of life,32 and this representation is unaffected in mice raised under gnotobiotic conditions.33 Hence, microbial antigen exposure does not appear to be essential for initial expansion of the T15 clonotype. While the possibility of endogenous selecting factors for T15 B cells has been controversial,14 more recent studies have suggested that there are also PC-containing ligands that represent altered-self antigens.
Studies in atherosclerosis-prone mice have provided important insights into the immunobiology of anti-PC responses.20 In hyperlipidemic mice, the extent of atherosclerotic disease is roughly proportional to levels of spontaneously arising circulating antibodies to oxidation-associated changes in the lipid components of low density lipoprotein (LDL). To investigate the natural antibodies arising in hyperlipidemic mice, Witztum and colleagues surveyed the in vivo repertoire through the generation of B cell hybridomas made from cell fusions of splenocytes from hyperlipidemic apolipoprotein E (ApoE)–deficient mice.34 Molecular characterization of these ApoE hybridomas led to the unexpected discovery of major clonal expansion of B cells with antibody genes identical to and indistinguishable from the classical T15 antibody.35
The nature of putative in vivo selecting antigens for the NAbs expanded in hyperlipidemic individuals was initially a mystery. Studies of the associated inflammatory response found that oxidized LDL (OxLDL) is a target of the host response in chronic vascular syndromes due to atherosclerosis (reviewed in Ref. 36). In fact, PC-containing antigens, such as 1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphocholine (POVPC), are prevalent in the OxLDL found in atherosclerotic plaques.37 Plaques are also rich in deposited host IgM and IgG, as well as the soluble components of the innate immune system, including complement factors and C-reactive protein (CRP), which themselves directly bind PC-containing substances and dying and dead myeloid cells. Oxidation-associated altered-self determinants appear to be important targets for the emerging B cell repertoire,38 and, in addition to anti-PC responses, there are no doubt parallel sets of altered-self non-protein epitopic ligands that also select for clonal sets of innate-like B cells.39
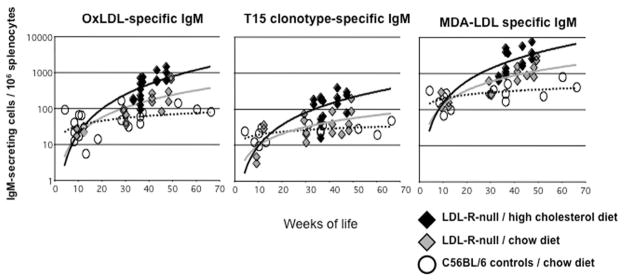
We wondered whether, in fact, simple dietary manipulations could themselves affect the levels of NAb-producing B cells clonally related to the T15 set. In earlier studies, we therefore set out to measure the levels of antibody-producing cells in LDL receptor–deficient mice on a C57BL/6 (i.e., wild-type) background. When fed regular chow, these mice have only minor lipid abnormalities and little or no evidence of atherosclerotic arterial plaques, while plaques rapidly develop in mice on high-fat diets, such as Western chow, which raise serum cholesterol levels to more than 1000 mg/dL. Indeed, compared to mice with normal cholesterol levels, antibody surveys and ELISpot assays of IgM-secreting cells have shown that hyperlipidemic mice develop high levels of anti-PC antibodies that bear T15 clonotypic markers (Fig. 1).40 There are also parallel induced expansions of IgM-secreting cells that recognize malondialdehyde (MDA) determinants that are also prominent epitopes on apoptotic cells.40 These studies in part highlight that environmental influences, deriving from dietary changes (i.e., high fat), can significantly alter the in vivo expression of a B-1 cell–linked clonotype. However, it remains unclear whether these pathologic vascular changes are induced solely by elevations of blood lipids, and we speculate that associated defects in apoptotic clearance are also contributory.
Figure 1.
Atherosclerosis-prone LDL receptor–deficient mice display progressive increases in splenic T15 IgM-secreting cells. C57BL/6 mice or congenic LDL receptor–deficient mice were raised under specific pathogen-free conditions. LDLR-null mice were fed either a high-cholesterol (i.e., 1.25%) Western chow or a regular chow, and the former group developed hypercholesterolemia (i.e., > 2000 mg/dL). Mice were sacrificed after a minimum of 16 weeks on the diet, at the indicted ages. Mice fed high-cholesterol diet had significantly elevated levels of IgM-secreting cells to MDA-derivatized LDL and copper-oxidized LDL, with higher frequency of T15 clonotypic IgM secreting cells (P < 0.05, unpaired t-test). T15 clonotype was identified, using the AB1-2 anti-idiotypic marker, while there was little binding to the isotype control (not shown). Adapted from Ref. 40.
To test whether induced B cell responses might in fact affect the pathogenesis of the vascular disease, we fed LDL-R–deficient mice a Western diet to initiate the development of aortic atherosclerosis, then subsequently used a standard regimen for immunizations with an extract of pronase-treated R36a pneumococci.40 Indeed, pneumococcal vaccination, which induces antibody responses to PC determinants that bear T15 clonotypic markers, was shown to arrest plaque progression in LDL receptor–deficient mice with cholesterol levels over 1000 mg/dl.40 These findings have therefore strengthened the hypothesis that some anti-PC IgM NAbs can play protective roles in inflammatory disease.
We therefore wondered whether there are other functionally equivalent endogenous factors that can serve as selecting ligands for T15-related clones. We have shown that PC antibodies and the prototypic T15 clonotypic antibodies can be considered autoantibodies to phospholipid-containing modified self-antigens.41 In fact, PC-containing phospholipid moieties are also prominent components in the cell membranes of mammalian cells. During the process of apoptotic cell death, different cell membrane–associated phospholipids can undergo selective enzyme-mediated and oxidation-associated modifications, and these cell membrane altered-self determinants become available for recognition by the immune system. Among these, phosphatidylserine (PS) becomes oxidized and rapidly translocates from the inner to the outer leaflet of the cell membrane upon the initiation of apoptosis, where it can serve as a recognition signal (i.e., “eat me” signal) for ingestion by professional phagocytes. Oxidative modifications of the abundantly distributed neutral phospholipid phosphatidylcholine (PtC) also affect the distribution and/or conformation of the PC head group,42 which renders it accessible for antibody recognition.
Intravenous infusions into immunocompetent mice of apoptotic autologous thymocytes induces significant expansion of IgM-secreting cells, which are dominated by B cells that recognize PC-containing determinants and bear T15-related clonotypic markers.23 There is also an expansion of IgM-secreting B cells that recognize MDA-containing antigens, which are further boosted by intravenous infusions of ACs.23 Notably, in mice deficient in the inherited VHS107.1 gene segment required for the canonical T15 VH rearrangements,25 the representation of IgM-secreting splenic cells to AC induced anti-PC determinants were decreased more than an order of magnitude.23 In fact, the response in VHS107.1-deficient mice was shifted to enhanced recognition of the structurally unrelated and distinct oxidation-associated ligand MDA,23 which is generated during apoptosis (and other types of injury) and acts as an adduct that can derivatize self proteins. We speculate that the VHS107.1 gene segment enables preferential generation of a binding site well suited to recognition of tethered PC determinants as occur on the surface of ACs. The VHS07.1 gene may therefore have been molded and selected during evolution of the murine immune system, owing to benefits for maintaining homeostasis that be linked to the roles of innate-like B cells.
Functional contributions of IgM NAbs to homeostasis
T15 IgM NAbs may also modulate the functional activities of professional phagocytes during responses of the innate immune system. Earlier studies have shown that C1q can directly bind to AC membranes and then serve as an signal for the phagocytic clearance of these dying cells.43,44 In fact, C1q may directly interact with externalized PS on damaged cells.45 In some settings, the deposition of C1q onto ACs can subsequently have immunomodulatory effects that inhibit the secretion of proinflammatory cytokines.46 Similar properties have also been associated with deposition onto ACs of the mannose-binding lectin (MBL), which triggers the lectin pathway of complement activation. MBL is structurally related to C1q, and these two recognition molecules share a common ancestral genetic origin.47 This may suggest that initiation of apoptosis is associated with a change in the distribution of high-mannose glycoconjugates on the cell membrane.48 These findings are consistent with reports that phagocytes of C1q-deficient mice, as well as MBL-deficient mice, display defects in AC clearance.48,49 As mentioned above, while the T15 natural antibodies do not bind healthy cells, these antibodies can recognize exposed PC determinants on ACs and form complexes.23,50 Importantly, complexes of T15 IgM with ACs greatly enhanced capacity to recruit the early complement factors, C1q and the structurally related MBL, at levels several-fold higher than in the absence of bound IgM. Notably, IgM constant regions themselves can contain high-mannose glycoconjugates.51 As a consequence, the recruitment of C1q or MBL by IgM–NAb complexes amplifies several-fold the capacity of professional phagocytes for clearance of apoptotic cells,23,50 which is a fundamental homeostatic function of the innate immune system––to clear damaged cells before they can progress to secondary necrosis and release of inflammatory substances and autoantigens. Apoptotic cell–reactive polymeric IgM may therefore serve to integrate and amplify the efficiency of these complement-associated innate immune functions.23,49,50
The formation of IgM NAb complexes with ACs can also result in strong suppression of in vivo and in vitro inflammatory responses, including those induced by ligands for both membrane-associated and endosomal Toll-like receptors (TLRs), which include TLR3, TLR4, TLR7, and TLR9.50 These inhibitory activities are also dependent on the recruitment of C1q and MBL, which are postulated to serve as bridging molecules that trigger phagocyte functions in a way that does not require activation of the complement cascade.50 Hence, both the enhancement of apoptotic clearance and the down-modulation of inflammatory responses are therefore pathways by which some NAbs may augment and amplify housekeeping functions that serve to protect the host. Studies of myeloid dendritic cells (DCs) have shown that the anti-inflammatory effects of the T15 IgM anti-AC antibody are mediated by induction, at a transcript and a protein level, of the prototypic dual-specificity phosphatase-1 (DUSP-1), also termed mitogen-activated protein kinase-1 (MKP-1), which can block activation of all three primary MAP kinases implicated in inflammatory responses.52
In vitro studies have shown that anti-AC IgM antibodies can directly block the activating effects of lupus-associated IgG autoantibodies on bone marrow–derived DCs.53 In fact, the inflammatory effects of both anti-DNA– and anti-RNA IgG–nucleic acid immune complexes in myeloid DCs were inhibited by suppression of the secretion of inflammatory cytokines IL-6 and TNF-α.53 This T15 IgM NAb also suppressed IC-mediated induction of cell surface expression of CD80 and CD86, as well as CD40 and other co-stimulatory molecules.
The immune-modulatory properties of IgM natural antibodies to ACs can also oppose the in vivo pathogenic influence of IgG autoantibody ICs. In vivo studies have shown that administration of anti-PC IgM greatly attenuates disease severity in a murine model of collagen-induced arthritis (CIA).50 In this model, immunization with xenogenic collagen type II (CII) emulsified in complete Freund’s adjuvant induces a pathogenic autoimmune response to CII,54 with tissue injury in part mediated through the activating Fcγ receptors.55 Infusions of IgM NAbs to ACs also blocked the disease process induced by passive transfer of anti–type II collagen autoantibodies,50 in which inflammatory arthritis is mediated by FcγR and innate immune cells. After antibodies/immune complexes are generated, lymphocytes do not play central roles. We have also found that infusions of purified T15 IgM antibodies, but not isotype control or saline, also significantly improve the survival of male NZW × BXSB F1 mice that otherwise develop an accelerated lupus-like syndrome with prominent autoimmune renal and cardiac pathology (manuscript in preparation).
Studies of human anti-PC responses
Our current knowledge of the structural features of human anti-PC antibodies is currently limited. Natural anti-PC antibodies are ubiquitous, but levels vary more than 100-fold among adults,56–58 and levels of anti-PC IgM are reported to directly correlate with recognition by serum antibodies of membrane determinants of ACs, suggesting these are a major source of human AC-binding antibodies.59 Moreover, high levels of anti-PC IgM have been correlated with protection from atherosclerotic cardiovascular events. To determine whether there is potential clinical relevance, Frostegard and coworkers studied a cohort of Swedish lupus patient60 and found that individuals with lower anti-PC IgM levels more frequently had cardiovascular events that included myocardial infarction and cerebrovascular events.61,62 In an adult SLE cohort from Johns Hopkins, we independently confirmed that lower levels of IgM PC antibodies, but not other IgM antibody specificities, were significantly correlated with a clinical history of cardiovascular events.57 We also found that lower levels of anti-PC IgM correlated with higher overall disease activity in SLE patients, based on the SLEDAI score (reviewed in Ref. 63).
To independently evaluate whether circulating anti-PC IgM could exert a protective influence that opposes the development of the vascular lesions of atherosclerotic cardiovascular disease, we looked for associations with measurement of subclinical disease using noninvasive carotid ultrasonography measurements, which have proven value in the estimation of future cardiovascular outcomes.64 In this cross-sectional SLE cohort, we confirmed that subclinical CV disease, as detected by carotid ultrasound, was associated with lower levels of anti-PC IgM, as well as lower levels of the ratio of anti-PC IgM/total IgM, compared to patients without plaque (P = 0.004 and P = 0.02, respectively). Moreover, the anti-PC IgM/total IgM ratio remained significant even after adjusting for age, cholesterol, and hypertension. Levels of adiponectin and soluble E-selectin (sE-selectin) were also significantly elevated in the patients with carotid plaque. E-selectin is known to play a role in mediating adhesion between endothelial cells and leukocytes, and increased levels of sE-selectin may reflect endothelial activation that occurs in inflammatory diseases. In contrast, the adipose-derived factor adiponectin is generally considered to be anti-inflammatory and atheroprotective, yet elevated adiponectin levels are often found in SLE patients, although the mechanistic implications are unclear. Notably, our statistical models showed that combining evaluating adiponectin and sE-selectin along with the anti-PC IgM/total IgM ratio was better at predicting plaque than the individual tests alone.64 These results support the hypothesis that IgM-natural autoantibodies may have the capacity to inhibit atherogenesis. Taken together, these data potentially further support the utility of IgM anti-PC levels as a biomarker for subclinical CV disease.
To investigate the structure–function relationships among human antibodies and PC determinants on ACs, we reasoned that the gene rearrangements responsible for these highly prevalent natural antibodies should be highly represented in all healthy adults. To isolate human antibody clones that recognize ACs, we used proven phage-display antibody technology in which there is a physical linkage between antigen-binding particles and the encoding somatically rearranged antibody genes.65 We therefore sought to select antibodies from a large library generated from human bone marrow that contains a cellular immune record of an individual’s lifetime antigenic experiences.66
Sequential rounds of selection were then performed with a library of phagemids displaying Fab antibodies with the repertoire of Ig transcripts expressed in the bone marrow of six healthy adult donors.66 The first round of selection used immobilized PC-protein antigen, with later rounds of selection using the surface of intact cells undergoing apoptotic death. Focusing on four of these selected phagemid clones, we first confirmed the PC binding reactivity and also showed binding to both early stage (7AAD− Annexin V+) and late stage (7AAD+ Annexin V+) ACs, while none of these antibodies showed detectable binding to freshly isolated healthy thymocytes.66 Interestingly, with features reminiscent of the T15 antibody, three of the four identified AC-reactive antibody clones had VH3 region rearrangements with germline configuration (although two had single mutations introduced by primers).66 Among the immune system differences between mice and humans, in the latter TdT appears to be expressed at all stages of B cell development,67 which could result in a more diversified human repertoire. One of the AC-reactive antibody clones was expressed as a complete polymeric IgM and retained binding reactivity for apoptotic thymocytes; AC binding by this antibody could be inhibited by soluble PC–BSA antigen but not control antigens.66
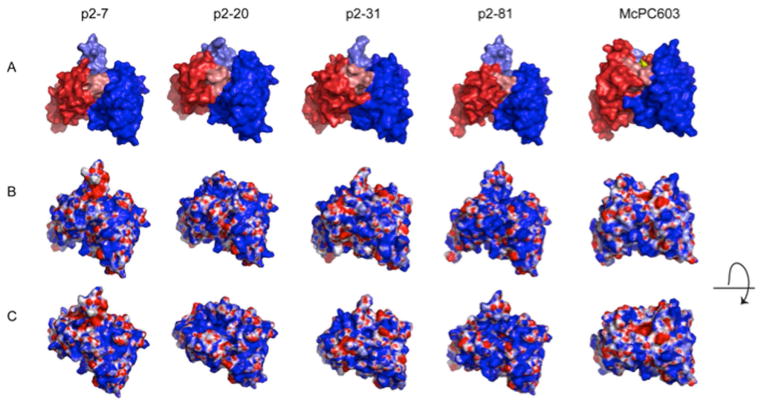
The physiologic relevance of these results was further documented when we found that polyclonal IgM antibodies in human umbilical cord plasma (from a newborn) bound to ACs, with binding significantly reduced by PC–BSA antigen blockade.66 These findings are therefore consistent with evidence that PC-reactive natural IgM antibodies contribute to AC binding in humans at birth. However, molecular modeling studies suggested that the topographic features, including the electrostatic surface features and the nature of the binding groove of these human anti-PC antibodies, were quite dissimilar to the archetypic T15 antibody (Fig. 2).66 These findings therefore suggest that there is a variety of ways for the human immune system to produce PC-reactive anti-AC antibodies. However, this specific set of human antibodies may have been biased in part by the initial round of phage-display selection, the bottleneck for in vitro clonal selection, as we used a non-physiologic experimental antigen, an albumin–PC conjugate. While we have not yet identified a human antibody that binds PC epitopes in exactly the same manner (and same epitope–paratope interaction) as the murine T15 antibody, we speculate that alternate selection strategies may enable isolation of anti-PC clones with very different structural and functional properties.
Figure 2.
Structure models of the variable region of the selected human PC antibodies. Models are shown for the anti-PC clones: p2–7, encoded by a VH3-30 and Vκ3-20; p2–20, encoded by a VH3-33 and Vλ1-44; p2–31, encoded by a VH1-2 and Vλ1-47, and p2–81, encoded by VH3-30 and Vκ1-5 rearrangements. The models are compared to the crystal structure of the PC-binding S107.1-encoded murine antibody McPC603, with the PC antigen in the binding pocket (PDB ID: 2MCP). (A) The VL region is visualized in red and the VH region in blue. HCDR3 is highlighted in lighter blue shade and LCDR3 in lighter red shade. (B) Electrostatic surface models of the variable regions. Blue color represents positively charged surface residues, red negatively charged residues. (C) Electrostatic surface models with a view looking into the potential antigen-binding site. Taken from Ref. 66.
Conclusions
The emergence within the B-1 cell tier of lymphocytic clonotypic sets with potential dual responsibilities for housekeeping functions and for anti-microbial protection appears to be integral to immune development. The T15 clone has recently been rediscovered, owing to expansions in mice with altered internal milieus (i.e., hyperlipidemia). Subsequent studies have illuminated homeostatic roles for enhancing clearance of damaged cells, and also for ameliorating inflammatory responses. We therefore hypothesize that certain recurrent clones within the B-1 pool may provide an added and overlaid regulatory layer that may serve to resist the development of inflammatory and autoimmune disease.
The specialized properties of these protective NAbs are linked to the right combination of antigen-binding site and antibody constant regions that provide effector functions. These autoreactive antibodies recognize altered-self determinants on ACs but not healthy cells, while optimal functional properties may be linked to the mu regions of these polymeric antibodies.68 Using phage-display technology, we recovered PC-reactive AC-binding antibodies from the repertoire of healthy humans.66 Like the murine T15 clone, these anti-PC antibodies can represent germline configuration of human VHIII clan genes, which share general homology with VHS107.1. Further investigations of fine specificity are required, as B cells can potentially recognize distinct sets of PC epitopes on damaged and dying cells.41
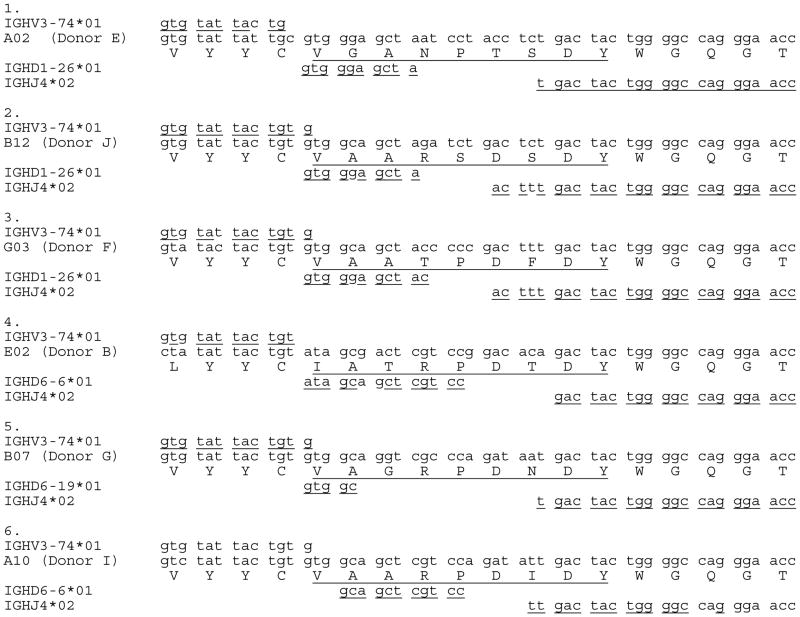
An alternative approach to recovery of anti-PC antibodies has also been reported by Fiskesund and coworkers, who used flow cytometric sorting of PC-reactive human peripheral B cells to isolate a panel of human MAbs.69 Aside from those derived from B cells with a naive phenotype, these anti-PC clones generally displayed significant levels of hypermutation. Yet the capacity of these antibodies to bind altered antigens, including ACs, was not investigated, so the effect on hypermutation recognition of self and microbial PC antigens is currently unknown. Unexpectedly, different adult donors had clones with very similar gene usage and HCDR3 amino acid sequences despite different somatically-generated CDR3 splice sites at a DNA level69 (Table 1). Hence, it appears that formation of human anti-PC antibodies generally requires the influence of somatic mechanisms for creating N insertions for non-templated junctional diversity.
Table I.
Junctional diversification and HCDR3 for stereotypic set of human anti-PC antibodies.
NOTE: Human monoclonal antibodies were recovered by flow cytometric sorting of phosphorylcholine-binding peripheral blood B cells from healthy donors, as recently reported.69 These anti-PC antibodies from different adult donors are proposed to be part of the same stereotypic set. While these share conserved HCDR3 amino acid structural features, each of these six human VH rearrangements appear to have been generated by a distinct molecular junctional diversification event, which include N insertions, which were not primary sequence–dependent somatic events. Here, HCDR3 is defined as starting after the invariant cysteine and before the invariant tryptophan. Alignments are shown with closest germline VH, DH, and JH gene segments, using Igblast. I thank Dr. Roland Fiskesund (Karolinska) for providing these DNA sequences, which are also depicted in Table II from Ref. 69.
These studies of human anti-PC antibodies evoke patterns first recognized in B cell chronic lymphocytic leukemia (CLL), a B-cell malignancy that has been argued to derive from a human B-1 cell analogue. Notably, most CLL clones express germline-configuration BCR genes, and there are major sets of CLL, from unrelated patients, with binding reactivity for the same apoptosis-associated set of antigens.70 Many CLL clones have highly similar stereotypical antibody gene sequences, even though these arise in different individuals. Although these do not display primary DNA sequence–directed rearrangements, such as described in the archetypic T15 clone, these recurrent sets of stereotypical sequences have features of convergent protein sequences in their CDR3s despite evidence that they arose from different DNA rearrangement events. Hence, these recurrent stereotypic sets of CLL BCR share CDR3 homology at an amino acid level, which suggests there has been in vivo selection by antigen interaction.69 Notably, in CLL the stereotypical clones often recognize MDA-containing compounds, not PC antigens, that are expressed also on ACs, with evidence that some clones are polyreactive for many other self-antigens.70 It has therefore been argued that during the pathogenesis of the disease an altered self-antigen(s) could be selecting for these leukemic clones.71
Our current understanding of the influences that potentially mold immune repertoire is highly biased by the methodologic experimental strategies that we use in our investigations. From one perspective, our findings provide clear evidence that PC reactivity by human antibodies can be encoded by germline configuration genes without hypermutation, indicating that these antibodies can be truly natural antibodies. However, phage-display methods, which randomly combine VH and VL regions, may be biased toward selection of antibodies in which one chain dominates the binding specificity, while the partner chain is permissive. Moreover, phage-display cloning may more readily recover antibodies that are highly represented among the sampled transcripts,72,73 and especially from plasma cells that are highly prevalent in the bone marrow, as used in this study.66 In contrast, for the flow cytometric approach used by Fiskesund and coworkers, the bias is based on B cell clonal frequency in the bloodstream.69 B cells bearing memory phenotypic markers would be predicted to often express hypermutated antibody genes.
The physicochemical properties of the specific form of PC antigen used for selection may also be important. For the PC–albumin conjugate, much of the surface of the albumin molecule displays an anionic charge, which may have contributed to the selection by phage display of antibodies with cationic surfaces (Fig. 2). This may therefore not reliably reproduce the subtle structural features of many apoptosis-associated PC determinants. Indeed we speculate that apoptosis-associated oxidative modification of PtC likely exposes PC determinants that are tethered by a long aliphatic chain. In the mouse, this may explain the apparent evolutionary selection of VHS107.1-encoded T15-related clones that generally have tunnel-like antigen-binding pits for PC determinants.24 Hence, each of these technical approaches may lead to biases in the antibodies selected; more studies are needed to provide a more complete understanding of the genetic and structural features of natural antibodies that recognize apoptotic-cell membrane determinants.
In recent years, advances in DNA-sequencing technology have enabled much more detailed surveys of the diversity within B cell repertoires. Identical twins, which share both inheritance of immunogenetic elements and presumably common antigenic exposures, display even higher levels of convergent IgH sequences (with public HCDR3) within their mutated antigen-experienced memory B cell compartments.74 Convergent somatic evolution has also been found in VH gene-sequencing studies of blood B cells from unrelated patients infected by dengue, as well as after vaccination for influenza.75,76 Becasue HCDR3 are often the most important contributors to antigen binding specificity,77 these convergent VH sequences may reflect in vivo antigenic selection by microbial antigens.
Convergent somatic evolution of B cell clones may therefore represent a common feature of the human repertoire in responses to both damaged and dying cells and to microbial pathogens, and in some cases also for clones with duality in their functional roles. Further investigation is therefore merited to determine whether some PC-reactive human antibodies that retain highly refined molecular specificity for epitopes on the membranes of ACs can convey the same levels of protective homeostatic and immunoregulatory properties as the prototypic murine B-1 cell NAb. Utilization of next-generation DNA sequencing approaches promises to advance our understanding of how B cell repertoires diversify during the perinatal to adult stages of immune development, in addition to better understanding the contributions of mechanisms responsible for somatic immune evolution of convergent stereotypic clones.
Acknowledgments
This work was supported by the National Institutes of Health (R0141090118, R01A1068063, American Recovery and Reinvestment Act supplement); the American College of Rheumatology Research Education Foundation Within Our Reach campaign; the Alliance for Lupus Research; the Arthritis Foundation; and the P. Robert Majumder Charitable Trust. Also, biostatistical support was provided in part by the NYU CTSA Grant ULlTR000038 from the NIH National Center for Advancing Translation Sciences (NCATS). We acknowledge the contributions of current and past collaborators, and especially Joseph Witztum, Christof Binder, and Wulf Palinski for studies performed at the University of California San Diego.
References
- 1.Avrameas S, Selmi C. Natural autoantibodies in the physiology and pathophysiology of the immune system. Journal of autoimmunity. 2013;41:46–49. doi: 10.1016/j.jaut.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nature reviews. Immunology. 2011;11:34–46. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- 3.Herzenberg LA, Herzenberg LA. Toward a layered immune system. Cell. 1989;59:953–954. doi: 10.1016/0092-8674(89)90748-4. [DOI] [PubMed] [Google Scholar]
- 4.Hayakawa K, et al. Positive selection of natural autoreactive B cells. Science. 1999;285:113–116. doi: 10.1126/science.285.5424.113. [DOI] [PubMed] [Google Scholar]
- 5.Cyster JG, et al. Regulation of B-lymphocyte negative and positive selection by tyrosine phosphatase CD45. Nature. 1996;381:325–328. doi: 10.1038/381325a0. [DOI] [PubMed] [Google Scholar]
- 6.Dighiero G, et al. High frequency of natural autoantibodies in normal newborn mice. J Immunol. 1985;134:765–771. [PubMed] [Google Scholar]
- 7.Merbl Y, et al. Newborn humans manifest autoantibodies to defined self molecules detected by antigen microarray informatics. J Clin Invest. 2007;117:712–718. doi: 10.1172/JCI29943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Griffin DO, Holodick NE, Rothstein TL. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+ CD27+ CD43+ CD70. J Exp Med. 2011;208:67–80. doi: 10.1084/jem.20101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li W, Batliwalla F, Rothstein TL. Human B-1 cells are not preplasmablasts: analysis of microarray data and other issues. Blood. 2013;122:3691–3693. doi: 10.1182/blood-2013-08-520031. [DOI] [PubMed] [Google Scholar]
- 10.Cohn M, Notani G, Rice SA. Characterization of the antibody to the C-carbohydrate produced by a transplantable mouse plasmacytoma. Immunochemistry. 1969;6:111–123. doi: 10.1016/0019-2791(69)90183-9. [DOI] [PubMed] [Google Scholar]
- 11.Potter M, Leon MA. Three IgA myeloma immunoglobulins from the BALB/mouse: precipitation with pneumococcal C polysaccharide. Science. 1968;162:369–371. doi: 10.1126/science.162.3851.369. [DOI] [PubMed] [Google Scholar]
- 12.Potter M. Immunoglobulin-producing tumors and myeloma proteins of mice. Physiological reviews. 1972;52:631–719. doi: 10.1152/physrev.1972.52.3.631. [DOI] [PubMed] [Google Scholar]
- 13.Feeney AJ. Predominance of the prototypic T15 anti-phosphorylcholine junctional sequence in neonatal pre-B cells. J Immunol. 1991;147:4343–4350. [PubMed] [Google Scholar]
- 14.Vakil M, Briles DE, Kearney JF. Antigen-independent selection of T15 idiotype during B-cell ontogeny in mice. Developmental immunology. 1991;1:203–212. doi: 10.1155/1991/45352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Solvason N, et al. The fetal omentum in mice and humans. A site enriched for precursors of CD5 B cells early in development. Annals of the New York Academy of Sciences. 1992;651:10–20. doi: 10.1111/j.1749-6632.1992.tb24589.x. [DOI] [PubMed] [Google Scholar]
- 16.Feeney AJ. Predominance of VH-D-JH junctions occurring at sites of short sequence homology results in limited junctional diversity in neonatal antibodies. J Immunol. 1992;149:222–229. [PubMed] [Google Scholar]
- 17.Perlmutter RM, et al. Developmentally controlled expression of immunoglobulin VH genes. Science. 1985;227:1597–1601. doi: 10.1126/science.3975629. [DOI] [PubMed] [Google Scholar]
- 18.Benedict CL, Kearney JF. Increased junctional diversity in fetal B cells results in a loss of protective anti-phosphorylcholine antibodies in adult mice. Immunity. 1999;10:607–617. doi: 10.1016/s1074-7613(00)80060-6. [DOI] [PubMed] [Google Scholar]
- 19.Masmoudi H, et al. All T15 Id-positive antibodies (but not the majority of VHT15+ antibodies) are produced by peritoneal CD5+ B lymphocytes. International immunology. 1990;2:515–520. doi: 10.1093/intimm/2.6.515. [DOI] [PubMed] [Google Scholar]
- 20.Silverman GJ, et al. Neo-self antigens and the expansion of B-1 cells: lessons from atherosclerosis-prone mice. Current topics in microbiology and immunology. 2000;252:189–200. doi: 10.1007/978-3-642-57284-5_20. [DOI] [PubMed] [Google Scholar]
- 21.Kearney JF. Innate-like B cells. Springer Semin Immunopathol. 2005;26:377–383. doi: 10.1007/s00281-004-0184-0. [DOI] [PubMed] [Google Scholar]
- 22.Lieberman R, et al. Genetics of a new IgVH (T15 idiotype) marker in the mouse regulating natural antibody to phosphorylcholine. J Exp Med. 1974;139:983–1001. doi: 10.1084/jem.139.4.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y, et al. IgM antibodies to apoptosis-associated determinants recruit C1q and enhance dendritic cell phagocytosis of apoptotic cells. J Immunol. 2009;182:6031–6043. doi: 10.4049/jimmunol.0804191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Satow Y, et al. Phosphocholine binding immunoglobulin Fab McPC603. An X-ray diffraction study at 2.7 A. Journal of molecular biology. 1986;190:593–604. doi: 10.1016/0022-2836(86)90245-7. [DOI] [PubMed] [Google Scholar]
- 25.Mi QS, et al. Highly reduced protection against Streptococcus pneumoniae after deletion of a single heavy chain gene in mouse. Proc Natl Acad Sci U S A. 2000;97:6031–6036. doi: 10.1073/pnas.110039497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDaniel LS, et al. Blood clearance by anti-phosphocholine antibodies as a mechanism of protection in experimental pneumococcal bacteremia. J Immunol. 1984;133:3308–3312. [PubMed] [Google Scholar]
- 27.Claflin JL, Lieberman R, Davie JM. Clonal nature of the immune response to phosphorylcholine. I. Specificity, class, and idiotype of phosphorylcholine-binding receptors on lymphoid cells. J Exp Med. 1974;139:58–73. doi: 10.1084/jem.139.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leon MA, Young NM. Specificity for phosphorylcholine of six murine myeloma proteins reactive with Pneumococcus C polysaccharide and beta-lipoprotein. Biochemistry. 1971;10:1424–1429. doi: 10.1021/bi00784a024. [DOI] [PubMed] [Google Scholar]
- 29.Claflin JL, Berry J. Genetics of the phosphocholine-specific antibody response to Streptococcus pneumoniae. Germ-line but not mutated T15 antibodies are dominantly selected. J Immunol. 1988;141:4012–4019. [PubMed] [Google Scholar]
- 30.Clark SE, Weiser JN. Microbial modulation of host immunity with the small molecule phosphorylcholine. Infection and immunity. 2013;81:392–401. doi: 10.1128/IAI.01168-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schenkein HA, et al. Phosphorylcholine-dependent cross-reactivity between dental plaque bacteria and oxidized low-density lipoproteins. Infection and immunity. 2001;69:6612–6617. doi: 10.1128/IAI.69.11.6612-6617.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sigal NH, et al. Expression of phosphorylcholine-specific B cells during murine development. J Exp Med. 1977;146:933–948. doi: 10.1084/jem.146.4.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sigal NH, Gearhart PJ, Klinman NR. The frequency of phosphorylcholine-specific B cells in conventional and germfree BALB/C mice. J Immunol. 1975;114:1354–1358. [PubMed] [Google Scholar]
- 34.Palinski W, et al. Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasma. J Clin Invest. 1996;98:800–814. doi: 10.1172/JCI118853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shaw PX, et al. Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. J Clin Invest. 2000;105:1731–1740. doi: 10.1172/JCI8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Binder CJ, Silverman GJ. Natural antibodies and the autoimmunity of atherosclerosis. Springer Semin Immunopathol. 2005;26:385–404. doi: 10.1007/s00281-004-0185-z. [DOI] [PubMed] [Google Scholar]
- 37.Boullier A, et al. The binding of oxidized low density lipoprotein to mouse CD36 is mediated in part by oxidized phospholipids that are associated with both the lipid and protein moieties of the lipoprotein. The Journal of biological chemistry. 2000;275:9163–9169. doi: 10.1074/jbc.275.13.9163. [DOI] [PubMed] [Google Scholar]
- 38.Chou MY, et al. Oxidation-specific epitopes are dominant targets of innate natural antibodies in mice and humans. J Clin Invest. 2009;119:1335–1349. doi: 10.1172/JCI36800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang C, et al. Natural antibodies of newborns recognize oxidative stress-related malondialdehyde acetaldehyde adducts on apoptotic cells and atherosclerotic plaques. International immunology. 2013;25:575–587. doi: 10.1093/intimm/dxt022. [DOI] [PubMed] [Google Scholar]
- 40.Binder CJ, et al. Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nature medicine. 2003;9:736–743. doi: 10.1038/nm876. [DOI] [PubMed] [Google Scholar]
- 41.Shaw PX, et al. The autoreactivity of anti-phosphorylcholine antibodies for atherosclerosis-associated neo-antigens and apoptotic cells. J Immunol. 2003;170:6151–6157. doi: 10.4049/jimmunol.170.12.6151. [DOI] [PubMed] [Google Scholar]
- 42.Friedman P, et al. Correlation of antiphospholipid antibody recognition with the structure of synthetic oxidized phospholipids. Importance of Schiff base formation and aldol condensation. The Journal of biological chemistry. 2002;277:7010–7020. doi: 10.1074/jbc.M108860200. [DOI] [PubMed] [Google Scholar]
- 43.Korb LC, Ahearn JM. C1q binds directly and specifically to surface blebs of apoptotic human keratinocytes: complement deficiency and systemic lupus erythematosus revisited. J Immunol. 1997;158:4525–4528. [PubMed] [Google Scholar]
- 44.Ogden CA, et al. C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J Exp Med. 2001;194:781–795. doi: 10.1084/jem.194.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paidassi H, et al. C1q binds phosphatidylserine and likely acts as a multiligand-bridging molecule in apoptotic cell recognition. J Immunol. 2008;180:2329–2338. doi: 10.4049/jimmunol.180.4.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fraser DA, et al. C1q differentially modulates phagocytosis and cytokine responses during ingestion of apoptotic cells by human monocytes, macrophages, and dendritic cells. J Immunol. 2009;183:6175–6185. doi: 10.4049/jimmunol.0902232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matsushita M, et al. Origin of the classical complement pathway: Lamprey orthologue of mammalian C1q acts as a lectin. Proc Natl Acad Sci U S A. 2004;101:10127–10131. doi: 10.1073/pnas.0402180101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stuart LM, et al. Mannose-binding lectin-deficient mice display defective apoptotic cell clearance but no autoimmune phenotype. J Immunol. 2005;174:3220–3226. doi: 10.4049/jimmunol.174.6.3220. [DOI] [PubMed] [Google Scholar]
- 49.Quartier P, et al. Predominant role of IgM-dependent activation of the classical pathway in the clearance of dying cells by murine bone marrow-derived macrophages in vitro. European journal of immunology. 2005;35:252–260. doi: 10.1002/eji.200425497. [DOI] [PubMed] [Google Scholar]
- 50.Chen Y, et al. Regulation of dendritic cells and macrophages by an anti-apoptotic cell natural antibody that suppresses TLR responses and inhibits inflammatory arthritis. J Immunol. 2009;183:1346–1359. doi: 10.4049/jimmunol.0900948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arnold JN, et al. Human serum IgM glycosylation: identification of glycoforms that can bind to mannan-binding lectin. The Journal of biological chemistry. 2005;280:29080–29087. doi: 10.1074/jbc.M504528200. [DOI] [PubMed] [Google Scholar]
- 52.Gronwall C, et al. MAPK phosphatase-1 is required for regulatory natural autoantibody-mediated inhibition of TLR responses. Proc Natl Acad Sci U S A. 2012;109:19745–19750. doi: 10.1073/pnas.1211868109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vas J, et al. Natural antibody to apoptotic cell membranes inhibits the proinflammatory properties of lupus autoantibody immune complexes. Arthritis Rheum. 2012;64:3388–3398. doi: 10.1002/art.34537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Terato K, et al. Induction of arthritis with monoclonal antibodies to collagen. J Immunol. 1992;148:2103–2108. [PubMed] [Google Scholar]
- 55.Kleinau S, Martinsson P, Heyman B. Induction and suppression of collagen-induced arthritis is dependent on distinct fcgamma receptors. J Exp Med. 2000;191:1611–1616. doi: 10.1084/jem.191.9.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brown M, Schiffman G, Rittenberg MB. Subpopulations of antibodies to phosphocholine in human serum. J Immunol. 1984;132:1323–1328. [PubMed] [Google Scholar]
- 57.Gronwall C, et al. IgM autoantibodies to distinct apoptosis-associated antigens correlate with protection from cardiovascular events and renal disease in patients with SLE. Clin Immunol. 2012;142:390–398. doi: 10.1016/j.clim.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Silverman GJ, et al. Genetic imprinting of autoantibody repertoires in systemic lupus erythematosus patients. Clin Exp Immunol. 2008;153:102–116. doi: 10.1111/j.1365-2249.2008.03680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Padilla ND, et al. Levels of natural IgM antibodies against phosphorylcholine in healthy individuals and in patients undergoing isolated limb perfusion. Journal of immunological methods. 2004;293:1–11. doi: 10.1016/j.jim.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 60.Su J, et al. Natural antibodies against phosphorylcholine as potential protective factors in SLE. Rheumatology (Oxford) 2008;47:1144–1150. doi: 10.1093/rheumatology/ken120. [DOI] [PubMed] [Google Scholar]
- 61.de Faire U, et al. Low levels of IgM antibodies to phosphorylcholine predict cardiovascular disease in 60-year old men: effects on uptake of oxidized LDL in macrophages as a potential mechanism. Journal of autoimmunity. 2010;34:73–79. doi: 10.1016/j.jaut.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 62.Fiskesund R, et al. Low levels of antibodies against phosphorylcholine predict development of stroke in a population-based study from northern Sweden. Stroke; a journal of cerebral circulation. 2010;41:607–612. doi: 10.1161/STROKEAHA.109.558742. [DOI] [PubMed] [Google Scholar]
- 63.Gronwall C, Vas J, Silverman GJ. Protective Roles of Natural IgM Antibodies. Frontiers in immunology. 2012;3:66. doi: 10.3389/fimmu.2012.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gronwall C, et al. Relation of carotid plaque with natural IgM antibodies in patients with systemic lupus erythematosus. Clin Immunol. 2014;157:1–7. doi: 10.1016/j.clim.2014.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smith GP. Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Science. 1985;228:1315–1317. doi: 10.1126/science.4001944. [DOI] [PubMed] [Google Scholar]
- 66.Gronwall C, et al. Selection of apoptotic cell specific human antibodies from adult bone marrow. PLoS One. 2014;9:e95999. doi: 10.1371/journal.pone.0095999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Asma GE, van den Bergh RL, Vossen JM. Characterization of early lymphoid precursor cells in the human fetus using monoclonal antibodies and anti-terminal deoxynucleotidyl transferase. Clin Exp Immunol. 1986;64:356–363. [PMC free article] [PubMed] [Google Scholar]
- 68.Ehrenstein MR, Notley CA. The importance of natural IgM: scavenger, protector and regulator. Nature reviews. Immunology. 2010;10:778–786. doi: 10.1038/nri2849. [DOI] [PubMed] [Google Scholar]
- 69.Fiskesund R, et al. Naturally Occurring Human Phosphorylcholine Antibodies Are Predominantly Products of Affinity-Matured B Cells in the Adult. J Immunol. 2014;192:4551–4559. doi: 10.4049/jimmunol.1303035. [DOI] [PubMed] [Google Scholar]
- 70.Catera R, et al. Chronic lymphocytic leukemia cells recognize conserved epitopes associated with apoptosis and oxidation. Mol Med. 2008;14:665–674. doi: 10.2119/2008-00102.Catera. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chu CC, et al. Chronic lymphocytic leukemia antibodies with a common stereotypic rearrangement recognize nonmuscle myosin heavy chain IIA. Blood. 2008;112:5122–5129. doi: 10.1182/blood-2008-06-162024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Silverman GJ, et al. Repertoire cloning of human lupus autoantibodies. Annals of the New York Academy of Sciences. 1995;764:565–566. doi: 10.1111/j.1749-6632.1995.tb55882.x. [DOI] [PubMed] [Google Scholar]
- 73.Roben P, et al. Repertoire cloning of lupus anti-DNA autoantibodies. J Clin Invest. 1996;98:2827–2837. doi: 10.1172/JCI119111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang C, et al. B-cell repertoire responses to varicella-zoster vaccination in human identical twins. Proc Natl Acad Sci U S A. 2015;112:500–505. doi: 10.1073/pnas.1415875112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Parameswaran P, et al. Convergent antibody signatures in human dengue. Cell host & microbe. 2013;13:691–700. doi: 10.1016/j.chom.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jackson KJ, et al. Human responses to influenza vaccination show seroconversion signatures and convergent antibody rearrangements. Cell host & microbe. 2014;16:105–114. doi: 10.1016/j.chom.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xu JL, Davis MM. Diversity in the CDR3 region of V(H) is sufficient for most antibody specificities. Immunity. 2000;13:37–45. doi: 10.1016/s1074-7613(00)00006-6. [DOI] [PubMed] [Google Scholar]